Abstract
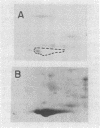
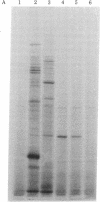
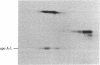
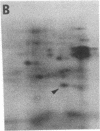
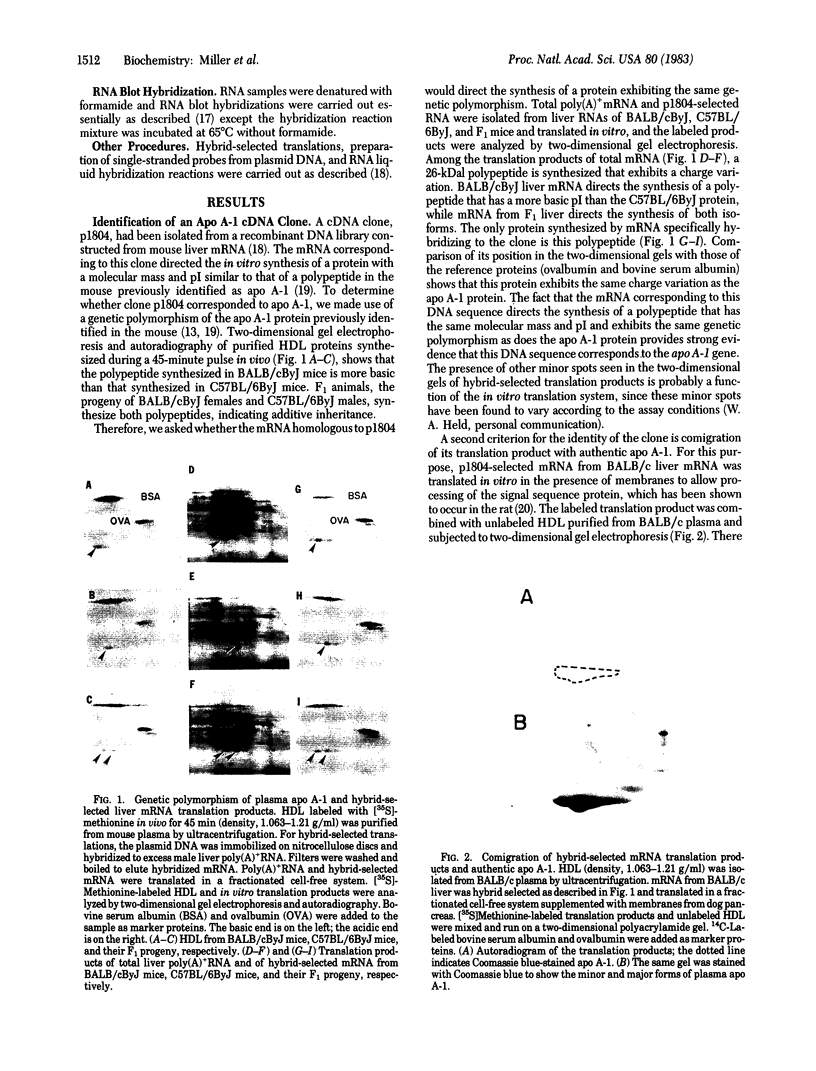
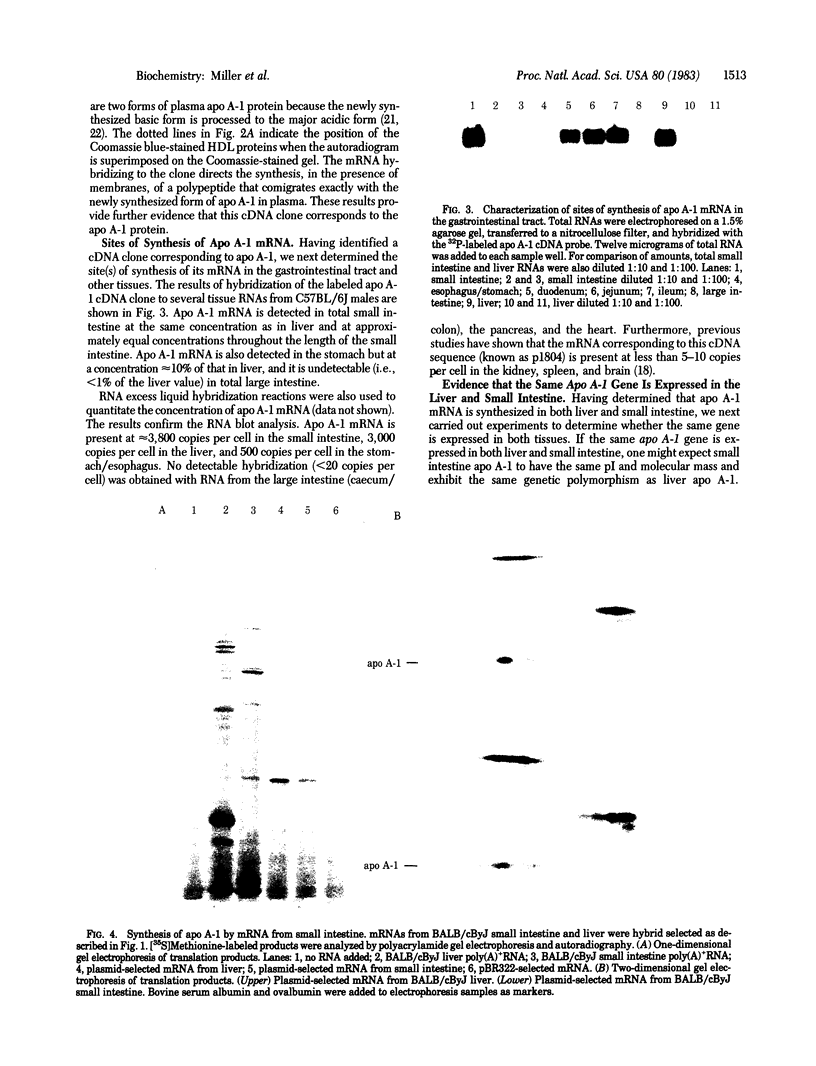
A cDNA clone for mouse apoprotein A-1 (apo A-1), the major apoprotein of plasma high density lipoproteins, has been identified. In addition to structural and physiological evidence, a genetic polymorphism for mouse plasma apo A-1 has been used to confirm that this DNA sequence corresponds to the apo A-1 gene. Use of this clone in molecular hybridization studies has shown that the concentration of apo A-1 mRNA is similar in liver and small intestine and is constant along the entire length of the small intestine. We provide evidence that the same apo A-1 gene is expressed in both liver and small intestine. Apo A-1 mRNA is also present in the stomach and esophagus at 10-15% the concentration found in small intestine but is undetectable in other tissues (such as large intestine, pancreas, heart, kidney, spleen, and brain). Finally, we show that there is a differential effect of a diet high in saturated fat and cholesterol on apo A-1 mRNA levels in liver and small intestine.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barth R. K., Gross K. W., Gremke L. C., Hastie N. D. Developmentally regulated mRNAs in mouse liver. Proc Natl Acad Sci U S A. 1982 Jan;79(2):500–504. doi: 10.1073/pnas.79.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. K., Brown M. S., Ho Y. K., Havel R. J., Goldstein J. L. Mouse macrophages synthesize and secrete a protein resembling apolipoprotein E. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7545–7549. doi: 10.1073/pnas.78.12.7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg K., Borresen A. L. Serum-high-density-lipoprotein and atherosclerotic heart-disease. Lancet. 1976 Mar 6;1(7958):499–501. doi: 10.1016/s0140-6736(76)90291-9. [DOI] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Eisenberg S., Levy R. I. Lipoprotein metabolism. Adv Lipid Res. 1975;13:1–89. [PubMed] [Google Scholar]
- Elliott R. W. Use of two-dimensional electrophoresis to identify and map new mouse genes. Genetics. 1979 Feb;91(2):295–308. doi: 10.1093/genetics/91.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding C. J., Shore V. G., Fielding P. E. A protein cofactor of lecithin:cholesterol acyltransferase. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1493–1498. doi: 10.1016/0006-291x(72)90776-0. [DOI] [PubMed] [Google Scholar]
- Glickman R. M., Green P. H. The intestine as a source of apolipoprotein A1. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2569–2573. doi: 10.1073/pnas.74.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glomset J. A. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968 Mar;9(2):155–167. [PubMed] [Google Scholar]
- Gordon J. I., Smith D. P., Andy R., Alpers D. H., Schonfeld G., Strauss A. W. The primary translation product of rat intestinal apolipoprotein A-I mRNA is an unusual preproprotein. J Biol Chem. 1982 Jan 25;257(2):971–978. [PubMed] [Google Scholar]
- Gordon T., Castelli W. P., Hjortland M. C., Kannel W. B., Dawber T. R. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977 May;62(5):707–714. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie N. D., Held W. A., Toole J. J. Multiple genes coding for the androgen-regulated major urinary proteins of the mouse. Cell. 1979 Jun;17(2):449–457. doi: 10.1016/0092-8674(79)90171-5. [DOI] [PubMed] [Google Scholar]
- Jackson R. L., Gotto A. M., Stein O., Stein Y. A comparative study on the removal of cellular lipids from Landschütz ascites cells by human plasma apolipoproteins. J Biol Chem. 1975 Sep 25;250(18):7204–7209. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Shapiro D. J., Taylor J. M., McKnight G. S., Palacios R., Gonzalez C., Kiely M. L., Schimke R. T. Isolation of hen oviduct ovalbumin and rat live albumin polysomes by indirect immunoprecipitation. J Biol Chem. 1974 Jun 25;249(12):3665–3671. [PubMed] [Google Scholar]
- Soutar A. K., Garner C. W., Baker H. N., Sparrow J. T., Jackson R. L., Gotto A. M., Smith L. C. Effect of the human plasma apolipoproteins and phosphatidylcholine acyl donor on the activity of lecithin: cholesterol acyltransferase. Biochemistry. 1975 Jul 15;14(14):3057–3064. doi: 10.1021/bi00685a003. [DOI] [PubMed] [Google Scholar]
- Stein O., Stein Y. The removal of cholesterol from Landschütz ascites cells by high-density apolipoprotein. Biochim Biophys Acta. 1973 Nov 29;326(2):232–244. doi: 10.1016/0005-2760(73)90249-x. [DOI] [PubMed] [Google Scholar]
- Stein O., Vanderhoek J., Stein Y. Cholesterol content and sterol synthesis in human skin fibroblasts and rat aortic smooth muscle cells exposed to lipoprotein-depleted serum and high density apolipoprotein/phospholipid mixtures. Biochim Biophys Acta. 1976 May 27;431(2):347–358. doi: 10.1016/0005-2760(76)90155-7. [DOI] [PubMed] [Google Scholar]
- Stein Y., Glangeaud M. C., Fainaru M., Stein O. The removal of cholesterol from aortic smooth muscle cells in culture and Landschutz ascites cells by fractions of human high-density apolipoprotein. Biochim Biophys Acta. 1975 Jan 24;380(1):106–118. doi: 10.1016/0005-2760(75)90049-1. [DOI] [PubMed] [Google Scholar]
- Zannis V. I., Breslow J. L., Katz A. J. Isoproteins of human apolipoprotein A-I demonstrated in plasma and intestinal organ culture. J Biol Chem. 1980 Sep 25;255(18):8612–8617. [PubMed] [Google Scholar]