Abstract
Anorexia nervosa (AN), a psychiatric disorder predominantly affecting young women, is characterized by self-imposed chronic nutritional deprivation and distorted body image. AN is associated with a number of medical co-morbidities including low bone mass. The low bone mass in AN is due to an uncoupling of bone formation and bone resorption, which is the result of hormonal adaptations aimed at decreasing energy expenditure during periods of low energy intake. Importantly, the low bone mass in AN is associated with a significant risk of fractures and therefore treatments to prevent bone loss are critical. In this review, we discuss the hormonal determinants of low bone mass in AN and treatments that have been investigated in this population.
Keywords: Anorexia nervosa, bone mineral density
Introduction
Anorexia nervosa (AN) is a psychiatric disease characterized by self-induced starvation coupled with a fear of gaining weight and a distorted body image [1]. AN predominantly affects young women and has a lifetime prevalence of 2.2% [2] but the disease also affects adolescent and adult males [3]. An increasing number of women over the age of 35 are also being treated for AN [4] and therefore the epidemiology of the disease may be shifting in the coming years.
AN has the one of the highest mortality rates of any psychiatric disorder [5] and is associated with a significant number of medical complications, including bradycardia, hypotension, anemia and the most common finding -- low bone mass [6]. Nearly 50% of women with AN have bone mineral density (BMD) values more than one-standard deviation below an age-comparable mean and an additional 30% have BMD values more than 2.5 standard deviations below an age-comparable mean [7, 6]. This low bone mass is associated with an increased risk of fracture. Thirty percent of women with AN report a history of a fracture [6] and a population-based retrospective study demonstrated the cumulative incidence of fracture to be 57%, even many years after the diagnosis of AN [8]. Importantly, a prospective study of young-women with AN demonstrated a seven-fold increased risk of fracture as compared to normal-weight controls [9]. Therefore, the low bone mass in AN is clinically significant.
There are a number of mechanisms which contribute to this low bone mass. As AN in characterized by decreased nutrient intake, it has been hypothesized that decreased intake of calcium and vitamin D may be important mediators of bone loss. However, girls and women with AN have higher intakes of both calcium and vitamin D, predominantly through supplements, as compared to normal weight controls and therefore this is not an important cause of bone loss in this disease [10, 11]. The bone loss in AN is the consequence of a response by hormonal pathways, including neuroendocrine axes and appetite-regulating hormones, to preserve energy and decrease energy expenditure in a state of nutritional deprivation. In adults with AN, the result is an uncoupling of bone formation and bone resoprtion with a profound decrease in bone formation and an increase in bone resorption [12, 13]. We will review these hormonal changes, their effects on BMD and potential treatment options below.
Hormonal mediators of the low bone mass in AN
Anorexia nervosa is a state of growth hormone resistance
States of nutritional deprivation are characterized by growth hormone (GH) resistance. Individuals with AN have elevated levels of GH coincident with low levels of insulin-like growth factor I (IGF-I) – an important anabolic hormone which mediates many of the growth-promoting actions of GH in the periphery, including bone metabolism [14-17]. IGF-I levels are extremely sensitive to nutritional cues – acute nutritional deprivation, in the form of four days of fasting, results in a 50% decrease in IGF-I levels [18]. Chronic nutritional deprivation, as seen in AN, also results in similarly low IGF-I levels – levels in AN are approximately 50% of those of normal-weight women [19]. The low IGF-I levels in AN also respond acutely to re-feeding with IGF-I levels increasing by approximately 50% after only three days of hyper-alimentation therapy [20]. Growth hormone resistance is likely an adaptive response to the state of under-nutrition. Low IGF-I levels preserve energy by decreasing expenditures on growth, including expenditures on the growth and maintenance of bone mass. Elevated GH levels are likely the result of both positive feedback on the pituitary from the low IGF-I levels [21], as well as ghrelin-stimulated GH secretion. Levels of ghrelin – an appetite-stimulating (orexigenic) hormone secreted by cells in the fundus of the stomach – are elevated in AN [22-24]. Ghrelin receptors stimulate GH secretion and the elevated ghrelin levels in states of fasting and chronic under-nutrition may be an important means of maintaining euglycemia [25]. Elevated GH levels may also be important for mobilizing fat stores in states of nutritional deprivation [26]. Therefore elevated GH levels in AN may be necessary for energy mobilization, while the low IGF-I levels help decrease energy expenditure.
IGF-I has significant bone anabolic effects and the low levels of IGF-I are therefore an important contributor to the low bone mass in AN. IGF-I is a chemotactic factor which induces osteoblast recruitment [27] and in cultured rat calvaria, stimulates DNA synthesis and increases bone collagen content [28]. The effects of treatment with recombinant human IGF-I on bone turnover markers and BMD have been investigated in AN. Recombinant human IGF-I increases levels of bone formation markers in girls and women with AN [29-31], and in women with AN increases lumbar spine BMD by 1.8% when used in conjunction with oral contraceptive pills. [29].
The mechanism responsible for the GH resistance state in AN is unknown, but recent studies suggest that fibroblast growth factor 21 (FGF-21) and sirtuin 1 (SIRT1) may play important roles. FGF-21 is a hormone produced in the liver [32] and adipocytes [33]. Transgenic FGF-21 mice have phenotypic characteristics similar to women with AN – they weigh less and have a lower core body temperature than wild-type litter mates [34, 35], and importantly, they are GH resistant with elevated levels of GH and low levels of IGF-I [36]. The mechanism by which FGF-21 transgenic mice become GH resistant is thought to be a decrease in STAT5 – a transcription factor important in GH signaling [36]. In adolescent girls with AN, FGF-21 levels are higher than normal-weight controls after controlling for body fat and insulin resistance – two important factors which may affect FGF-21 levels [37]. There is also a significant association between FGF-21 levels and GH area under the curve and an inverse association between elevated FGF-21 levels and IGF-I [37], suggesting that FGF-21 may be an important mediator of GH resistance in AN.
SIRT1 is a histone deacetylase which promotes fatty acid oxidation and gluconeogenesis during fasting and nutrient deprivation [38]. Similar to FGF-21, SIRT1 is thought to be an important mediator of GH resistance by decreasing STAT5 phosphorylation [39]. During a 48 hour fast, mice that lacked SIRT1 had higher GH-dependent increases in IGF-I and higher IGF-I mRNA levels compared to wild-type mice, suggesting that SIRT1 may be an important factor in GH resistance during starvation [39].
Lastly, low insulin levels, which are characteristic of states of under-nutrition, may also play a role in GH resistance. In animal models, GH receptor expression is reduced in low insulin states and expression is increased in response to insulin therapy [40]. GH receptor up-regulation by insulin has also been demonstrated in in vitro models [41]. Therefore low insulin levels may also play a role in the GH resistance state of AN.
Hypogonadotropic hypogonadism
Amenorrhea due to hypogonadotropic hypogonadism is a common finding in women with AN. Amenorrheic women with AN have low amplitude luteinizing hormone (LH) pulsatility, which is a secretory pattern similar to that of pre-pubertal girls and this abnormal pattern normalizes with weight recovery [42]. The LH response to gonadotropin-releasing hormone (GnRH) is normal with a normal or exaggerated follicle-stimulating hormone response and therefore an abnormal GnRH secretory pattern is the likely cause of amenorrhea in AN [43-46]. Similar to the effects of estrogen deficiency in post-menopausal women, hypogonadotropic hypogonadism results in an increase bone resorption [47]. One might expect that estrogen replacement in women with AN would result in increases in BMD but two randomized, placebo-controlled studies have demonstrated that oral contraceptives do not improve BMD [48, 29], except in the subset of women who were very low weight (less than 70% of ideal body weight) in a post-hoc analysis. Recently, a study investigating the effects of physiologic estrogen replacement in adolescent girls with AN demonstrated a 2.6% increase in spine BMD after 18 months of treatment [49] (Figure 1). This finding suggests that physiologic doses of estrogen – which in the majority of girls in the study consisted of 100 μg of transdermal 17β-estradiol applied twice weekly -- but not the supraphysiologic doses of estrogen in oral contraceptive pills, may be beneficial for the treatment of the low bone mass in AN.
Figure 1.
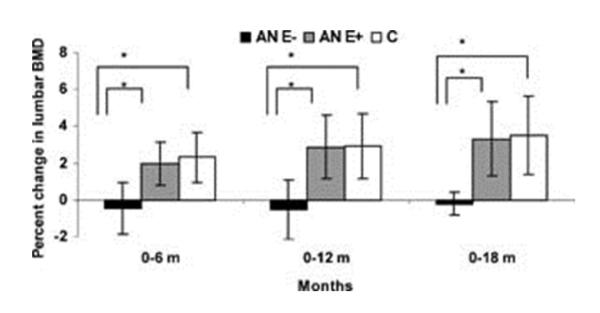
Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. Percent change in lumbar spine bone mineral density (LBMD) in adolescent girls with anorexia nervosa (AN) randomized to placebo (AN E–; black bars), girls with AN randomized to estrogen (AN E+; gray bars), and normal-weight control girls (C; white bars). AN E+ girls had significant increases in LBMD at 6, 12, and 18 months compared with AN E– girls. When compared with control girls, AN E– girls had significant decreases in LBMD at 6, 12, and 18 months, whereas AN E+ girls did not differ from control girls for changes in BMD over time. Analysis was performed for differences between means for pairs. *p < 0.05. Borrowed with permission from [49] © 2011, John Wiley and Sons.
Levels of testosterone, another gonadal steroid, are also low in women with AN [50, 51] and these low levels predict low BMD [51]. Therefore, one might expect that testosterone replacement would result in increases in BMD in women with AN. A randomized, placebo-controlled study investigating the effects of 12 months of testosterone replacement in women with AN demonstrated no improvements in BMD [52], suggesting that other factors may play a more significant role in the bone loss in AN.
Disruption of the hypothalamic-pituitary-adrenal axis
Adrenal androgen precursor levels are also low in AN. Dehydroepiandrosterone (DHEA) levels are decreased in women with AN [53] and are a predictor of low BMD [51] and increased bone resorption [54]. The effects of DHEA replacement on BMD in AN have been investigated. Twelve months of treatment with DHEA does not improve BMD as compared to placebo [55], whereas DHEA given in conjunction with oral contraceptives for 18 months results in maintenance of BMD in girls and women with AN [56], again suggesting that factors other than androgens play a more significant role in the low bone mass state of AN.
Girls and women with AN have elevated cortisol levels [57, 58], which are an important contributor to the low bone mass in AN; elevated 12-hour pooled cortisol levels are associated with low BMD in AN even after controlling for duration of amenorrhea and IGF-I levels, suggesting an important independent effect of hypercortisolemia [58]. Cortisol levels are likely increased due to activation of a stress response induced by chronic nutritional deprivation and are also a means of maintaining euglycemia via a counter-regulatory response pathway [59], but decreased metabolic clearance of cortisol has also been reported [60]. Elevated cortisol levels affect BMD in a number of ways. First, hypercortisolemia may decrease calcium absorption in the intestine [61]. Elevated cortisol levels also decrease bone formation; glucocorticoid receptors found on osteoblasts may reduce osteoblast proliferation [62] and elevated cortisol levels may inhibit GH secretion and decrease IGF-I synthesis in bone [63]. In adolescent girls with AN, the relationship between elevated cortisol levels and decreased bone formation is evident from the fact that high cortisol levels are associated with lower levels of osteocalcin and C-terminal propeptide Type 1 procollagen – markers of bone formation [57]. Lastly, hypercortisolemia also increases bone resorption; elevated cortisol levels may increase bone resorption via parathyroid hormone (PTH) by increasing PTH receptor expression on osteoblasts [64] and also by decreasing gonadotropin secretion [65], thereby contributing to the state of hypogonadotropic hypogonadism characteristic of AN.
Non-thyroidal illness syndrome
Women with AN have thyroid hormone levels consistent with the non-thyroidal illness syndrome, previously called the euthyroid-sick syndrome. In AN, levels of T4 and T3 are low or low-normal, with elevated levels of reverse T3 and normal levels of thyroid stimulating hormone (TSH) [66-69]. In AN, low T3 levels are due in part to decreased peripheral conversion of T4 to T3 [66, 67]. Exogenous TRH stimulation results in a delayed but quantitatively appropriate TSH response [67, 70, 71], suggesting that decreased TRH stimulation from the hypothalamus is contributing to the non-thyroidal illness state. These mechanisms -- which are likely an adaptive response allowing the body to decrease its metabolic rate and therefore energy expenditure, as suggested by the fact that increases in T3 with weight recovery are associated with increases in resting energy expenditure [72] -- reverse with weight gain [70, 73].
States of hyperthyroidism have been associated with loss of bone mass [74], therefore whether the non-thyroidal illness syndrome contributes to the low bone mass in AN is controversial. Thyroid hormone receptor knockout mice exhibit decreased trabecular BMD and elevated levels of marrow fat [75], similar to women with anorexia nervosa [76]. Thyroid hormone receptors are found on osteoblasts [77] and levels of IGF-I, an important stimulator of bone formation, increase after treatment of hypothyroidism [78], both of which suggest that thyroid hormone may be an important contributor to bone mass. Yet, although there is some evidence suggesting that low levels of thyroid hormone may contribute to the low bone mass in AN, the fact that that the non-thyroidal illness syndrome results in a decrease in energy expenditure and treatment would disadvantageously increase energy expenditure in a state of under-nutrition, makes thyroid hormone a highly undesirable treatment option in AN.
Appetite regulating hormones
Hypoleptinemia
Girls and women with AN have decreased levels of subcutaneous and visceral adipose tissue. Levels of leptin, an anorexigenic hormone secreted by adipose tissue, primarily subcutaneous adipose tissue, are low in AN [79, 80]. Leptin is likely the key mediator between energy expenditure and hypogonadotropic hypogonadism in AN; women with AN who are not amenorrheic have higher leptin levels as compared to those who are amenorrheic [81]. Importantly, low leptin levels are associated with low BMD [82] and abnormalities in microarchitectural parameters [83] in AN. Administration of recombinant human leptin to women with hypothalamic amenorrhea results in increased levels of osteocalcin and bone-specific alkaline phosphatase – two markers of bone formation, although a major effect of treatment was weight loss [84]. Therefore, although leptin may be a mediator of the low bone mass in AN and treatment with recombinant human leptin may stimulate bone formation, this is not an appropriate potential treatment for individuals with AN, given the associated weight loss.
Elevated Peptide YY levels
Peptide YY (PYY) is another anorexigenic hormone but contrary to what we might expect in normal physiology, levels are elevated in girls and women with AN [85, 86] and therefore may contribute to the decreased nutrient intake characteristic of AN. In animal models, mice lacking PYY’s receptor – the Y2 receptor – have increased trabecular bone parameters [87], suggesting that PYY may be a negative regulator of trabecular bone. In girls and women with AN, PYY has also been shown to be inversely associated with BMD [86, 88] suggesting that this may be an important mediator of the low bone mass in AN.
Elevated ghrelin levels
Levels of ghrelin, an appetite-stimulating hormone secreted by cells in the stomach, are elevated in girls and women with AN [22-24] and ghrelin levels decrease with weight gain in AN [22]. Ghrelin induces GH secretion and therefore the elevated ghrelin levels in AN also contribute to the higher GH levels characteristic of the GH resistance state. In animal and in vitro models, ghrelin stimulates bone formation. Ghrelin’s receptor, the growth hormone secretagogue receptor 1a, is present in rat osteoblast-like cells and treatment with ghrelin results in a dose-dependent increase in the osteoblast-like cells [89]. In rats, ghrelin administration also increases BMD [89] and in humans, a significant positive association between BMD and ghrelin is observed in normal-weight adolescent girls -- even after controlling for cortisol levels, body composition and GH/IGF-I levels -- but not in girls with AN [90]. In fact, in girls with AN, ghrelin is inversely associated with BMD [88]. Therefore the association between ghrelin and BMD may be disrupted in AN.
Adiponectin
Although adiponectin is secreted by adipocytes, levels are lower in obesity as compared to normal-weight individuals. In AN, levels of adiponectin have been shown to be higher, lower and comparable to normal-weight controls [91-93] but after controlling for fat-mass, levels have been shown to be higher in AN [91]. Adiponectin may also be a mediator of low bone mass in AN. Adiponectin stimulates RANK-ligand, an osteoclast activator, and also decreases osteoprotegrin – a decoy receptor for RANK-ligand which inhibits its osteoclast-activating effects [94]. In girls with AN, adiponectin has been shown to be inversely associated with BMD [91], again suggesting that this adipokine may be an important contributor to the bone loss characteristic of AN.
Potential treatments for the low bone mass in AN (Table 1)
Table 1.
Therapies that have been investigated to treat the low bone mass in anorexia nervosa and their effect on bone mineral density (BMD)
| Therapy | Change in BMD | Duration of treatment | References |
|---|---|---|---|
| Oral contraceptives | No change compared to placebo except in women less than 70% ideal body weight |
Mean of 1-1.5 years | [48, 29] |
| Physiologic estrogen replacement | 2.6% increase in spine BMD | 1.5 years | [49] |
| IGF-I + oral contraceptives | 1.8% increase in spine BMD | 9 months | [29] |
| Testosterone | No change compared to placebo | 1 year | [52] |
| DHEA | No change compared to placebo | 1 year | [55] |
| DHEA + oral contraceptives | Maintenance of BMD as compared to loss of BMD in placebo group |
1.5 years | [55] |
| Bisphosphonates | 2% increase in hip BMD 3-4% increase in spine BMD |
1 year | [52] |
AN is a chronic disease and only approximately 50% of women with AN recover even many years after their initial diagnosis [95]. In those who gain weight and resume their menses, the mean annual increase in BMD is 1.8% at the hip and 3.1% at the spine [96]. In those who remain low weight and amenorrheic, the annual rate of decline is -2.4% at the hip and -2.6% at the spine [96]. Therefore finding an effective therapy for the treatment of bone loss in AN is critical. As previously discussed, supra-physiologic doses of estrogen, in the form of oral contraceptives, do not improve BMD as compared to placebo except in women who are very low weight [48, 29], whereas physiologic, primarily transdermal estrogen replacement in adolescents increases spine BMD by approximately 2.6% after 18 months of treatment [49] (Figure 1). Replacement of androgens, in the form of testosterone or DHEA, a pre-androgen, does not improve BMD as compared to placebo [52], although, when combined with an oral contraceptive, DHEA is able to maintain BMD in girls and women with AN [56]. The only other treatments which have demonstrated a benefit in adult women with AN are the anabolic agent, IGF-I, in combination with oral contraceptives, which increased spine BMD by 1.8% after 9 months [29] and risedronate, a bisphosphonate, which demonstrated a 2-4% increase in spine and hip BMD after 12 months of treatment [52]. Importantly, none of these therapies have led to normalization of BMD in girls or women with AN, and the effect of these treatments on fracture risk has not been investigated.
Conclusions
Anorexia nervosa, a state of chronic under-nutrition, is characterized by hormonal dysregulation which results in significant bone loss. The associated hormonal abnormalities, which include GH resistance, hypogonadotropic hypogonadism, hypercortisolemia, and the non-thyroidal illness syndrome, are likely an adaptive response to preserve energy in a state of chronic under-nutrition. The low bone mass, which is a consequence of this hormonal dysregulation, is clinically very important and associated with a significant increased risk of fracture [8]. There are currently no approved treatments for the bone loss associated with AN but a number of potential therapies have been investigated. Physiologic estrogen replacement in adolescents , bisphosphonates and the combination of recombinant human IGF-I and oral contraceptives in adults have been shown to increase BMD in AN by 1.8% to 4%. These treatments do not result in normalization of BMD and importantly, we do not know the effect of treatment on risk of fracture. Therefore further studies, are necessary to investigate potential treatment options for the bone loss associated with AN.
Acknowledgments
PK Fazeli has received research support from the NIH (K23 DK094820).
A Klibanski has received research supplies from Ipsen (drug and placebo only for study) and Eli Lilly (drug and placebo only for study); and research support from the NIH (R24 DK092759).
Footnotes
Conflict of Interest
Human and Animal Rights and Informed Consent
All studies by PK Fazeli and A Klibanski involving animal and/or human subjects were performed after approval by the appropriate institutional review boards. When required, written informed consent was obtained from all participants.
References
Papers of particular interest, published recently, have been highlighted as:
•• Of major importance
• Of importance
- 1.Association AP. Diagnostic and statistical manual of mental disorders (DSM-IV) 4th Washington, DC: 1994. [Google Scholar]
- 2.Keski-Rahkonen A, Hoek HW, Susser ES, Linna MS, Sihvola E, Raevuori A, et al. Epidemiology and course of anorexia nervosa in the community. The American journal of psychiatry. 2007;164(8):1259–65. doi: 10.1176/appi.ajp.2007.06081388. doi:10.1176/appi.ajp.2007.06081388. [DOI] [PubMed] [Google Scholar]
- 3.Lucas AR, Crowson CS, O'Fallon WM, Melton LJ., 3rd The ups and downs of anorexia nervosa. The International journal of eating disorders. 1999;26(4):397–405. doi: 10.1002/(sici)1098-108x(199912)26:4<397::aid-eat5>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Trickey H. Eating disorders exact toll on adults, too. 2006 wwwcnncom.
- 5.Harris EC, Barraclough B. Excess mortality of mental disorder. The British journal of psychiatry : the journal of mental science. 1998;173:11–53. doi: 10.1192/bjp.173.1.11. [DOI] [PubMed] [Google Scholar]
- 6.Miller KK, Grinspoon SK, Ciampa J, Hier J, Herzog D, Klibanski A. Medical findings in outpatients with anorexia nervosa. Archives of internal medicine. 2005;165(5):561–6. doi: 10.1001/archinte.165.5.561. doi:10.1001/archinte.165.5.561. [DOI] [PubMed] [Google Scholar]
- 7.Grinspoon S, Thomas E, Pitts S, Gross E, Mickley D, Miller K, et al. Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Annals of internal medicine. 2000;133(10):790–4. doi: 10.7326/0003-4819-133-10-200011210-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucas AR, Melton LJ, 3rd, Crowson CS, O'Fallon WM. Long-term fracture risk among women with anorexia nervosa: a population-based cohort study. Mayo Clinic proceedings Mayo Clinic. 1999;74(10):972–7. doi: 10.4065/74.10.972. doi:10.4065/74.10.972. [DOI] [PubMed] [Google Scholar]
- 9.Rigotti NA, Neer RM, Skates SJ, Herzog DB, Nussbaum SR. The clinical course of osteoporosis in anorexia nervosa. A longitudinal study of cortical bone mass. JAMA : the journal of the American Medical Association. 1991;265(9):1133–8. [PubMed] [Google Scholar]
- 10.Misra M, Tsai P, Anderson EJ, Hubbard JL, Gallagher K, Soyka LA, et al. Nutrient intake in community-dwelling adolescent girls with anorexia nervosa and in healthy adolescents. The American journal of clinical nutrition. 2006;84(4):698–706. doi: 10.1093/ajcn/84.4.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haagensen AL, Feldman HA, Ringelheim J, Gordon CM. Low prevalence of vitamin D deficiency among adolescents with anorexia nervosa. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2008;19(3):289–94. doi: 10.1007/s00198-007-0476-z. doi:10.1007/s00198-007-0476-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stefanis N, Mackintosh C, Abraha HD, Treasure J, Moniz C. Dissociation of bone turnover in anorexia nervosa. Annals of clinical biochemistry. 1998;35:709–16. doi: 10.1177/000456329803500602. Pt 6. [DOI] [PubMed] [Google Scholar]
- 13.Soyka LA, Grinspoon S, Levitsky LL, Herzog DB, Klibanski A. The effects of anorexia nervosa on bone metabolism in female adolescents. The Journal of clinical endocrinology and metabolism. 1999;84(12):4489–96. doi: 10.1210/jcem.84.12.6207. [DOI] [PubMed] [Google Scholar]
- 14.Garfinkel PE, Brown GM, Stancer HC, Moldofsky H. Hypothalamic-pituitary function in anorexia nervosa. Archives of general psychiatry. 1975;32(6):739–44. doi: 10.1001/archpsyc.1975.01760240067005. [DOI] [PubMed] [Google Scholar]
- 15.Scacchi M, Pincelli AI, Caumo A, Tomasi P, Delitala G, Baldi G, et al. Spontaneous nocturnal growth hormone secretion in anorexia nervosa. The Journal of clinical endocrinology and metabolism. 1997;82(10):3225–9. doi: 10.1210/jcem.82.10.4275. [DOI] [PubMed] [Google Scholar]
- 16.Stoving RK, Veldhuis JD, Flyvbjerg A, Vinten J, Hangaard J, Koldkjaer OG, et al. Jointly amplified basal and pulsatile growth hormone (GH) secretion and increased process irregularity in women with anorexia nervosa: indirect evidence for disruption of feedback regulation within the GH-insulin-like growth factor I axis. The Journal of clinical endocrinology and metabolism. 1999;84(6):2056–63. doi: 10.1210/jcem.84.6.5734. [DOI] [PubMed] [Google Scholar]
- 17.Misra M, Miller KK, Bjornson J, Hackman A, Aggarwal A, Chung J, et al. Alterations in growth hormone secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. The Journal of clinical endocrinology and metabolism. 2003;88(12):5615–23. doi: 10.1210/jc.2003-030532. [DOI] [PubMed] [Google Scholar]
- 18.Grinspoon SK, Baum HB, Peterson S, Klibanski A. Effects of rhIGF-I administration on bone turnover during short-term fasting. The Journal of clinical investigation. 1995;96(2):900–6. doi: 10.1172/JCI118137. doi:10.1172/JCI118137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Counts DR, Gwirtsman H, Carlsson LM, Lesem M, Cutler GB., Jr The effect of anorexia nervosa and refeeding on growth hormone-binding protein, the insulin-like growth factors (IGFs), and the IGF-binding proteins. The Journal of clinical endocrinology and metabolism. 1992;75(3):762–7. doi: 10.1210/jcem.75.3.1381372. [DOI] [PubMed] [Google Scholar]
- 20.Hotta M, Fukuda I, Sato K, Hizuka N, Shibasaki T, Takano K. The relationship between bone turnover and body weight, serum insulin-like growth factor (IGF) I, and serum IGF-binding protein levels in patients with anorexia nervosa. The Journal of clinical endocrinology and metabolism. 2000;85(1):200–6. doi: 10.1210/jcem.85.1.6321. [DOI] [PubMed] [Google Scholar]
- 21.Gianotti L, Pincelli AI, Scacchi M, Rolla M, Bellitti D, Arvat E, et al. Effects of recombinant human insulin-like growth factor I administration on spontaneous and growth hormone (GH)-releasing hormone-stimulated GH secretion in anorexia nervosa. The Journal of clinical endocrinology and metabolism. 2000;85(8):2805–9. doi: 10.1210/jcem.85.8.6743. [DOI] [PubMed] [Google Scholar]
- 22.Otto B, Cuntz U, Fruehauf E, Wawarta R, Folwaczny C, Riepl RL, et al. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. European journal of endocrinology / European Federation of Endocrine Societies. 2001;145(5):669–73. [PubMed] [Google Scholar]
- 23.Misra M, Miller KK, Herzog DB, Ramaswamy K, Aggarwal A, Almazan C, et al. Growth hormone and ghrelin responses to an oral glucose load in adolescent girls with anorexia nervosa and controls. The Journal of clinical endocrinology and metabolism. 2004;89(4):1605–12. doi: 10.1210/jc.2003-031861. [DOI] [PubMed] [Google Scholar]
- 24.Misra M, Miller KK, Kuo K, Griffin K, Stewart V, Hunter E, et al. Secretory dynamics of ghrelin in adolescent girls with anorexia nervosa and healthy adolescents. American journal of physiology Endocrinology and metabolism. 2005;289(2):E347-56. doi: 10.1152/ajpendo.00615.2004. doi:10.1152/ajpendo.00615.2004. [DOI] [PubMed] [Google Scholar]
- 25.Zhao TJ, Liang G, Li RL, Xie X, Sleeman MW, Murphy AJ, et al. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(16):7467–72. doi: 10.1073/pnas.1002271107. doi:10.1073/pnas.1002271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gahete MD, Cordoba-Chacon J, Luque RM, Kineman RD. The rise in growth hormone during starvation does not serve to maintain glucose levels or lean mass but is required for appropriate adipose tissue response in female mice. Endocrinology. 2013;154(1):263–9. doi: 10.1210/en.2012-1849. doi:10.1210/en.2012-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakasaki M, Yoshioka K, Miyamoto Y, Sasaki T, Yoshikawa H, Itoh K. IGF-I secreted by osteoblasts acts as a potent chemotactic factor for osteoblasts. Bone. 2008;43(5):869–79. doi: 10.1016/j.bone.2008.07.241. doi:10.1016/j.bone.2008.07.241. [DOI] [PubMed] [Google Scholar]
- 28.Canalis E. Effect of insulinlike growth factor I on DNA and protein synthesis in cultured rat calvaria. The Journal of clinical investigation. 1980;66(4):709–19. doi: 10.1172/JCI109908. doi:10.1172/JCI109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grinspoon S, Thomas L, Miller K, Herzog D, Klibanski A. Effects of recombinant human IGF-I and oral contraceptive administration on bone density in anorexia nervosa. The Journal of clinical endocrinology and metabolism. 2002;87(6):2883–91. doi: 10.1210/jcem.87.6.8574. [DOI] [PubMed] [Google Scholar]
- 30.Grinspoon S, Baum H, Lee K, Anderson E, Herzog D, Klibanski A. Effects of short-term recombinant human insulin-like growth factor I administration on bone turnover in osteopenic women with anorexia nervosa. The Journal of clinical endocrinology and metabolism. 1996;81(11):3864–70. doi: 10.1210/jcem.81.11.8923830. [DOI] [PubMed] [Google Scholar]
- 31.Misra M, McGrane J, Miller KK, Goldstein MA, Ebrahimi S, Weigel T, et al. Effects of rhIGF-1 administration on surrogate markers of bone turnover in adolescents with anorexia nervosa. Bone. 2009;45(3):493–8. doi: 10.1016/j.bone.2009.06.002. doi:10.1016/j.bone.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochimica et biophysica acta. 2000;1492(1):203–6. doi: 10.1016/s0167-4781(00)00067-1. [DOI] [PubMed] [Google Scholar]
- 33.Zhang X, Yeung DC, Karpisek M, Stejskal D, Zhou ZG, Liu F, et al. Serum FGF21 levels are increased in obesity and are independently associated with the metabolic syndrome in humans. Diabetes. 2008;57(5):1246–53. doi: 10.2337/db07-1476. doi:10.2337/db07-1476. [DOI] [PubMed] [Google Scholar]
- 34.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, et al. FGF-21 as a novel metabolic regulator. The Journal of clinical investigation. 2005;115(6):1627–35. doi: 10.1172/JCI23606. doi:10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell metabolism. 2007;5(6):415–25. doi: 10.1016/j.cmet.2007.05.003. doi:10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Inagaki T, Lin VY, Goetz R, Mohammadi M, Mangelsdorf DJ, Kliewer SA. Inhibition of growth hormone signaling by the fasting-induced hormone FGF21. Cell metabolism. 2008;8(1):77–83. doi: 10.1016/j.cmet.2008.05.006. doi:10.1016/j.cmet.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fazeli PK, Misra M, Goldstein M, Miller KK, Klibanski A. Fibroblast growth factor-21 may mediate growth hormone resistance in anorexia nervosa. The Journal of clinical endocrinology and metabolism. 2010;95(1):369–74. doi: 10.1210/jc.2009-1730. doi:10.1210/jc.2009-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gillum MP, Erion DM, Shulman GI. Sirtuin-1 regulation of mammalian metabolism. Trends in molecular medicine. 2010 doi: 10.1016/j.molmed.2010.09.005. doi:10.1016/j.molmed.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamamoto M, Iguchi G, Fukuoka H, Suda K, Bando H, Takahashi M, et al. SIRT1 regulates adaptive response of the growth hormone--insulin-like growth factor-I axis under fasting conditions in liver. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(37):14948–53. doi: 10.1073/pnas.1220606110. doi:10.1073/pnas.1220606110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baxter RC, Turtle JR. Regulation of hepatic growth hormone receptors by insulin. Biochemical and biophysical research communications. 1978;84(2):350–7. doi: 10.1016/0006-291x(78)90177-8. [DOI] [PubMed] [Google Scholar]
- 41.Leung KC, Doyle N, Ballesteros M, Waters MJ, Ho KK. Insulin regulation of human hepatic growth hormone receptors: divergent effects on biosynthesis and surface translocation. The Journal of clinical endocrinology and metabolism. 2000;85(12):4712–20. doi: 10.1210/jcem.85.12.7017. [DOI] [PubMed] [Google Scholar]
- 42.Boyar RM, Katz J, Finkelstein JW, Kapen S, Weiner H, Weitzman ED, et al. Anorexia nervosa. Immaturity of the 24-hour luteinizing hormone secretory pattern. The New England journal of medicine. 1974;291(17):861–5. doi: 10.1056/NEJM197410242911701. doi:10.1056/NEJM197410242911701. [DOI] [PubMed] [Google Scholar]
- 43.Wiegelmann W, Solbach HG. Effects of LH-RH on plasma levels of LH and FSH in anorexia nervosa. Hormone and metabolic research = Hormonund Stoffwechselforschung = Hormones et metabolisme. 1972;4(5):404. doi: 10.1055/s-0028-1097104. doi:10.1055/s-0028-1097104. [DOI] [PubMed] [Google Scholar]
- 44.Mecklenburg RS, Loriaux DL, Thompson RH, Andersen AE, Lipsett MB. Hypothalamic dysfunction in patients with anorexia nervosa. Medicine. 1974;53(2):147–59. doi: 10.1097/00005792-197403000-00003. [DOI] [PubMed] [Google Scholar]
- 45.Travaglini P, Beck-Peccoz P, Ferrari C, Ambrosi B, Paracchi A, Severgnini A, et al. Some aspects of hypothalamic-pituitary function in patients with anorexia nervosa. Acta endocrinologica. 1976;81(2):252–62. doi: 10.1530/acta.0.0810252. [DOI] [PubMed] [Google Scholar]
- 46.Nillius SJ, Fries H, Wide L. Successful induction of follicular maturation and ovulation by prolonged treatment with LH-releasing hormone in women with anorexia nervosa. American journal of obstetrics and gynecology. 1975;122(8):921–8. doi: 10.1016/0002-9378(75)90349-x. [DOI] [PubMed] [Google Scholar]
- 47.Riis BJ, Rodbro P, Christiansen C. The role of serum concentrations of sex steroids and bone turnover in the development and occurrence of postmenopausal osteoporosis. Calcified tissue international. 1986;38(6):318–22. doi: 10.1007/BF02555743. [DOI] [PubMed] [Google Scholar]
- 48.Klibanski A, Biller BM, Schoenfeld DA, Herzog DB, Saxe VC. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. The Journal of clinical endocrinology and metabolism. 1995;80(3):898–904. doi: 10.1210/jcem.80.3.7883849. [DOI] [PubMed] [Google Scholar]
- 49.Misra M, Katzman D, Miller KK, Mendes N, Snelgrove D, Russell M, et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26(10):2430–8. doi: 10.1002/jbmr.447. doi:10.1002/jbmr.447. Demonstrates an increase in bone mineral density in response to physiologic estrogen replacement in adolescent girls with anorexia nervosa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van Binsbergen CJ, Coelingh Bennink HJ, Odink J, Haspels AA, Koppeschaar HP. A comparative and longitudinal study on endocrine changes related to ovarian function in patients with anorexia nervosa. The Journal of clinical endocrinology and metabolism. 1990;71(3):705–11. doi: 10.1210/jcem-71-3-705. [DOI] [PubMed] [Google Scholar]
- 51.Miller KK, Lawson EA, Mathur V, Wexler TL, Meenaghan E, Misra M, et al. Androgens in women with anorexia nervosa and normal-weight women with hypothalamic amenorrhea. The Journal of clinical endocrinology and metabolism. 2007;92(4):1334–9. doi: 10.1210/jc.2006-2501. doi:10.1210/jc.2006-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller KK, Meenaghan E, Lawson EA, Misra M, Gleysteen S, Schoenfeld D, et al. Effects of risedronate and low-dose transdermal testosterone on bone mineral density in women with anorexia nervosa: a randomized, placebo-controlled study. The Journal of clinical endocrinology and metabolism. 2011;96(7):2081–8. doi: 10.1210/jc.2011-0380. doi:10.1210/jc.2011-0380. Demonstrates an increase in bone mineral density in response to one year of bisphosphonate therapy in anorexia nervosa. The 2-4% increase in bone mineral density is the greatest increase reported to date in response to a treatment in women with anorexia nervosa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zumoff B, Walsh BT, Katz JL, Levin J, Rosenfeld RS, Kream J, et al. Subnormal plasma dehydroisoandrosterone to cortisol ratio in anorexia nervosa: a second hormonal parameter of ontogenic regression. The Journal of clinical endocrinology and metabolism. 1983;56(4):668–72. doi: 10.1210/jcem-56-4-668. [DOI] [PubMed] [Google Scholar]
- 54.Gordon CM, Goodman E, Emans SJ, Grace E, Becker KA, Rosen CJ, et al. Physiologic regulators of bone turnover in young women with anorexia nervosa. The Journal of pediatrics. 2002;141(1):64–70. doi: 10.1067/mpd.2002.125003. doi:10.1067/mpd.2002.125003. [DOI] [PubMed] [Google Scholar]
- 55.Gordon CM, Grace E, Emans SJ, Feldman HA, Goodman E, Becker KA, et al. Effects of oral dehydroepiandrosterone on bone density in young women with anorexia nervosa: a randomized trial. The Journal of clinical endocrinology and metabolism. 2002;87(11):4935–41. doi: 10.1210/jc.2002-020545. [DOI] [PubMed] [Google Scholar]
- 56.Divasta AD, Feldman HA, Giancaterino C, Rosen CJ, Leboff MS, Gordon CM. The effect of gonadal and adrenal steroid therapy on skeletal health in adolescents and young women with anorexia nervosa. Metabolism: clinical and experimental. 2012;61(7):1010–20. doi: 10.1016/j.metabol.2011.11.016. doi:10.1016/j.metabol.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Misra M, Miller KK, Almazan C, Ramaswamy K, Lapcharoensap W, Worley M, et al. Alterations in cortisol secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. The Journal of clinical endocrinology and metabolism. 2004;89(10):4972–80. doi: 10.1210/jc.2004-0723. doi:10.1210/jc.2004-0723. [DOI] [PubMed] [Google Scholar]
- 58.Lawson EA, Donoho D, Miller KK, Misra M, Meenaghan E, Lydecker J, et al. Hypercortisolemia is associated with severity of bone loss and depression in hypothalamic amenorrhea and anorexia nervosa. The Journal of clinical endocrinology and metabolism. 2009;94(12):4710–6. doi: 10.1210/jc.2009-1046. doi:10.1210/jc.2009-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Misra M, Klibanski A. The neuroendocrine basis of anorexia nervosa and its impact on bone metabolism. Neuroendocrinology. 2011;93(2):65–73. doi: 10.1159/000323771. doi:10.1159/000323771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boyar RM, Hellman LD, Roffwarg H, Katz J, Zumoff B, O'Connor J, et al. Cortisol secretion and metabolism in anorexia nervosa. The New England journal of medicine. 1977;296(4):190–3. doi: 10.1056/NEJM197701272960403. doi:10.1056/NEJM197701272960403. [DOI] [PubMed] [Google Scholar]
- 61.Canalis E. Clinical review 83: Mechanisms of glucocorticoid action in bone: implications to glucocorticoid-induced osteoporosis. The Journal of clinical endocrinology and metabolism. 1996;81(10):3441–7. doi: 10.1210/jcem.81.10.8855781. [DOI] [PubMed] [Google Scholar]
- 62.Rauch A, Seitz S, Baschant U, Schilling AF, Illing A, Stride B, et al. Glucocorticoids suppress bone formation by attenuating osteoblast differentiation via the monomeric glucocorticoid receptor. Cell metabolism. 2010;11(6):517–31. doi: 10.1016/j.cmet.2010.05.005. doi:10.1016/j.cmet.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 63.McCarthy TL, Centrella M, Canalis E. Cortisol inhibits the synthesis of insulin-like growth factor-I in skeletal cells. Endocrinology. 1990;126(3):1569–75. doi: 10.1210/endo-126-3-1569. [DOI] [PubMed] [Google Scholar]
- 64.Urena P, Iida-Klein A, Kong XF, Juppner H, Kronenberg HM, Abou-Samra AB, et al. Regulation of parathyroid hormone (PTH)/PTH-related peptide receptor messenger ribonucleic acid by glucocorticoids and PTH in ROS 17/2.8 and OK cells. Endocrinology. 1994;134(1):451–6. doi: 10.1210/endo.134.1.8275958. [DOI] [PubMed] [Google Scholar]
- 65.Padmanabhan V, Keech C, Convey EM. Cortisol inhibits and adrenocorticotropin has no effect on luteinizing hormone-releasing hormone-induced release of luteinizing hormone from bovine pituitary cells in vitro. Endocrinology. 1983;112(5):1782–7. doi: 10.1210/endo-112-5-1782. [DOI] [PubMed] [Google Scholar]
- 66.Moshang T, Jr., Parks JS, Baker L, Vaidya V, Utiger RD, Bongiovanni AM, et al. Low serum triiodothyronine in patients with anorexia nervosa. The Journal of clinical endocrinology and metabolism. 1975;40(3):470–3. doi: 10.1210/jcem-40-3-470. [DOI] [PubMed] [Google Scholar]
- 67.Miyai K, Yamamoto T, Azukizawa M, Ishibashi K, Kumahara Y. Serum thyroid hormones and thyrotropin in anorexia nervosa. The Journal of clinical endocrinology and metabolism. 1975;40(2):334–8. doi: 10.1210/jcem-40-2-334. [DOI] [PubMed] [Google Scholar]
- 68.Croxson MS, Ibbertson HK. Low serum triiodothyronine (T3) and hypothyroidism in anorexia nervosa. The Journal of clinical endocrinology and metabolism. 1977;44(1):167–74. doi: 10.1210/jcem-44-1-167. [DOI] [PubMed] [Google Scholar]
- 69.Leslie RD, Isaacs AJ, Gomez J, Raggatt PR, Bayliss R. Hypothalamo-pituitary-thyroid function in anorexia nervosa: influence of weight gain. British medical journal. 1978;2(6136):526–8. doi: 10.1136/bmj.2.6136.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Casper RC, Frohman LA. Delayed TSH release in anorexia nervosa following injection of thyrotropin-releasing hormone (TRH) Psychoneuroendocrinology. 1982;7(1):59–68. doi: 10.1016/0306-4530(82)90055-5. [DOI] [PubMed] [Google Scholar]
- 71.Kiyohara K, Tamai H, Takaichi Y, Nakagawa T, Kumagai LF. Decreased thyroidal triiodothyronine secretion in patients with anorexia nervosa: influence of weight recovery. The American journal of clinical nutrition. 1989;50(4):767–72. doi: 10.1093/ajcn/50.4.767. [DOI] [PubMed] [Google Scholar]
- 72.Onur S, Haas V, Bosy-Westphal A, Hauer M, Paul T, Nutzinger D, et al. L-tri-iodothyronine is a major determinant of resting energy expenditure in underweight patients with anorexia nervosa and during weight gain. European journal of endocrinology / European Federation of Endocrine Societies. 2005;152(2):179–84. doi: 10.1530/eje.1.01850. doi:10.1530/eje.1.01850. [DOI] [PubMed] [Google Scholar]
- 73.Moore R, Mills IH. Serum T3 and T4 levels in patients with anorexia nervosa showing transient hyperthyroidism during weight gain. Clinical endocrinology. 1979;10(5):443–9. doi: 10.1111/j.1365-2265.1979.tb02100.x. [DOI] [PubMed] [Google Scholar]
- 74.Fraser SA, Anderson JB, Smith DA, Wilson GM. Osteoporosis and fractures following thyrotoxicosis. Lancet. 1971;1(7707):981–3. doi: 10.1016/s0140-6736(71)91383-3. [DOI] [PubMed] [Google Scholar]
- 75.Kindblom JM, Gevers EF, Skrtic SM, Lindberg MK, Gothe S, Tornell J, et al. Increased adipogenesis in bone marrow but decreased bone mineral density in mice devoid of thyroid hormone receptors. Bone. 2005;36(4):607–16. doi: 10.1016/j.bone.2005.01.017. doi:10.1016/j.bone.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 76.Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, et al. Increased bone marrow fat in anorexia nervosa. The Journal of clinical endocrinology and metabolism. 2009;94(6):2129–36. doi: 10.1210/jc.2008-2532. doi:10.1210/jc.2008-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abu EO, Bord S, Horner A, Chatterjee VK, Compston JE. The expression of thyroid hormone receptors in human bone. Bone. 1997;21(2):137–42. doi: 10.1016/s8756-3282(97)00097-5. [DOI] [PubMed] [Google Scholar]
- 78.Miell JP, Taylor AM, Zini M, Maheshwari HG, Ross RJ, Valcavi R. Effects of hypothyroidism and hyperthyroidism on insulin-like growth factors (IGFs) and growth hormone- and IGF-binding proteins. The Journal of clinical endocrinology and metabolism. 1993;76(4):950–5. doi: 10.1210/jcem.76.4.7682563. [DOI] [PubMed] [Google Scholar]
- 79.Grinspoon S, Gulick T, Askari H, Landt M, Lee K, Anderson E, et al. Serum leptin levels in women with anorexia nervosa. The Journal of clinical endocrinology and metabolism. 1996;81(11):3861–3. doi: 10.1210/jcem.81.11.8923829. [DOI] [PubMed] [Google Scholar]
- 80.Misra M, Miller KK, Kuo K, Griffin K, Stewart V, Hunter E, et al. Secretory dynamics of leptin in adolescent girls with anorexia nervosa and healthy adolescents. American journal of physiology Endocrinology and metabolism. 2005;289(3):E373–81. doi: 10.1152/ajpendo.00041.2005. doi:10.1152/ajpendo.00041.2005. [DOI] [PubMed] [Google Scholar]
- 81.Miller KK, Grinspoon S, Gleysteen S, Grieco KA, Ciampa J, Breu J, et al. Preservation of neuroendocrine control of reproductive function despite severe undernutrition. The Journal of clinical endocrinology and metabolism. 2004;89(9):4434–8. doi: 10.1210/jc.2004-0720. doi:10.1210/jc.2004-0720. [DOI] [PubMed] [Google Scholar]
- 82.Legroux-Gerot I, Vignau J, Biver E, Pigny P, Collier F, Marchandise X, et al. Anorexia nervosa, osteoporosis and circulating leptin: the missing link. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2010;21(10):1715–22. doi: 10.1007/s00198-009-1120-x. doi:10.1007/s00198-009-1120-x. [DOI] [PubMed] [Google Scholar]
- 83.Lawson EA, Miller KK, Bredella MA, Phan C, Misra M, Meenaghan E, et al. Hormone predictors of abnormal bone microarchitecture in women with anorexia nervosa. Bone. 2010;46(2):458–63. doi: 10.1016/j.bone.2009.09.005. doi:10.1016/j.bone.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM, et al. Recombinant human leptin in women with hypothalamic amenorrhea. The New England journal of medicine. 2004;351(10):987–97. doi: 10.1056/NEJMoa040388. doi:10.1056/NEJMoa040388. [DOI] [PubMed] [Google Scholar]
- 85.Misra M, Miller KK, Tsai P, Gallagher K, Lin A, Lee N, et al. Elevated peptide YY levels in adolescent girls with anorexia nervosa. The Journal of clinical endocrinology and metabolism. 2006;91(3):1027–33. doi: 10.1210/jc.2005-1878. doi:10.1210/jc.2005-1878. [DOI] [PubMed] [Google Scholar]
- 86.Utz AL, Lawson EA, Misra M, Mickley D, Gleysteen S, Herzog DB, et al. Peptide YY (PYY) levels and bone mineral density (BMD) in women with anorexia nervosa. Bone. 2008;43(1):135–9. doi: 10.1016/j.bone.2008.03.007. doi:10.1016/j.bone.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baldock PA, Sainsbury A, Couzens M, Enriquez RF, Thomas GP, Gardiner EM, et al. Hypothalamic Y2 receptors regulate bone formation. The Journal of clinical investigation. 2002;109(7):915–21. doi: 10.1172/JCI14588. doi:10.1172/JCI14588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Misra M, Prabhakaran R, Miller KK, Goldstein MA, Mickley D, Clauss L, et al. Prognostic indicators of changes in bone density measures in adolescent girls with anorexia nervosa-II. The Journal of clinical endocrinology and metabolism. 2008;93(4):1292–7. doi: 10.1210/jc.2007-2419. doi:10.1210/jc.2007-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fukushima N, Hanada R, Teranishi H, Fukue Y, Tachibana T, Ishikawa H, et al. Ghrelin directly regulates bone formation. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2005;20(5):790–8. doi: 10.1359/JBMR.041237. doi:10.1359/JBMR.041237. [DOI] [PubMed] [Google Scholar]
- 90.Misra M, Miller KK, Stewart V, Hunter E, Kuo K, Herzog DB, et al. Ghrelin and bone metabolism in adolescent girls with anorexia nervosa and healthy adolescents. The Journal of clinical endocrinology and metabolism. 2005;90(9):5082–7. doi: 10.1210/jc.2005-0512. doi:10.1210/jc.2005-0512. [DOI] [PubMed] [Google Scholar]
- 91.Misra M, Miller KK, Cord J, Prabhakaran R, Herzog DB, Goldstein M, et al. Relationships between serum adipokines, insulin levels, and bone density in girls with anorexia nervosa. The Journal of clinical endocrinology and metabolism. 2007;92(6):2046–52. doi: 10.1210/jc.2006-2855. doi:10.1210/jc.2006-2855. [DOI] [PubMed] [Google Scholar]
- 92.Tagami T, Satoh N, Usui T, Yamada K, Shimatsu A, Kuzuya H. Adiponectin in anorexia nervosa and bulimia nervosa. The Journal of clinical endocrinology and metabolism. 2004;89(4):1833–7. doi: 10.1210/jc.2003-031260. [DOI] [PubMed] [Google Scholar]
- 93.Housova J, Anderlova K, Krizova J, Haluzikova D, Kremen J, Kumstyrova T, et al. Serum adiponectin and resistin concentrations in patients with restrictive and binge/purge form of anorexia nervosa and bulimia nervosa. The Journal of clinical endocrinology and metabolism. 2005;90(3):1366–70. doi: 10.1210/jc.2004-1364. doi:10.1210/jc.2004-1364. [DOI] [PubMed] [Google Scholar]
- 94.Luo XH, Guo LJ, Xie H, Yuan LQ, Wu XP, Zhou HD, et al. Adiponectin stimulates RANKL and inhibits OPG expression in human osteoblasts through the MAPK signaling pathway. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2006;21(10):1648–56. doi: 10.1359/jbmr.060707. doi:10.1359/jbmr.060707. [DOI] [PubMed] [Google Scholar]
- 95.Lowe B, Zipfel S, Buchholz C, Dupont Y, Reas DL, Herzog W. Long-term outcome of anorexia nervosa in a prospective 21-year follow-up study. Psychological medicine. 2001;31(5):881–90. doi: 10.1017/s003329170100407x. [DOI] [PubMed] [Google Scholar]
- 96.Miller KK, Lee EE, Lawson EA, Misra M, Minihan J, Grinspoon SK, et al. Determinants of skeletal loss and recovery in anorexia nervosa. The Journal of clinical endocrinology and metabolism. 2006;91(8):2931–7. doi: 10.1210/jc.2005-2818. doi:10.1210/jc.2005-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]


