Abstract
The capacity of varicella zoster virus (VZV) to cause varicella (chickenpox) relics upon multiple steps, beginning with inoculation of the host at mucosal sites with infectious virus in respiratory droplets. Despite the presence of a powerful immune defense system, this virus is able to disseminate from the site of initial infection to multiple sites, resulting in the emergence of distinctive cutaneous vesiculopustular lesions. Most recently, it has been proposed that the steps leading to cutaneous infection include VZV infecting human tonsillar CD4+ T cells that express skin homing markers that allow them to transport VZV directly from the lymph node to the skin during the primary viremia. It has also been proposed that dendritic cells (DC) of the respiratory mucosa may be among the first cells to encounter VZV and these cells may transport virus to the draining lymph node. These various virus-host cell interactions would all need to occur in the face of an intact host immune response for the virus to successfully cause disease. Significantly, following primary exposure to VZV, there is a prolonged incubation period before emergence of skin lesions, during which time the adaptive immune response is delayed. For these reasons, it has been proposed that VZV must encode functions which benefit the virus by evading the immune response. This chapter will review the diverse array of immunomodulatory mechanisms identified to date that VZV has evolved to at least transiently limit immune recognition.
1 Introduction
Primary varicella zoster virus (VZV) infection leading to varicella (chickenpox) is initiated by inoculation of mucosal sites, most frequently the upper respiratory tract, with infectious virus in respiratory droplets (Grose 1981). From the time of virus inoculation of the host to development of the cutaneous rash, there are multiple steps and many virus–cell interactions. For example, VZV may enter directly into a primary viremia, following infection of human tonsillar CD4+ T cells that express skin homing markers that allow them to transport VZV directly from the lymph node to the skin during the primary viremia (Ku ct al. 2002, 2004). Furthermore, it has been proposed that dendritic cells (DCs) of the respiratory mucosa may be among the first cells to encounter VZV during primary infection and may serve as a means for viral transport to the draining lymph node (Abendroth et al. 2001b; Morrow et al. 2003), where a primary cell-associated viremia initiates, during which time virus is transported to the reticuloendothelial organs where it undergoes another period of replication that results in a secondary cell-associated viremia and virus transport via T cells to the skin (Arvin et al. 1996; Grose 1981). At the skin, deep cutaneous infections are established which are maintained and contained during the incubation period, followed by emergence at the surface into distinctive vesiculopustular lesions. VZV also gains access to nerve axonal termini, and establishes a persistent latent state in which some viral antigens appear to be expressed. These different virus–host cell interactions would all need to occur in the face of an intact host immune response for the virus to successfully cause disease. It is, therefore, reasonable to predict that VZV encoded immune evasion mechanisms manifested during the first stages of primary infection as well as during latency and following reactivation so as to benefit virus by limiting and/or delaying immune recognition. Indeed, after primary exposure to VZV there is a prolonged incubation period of 10–21 days before appearance of skin lesions, during which time the adaptive response is delayed or virus remains initially undetected by the developing adaptive immune response. This suggests that VZV must encode immunomodulatory strategies to delay immune detection (Arvin 2001). This chapter will review the diverse array of mechanisms identified to date that VZV has evolved to at least transiently evade the immune response.
2 VZV Interference with Interferons
Immune control of productive VZV infection involves both innate and adaptive host responses (Abendroth and Arvin 1999). The innate host immune response, involving natural killer (NK) cells, NK-T cells, and type 1 (α and β) and type II (γ) interferon (IFN), is an important early host response designed to prevent or limit virus spread within the host (Abendroth and Arvin 2001; Arvin et al. 1986). In response to virus infection, a variety of signal transduction pathways are activated by the innate responses, including the expression of type I IFNs (i.e., IFN α/β), that induce a large number of IFN-stimulated genes, leading to an antiviral state. These genes include those encoding proteins such as protein kinase R (PKR), 2–5 oligoadenylate synthetase (2–5 OAS), and the Mx proteins that induce an antiviral response by interfering with viral transcription, translation, and likely other viral processes such as viral DNA replication and assembly (Muller et al. 1994; Sadler and Williams 2008). Many viruses have evolved mechanisms that impair the synthesis of IFNs or interfere with the downstream antiviral effects of IFNs (Garcia-Sastre and Biron 2006; Haller et al. 2006; Katze et al. 2002).
Both type I and type II IFNs can inhibit VZV replication in vitro (Balachandra et al. 1994; Desloges et al. 2005). The importance of type I IFNs in vivo is highlighted by the observation that treatment of immunocompromised individuals with IFNα can reduce the severity of varicella (Arvin et al. 1982). Thus, VZV induced control of expression of IFNs and/or the downstream IFN signaling events would be likely to provide a survival advantage to the virus during replication. Ku et al. assessed IFNα expression during VZV infection of human skin xenografts in SCID mice (Ku et al. 2004) by immunohistochemical detection of IFNα and reported its expression in epidermal skin cells in uninfected skin (Ku et al. 2004). In contrast, IFNα expression was downregulated in VZV infected cells within VZV infected skin xenografts, and upregulatcd in bystander uninfected epidermal cells, suggesting that local proximity to the infection were in an antiviral state, but that VZV modulated the expression of IFNα. To verify block in IFNα signaling in VZV infected skin cells, immunohistochemical staining for Stat-1 phosphorylation revealed that Stat1 was phosphorylated and translocated to the nuclei in the neighboring uninfected cells, but not in the VZV-infected cells. In combination, these observations suggest that VZV infection of human skin cells impairs Stat1 activation and IFNα production in VZV infected skin cells, but may not be able to prevent signaling to bystander cells, which in turn may contain the spreading VZV lesion. This is supported by the observation that inhibition of Type I IFN activity by administration of an IFN α/β receptor neutralizing antibody (Colamonici and Domanski 1993) to SCTD-hu skin mice resulted in more extensive viral replication and lesion formation compared with mice that received no antibody (Ku et al. 2004). These findings demonstrate that IFNα can modulate cutaneous VZV replication in vivo but that VZV can inhibit IFNα and in doing so enhance the capacity to replicate in the skin.
In the context of immune function in the presence of IFNα, Ambagala and Cohen (2007) reported that VZV IE63 is required to inhibit IFNα induced antiviral responses in vitro (Ambagala and Cohen 2007). This study utilized a viable ORF63 deletion virus to infect human melanoma cells and showed that this virus was hypersensitive to the antiviral effects of human IFNα (i.e., replication was severely inhibited in the presence of IFNα), compared to parent virus or other viral gene mutants. The ORF63 deletion mutant was hypersensitive to IFNα but not IFNγ, with IFNα inhibiting viral gene expression at a posttranscriptional level in ORF63 deletion virus-infected cells.
An important component of the innate response which is enhanced by the activity of IFNs is signaling by the double stranded RNA sensor PKR. Unless blocked, activated PKR phopshorylates the α-subunit of eukaryotic initiation factor 2 (eIF-2α) and effectively inhibits initiation of translation. In the closely related herpesvirus HSV-I, PKR is blocked by several viral genes, predominantly by the γ34.5 gene, which redirects the protein phosphatase 2 (PP2A) to dephosphorylate EIF2α. An increased level of phosphorylated eIF-2α was reported in VZV ORF63 deletion virus-infected cells compared to those infected with parent virus (Ambagala and Cohen 2007). In the same study, cells transiently transfected with a plasmid expressing ORF63 showed a decrease in basal levels of phosphorylated eIF-2α, demonstrating that IE63 is sufficient to inhibit this phosphorylation. Taken together, these results indicated that IE63 play a role in modulating innate immune response to VZV by interfering with IFNα induced signaling and the activity of the PKR sensor. Interestingly, Desloges et al. reported that productive VZV infection does not significantly alter levels of PKR in human melanoma cells (Desloges et al. 2005). Thus, there is uncertainty as to how VZV induces phosphorylation of eIF-2α in the absence of IE63, but it has been postulated that VZV ORF63 mediated disruption of eIF-2α phosphorylation may occur in a PKR-independent manner (Ambagala and Cohen 2007).
Thus, it appears that VZV can both impair expression of IFNα as well as inhibit antiviral signaling induced by IFNα. Additional studies will be required to identify the viral gene(s) that suppress IFNα expression. Similarly, further work examining PKR expression and function during infection with ORF63 deleted virus will be required to reveal the precise mechanism by which this viral gene product disrupts eIF-2α phosphorylation.
VZV ORF66 protein has been shown to block the induction of the IFN signaling pathway in T cells following IFNγ exposure (Schaap et al. 2005). Schaap et al. (2005) reported that following IFNγ treatment Stat-1 phosphorylation was significantly reduced in T cells infected with parental virus as compared to cells infected without a functional ORF66 (Schaap et al. 2005). The mechanism by which ORF66 modulates IFNγ signaling has yet to be reported, although it may be possible that ORF66 functions in a manner similar to its HSV-1 related gene product Us3 which phosphorylates the IFNg receptor (Liang and Roizman 2008).
3 Interference with Antigen Presentation by VZV
During primary infection, both VZV specific CD8+ and CD4+ T cells develop and function in the resolution of varicella (Abendroth and Arvin 1999). Individuals with impaired cell-mediated immunity have an increased risk of more severe varicella (Gershon et al. 1997; Jura et al. 1989). A decline of cell-mediated immunity has also been associated with the increased risk of herpes zoster in the elderly, high-lighting the significance of VZV-specific T cells in reactivation from latency (Levin and Hayward 1996). Evaluation of the kinetics of the VZV specific CD4+ T cell response during varicella revealed that VZV specific T cells were rarely detected until varicella rash onset (Arvin et al. 1986). These observations are consistent with the hypothesis that VZV evades host recognition by T cells during the prolonged incubation period following initial infection (Abendroth and Arvin 2001), enhancing virus access to skin sites of replication, thus enabling transmission to others. A delay in the acquisition of VZV-specific T cells for > 72 h was associated with persistent viremia, more lesions, and in extreme cases, potentially fatal virus dissemination (Abendroth and Arvin 2001). These observations have led to the analysis of major histocompatibility complex (MHC) class I and class II expression in VZV infected cells.
3.1 Downregulation of MHC Class I Molecules by VZV
Surface MHC class I, consisting of heterotrimers of a membrane bound heavy chain (αC), a light chain β2microglobulin (β2m), and antigenic peptides, are required for target cell recognition by CD8+ T cells (Hansen and Bouvier 2009). Based upon the hypothesis that VZV may evade immune detection by T cells, Cohen (1998) reported that both wild-type VZV (Emily) and the vaccine virus (Oka) could downregulate cell-surface levels of MHC class I heavy chain on human fibroblasts (HFs) (Cohen 1998). Radioactive labeling experiments and western blotting for MHC class heavy chain revealed that the amount of newly synthesized and total cellular MHC class I protein was comparable in VZV-infected and uninfected cells, suggesting that VZV may interfere with the MHC class I biosynthesis pathway at a posttranslational level (Cohen 1998).
Similar findings were reported by Abendroth et al. following VZV infection of HFs and T cells (Abendroth et al. 2001a). Flow cytometric analysis revealed that a clinical VZV strain (Schenke) and the vaccine virus (Oka) selectively downregulated cell-surface expression of MHC class I on cultured HFs and also on VZV-infected T cells derived from infected SCID-hu thymus/liver mice. To identify potential mechanisms, biochemical analyses and immunofluorescent staining and confocal microscopy of VZV infected HFs were used. These approaches revealed that VZV interferes with MHC class I transport from the Golgi to the cell-surface (Abendroth et al. 2001a), suggesting the pathway by which VZV downmodulates cell-surface MHC class I expression is different from that of other α-herpesviruses such as herpes simplex virus (HSV) and bovine herpes virus (BHV). The latter express proteins (HSV ICP47 and BHV UL49.5) that interfere with the transporter associated with antigen presentation (TAP), thus blocking the transport of antigenic peptides from the cytoplasm into the endoplasmic reticulum (ER) lumen (Ahn et al. 1996; Koppers-Lalic et al. 2005, 2008; Tomazin et al. 1996; Verweij et al. 2008).
To elucidate viral gene classes responsible for MHC class I downmodulation in VZV infected cells, phosphonoacetic acid (PAA), an inhibitor of viral DNA replication and VZV late gene expression, was added to VZV infected cells and cell-surface MHC class I expression was measured by flow cytometry. In the presence of PAA, MHC class I expression at cell surfaces was not reduced compared to untreated cells, suggesting that VZV immediate early or early gene product(s), or a virion component(s) maybe involved in downregulation of cell-surface MHC class I on infected cells. Furthermore, a transient transfection approach using a variety of VZV expression constructs in HFs was then utilized to better define the viral gene responsible for this phenotype. In cells transiently transfected with a plasmid encoding the VZV ORF66 protein kinase, there was a significant decrease in cell-surface MHC class I expression, suggesting that ORF66 was sufficient to downregulate cell-surface MHC class I expression (Abendroth et al. 2001a).
Eisfeld et al. (2007) went on to examine the effects of the ORF66 protein kinase on cell surface MHC class I expression alone and in the context of VZV infection using a panel of recombinant replication defective adenoviruses and VZV expressing functional or altered ORF66 protein kinase genes tagged with green fluorescent protein (Eisfeld et al. 2007). In VZV infected MRC-5 cells, downregulation of cell-surface MHC class I required the expression of a functional ORF66 protein kinase domain. This represents a novel role for VZV ORF66 protein kinase in immune evasion. The expression of a functional ORF66 kinase impaired MHC class I maturation in the absence of influencing MHC class I synthesis, degradation or association with β2m, and suggested the kinase induced a delay in the processing of MHC class I trimeric complexes through the Golgi to an endoglycosidase H resistant form. Using a combination of immunoprecipitation experiments and immunofluorescent staining and confocal microscopy there was little evidence suggesting a close or direct association of ORF66 with folded MHC class I molecules or MHC class I heavy chains (Eisfeld et al. 2007). However, VZV lacking the kinase activity still downmodulated MHC class I expression to a lesser extent, suggesting additional proteins may be involved. In the same study, the role of VZV ORF9a protein, which is analogous to the BHV UL49.5 gene which modulates MHC class I, was also assessed. HFK293T cells transiently transfected with an ORF9a expression construct showed no decrease in cell-surface MHC class I, indicating that the MHC class I downmodulatory function of ORF9a is not conserved between BHV and VZV. Additional detail on the function of the ORF66 protein kinase is provided in (Erazo et al. 2009).
VZV specific cell-mediated immunity would be well established in the skin during the development of varicella skin lesions and ORF66 encoded MHC class I downregulation may play a role in allowing skin cells to transiently evade CD8+ T cell surveillance, facilitating local virus replication and transmission during the first few days of cutaneous lesion formation. The assessment of MHC class I expression on human skin cells following inoculation of SCID-hu skin mice or of human skin explants with an ORF66 mutant virus would assist in elucidating the in vivo immunomodulatory roles of ORF66. It also remains to be determined whether ORF66 causes the downregulation of MHC class I observed on VZV infected T cells (Abendroth et al. 2001a), but it is interesting to note that ORF66 mutant viruses are impaired for growth in T cells and this has been attributed to an increased susceptibility of infected T cells to apoptosis (Schaap et al. 2005). Thus, ORF66 may employ multiple mechanisms during VZV infection to enable immune evasion and promote T cell survival to enable virus spread and transfer to the skin.
VZV and other viruses that modulate cell-surface MHC class I expression may evade CD8+ T cell recognition; however, the overall reduction of cell-surface MHC class I may make these infected cells more sensitive to NK cell mediated killing (Farrell and Davis-Poynter 1998; Tortorella et al. 2000). In this respect, VZV infected HFs are susceptible to NK cell mediated lysis (Bowden et al. 1985; Ihara et al. 1984; Ito et al. 1996), although any contribution of downregulated MHC class I to this killing remains to be examined. Prior studies on human cytomegalovirus (HCMV) and murine cytomegalovirus (MCMV) have revealed that these herpesviruses have evolved a variety of mechanisms to combat NK cell recognitions as well as CD8+ T cell recognition and killing (Miller-Kittrell and Sparer 2009; Wilkinson et al. 2008). In contrast, to date VZV has not been reported to encode an MHC class I homolog, although it remains possible that VZV may subvert NK cell-mediated killing either via expression of an as yet unidentified MHC class I homolog or downmodulation of selective MHC class I alleles. Human immunodeficiency virus (HIV) has been previously shown to selectively downregulate the cell-surface expression of specific MHC class I alleles and not others, preventing NK mediated killing (Cohen et al. 1999). To date, there have been no reports examining whether VZV may also cause an allele specific downmodulation of MHC class I molecules which may enable VZV to evade NK cell mediated killing.
3.2 VZV Interference with MHC Class II Expression
Unlike MHC class I molecules, constitutive expression of MHC class II is restricted to B cells, monocytes, DCs, and thymic epithelium. However, IFNγ treatment can stimulate MHC class II expression by many cell types, including HFs (Collins et al. 1984; Pober et al. 1983). The importance of CD4+ T cells for resolution of varicella lead to the postulation that VZV may encode an immunomodulatory function that allows the virus to inhibit the induction of MHC class II expression by IFNγ (Abendroth et al. 2000). VZV strain Schenke infected HFs were treated with IFNγ to stimulate MHC class II expression and then analyzed by How cytometry, Upregulation of cell-surface MHC class II expression was impaired in VZV infected cells compared with mock infected counterparts. In contrast, cells that were treated with IFNγ prior to VZV infection expressed comparable levels of MHC class II to mock infected cells treated with IFNγ. Taken together, these results demonstrated that VZV inhibited IFNγ-mediated upregulation of MHC class II but could not downregulate MHC class II already induced by IFNγ. Northern blot and in situ hybridization for MHC class II α-chain transcripts in infected cells treated with IFNγ revealed that VZV suppressed upregulation of MHC class II at the level of mRNA transcription (Abendroth et al. 2000). VZV infection inhibited the expression of Stat1α and Jak2 proteins, but had no effect on Jak1. Furthermore, VZV infection inhibited transcription of the interferon regulatory factor (IRF-1) and class II transactivator (CIITA). Collectively these data demonstrated that VZV encodes an immunomodulatory function which directly interferes with the IFNγ signal transduction via the Jak/Stat pathway and enabled the virus to inhibit IFNγ induction of cell-surface MHC class II expression (Abendroth et al. 2000). The significance of these in vitro based studies was further confirmed by examination of varicella and herpes zoster skin biopsies for MHC class II and VZV RNA synthesis by in situ hybridization. These experiments demonstrated that during natural cutaneous infection, dermal and epidermal cells infected with VZV do not express MHC class II transcripts in vivo, whereas MHC class II transcripts were readily detected in the uninfected bystander uninfected cells (Abendroth et al. 2000).
More recently, analyses of VZV encoded MHC class II modulation was extended to VZV infected human keratinocytes. Black et al. (2009) demonstrated that immortalized human keratinocytes infected with cell-free VZV virus (Oka) and subsequently treated with IFNγ failed to upregulate MHC class II molecules to the same level as the IFNγ treated mock infected keratinocytes (Black et al. 2009). This supports the notion that despite attenuation, the vaccine virus still retains its capacity to interfere with IFNγ induced MHC class II upregulation, as is the case for downregulation of MHC class I (Abendroth et al. 2001a; Cohen 1998). Significantly, VZV infected keratinocytes treated with IFNγ were impaired in their ability to stimulate antigen specific CD4+ and CD8+ T cells in vitro compared with IFN treated uninfected keratinocytes (Black et al. 2009). The mechanisms involved in modulating the IFNγ upregulation of MHC class II in keratinocytes may be similar to that reported in VZV infected HFs (Abendroth et al. 2000), although this remains to be determined. Furthermore, the viral gene product(s) responsible for inhibiting IFNγ induced MHC class II in keratinocytes or HFs has yet to be elucidated. It has been shown that ORF66 blocks the induction of IFN signaling in human T cells following IFN treatment (Schaap et al. 2005). Given that HFs and keratinocytes are important cell types for viral replication in the skin (Nikkels et al. 1995), the capacity of VZV to inhibit IFNγ induced upregulation of MHC class II in these cells is likely to provide the virus with an important strategy for evasion of CD4+ T cell recognition during both varicella and herpes zoster.
4 VZV Interference with the NFkB Pathway and Intercellular Adhesion Molecule 1 Expression
NFκB is a potent transcription factor that is normally present within cells in an inactivate state in the cytoplasm due to its association with the inhibitory protein IκBα Degradation of IκBα results in translocation of NFκB proteins to the nucleus where they stimulate expression of a wide range of genes, including those involved in the immune response (Ghosh and Hayden 2008; Hayden and Ghosh 2008). In a microarray based study of VZV infected HFs, Jones and Arvin (2005) reported that many NFκB responsive genes were downregulated following infection (Jones and Arvin 2005). In a subsequent report, the same authors performed an analysis of the NFκB activation pathway in VZV infected HFs and revealed that after a transient nuclear localization VZV interferes with this pathway by sequestering NFκB proteins (p50 and p65) in the cytoplasm of VZV infected cells (Jones and Arvin 2006). The cytoplasmic sequestration of p50 and p65 required VZV protein expression, as UV-inactivated virus did not inhibit the nuclear translocation of these NFκB proteins. In addition, while IκBα is normally degraded to enable translocation of NFκB proteins to the nucleus, VZV infection of HFs inhibited this degradation. The inhibition of nuclear import of NFκB proteins was confirmed in vivo, where in epidermal cells in skin xenografts of SCID-hu mice infected with VZV, p50 and p65 remained in the cytoplasm of VZV infected cells, yet neighboring uninfected epidermal cells displayed normal nuclear accumulation of these NFκB proteins (Jones and Arvin 2006). This finding is consistent with other work from the Arvin group demonstrating that VZV infected skin cells in vivo lacked IFNα expression and Stat-1 remained localized to the cytoplasm, whereas surrounding uninfected bystander cells expressed IFNα and Stat-1 was phosphorylated and translocated into the nucleus (Ku et al. 2004). Given that NFκB is a major inducer of IFNα transcription, viral modulation of the NFκB signaling pathway would likely limit IFNα production within VZV infected cells. The VZV gene product(s) responsible for the modulation of the NFκB pathway in HFs or any other cell-type is yet to be elucidated. Given the pivotal role the NFκB signaling pathway plays in both the innate and adaptive arms of the immune response, it is likely that regulating the actions of this transcription factor may be central in other cell-types infected with VZV such as DCs.
In the presence of proinflammatory cytokines such as IFNγ and tumor necrosis factor (TNF), keratinocytes can be induced to express not only MHC class II molecules but also surface intercellular adhesion molecule (ICAM-1) which is the ligand for leukocyte function antigen (LFA-1) expressing T cells (Rothlein et al. 1986). Nikkels et al. (2004) performed an immunohistochemical analysis for a variety of different immune cell markers and cytokines on frozen sections from herpes zoster skin biopsies. Despite increased expression of IFNγ, TNFα, and IL-6 in VZV infected skin there was a decrease in expression of both ICAM-1 and MHC class II in VZV infected keratinocytes within the center of the herpes zoster lesions (Nikkels et al. 2004). This was the first demonstration that VZV could modulate ICAM-1 expression. Black et al. (2009) also examined ICAM-1 expression on uninfected keratinocytes in comparison to VZV infected keratinocytes in vitro. Similar to their assessment of cell-surface MHC class II expression, human keratinocytes infected with VZV inhibited IFNγ mediated upregulation of ICAM-1. These reports demonstrate that VZV encodes an immunoevasive strategy targeting the expression of ICAM-1 in both keratinocytes in vitro and in vivo and identifies an additional mechanism by which VZV may evade T cell clearance.
The molecular basis of VZV mediated ICAM-1 inhibition was assessed in infected melanoma cells (MeWo) and human MRC5 cells (El Mjiyad et al. 2007). Consistent with the study by Jones and Arvin (2006), nuclear translocation of p50 was strongly decreased by VZV infection, although inhibition of p65 translocation was less marked as nuclear translocation of this subunit was still observed. Interestingly, using a coimmunoprecipitation approach, VZV infection induced the nuclear accumulation of the NFκB inhibitor p100. Significantly, in addition to the demonstration of inhibition of ICAM-1 mRNA synthesis, analysis of TNFα treated VZV infected cells using an electrophoretic mobility shift assay (EMSA) revealed that NFκB subunits present in VZV infected cells were unable to bind to ICAM-1 or IL-8 promoters, thus providing a mechanistic basis for the inhibition of ICAM-1 expression (and possibly other genes) by VZV mediated interference with NFκB activation.
5 Impact of VZV on Human dendritic cells
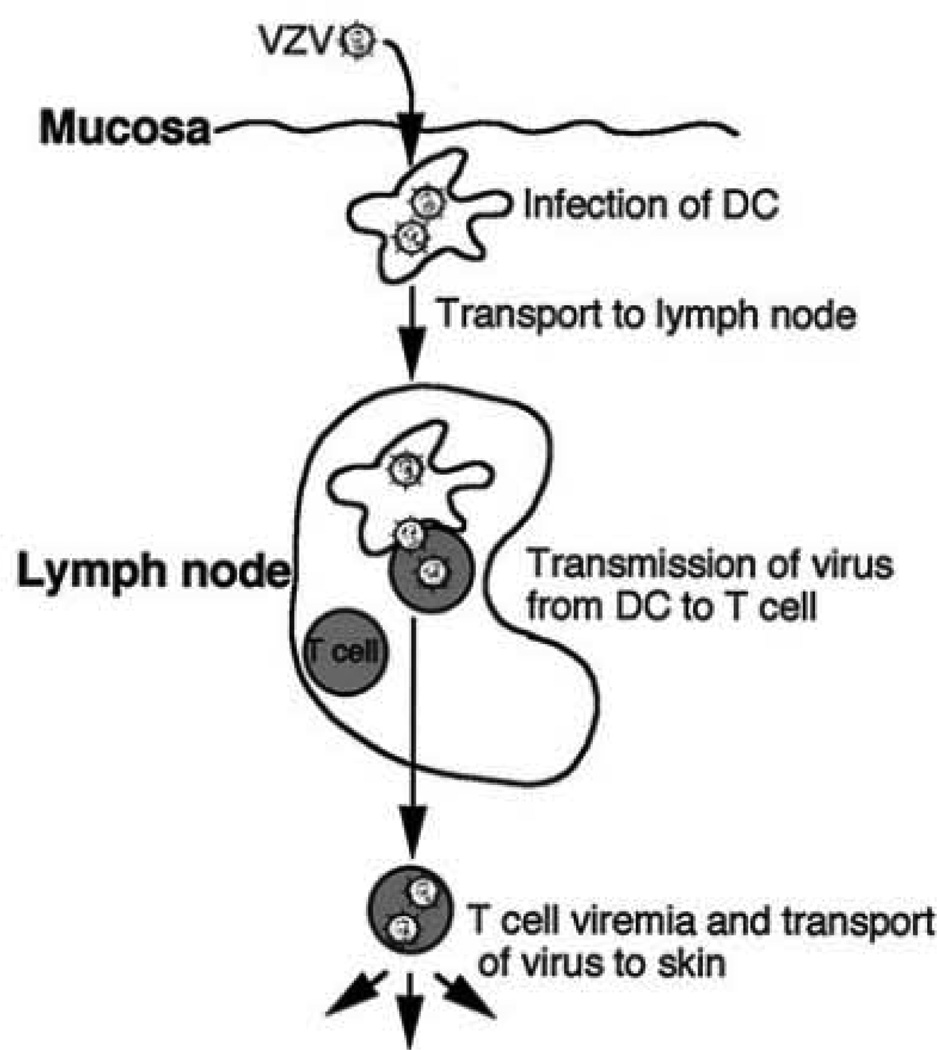
DCs are bone-marrow derived potent antigen presenting cells (APCs) that are located at many sites, including the skin, blood, lymph, and mucosal surfaces (Banchereau et al. 2000; Banchereau and Steinman 1998; Klagge and Schneider-Schaulies 1999). DCs uptake and process antigen in the periphery and transport viral antigens to T-cell rich areas of the lymph nodes, where they display MHC–peptide complexes together with costimulatory molecules. This results in the activation of naïve and resting antigen-specific T cells and effector T cell differentiation (Banchereau et al. 2000). The hypothesis that DCs of the respiratory mucosa may be the first cell type to encounter VZV during primary infection led to studies to investigate VZV–DC interactions. Human immature monocyte derived DCs were shown to be fully permissive to a productive VZV infection as immediate early (IE), early (E), and late (L) viral gene products are made in CD1a+ DCs and infectious virus can be recovered (Fig. 2). VZV infected immature DCs showed no significant decrease in cell viability or evidence of apoptosis and did not exhibit altered cell surface levels of the immune molecules MHC class I, MHC class II, CD86, CD40, or CD1a. Significantly, when autologous T cells were incubated with VZV infected DCs, VZV antigens were readily detected in CD3+ T cells and infectious virus was recovered from these cells (Abendroth et al. 2001b). This work provided the first evidence that immature DCs were permissive to VZV and that DC infection could lead to virus transmission to T cells, supporting the hypothesis that DC may mediate virus dissemination in the initial viremia following infection (Fig. 1).
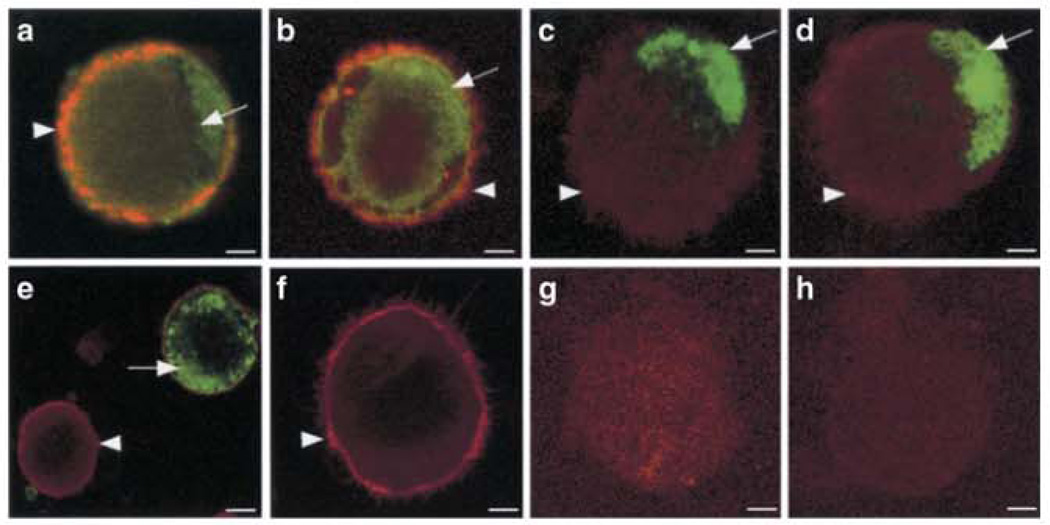
Fig. 2.
Varicella zoster virus infection of human immature DCs in vitro. Immunofluorescent Staining of immature DCs at 48 h postinfection (a–e, g) and mock infected (f and h) for CD1a (red staining) and VZV antigens ORF62 (a and f), ORF4 (b), ORF29 (c), ORF61 (d), and glycoprotein C (e). Copyright © American Society for Microbiology, J Virol, July 2001, pp. 6183–6192, vol. 75, no. 13. DOI: 10.1128/JV1.75.13.6183–6192.2001
Fig. 1.
Proposed model of VZV transport from mucosal sites to the T cells in lymph nodes during primary VZV infection. Copyright © American Society for Microbiology, J Virol, July 2001, pp. 6183–6192, vol. 75, no. 13. DOI: 10.1128/JVI.75.13.6183–6192.2001
As immature DC become mature their ability to stimulate T cells increases as a result of upregulation of MHC I, MHC II, CD86, CD83, and CD80 (Banchereau and Steinman 1998). Maturation of DC leading to migration and increased T cell stimulation is essential for initiation of the immune response (Banchereau et al. 2000; Banchereau and Steinman 1998), and so may represent an area targeted for disruption by VZV. While the impact of VZV infection on the maturation of DCs has not yet been published, this is currently an area of active investigation. As mature DCs are potent APCs essential for initiating successful antiviral immune responses (Banchereau and Steinman 1998), they would also serve as an ideal target for viruses such as VZV seeking to evade or delay the immune response by disrupting their immune function. In this respect, Morrow et al. showed that VZV productively infects mature monocyte derived DC, and in doing so, impairs their ability to function properly by the selective downregulation of functionally important cell-surface immune molecules MHC class I, CD80, CD83 and CD86 (Morrow et al. 2003). Importantly, the same study demonstrated that VZV infection of mature DCs significantly reduced their capacity to stimulate the proliferation of allogeneic T cells. Although the precise molecular mechanisms responsible for VZV mediated downregulation of cell-surface MHC class I, CD80, CD83, and CD86 remain to be elucidated, current evidence suggests that direct virus infection and not soluble factors are required for this phenotype (Morrow et al. 2003). The identity of the VZV gene(s) responsible for the disruption of immune molecules during productive infection of mature DCs has yet to be determined.
Hu and Cohen (2005) utilized a VZV ORF47 deletion virus to show that ORF47 was critical for virus replication in immature DC, but not in mature DC. VZV ORF47 was however, required for the full replicative cycle and for transmission of virus from these cells to other cells. This study also demonstrated that both immature and mature DCs infected with VZV had reduced surface Fas expression, which may protect infected cells from apoptosis, a finding consistent with the previous report of a lack of apoptosis in VZV infected immature DCs (Abendroth et al. 2001b).
The skin is a critical site of VZV infection during both varicella and herpes zoster, and skin DCs play a pivotal role in the induction of antiviral immunity, so there is good reason to study infection and modulation of DCs in human skin during natural cutaneous VZV infection. The two subsets of DC that are normally present in human skin and which may therefore be involved in the pathogenesis of VZV infection are the Langerhans cells (LC) of the epidermis and dermal DC (DDC) (Valladeau and Saeland 2005). These CD1a+ DC are found in an immature state in uninfected skin and following antigen capture have the capacity to migrate from the periphery to the lymph nodes, where they interact with T cells to initiate an immune response (Valladeau and Saeland 2005). There are also other types of DC, such as the blood derived myeloid DC (MDC) and plasmacytoid DC (PDC) that may also play a role in the pathogenesis of VZV infection. Of particular interest is the importance of PDC in innate antiviral immune responses due to their ability to recruit to sites of inflammation and secrete high levels of IFNα (Liu 2005; Siegal et al. 1999). PDC also participate in adaptive immune responses through their secretion of cytokines and chemokines that promote activation of effector cells, including NK, NKT, B, and T cells, and also antigen presentation to T cells (Colonna et al. 2004; Salio et al. 2004; Zhang and Wang 2005).
There have been several studies examining the impact of VZV infection on different DC subsets. It has been reported that the frequency of CD1a+ DCs is reduced in VZV infected skin epidermis compared to uninfected skin, demonstrating an alteration in the distribution of DC in response to VZV infection of the skin (Nikkels et al. 2004). It has also been reported that PDCs infiltrate into the dermis of varicella lesions, suggesting that these cells contribute to the immune control of VZV infection (Gerlini et al. 2006).
Recent work from the Abendroth group has examined in more detail DC subsets in skin biopsies from varicella and herpes zoster cases (Huch et al. 2010). Immunostaining and microscopy analysis of VZV infected lesions of both varicella and herpes zoster showed that in comparison to normal uninfected skin, the proportion of cells expressing DC-SIGN (DDC marker) or DC-LAMP and CD83 (mature DC markers) were not significantly altered. In contrast, the frequency of LCs was significantly decreased in VZV infected skin, concomitant with a striking influx of PDC into VZV infected skin. The authors suggested that this loss of LC from the skin was most likely to be a consequence of migration of these cells to distal sites, such as lymph nodes. Within infected skin the LCs and PDC were closely associated with VZV antigen positive cells, and a small proportion of both LC and PDC showed evidence of VZV infection. Despite only sporadic detection of VZV infected PDC and LC during natural cutaneous infection this finding is thought to be important when considered in the context of the frequency of infection of other cell types which have been shown to play crucial roles in the course of natural VZV infection. In this respect, the proportion of VZV infected lymphocytes in peripheral blood during natural VZV infection is very small, with estimates in the range of 1 in 100,000 PBMCs from healthy varicella patients becoming infected, yet the role of peripheral blood T cells in transporting virus to distal sites is regarded as a significant event in VZV pathogenesis (Koropchak et al. 1989, 1991).
In an extension of these in vivo observations, PDC isolated from human blood and LC derived from the MUTZ-3 cell line were shown to be permissive to VZV infection (Huch et al. 2010). Interestingly, significant induction of IFNα by PDC did not occur following VZV infection and infected PDC cultures remained refractory to IFNα induction even when stimulated with a TLR9 agonist which stimulates IFNα production by PDC. Additional work will be required to define the mechanism of VZV encoded modulation of IFNα production by PDC and identification of any viral gene(s) that encode this function. Furthermore, it remains to be determined whether infected PDC or LC are impaired in other functions such as antigen presentation.
In summary, definition of changes that occur to the distribution of multiple DC subsets in the skin of individuals suffering from primary and recurrent VZV disease, with the identification of LC and PDC as subsets most affected during infection, implicates these DCs as playing important roles in VZV pathogenesis. Furthermore, the capacity of VZV to infect and impair function of different DC subsets highlights VZV mediated immune control of these cells.
6 Concluding Remarks and Future Perspectives
Modulation of immune function has emerged as a powerful strategy by which the virus is likely to evade or delay host defenses during critical stages of infection. This chapter has highlighted the plethora of VZV encoded immune evasion strategies that are particularly relevant to those who suffer from either varicella or herpes zoster, and ongoing investigations will better define the relationship between VZV and the host immune system. A significant outcome of elucidating mechanism and identifying viral genes that modulate host immune surveillance and infection will be for development of a better “second generation” vaccine against VZV disease. This vaccine should consist of specifically targeted modifications, such that it replicates at the inoculation site without causing a lesion and lacks viral genes that permit evasion of host defense mechanisms or infectivity for DCs, yet induces immunity as effectively as natural infection.
The study of VZV encoded immune modulation poses several significant challenges. Firstly, the high species specificity and the lack animal models to study VZV infection in the context of a fully in intact immune response limits the capacity to study VZV control of immune function. Secondly, the study of naturally infected individuals is complicated by the difficulty in obtaining tissues from different anatomical sites and/or low levels of infection, e.g., T cells in the blood. For these reasons, experimental models of infection using primary cultured human cell types or tissues implanted into SCID mice will continue to play a critical role in the analysis of VZV mediated immunomodulation and in driving analysis of naturally infected cell and tissue samples.
Acknowledgments
AA and BS were supported by NHMRC grant 457356. PRK acknowledges support for this work by Public Health Service NIH grants NS064022 and EY08098, and funds from the Research to Prevent Blindness Inc. and the Eye and Ear Institute of Pittsburgh.
Contributor Information
Allison Abendroth, Email: allison.abendroth@sydney.edu.au, Department of Infectious Diseases and Immunology, University of Sydney, Blackburn Building, Room 601, Camperdown, NSW 2006, Australia; Centre for Virus Research, Westmead Millennium Institute, Westmead, NSW 2145, Australia.
Paul R. Kinchington, Email: kinchingtonp@upmc.edu, Department of Ophthalmology, School of Medicine, University of Pittsburgh, Pittsburgh, USA; Department of Molecular Microbiology and Genetics, School of Medicine, University of Pittsburgh, Pittsburgh, USA.
Barry Slobedman, Email: barry.slobeman@sydney.edu.au, Centre for Virus Research, Westmead Millennium Institute, Westmead, NSW 2145, Australia.
References
- Abendroth A, Arvin A. Varicella-zoster virus immune evasion. Immunol Rev. 1999;168:143–156. doi: 10.1111/j.1600-065x.1999.tb01289.x. [DOI] [PubMed] [Google Scholar]
- Abendroth A, Arvin AM. Immune evasion as a pathogenic mechanism of varicella zoster virus. Semin Immunol. 2001;13:27–39. doi: 10.1006/smim.2001.0293. [DOI] [PubMed] [Google Scholar]
- Abendroth A, Slobedman B, Lee E, Mellins E, Wallace M, Arvin AM. Modulation of major histocompatibility class II protein expression by varicella-zoster virus. J Virol. 2000;74:1900–1907. doi: 10.1128/jvi.74.4.1900-1907.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abendroth A, Lin I, Slobedman B, Ploegh H, Arvin AM. Varicella-zoster virus retains major histocompatibility complex class I proteins in the Golgi compartment of infected cells. J Virol. 2001a;75:4878–4888. doi: 10.1128/JVI.75.10.4878-4888.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abendroth A, Morrow G, Cunningham AL, Slobedman B. Varicella-zoster virus infection of human dendritic cells and transmission to T cells: implications for virus dissemination in the host. J Virol. 2001b;75:6183–6192. doi: 10.1128/JVI.75.13.6183-6192.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K, Meyer TH, Uebel S, Sempe P, Djaballah H, Yang Y, Peterson PA, Fruh K, Tampe R. Molecular mechanism and species specificity of TAP inhibition by herpes simplex virus ICP47. EMBO J. 1996;15:3247–3255. [PMC free article] [PubMed] [Google Scholar]
- Ambagala AP, Cohen JI. Varicella-zoster virus IE63, a major viral latency protein, is required to inhibit the alpha interferon-induced antiviral response. J Virol. 2007;81:7844–7851. doi: 10.1128/JVI.00325-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvin A. Varicella zoster virus. In: Knipe DaHP., editor. Fields virology. 4th edn. vol 2. Philadelphia: Lippincott Williams and Wilkins; 2001. pp. 2731–2767. [Google Scholar]
- Arvin AM, Schmidt NJ, Cantell K, Merigan TC. Alpha interferon administration to infants with congenital rubella. Antimicrob Agents Chemother. 1982;21:259–261. doi: 10.1128/aac.21.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvin AM, Koropchak CM, Williams BR, Grumet FC, Foung SK. Early immune response in healthy and immunocompromised subjects with primary varicella-zoster virus infection. J Infect Dis. 1986;154:422–429. doi: 10.1093/infdis/154.3.422. [DOI] [PubMed] [Google Scholar]
- Arvin AM, Moffat JK, Redman R. Varicella-zoster virus: aspects of pathogenesis and host response to natural infection and varicella vaccine. Adv Virus Res. 1996;46:263–309. doi: 10.1016/s0065-3527(08)60074-3. [DOI] [PubMed] [Google Scholar]
- Balachandra K, Thawaranantha D, Ayuthaya PI, Bhumisawasdi J, Shiraki K, Yamanishi K. Effects of human alpha, beta and gamma interferons on varicella zoster virus in vitro. South-east Asian J Trop Med Public Health. 1994;25:252–257. [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. lmmunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Black AP, Jones L, Malavige GN, Ogg GS. Immune evasion during varicella zoster virus infection of keratinocytes. Clin Exp Dermatol. 2009;34(8):941–944. doi: 10.1111/j.1365-2230.2009.03350.x. [DOI] [PubMed] [Google Scholar]
- Bowden RA, Levin MJ, Giller RH, Tubergen DG, Hayward AR. Lysis of varicella zoster virus infected cells by lymphocytes from normal humans and immunosuppressed pediatric leukaemic patients. Clin Exp Immunol. 1985;60:387–395. [PMC free article] [PubMed] [Google Scholar]
- Cohen JI. Infection of cells with varicella-zoster virus down-regulates surface expression of class I major histocompatibility complex antigens. J Infect Dis. 1998;177:1390–1393. doi: 10.1086/517821. [DOI] [PubMed] [Google Scholar]
- Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, Baltimore D. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity. 1999;10:661–671. doi: 10.1016/s1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- Colamonici OR, Domanski P. Identification of a novel subunit of the type I interferon receptor localized to human chromosome 21. J Biol Chem. 1993;268:10895–10899. [PubMed] [Google Scholar]
- Collins T, Korman AJ, Wake CT, Boss JM, Kappes DJ, Fiers W, Ault KA, Gimbrone MA, Jr, Strominger JL, Pober JS. Immune interferon activates multiple class II major histocompatibility complex genes and the associated invariant chain gene in human endothelial cells and dermal fibroblasts. Proc Natl Acad Sci USA. 1984;81:4917–4921. doi: 10.1073/pnas.81.15.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- Desloges N, Rahaus M, Wolff MH. Role of the protein kinase PKR in the inhibition of varicella-zoster virus replication by beta interferon and gamma interferon. J Gen Virol. 2005;86:1–6. doi: 10.1099/vir.0.80466-0. [DOI] [PubMed] [Google Scholar]
- Eisfeld AJ, Yee MB, Erazo A, Abendroth A, Kinchington PR. Downregulation of class I major histocompatibility complex surface expression by varicella-zoster virus involves open reading frame 66 protein kinase-dependent and -independent mechanisms. J Virol. 2007;81:9034–9049. doi: 10.1128/JVI.00711-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Mjiyad N, Bontems S, Gloire G, Horion J, Vandevenne P, Dejardin E, Piette J, Sadzot-Delvaux C. Varicella-zoster virus modulates NF-kappaB recruitment on selected cellular promoters. J Virol. 2007;81:13092–13104. doi: 10.1128/JVI.01378-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erazo A, Kinchington PR. Varicella-zoster virus open reading frame 66 protein kinase and its relationship to alphaherpesvirus US3 kinases. Curr Top Microbiol Immunol. 2009 doi: 10.1007/82_2009_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell HE, Davis-Poynter NJ. From sabotage to camouflage: viral evasion of cytotoxic T lymphocyte and natural killer cell-mediated immunity. Semin Cell Dev Biol. 1998;9:369–378. doi: 10.1006/scdb.1998.0246. [DOI] [PubMed] [Google Scholar]
- Garcia-Sastre A, Biron CA. Type 1 interferons and the virus–host relationship: a lesson in detente. Science. 2006;312:879–882. doi: 10.1126/science.1125676. [DOI] [PubMed] [Google Scholar]
- Gerlini G, Mariotti G, Bianchi B, Pimpinelli N. Massive recruitment of type I interferon producing plasmacytoid dendritic cells in varicella skin lesions. J Invest Dermatol. 2006;126:507–509. doi: 10.1038/sj.jid.5700052. [DOI] [PubMed] [Google Scholar]
- Gershon AA, Mervish N, LaRussa P, Steinberg S, Lo SH, Hodes D, Fikrig S, Bonagura V, Bakshi S. Varicella-zoster virus infection in children with underlying human immunodeficiency virus infection. J Infect Dis. 1997;176:1496–1500. doi: 10.1086/514147. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nut Rev Immunol. 2008;8:837–848. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- Grose C. Variation on a theme by Fenner: the pathogenesis of chickenpox. Pediatrics. 1981;68:735–737. [PubMed] [Google Scholar]
- Haller O, Kochs G, Weber F. The interferon response circuit: induction and suppression by pathogenic viruses. Virology. 2006;344:119–130. doi: 10.1016/j.virol.2005.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen TH, Bouvier M. MHC class I antigen presentation: learning from viral evasion strategies. Nal Rev Immunol. 2009;9:503–513. doi: 10.1038/nri2575. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Hu H, Cohen JI. Varicella-zoster virus open reading frame 47 (ORF47) protein is critical for virus replication in dendritic cells and for spread to other cells. Virology. 2005;337:304–311. doi: 10.1016/j.virol.2005.04.024. [DOI] [PubMed] [Google Scholar]
- Huch J, Cunningham A, Arvin A, Nasr N, Santegoets S, Slobedman E, Slobedman B, Abendroth A. Impact of varicella zoster virus on dendritic cell subsets in human skin during natural infection. J Virol. 2010;84:4060–4072. doi: 10.1128/JVI.01450-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara T, Starr SE, Ito M, Douglas SD, Arbeter AM. Human polymorphonuclear leukocyte-mediated cytotoxicity against varicella-zoster virus-infected fibroblasts. J Virol. 1984;51:110–116. doi: 10.1128/jvi.51.1.110-116.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Watanabe M, Kamiya H, Sakurai M. Inhibition of natural killer (NK) cell activity against varicella-zoster virus (VZV)-infected fibroblasts and lymphocyte activation in response to VZV antigen by nitric oxide-releasing agents. Clin Exp Immunol. 1996;106:40–44. doi: 10.1046/j.1365-2249.1996.d01-807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JO, Arvin AM. Viral and cellular gene transcription in fibroblasts infected with small plaque mutants of varicella-zoster virus. Antiviral Res. 2005;68:56–65. doi: 10.1016/j.antiviral.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Jones JO, Arvin AM. Inhibition of the NF-kappaB pathway by varicella-zoster virus in vitro and in human epidermal cells in vivo. J Virol. 2006;80:5113–5124. doi: 10.1128/JVI.01956-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jura E, Chadwick EG, Josephs SH, Steinberg SP, Yogev R, Gershon AA, Krasinski KM, Borkowsky W. Varicella-zoster virus infections in children infected with human immunodeficiency virus. Pediatr Infect Dis J. 1989;8:586–590. doi: 10.1097/00006454-198909000-00003. [DOI] [PubMed] [Google Scholar]
- Katze MG, He Y, Gale M., Jr Viruses and interferon: a fight for supremacy. Nat Rev Immunol. 2002;2:675–687. doi: 10.1038/nri888. [DOI] [PubMed] [Google Scholar]
- Klagge IM, Schneider-Schaulies S. Virus interactions with dendritic cells. J Gen Virol. 1999;80:823–833. doi: 10.1099/0022-1317-80-4-823. [DOI] [PubMed] [Google Scholar]
- Koppers-Lalic D, Reits EA, Ressing ME, Lipinska AD, Abele R, Koch J, Marcondes Rezende M, Admiraal P, van Leeuwen D, Bienkowska-Szewczyk K, Mettenleiter TC, Rijsewijk FA, Tampe R, Neefjes J, Wiertz FJ. Varicelloviruses avoid T cell recognition by UL49.5-mediated inactivation of the transporter associated with antigen processing. Proc Natl Acad Sci USA. 2005;102:5144–5149. doi: 10.1073/pnas.0501463102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppers-Lalic D, Verweij MC, Lipinska AD, Wang Y, Quinten E, Reits EA, Koch J, Loch S, Marcondes Rezende M, Daus F, Bienkowska-Szewczyk K, Osterrieder N, Mettenleiter TC, Heemskerk MH, Tampe R, Neefjes JJ, Chowdhury SI, Ressing ME, Rijsewijk FA, Wiertz EJ. Varicellovirus UL 49.5 proteins differentially affect the function of the transporter associated with antigen processing, TAP. PLoS Pathog. 2008;4:e1000080. doi: 10.1371/journal.ppat.1000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koropchak CM, Solem SM, Diaz PS, Arvin AM. Investigation of varicella-zoster virus infection of lymphocytes by in situ hybridization. J Virol. 1989;63:2392–2395. doi: 10.1128/jvi.63.5.2392-2395.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koropchak CM, Graham G, Palmer J, Winsberg M, Ting SF, Wallace M, Prober CG, Arvin AM. Investigation of varicella-zoster virus infection by polymerase chain reaction in the immunocompetent host with acute varicella. J Infect Dis. 1991;163:1016–1022. doi: 10.1093/infdis/163.5.1016. [DOI] [PubMed] [Google Scholar]
- Ku CC, Padilla JA, Grose C, Butcher EC, Arvin AM. Tropism of varicella-zoster virus for human tonsillar CD4(+) T lymphocytes that express activation, memory, and skin homing markers. J Virol. 2002;76:11425–11433. doi: 10.1128/JVI.76.22.11425-11433.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku CC, Zerboni L, lto H, Graham BS, Wallace M, Arvin AM. Varicella-zoster virus transfer to skin by T Cells and modulation of viral replication by epidermal cell interferon-alpha. J Exp Med. 2004;200:917–925. doi: 10.1084/jem.20040634. Epub 2004 Sep 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin MJ, Hayward AR. The varicella vaccine. Prevention of herpes zoster. Infect Dis Clin North Am. 1996;10:657–675. doi: 10.1016/s0891-5520(05)70319-6. [DOI] [PubMed] [Google Scholar]
- Liang L, Roizman B. Expression of gamma interferon-dependent genes is blocked independently by virion host shutoff RNase and by US3 protein kinase. J Virol. 2008;82:4688–4696. doi: 10.1128/JVI.02763-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- Miller-Kittrell M, Sparer TE. Feeling manipulated: cytomegalovirus immune manipulation. Virol J. 2009;6:4. doi: 10.1186/1743-422X-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow G, Slobedman B, Cunningham AL, Abendroth A. Varicella-zoster virus productively infects mature dendritic cells and alters their immune function. J Virol. 2003;77:4950–4959. doi: 10.1128/JVI.77.8.4950-4959.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkemagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- Nikkels AF, Debrus S, Sadzot-Delvaux C, Piette J, Rentier B, Pierard GE. Localization of varicella-zoster virus nucleic acids and proteins in human skin. Neurology. 1995;45:S47–S49. doi: 10.1212/wnl.45.12_suppl_8.s47. [DOI] [PubMed] [Google Scholar]
- Nikkels AF, Sadzot-Delvaux C, Pierard GE. Absence of intercellular adhesion molecule 1 expression in varicella zoster virus-infected keratinocytes during herpes zoster: another immune evasion strategy? Am J Dermatopathol. 2004;26:27–32. doi: 10.1097/00000372-200402000-00005. [DOI] [PubMed] [Google Scholar]
- Pober JS, Gimbrone MA, Jr, Cotran RS, Reiss CS, Burakoff SJ, Fiers W, Ault KA. Ia expression by vascular endothelium is inducible by activated T cells and by human gamma interferon. J Exp Med. 1983;157:1339–1353. doi: 10.1084/jem.157.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlein R, Dustin ML, Marlin SD, Springer TA. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986;137:1270–1274. [PubMed] [Google Scholar]
- Sadler AJ, Williams BR. lnterferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salio M, Palmowski MJ, Atzberger A, Hermans IF, Cerundolo V. CpG-matured murine plasmacytoid dendritic cells are capable of in vivo priming of functional CD8 T cell responses to endogenous but not exogenous antigens. J Exp Med. 2004;199:567–579. doi: 10.1084/jem.20031059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap A, Fortin JF, Sommer M, Zerboni L, Stamatis S, Ku CC, Nolan GP, Arvin AM. T-cell tropism and the role of ORF66 protein in pathogenesis of varicella-zoster virus infection. J Virol. 2005;79:12921–12933. doi: 10.1128/JVI.79.20.12921-12933.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, Ho S, Antonenko S, Liu YJ. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- Tomazin R, Hill AB, Jugovic P, York I, van Endert P, Ploegh HL, Andrews DW, Johnson DC. Stable binding of the herpes simplex virus ICP47 protein to the peptide binding site of TAP. EMBO J. 1996;15:3256–3266. [PMC free article] [PubMed] [Google Scholar]
- Tortorella D, Gewurz BE, Furman MH, Schust DJ, Ploegh HL. Viral subversion of the immune system. Annu Rev Immunol. 2000;18:861–926. doi: 10.1146/annurev.immunol.18.1.861. [DOI] [PubMed] [Google Scholar]
- Valladeau J, Saeland S. Cutaneous dendritic cells. Semin Immunol. 2005;17:273–283. doi: 10.1016/j.smim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Verweij MC, Koppers-Lalic D, Loch S, Klauschies F, de la Salle H, Quinten E, Lehner PJ, Mulder A, Knittler MR, Tampe R, Koch J, Ressing ME, Wiertz EJ. The varicellovirus UL49.5 protein blocks the transporter associated with antigen processing (TAP) by inhibiting essential conformational transitions in the 6+6 transmembrane TAP core complex. J Immunol. 2008;181:4894–4907. doi: 10.4049/jimmunol.181.7.4894. [DOI] [PubMed] [Google Scholar]
- Wilkinson GW, Tomasec P, Stanton RJ, Armstrong M, Prod’homme V, Aicheler R, McSharry BP, Rickards CR, Cochrane D, Llewellyn-Lacey S, Wang EC, Griffin CA, Davison AJ. Modulation of natural killer cells by human cytomegalovirus. J Clin Virol. 2008;41:206–212. doi: 10.1016/j.jcv.2007.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wang FS. Plasmacytoid dendritic cells act as the most competent cell type in linking antiviral innate and adaptive immune responses. Cell Mol Immunol. 2005;2:411–417. [PubMed] [Google Scholar]