Abstract
A growing body of evidence suggests the involvement of inflammatory processes in the pathophysiology of schizophrenia. Four to eight-week exposure to cuprizone, a copper chelator, causes robust demyelination and has been used to build a model for multiple sclerosis. In contrast, we report here the effects of one-week cuprizone exposure in mice. This short-term cuprizone exposure elicits behavioral changes that include augmented responsiveness to methamphetamine and phencyclidine, as well as impaired working memory. The cellular effects of one-week cuprizone exposure differ substantially from the longer-term exposure; perturbation of astrocytes and microglia is induced without any sign of demyelination. Furthermore, the proinflammatory cytokine interleukin-6 was significantly up-regulated in glial fibrillary acidic protein (GFAP)-positive cells. We propose that this cuprizone short-term exposure may offer a model to study some aspects of biology relevant to schizophrenia and related conditions.
Keywords: inflammation, astrocyte, cytokine, interleukin-6, schizophrenia
Introduction
Although we are still far from a complete picture of the cause of schizophrenia, our understanding of this disease has come a long way. A mounting body of epidemiological data suggests that inflammatory dysregulation may underlie the pathology of some cases of schizophrenia (Benros et al., 2011; Yolken and Torrey, 2008). Genome-wide association studies have added weight to the idea of immune involvement in the pathology by revealing an association between schizophrenia susceptibility loci and the major histocompatibility complex (MHC) locus on chromosome 6p, a well-replicated common variant locus (Duan et al., 2010; Purcell et al., 2009; Shi et al., 2009; Stefansson et al., 2009). Immune system-related cytokines, including interleukin 6 (IL-6), are reportedly elevated in serum and cerebrospinal fluid (CSF) of schizophrenia patients (Coughlin et al., 2013; Garver et al., 2003; Lin et al., 1998; Miller et al., 2011; Potvin et al., 2008; van Kammen et al., 1999). Furthermore, recent positron emission tomography studies with radioligands for translocator protein (peripheral benzodiazepine receptor) support the presence of excess neuroinflammation in the brains of patients with schizophrenia or early psychosis compared with those of normal controls (Doorduin et al., 2009; Kreisl et al., 2012).
Here we propose that a short-term (one-week) systemic exposure of mice to a copper chelator, cuprizone, may emulate an important aspect of biology underlying schizophrenia and related disorders. Long-term exposure (4-8 weeks) to cuprizone has been used to generate a model for multiple sclerosis, in which robust demyelination is elicited (Koutsoudaki et al., 2009; Liu et al., 2010; Matsushima and Morell, 2001). In contrast, we demonstrate that short-term (one-week) exposure to this compound leads to a combination of glial activation and behavioral changes without inducing demyelination.
Materials and methods
Animals and cuprizone exposure
All experimental procedures followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals, the Johns Hopkins University Animal Care and Use Guidelines, and the guidelines provided by Mitsubishi Tanabe Pharma Corporation. C57BL/6J male mice were obtained from Charles River Japan. They were kept in quiet facilities under controlled temperatures (23 ± 3°C) and a controlled light schedule (lights were on from 7am to 7pm) with unlimited food and water. Eight-week-old mice were randomly allocated to two groups. For one week, mice were fed either a diet containing 0.2% cuprizone (Sigma-Aldrich), or a control diet consisting of standard mouse chow. The diets of the two groups were identical except for the presence of cuprizone. All experiments described below were performed on the final day of cuprizone exposure.
Behavioral analyses
Each of the behavioral tests described below were performed with a different cohort of mice. Animal behavior and movement were monitored with the SCANET system (Melquest). In testing hyperlocomotion induced by metamphetamine (0.3 mg/kg: from Dainippoin Pharma) or phencyclidine (2.5 mg/kg: synthesized by Mitsubishi Tanabe Pharma), animals were habituated in the apparatus for 30 min and then injected subcutaneously with saline, or one of the above drugs. Ambulatory activity, measured as “counts” passing a set of infrared beams in the SCANET system, was measured in 5 min intervals for 60 min after the injection. The Y-maze (22 cm long, 20 cm high and 6.5 cm wide, with arms positioned 120° degrees apart) was used according to a published protocol (Fukumoto et al., 2010). Briefly, each mouse was placed at the end of one arm and allowed to move freely through the maze for 5 min. The sequence of arm entries was recorded manually. A spontaneous alternation was defined as entry into all three arms on consecutive occasions. Therefore, the possible maximum alternation was the total number of arm entries minus two, and the ratio of spontaneous alternation was calculated as actual alternations/maximum alternations. The total number of arms entered during the sessions was also determined. The novel object recognition test was conducted according to a published protocol (Barker et al., 2007). Briefly, mice were habituated to the arena (45.5 × 25 × 35 cm) without any objects for 5 min. An acquisition phase was conducted 24 hours later, followed by a recognition test 3h after acquisition. The SCANET MV-10 system (Melquest) was used to measure movements. During acquisition, two identical objects were placed in the arena and the animal was allowed to explore for 5 min. Exploratory behavior was defined as the animal biting, sniffing or touching the objects. Other behaviors, such as looking around while sitting on or resting against the object, were not considered as exploration. In the recognition test the animal was presented with a familiar object and a novel object, distinct in color and shape, in the same positions as during acquisition for 5 min. The percentage of exploratory preference was defined as the ratio of the time spent exploring the novel object (Tn) over the time spent exploring the novel and the familiar objects (Tf), [Tn/(Tn + Tf)] × 100. Prepulse inhibition was assessed using the San Diego Instruments apparatus according to a published protocol (Kopec et al., 2010). Briefly, mice were habituated for 5 min with background noise levels (70 dB) prior to testing. The 20 min test sessions consisted of 5 trial types: (1) pulse alone (120 dB for 40 ms); a 20 ms prepulse at (2) 10 dB, (3) 15 dB, or (4) 20 dB above background levels, followed 100ms later by the main pulse; or (5) background only (70 dB, no stimulus). The inter-trial interval was 12-18 s (average is 15 s), and each session consisted of 12 sets of each 5 trials, thus a total of 60 trials. Startle amplitude was recorded for each trial except for the first set of trials. The prepulse inhibition ratio at each prepulse sound level was calculated as 100 x (averaged response amplitude in trials with a prepulse stimulus and startle stimulus/averaged response amplitude in trials with the startle stimulus alone). The numbers of animals used for each assay is described in the figure legends.
Black-Gold staining
Mice were perfused with 10% neutral phosphate buffered formalin. The brains were fixed overnight in the same solution, then treated with 25% sucrose solution, and mounted with O.C.T. compound. Sections were cut in the vicinity of bregma -2.0 mm at a thickness of 20 μm on a freezing sliding microtome. Black-Gold staining was performed on control (n=3) and one-week cuprizone exposure mice (n=4). Four sections were stained per mouse (control: 12 sections, cuprizone: 16 sections) using the Black-Gold II myelin staining kit (Millipore) with minor adjustments to the incubation period stated in the manufacturer’s protocol. Brain sections were imaged using TE300 (Nikon) and DP controller (Olympus). Four images were analyzed per mouse. The average light intensity within the corpus callosum was quantified using Image J software.
Electron microscopy
Electron microscopy was conducted on control (n=3) and one-week cuprizone exposure mice (n=3). Mice were perfused with 2.5% glutaraldehyde. Sections were postfixed in 2.5% glutaraldehyde and 2% osmium tetroxide solution, then dehydrated and mounted with epoxy resin. Sections of 100 nm thickness were cut using an EM UC7 ultramicrotome (Leica) then stained with uranyl acetate and lead citrate. Quantification was conducted using eight to thirteen sections per mouse imaged with a CCD camera. All fibers within each 3 μm2 image were digitized. A total of 40-70 digitized fibers were analyzed per mouse. Image J software was used to calculate G-ratios (defined as the diameter of the axon divided by the diameter of the axon and myelin) based on the widest point for each axon.
Molecular histology
Immunohistochemistry was performed according to a published protocol (Kousaka et al., 2009). Briefly, 8 μm thick coronal sections were incubated with antibodies against GFAP (rabbit polyclonal antibody, Dako, 1:50) and Iba1 (rabbit polyclonal, Wako, 1:16,000), followed by secondary antibodies with horseradish peroxidase (Nichirei) and 3, 3’-diaminobenzidine for signal detection. In situ hybridization was conducted as published (Kikawada et al., 2007). Briefly, a 500 bp DNA fragment corresponding to nucleotide positions 66-461 of mouse interleukin 6 (NM_031168.1) was subcloned into pGEMT-Easy vector (Promega) and used to generate the RNA probe. Brain tissue was fixed with Tissue Fixative (Genostaff), embedded in paraffin, then sectioned at 6 μm. Hybridization was performed with probes at a concentration of 300 ng/ml in Probe Diluent-1 (Genostaff) at 60°C for 16 h. Digoxygenin (DIG)-labeled probes were detected using NBT/BCIP solution (Sigma-Aldrich) for color reactions. Cell counting was conducted blind to treatment.
Quantitative real-time PCR
Total RNA was prepared using the RNeasy Mini Kit (Qiagen), which was reverse transcribed to cDNA with ReverTra Ace (Toyobo) with random primer pd(N)9 (TAKARA BIO, Shiga, Japan). Quantitative real-time PCR was performed using Taqman reagents and the ABI 7500 Fast Sequence Detection System (Life technologies). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal standard. Primer and probe information is shown in Supplementary Table 1.
Copper measurement
Total copper levels in brain were measured in control (n=3) and one-week cuprizone exposure mice (n=3). Brains were homogenized in 0.01 M HCl, centrifuged at 4°C and the supernatant was assessed using the Metallo Assay Low Copper LS (Metallogenics) kit according to the manufacturer’s instructions. Briefly, color reagent was added to samples, copper standard solution (40 μg/dL) and distilled water in a 96 well plate and incubated at room temperature for 10 min. Copper concentrations in samples were calculated from the standard by measuring the absorbance at 582 and 675 nm using Spectra Max M2 (Molecular Devices).
Statistical analysis
Behavioral data of two groups were analyzed by Student’s t-test. Prior to statistical analysis of quantitative RT-PCR data, the mRNA expression level in the cuprizone group was normalized to the control group for each brain region. Analysis was conducted using Student’s t-tests within each brain region and results were considered significant at a Bonferroni adjusted p value of < 0.05. The data are shown as mean ± S.E.M.
Results
Exposure of mice to cuprizone for one week results in abnormal behaviors
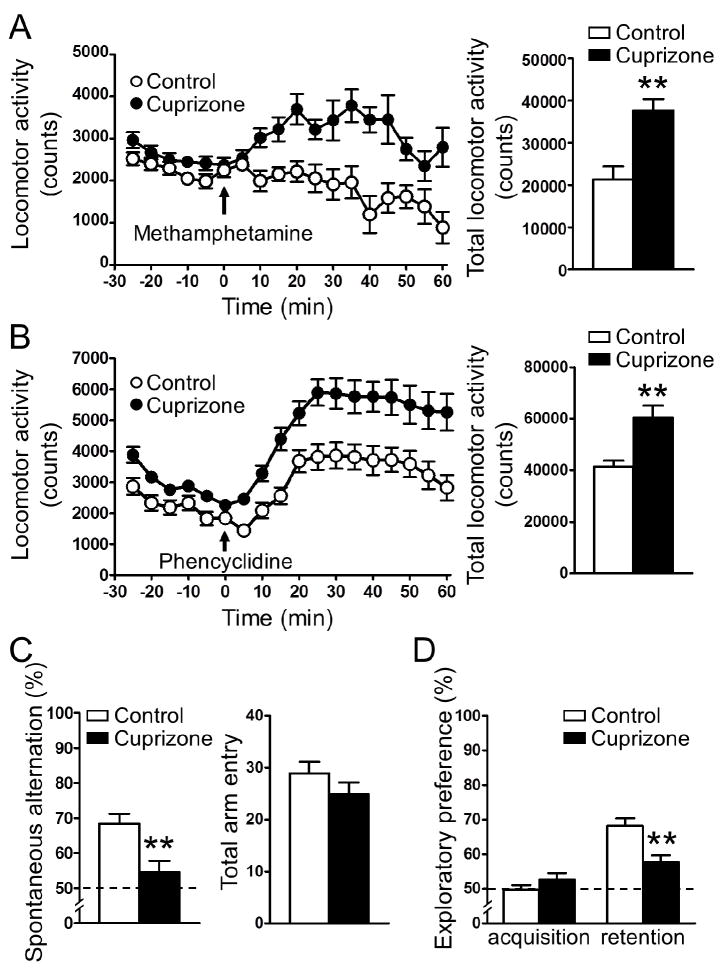
To explore the behavioral outcome of short-term (one-week) exposure to cuprizone, we performed several behavioral tests. Mice exposed to the cuprizone-containing diet for one week showed increased locomotor activity in response to a low dose of psychostimulant (0.3 mg/kg methamphetamine) whereas mice fed the control diet showed no increase in locomotion (Fig. 1a). At higher doses of methamphetamine (1.0 and 3.0 mg/kg), both groups responded with similar levels of hyperlocomotion. (Supplementary Fig. 1). The short-term exposure of mice to cuprizone also augmented locomotor response to phencyclidine, a N-methyl-D-aspartate (NMDA)-type glutamate receptor antagonist (Fig. 1b). There was no difference between groups in baseline locomotor activity (Supplementary Fig. 2). Evaluation of working memory using the Y-maze test demonstrated that short-term cuprizone exposure significantly decreased spontaneous alternation without affecting total arm entry (Fig. 1c). The cuprizone mice also displayed decreased preference for the novel object during the retention phase of the novel object recognition test (Fig. 1d). The acoustic startle response and prepulse inhibition were measured, however, no significant difference was observed between control and cuprizone mice (Supplementary Fig. 3).
Figure 1. Short-term (one-week) exposure to cuprizone results in abnormal behaviors in mice.
(A, B) Short-term cuprizone exposure augmented the locomotor response to 0.3 mg/kg methamphetamine (A), and 2.5 mg/kg phencyclidine (B). The left panels depict the time course of locomotion in 5 min blocks. Arrow indicates time of psychostimulant injection. The right panels show total locomotion (cumulative horizontal movement) from t = 0 to 60. (C) Short-term cuprizone exposure caused decreased spontaneous alternation in the Y-maze (left), while total arm entry remained unchanged (right). (D) Short-term cuprizone exposure decreased exploratory preference for the novel object in the retention phase of the novel object recognition test. The dashed line represents interaction with the novel object at the level of chance. n = 10. **p < 0.01: Student’s t-test. Data are mean ± S.E.M.
Pharmacokinetics for PCP and methamphetamine following cuprizone exposure
Methamphetamine and PCP concentrations within the brain and blood plasma were measured to determine whether there was any difference in the pharmacokinetics of drug metabolism following cuprizone exposure. At the dose utilized in the locomotor assay (methamphetamine, 0.3 mg/kg and phencyclidine, 2.5 mg/kg), drug concentrations in the brain, 0.5 and 1 h after administration, did not differ between control and cuprizone mice (Supplementary Fig 4). The plasma concentration of PCP was significantly lower in the cuprizone mice 1 h after injection. No difference was observed at 0.5 h for PCP or either time point for methamphetamine (data not shown). Therefore, it seems that the observed cuprizone-induced hypersensitivity to psychostimulants does not result from increased exposure to the drugs.
Short-term (one-week) exposure to cuprizone does not cause demyelination
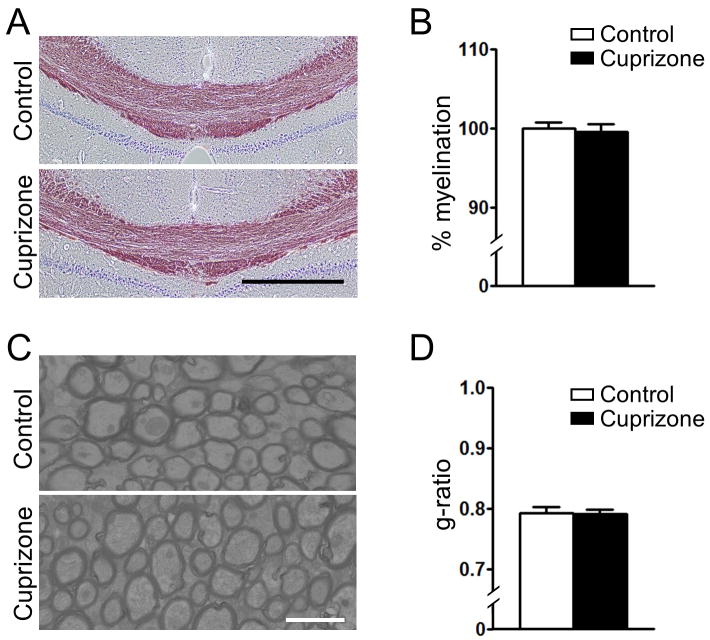
To investigate whether short-term (one-week) exposure to cuprizone induces demyelination, we performed Black-Gold myelin staining and electron microscopy studies in the corpus callosum of control and cuprizone-exposed mice. The Black-Gold stain showed no gross differences in myelination levels between the control and one-week cuprizone-exposed groups (Fig. 2a, b). The G-ratios obtained by measuring individual axons using electron microscopy further support the lack of demyelination after a one-week exposure to cuprizone (Fig. 2c, d). Of note, the level of copper in the brain was significantly augmented following short-term cuprizone-exposure (Supplementary Fig 5). An increased copper level in various tissues, including brain, was previously reported in long-term (3-9 months) cuprizone-exposed mice (Zatta et al., 2005).
Figure 2. Short-term (one-week) exposure to cuprizone does not induce demyelination.
(A) Representative black-gold staining of the corpus callosum of control and short-term cuprizone exposed mice. Scale bar: 500 μm. (B) Quantification of black-gold staining showed no demyelination in the short-term cuprizone-exposed mice. (C) Electron microscopy of axons in the corpus callosum of control and short-term cuprizone-exposed mice (representative image). Scale bar: 2 μm. (D) Analysis of the g-ratio in the corpus callosum revealed no demyelination in the short-term cuprizone-exposed mice. n = 3.
Short-term exposure to cuprizone robustly activates astrocytes and microglia
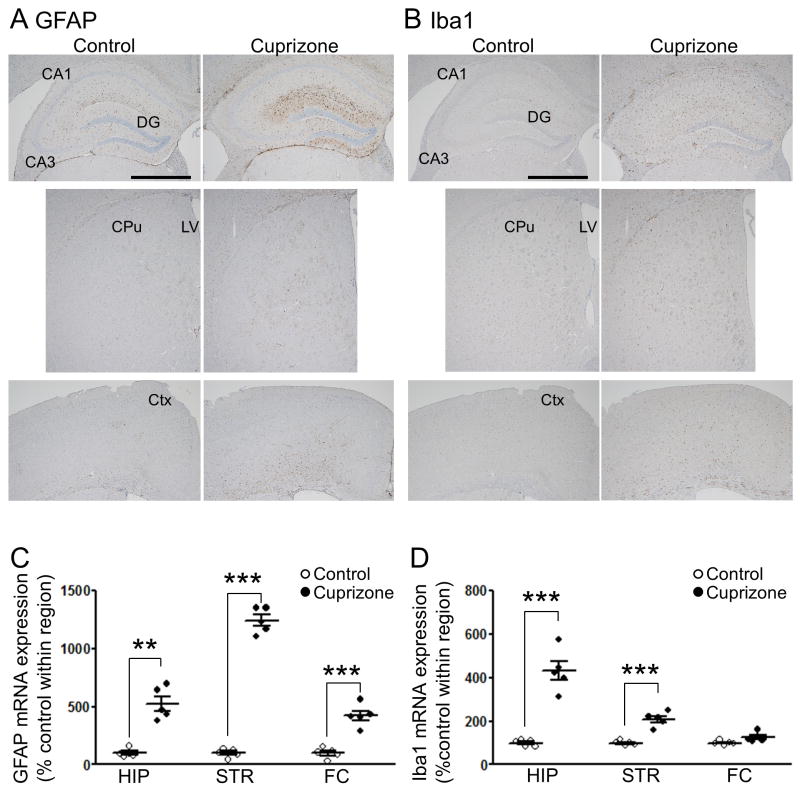
Given the absence of demyelination, we wondered if there were alterations in other types of glia in the mice. Immunohistochemical staining revealed a robust increase in the number of glial fibrillary acidic protein (GFAP)-positive cells in mice exposed to cuprizone, indicating the activation of astrocytes (Fig. 3a). Cells positive for ionized calcium binding adaptor molecule 1 (Iba1), a marker of activated microgila, were also increased in cuprizone-exposed mice (Fig. 3b). Consistent with the immunostaining, GFAP mRNA expression was augmented in the hippocampus, striatum and frontal cortex of the cuprizone mice (Fig. 3c). Likewise, Iba1 mRNA was also up-regulated in the hippocampus and striatum of the cuprizone-exposed mice (Fig. 3d).
Figure 3. Astrocytes and microglia are activated in short-term (one-week) cuprizone-exposed mice.
Representative images of immunohistochemistry for GFAP (A) and Iba1 (B). Scale bars,1 mm. DG, dentate gyrus; CPu, caudate putamen; LV, lateral ventricle; and Ctx, cortex. (C) GFAP mRNA was significantly up-regulated in the hippocampus, striatum, and frontal cortex of cuprizone-exposed mice compared to control. (D) Iba1 mRNA was significantly up-regulated in the hippocampus and striatum of cuprizone-exposed mice. HIP, hippocampus; STR/CPu, striatum; and FC, frontal area of Ctx. n = 5. **p < 0.01, ***p < 0.001: Student’s t-test, significant at the indicated Bonferroni adjusted p values. Data are mean ± S.E.M.
Short-term exposure to cuprizone induces interleukin 6 (IL-6)
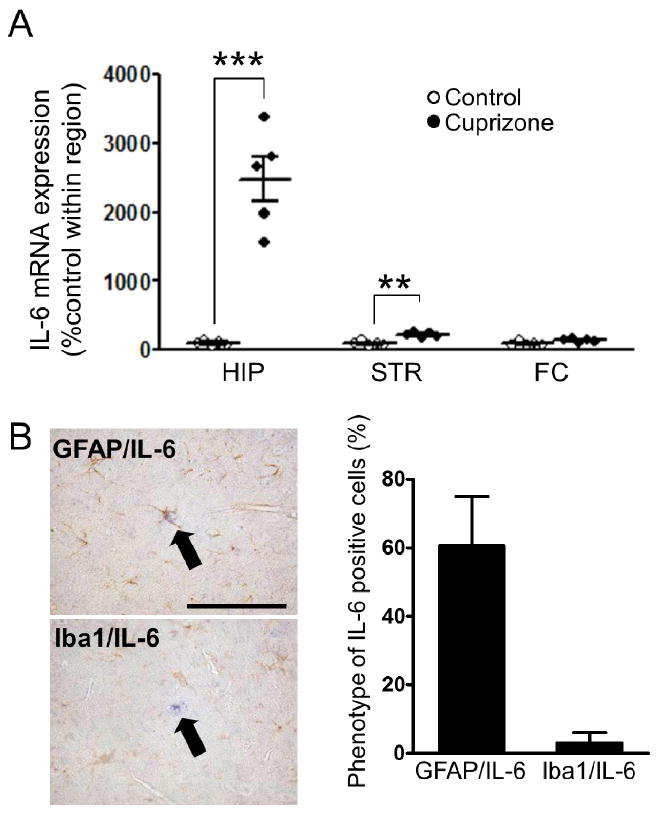
Glial cell activation is known to induce cytokine and chemokine expression and release, which modulate neural plasticity (Ben Achour and Pascual, 2010; Glass et al., 2010; Singh et al., 2011). Among such molecules, an increase in IL-6 is reported in the blood of patients with schizophrenia (Coughlin et al., 2013; Potvin et al., 2008). Measurement of IL-6 mRNA levels revealed elevation in the hippocampus and striatum of mice exposed to short-term cuprizone (Fig. 4a). Histological co-staining revealed that the majority of IL-6-positive cells were co-labeled with GFAP-, but not with Iba1-immunoreactivity (Fig. 4b).
Figure 4. Interleukin 6 is markedly induced by short-term exposure to cuprizone.
(A) Cuprizone exposure significantly increased expression of IL-6 mRNA in the hippocampus and striatum. n = 5. **p < 0.01, ***p < 0.001: Student’s t-test, significant at the indicated Bonferroni adjusted p values. HIP, hippocampus; STR/CPu, striatum; and FC, frontal area of the cortex. (B) Left, representative images of IL-6 in situ hybridization (blue) co-stained with GFAP or Iba1 immunohistochemistry (brown) in cuprizone-exposed mice. Right, percentage of IL-6-positive cells co-labeled with GFAP or Iba1 (63 were cells counted). HIP, hippocampus; STR/CPu, striatum; and FC, frontal area of Ctx. Scale bar, 50 μm. n = 3.
Discussion
The present study shows that short-term (one-week), systemic exposure of mice to a copper chelator, cuprizone, elicits unique cellular changes that are accompanied by behavioral deficits, such as hypersensitivity to methamphetamine and phencyclidine as well as working memory deficits. The phenotype of this model, especially lack of demyelination, is a clear contrast to mice exposed to cuprizone for longer durations (4-8 weeks), which display robust demyelination and are used as a model for multiple sclerosis (Koutsoudaki et al., 2009; Liu et al., 2010; Matsushima and Morell, 2001). Although elevated expression of mRNA and protein for pro-inflammatory cytokines has been reported in mice displaying robust demyelination following cuprizone exposure (Arnett et al., 2001; Yoshikawa et al., 2011), our study is the first to examine cytokine levels in cuprizone-exposed mice prior to the onset of demyelination. Given that inflammatory cascades are thought to be involved in major mental illnesses, cuprizone short-term (one-week) exposure may offer a promising model to study some aspects of schizophrenia and related conditions.
A possible association between copper and schizophrenia has been historically suggested (Bowman and Lewis, 1982), however no consistent relationship has been established: some studies have reported increased copper in serum or plasma (Herran et al., 2000; Wolf et al., 2006; Yanik et al., 2004), whereas others reported no difference in CSF and postmortem brains (Kornhuber et al., 1994; Shore et al., 1983). Copper acts as a modulator of neuronal transmission, and its release from neurons may regulate N-methyl-D-aspartate receptor activity (Gaier et al., 2013; Schlief and Gitlin, 2006). Copper is also a component of several metalloenzymes linked to dopamine synthesis, in biochemical pathways involving either antagonism of dopamine production or catalysis of its breakdown. It has been suggested that chronic copper exposure may result in overcompensation for dopamine deficiency, via an increased number or sensitivity of post-synaptic dopamine receptors in certain areas (Bowman and Lewis, 1982; Wolf et al., 2006).
Animal models are useful for understanding some aspects of the pathophysiology of psychiatric disorders and for testing pharmacological interventions based on specific hypotheses. Although drug-induced models may not have etiological validity, their ease of generation and high throughput makes them valuable when focusing on specific endophenotypes or pathophysiology relevant to diseases. Previous works have observed behavioral changes in rodents that may be reminiscent of psychiatric disorders upon long-term cuprizone exposure (Gregg et al., 2009; Xu et al., 2009). Some of these changes were ameliorated by neuroleptic treatment (Xu et al., 2010; Xu et al., 2011; Zhang et al., 2008), which suggests that the long-term cuprizone exposure model may mimic psychiatric manifestations associated with multiple sclerosis and related conditions that involve robust white matter damage. Pioneering work indicates that the atypical antipsychotic, quetiapine, facilitated oligodendrocyte development by directing the differentiation of neural progenitors towards the oligodendrocyte lineage (Xiao et al., 2008). In contrast, the short-term cuprizone exposure model presented here may model psychotic conditions that occur in the absence of demyelination.
The short-term cuprizone exposure model raises at least two major questions. The first issue is the cause of the astroglial changes, including IL-6 activation. Although it is beyond the scope of this study, future experiments will be needed to explore the neurochemical and behavioral consequence of region-specific IL-6 modulation in astrocytes. Region-specific difference in the response to cuprizone may be related to heterogeneity in the age of maturation of white matter tracts (Yang et al., 2009). Indeed, the effects of cuprizone are more severe when mice are exposed at earlier ages (Makinodan et al., 2009; Wang et al., 2013). The second issue is how to reconcile recent reports supporting the involvement of inflammation in schizophrenia (Doorduin et al., 2009; Kreisl et al., 2012), with classic observations showing no robust gliosis in autopsied brains from patients (Harrison, 1999). Although further studies are needed, one possible working hypothesis is the transient activation of glial cells in a state-dependent fashion, preferentially associated with the onset and several relapse phases during the course of the disease.
Supplementary Material
Acknowledgments
We thank Dr. Jeff Leek for advice on statistical analysis and Ms. Yukiko Y. Lema for organizing the manuscript. This work was supported by research funds from Mitsubishi Tanabe Parma Corporation, U.S. Public Health Service Grant MH-069853 (A.S.), Silvio O. Conte Center grant MH-094268 (A.S.), MH-084018 (A.S.), MH-088753 (A.S.), MH-085226 (A.S.), MH-092443 (A.S.), and foundation grants from SMRI (A.S.), S-R (A.S.), RUSK (A.S.), MSCRF (A.S.), and NARSAD (A.S). S.K. was supported by fellowships from Uehara Memorial foundation, Kanae foundation, and JSPS, and is currently supported by NIMH K99 award (MH-093458).
References
- Arnett HA, et al. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4:1116–22. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- Barker GR, et al. Recognition memory for objects, place, and temporal order: a disconnection analysis of the role of the medial prefrontal cortex and perirhinal cortex. J Neurosci. 2007;27:2948–57. doi: 10.1523/JNEUROSCI.5289-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Achour S, Pascual O. Glia: the many ways to modulate synaptic plasticity. Neurochem Int. 2010;57:440–5. doi: 10.1016/j.neuint.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Benros ME, et al. Autoimmune diseases and severe infections as risk factors for schizophrenia: a 30-year population-based register study. Am J Psychiatry. 2011;168:1303–10. doi: 10.1176/appi.ajp.2011.11030516. [DOI] [PubMed] [Google Scholar]
- Bowman MB, Lewis MS. The copper hypothesis of schizophrenia: a review. Neurosci Biobehav Rev. 1982;6:321–8. doi: 10.1016/0149-7634(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Coughlin JM, et al. Marked reduction of soluble superoxide dismutase-1 (SOD1) in cerebrospinal fluid of patients with recent-onset schizophrenia. Mol Psychiatry. 2013;18:10–1. doi: 10.1038/mp.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doorduin J, et al. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med. 2009;50:1801–7. doi: 10.2967/jnumed.109.066647. [DOI] [PubMed] [Google Scholar]
- Duan J, et al. Genome-wide approaches to schizophrenia. Brain Res Bull. 2010;83:93–102. doi: 10.1016/j.brainresbull.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto H, et al. A noncompetitive BACE1 inhibitor TAK-070 ameliorates Abeta pathology and behavioral deficits in a mouse model of Alzheimer’s disease. J Neurosci. 2010;30:11157–66. doi: 10.1523/JNEUROSCI.2884-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaier ED, et al. Copper signaling in the mammalian nervous system: synaptic effects. J Neurosci Res. 2013;91:2–19. doi: 10.1002/jnr.23143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garver DL, et al. Elevated interleukin-6 in the cerebrospinal fluid of a previously delineated schizophrenia subtype. Neuropsychopharmacology. 2003;28:1515–20. doi: 10.1038/sj.npp.1300217. [DOI] [PubMed] [Google Scholar]
- Glass CK, et al. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–34. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg JR, et al. Downregulation of oligodendrocyte transcripts is associated with impaired prefrontal cortex function in rats. Schizophr Res. 2009;113:277–87. doi: 10.1016/j.schres.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122(Pt 4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Herran A, et al. Higher levels of serum copper in schizophrenic patients treated with depot neuroleptics. Psychiatry Res. 2000;94:51–8. doi: 10.1016/s0165-1781(00)00126-8. [DOI] [PubMed] [Google Scholar]
- Kikawada T, et al. Trehalose transporter 1, a facilitated and high-capacity trehalose transporter, allows exogenous trehalose uptake into cells. Proc Natl Acad Sci U S A. 2007;104:11585–90. doi: 10.1073/pnas.0702538104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec K, et al. Glycine transporter (GlyT1) inhibitors with reduced residence time increase prepulse inhibition without inducing hyperlocomotion in DBA/2 mice. Biochem Pharmacol. 2010;80:1407–17. doi: 10.1016/j.bcp.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Kornhuber J, et al. Iron, copper, zinc, magnesium, and calcium in postmortem brain tissue from schizophrenic patients. Biol Psychiatry. 1994;36:31–4. doi: 10.1016/0006-3223(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Kousaka A, et al. The distribution and characterization of endogenous protein arginine N-methyltransferase 8 in mouse CNS. Neuroscience. 2009;163:1146–57. doi: 10.1016/j.neuroscience.2009.06.061. [DOI] [PubMed] [Google Scholar]
- Koutsoudaki PN, et al. Demyelination of the hippocampus is prominent in the cuprizone model. Neurosci Lett. 2009;451:83–8. doi: 10.1016/j.neulet.2008.11.058. [DOI] [PubMed] [Google Scholar]
- Kreisl WC, et al. A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J Cereb Blood Flow Metab. 2012 doi: 10.1038/jcbfm.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A, et al. The inflammatory response system in treatment-resistant schizophrenia: increased serum interleukin-6. Schizophr Res. 1998;32:9–15. doi: 10.1016/s0920-9964(98)00034-6. [DOI] [PubMed] [Google Scholar]
- Liu L, et al. CXCR2-positive neutrophils are essential for cuprizone-induced demyelination: relevance to multiple sclerosis. Nat Neurosci. 2010;13:319–26. doi: 10.1038/nn.2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan M, et al. Demyelination in the juvenile period, but not in adulthood, leads to long-lasting cognitive impairment and deficient social interaction in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:978–85. doi: 10.1016/j.pnpbp.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Matsushima GK, Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain Pathol. 2001;11:107–16. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, et al. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–71. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin S, et al. Inflammatory cytokine alterations in schizophrenia: a systematic quantitative review. Biol Psychiatry. 2008;63:801–8. doi: 10.1016/j.biopsych.2007.09.024. [DOI] [PubMed] [Google Scholar]
- Purcell SM, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–52. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlief ML, Gitlin JD. Copper homeostasis in the CNS: a novel link between the NMDA receptor and copper homeostasis in the hippocampus. Mol Neurobiol. 2006;33:81–90. doi: 10.1385/MN:33:2:81. [DOI] [PubMed] [Google Scholar]
- Shi J, et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460:753–7. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore D, et al. CSF copper concentrations in chronic schizophrenia. Am J Psychiatry. 1983;140:754–7. doi: 10.1176/ajp.140.6.754. [DOI] [PubMed] [Google Scholar]
- Singh S, et al. Astrocytes and microglia: responses to neuropathological conditions. Int J Neurosci. 2011;121:589–97. doi: 10.3109/00207454.2011.598981. [DOI] [PubMed] [Google Scholar]
- Stefansson H, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–7. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kammen DP, et al. Elevated interleukin-6 in schizophrenia. Psychiatry Res. 1999;87:129–36. doi: 10.1016/s0165-1781(99)00053-0. [DOI] [PubMed] [Google Scholar]
- Wang H, et al. Cuprizone-induced demyelination in mice: age-related vulnerability and exploratory behavior deficit. Neurosci Bull. 2013;29:251–9. doi: 10.1007/s12264-013-1323-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf TL, et al. Plasma copper, iron, ceruloplasmin and ferroxidase activity in schizophrenia. Schizophr Res. 2006;86:167–71. doi: 10.1016/j.schres.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Xiao L, et al. Quetiapine facilitates oligodendrocyte development and prevents mice from myelin breakdown and behavioral changes. Mol Psychiatry. 2008;13:697–708. doi: 10.1038/sj.mp.4002064. [DOI] [PubMed] [Google Scholar]
- Xu H, et al. Behavioral and neurobiological changes in C57BL/6 mouse exposed to cuprizone: effects of antipsychotics. Front Behav Neurosci. 2010;4:8. doi: 10.3389/fnbeh.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, et al. Recovery of behavioral changes and compromised white matter in C57BL/6 mice exposed to cuprizone: effects of antipsychotic drugs. Front Behav Neurosci. 2011;5:31. doi: 10.3389/fnbeh.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, et al. Behavioral and neurobiological changes in C57BL/6 mice exposed to cuprizone. Behav Neurosci. 2009;123:418–29. doi: 10.1037/a0014477. [DOI] [PubMed] [Google Scholar]
- Yang HJ, et al. Region-specific susceptibilities to cuprizone-induced lesions in the mouse forebrain: Implications for the pathophysiology of schizophrenia. Brain Res. 2009;1270:121–30. doi: 10.1016/j.brainres.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Yanik M, et al. Plasma manganese, selenium, zinc, copper, and iron concentrations in patients with schizophrenia. Biol Trace Elem Res. 2004;98:109–17. doi: 10.1385/BTER:98:2:109. [DOI] [PubMed] [Google Scholar]
- Yolken RH, Torrey EF. Are some cases of psychosis caused by microbial agents? A review of the evidence. Mol Psychiatry. 2008;13:470–9. doi: 10.1038/mp.2008.5. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K, et al. Inhibition of 5-lipoxygenase activity in mice during cuprizone-induced demyelination attenuates neuroinflammation, motor dysfunction and axonal damage. Prostaglandins Leukot Essent Fatty Acids. 2011;85:43–52. doi: 10.1016/j.plefa.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatta P, et al. Copper and zinc dismetabolism in the mouse brain upon chronic cuprizone treatment. Cell Mol Life Sci. 2005;62:1502–13. doi: 10.1007/s00018-005-5073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, et al. Quetiapine alleviates the cuprizone-induced white matter pathology in the brain of C57BL/6 mouse. Schizophr Res. 2008;106:182–91. doi: 10.1016/j.schres.2008.09.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.