Abstract
Background and Aims
The 7α-dehydroxylation of primary bile acids (BAs), chenodeoxycholic (CDCA) and cholic acid (CA) into the secondary BAs, lithocholic (LCA) and deoxycholic acid (DCA) is a key function of the gut microbiota. We aimed to study the linkage between fecal BAs and gut microbiota in cirrhosis since this could help understand cirrhosis progression.
Methods
Fecal microbiota were analyzed by culture-independent multitagged-pyrosequencing, fecal BAs using HPLC and serum BAs using LC-MS in controls, early (Child A), and advanced cirrhotics(Child B/C). A subgroup of early cirrhotics underwent BA and microbiota analysis before/after eight weeks of rifaximin.
Results
Cross-sectional: 47 cirrhotics(24 advanced) and 14 controls were included. In feces, advanced cirrhotics had the lowest total, secondary, secondary/primary BA ratios, and highest primary BAs compared to early cirrhotics and controls. Secondary fecal BAs were detectable in all controls but in a significantly lower proportion of cirrhotics (p<0.002). Serum primary BAs were higher in advanced cirrhotics compared to the rest. Cirrhotics, compared to controls, had a higher Enterobacteriaceae (potentially pathogenic) but lower Lachonospiraceae, Ruminococcaceae and Blautia (7α-dehydroxylating bacteria) abundance. CDCA was positively correlated with Enterobacteriaceae(r=0.57, p<0.008) while Ruminococcaceae were positively correlated with DCA(r=0.4, p<0.05). A positive correlation between Ruminococcaceae and DCA/CA (r=0.82, p<0.012) and Blautia with LCA/CDCA (r=0.61, p<0.03) was also seen. Prospective study: Post-rifaximin, six early cirrhotics had reduction in Veillonellaceae and in the secondary/primary BA ratios.
Conclusions
Cirrhosis, especially advanced disease, is associated with a decreased conversion of primary to secondary fecal BAs which is linked with abundance of key gut microbiome taxa.
Keywords: gut microbiota, complications of cirrhosis, bile acid dehydroxylation, dysbiosis, rifaximin, pathogenesis
Introduction
There is emerging evidence that the gut milieu plays an important role in the progression of the complications of cirrhosis [1–3]. Studies have found dysbiosis in the gut microbiota in patients with cirrhosis that has the potential to influence complications such as hepatic encephalopathy[1, 2]. The gut milieu in cirrhosis involves the interaction between the microbiota and secreted factors such as bile acids (BAs) that can also modulate the gut barrier[4]. The gut microbiota are known to convert 7α-dehydroxylate primary BAs and chenodeoxycholic acid (CDCA) & cholic acid (CA) into secondary bile acids lithocholic acid (LCA) & deoxycholic acid (DCA), respectively[5]. Cirrhotic patients have been shown to have a lower proportion of secondary BAs in their bile but the mechanism for this is not clear[6]. Since BAs have important downstream pathophysiologic effects, a better understanding of the interaction between the intestinal microbiome and BAs is important to gain insight into the pathophysiology of cirrhosis[7, 8]. We hypothesized that the gut dysbiosis in cirrhosis may be related to this altered bile acid profile and that modulating the gut microbiota using a non-absorbable antibiotic may further affect the conversion of primary to secondary bile acids.
The aim was to study the linkage between fecal bile acid concentrations and the gut microbiota in cirrhotic patients with differing disease severity compared to healthy controls and to define the changes in this correlation after the administration of gut-selective, non-absorbable antibiotic, rifaximin.
Patients and Methods
This study was divided into cross-sectional (comparison between cirrhotic patients and age-matched controls) and longitudinal (a sub-group of patients with early cirrhosis were studied before and after rifaximin therapy) components.
Subjects
Subjects were prospectively recruited after informed consent. Cirrhotic patients (diagnosed using compatible biopsy, radiological or endoscopic evidence) and age-comparable controls were enrolled the cross-sectional study. We excluded patients who had been abusing alcohol/illicit drugs over the past 3 months, those on absorbable antibiotics, those unable to give consent or samples, with inflammatory bowel disease or diagnosed irritable bowel syndrome and on ursodeoxycholic acid or probiotics. Healthy controls without chronic diseases or on regular medications were recruited using advertisements or word of mouth referral. We divided patients with cirrhosis into early cirrhosis (Child Class A without history of decompensation) and advanced cirrhosis on the basis of decompensation (hepatic encephalopathy, ascites, variceal bleeding, hepatic hydrothorax or Child Class B and C). All subjects gave a detailed dietary history evaluation (prior seven days), a physical examination and collection of serum and fresh fecal samples on the day of the study while cirrhotics were also checked for venous ammonia and MELD score laboratory values.
The longitudinal study was carried out on a subgroup of patients with early cirrhosis, without any decompensation or prior HE who were treated with rifaximin 550mg BID for eight weeks. Stool samples were collected and analyzed before and after rifaximin therapy. Dietary intake was kept constant throughout the trial.
Serum was stored at −80°C for subsequent serum analysis of bile acids. Stool was freshly collected and divided into 2 parts (1) frozen at −80°C for subsequent bile acid analysis and (2) a portion for DNA extraction microbiome analysis.
Bile acid analysis
The initial analysis was performed using HPLC (supplementary data) and results were confirmed using liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) and gas chromatography mass spectrometry (GC-MS). Serum bile acid analysis was performed according LC-ESI-MS/MS as described by Muto et al [9].
Interrogation of the Microbiome
Stool was collected and DNA extracted for microbiome analysis using published techniques [1, 10]. We first use Length Heterogeneity PCR (LH-PCR) fingerprinting of the 16S rRNA to rapidly survey our samples and standardize the community amplification. We then interrogated the microbial taxa associated with the gut fecal microbiome using Multitag Pyrosequencing (MTPS).
Microbiome Community Fingerprinting
LH-PCR was done to standardize the community analysis as previously published[10]. Briefly, total genomic DNA was using Bio101 kit from MP Biomedicals Inc., Montreal, Quebec as per the manufacturer’s instructions. About 10 ng of extracted DNA was amplified by PCR using a fluorescently labeled forward primer 27F (5′-(6FAM) AGAGTTTGATCCTGGCTCA G-3′) and unlabeled reverse primer 355R′ (5′-GCTGCCTCCCGTAGGAGT-3′). Normalized peak areas were calculated using a custom PERL script and operational taxonomic units (OTUs) constituting <1% of the community from each sample were eliminated from the analysis.
MTPS
Specifically, we have generated a set of 96 emulsion PCR fusion primers that contain the 454 emulsion PCR linkers on the 27F and 355R primers and a different 8 base “barcode” between the A adapter and 27F primer. Thus, each fecal sample was amplified with unique bar-coded forward 16S rRNA primers and then up to 96 samples were pooled and subjected to emulsion PCR and pyrosequenced using a GS-FLX pyrosequencer (Roche). Data from each pooled sample were “deconvoluted” by sorting the sequences into bins based on the barcodes using custom PERL scripts. Thus, we were able to normalize each sample by the total number of reads from each barcode.
Microbiome Community Analysis
We identified the taxa present using the Bayesian analysis tool in Version 10 of the Ribosomal Database Project (RDP10). The abundances of the bacterial identifications were then normalized using a custom PERL script and genera present at >1% of the community were tabulated.
Statistical analysis
The ratio of mean secondary/primary BAs from the fecal samples derived from the values obtained was performed to reflect the relative concentrations of DCA vs CA (DCA/CA), LCA vs CDCA (LCA/CDCA) and mean total secondary/primary BAs (DCA+LCA/CDCA+CA). Comparisons were made between controls and cirrhosis and between early and advanced cirrhosis using t-tests for continuous data and chi-square and Kruskall-Wallis tests for non-parametric data. Analyses were also performed on the serum BA results between controls, early and advanced cirrhotic patients. We also compared patients with/without rifaximin within the cirrhosis group. Correlations between BAs and microbiome abundances were performed using Spearman’s correlation analysis. Analysis of BAs before and after rifaximin therapy was performed using Wilcoxon matched-pairs rank tests.
Results
In the cross-sectional study, 47 cirrhotic patients (age 56±4 years, 32 men) and 14 age-matched controls (age 54±3 years, 10 men) were included. The mean MELD was 12.3±6.5 and the majority had hepatitis C (66%) while 15% had alcoholic liver disease. Advanced cirrhosis was found in 24 patients; all had treated hepatic encephalopathy (100% on lactulose alone, 25% with additional rifaximin) while 88% had ascites on therapy with diuretics (Table 1). Dietary constituents were similar between controls (mean daily intake 2320 Kcal and 21% protein) and cirrhotics (2185 Kcal and 18% protein) over the prior seven days without probiotics.
Table 1.
Baseline description of the study groups
| Controls (n=14) | Early Cirrhosis (n=23) | Advanced Cirrhosis (n=24) | |
|---|---|---|---|
| Age (years) | 52±5 | 55±2 | 54±5 |
| Gender (Male/Female) | 10/4 | 17/6 | 20/4 |
| Race (Cauc/AA/Other) | 12/2/0 | 15/6/2 | 14/9/1 |
| BMI | 29±6 | 32±4 | 30±4 |
| MELD score | - | 8.1±2.3 | 16.0± 6.8* |
| Controlled ascites | - | 0 | 21 (87.5%) |
| Controlled HE | - | 0 | 24 (100%) |
| Lactulose use | - | - | 24 (100%) |
| Rifaximin use | - | - | 12 (50%) |
| Median daily stools | 1 | 1 | 1.5 |
p<0.05 between groups,
Cauc: Caucasian, AA: African American, BMI: body mass index, HE: hepatic encephalopathy
Fecal Bile acids
Total bile acids were significantly lower in advanced cirrhotics compared to the other groups (Table 2). We did not find the same spectrum of BAs or appreciable concentrations of 7-oxo-bile acids in all groups. Of the primary BAs, CA was present in a significantly higher percent of cirrhotics while CDCA was detected similarly in all groups (Table 1). In contrast, secondary BAs were present in a significantly lower percentage of cirrhotics. Secondary BA levels were highest in controls and lowest in advanced cirrhosis (Table 2). As follows, the ratios of all secondary to primary BAs and specifically of DCA/CA & LCA/CDCA were also lowest in advanced cirrhosis (Table 2).
Table 2.
Distribution of fecal bile acids in entire population
| Median values in μg/100 mg dry stool | Controls (n=14) | Early Cirrhosis (n=23) | Advanced Cirrhosis (n=24) |
|---|---|---|---|
| Total Bile Acids | 206.5 | 156.1 | 39.0* |
| Primary | |||
| CA (% in whom detected) | 36.0 (14%) | 64.6 (22%) | 16.0 (71%)*† |
| CDCA (% in whom detected) | 3.1 (51%) | 10.1 (57%) | 12.4 (83%)*† |
| Secondary | |||
| LCA (% in whom detected) | 83.2 (100%) | 63.8 (87%) | 12.7 (46%)*† |
| DCA (% in whom detected) | 110.7 (100%) | 35.8 (83%) | 8.3 (50%)*† |
| Secondary/Primary Ratios | |||
| LCA / CDCA | 39.7 | 7.6 | 2.2* |
| DCA / CA | 6.3 | 3.2 | 0.9* |
| LCA+DCA / CDCA+CA | 79.8 | 9.6 | 0.004* |
p<0.05 between groups on Kruskall-Wallis tests on median concentrations,
p<0.05 between groups on percent in whom the respective bile acids were detected,
CA: cholic acid, CDCA: chenodeoxycholic acid, LCA: lithocholic acid, DCA: deoxycholic acid.
Comparison within the cirrhosis group
The median MELD score was significantly higher in rifaximin group (16.5) compared to those on lactulose alone (13) and those without HE (6.5, p<0.0001). Since the patients on rifaximin in the cross-sectional group were more advanced, it followed that there was a reduction of total BAs in that group. We found that in those on rifaximin and advanced cirrhosis there was a reduction in the ratio of secondary/primary bile acids (Table 3) compared to other groups, independent of bowel movement frequency.
Table 3.
Distribution of fecal bile acids within the cirrhosis group
| Median values in μg/100 mg dry stool | Early Cirrhosis (n=23) | Advanced cirrhosis on lactulose alone (n=12) | Advanced cirrhosis on lactulose +rifaximin (n=12) |
|---|---|---|---|
| Total Bile Acids | 156.1 | 59.0 | 14.2* |
| Primary | |||
| CA (% in whom detected) | 64.7 (22%) | 20.4 (58%) | 13.9 (92%)*† |
| CDCA (% in whom detected) | 10.1 (56%) | 25.9(75%) | 11.6(92%) |
| Secondary | |||
| LCA (% in whom detected) | 63.8 (87%) | 26.5(67%) | 4.1(25%)*† |
| DCA (% in whom detected) | 35.8(83%) | 35.6(67%) | 5.7(33%)*† |
| Secondary/Primary Ratios | |||
| LCA/CDCA | 7.5 | 2.2 | 2.2 |
| DCA/CA | 3.2 | 1.04 | 0.4* |
| LCA+DCA / CDCA+CA | 9.6 | 0.7 | 0.0* |
p<0.05 between groups on Kruskall-Wallis tests on median concentrations,
p<0.05 between groups on percent in whom the respective bile acids were detected; the median daily bowel movement frequency was similar in advanced cirrhotics with and without rifaximin (1.5 in each group). Early cirrhosis group consists of cirrhotics with Child A cirrhosis without prior/current complications and were not on lactulose or rifaximin.
CA: cholic acid, CDCA: chenodeoxycholic acid, LCA: lithocholic acid, DCA: deoxycholic acid
Serum Bile acids
The majority of serum BAs were conjugated and there was a significantly higher level in cirrhotic groups, especially advanced patients (Table 4). Levels of unconjugated primary BAs were also higher in cirrhotics but unconjugated DCA was lower in this group compared to controls.
Table 4.
Distribution of serum bile acids within groups
| Median values in μm/L | Control (n=14) | Early Cirrhosis (n=23) | Advanced Cirrhosis (n=24) | P value |
|---|---|---|---|---|
| Conjugated | ||||
| GUDCA | 0.00 | 0.30 | 0.75 | 0.003 |
| TUDCA | 0.00 | 0.00 | 0.12 | 0.02 |
| GCA | 0.05 | 2.50 | 5.65 | <0.0001 |
| TCA | 0.00 | 1.20 | 5.93 | <0.0001 |
| GCDCA | 0.70 | 5.20 | 16.8 | <0.0001 |
| TCDCA | 0.00 | 3.10 | 2.45 | <0.0001 |
| GDCA‡ | 0.49 | 0.15 | 0.00 | 0.37 |
| Double conjugated | ||||
| TCDCA3S | 0.00 | 0.20 | 1.00 | <0.0001 |
| GCDCA3S | 0.00 | 0.65 | 1.30 | <0.0001 |
| Unconjugated | ||||
| CDCA | 0.00 | 0.58 | 0.62 | 0.05 |
| CA | 0.00 | 0.00 | 0.15 | 0.04 |
| DCA‡ | 0.53 | 0.10 | 0.00 | 0.05 |
| LCA‡ | 0.00 | 0.00 | 0.00 | 1.0 |
secondary bile acids; a significantly higher concentration was seen in patients with cirrhosis, especially those with advanced cirrhosis in the serum compared to controls using the Kruskall-Wallis test.
CA: cholic acid, CDCA: chenodeoxycholic acid, LCA: lithocholic acid, DCA: deoxycholic acid, TCA: taurocholic acid, TCDCA: taurochenodeoxycholic acid, TLCA: tauro lithocholic acid, TDCA: taurodeoxycholic acid, GCA: glycocholic acid, GCDCA: glycochenodeoxycholic acid, GLCA: glycolithocholic acid, GDCA: glycodeoxycholic acid, TCDCA3S: taurochenodeoxycholic acid 3-sulphate, GCDCA3S: glycochenodeoxycholic acid 3-sulphate.
Prospective study of rifaximin
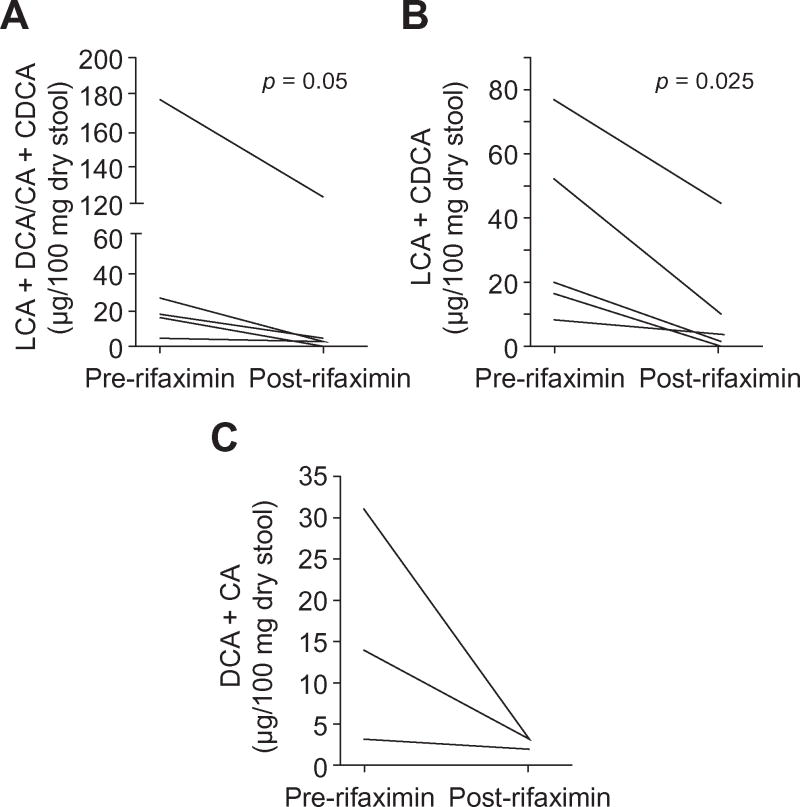
We studied six early cirrhotic patients before and after use of rifaximin 550mg PO BID for 8 weeks. The mean age was 52±5 years and four patients were men. The mean MELD score was 10 and the etiologies were hepatitis C (n=4) and cryptogenic cirrhosis (n=2). All patients were able to tolerate rifaximin (adherence was 94%) and were on a stable diet (pre 2104 Kcal vs. post 2185 Kcal/day) during the trial. Stool was collected for BA and microbiota analysis at baseline and after 8 weeks of rifaximin therapy. The MELD score (pre 10±2 and post 10±4) and the median daily stool frequency (1 vs. 1, p=0.85) did not significantly change with rifaximin use. We found a significant reduction in the ratio of mean total secondary/primary bile acids and LCA/CDCA with a trend towards this reduction in the DCA/CA ratio in the group after rifaximin compared to baseline (Table 5, Figures 1A, B and C).
Table 5.
Comparison of median fecal bile acid values pre and post rifaximin
| Median values in μg/100 mg dry stool | Prospective trial | |
|---|---|---|
| Pre rifaximin (n=6) | Post-rifaximin (n=6) | |
| Total Bile Acids | 581 | 526 |
| Primary | ||
| CA | 21.5 | 39.3* |
| CDCA | 2.6 | 19.2* |
| Secondary | ||
| LCA | 113.9 | 118.9 |
| DCA | 377.6 | 354.2 |
| Secondary/Primary Ratios | ||
| LCA/CDCA | 20.1 | 3.4* |
| DCA/CA | 13.9 | 2.5 |
| LCA+DCA / CDCA+CA | 17.5 | 2.5* |
p<0.05 in this open-label trial of rifaximin in early cirrhotic patients, there was a net decrease in the secondary/primary BA ratio after rifaximin using the Wilcoxon matched-pairs test.
CA: cholic acid, CDCA: chenodeoxycholic acid, LCA: lithocholic acid, DCA: deoxycholic acid
Fig 1. Change in fecal secondary/primary bile acid concentration ratios after rifaximin therapy in early cirrhotic patients.
Figure 1a: LCA+DCA/CDCA+CA ratio, Figure 1b: LCA/CDCA ratio and Figure 1c: DCA/CA ratio change; there was a significant decrease in the fecal secondary to primary bile acid ratios after rifaximin therapy in early cirrhotic patients
Microbiome changes
The composition of the microbiota was different between groups with significant differences concentrated on the phyla Firmicutes, Bacteriodetes and Proteobacteria (Table 6). A significantly higher Enterobacteriaceae and Veillonellaceae and lower autochthonous bacteria (Blautia, Ruminococcaceae, Lachnospiraceae) abundance in cirrhotics, especially advanced cirrhotics compared to controls was observed. When the entire group (controls and cirrhotics) were studied, a significant positive correlation was found within autochthonous bacteria abundance (Lachnospiraceae with Ruminococcaceae r=0.58, p=0.001 and with Blautia r=0.42, p=0.018, Ruminococcaceae with Blautia, r=0.57, p=0.001) while there was a negative correlation between Bacterioideaceae and Lachnospiraceae (r=−0.40, p=0.05) and between Bacterioideceae and Ruminococceae (r=−0.37, p=0.05). Coriobacteriaceae were positively correlated with Enterobacteriaceae (r=0.58, p=0.001) and Streptococcaceae (p=0.4, p=0.03).
Table 6.
Microbiome analysis
| Phylum_Taxon (median %) | Control (n=14) | Early Cirrhosis (n=23) | Advanced Cirrhosis (n=24) | P value |
|---|---|---|---|---|
| Firmicutes | ||||
| Veillonellaceae | 2.5 | 0.0 | 3.4 | 0.013 |
| Lachnospiraceae | 18.1 | 13.5 | 3.3 | 0.04 |
| Ruminococcaceae | 12.8 | 3.6 | 1.9 | 0.001 |
| Blautia | 4.7 | 2.9 | 0.0 | 0.01 |
| Streptococcaceae | 0.0 | 0.0 | 0.0 | |
| Clostridiaceae | 0.0 | 0.0 | 0.0 | |
| Bacteroidetes | ||||
| Bacteroidaceae | 28.0 | 12.6 | 44.3 | 0.34 |
| Rikenellaceae | 2.3 | 1.2 | 0.0 | 0.02 |
| Porphyromonadaceae | 1.3 | 0 | 1.5 | 0.41 |
| Prevotellaceae | 0.0 | 0.0 | 0.0 | |
| Proteobacteria | ||||
| Alcaligenaceae | 0.0 | 0.0 | 0.0 | |
| Enterobacteriaceae | 0.0 | 0.0 | 0.20 | 0.02 |
The median concentrations of taxa are shown with significantly higher Enterobacteriaceae and Veillonellaceae and lower autochthonous bacteria (Blautia, Ruminococcaceae, Lachnospiraceae) in cirrhotics, especially advanced cirrhotics compared to controls. P values only shown for key taxa that were present as median >0.1% abundance in at least one group
When correlations were restricted to cirrhotics, the positive linkages within the autochthonous families increased in magnitude (Lachnospiraceae with Ruminococcaceae r=0.81, p<0.001, and with Blautia r=0.51, p=0.01 and Ruminococcaceae with Blautia, r=0.53, p=0.01). Similar to prior studies Alcaligenaceae and Porphyromonadaceae were highly correlated (r=0.67, p=0.001). The correlation between Coriobacteriaceae and Enterobacteriaceae (r=0.82, p<0.0001) and Streptococcaceae (r=0.6, p=0.001) increased in magnitude. However the negative relationship between Bacterioideaceae and Lachnospiraceae (r=−0.39, p=0.07) and between Bacterioideceae and Ruminococcaceae (r=−0.32, p=0.05) relatively weakened compared to when the entire group (control+cirrhosis) correlations.
After rifaximin, there were no significant changes in overall microbiome composition (Bacterioidetes 37% vs 32%, Firmicutes, 36% vs 35%, Proteobacteria 5% vs. 6%) while at the taxon level the only significant difference was a reduction in Veillonellaceae abundance(2.5% vs. 1%).
Correlation between stool BAs and microbiome
The fecal primary BA, CDCA was significantly positively correlated with Enterobacteriaceae (r=0.57, p=0.008) and negatively with Bacterioideceae (r=−0.5, p=0.026) abundance. No correlation between CA concentrations with microbiome was found. On the other hand, Ruminococcaceae were positively correlated with the secondary BA, DCA (r=0.4, p=0.05). There was also a significant positive correlation between Ruminococcaceae and DCA/CA (r=0.82, p=0.012) and between Blautia and LCA/CDCA (r=0.61, p=0.03) (Supplementary figures).
Discussion
We found a decrease in total fecal BA concentration and reduction in the ratio of secondary to primary BAs with worsening of liver disease severity. There was a significant reduction in the ratio of fecal secondary to primary BA concentrations in those on rifaximin in the cross-sectional study and also after rifaximin therapy in the prospective portion of the study. The microbiome in cirrhosis showed dysbiosis with overgrowth of Enterobacteriaceae and decrease in autochthonous genera compared to controls. Autochthonous genera were positively correlated with secondary BAs and the secondary/primary fecal BAs ratios, while the potentially pathogenic ones were correlated with primary BAs.
Apart from their digestive role, bile acids can influence the intestinal milieu, the integrity of the mucosal barrier and the synthesis of anti-bacterial peptides and lectins[6, 7]. They also have direct anti-microbial effect and can cause membrane damage to both microbial cells and the intestinal barrier[4, 8]. The conversion from primary to secondary BAs is usually performed by a restricted group of bacteria in the order Clostridiales[5]. This conversion is dependent on several factors, i.e. availability of the bacteria, adequate bile flow, adequate contact of the bacteria with the bile, and diet[11]. Therefore to account for these factors, instead of the absolute fecal concentrations, the ratios of secondary to primary BAs are useful and are also described throughout our paper. We found a lower absolute total BA value which was accompanied by a reduction in the secondary/primary BA ratio with the progression of liver disease severity. As expected, serum concentrations of BAs were significantly lower than fecal levels and there was a corresponding increase in conjugated and unconjugated primary BAs in the serum of cirrhotics, especially advanced patients, with a reduction in serum DCA in compared to controls. This is consistent with the cholestasis seen in cirrhotic patients in which BAs, even after sulphation, are refluxed into the circulation without reaching the intestinal lumen and influencing the microbiota[12].
When the detection rate of fecal BAs was studied between groups, we found a higher proportion of controls had secondary BAs present compared to cirrhotics and vice versa with respect to CA but not CDCA. Replicating prior studies in the cirrhosis group, we found the major deficit to be in the CA rather than CDCA pathway[13]. An imbalance in the ratio between secondary to primary BAs in advanced cirrhosis was found even when the group that was on rifaximin and lactulose was compared to the group on lactulose alone, negating the possible effect of bowel movement frequency on this result. In prior studies the exact cause of this imbalance was not clear but the current study shows an association between gut dysbiosis and these changes in the bile acids.
The bacterial communities in cirrhosis showed dysbiosis; there was a significant reduction in families belonging to the order Clostridiales such as Lachnospiraceae, Ruminococcaceae and Blautia which are capable of performing 7α dehydroxylation and a significantly higher abundance of the potentially pathogenic family Enterobacteriaceae [1, 3, 14, 15]. This was also accompanied by a direct correlation between secondary bile acids and ratios with the autochthonous families. This dysbiosis in cirrhosis has been associated with complications such as hepatic encephalopathy and is only partly affected by drugs such as lactulose[16]. The enumeration of bacteria may in itself not explain the consequences of their overgrowth in cirrhosis, rather it may be the expression of metabolic function that can result in the production of ammonia, indoles, short-chain fatty acids and the conversion of primary to secondary bile acids[8, 17]. This conversion could be considered a “key functional test” of the gut microbiota which is the most robust in healthy individuals but least in the advanced cirrhotic patients. DCA is the major secondary BA that is re-absorbed into the entero-hepatic circulation; therefore the likely explanation of the lower DCA found in cirrhotic patients’ bile is the lower conversion by the microbiota[13]. There could be other explanations for the decrease in secondary/primary BA ratio; a higher stool frequency could decrease contact time of BAs with bacteria in cirrhotics on lactulose and stool acidification by lactulose could potentially reduce 7α-dehydroxylation[8].
Therefore to further study this mechanism, we conducted pre-post analysis after rifaximin, a broad-spectrum antibiotic that has shown to be efficacious in hepatic encephalopathy and travelers’ diarrhea and has activity against Clostridia, especially C. difficile [18, 19]. Rifaximin therapy has been shown to change the functional activity and bacterial virulence, rather than being bactericidal and its action is promoted by adequate bile in in-vitro studies[20–22]. Confirming this, we found that despite a stable MELD score, diet and stool frequency, rifaximin resulted in a reduction of the secondary/primary BA ratio without a significant change in bacterial abundance apart from reduced Veillonellaceae. This indicates a change in the microbiota function is likely associated with this decreased conversion. Also the ratios achieved in early cirrhotics after rifaximin were similar to those seen in advanced cirrhotics (were also on lactulose) on rifaximin in the cross-sectional study. A recent study showed an increased primary BA profile in diarrhea-predominant irritable bowel syndrome that was closely linked to symptoms; in cirrhosis however, the bowel movement frequency was not related to primary BA conversion[23].
The question now arises – what is the utility of this reduction in secondary/primary bile acid ratio in these situations? It could be argued that the bacteria that are normally suppressed by rifaximin could be responsible for not only secondary BA production, but also the production of other toxic molecules such as indoles and ammonia that can result in harmful effects to the host and part of its mechanism of action may be to reduce their pathogenicity[17, 18]. Bile acids especially CA and DCA can modulate the population of colonic bacteria due to their strong antimicrobial properties[24]. Given the synthetic deficits of CA in bile of cirrhotics, it is likely that overgrowth of bacteria that would usually be suppressed in situations of normal bile synthesis and flow would be promoted i.e. Enterobacteriaceae in the current study[22]. This in turn could potentiate several complications related to bacterial translocation due to deficient gut barrier function which are pre-mortal events in advanced cirrhosis[25, 26]. The correlation of secondary/primary BA ratios and secondary BAs with families present in healthy human flora such as Lachnospiraceae, Ruminococcaceae and Blautia is likely due to the ability of some species in these families to perform 7α-dehydroxylation and indicating the presence of a “normal flora”. It has been shown that 7α-dehydroxylation from primary to secondary BAs provides an energy advantage to the bacteria[5]. However the exact role of secondary BAs in humans is still unclear. Secondary BAs have membrane destabilizing actions on the microbiota and on the gut epithelium[24]. Intestinal permeability increases and barrier function deteriorates with worsening cirrhosis; therefore it could be speculated that the reduction in secondary BAs could be a result of an adaptation to prevent this from occurring[27]. It could also be hypothesized that rifaximin which is clearly associated with good outcomes in early and advanced cirrhosis, acts in part by changing the behavior of the gut microbiota by reducing the production of secondary BAs, that could have a negative influence on the gut barrier. However, further studies are needed to understand this complex interaction between microbiota and bile acids in affecting the intestinal milieu. The findings, also, are not generalizable to severely advanced cirrhotics on absorbable antibiotics since they were excluded.
Therefore, we conclude that cirrhosis is associated with a decrease in total fecal BA concentration and a decrease in secondary BA conversion from primary BAs compared to controls, which worsens with the severity of the liver disease. There was also a significant increase in serum BAs in cirrhotic patients. This change in fecal bile acid patterns is modulated by the differential presence of key microbial families that may have important implications for the pathogenesis of cirrhosis. Further studies are needed to identify BAs as a therapeutic target for gut bacterial modification to prevent progression and complications of cirrhosis.
Supplementary Material
Acknowledgments
Financial support: This work was supported by grant U01AT004428 from the National Center for Complementary and Alternative Medicine, grant RO1AA020203 from the National Institute on Alcohol Abuse and Alcoholism, grant RO1DK087913 from the National Institute of Diabetes and Digestive and Kidney Diseases, VA Merit Review grant BX0013280-01, the McGuire Research Institute and partly by an investigator-initiated grant from Salix Pharmaceuticals. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- HE
hepatic encephalopathy
- MELD
model for end-stage liver disease
- BA
bile acids
- CA
cholic acid
- CDCA
chenodeoxycholic acid
- LCA
lithocholic acid
- DCA
deoxycholic acid
- UDCA
ursodeoxycholic acid
- GBA
glycine conjugated bile acid
- TBA
taurine conjugated bile acid
- BA3S
bile acid 3-sulphate
- LH-PCR
Length Heterogeneity PCR
- MTPS
Multitag Pyrosequencing
- HPLC
high-performance liquid chromatography
- LC-ESI-MS/MS
liquid chromatography-electrospray ionization tandem mass spectrometry
Footnotes
Presentation: Portions of this manuscript were an oral presentation the International Liver Congress by EASL held in 2012 in Barcelona
Conflicts of interest: JSB is a consultant for Salix Pharmaceuticals
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bajaj JS, Ridlon JM, Hylemon PB, Thacker LR, Heuman DM, Smith S, et al. Linkage of gut microbiome with cognition in hepatic encephalopathy. Am J Physiol Gastrointest Liver Physiol. 2012;302:G168–175. doi: 10.1152/ajpgi.00190.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, et al. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. Am J Physiol Gastrointest Liver Physiol. 2012;303:G675–685. doi: 10.1152/ajpgi.00152.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Yang F, Lu H, Wang B, Lei D, Wang Y, et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology. 2011;54:562–572. doi: 10.1002/hep.24423. [DOI] [PubMed] [Google Scholar]
- 4.Islam KB, Fukiya S, Hagio M, Fujii N, Ishizuka S, Ooka T, et al. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology. 2011;141:1773–1781. doi: 10.1053/j.gastro.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 5.Ridlon JM, Kang DJ, Hylemon PB. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Vlahcevic ZR, Buhac I, Bell CC, Jr, Swell L. Abnormal metabolism of secondary bile acids in patients with cirrhosis. Gut. 1970;11:420–422. doi: 10.1136/gut.11.5.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swann JR, Want EJ, Geier FM, Spagou K, Wilson ID, Sidaway JE, et al. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc Natl Acad Sci U S A. 2011;108 (Suppl 1):4523–4530. doi: 10.1073/pnas.1006734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamer HM, De Preter V, Windey K, Verbeke K. Functional analysis of colonic bacterial metabolism: relevant to health? Am J Physiol Gastrointest Liver Physiol. 2011;302:G1–9. doi: 10.1152/ajpgi.00048.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muto A, Takei H, Unno A, Murai T, Kurosawa T, Ogawa S, et al. Detection of Delta4-3-oxo-steroid 5beta-reductase deficiency by LC-ESI-MS/MS measurement of urinary bile acids. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;900:24–31. doi: 10.1016/j.jchromb.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 10.Gillevet P, Sikaroodi M, Keshavarzian A, Mutlu EA. Quantitative assessment of the human gut microbiome using multitag pyrosequencing. Chem Biodivers. 2010;7:1065–1075. doi: 10.1002/cbdv.200900322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devkota S, Wang Y, Musch MW, Leone V, Fehlner-Peach H, Nadimpalli A, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature. 2012;487:104–108. doi: 10.1038/nature11225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maillette de Buy Wenniger L, Beuers U. Bile salts and cholestasis. Dig Liver Dis. 2010;42:409–418. doi: 10.1016/j.dld.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 13.McCormick WC, 3rd, Bell CC, Jr, Swell L, Vlahcevic ZR. Cholic acid synthesis as an index of the severity of liver disease in man. Gut. 1973;14:895–902. doi: 10.1136/gut.14.11.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology. 2004;39:1441–1449. doi: 10.1002/hep.20194. [DOI] [PubMed] [Google Scholar]
- 15.Duboc H, Rainteau D, Rajca S, Humbert L, Farabos D, Maubert M, et al. Increase in fecal primary bile acids and dysbiosis in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24:513–520. e246–517. doi: 10.1111/j.1365-2982.2012.01893.x. [DOI] [PubMed] [Google Scholar]
- 16.Bajaj JS, Gillevet PM, Patel NR, Ahluwalia V, Ridlon JM, Kettenmann B, et al. A longitudinal systems biology analysis of lactulose withdrawal in hepatic encephalopathy. Metab Brain Dis. 2012;27:205–215. doi: 10.1007/s11011-012-9303-0. [DOI] [PubMed] [Google Scholar]
- 17.Riggio O, Mannaioni G, Ridola L, Angeloni S, Merli M, Carla V, et al. Peripheral and splanchnic indole and oxindole levels in cirrhotic patients: a study on the pathophysiology of hepatic encephalopathy. Am J Gastroenterol. 2010;105:1374–1381. doi: 10.1038/ajg.2009.738. [DOI] [PubMed] [Google Scholar]
- 18.Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071–1081. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- 19.Flores J, Dupont HL, Jiang ZD, Okhuysen PC, Melendez-Romero JH, Gonzalez-Estrada A, et al. A randomized, double-blind, pilot study of rifaximin 550 mg versus placebo in the prevention of travelers’ diarrhea in Mexico during the dry season. J Travel Med. 2011;18:333–336. doi: 10.1111/j.1708-8305.2011.00549.x. [DOI] [PubMed] [Google Scholar]
- 20.Brown EL, Xue Q, Jiang ZD, Xu Y, Dupont HL. Pretreatment of epithelial cells with rifaximin alters bacterial attachment and internalization profiles. Antimicrob Agents Chemother. 2009;54:388–396. doi: 10.1128/AAC.00691-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darkoh C, Lichtenberger LM, Ajami N, Dial EJ, Jiang ZD, DuPont HL. Bile acids improve the antimicrobial effect of rifaximin. Antimicrob Agents Chemother. 2010;54:3618–3624. doi: 10.1128/AAC.00161-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang ZD, Ke S, Dupont HL. Rifaximin-induced alteration of virulence of diarrhoea-producing Escherichia coli and Shigella sonnei. Int J Antimicrob Agents. 2010;35:278–281. doi: 10.1016/j.ijantimicag.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Duboc H, Rainteau D, Rajca S, Humbert L, Farabos D, Maubert M, et al. Increase in fecal primary bile acids and dysbiosis in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24:513–e247. doi: 10.1111/j.1365-2982.2012.01893.x. [DOI] [PubMed] [Google Scholar]
- 24.Kurdi P, Kawanishi K, Mizutani K, Yokota A. Mechanism of growth inhibition by free bile acids in lactobacilli and bifidobacteria. J Bacteriol. 2006;188:1979–1986. doi: 10.1128/JB.188.5.1979-1986.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008;28:26–42. doi: 10.1055/s-2008-1040319. [DOI] [PubMed] [Google Scholar]
- 26.Teltschik Z, Wiest R, Beisner J, Nuding S, Hofmann C, Schoelmerich J, et al. Intestinal bacterial translocation in cirrhotic rats is related to compromised paneth cell antimicrobial host defence. Hepatology. 2011 doi: 10.1002/hep.24789. [DOI] [PubMed] [Google Scholar]
- 27.Scarpellini E, Valenza V, Gabrielli M, Lauritano EC, Perotti G, Merra G, et al. Intestinal permeability in cirrhotic patients with and without spontaneous bacterial peritonitis: is the ring closed? Am J Gastroenterol. 2010;105:323–327. doi: 10.1038/ajg.2009.558. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.