Abstract
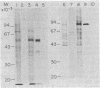
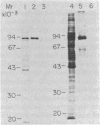
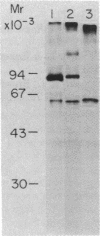
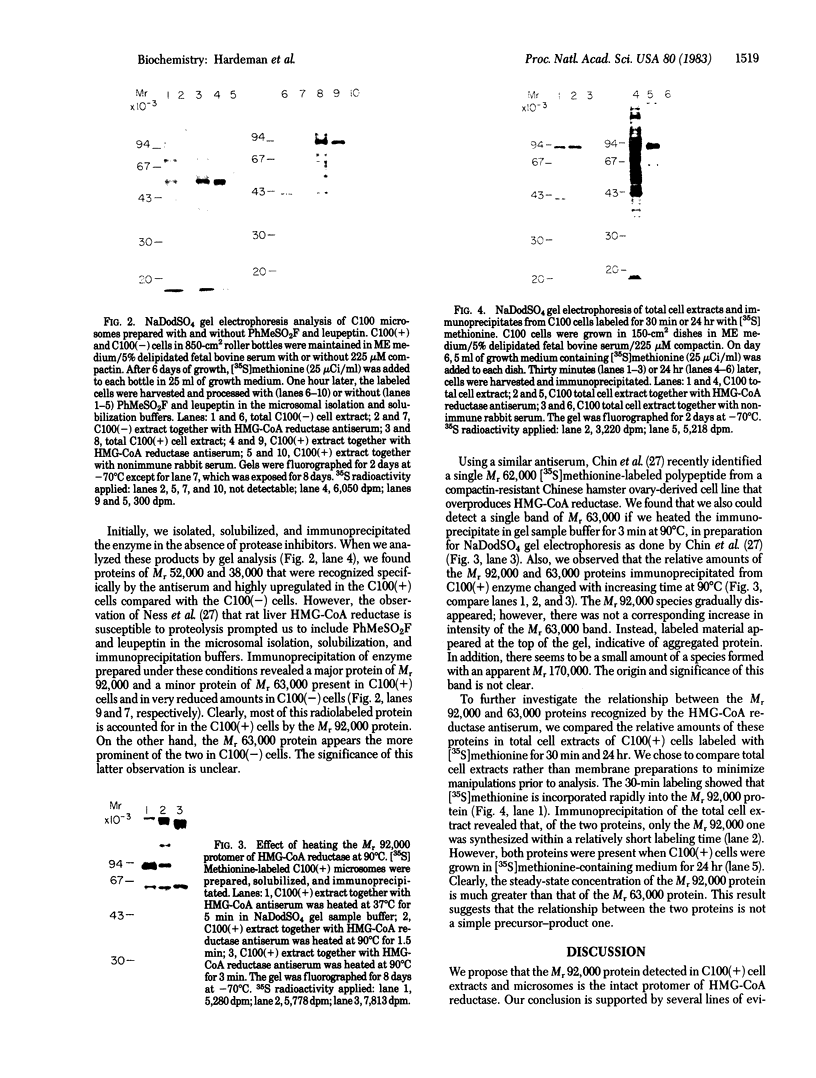
We describe a cell line, designated C100, that displays a 100-fold increase in the major regulatory enzyme of the cholesterol biosynthetic pathway, 3-hydroxy-3-methylglutaryl-coenzyme A reductase [HMG-CoA; mevalonate:NADP+ oxido-reductase (CoA-acylating), EC 1.1.1.34]. Immunoprecipitation of [35S]methionine-labeled enzyme from C100 microsomal membranes prepared in the presence of the protease inhibitors phenyl-methylsulfonyl fluoride and leupeptin revealed two up regulated proteins: a major band of Mr 92,000 and a minor band of Mr 63,000. We conclude that the Mr 92,000 protein is probably the intact form of HMG-CoA reductase protomer based on the following criteria. (i) It is a highly up regulated microsomal membrane protein that coincides with the increase in HMG-CoA reductase specific activity in this cell line. (ii) It is recognized by a specific HMG-CoA reductase antiserum under a variety of stringencies. (iii) Isolation and solubilization of [35S]methionine-labeled C100 microsomal membranes in the absence of protease inhibitors resulted in the disappearance of the Mr 92,000 protein and the appearance of two proteins of Mr 52,000 and 38,000. (iv) Analysis of cells labeled for 30 min with [35S]methionine, well under the half-life of HMG-CoA reductase, revealed only the Mr 92,000 protein to be present in total cell extract. (v) The previously reported single immunoprecipitation polypeptide for HMG-CoA reductase of Mr 62,000 [Chin, D. J., Luskey, K. L., Anderson, R. G. W., Faust, J. R., Goldstein, J. L. & Brown, M. S. (1982) Proc. Natl. Acad. Sci. USA 79, 1185-1189] can be isolated and appears to be the result of both proteolysis and sample preparation for NaDodSO4 gel electrophoresis. Analysis of C100 cells labeled with [35S]methionine for 24 hr indicates that the predominant steady-state form of the enzyme is the Mr 92,000, rather than the Mr 63,000, protein, further suggesting that the two proteins do not have a classical precursor-product relationship.
Keywords: undegraded protomer, regulation of cholesterol synthesis
Full text
PDF

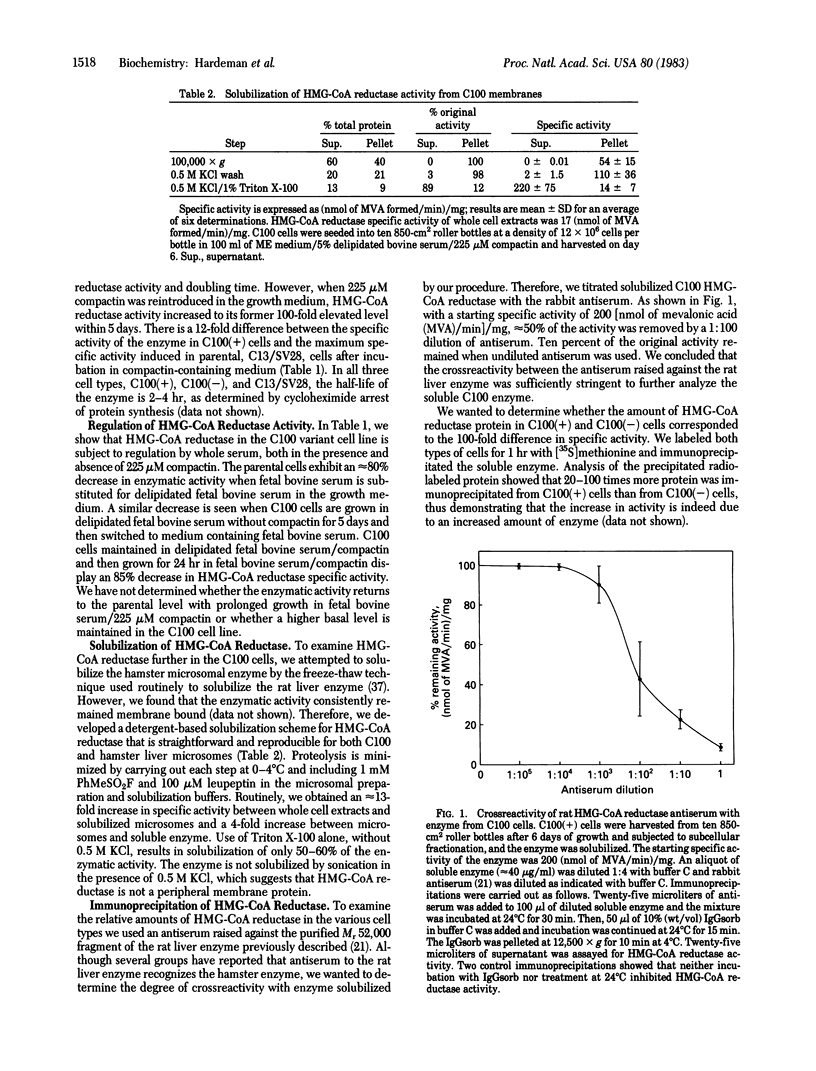

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arebalo R. E., Hardgrave J. E., Scallen T. J. The in vivo regulation of rat liver 3-hydroxy-3-methylglutaryl coenzyme A reductase. Phosphorylation of the enzyme as an early regulatory response following cholesterol feeding. J Biol Chem. 1981 Jan 25;256(2):571–574. [PubMed] [Google Scholar]
- Arebalo R. E., Tormanen C. D., Hardgrave J. E., Noland B. J., Scallen T. J. In vivo regulation of rat liver 3-hydroxy-3-methylglutaryl-coenzyme A reductase: immunotitration of the enzyme after short-term mevalonate or cholesterol feeding. Proc Natl Acad Sci U S A. 1982 Jan;79(1):51–55. doi: 10.1073/pnas.79.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg Z. H., Stonik J. A., Brewer H. B., Jr In vitro and in vivo phosphorylation of rat liver 3-hydroxy-3-methylglutaryl coenzyme A reductase and its modulation by glucagon. J Biol Chem. 1980 Sep 25;255(18):8541–8545. [PubMed] [Google Scholar]
- Brown M. S., Faust J. R., Goldstein J. L., Kaneko I., Endo A. Induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts incubated with compactin (ML-236B), a competitive inhibitor of the reductase. J Biol Chem. 1978 Feb 25;253(4):1121–1128. [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L., Dietschy J. M. Active and inactive forms of 3-hydroxy-3-methylglutaryl coenzyme A reductase in the liver of the rat. Comparison with the rate of cholesterol synthesis in different physiological states. J Biol Chem. 1979 Jun 25;254(12):5144–5149. [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J Lipid Res. 1980 Jul;21(5):505–517. [PubMed] [Google Scholar]
- Cadman E., Bostwick J. R., Eichberg J. Determination of protein by a modified Lowry procedure in the presence of some commonly used detergents. Anal Biochem. 1979 Jul 1;96(1):21–23. doi: 10.1016/0003-2697(79)90548-7. [DOI] [PubMed] [Google Scholar]
- Cavenee W. K., Chen H. W., Kandutsch A. A. Regulation of cholesterol biosynthesis in enucleated cells. J Biol Chem. 1981 Mar 25;256(6):2675–2681. [PubMed] [Google Scholar]
- Chang T. Y., Limanek J. S., Chang C. C. Evidence indicating that inactivation of 3-hydroxy-3-methylglutaryl coenzyme A reductase by low density lipoprotein or by 25-hydroxycholesterol requires mediator protein(s) with rapid turnover rate. J Biol Chem. 1981 Jun 25;256(12):6174–6180. [PubMed] [Google Scholar]
- Chin D. J., Luskey K. L., Anderson R. G., Faust J. R., Goldstein J. L., Brown M. S. Appearance of crystalloid endoplasmic reticulum in compactin-resistant Chinese hamster cells with a 500-fold increase in 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1185–1189. doi: 10.1073/pnas.79.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin D. J., Luskey K. L., Faust J. R., MacDonald R. J., Brown M. S., Goldstein J. L. Molecular cloning of 3-hydroxy-3-methylglutaryl coenzyme a reductase and evidence for regulation of its mRNA. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7704–7708. doi: 10.1073/pnas.79.24.7704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards P. A., Lemongello D., Kane J., Shechter I., Fogelman A. M. Properties of purified rat hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase and regulation of enzyme activity. J Biol Chem. 1980 Apr 25;255(8):3715–3725. [PubMed] [Google Scholar]
- Faust J. R., Goldstein J. L., Brown M. S. Synthesis of ubiquinone and cholesterol in human fibroblasts: regulation of a branched pathway. Arch Biochem Biophys. 1979 Jan;192(1):86–99. doi: 10.1016/0003-9861(79)90074-2. [DOI] [PubMed] [Google Scholar]
- Faust J. R., Luskey K. L., Chin D. J., Goldstein J. L., Brown M. S. Regulation of synthesis and degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase by low density lipoprotein and 25-hydroxycholesterol in UT-1 cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5205–5209. doi: 10.1073/pnas.79.17.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E., Rothman J. E. Transport of vesicular stomatitis virus glycoprotein in a cell-free extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3870–3874. doi: 10.1073/pnas.77.7.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Havel C., Hansbury E., Scallen T. J., Watson J. A. Regulation of cholesterol synthesis in primary rat hepatocyte culture cells. Possible regulatory site at sterol demethylation. J Biol Chem. 1979 Oct 10;254(19):9573–9582. [PubMed] [Google Scholar]
- Heller R. A., Gould R. G. Solubilization and partial purification of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase. Biochem Biophys Res Commun. 1973 Feb 5;50(3):859–865. doi: 10.1016/0006-291x(73)91324-7. [DOI] [PubMed] [Google Scholar]
- Huneeus V. Q., Wiley M. H., Siperstein M. D. Isopentenyladenine as a mediator of mevalonate-regulated DNA replication. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5842–5846. doi: 10.1073/pnas.77.10.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingebritsen T. S., Geelen M. J., Parker R. A., Evenson K. J., Gibson D. M. Modulation of hydroxymethylglutaryl-CoA reductase activity, reductase kinase activity, and cholesterol synthesis in rat hepatocytes in response to insulin and glucagon. J Biol Chem. 1979 Oct 25;254(20):9986–9989. [PubMed] [Google Scholar]
- Ingebritsen T. S., Parker R. A., Gibson D. M. Regulation of liver hydroxymethylglutaryl-CoA reductase by a bicyclic phosphorylation system. J Biol Chem. 1981 Feb 10;256(3):1138–1144. [PubMed] [Google Scholar]
- James M. J., Kandutsch A. A. Inter-relationships between dolichol and sterol synthesis in mammalian cell cultures. J Biol Chem. 1979 Sep 10;254(17):8442–8446. [PubMed] [Google Scholar]
- Jenke H. S., Löwel M., Berndt J. In vivo effect of cholesterol feeding on the short term regulation of hepatic hydroxymethylglutaryl coenzyme A reductase during the diurnal cycle. J Biol Chem. 1981 Sep 25;256(18):9622–9625. [PubMed] [Google Scholar]
- Kaneko I., Hazama-Shimada Y., Endo A. Inhibitory effects on lipid metabolism in cultured cells of ML-236B, a potent inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme-A reductase. Eur J Biochem. 1978 Jun 15;87(2):313–321. doi: 10.1111/j.1432-1033.1978.tb12380.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laskey R. A. The use of intensifying screens or organic scintillators for visualizing radioactive molecules resolved by gel electrophoresis. Methods Enzymol. 1980;65(1):363–371. doi: 10.1016/s0076-6879(80)65047-2. [DOI] [PubMed] [Google Scholar]
- Nambudiri A. M., Ranganathan S., Rudney H. The role of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in the regulation of ubiquinone synthesis in human fibroblasts. J Biol Chem. 1980 Jun 25;255(12):5894–5899. [PubMed] [Google Scholar]
- Ness G. C., Spindler C. D., Benton G. A. Characteristics of magnesium-adenosine triphosphate-dependent inactivators of 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Biol Chem. 1980 Oct 10;255(19):9013–9016. [PubMed] [Google Scholar]
- Ness G. C., Way S. C., Wickham P. S. Proteinase involvement in the solubilization of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Biochem Biophys Res Commun. 1981 Sep 16;102(1):81–85. doi: 10.1016/0006-291x(81)91491-1. [DOI] [PubMed] [Google Scholar]
- Rodwell V. W., Nordstrom J. L., Mitschelen J. J. Regulation of HMG-CoA reductase. Adv Lipid Res. 1976;14:1–74. doi: 10.1016/b978-0-12-024914-5.50008-5. [DOI] [PubMed] [Google Scholar]
- Rothblat G. H., Arbogast L. Y., Ouellette L., Howard B. V. Preparation of delipidized serum protein for use in cell culture systems. In Vitro. 1976 Aug;12(8):554–557. doi: 10.1007/BF02797438. [DOI] [PubMed] [Google Scholar]
- Ryan J., Hardeman E. C., Endo A., Simoni R. D. Isolation and characterization of cells resistant to ML236B (compactin) with increased levels of 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Biol Chem. 1981 Jul 10;256(13):6762–6768. [PubMed] [Google Scholar]
- Schroepfer G. J., Jr Sterol biosynthesis. Annu Rev Biochem. 1981;50:585–621. doi: 10.1146/annurev.bi.50.070181.003101. [DOI] [PubMed] [Google Scholar]
- Shapiro D. J., Nordstrom J. L., Mitschelen J. J., Rodwell V. W., Schimke R. T. Micro assay for 3-hydroxy-3-methylglutaryl-CoA reductase in rat liver and in L-cell fibroblasts. Biochim Biophys Acta. 1974 Dec 29;370(2):369–377. doi: 10.1016/0005-2744(74)90098-9. [DOI] [PubMed] [Google Scholar]
- Sinensky M., Armagast S., Mueller G., Torget R. Somatic cell genetic analysis of regulation of expression of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6621–6623. doi: 10.1073/pnas.77.11.6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinensky M., Torget R., Edwards P. A. Radioimmune precipitation of 3-hydroxy-3-methylglutaryl coenzyme A reductase from Chinese hamster fibroblasts. Effect of 25-hydroxycholesterol. J Biol Chem. 1981 Nov 25;256(22):11774–11779. [PubMed] [Google Scholar]
- Sinensky M., Torget R., Schnitzer-Polokoff R., Edwards P. A. Analysis of regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase in a somatic cell mutant auxotrophic for mevalonate. J Biol Chem. 1982 Jul 10;257(13):7284–7286. [PubMed] [Google Scholar]