Abstract
Multiple studies have shown that bipolar (BP) electric pulses in the microsecond range are more effective at permeabilizing cells while maintaining similar cell survival rates as compared to monopolar (MP) pulse equivalents. In this paper, we investigated whether the same advantage existed for BP nanosecond-pulsed electric fields (nsPEF) as compared to MP nsPEF. To study permeabilization effectiveness, MP or BP pulses were delivered to single Chinese hamster ovary (CHO) cells and the response of three dyes, Calcium Green-1, Propidium Iodide (PI), and FM1-43, was measured by confocal microscopy. Results show that BP pulses were less effective at increasing intracellular calcium concentration or PI uptake and cause less membrane reorganization (FM1-43) than MP pulses. Twenty-four hour survival was measured in three cell lines (Jurkat, U937, CHO) and over ten times more BP pulses were required to induce death as compared to MP pulses of similar magnitude and duration. Flow cytometry analysis of CHO cells after exposure (15 minutes) revealed that to achieve positive FITC-Annexin V and PI expression, ten times more BP pulses were required than MP pulses. Overall, unlike longer pulse exposures, BP nsPEF exposures proved far less effective at both membrane permeabilization and cell killing than MP nsPEF.
Keywords: Bipolar, nanosecond electric pulse, calcium, monopolar, CHO, FM1-43
INTRODUCTION
Cell permeabilization is traditionally accomplished by application of micro- and millisecond monopolar (MP) electric pulses at electric field amplitudes of hundreds to thousands of volts per centimeter [1, 2]. High-amplitude exposures cause irreversible electroporation (IRE), resulting in cell death, which has been used successfully in vivo to kill unwanted tissue [3, 4]. Symmetric biphasic (BP) pulses, which are distinguished by a reversal of polarity halfway through pulse duration, have been investigated as a method to improve permeabilization of cell membrane by efficiently porating both sides of the cell. Tekle et al. showed that 400-µs BP pulses increased transfection efficiency while reducing cell death [5]. Related work by Kotnik et al. studied the impact of 1000-µs (total duration) MP and BP pulses on the permeabilization of cells to bleomycin, survival, and uptake of Lucifer yellow [6]. Results showed that BP pulses (both equivalent (500+500-µs) and double duration (1000+1000-µs) increased the permeabilization of cells to bleomycin and Lucifer yellow, but had the same effect on survival. They concluded that BP pulses offer the advantage of increased cell permeabilization without the downside of increased cellular mortality. Furthermore, the use of BP pulses for in vivo brain tissue ablation (IRE-based) was shown to be advantageous for reducing muscle contractions despite requiring higher-amplitude exposure to achieve a similar lethal effect [7]. Cleaner regions of ablation and more defined survival borders were also seen with BP IRE. Similar reduction in muscle contraction and maintained permeabilization efficiency was observed during electrochemotherapeutic treatment of skin cancer with BP pulses [8]. In a recent theoretical paper, Arena et al. predicted that pulses with polarity shifts in the nanosecond range would be advantageous in limiting joule heating and penetrating epithelial layers, resulting in more efficient electroporation of underlying tissues [9]. Taken together, these studies suggest an advantage to using BP pulses for electropermeabilization and IRE.
Nanosecond pulsed electric fields (nsPEF) have been shown to permeabilize the plasma membrane (PM), albeit with a larger population of smaller pores (i.e. nanopores), and have been speculated to produce intracellular permeabilization due to inherently higher frequency composition and faster rise times [10–14]. However, few papers have examined whether exposing cells to a BP nsPEF will offer the same advantages as observed with longer pulses. Vernier et al. used bursts of extremely short MP and BP nsPEF (15-ns, 50-pulses 28-kV/cm) to qualitatively show with FM1-43 that membrane reorganization occurred symmetrically with BP pulses and asymmetrically with MP pulses [15]. However, little further assessment of the effectiveness of the exposure was presented. French et al. also showed that extremely short (total duration 1.6-ns at full width half maximum) BP (capacitively-coupled) pulses were less effective than MP (conductively-connected) pulses at permeabilizing cells, as measured by bleomycin uptake [16]. Given such limited data on BP nsPEF exposures and the potential advantages for in vitro and in vivo nsPEF applications, we measured the cellular impact of 600-ns BP nsPEF exposures as compared to MP exposures.
MATERIALS AND METHODS
Cell lines and propagation
Chinese Hamster Ovarian-K1 (CHO), U937, and Jurkat cell lines were obtained from American Type Culture Collection (ATCC, Manassas, VA). CHO cells were propagated at 37°C with 5% CO2 in air, in F12K medium supplemented with 10% fetal bovine serum, 2-mM L-glutamine, and 100-U/mL penicillin/streptomycin. Jurkat and U937 cells were grown in RPMI-1640 medium supplemented with 10% fetal bovine serum, 2-mM L-glutamine, and 100-U/mL penicillin/streptomycin. The media and its components were purchased from Mediatech Cellgro.
Microscope Exposure System
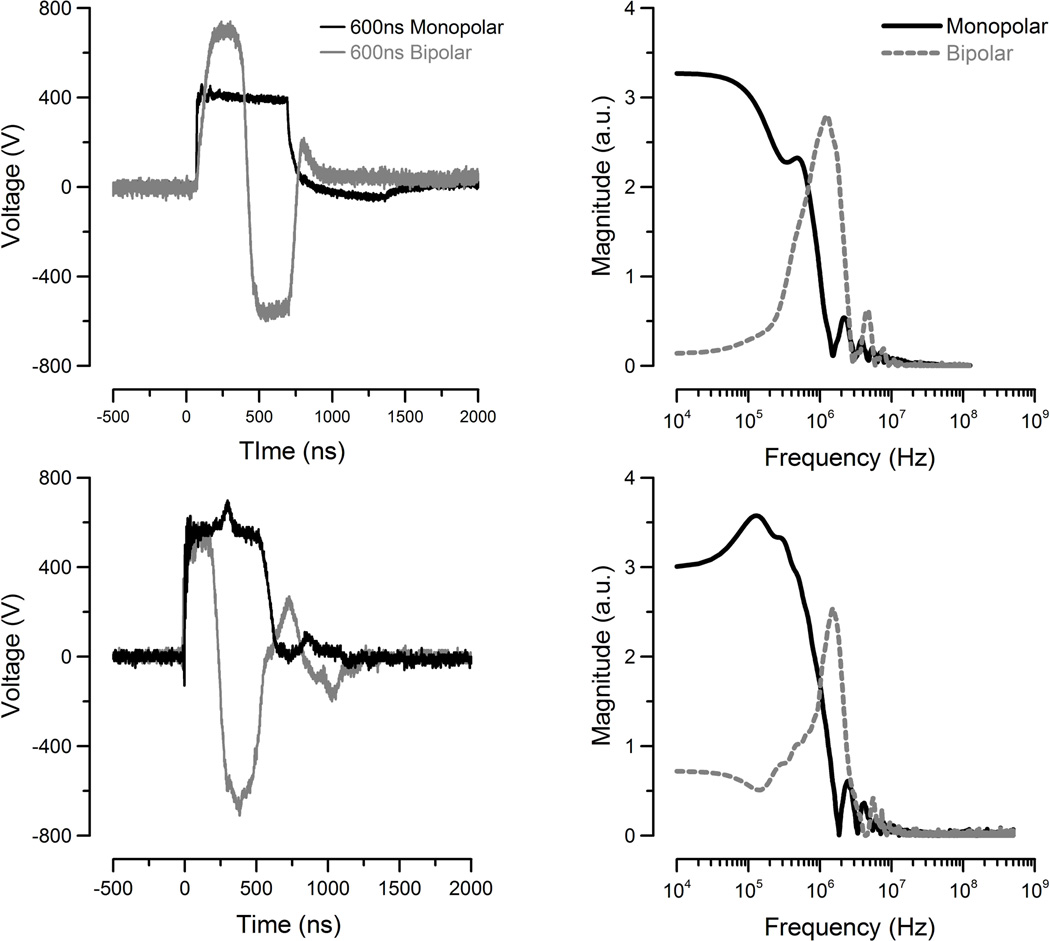
Monopolar pulses were delivered to the cells as described in previous publications [11]. A custom bipolar (BP) pulse generator was constructed to generate bipolar pulses using a full-bridge voltage-source inverter (VSI) consisting of four MOSFETs (IXYS, IXFB38N100Q2, 1 kV, Milpitas, CA). Either the top or the bottom switch of each leg was turned on to allow generation of one polarity of the pulse. Timing was controlled by a function generator (Stanford, DG535, Sunnyvale, CA), which generated two control pulses to trigger the diagonal switches sequentially. Single MP and BP exposures were delivered to cells using a pair of tungsten electrodes (125-µm diameter, 135-µm separation) positioned 50-µm above the glass surface by a micromanipulator (Sutter MP285, Novato, CA). The BP and MP pulse shapes are shown in Figure 1A along with their Fast Fourier Transform (FFT) spectrum (Fig 1B) as computed by MATLAB® (Mathworks, Natlick, MA). Finite Difference Time Domain (FDTD) modeling, as in previous publications, predicted the resulting electric field amplitude for MP exposures to be 1.8–14 kV/cm (125V-1kV charging voltage) at the cell. BP exposures ranged from 3–24 kV/cm (125-1kV charging voltage) due to the higher peak amplitude of ~700V.
Figure 1.
Oscilloscope traces of the MP and BP nsPEF waveforms for microscope (top) and cuvette (bottom) exposures. The FFT spectrum of each pulse shape is compared (right).
Fluorescence Staining
For microscopy experiments, CHO cells were trypsinized and plated onto poly-L-lysine-coated 35-mm dishes with a glass coverslip bottom (MatTek, Ashland, MA). To stain cells, culture medium was removed, cells were twice washed with calcium free and magnesium free Dulbecco’s phosphate buffered saline ((DPBS), Gibco, Grand Island, NY). A buffered solution containing 135-mM NaCl, 5-mM KCL, 10-mM HEPES, 10-mM Glucose, 2-mM CaCl2, and 2-mM MgCl2 with a pH of 7.4 and osmolality of 290–310 mOsm/kg was then placed on the cells (Sigma, St. Louis MO). Calcium Green-1 AM ester (Molecular Probes, Eugene, OR) was added at a final concentration of 3-µM and incubated at room temperature for 30-min to allow for cellular uptake. Prior to imaging, cells were washed again with the buffer and allowed to rest for an additional 30-min. At the beginning of the experimentation, 3-µM propidium iodide (PI) was added to the cell dish. Due to spectral overlap, FM1-43 exposures were done independently by adding 9 µM FM1-43 (Molecular Probes, Eugene, OR) in deionized water to the cells in buffer. Cells were incubated with FM1-43 for 15-min prior to experimentation at room temperature.
Cuvette Exposure System
To expose a large population of cells using an electroporation cuvette, CHO were removed from the culture dish with Trypsin-EDTA and suspended in fresh full F12K medium at a density of 1200-cells/µL (Gibco, Grand Island, NY). Jurkat and U937 cells were centrifuged and re-suspended in fresh media at a density of 1200-cells/µL. 90uL of cells in a 1-mm aluminum electroporation cuvette were exposed to 10-kV/cm MP or BP pulses at 1-Hz repetition rate. Two Marx bank capacitor systems were used to generate either a 600-ns MP or 600-ns BP pulse. A high voltage power supply (0–10-KV/cm) was used to charge the Marx bank capacitors. Delivery to the cuvette was achieved by a spark gap switch that discharged over an air gap between two conductive plates completing the circuit. The rate of discharge and amplitude was set by adjusting the charging voltage and the distance between the plates. The pulse delivered to the cuvette was measured using a high voltage probe connected to a high speed oscilloscope (TDS3052B, Tektronix, Beaverton, OR). Removal of the charging voltage controlled the number of pulses delivered, which we counted manually. The resultant pulse shapes are shown in Figure 1C along with their FFT spectrum (Fig 1D).
MTT
Cell viability was measured at 24 hours post exposure using MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay (Gibco, Grand Island, NY). To perform the assay, 30 µL of exposed cells were transported to 96 well dishes containing 70 µL of fresh media, and incubated at 37 °C for 24 hours. Then, 10µL MTT reagent was added and incubated for 2-h until a precipitate was visible. 100µL detergent was added to each well, and the plate was left at room temperature for 2 h. The absorbance was then measured at 570 nm with a Synergy Plate Reader (Biotech).
Cell Flow Cytometry
Fluorescence and scattering measurements were made in CHO cells using an Acurri C-Flow flow cytometer. First, a staining solution containing fresh medium supplemented with 20-µL/ml of FITC-Annexin V (Molecule Probes, Eugene, OR) and 4-µL/mL PI (Molecule Probes, Eugene, OR) was made. Amber centrifuge tubes were filled with 50-µL of the staining solution to which 50 µL of exposed cells were added immediately after exposure. The contents were mixed gently using a 1-mL pipette and allowed to rest for 15-min at room temperature. Immediately before measurement, the cells were mixed to resuspend them evenly throughout the solution. The 50µL cell solution resulted in cell counts of ~55,000–60,000 cells within the forward and side scatter gated region. Analysis of the data was performed using both CFlow software (BD Biosciences, San Jose, Ca) and FCS Express (De Novo Software, Los Angeles, CA). Data were gated to remove background counts and positive expression was measured as fluorescence intensities above the sham population. Digitonin (0.4%) (Sigma, St. Louis, MO) was added to unexposed cells as a positive control for both FITC-Annexin V and PI.
RESULTS
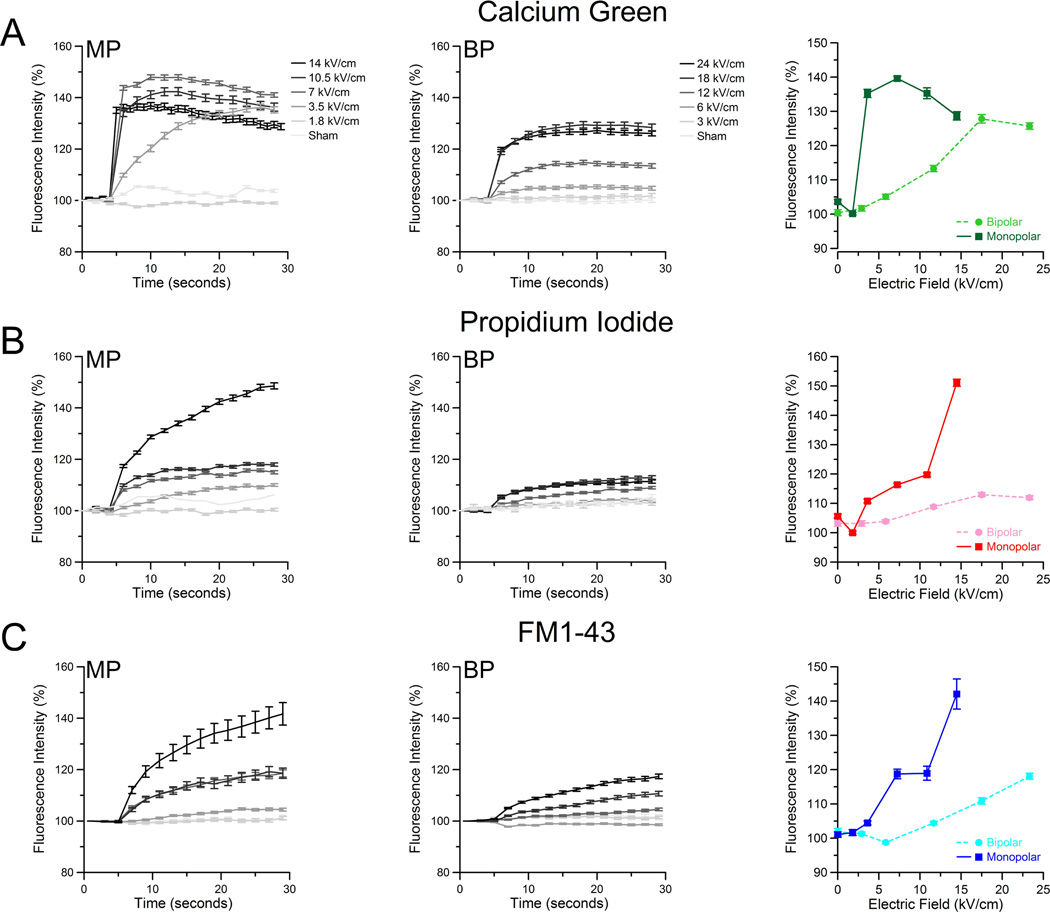
Intracellular calcium increase and PI uptake were observed following cellular exposure to either a single 600ns BP or MP pulse. CHO cells were exposed to either MP or BP pulses at five discrete voltages. Whole-cell fluorescence changes before and after exposure (Fig 2A right) were measured using ImageJ software. For higher-amplitude MP exposures (7.5/14 kV/cm), we observed a rapid 40% increase in fluorescence intensity. Similar BP exposures yielded only a maximum 25% increase, with the 12-kV/cm exposure only achieving 10% total increase (Fig 2A, middle). The total change in fluorescence intensity after 30-sec is presented in Fig 2A (right). Overall, a higher amplitude BP exposure is needed to observe a change in intracellular calcium as compared to MP. A slower rise in the intracellular concentration is also observed following BP pulses. In addition to measuring intracellular calcium changes, we also monitored the uptake of PI. At all amplitudes, MP pulses generate a larger influx of PI than BP pulse exposures (Fig 2B left and middle). It is important to note that the PI signal is very weak compared to positive controls treated with digitonin (data not shown) which routinely achieves 100-fold or higher increases in fluorescence intensity. The final fluorescence increase after 30-sec shows a significant difference between MP and BP exposures (Fig 2B, right).
Figure 2.
BP pulses (300+300-ns) cause less intracellular calcium increase, PI uptake, and increase in FM1-43 fluorescence than MP pulses (600-ns). Cells were imaged at 1 frame per second for 30 seconds with the nsPEF exposure occurring at 5 seconds. Whole cell fluorescence was measured for each cell (n >100 cells per exposure per dye) and the average fluorescence response was calculated along with the standard error. The frequency of error bar plotting was reduced for clarity to every third data point. The temporal response of Calcium Green (A), PI (B), and FM1-43 (C) is shown for MP (left) and BP (middle) exposures. Bar graphs (right) show the a comparison of MP to BP fluorescence response 30 seconds after nsPEF exposure.
The extent of disruption of the cell membrane by BP and MP pulses was evaluated by incorporation of FM1-43 dye into cell membranes. This FM1-43 fluorescence increase has been shown to track the externalization of phosphatidylserine, and thus has been used as a calcium-independent analogue for Annexin V [15]. Additionally, this dye is soluble in deionized water, thus removing any destabilizing effects produced by organic solvents such as dimethyl sulfoxide. Following exposure, we observe that FM1-43 fluorescence increases significantly after MP exposure, mirroring intracellular calcium increases and PI uptake data (Fig 2C, left). BP pulses evoked a weaker response (Fig 2C, middle). The final fluorescence intensity values for FM1-43 after 30 seconds, for each amplitude, are compared (Fig 2C, right). Overall, all employed assays consistently showed a profoundly weaker effect of BP pulses.
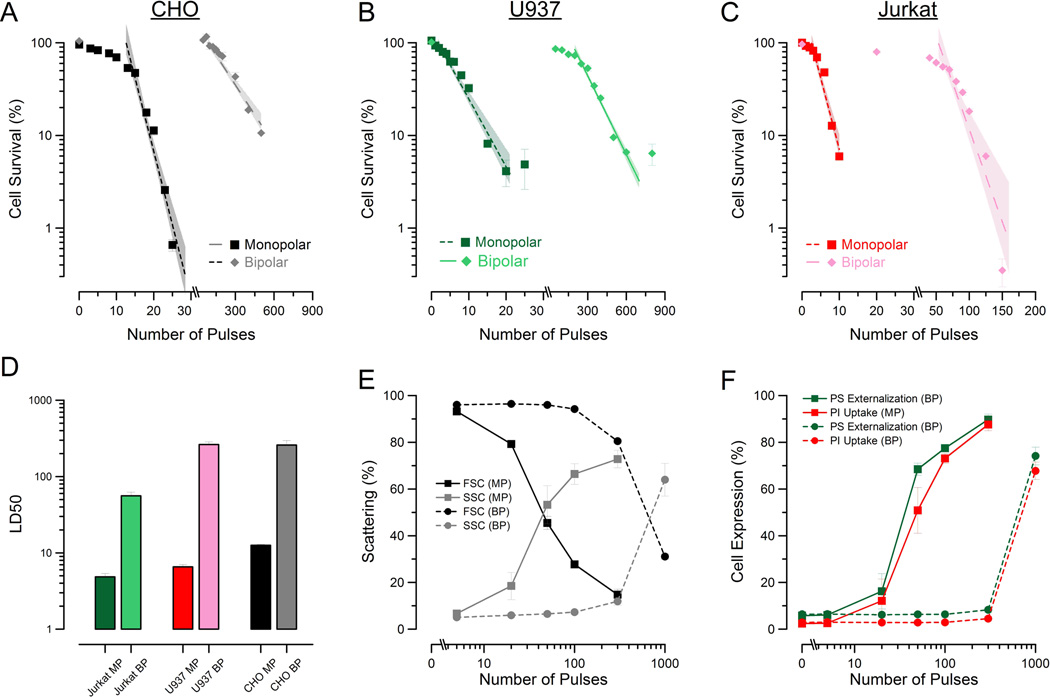
To evaluate lethality, we delivered a discrete number or either BP or MP pulses (10kV/cm) to CHO cells suspended within an electroporation cuvette. The cells were then allowed to recover (or die) for 24-h, at which time MTT assays were performed. Figure 3A shows the decrease in cell survival with increasing MP and BP pulse exposures. A rapid decrease in viability with increasing number of MP pulse exposures was observed. BP exposures required significantly more exposures to cause cell death. To determine whether this effect was unique to CHO cells, the survival experiment was replicated in Jurkat (Fig. 3B) and U937 (Fig. 3C) cells due to their extensive use in previous MP nsPEF experiments. Similar results were observed: MP pulses were substantially more effective at killing cells. The LD50 was estimated from these data by a logarithmic fit for each cell type (Fig 3D). BP exposures required 20-times the number of pulses compared to MP exposures of equal amplitude and duration to kill 50% of the CHO cells. Jurkat BP exposures required a 10-fold increase in pulse number(50 BP pulses to 5 MP pulses) and U937 BP exposures required a nearly 40-fold increase (260 BP pulses to 6 MP pulses) as compared to MP exposures. From these data it appears that the difference between MP and BP exposure lethality is not specifically related to cell type. In a previous publication, we showed that for 10-ns MP pulse exposures, different cells appeared to exhibit different sensitivity to the stressor [28]. While BP pulses were significantly less effective than MP pulses, quantifiable sensitivity differences that mirror those observed with MP (CHO, Jurkat, and U937) are still observed.
Figure 3.
BP pulses are less effective at killing and permeabilizing cells than MP pulses. Survival curves for CHO (A), U937 (B), and Jurkat (C) cells exposed to an increasing number of 10 kV/cm, 600ns total duration BP and MP pulses. Error bars represent the mean of 3 samples per exposure ± S.E. The survival data were exponentially fit to determine the LD50 for number of pulses (D) showing the decreased response of cells to BP exposures. Error bars represent the mean ± 95% C.I. Forward and side scattering intensity of cells (E) exposed to 5-kV/cm BP and MP pulses shows BP-exposed population requiring significantly more exposure before morphological changes are observed. In panel F, the percent of positively expressing cells for both PI (red) and PS (green) for both MP (square) and BP (circle) exposures show BP pulses to be less effective at PM injury. Error bars for (E) and (F) represent mean of 3 samples per exposure ± S. D.
To investigate whether damage to the PM via poration played a role in MP and BP pulse-induced cell death, we performed flow cytometry analysis of CHO cells. Cells were analyzed 15 minutes after exposure to allow FITC-Annexin V time to bind to externalized PS residues, a well-reproduced effect of nsPEF exposure. In addition to tracking the pulse-induced externalization of PS residues to the outer membrane surface, we also added PI to observe PM disruption in exposed cells. For this study, cells were exposed to one-half the amplitude (5-kV/cm) of the survival studies to better resolve the plasma membrane effects for MP exposures given the few pulses needed to kill cells at 10kV/cm. Figure 3E shows the normalized scattering signal for both the forward- and side-scattering channels. As in previous publications, a reduction in forward scattering and increase in side scattering is seen in cells following exposure to nsPEF [23]. This effect is also observed in BP exposures, albeit only after 300 and 1000 pulses. A comparison of the number of pulses versus the percent of positive population for both FITC and PI is presented in Fig. 3F. It is clear from these data that BP pulses have less impact on the PM than MP pulses. We observe that significantly more BP pulses (10-fold) are required to obtain positive pulse-induced externalization of PS and uptake of PI. These flow results mirror those observed in the acute (30-sec) microscopy experiments (Fig 2) where BP pulses were less effective at inducing membrane damage than MP pulses.
DISCUSSION
Studies involving electric pulse exposures with durations >1-µs have shown greater membrane permeabilization with similar cell survival for BP exposures as compared to MP exposures. In this study, we explore whether this observation is maintained for 600-ns BP pulses as compared to 600-ns MP pulses. We found quite the opposite: nanosecond BP pulses are much less effective at causing membrane injury and cell death. Previous publications predict that the time for the PM to reach critical breakdown voltage or charging time (τcrit) is on the order of 100ns or shorter [13, 17, 18]. In this study, we used 600-ns MP and 300+300-ns BP pulses. In both cases, the requisite τcrit should have been achieved. From our microscopy data, it is obvious that both BP and MP pulses appear to have created some injury (presumably pore formation) to the plasma membrane, albeit to a lesser degree with BP exposures. The remaining duration beyond τcritthe pass-band of the pulse, has been attributed to pore expansion time [19, 20]. The effect of pass-band difference between MP and BP pulses is observed in all the microscopy results, but most notably for PI uptake. The rapid depolarization and repolarization of the BP pulse reduces the holding time (passband) that would otherwise result in expansion of created pores. In contrast, for much longer pulses (micro- and millisecond), poration is readily achieved on both poles of the cell and, due to the very long passbands of such BP pulses, considerable expansion can take place. This advantage was shown by Tekle et al. who spatially observed that PI enters from predominantly one pole of a cell upon MP exposure, and nearly equivalently from both poles upon BP exposure[5]. From the data, we postulate that 600ns MP and BP pulses are both effective at poration of the PM, but due to the polarity shift, BP pulses are less effective at expanding pores.
The frequency composition of nsPEF BP pulses is quite different from both longer-pulse exposures and nsPEF MP pulses. Specifically, as presented in Fig. 1, the frequency content within the BP pulse shifts to >1 MHz. The circuit model of the cell predicts that higher frequencies are less effective at permeabilizing the PM [14]. Therefore, BP pulse exposures, with inherently higher frequency composition and less energy at lower frequencies, are predicted to be less effective. The data presented in this paper appears to support this prediction. Difference in cellular response has been attributed to changes in frequency composition for MP pulses. Specifically, when exploring the impact of rise-time on cellular response to nsPEF, Beebe et al. found that 15-ns rise-time pulses (inherently higher frequency composition) had less effect on the PM than 150-ns rise-time pulses with the same total duration [10]. They concluded this difference was due to the higher-frequency-composition of the faster-rise-time pulse.
While the high-frequency components present with short-duration pulses are believed to be less effective at PM permeabilization they are hypothesized to cause intracellular membrane permeabilization [14, 21, 22]. Direct effects of nsPEF on the mitochondrial membrane have been speculated to initiate intrinsic apoptosis [23, 24]. Additional work has supported these hypotheses by showing uptake of barium ions into the mitochondria after exposure [25]. Very recent work has also shown evidence for direct permeabilization of the endoplasmic reticulum, albeit at ten-fold shorter durations than were used in this study [26, 27]. Beebe et al. also found that faster-rise-time pulses have greater impact on the mitochondrial membrane potential than slower-rise-time pulses [10]. Based on these hypotheses, we expected BP nsPEF to exacerbate high-frequency-specific effects on intracellular membranes. However, the data presented, both survival and flow cytometry, suggest the opposite, that BP pulses are not only less effective overall, but also do not appear to cause cell death without causing PM injury. A similar observation was also made in previous work showing that 10-ns MP (higher frequency components than 600ns MP used in this study) pulses killed most cell types (immune cells excluded) at doses exceeding that required to cause PI uptake within 15-min of exposure [28]. Despite the dependence of cell death on PM effects, a recent and surprising result by Pakhomova et al. showed that by restricting cell swelling (necrotic death), no increase in 24-hour survival was seen in U937 cells; they just died by apoptosis instead [29]. These findings, combined with the findings in this paper, suggest that the PM may be the epicenter for cell-death induction, but the mechanism by which these death mechanism(s) are being activated remains unclear.
In summary, we explored the effectiveness of nanosecond-duration BP pulses as compared to MP pulses. We found, using single cell microscopy, that membrane damage and uptake of ions was significantly reduced in BP exposures. By exposing populations of cells in an electroporation cuvette, we found that BP pulses were universally less effective at killing cells across three cell types. Cell flow cytometry results connected microscopy observations to survival, showing that PM injury appeared to be a prerequisite for cell death for both BP and MP pulse exposures. Lastly, we used existing theoretical concepts to provide an explanation as to why nsPEF BP pulses display diminished effectiveness, despite previous observations that longer BP pulses are more effective at killing and porating cells. Further exploration into the effect of BP pulses on cells and cell membranes is warranted, specifically for shorter-duration exposures, to fully explore whether the frequency composition of an electric pulse truly plays a role in cell injury and survival.
Highlights.
Bipolar nanosecond pulses are less effective plasma membrane permeabilization
Bipolar nanosecond pulses are much less effective at killing cells
Cell electropermeabilization is not solely dependent on peak electric peak/voltage
ACKNOWLEDGEMENTS
This research was supported by intramural funds from the Air Force Surgeon General’s Office, Medical Research Program (to B.L.I.) and by a National Institutes of Health grant R01GM088303 (to A.G.P.). Mr. Roth is funded by the SMART Scholarship: OSDT&E/ PE0601120D8Z National Defense Education Program (NDEP) / BA-1, Basic Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Teissie J, et al. Electropermeabilization of cell membranes. Adv Drug Deliv Rev. 1999;35(1):3–19. doi: 10.1016/s0169-409x(98)00060-x. [DOI] [PubMed] [Google Scholar]
- 2.Weaver JC, Chizmadzhev YA. Theory of electroporation: A review. Bioelectrochemistry and Bioenergetics. 1996;41:135–160. [Google Scholar]
- 3.Davalos R, Mir L, Rubinsky B. Tissue Ablation with Irreversible Electroporation. Annals of Biomedical Engineering. 2005;33(2):223–231. doi: 10.1007/s10439-005-8981-8. [DOI] [PubMed] [Google Scholar]
- 4.Rubinsky B, editor. Series in Biomedical Engineering. Berlin Heidelberg: Springer-Verlag; 2010. Irreversible Electroporation. [Google Scholar]
- 5.Tekle E, Astumian RD, Chock PB. Electroporation by using bipolar oscillating electric field: an improved method for DNA transfection of NIH 3T3 cells. Proceedings of the National Academy of Sciences. 1991;88(10):4230–4234. doi: 10.1073/pnas.88.10.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotnik T, et al. Cell membrane electropermeabilization by symmetrical bipolar rectangular pulses: Part I. Increased efficiency of permeabilization. Bioelectrochemistry. 2001;54(1):83–90. doi: 10.1016/s1567-5394(01)00114-1. [DOI] [PubMed] [Google Scholar]
- 7.Arena CB, et al. High-frequency irreversible electroporation (H-FIRE) for non-thermal ablation without muscle contraction. Biomedical engineering online. 10(1):102. doi: 10.1186/1475-925X-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daskalov I, Mudrov N, Peycheva E. Exploring new instrumentation parameters for electrochemotherapy. Attacking tumors with bursts of biphasic pulses instead of single pulses. Engineering in Medicine and Biology Magazine, IEEE. 1999;18(1):62–66. doi: 10.1109/51.740982. [DOI] [PubMed] [Google Scholar]
- 9.Arena CB, et al. Theoretical considerations of tissue electroporation with high-frequency bipolar pulses. Biomedical Engineering, IEEE Transactions on. 58(5):1474–1482. doi: 10.1109/TBME.2010.2102021. [DOI] [PubMed] [Google Scholar]
- 10.Beebe SJ, et al. Transient Features in Nanosecond Pulsed Electric Fields Differentially Modulate Mitochondria and Viability. PLoS ONE. 2012;7(12):e51349. doi: 10.1371/journal.pone.0051349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pakhomov AG, et al. Lipid nanopores can form a stable, ion channel-like conduction pathway in cell membrane. Biochem Biophys Res Commun. 2009;385(2):181–186. doi: 10.1016/j.bbrc.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pakhomov AG, Pakhomova ON. Nanopores: A distinct transmembrane passageway in electroporated cells. In: Pakhomov AG, Miklavcic D, Markov MS, editors. Advanced Electroporation Techniques in Biology in Medicine. CRC Press; 2010. [Google Scholar]
- 13.Schoenbach KS, et al. Bioelectric Effects of Nanosecond Pulses. IEEE Transactions on Dielectrics and Electrical Insulation. 2007;14(5):1088–1109. [Google Scholar]
- 14.Schoenbach KH, et al. Bioelectrics - new applications for pulsed power technology. IEEE Trans. Plasma Sci. 2002;30(1):293–300. [Google Scholar]
- 15.Vernier PT, Sun Y, Gundersen MA. Nanoelectropulse-driven membrane perturbation and small molecule permeabilization. BMC Cell Biol. 2006;7:37. doi: 10.1186/1471-2121-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.French DM, et al. Conductive versus capacitive coupling for cell electroporation with nanosecond pulses. Journal of Applied Physics. 2009;106(7):074701-074701-4. [Google Scholar]
- 17.Smith KC, Weaver JC. Active Mechanisms Are Needed to Describe Cell Responses to Submicrosecond, Megavolt-per-Meter Pulses: Cell Models for Ultrashort Pulses. Biophysical Journal. 2008;95(4):1547–1563. doi: 10.1529/biophysj.107.121921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weaver JC, et al. A brief overview of electroporation pulse strength duration space: A region where additional intracellular effects are expected. Bioelectrochemistry. 2012;87(0):236–243. doi: 10.1016/j.bioelechem.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith KC, Neu JC, Krassowska W. Model of creation and evolution of stable electropores for DNA delivery. Biophysical journal. 2004;86(5):2813–2826. doi: 10.1016/S0006-3495(04)74334-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotnik T, et al. Role of pulse shape in cell membrane electropermeabilization. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2003;1614(2):193–200. doi: 10.1016/s0005-2736(03)00173-1. [DOI] [PubMed] [Google Scholar]
- 21.Kotnik T, Miklavcic D. Theoretical Evaluation of Voltage Inducement on Internal Membranes of Biological Cells Exposed to Electric Fields. Biophysical journal. 2006;90(2):480–491. doi: 10.1529/biophysj.105.070771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoenbach KH, Beebe SJ, Buescher ES. Intracellular effect of ultrashort electrical pulses. Bioelectromagnetics. 2001;22(6):440–448. doi: 10.1002/bem.71. [DOI] [PubMed] [Google Scholar]
- 23.Beebe SJ, et al. Nanosecond, high-intensity pulsed electric fields induce apoptosis in human cells. Faseb J. 2003;17(11):1493–1495. doi: 10.1096/fj.02-0859fje. [DOI] [PubMed] [Google Scholar]
- 24.Beebe S, Sain N, Ren W. Induction of Cell Death Mechanisms and Apoptosis by Nanosecond Pulsed Electric Fields (nsPEFs) Cells. 2013;2(1):136–162. doi: 10.3390/cells2010136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Napotnik TB, et al. Nanosecond electric pulses cause mitochondrial membrane permeabilization in Jurkat cells. Bioelectromagnetics. 2012;33(3):257–264. doi: 10.1002/bem.20707. [DOI] [PubMed] [Google Scholar]
- 26.Semenov I, Xiao S, Pakhomov AG. Primary pathways of intracellular Ca 2+ mobilization by nanosecond pulsed electric field. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2012 doi: 10.1016/j.bbamem.2012.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semenov I, et al. Recruitment of the intracellular Ca<sup>2+</sup> by ultrashort electric stimuli: The impact of pulse duration. Cell Calcium. 2013 doi: 10.1016/j.ceca.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibey BL, et al. Dose-Dependent Thresholds of 10-ns Electric Pulse Induced Plasma Membrane Disruption and Cytotoxicity in Multiple Cell Lines. PLoS ONE. 2011;6(1):e15642. doi: 10.1371/journal.pone.0015642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pakhomova ON, et al. Two Modes of Cell Death Caused by Exposure to Nanosecond Pulsed Electric Field. PLoS ONE. 2013;8(7):e70278. doi: 10.1371/journal.pone.0070278. [DOI] [PMC free article] [PubMed] [Google Scholar]