Abstract
Everyday action impairments often are observed in demented older adults, and they are common potential barriers to functional independence. We evaluated whether the ability to segment and efficiently encode activities is related to the ability to execute activities. Further, we evaluated whether brain regions important for segmentation also were important for action performance. Cognitively healthy older adults and those with very mild or mild dementia of the Alzheimer's type watched and segmented movies of everyday activities and then completed the Naturalistic Action Test. Structural MRI was used to measure volume in the dorsolateral prefrontal cortex (DLPFC), medial temporal lobes (MTL), posterior cortex, and anterior cingulate cortex (ACC). Dementia status and the ability to segment everyday activities strongly predicted naturalistic action performance, and MTL volume largely accounted for this relationship. In addition, the current results supported the Omission-Commission Model: Different cognitive and neurological mechanisms predicted different types of action error. Segmentation, dementia severity, and MTL volume predicted everyday omission errors, DLPFC volume predicted commission errors, and ACC volume predicted action additions. These findings suggest that event segmentation may be critical for effective action production, and that the segmentation and production of activities may recruit the same event representation system.
Keywords: Perception, Segmentation, Action performance, MTL volume, Dementia
1. Introduction
Alzheimer's disease (AD) is associated with impairments in memory and attention. These impairments are salient and have been well studied. AD also impairs one's ability to perform everyday tasks. These impairments are less well studied but perhaps equally important. Clinicians often collect reports of instrumental activities of daily living to assess an individual's ability to live independently. The patient or caregiver answers questions about complex activities related to preparing food, housekeeping, taking medications, and managing finances (Lawton & Brody, 1969). Individuals who are unable to perform these types of instrumental activities independently meet the criteria for AD. Although these qualitative reports are important for diagnosis, they cannot distinguish the cognitive mechanisms underlying the functional deficit (Schwartz, Segal, Veramonti, Ferraro, & Buxbaum, 2002). Further, their subjective nature calls into question their accuracy, particularly in the earliest stages of dementia (see Gold, 2012 for a review).
Direct measurements of everyday action performance are a valuable complement to subjective reports, and have provided further evidence for action impairments in AD. The Naturalistic Action Test (NAT) was created to simulate the complex nature of real-world activities of daily living by requiring participants to complete naturalistic actions (Schwartz et al., 2002), and performance is correlated with subjective reports of daily living (Giovannetti, Libon, Buxbaum, & Schwartz, 2002; Schwartz et al., 2002). Naturalistic actions are everyday tasks that often require using objects to complete a series of steps in order to achieve a goal. One advantage of the NAT is that it explicitly assays different types of error. Error types include omitting parts of an activity (omissions), completing parts of an activity incorrectly (commissions), and performing task-irrelevant activities (action additions). Importantly, this taxonomy of error types provides a more specific method of assessing everyday action deficits that are consistent with different neurological conditions such as traumatic brain injuries (Schwartz et al., 1998), strokes (Buxbaum, Schwartz, & Montgomery, 1998; Schwartz et al., 1999), and AD (Giovannetti et al., 2002).
In particular, older adults with varying degrees of AD demonstrate different error patterns: cognitively healthy older adults and those with mild cognitive impairment produce a higher proportion of commission than omission errors, whereas participants with AD produce a similar proportion of omission and commission errors (Giovannetti et al., 2008). This dissociation in error patterns indicates that omissions and commissions are fundamentally different measures of action performance, separable by data reduction techniques. Further, these error types are largely associated with different cognitive mechanisms–omissions are related to memory and global cognitive functioning (i.e., MMSE scores), whereas commissions are related to measures of executive function and working memory (Giovannetti et al., 2008, 2012; Kessler, Giovannetti, & MacMulen, 2007).
Some researchers have speculated that omission errors are due to a semantic memory deficit (Bier & Macoir, 2010; Buxbaum et al., 1998; Ochipa, Rothi, & Heilman, 1992). That is, demented participants (or any other group that demonstrates high rates of omission errors) may have insufficient task knowledge, a poor representation of the objects needed to complete the task, or both (e.g., De Renzi & Lucchelli, 1988, Hartmann, Goldenberg, Daumuller, & Hermsdorfer, 2005). Commission errors, on the other hand, could be due to age-related declines in executive control and working memory capacity (e.g., Mahurin, DeBettignies, & Pirozzolo, 1991). In other words, cognitively healthy older adults should have the appropriate task knowledge and the ability to keep the goal in mind, but working memory limitations may lead to an individual performing the task inappropriately. Giovannetti, Schwartz, and Buxbaum (2007) further evaluated the role of working memory in commission errors by assessing errors for young adults who performed actions either under full attention or divided attention conditions. Young adults produced more commission errors under divided attention (i.e., when working memory was taxed) than under full attention conditions.
Successful performance of everyday tasks requires action planning and organization, which likely depends on general cognitive abilities including working memory efficiency and semantic knowledge. Effective planning also may depend on the ability to construct an effective representation of the parts and subparts of the activity being planned. We hypothesized that some of the same event representations processing mechanisms that are used in effective action planning also are used during action perception. For example, representations (or scripts) of learned actions may help us predict what other people will do and they may guide our own preparations to perform an action (e.g., Barbey, Krueger, & Grafman, 2009). Lesions in the frontal lobes often affect the organization of these action representations, and thus action planning abilities (e.g., Sirigu et al., 1995); however, these representations may also be affected by the neuropathology associated with Alzheimer's disease. Thus, we asked whether the ability to perceive event structure when observing goal-directed activity is related to the ability to organize and execute goal-directed activity. In other words, in people at risk of disorders of action performance, is action performance related to action perception?
1.1. Segmenting continuous activity
Individuals perceive a continuous stream of activity on a daily basis; however, this activity is not stored as a continuous reel but rather as discrete events. For instance, when thinking about what happened last weekend, an individual will recount the activity in separate events (e.g., went to the gym, went grocery shopping, did laundry, went out to dinner). According to Event Segmentation Theory (EST), this process of segmenting activity into events occurs spontaneously during perception (Zacks, Speer, Swallow, Braver, & Reynolds, 2007). EST proposes that event boundaries result from updating working memory representations in response to errors in perception prediction. Information relevant to the current event is captured by an event model, which is a representation of the current activity that is held active in working memory. Event models are comprised of current perceptual input as well as relevant information from episodic and semantic memory. The maintenance and manipulation of this information may be supported by medial temporal structures (Bailey et al., in press) and by lateral prefrontal cortex (PFC; Grafman, 1995; Zacks et al., 2007). The contents of an event model can influence how the perceptual information is processed in posterior regions including the inferior temporal cortex (IT), the human MT complex (MT+), and posterior superior temporal sulcus (pSTS; Zacks et al., 2007). Further, information in an event model aids in accurate predictions about what will happen in the near future. For example, when watching a man set a table for dinner, individuals use episodic memories of setting a table as well as semantic memory (e.g., scripts and schemas related to preparing for a meal) to help them make predictions about what the man will do next. As the man is in the middle of one part of the activity, such as arranging the dinner plates, information in the event model remains stable and the activity is predictable. Individuals likely predict that after the man places the plate in front of the first chair, he will do the same for the next chair; thus, the predictions are fairly accurate. EST proposes that the anterior cingulate cortex (ACC) is responsible for maintaining these predictions and also for assessing their accuracy by comparing them to what actually happens. This comparison process then produces an error signal, which accumulates as the activity becomes less predictable. For instance, after all of the plates are arranged, it is more difficult to predict what the man will do next. Will he arrange the silverware? Will he arrange the glasses? Will he walk into the kitchen? Prediction error increases because information relevant to arranging the dinner plates is no longer useful, thus the event model must be updated to match the current event. If he began placing the silverware, then information relevant to the proper arrangement of silverware should now be contained in the event model. It is at these points in an activity (i.e., when prediction error spikes and event models are updated) that an event boundary is perceived.
Importantly, event boundaries help people chunk activity into meaningful events, which has consequences for later retrieval. Individuals who are better able to identify these event boundaries are better able to remember the activity at a later point (Bailey et al., in press; Kurby & Zacks, 2011; Sargent et al., in preparation; Zacks, Speer, Vettel, & Jacoby, 2006). If the ability to organize and chunk activity during perception has an effect on how that activity is remembered, could that ability be related to how well one performs everyday tasks? And if perception and action are related, which neural mechanisms mediate this relationship? We evaluated whether the integrity of several brain regions thought to be involved in event segmentation also was related to NAT performance. Finally, we examined whether cognitive variables were related to different aspects of action performance. Specifically, we examined working memory, semantic memory, and script knowledge given their relationships with action representations in individuals with Alzheimer's disease (e.g., Allain et al., 2008; Giovannetti et al., 2008; Grafman et al., 1991).
1.2. Current study
To address these questions, we asked cognitively healthy older adults and those with mild or mild AD to watch and segment three movies of everyday activities into events. Then they completed the NAT, which involved performing activities that were different from those in the movie. The participants also underwent structural MRI scans. The two main goals of the current study were to evaluate (1) whether segmenting an activity during perception is related to performing an activity and, if so, (2) which brain regions mediate the action perception and action performance relationship.
2. Method
This study was conducted as a part of a larger investigation of event segmentation in healthy older adults and those with very mild or mild AD. For data regarding the neural correlates of event segmentation and everyday memory (see Bailey et al., in press); for data regarding genetic predictors of everyday memory (see Bailey et al., in preparation).
2.1. Participants
All participants were recruited through the Knight Alzheimer's Disease Research Center (ADRC) at Washington University in St. Louis. The presence of AD was assessed according to NINCDS-ADRDA standards (Jack Jr. et al., 2001; McKhann et al., 1984). The Clinical Dementia Rating (CDR) scale (Morris, 1993) was then used as a global dementia staging instrument. The CDR is based on a 90-min clinical interview of both the participant and a collateral source (often a spouse, child, or close friend) conducted by a neurologist or a psychiatrist (Morris et al., 2001). This interview assesses changes in participants’ cognitive and functional abilities in the areas of memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care. CDR scores can be 0, 0.5, 1, or 2 indicating no, questionable/very mild, mild, and moderate AD, respectively. Information from the clinical interview and from the collateral source is used to arrive at an etiological diagnosis. Diagnosis and staging of AD is conducted independent of neuropsychological data and is based on intra-individual decline. A staging of CDR 0.5 corresponds closely to the diagnostic category of mild cognitive impairment (Gauthier et al., 2006), and is strongly associated with postmortem Alzheimer's neuropathology (Berg et al., 1998). Data for the Geriatric Depression Scale (GDS), Mini-Mental State Exam (MMSE), and Short Blessed Test as well as performance on other cognitive measures from our current sample are presented in Table 1.
Table 1.
Descriptive statistics for age, mental status, segmentation agreement, NAT, psychometric, and brain volume variables.
| CDR 0 |
CDR 0.5 |
CDR 1 |
||||
|---|---|---|---|---|---|---|
| M | SE | M | SE | M | SE | |
| Age | 75.12 | 1.04 | 78.35 | 1.01 | 76.83 | 2.82 |
| GDS | 0.66 | 0.16 | 1.52 | 0.31 | 2.94 | 0.67 |
| MMSE | 29.18 | 0.23 | 27.89 | 0.42 | 22.53 | 0.74 |
| Short blessed test | 7.97 | 0.24 | 10.26 | 0.72 | 15.65 | 1.44 |
| Breakfast agreement | 0.39 | 0.02 | 0.38 | 0.02 | 0.29 | 0.03 |
| Library agreement | 0.46 | 0.01 | 0.39 | 0.02 | 0.32 | 0.04 |
| Party agreement | 0.43 | 0.02 | 0.38 | 0.02 | 0.29 | 0.03 |
| NAT score | 4.97 | 0.24 | 3.48 | 0.35 | 0.75 | 0.31 |
| NAT omissions | 4.85 | 1.36 | 12.58 | 2.68 | 36.67 | 6.67 |
| NAT commissions | 10.74 | 2.27 | 14.56 | 2.38 | 25.70 | 6.17 |
| NAT action additions | 1.91 | 0.17 | 1.90 | 0.19 | 3.17 | 1.22 |
| Script knowledge | 7.13 | 0.48 | 5.12 | 0.42 | 3.64 | 0.86 |
| WAIS information | 22.09 | 0.92 | 19.29 | 0.90 | 12.75 | 2.24 |
| Boston naming | 55.47 | 1.08 | 53.16 | 1.02 | 40.33 | 4.42 |
| Animal naming | 21.03 | 0.87 | 16.81 | 0.95 | 12.42 | 1.43 |
| Mental control | 7.71 | 0.29 | 7.19 | 0.30 | 4.75 | 0.98 |
| Digit span forward | 7.09 | 0.17 | 6.74 | 0.17 | 6.33 | 0.43 |
| Digit span backward | 5.12 | 0.22 | 4.52 | 0.20 | 3.83 | 0.39 |
| Letter fluency | 9.09 | 0.36 | 7.35 | 0.61 | 3.25 | 1.26 |
| Entorhinal volume | 3853.85 | 132.56 | 3443.82 | 130.55 | 2552.15 | 287.57 |
| Hippocampal volume | 7458.54 | 163.52 | 6513.32 | 186.87 | 5665.65 | 294.22 |
| Parahippocampal volume | 3596.98 | 92.67 | 3455.41 | 123.10 | 3449.77 | 495.39 |
| DLPFC volume | 27107.17 | 528.26 | 25830.57 | 670.55 | 26318.39 | 1226.49 |
| Rostral ACC volume | 4119.95 | 207.16 | 3896.44 | 141.09 | 3553.34 | 380.91 |
| Caudal ACC volume | 3684.50 | 128.31 | 3509.70 | 123.13 | 3223.02 | 267.29 |
| Posterior cingulate volume | 5927.69 | 144.94 | 5699.61 | 145.52 | 5265.80 | 354.30 |
| Precuneus volume | 16871.99 | 373.68 | 16412.04 | 457.55 | 14828.08 | 432.76 |
| Cuneus volume | 5736.99 | 173.68 | 5440.73 | 174.47 | 5636.17 | 237.17 |
Note: GDS = Geriatric Depression Scale, which range from 0 to 30. Scores on the short blessed test can range from 0 to 28. MMSE = Mini mental state examination, which can range from 0 to 30. WAIS information = information subtest from the Wechsler Adult Intelligence Scale. ACC = anterior cingulate cortex.
Participants were excluded based on the presence of confounding neurological disorders (e.g., Parkinson's disease, Huntington's disease), neurological damage (e.g., due to seizures or head trauma), other types of dementia (e.g., vascular, Lewy Bodies), cerebrovascular disease, and mood disorders. We recruited 34 (20 female) CDR 0 individuals, 31 (9 female) CDR 0.5 individuals (very mild AD), and 12 (3 female) CDR 1 individuals (mild AD). Participants’ mean age did not differ significantly by CDR group (CDR 0: M = 75.1, SE = 1.0; CDR 0.5: M = 78.3, SE = 1.0; CDR 1: M = 76.8, SE = 2.3), F(2,76) = 2.20, p = 0.12.
2.2. Materials
2.2.1. Segmentation
Participants watched four movies: one practice movie and three experimental movies. The practice movie involved a male actor building a ship out of Legos (155 s duration). The experimental movies involved a female actor preparing breakfast (329 s), a male actor decorating a room for a party (376 s), and a female actor checking out a book at a library (249 s). Fig. 1 depicts still frames from each of the experimental movies. Participants watched and segmented each movie twice – once at a coarse grain level and once at a fine grain level.1 As they watched the movies, participants were instructed to press the spacebar each time they thought one large (coarse grain) or small (fine grain) meaningful unit of activity ended and another began. Segmentation agreement is the extent to which a participant's segmentation locations correlated with the normative2 segmentation locations of the entire sample. Segmentation agreement was calculated with point-biserial correlations that were scaled to control for individual differences in the number of times they pressed the spacebar (see Kurby & Zacks, 2011). Segmentation agreement was averaged across all three movies and both grain sizes. Values ranged from 0 to 1, with larger values indicating better agreement with the group. Participants also completed three memory measures following each movie: free recall of the activity, forced-choice recognition of still frames from the movie, and an order memory test with still frames from the movie.
Fig. 1.
Stills taken from each of the three experimental movies: making breakfast, decorating for a party, and planting window boxes. Durations were 329 s, 376 s, and 354 s.
2.2.2. Naturalistic action test (NAT)
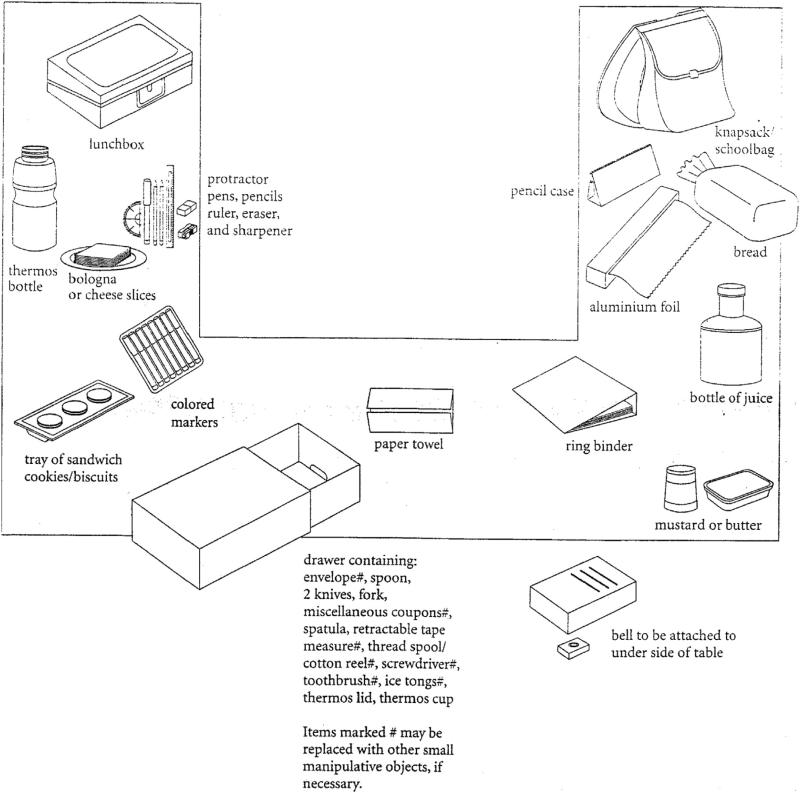
The NAT consists of three subtasks; however in the current study we only administered the third task because previous research has shown it to be the most challenging, and thus the most discriminative for a population including non-demented people (Schwartz et al., 2002). Participants were instructed to pack (1) a lunchbox with a sandwich, drink, and snack and (2) a schoolbag with school supplies. Participants were seated at a U-shaped table on which necessary objects were arranged (see Fig. 2). An opaque drawer, which contained more necessary objects (e.g., thermos lid, thermos cap, knife) and distractor objects (e.g., screwdriver, spatula, envelope), was also positioned on the table. Participants also were instructed to ring a doorbell attached underneath the tabletop after they packed the lunchbox and after they packed the schoolbag. Performance on the NAT was scored for accomplishment and for errors.
Fig. 2.
Schematic for how the objects were arranged for the NAT Task 3.
2.2.2.1. NAT score
The NAT score was calculated by combining the number of steps accomplished and the number of key errors committed. Task 3 had a total of 10 possible steps that could be accomplished. Six of these steps were for the lunchbox portion, 2 steps were for the schoolbag portion, and 2 were for ringing the doorbell. The NAT score ranged from 0 – less than 5 steps accomplished and 0 or more errors to 6–10 steps accomplished and less than 2 errors.
2.2.2.2. Comprehensive error score
All errors committed on the NAT were recorded regardless of whether they were later corrected. These errors can be classified into one of three error categories: omissions, commissions, and action additions. Errors were scored as omissions when a step or part of a step was not completed (e.g., omitted wrapping the sandwich in aluminum foil or omitted packing the cookies). Errors were scored as commissions when a step was performed, but it was performed inaccurately (e.g., packing more than 8 cookies, packing sandwich then wrapping it inside the lunchbox, or packing pencils into the lunchbox). Errors were scored as action additions when an extra step that was not immediately relevant or necessary to the task was performed (e.g., eating the bologna or packing the lunchbox into the schoolbag). See Giovannetti et al. (2002) for a complete list and description of NAT errors. Omission and commission errors were scored as a standardized error rate as described in the test manual (Schwartz, Buxbaum, Ferraro, Veramonti, & Segal, 2003), whereas action additions were scored as total number committed.
2.2.3. Script knowledge
Participants completed a script knowledge task based on the procedure described by Rosen, Caplan, Sheesley, Rodriguez, and Grafman (2003). In this task, participants were given three minutes to write down, from beginning to end, all the steps involved in each of three everyday activities: shopping for groceries, getting ready for work, and going out to eat. These three activities were chosen from the list of 15 activities because they were the highest frequency, and therefore the most “everyday”, activities (study 1; Rosen et al., 2003). A step was counted as correct if it corresponded to one of the 16 most commonly reported steps for that activity as defined by norms reported by Rosen et al. (2003). Performance was scored as a total number of commonly reported steps mentioned.
2.2.4. Psychometric battery
A cognitive battery (ELSMEM; Storandt, Balota, & Salthouse, 2009) designed to assess a broad spectrum of abilities was administered to all participants, usually a week or two after their annual clinical assessment. Previous work has implicated semantic memory and working memory in action performance; thus, we assessed these two domains. The semantic memory tasks included the participants’ scores on the Information subtest from the Wechsler Adult Intelligence Scale (WAIS; Wechsler, 1997), the Boston Naming Test (Kaplan, Goodglass, & Weintraub, 1983), and Animal Naming (Kaplan et al., 1983). The working memory tasks included the Wechsler Memory Scale (WMS) Mental Control, Digit Span Forward and Backward, and Letter Fluency (Thurstone & Thurstone, 1949).
2.2.5. MRI acquisition and analysis
Participation at the ADRC includes structural MRI scans every other year. Some participants in our current study had undergone multiple scans, and we accessed the scan that occurred closest to their behavioral session. However, some participants (n = 22) had never completed a scan, so the sample included volume estimates for 23 CDR 0, 24 CDR 0.5, and 8 CDR 1 participants.3 T1-weighted MP-RAGE scans (TR = 9.7 ms, TE = 4 ms, TI = 20 ms, 1 mm × 1 mm × 1.25 mm resolution) were obtained for each subject.
Gray matter volume estimates were obtained using FreeSurfer 5.1 image analysis suite and regions of interest (ROIs) were based on the Desikan–Killiany atlas (Desikan et al., 2006). ROIs were the DLPFC, MTL, ACC, and posterior regions. DLPFC was defined as the rostral middle frontal gyrus. MTL was defined as the entorhinal cortex, hippocampus, and parahippocampal gyrus. ACC was defined as the rostral anterior and caudal anterior cingulate cortex. The posterior region was defined as the posterior cingulate cortex, precuneus, and cuneus. Volumes were summed across hemispheres and then normalized to control for intracranial volume using linear regression (e.g., Buckner et al., 2004).
2.3. Procedure
Participants were seated in front of a laptop computer and practiced the coarse-grained event segmentation task using the example movie. After they finished the practice, the experimenter answered any questions and restated the coarse-grained instructions. Then, they segmented the breakfast, party, and library movies, respectively. Immediately following each movie, the participants completed the recall task, the forced-choice recognition task, and finally the order memory task. After the third movie, they completed the NAT Task 3, followed by the script knowledge test. Next, participants watched each movie again – including the example movie – and segmented at a fine grain. (No memory tests followed this viewing.) Finally, participants provided a saliva sample for DNA analyses. (The event memory tests and DNA analyses were collected for separate projects and will not be discussed further.)
2.4. Data preparation
We screened each cognitive and action variable for values more than 3.5 standard deviations different from the total sample mean (11 bivariate outliers); 2 values (<.1% of the data) met this criterion. Along with missing values (<2% of the data), we replaced these extreme values using the expectation maximization (EM) procedure in SPSS 19.0. All variables were approximately normally distributed (skewness <|1.5|, kurtosis <|1.5|). Descriptive statistics for age, the cognitive variables, and the brain variables are presented in Table 1.
3. Results
Segmentation agreement scores for the three movies were strongly correlated (rs = 0.57–0.61), as were scores on the three semantic memory tasks (rs = .62–.83) and the four working memory tasks (rs = 0.37–0.53). Given that variables representing each construct correlated positively with each other, composite variables were created. The segmentation agreement composite was the average of the z scored agreement values for the breakfast, library, and party movies. The semantic memory composite was the average of the z scored values for the WAIS Information, Boston Naming, and Animal Naming tests. The working memory composite was the average of the z scored values for the WMS Mental Control, Digit Forward, Digit Backward, and Letter Fluency tests. Psychometric tests were organized into these composites based on large-scale confirmatory-factor analyses that have been cross-validated in demented and non-demented samples (Johnson, Storandt, Morris, Langford, & Galvin, 2008).
3.1. Segmentation agreement and NAT performance
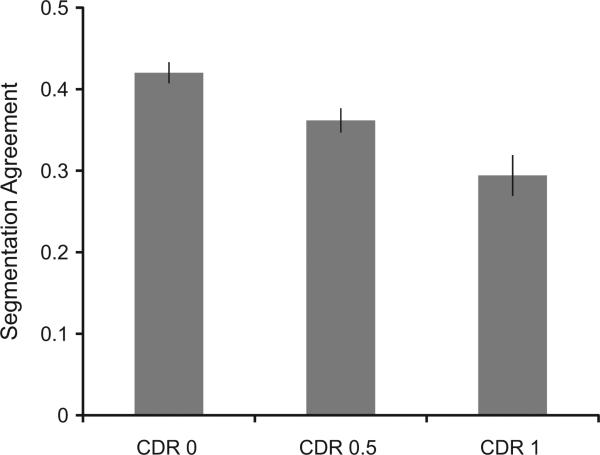
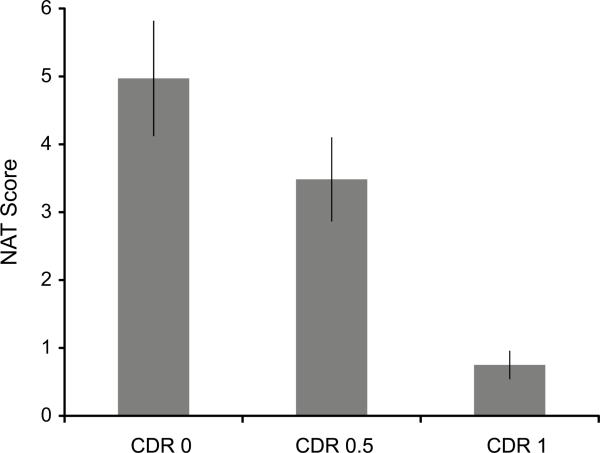
Segmentation agreement scores are presented by CDR group in Fig. 3, and NAT scores are presented by CDR group in Fig. 4. On both measures CDR 0 participants performed better than CDR .5 participants (ts > 2.72, ps < 0.005), who performed better than CDR 1 participants (ts > 3.08, ps < 0.002).4
Fig. 3.
Segmentation agreement scores by CDR group. Error bars are standard errors of the mean.
Fig. 4.
NAT scores ranging from 0–6 by CDR group. Error bars are standard errors of the mean.
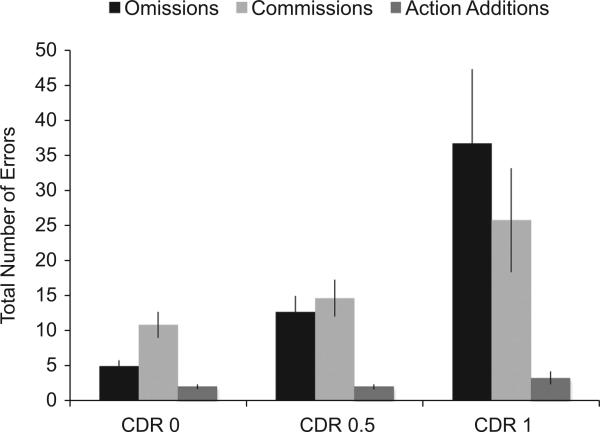
The mean total number of each error type committed on the NAT is presented by CDR group in Fig. 5. A Group (CDR 0, 0.5, 1) × Error Type (omissions, commissions, action additions) mixed ANOVA revealed a significant main effect of Group, F(2,74) = 23.38, p < 0.001, η2 = 0.15, a significant main effect of error type, F(2,74) = 35.16, p < 0.001, η2 = 0.28, and a significant group × error type interaction, F(4,148) = 7.63, p < 0.001, η2 = 0.12. Planned t-test comparisons indicated that the CDR 1 group had the highest number of errors (M = 65.53, SE = 8.25), followed by the CDR 0.5 group (M = 29.05, SE = 3.98), and then the CDR 0 group (M = 17.50, SE = 2.78). This group difference was significant for omission errors, F(2,74) = 22.71, p < 0.001, and for commission errors, F(2,74) = 4.57, p = 0.013, but not for action additions, F(2,74) = 2.28, p = 0.11.
Fig. 5.
Mean number of errors (omission, commissions, and action additions) committed on the NAT. Error bars are standard errors of the mean.
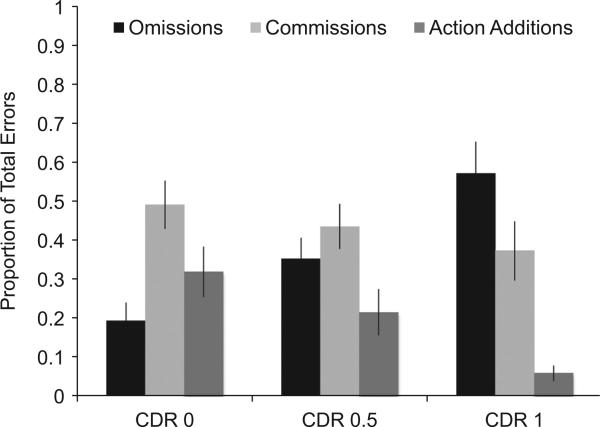
Because the total number of errors differed significantly between CDR groups, we computed their errors as proportion scores to better evaluate the pattern of errors by group. For each participant, we calculated the proportion of the total errors that were omissions, commissions, and action additions (see Fig. 6). A one-way MANOVA revealed a significant multivariate main effect of CDR group, Wilks’ λ = 0.807, F(4, 142) = 4.01, p = 0.004, ηp2 = 0.102. Given the significance of the overall test, the univariate main effects were evaluated. A significant univariate main effect of CDR group was observed for proportion of omissions, F(2,74) = 8.26, p = 0.001, η2 = 0.187, and the main effect of CDR group for proportion of action additions approached significance, F(2,74) = 3.01, p = 0.055, η2 = 0.077. A Tukey B post hoc test revealed that CDR 1 participants (M = 0.57, SE = 0.08) committed a significantly higher proportion of omission errors than did the CDR 0 (M = 0.19, SE = 0.05) and CDR 0.5 participants (M = 0.35, SE = 0.05).
Fig. 6.
Mean proportion of each error type from total errors. Error bars are standard errors of the mean.
3.2. Relations between segmentation agreement and NAT performance
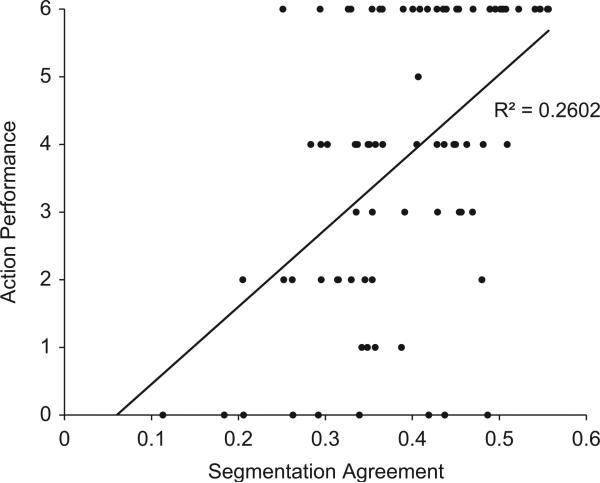
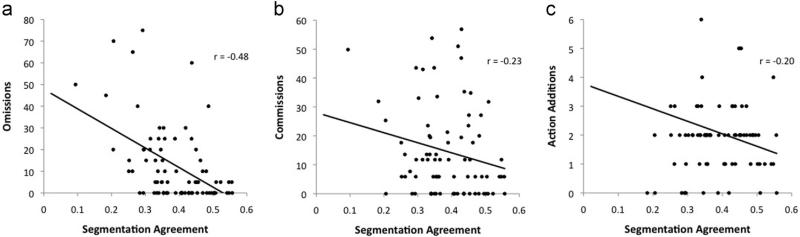
Table 2 shows the correlations between segmentation agreement and (1) NAT score and (2) NAT error types for the entire sample and for each CDR group. Across the entire sample, segmentation agreement was significantly correlated with NAT score (r = 0.51, p < 0.001; see Figs. 7 and 8). Segmentation agreement also significantly correlated with omissions (r = –0.49, p < 0.001), commissions (r = –0.22, p = 0.03), and action additions (r = –0.22, p = 0.03) in the full sample. However, when broken down by CDR group, agreement correlated only with NAT score (r = 0.45, p = 0.004) and omissions (r = –0.34, p = 0.025) for the CDR 0 participants and with omissions (r = –0.32, p = 0.039) for the CDR 0.5 participants. The remaining correlations were in the predicted direction, but most likely did not reach significance because of the sample size within each CDR group.
Table 2.
Correlations with segmentation agreement.
| Group | NAT score | Omissions | Commissions | Action additions |
|---|---|---|---|---|
| All (n = 76) | 0.51 | –0.49 | –0.22 | –0.22 |
| CDR 0 (n = 33) | 0.45 | –0.34 | –0.18 | –0.10 |
| CDR 0.5 (n = 31) | 0.28 | –0.32 | –0.05 | 0.07 |
| CDR 1 (n = 12) | 0.21 | –0.32 | 0.01 | –0.33 |
Note: Bolded values are significant at p < 0.05.
Fig. 7.
Scatter plot for the relationship between segmentation agreement and NAT scores.
Fig. 8.
Scatter plots for the relationships between segmentation agreement and (a) omission errors, (b) commission errors, and (c) action additions.
3.3. Relations between all cognitive variables and NAT performance
To examine the relationships amongst the NAT variables and the cognitive variables, we calculated the zero-order correlations (see Table 3). Given the high correlations amongst the cognitive variables and between the cognitive variables and the NAT variables, we conducted a series of linear regressions to identify which cognitive variables uniquely predicted NAT performance and errors. Segmentation agreement, CDR group, working memory, semantic memory, and script knowledge were entered simultaneously into separate regressions as predictors of (1) NAT score, (2) omission errors, (3) commission errors, and (4) action additions.
Table 3.
Correlations between NAT and cognitive variables.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| NAT | |||||||
| 1 NAT score | – | ||||||
| 2 Omissions | –0.80 | – | |||||
| 3 Commissions | –0.38 | 0.24 | – | ||||
| 4 Action additions | –0.16 | 0.03 | 0.24 | – | |||
| Psychometrics | |||||||
| 5 Working memory | 0.52 | –0.46 | –0.28 | –0.40 | – | ||
| 6 Semantic memory | 0.53 | –0.44 | –0.22 | –0.38 | 0.77 | – | |
| 7 Script knowledge | 0.48 | –0.36 | –0.19 | –0.27 | 0.49 | 0.46 | – |
| 8 Segmentation agreement | 0.51 | –0.48 | –0.23 | –0.20 | 0.44 | 0.50 | 0.51 |
Note: N = 77. Composite variables created by averaging z scores for variables representing each construct. Significant correlations (p < 0.05) are bolded.
The results of the regression analyses are presented in Table 4. In the first regression, these predictors accounted for 53.3% of the variance in NAT scores, F(5,76) = 16.18, p < 0.001; however, the only significant predictors were CDR group (β = –0.44, p < 0.001) and segmentation agreement (β = 0.20, p = 0.048). In the second regression, the predictors accounted for 41.9% of the variance in omission errors, F(5,76) = 10.22, p < 0.001. Again, the only significant predictors were CDR group (β = 0.39, p = 0.001) and segmentation agreement (β = –0.28, p = 0.015). Although the predictors accounted for 12.1% of the variance in commission errors, F(5,76) = 1.92, p = 0.097, and 18.5% of the variance in action additions, F(5,76) = 3.22, p = 0.011, no individual predictor significantly predicted unique variance in either error type.
Table 4.
Regression analyses predicting NAT score and error types.
| R2 | F | p | df | β | t | |
|---|---|---|---|---|---|---|
| NAT score | 0.533 | 16.18 | < 0.001 | 5,76 | ||
| Predictor | ||||||
| CDR | –0.439 | –4.243 | ||||
| Segmentation agreement | 0.200 | 2.009 | ||||
| Working memory | 0.087 | 0.638 | ||||
| Semantic memory | 0.085 | 0.624 | ||||
| Script knowledge | 0.101 | 0.978 | ||||
| Omission errors | 0.419 | 10.22 | < 0.001 | 5,76 | ||
| Predictor | ||||||
| CDR | 0.389 | 3.374 | ||||
| Segmentation agreement | –0.276 | –2.484 | ||||
| Working memory | –0.153 | –1.003 | ||||
| Semantic memory | –0.003 | –0.019 | ||||
| Script knowledge | 0.035 | 0.305 | ||||
| Commission errors | 0.121 | 1.95 | 0.097 | 5,76 | ||
| Predictor | ||||||
| CDR | 0.223 | 1.574 | ||||
| Segmentation agreement | –0.088 | –0.645 | ||||
| Working memory | –0.196 | –1.044 | ||||
| Semantic memory | 0.082 | 0.439 | ||||
| Script knowledge | 0.021 | 0.151 | ||||
| Action additions | 0.185 | 3.22 | 0.011 | 5,76 | ||
| Predictor | ||||||
| CDR | –0.112 | –0.818 | ||||
| Segmentation agreement | –0.276 | –0.270 | ||||
| Working memory | –0.250 | –1.378 | ||||
| Semantic memory | –0.195 | –1.082 | ||||
| Script knowledge | –0.088 | –0.646 |
Note: Composite variables created by averaging z scores for variables representing each construct. Bolded values are significant at the p < 0.05 level.
3.4. Regional brain volume
Given the strong correlations between segmentation agreement, action performance, and action errors (Table 2), we evaluated whether the brain regions important for segmentation were important for action performance. Correlations between brain volume, NAT performance, and NAT error types are presented in Table 5. First, NAT score was significantly correlated with MTL volume and posterior volume. When volume from both brain regions was entered into a regression predicting NAT score, only MTL volume was a significant predictor (β = 0.46, p = 0.002). Second, omission errors correlated significantly with MTL volume and ACC volume, but again MTL volume was the only significant predictor of omission errors when entered into a regression (β = –0.39, p = 0.004). However, MTL volume no longer significantly predicted NAT score or omission errors after controlling for CDR group (Rs < 0.022, βs < 0.19, ps > 0.20).
Table 5.
Correlations between NAT performance and structural MRI volumetric estimates (n = 55).
| Region of Interest | NAT score | Omissions | Commissions | Action additions |
|---|---|---|---|---|
| DLPFC volume | 0.19 | –0.18 | 0.27 | 0.11 |
| MTL volume | 0.47 | –0.43 | –0.21 | –0.22 |
| Posterior volume | 0.25 | –0.19 | –0.02 | –0.33 |
| ACC volume | 0.17 | –0.25 | –0.03 | –0.38 |
Note: Bolded values are significant at p < 0.05.
Next, commission errors were related to DLPFC volume, but this was a positive correlation.5 We examined this relationship further because it was opposite of the predicted direction. The correlation remained significant and even increased numerically after controlling for CDR (r = 0.34, p = 0.013). We also examined whether commission errors were related to the total time to complete the NAT. We reasoned that participants might commit more substitution or sequence errors if they rushed through the task, but commission errors were not correlated with total time on task (r = 0.085, p = 0.235), and time on task did not differ significantly by CDR group, F(2,76) = 0.76, p = 0.47.
Lastly, action additions were correlated with both posterior volume and ACC volume. After both posterior and ACC volume were entered into a regression, only ACC volume significantly predicted action additions (β = –0.30, p = 0.032). Further, this relationship remains significant after controlling for CDR group (β = –0.35, p = 0.010).
3.4.1. Mediating segmentation-NAT relationship
Results from the current study indicate that segmentation agreement is related to NAT performance. Further, MTL volume uniquely predicts NAT performance as well as segmentation agreement (Bailey et al., in press). Thus, we asked whether MTL volume mediated the relationship between segmentation agreement and NAT score. To do so, we regressed NAT score onto segmentation agreement in the first analysis. Then, in the second analysis we regressed NAT score onto segmentation agreement after entering MTL volume into the model. Segmentation agreement accounted for 26.3% of the variance in NAT performance. This number dropped to 10.4% [approximately a 60% reduction, F(1,55) = 10.44, p < 0.01] after controlling for MTL volume. We ran the same regressions, but with omissions as the dependent variable. Segmentation agreement accounted for 23.9% of the variance in omission errors, and after controlling for MTL volume, this dropped to 8.6% [approximately a 64% reduction F(1,55) = 10.63, p < 0.01]. Although MTL volume accounted for a large portion of the variance in these relationships, segmentation agreement still significantly predicted NAT performance (R2 = 0.104, β = 0.334, p = 0.007) and omissions (R2 = 0.086, β = –0.304, p = 0.017) after controlling for MTL volume.
4. Discussion
These results indicate that as dementia status worsens, people's ability to adaptively segment continuous activity declines (see also Zacks et al., 2006). Action performance also declines with worsening dementia. Importantly, the ability to segment everyday activities into meaningful chunks during perception was related to the ability to perform a different set everyday activity, even after controlling for working memory, semantic memory, script knowledge, and dementia status.
Why does event segmentation ability predict ability to produce naturalistic activity? Perception and action likely both involve a common ability to organize activity into goals and subgoals. Viewers comprehend goal-directed activity, in part, by building mental models of the events and segmenting those models at event boundaries. Event models contain information about who the actor is, what she is doing, why she is doing it, and the objects used to accomplish the task (Kurby & Zacks, 2008; Zacks et al., 2007). It is possible that event models guide the production of activity as well. Action plans are hierarchical structures that temporally organize goal and instrument information into basic actions for execution (Miller, Galanter, & Pribram, 1960; Spector & Grafman, 1994). If individuals tend to segment activity appropriately, then they likely create action plans that are effectively structured and result in fewer action errors.
Dementia-related MTL atrophy accounted for a large portion of the relationship between segmentation ability and action performance. Variance shared between MTL volume and dementia severity predicted these outcome measures most likely because MTL atrophy, which occurs in the earliest stages of AD (McDonald et al., 2009), leads to changes in cognition. Declines in memory, judgment making, and problem solving that are important for segmentation and action lead to higher CDR scores.
In regards to the perception and performance of everyday activities, could MTL be important for schema selection or associations within schema networks? In their account of action performance, Norman and Shallice (1980) propose that routine, naturalistic actions rely on a hierarchy of action schemas controlled by a contention scheduling system. Action schemas (e.g., making a sandwich) represent the highest level in the hierarchy and are made up of basic level actions (e.g., pick up) and object representations (e.g., bread). High-level schemas are excited by a supervisory attentional system, activation propagates down the hierarchy until a basic-level schema exceeds threshold, and an action is performed. Disruptions within this schema network lead to commission and omission errors (Cooper, Schwartz, Yule, & Shallice, 2005). For example, commissions may occur when a schema is activated in the incorrect order (sequence errors) or when inappropriate object representations are activated (object substitutions). Omission errors may occur when schemas are inappropriately deactivated. Cooper et al. (2005) speculated that noise in different aspects of the hierarchy explains patterns of action performance for patients with damage to different brain regions. Disruption of schema activation may explain impairments in patients with frontal damage, whereas disruption of object representation activation may explain impairments in patients with parietotemporal damage (see also Schwartz, 2006).
What then is the role of the MTL? One line of research has demonstrated that medial temporal structures support the rapid binding of features and the retrieval of those relations over time intervals as short as 5 s (Hannula, Tranel, & Cohen, 2006; Olson, Moore, Stark, & Chatterjee, 2006; Oztekin, Davachi, & McElree, 2010; Swallow, et al., 2011). This online feature binding is important for keeping track of what is currently happening during the perception and production of everyday activities. For instance, the ability to associate objects and locations (e.g., sandwich in the lunchbox) is critical for event perception because without the association, the object's function or the actor's intention may be difficult to comprehend. Further, losing the association (e.g., sandwich in the lunchbox) during action production may cause an individual to lose track of which step was executed leading to errors in action performance.
A second possibility is that the MTL is necessary for the retrieval of contextual details in previous episodic memories (Nadel & Moscovitch, 1997). According to the transformation hypothesis (e.g., Winocur & Moscovitch, 2011), multiple experiences with making a sandwich eventually lead to the abstraction and corticalization of schematic information. The hippocampus no longer represents the higher-level gist information of the activity such as remembering that a sandwich often is composed of bread and meat. However, the hippocampus is needed to retrieve specific instances and detailed information that often are important for completing subtasks within the activity. This hypothesis could explain why individuals with MTL atrophy receive less attention regarding action impairments. These individuals may retain the gist information, which allows them to functional somewhat independently. Under closer examination, though, we find that they do not perform these tasks efficiently (i.e., high rates omissions and commissions) perhaps because they are unable to retrieve task-specific information.
4.1. Distinct action errors
Results from the current study replicate previous findings that global cognitive functioning predicted action performance and omission errors (Giovannetti et al., 2002). Importantly, the current results also demonstrate that segmentation agreement predicted unique variance in action performance beyond dementia status; thereby indicating that segmentation is potentially critical in the appropriate production of behavior. Further, MTL volume was related to action performance and omission errors, but what are the cognitive and neurological correlates of the other types of action error?
Surprisingly, DLPFC volume was positively related to commission errors. We predicted the opposite: DLPFC supports working memory; thus, higher DLPFC volume should lead to fewer commission errors. One explanation for this positive relationship is that DLPFC is involved in error correction. Although the ACC often is associated with error monitoring, the lateral PFC also has been implicated (e.g., Dehaene, Posner, & Tucker, 1994), especially when the error monitoring involves commission errors (Kiehl, Liddle, & Hopfinger, 2000). Thus, individuals with higher DLPFC volume may better detect an error and correct their actions – leading to more commissions versus omissions. Sequence errors are a specific type of commission error in which steps are completed out of order or in reverse order such as sealing thermos without filling thermos or packing cookies into lunchbox then wrapping them in foil, and they represent approximately 70% of the commission errors made in the current study. Individuals with intact DLPFC may avoid committing an omission error (e.g., omit wrapping cookies in foil) by correcting the misstep (e.g., wrapping the cookies after packing them into lunchbox), which results in a commission error. To support this idea, we observed that DLPFC volume has a significant positive correlation with sequence errors (r = 0.24, p = 0.038) and a marginal negative correlation with omission errors (r = –0.18, p = 0.099).
Finally, we found that lower ACC volume predicted action additions. Giovannetti et al., 2008 described action additions as the inability to inhibit off-task actions, and the ACC is associated with response inhibition (Braver, Barch, Gray, Molfese, & Snyder, 2001). Thus, individuals with higher ACC volume may better inhibit irrelevant actions and commit fewer action additions.
The current pattern of results supports the Omission-Commission model (Giovannetti et al., 2012), which proposes that omission and commission errors are distinct everyday action impairments. We found that omissions were predicted by segmentation ability; commissions were not. Importantly, this study was the first to systematically evaluate which brain regions were associated with each error type. MTL volume predicted omissions, DLPFC predicted commissions, and ACC predicted action additions.
4.2. Conclusion
Segmentation of everyday activities is related to the performance of different activities in cognitively healthy older adults and those with mild AD, and the integrity of the MTL largely, but not completely, accounts for this relationship. Performance of everyday activities likely involves remembering the necessary task steps, monitoring where you are in the task sequence, and dividing the actions into meaningful steps – all of which are involved in segmenting ongoing activity. Finally, different brain structures were important for different error types, which was generally consistent with neuropsychological models of error. The segmentation of events may be critical for action production, in particular because producing temporally organized goal-directed behavior may depend on the same representational system used to perceive temporal organization in others’ behavior.
Acknowledgments
Thanks to Jason Hassenstaab, Denise Head, and John Morris for their input on the diagnostic procedures at the Knight Alzheimer's Disease Research Center. A special thanks to Melody Brenneisen for data entry and scoring procedures, Becky Fierberg for recruiting participants, and Betsy Grant for assistance with the psychometric data. This research was supported by NIH Grant R01 AG031150, PI Jeffrey M. Zacks; NIH Grant F32 AG039162, PI Heather Bailey; NIA Grant T32 AG000030-31, PI David Balota; NIA Grants P50 AG05681, P01 AG03991, P01 AG26276, PI John C. Morris; the generous support of Fred Simmons and Olga Mohan.
Footnotes
We included fine-grained segmentation because previous work has demonstrated that segmentation is hierarchically organized, with fine-grained events grouping into coarse-grained events. Kurby and Zacks (2011) found that older adults showed less hierarchical segmentation than younger adults. However, we did not find differences amongst the older adult groups tested here. Thus, all analyses regarding segmentation agreement collapses agreement across the coarse-segmentation and fine-segmentation conditions.
The participants in this study by definition are non-normative. Thus, we used normative segmentation locations from a group of healthy older adults reported in a previous study (Kurby, Sargent, Bailey, & Zacks, 2012).
Images for 6 participants were collected on a Siemens 1.5T Vision scanner, whereas images for 49 participants were collected on a Siemens 3T Trio scanner. Volume estimates did not differ by scanner type. Also, controlling for scanner type and the scanner × volume interaction did not change the amount of variance the volume measures accounted for in the cognitive variables. Thus, scanner type was not included in any further analyses.
To ensure that group differences in segmentation agreement were not influenced by slower reaction time to press the spacebar, we computed cross-correlations for the distribution of button presses for each CDR group. The maximal lag between the distributions was zero and the zero-order correlations between the group distributions were significant (rs = 0.35 to 0.66, ps < 0.001). These results indicate that neither the CDR 0.5 nor CDR 1 groups had poorer segmentation agreement scores because their distribution of segmentation locations were shifted by a constant value due to slowing.
Our region of interest for DLPFC consisted of the rostral middle frontal gyrus, but we also examined nearby regions (i.e., caudal middle frontal gyrus and the inferior frontal gyrus: pars orbitalis, pars triangularis, and pars opercularis). These prefrontal regions were not included in our DLPFC composite because volume estimates from these regions were only moderately correlated with one another. Similar to the rostral middle frontal gyrus though, we observed weak positive correlations between the volumes for these four regions and commission errors (rs ranging from –0.018 to 0.217).
References
- Allain P, Le G, Foucher C, Etcharry-Bouyx F, Barre J, Dubas F, et al. Script representation in patients with Alzheimer's disease. Cortex. 2008;44:294–304. doi: 10.1016/j.cortex.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Bailey HR, Zacks JM, Hambrick DZ, Zacks RT, Head D, Kurby CA, Sargent JQ. Medial temporal lobe volume predicts elders’ everyday memory. Psychological Science. 2013;24:1113–1122. doi: 10.1177/0956797612466676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey H, Zacks JM, Hambrick DZ, Zacks RT, Head D, Kurby CA, et al. Medial temporal lobe volume predicts elders' everyday memory. Psychological Science. 2013 doi: 10.1177/0956797612466676. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbey AK, Krueger F, Grafman J. An evolutionarily adaptive neural architecture for social exchange. Trends in Neurosciences. 2009;32:603–610. doi: 10.1016/j.tins.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg L, McKeel, Miller DW, Storandt JP, Rubin M, Morris EH, Baty JC, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer's disease: Relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Archives of Neurology. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- Bier N, Macoir J. How to make a spaghetti sauce with a dozen small things I cannot name: A review of the impact of semantic-memory deficits on everyday actions. Journal of Clinical and Experimental Neuropsychology. 2010;32:201–211. doi: 10.1080/13803390902927885. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: Effects of frequency, inhibition, and errors. Cerebral Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. NeuroImage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Buxbaum LJ, Schwartz MF, Montgomery M. Ideational apraxia and naturalistic action. Cognitive Neuropsychology. 1998;15:617–643. doi: 10.1080/026432998381032. [DOI] [PubMed] [Google Scholar]
- Cooper RP, Schwartz MF, Yule P, Shallice T. The simulation of action disorganization in complex activities of daily living. Cognitive Neuropsychology. 2005;22:959–1004. doi: 10.1080/02643290442000419. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Lucchelli F. Ideational apraxia. Brain. 1988;111:1173–1185. doi: 10.1093/brain/111.5.1173. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychological Science. 1994;5:303–305. [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K, et al. Mild cognitive impairment. Lancet. 2006;367:1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- Giovannetti T, Bettcher BM, Brennan L, Libon DJ, Burke M, Duey K, et al. Characterization of everyday functioning in mild cognitive impairment: A direct assessment approach. Dementia and Geriatric Cognitive Disorders. 2008;25:359–365. doi: 10.1159/000121005. [DOI] [PubMed] [Google Scholar]
- Giovannetti T, Bettcher BM, Brennan L, Libon DJ, Kessler RK, Duey K. Coffee with jelly or unbuttered toast: Commissions and omissions are dissociable aspects of everyday action impairment in Alzheimer's disease. Neuropsychology. 2008;22:235–245. doi: 10.1037/0894-4105.22.2.235. [DOI] [PubMed] [Google Scholar]
- Giovannetti T, Britnell P, Brennan L, Siderowf A, Grossman M, Libon DJ, et al. Everyday action impairments in Parkinson's disease dementia. Journal of the International Neuropsychological Society. 2012;18:787–798. doi: 10.1017/S135561771200046X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannetti T, Libon DJ, Buxbaum LJ, Schwartz MF. Naturalistic action impairments in dementia. Neuropsychologia. 2002;40:1220–1232. doi: 10.1016/s0028-3932(01)00229-9. [DOI] [PubMed] [Google Scholar]
- Giovannetti T, Schwartz MF, Buxbaum LJ. The coffee challenge: A new method for the study of everyday action errors. Journal of Clinical and Experimental Neuropsychology. 2007;29:690–705. doi: 10.1080/13803390600932286. [DOI] [PubMed] [Google Scholar]
- Gold DA. An examination of instrumental activities of daily living assessment in older adults and mild cognitive impairment. Journal of Clinical and Experimental Neuropsychology. 2012;34:11–34. doi: 10.1080/13803395.2011.614598. [DOI] [PubMed] [Google Scholar]
- Grafman J. Similarities and distinctions among current models of prefrontal cortical functions. Annals of the New York Academy of Sciences (Vol. Structure and functions of the human prefrontal cortex. New York Academy of Sciences; National Institute of Health, National Institute of Neurological Disorders & Stroke, Medical Neurology Branch, Cognitive Neuroscience Section; New York, NY: Bethesda, MD, US: 1995. pp. 337–368. [DOI] [PubMed] [Google Scholar]
- Grafman J, Thompson K, Weingartner H, Martinez R, Lawlor BA, Sunder-land T. Script generation as an indicator of knowledge representation in patients with Alzheimer's disease. Brain and Language. 1991;40:344–358. doi: 10.1016/0093-934x(91)90134-m. [DOI] [PubMed] [Google Scholar]
- Hannula DE, Tranel D, Cohen NJ. The long and the short of it: Relational memory impairments in amnesia, even at short lags. The Journal of Neuroscience. 2006;26:8352–8359. doi: 10.1523/JNEUROSCI.5222-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann K, Goldenberg G, Daumuller M, Hermsdorfer J. It takes the whole brain to make a cup of coffee: The neuropsychology of naturalistic actions involving technical devices. Neuropsychologia. 2005;43:625–637. doi: 10.1016/j.neuropsychologia.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Jack CR, Jr., Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, Thies B, Phelps CH. Introduction to the recommendations from the National Institute on Aging – Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & Dementia: The Journal of the Alzheimer's Association. 2011;7:257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DK, Storandt MS, Morris JC, Langford ZD, Galvin J. Cognitive profiles in dementia: Alzheimer's disease vs. healthy brain aging. Neurology. 2008;71:1600–1607. doi: 10.1212/01.wnl.0000335972.35970.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. Lea & Febiger; Philadelphia: 1983. [Google Scholar]
- Kessler RK, Giovannetti T, MacMulen L. Everyday action in schizophrenia: Performance patterns and neuropsychological correlates. Neuropsychology. 2007;21:448–457. doi: 10.1037/0894-4105.21.4.439. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: An event-related fMRI study. Psychophysiology. 2000;37:216–223. [PubMed] [Google Scholar]
- Kurby CA, Sargent JQ, Bailey HR, Zacks JM. Event segmentation and memory in younger and older adults: An fMRI investigation.. Poster presentation to be given at the 2012 Cognitive Aging Conference; Atlanta, GA.. 2012. [Google Scholar]
- Kurby CA, Zacks JM. Segmentation in the perception and memory of events. Trends in Cognitive Sciences. 2008;12:72–79. doi: 10.1016/j.tics.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurby CA, Zacks JM. Age differences in the perception of hierarchical structure. Memory & Cognition. 2011;39:75–91. doi: 10.3758/s13421-010-0027-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- Mahurin RK, DeBettignies BH, Pirozzolo FJ. Structured assessment of independent living skills: Preliminary report of a performance measure of functional abilities in dementia. Journal of Gerontology: Psychological Sciences. 1991;46:58–66. doi: 10.1093/geronj/46.2.p58. [DOI] [PubMed] [Google Scholar]
- McDonald CR, McEvoy LK, Gharapetian L, Fennema-Notestine C, Hagler DJ, Jr., Holland D, Koyama A, Brewer JB, Dale AM. Regional rates of neocortical atrophy from normal aging to early Alzheimer disease. Neurology. 2009;73:457–465. doi: 10.1212/WNL.0b013e3181b16431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Miller GA, Galanter E, Pribram KH. Plans and the structure of behavior. Holt; New York: 1960. [Google Scholar]
- Morris JC. The clinical dementia rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- Morris JC, Storandt M, Miller JP, McKeel DW, Jr., Price JL, Rubin EH, Berg L. Mild cognitive impairment represents early-stage Alzheimer's disease. Archives of Neurology. 2001;58:397–405. doi: 10.1001/archneur.58.3.397. [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Current Opinion in Neurobiology. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Norman DA, Shallice T. Center for human information processing (CHIP) technical report No. 99. University of California; San Diego: 1980. Attention to action: Willed and automatic control of behaviour. [Google Scholar]
- Ochipa C, Rothi LJG, Heilman KM. Conceptual apraxia in Alzheimer's disease. Brain. 1992;115:1061–1071. doi: 10.1093/brain/115.4.1061. [DOI] [PubMed] [Google Scholar]
- Olson IR, Moore KS, Stark M, Chatterjee A. Working memory is impaired when the medial temporal lobe is damaged. Journal of Cognitive Neuroscience. 2006;18:1087–1097. doi: 10.1162/jocn.2006.18.7.1087. [DOI] [PubMed] [Google Scholar]
- Oztekin I, Davachi L, McElree B. Are representations in working memory distinct from representations in long-term memory? Neural evidence in support of a single store. Psychological Science. 2010;21:1123–1133. doi: 10.1177/0956797610376651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen VM, Caplan L, Sheesley L, Rodriguez R, Grafman J. An examination of daily activities and their scripts across the adult lifespan. Behavior Research Methods, Instruments, & Computers. 2003;35:32–48. doi: 10.3758/bf03195495. [DOI] [PubMed] [Google Scholar]
- Sargent JQ, Zacks JM, Hambrick DZ, Zacks RT, Kurby CA, Bailey HR, et al. Event segmentation ability uniquely predicts memory across the lifespan. 2013 doi: 10.1016/j.cognition.2013.07.002. (in preparation) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Buxbaum LJ, Ferraro M, Veramonti T, Segal M. Naturalistic action test. Thames Valley Test Company; Bury St. Edmunds, United Kingdom: 2003. [Google Scholar]
- Schwartz MF, Buxbaum LJ, Montgomery MW, Fitzpatrick-DeSalme E, Hart T, Ferraro M, et al. Naturalistic action production following right hemisphere stroke. Neuropsychologia. 1999;37:51–66. doi: 10.1016/s0028-3932(98)00066-9. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Montgomery M, Buxbaum LJ, Lee S, Carew TG, Coslett HB, et al. Naturalistic action impairment in closed head injury. Neuropsychology. 1998;12:13–28. doi: 10.1037//0894-4105.12.1.13. [DOI] [PubMed] [Google Scholar]
- Schwartz MF, Segal M, Veramonti T, Ferraro M, Buxbaum LJ. The naturalistic action test: A standardized assessment for everyday action impairment. Neuropsychological Rehabitation. 2002;12:311–339. [Google Scholar]
- Schwartz MF. Cognitive neuropsychology of everyday action and planning. Cognitive Neuropsychology. 2006;23:202–221. doi: 10.1080/02643290500202623. [DOI] [PubMed] [Google Scholar]
- Sirigu A, Zalla T, Pillon B, Grafman J, Dubois B, Agid Y. Annals of the New York Academy of Sciences (Vol. Structure and functions of the human prefrontal cortex. New York Academy of Sciences; National Insts of Health, National Inst of Neurological Disorders & Stroke, Medical Neurology Branch, Cognitive Neuroscience Section; US New York, NY: Bethesda, MD: 1995. Planning and script analysis following prefrontal lobe lesions. pp. 277–288. [DOI] [PubMed] [Google Scholar]
- Spector L, Grafman J. Planning, neuropsychology and artificial intelligence: cross-fertilization. Vol. 9. Elsevier; 9. Amsterdam: 1994. pp. 377–392. [Google Scholar]
- Storandt M, Balota DA, Salthouse TA. ELSMEM: A computerized battery to assess executive, linguistic, spatial, and memory abilities. 2009 Available from: ( http://www.psych.wustl.edu/coglab/index.html.
- Swallow KM, Barch DM, Head D, Maley CJ, Holder D, Zacks JM. Changes in events alter how people remember recent information. Journal of Cognitive Neuroscience. 2011;23:1052–1064. doi: 10.1162/jocn.2010.21524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurstone LE, Thurstone TG. Examiner manual for the primary mental abilities test. Science Research Associates; Chicago: 1949. [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale—III. Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Winocur G, Moscovitch M. Memory transformation and systems consolidation. Journal of the International Neuropsychological Society. 2011;17:766–780. doi: 10.1017/S1355617711000683. [DOI] [PubMed] [Google Scholar]
- Zacks JM, Speer NK, Swallow KM, Braver TS, Reynolds JR. Event perception: A mind/brain perspective. Psychological Bulletin. 2007;133:273–293. doi: 10.1037/0033-2909.133.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks JM, Speer NK, Vettel JM, Jacoby LL. Event understanding and memory in healthy aging and dementia of the Alzheimer type. Psychology and Aging. 2006;21:466–482. doi: 10.1037/0882-7974.21.3.466. [DOI] [PubMed] [Google Scholar]