Abstract
Rationale
Existing animal models of impulsivity frequently use food restriction to increase subjects’ motivation. In addition, behavioral tasks that assess impulsive choice typically involve the use of reinforcers with dissimilar caloric content. These factors represent energy-homeostasis limitations, which may confound the interpretation of results and limit the applicability of these models.
Objectives
This study was aimed at validating face and convergent validities of a modified adjusting delay task, which assesses impulsive choice between isocaloric rein-forcers in ad libitum fed rats.
Methods
Male Wistar rats (n=18) were used to assess the preferredness and reinforcing efficacy of a “supersaccharin” solution (1.5% glucose/0.4% saccharin) over a 1.5% glucose solution. A separate group of rats (n=24) was trained in a modified adjusting delay task, which involved repeated choice between the glucose solution delivered immediately and the supersaccharin solution delivered after a variable delay. To pharmacologically validate the task, the effects of the 5-HT2A/C receptor agonist (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane [(±)-DOI] and the 5-HT1A receptor agonist (±)-8-hydroxy-2-(dipropylamino)tetralin hydrobromide [(±)-8-OH-DPAT] on impulsive choice were then evaluated.
Results
Supersaccharin was highly reinforcing and uniformly preferred over the glucose solution by all subjects. Rats quickly learned the task, and impulsivity was a very stable and consistent trait. DOI and 8-OH-DPAT significantly and dose dependently increased impulsive choice in this modified adjusting delay task.
Conclusions
We validated a rodent task of impulsive choice, which eliminates typical energy-homeostasis limitations and, therefore, opens new avenues in the study of impulsivity in preclinical feeding and obesity research.
Keywords: Impulsivity, Serotonin, Food restriction, Food deprivation, Delay discounting, Decision making
Introduction
Impulsivity is a multifaceted personality construct, which can be defined as a predisposition toward rapid, unplanned reactions to internal or external stimuli, with diminished regard to the negative consequences of these reactions to the impulsive individual or to others (Chamberlain and Sahakian 2007; Potenza 2007). In the last decade, the role of impulsivity in psychopathology has received increasing attention in both preclinical and clinical research (Chamberlain and Sahakian 2007; Dalley et al. 2008; Torregrossa et al. 2008). It has been proposed that impulsivity is an underlying multidimensional personality trait, which plays an important role in the vulnerability to certain psychiatric disorders, such as addiction, eating disorders, mania, personality disorders, attention deficit/hyperactivity disorder (ADHD), obesity, and obsessive– compulsive disorder (Belin et al. 2008; Cassin and von Ranson 2005; Crews and Boettiger 2009; Evenden 1999).
A variety of behavioral tasks have already been designed to evaluate the different dimensions of impulsivity trait in preclinical research (Dalley et al. 2008). Impulsive premature responding has been studied using the five-choice serial reaction time task (Belin et al. 2008; Robbins 2002), the differential reinforcement of low rate responding (O’Donnell and Seiden 1982; 1984), and the fixed consecutive number schedule (Evenden 1998a). Inability to withhold a response has been evaluated using the go/no go task (Schoenbaum et al. 1998; 2000) and the stop signal reaction time task (Eagle and Robbins 2003a, b). Finally, impulsive choice has been assessed using delay discounting procedures like the delay discounting task (Cardinal et al. 2001) and the adjusting delay task (Mazur 1986; 1988).
A commonly used experimental manipulation to increase subjects’ motivation toward reinforcers is the use of chronic food restriction (Beshel et al. 2007; Evenden 1998a) and, in some cases, water restriction (Schoenbaum et al. 1998; 2000). Although food restriction is generally imposed to all subjects as a controlled variable of the experimental procedure, it may still represent a confounding factor due to its profound effects on a variety of central neurotransmitter systems [e.g., monoamine, opioid, GABA, endocannabinoid, glutamate, neuropeptide Y (NPY), proopiomelanocortin (POMC), etc.] (Bi et al. 2003; Cheng et al. 2004; Di Marzo and Matias 2005; Wolinsky et al. 1996), as well as several peripherally released energy-homeostasis factors (e.g., leptin, insulin, ghrelin, etc.) (Carr et al. 2000; Fulton et al. 2000; Schoffelmeer et al. 2011; Skibicka et al. 2011), all critically involved in emotional and motivational processes. Moreover, food restriction may represent a significant limitation in all those experimental procedures where food consumption represents either a dependent or independent experimental variable.
Another common aspect of the procedures used thus far to assess rodent’s impulsive choice is the use of reinforcers with dissimilar caloric content (e.g., different amounts of food or different amounts of caloric solutions employed as reinforcers) (Evenden 1998a; Perry et al. 2005). This aspect may represent a drawback in studies where lever selection may be influenced by the metabolic or feeding state of the subjects (e.g., studies evaluating the effects of drugs that per se affect food intake or exert metabolic effects; studies testing groups of animals in different metabolic or feeding states, etc.).
The aforementioned energy-homeostasis limitations commonly observed in existing models of impulsive choice may be particularly relevant in the context of preclinical research on food intake and obesity. While the potential role of impulsivity as a risk factor for the development of certain forms of eating disorders and obesity in humans has been extensively demonstrated (Davis 2009; Davis et al. 2008; Puder and Munsch 2010; Waxman 2009), in preclinical research this aspect is far understudied. The development of animal models of impulsivity that minimize the potential interference given by energy-homeostasis confounding factors would therefore facilitate the study of impulsive behavior in food intake and obesity research.
This series of studies was aimed at developing a rodent task to assess impulsive choice between isocaloric reinforcers in ad libitum fed rats. For this purpose, we modified an existing adjusting delay task procedure (Perry et al. 2005) by using rats with unrestricted food and water, as well as by using two isocaloric solutions (1.5% w/v glucose solution vs. 1.5% w/v glucose and 0.4% w/v saccharin solution).We then pharmacologically validated our model by evaluating the effects of both a 5-HT2A/C receptor agonist, DOI, and a 5-HT1A receptor agonist, 8-OH-DPAT, on impulsive choice. The rationale for choosing DOI and 8- OH-DPAT is based on evidence that the serotonin system is highly involved in the control of impulsive behavior (Winstanley et al. 2006). In addition, we selected these two compounds to confirm the convergent validity of the modified adjusting delay task, as they have been extensively employed in several animal models of impulsivity.
Materials and methods
Subjects
Male Wistar rats (n=42), weighing 180–230 g and 41– 47 days old (Charles River, Wilmington, MA, USA) were double housed upon arrival in wire-topped, plastic cages (27×48×20 cm) on a 12-h reverse light cycle (lights off at 10:00AM), in an Association for the Assessment and Accreditation of Laboratory Animal Care approved humidity- (60%) and temperature-controlled (22°C) vivarium. Rats had access to corn-based chow {Harlan Teklad LM-485 Diet 7,012 [65% (kcal) carbohydrate, 13% fat, 21% protein, metabolizable energy 341 cal/100 g]; Harlan, Indianapolis, IN, USA} and water ad libitum at all times. Procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication number 85-23, revised 1996) and the Principles of Laboratory Animal Care and were approved by Boston University Medical Campus Institutional Animal Care and Use Committee.
Drugs
(±)-1-(2,5-Dimethoxy-4-iodophenyl)-2-aminopropane hydrochloride [(±)-DOI hydrochloride] and (±)-8-Hydroxy-2- (dipropylamino)tetralin hydrobromide [(±)-8-OH-DPAT] (Sigma Aldrich, St. Louis, MO, USA) were freshly dissolved in isotonic saline and injected using a volume of 1 ml/kg. The doses were calculated based on the salt weight. DOI binds with high affinity to 5-HT2A/C receptors (Ki=~0.7 nM and Ki=~2.4 nM, respectively) (De Vry et al. 1998; Nelson et al. 1999). 8-OH-DPAT binds with high affinity to 5-HT1A receptors (Ki=~1 nM) (Glennon et al. 1988; Helton and Colbert 1994). A moderate to weak binding of 8-OH-DPAT to 5-HT7 receptors has also been observed (Ki=~52–466 nM) (Bard et al. 1993; Ruat et al. 1993).
Apparatus
The test chambers (Med Associates Inc., St. Albans, VT, USA) used for operant oral self-administration had grid floors and were located in sound-attenuating and ventilated environmental cubicles (Cottone et al. 2009a; Cottone et al. 2007). Two syringe pumps dispensed solutions into two stainless steel drinking cups mounted 2 cm above the grid floor in the middle of an aluminum panel (Sabino et al. 2006). Two retractable levers were located 3.2 cm to either side of the drinking cups and two 28-V stimulus cue-lights were located above each lever. Liquid delivery and operant responses were recorded automatically by a microcomputer with 10-ms resolution.
Two-bottle choice preference test
To determine the relative preference for the two solutions, acclimated rats (n=8) were provided continuous two-bottle choice access to a 1.5% w/v glucose solution vs. a modified “supersaccharin” solution consisting of 1.5% w/v glucose and 0.4% w/v saccharin, in a counterbalanced design. Supersaccharin solution serves as a potent reinforcer and makes it unnecessary to water restrict animals to induce a very high liquid intake (Roberto et al. 2010; Sabino et al. 2009; Valenstein et al. 1967). Therefore, the two solutions were isocaloric, as glucose—the sole energetic ingredient— was present at the same concentration. Preference was calculated as percent of total (kilocalories) intake.
Two-lever choice preference test
To determine the relative reinforcing efficacy of the glucose vs. supersaccharin solutions, acclimated rats (n=10) were trained in an operant two-lever choice preference procedure in a counterbalanced design using a continuous fixed ratio reinforcement (FR1) schedule. Each response made on either lever resulted in the delivery of 0.1 ml of solution. Subjects were allowed a 30-min self-administration session 7 days per week until stable performance was attained. Preference was calculated as a percent of total lever presses within 30 min.
Modified adjusting delay task procedure to assess impulsive choice between isocaloric reinforcers in ad libitum fed rats
This modified adjusting delay task procedure was developed to evaluate impulsive choice between isocaloric reinforcers in ad libitum fed and watered rats. The procedure was adopted and modified from Dr. Perry and colleagues. Subjects (n=24) were allowed two overnight fixed ratio 1 (FR1) self-administration sessions (Sabino et al. 2011), with each of the two levers yielding 0.1 ml of 1.5% glucose solution. Rats were then moved to the next training phase. Beginning 3–4 h into the dark cycle, rats were transferred to the testing room daily and individually placed in the self-administration boxes. The procedure consisted of a 30-min pre-session and a 15-block session, the latter ending either after 2 h or after completion of the 15 blocks (whichever occurred first). Therefore, the procedure lasted no longer than 2.5 h. During the 30-min pre-session, levers were in the retracted position and the stimulus lights were off. This pre-session sought to acclimate subjects to the self-administration boxes and to help signal the imminent availability of the reinforcers. During the training phase, each of the 15 blocks consisted of four forced-choice trials. Forced-choice trials were signaled by the extension of the response-appropriate lever and the illumination of the stimulus light above it. In forced-choice trials, levers retracted immediately following a lever press response. A single response on one lever yielded 0.1 ml of the glucose solution delivered immediately, while a response on the other lever yielded 0.1 ml of the supersaccharin solution delivered after a constant delay of 6 s. Levers retracted at the end of each four-trial block. Each rat was then moved to the modified adjusting delay task as soon as it learned to complete at least 10 out of the 15 trials. In the modified adjusting delay task, the first and second trial of each block were forced-choice trials, whereas the third and fourth were free-choice trials. In the free-choice trials, rats were given the opportunity to choose between the two levers. The free-choice trials were signaled by illumination of the stimulus lights above both levers. The initial delay to the delivery of the more preferred reinforcer was 6 s, and it was adjusted only after the third and fourth trials in each block, depending on which lever was chosen by the subject. A response on the immediately reinforced lever resulted in a 1-s decrease in the delay, while a response on the delayed reinforced lever resulted in a 1-s increase in the delay. The lower and upper limits of the delay were set to 0 and 36 s, respectively. Following each lever press, an inter-trial interval (ITI) was imposed so that each trial would last 36 s (i.e., delay+ITI=36 s). During the ITI, the stimulus lights were turned off, and responses on the levers had no programmed consequences. During the delay, the stimulus lights above each lever were turned off. A mean adjusted delay (MAD) was calculated at the end of each session by averaging all adjusting delays on the free-choice trials, and this procedure was repeated until the MAD stabilized (varying by <3 s across 5 days with no consistently increasing or decreasing trends). The MAD ranged between 0.5 s (in the event that the immediately reinforced lever was always chosen during the 30 free-choice trials) and 21.5 s (in the event that the adjusting delay lever was always chosen). The MAD values were used as a quantitative measure of impulsive choice, with lower MADs indicating higher levels of impulsive choice. The MADs were transformed into impulsivity scores (ISs) using the following mathematical expressions:
Therefore, IS is a dimensionless variable in a linear inverse relationship with MAD. IS is a more intuitive variable relative to MAD and ranges between 0 and 1, representing the lowest and highest degree of impulsivity, respectively.
Drug treatments
To pharmacologically validate the modified adjusting delay task, the 24 rats used to establish the baseline were divided into two balanced groups of 12: The first group was administered DOI (vehicle, 0.01, 0.03, 0.1 and 0.3 mg/kg, 1 ml/kg s.c.); the second group was administered 8-OHDPAT (vehicle, 0.01, 0.03, 0.1, 0.3 and 1 mg/kg, 1 ml/kg s.c.). Both treatments were given 15 min prior to the beginning of the first block of the session (15 min into the pre-session). Treatments were given in full Latin square designs with one to three intervening treatment-free test days, in which the MAD returned to baseline. Rats were given 3 days of acclimation to daily saline injections before starting drug treatments.
Statistical analysis
Paired Student’s t tests were used to interpret significant within-subject differences in intake or preference ratio in the two-bottle choice and in the two-lever choice preference experiments. To evaluate the internal consistency of measurements and to determine whether rats stably differed in their individual performances, two-way, random effect intraclass correlations (ICC) of absolute agreement (Cottone et al. 2008; 2009b; Shrout and Fleiss 1979) were performed on MAD, IS, and latency during the last 5 days of training. ICC coefficients range between 0 and 1, with values closer to 1 meaning smaller within-subject variation across sessions. The effects of DOI and 8-OHDPAT on MAD were analyzed using one-way repeated measures analyses of variance (ANOVAs), where dose was a within-subject factor. Pairwise dose effects were interpreted using within-subject Newman–Keuls’s tests. Latency to respond was not a normally distributed variable and was therefore analyzed using the non-parametric Friedman’s test, followed by Dunn tests for post hoc comparisons. The software/graphic packages were Systat 11.0 and SigmaPlot 11.0 (Systat Software Inc., Chicago, IL, USA), InStat 3.0 (GraphPad, San Diego, CA, USA) and PASW Statistics 18.0 (SPSS Inc., Chicago, IL, USA).
Results
Two-bottle choice preference test
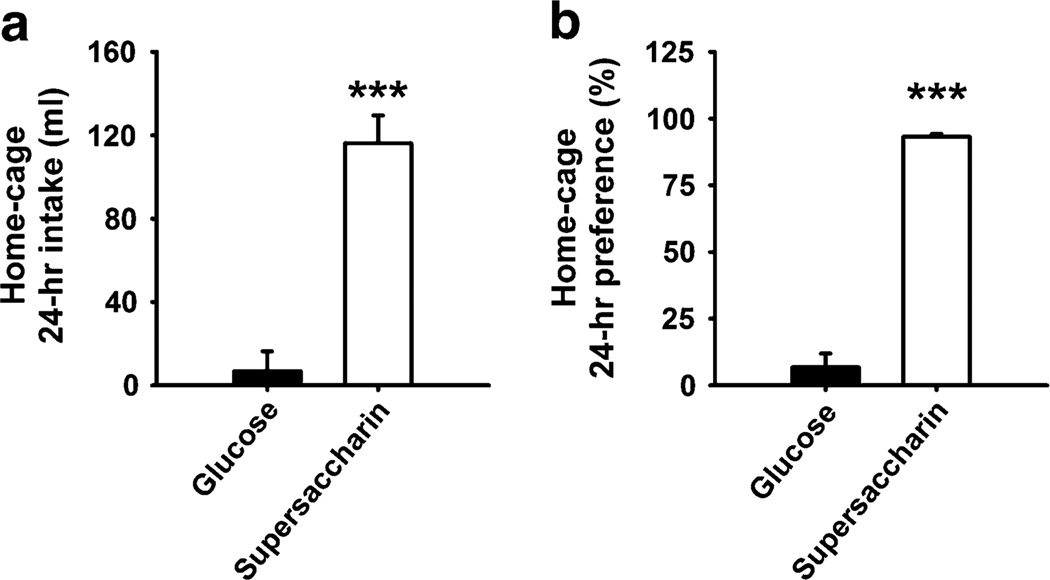
Figure 1 shows the intake and preference ratio of rats provided with two bottles containing the glucose and supersaccharin solutions in the home cages 24 h/day. Rats drank ~10-fold more of the supersaccharin compared to the glucose solution [intake: t(7)=6.58, p<0.001; preference ratio: t(7)=13.42, p<0.001]. The preference ratio ranged between 70.9% and 97.1%, indicating that all subjects uniformly preferred the supersaccharin solution over the glucose solution.
Fig. 1.
Twenty-four-hour preference test in male Wistar rats (n=8) provided in the home cages with two bottles containing the supersaccharin solution (1.5% w/v glucose and 0.4% w/v saccharin) and the glucose solution (1.5% w/v glucose). Panels show M±SEM of a intake and b preference, calculated as the % of total intake. ***p<0.001, difference from glucose (paired Student’s t tests)
Two-lever choice preference test
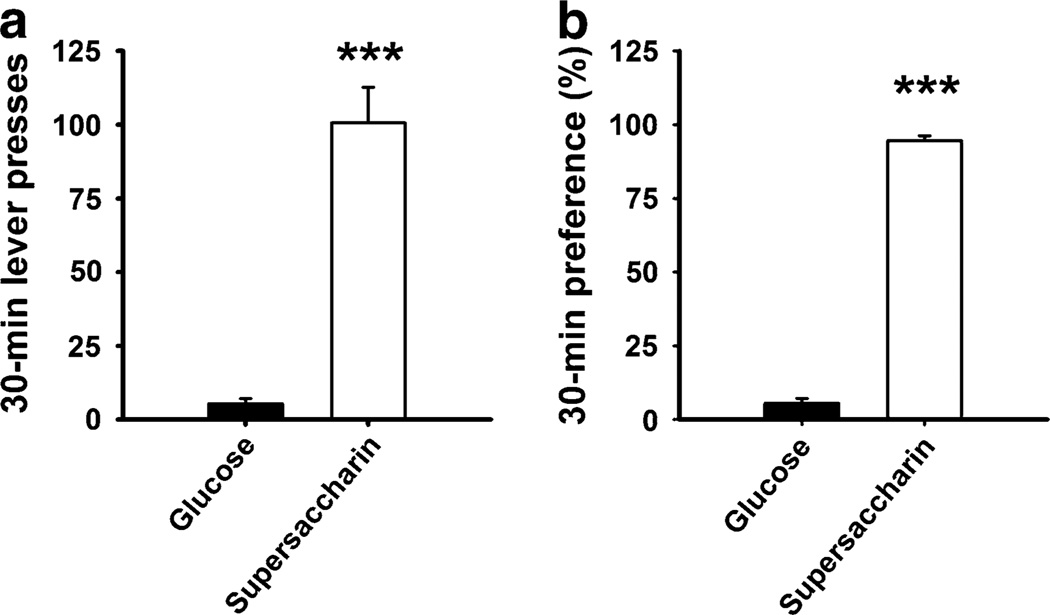
Figure 2 shows the number of lever presses and preference ratio for the glucose and supersaccharin solutions in a 30- min operant two-lever choice preference test. The supersaccharin solution was found to be ~17-fold more reinforcing than the glucose solution in operant self-administration [lever presses: t(9)=7.67, p<0.001; preference ratio: t(9)= 26.65, p<0.001]. The preference ratio ranged between 82.1% and 99.3%, indicating that all subjects uniformly preferred the supersaccharin solution over the glucose solution.
Fig. 2.
Thirty-minute preference test in male Wistar rats (n=10) allowed to self-administer the supersaccharin (1.5% w/v glucose and 0.4% w/v saccharin) and the glucose solution (1.5% w/v glucose) in a two-lever choice operant condition. Panels show M±SEM of a lever presses and b preference, calculated as the percent of total intake. ***p<0.001, difference from glucose (paired Student’s t tests)
Modified adjusting delay task procedure to assess impulsive choice between isocaloric reinforcers in ad libitum fed rats
All rats learned the modified adjusting delay task after 27 sessions (22 training sessions+5 baseline sessions). Table 1 shows the values of MAD, IS, number of completed trials, and latency during the 5-day baseline period. The orders of magnitude of MAD and latency were equivalent to those observed in previously published adjusting delay tasks (Perry et al. 2005). Intraclass correlation analysis showed very high internal consistency in MAD, IS, and latency [intraclass correlation, MAD, and IS: ICC (2,5)=0.80, F (23,92)=5.27, p<0.0001; latency: ICC (2,5)=0.73, F (23,92)=3.84, p<0.0001) across the 5 days. The very high intraclass correlation coefficients and the wide range of MADs or ISs (1.10<MAD<21.50 s, or 0.00<IS<0.97) revealed strong and stable individual differences. Rats completed 88% [26.43±0.83 (M±SEM)] of the 30 free-choice trials within the 2.5-h sessions.
Table 1.
MAD, IS, number of completed trials, and latency across a 5-day baseline period
| Parameter | Day | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| MAD (s) | 13.65±0.81 | 12.27±0.90 | 14.35±0.84 | 13.93±0.86 | 14.91±0.63 |
| IS (unitless) | 0.37±0.04 | 0.44±0.04 | 0.34±0.04 | 0.36±0.04 | 0.31±0.03 |
| Number of completed trials | 26.75±0.90 | 25.25±1.32 | 28.08±0.84 | 25.00±1.44 | 27.42±1.08 |
| Latency (s) | 39.47 (16.14, 55.04) | 43.82 (19.66, 104.95) | 24.46 (10.21, 55.54) | 33.37 (15.01, 102.02) | 21.92 (13.51, 45.30) |
Effects of the 5-HT2A/C receptor agonist DOI on impulsive behavior using a modified adjusting delay task
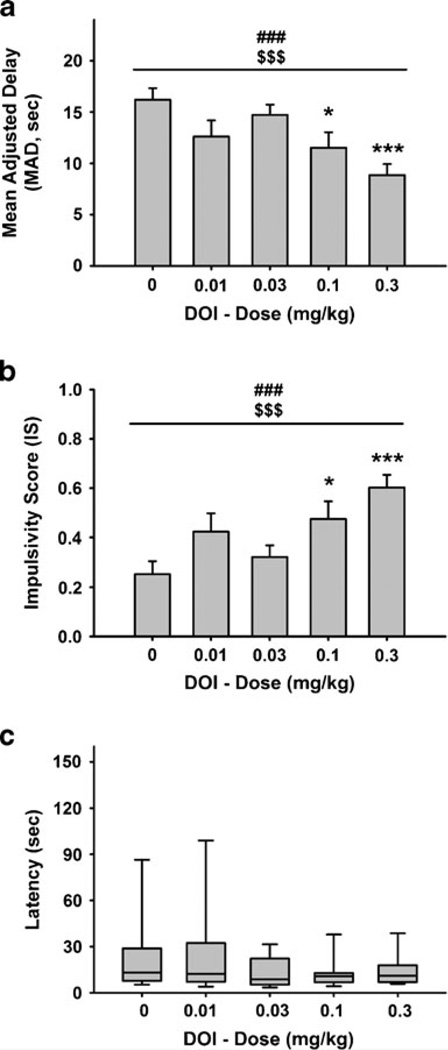
The 5-HT2A/C receptor agonist DOI significantly and dose-dependently reduced MAD and increased IS [overall effect of dose: F(4,44)=5.73, p<0.001; linear effect of dose: F (1,44)=17.60, p<0.001; Fig. 3a, b]. DOI significantly reduced MAD and increased IS when injected at the 0.1 and 0.3 mg/kg doses compared to vehicle condition, with a reduction of ~45% following the administration of the highest dose. Latencies to respond were not affected by drug treatment, as revealed by the Friedman’s test [χ2(4, N=12)=4.33, n.s.; Fig. 3c]. No effect of drug treatment was observed on the number of completed trials [overall effect of dose: F(4,44)=0.84, n.s.; linear effect of dose: F (1,44)=1.23, n.s.; Table 2].
Fig. 3.
Effect of pretreatment with DOI (n=12) (s.c., 15 min prior to the beginning of the first block of the session) on a mean adjusted delay (MAD) and b impulsivity score (IS), in male Wistar rats. a, b M ±SEM. c Box and whisker plots of latency where the boundaries indicate the 25th and 75th percentiles, the line within the box marks the median, and the whiskers (error bars) indicate the 90th and 10th percentiles. Symbols denote significant differences from vehicle: *p<0.05, ***p<0.001 (Newman–Keuls test or Dunn’s test); significant main effect of dose ###p<0.001, significant linear trend of dose, $$$p<0.001
Table 2.
Number of completed trials following DOI and 8-OH-DPAT treatments
| Drug | Dose | |||||
|---|---|---|---|---|---|---|
| 0 mg/kg | 0.01 mg/kg | 0.03 mg/kg | 0.1 mg/kg | 0.3 mg/kg | 1 mg/kg | |
| DOI | 29.33±0.51 | 27.66±2.33 | 29.50±0.50 | 30.00±0.00 | 30.00±0.00 | – |
| 8-OH-DPAT | 27.50±1.37 | 27.66±1.39 | 29.50±0.50 | 30.00±0.00 | 28.33±1.67 | 30.00±0.00 |
Effects of the 5-HT1A receptor agonist 8-OH-DPAT on impulsive behavior using a modified adjusting delay task
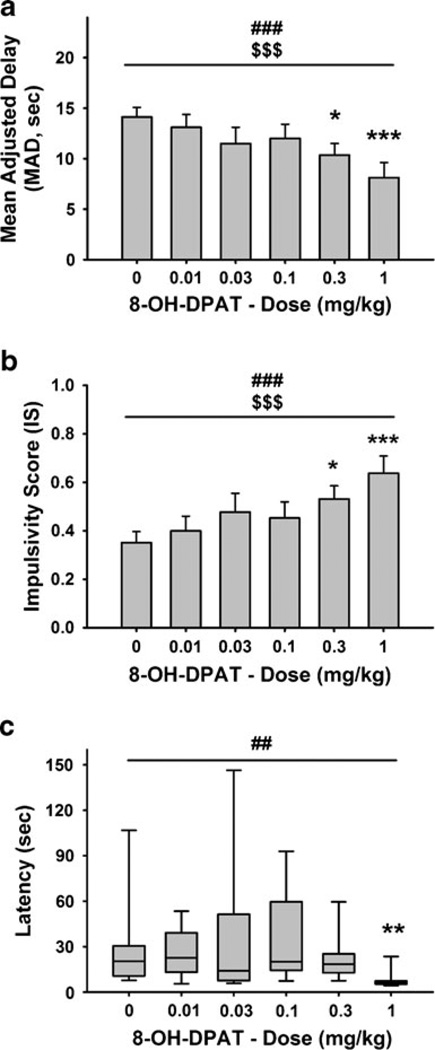
The 5-HT1A receptor agonist 8-OH-DPAT significantly and dose-dependently decreased MAD and increased IS [overall effect of dose: F(5,55)=7.25, p<0.0001; linear effect of dose: F(1,55)=32.91, p<0.0001; Fig. 4a, b]. Post hoc analysis revealed that rats showed significantly lower MAD and higher IS after administration of the 0.3 and 1 mg/kg doses compared to the vehicle condition. A 43% reduction in MAD was observed after the highest dose (1 mg/kg) of 8-OH-DPAT. Friedman’s test revealed a significant effect of dose on the latency [χ2(5, N=12)=15.90, p<0.01; Fig. 4c]. Dunn’s comparison revealed a significant decrease in the latency following the highest dose. No effect of drug treatment was observed on the number of completed trials [overall effect of dose: F(5,55)=1.15, n.s.; linear effect of dose: F(1,55)=2.81, n.s.; Table 2].
Fig. 4.
Effect of pretreatment with 8-OH-DPAT (n=12) (s.c., 15 min prior to the beginning of the first block of the session) on a mean adjusted delay (MAD), b impulsivity score (IS), and c latency in male Wistar rats. a, b M±SEM. c Box and whisker plots of latency where the boundaries indicate the 25th and 75th percentiles, the line within the box marks the median, and the whiskers (error bars) indicate the 90th and 10th percentiles. Symbols denote significant differences from vehicle: *p<0.05, **p<0.01 ***p<0.001 (Newman–Keuls test or Dunn’s test); significant main effect of dose #p<0.01, ###p<0.001, significant linear trend of dose $$$p<0.001
Discussion
The modified adjusting delay task proposed here exhibited robust face validity. In psychiatric research, face validity refers to the phenomenological similarity between animals’ and humans’ behavior (i.e., dependent variable; Markou 2000). Similar to what is observed in certain types of human personalities, our model proposed here interprets the tendency to choose the less preferred, more immediate reinforcer over the more preferred, delayed reinforcer as impulsive choice (Ainslie 1975). Indeed, by measuring the MAD, we measured the extent to which each subject discounted the delayed more preferred reinforcer (Mazur 1986, 1988; Perry et al. 2005). The order of magnitude of MAD in the present modified procedure was similar to the originally validated adjusting delay task.
MAD and the new variable IS, while varying heterogeneously between-subjects, revealed a very high within-subject consistency, suggesting that subjects showed stable individual differences in impulsive choice. Stable individual differences in MAD and IS were maintained until the end of the study, when an additional 3-day baseline period was evaluated (~1 month after the first baseline) [ICC (2,8)=0.83, F(23,161)=6.18 p<0.0001 including 5 days of the first baseline and 3 days of the second baseline]. This denotes an important feature of the face validity of the task: Impulsivity is a personality trait and, as such, represents an intrinsic aspect that remains stable throughout most of an individual’s life.
This modified adjusting delay task also eliminates certain energy-homeostasis limitations of existing impulsivity tasks induced by food restriction. Caloric restriction is a commonly used experimental procedure to increase subjects’ motivation towards reinforcers; to our knowledge most—if not all—behavioral tasks used to assess the different dimensions of impulsivity involve some degree of food restriction, which may represent a confounding factor given its profound influence on the central nervous system. For instance, alterations in the synthesis of serotonin, dopamine, noradrenaline, and the dopamine metabolite, 3,4 dihydroxyphenilacetic acid, were observed in hypothalamic and extrahypothalamic brain areas following food restriction (Carr et al. 2003; Haleem and Haider 1996; Knott and Curzon 1974; Pan et al. 2006). Food restriction was shown to induce a strong increase of messenger RNA levels of tyrosine hydroxylase and the dopamine transporter in the ventral tegmental area and in the nucleus accumbens of rats (Lindblom et al. 2006). In addition, in the nucleus arcuate of the hypothalamus, an important brain area responsible for the integration of peripherally released signals, food restriction decreases the expression of POMC, galanin-like, neuropeptide B, and agouti-related protein, while increasing the expression of NPY (Johansson et al. 2008). Therefore, these potential confounds were minimized in the proposed modified adjusting delay task by the use of ad libitum fed rats.
Therefore, in order to increase subjects’ motivation towards reinforcers, we used a glucose/saccharin (supersaccharin) solution, the highly rewarding and reinforcing properties of which are well-known, (Sabino et al. 2006; Sabino et al. 2009; Valenstein et al. 1967). We found that the supersaccharin solution was highly reinforcing and uniformly preferred by all rats when offered concurrently with the isocaloric glucose solution. The 24-h intake of the supersaccharin solution in the two-bottle choice preference test was exceptionally high, as expected, being three times higher than the average daily intake of water of rats of the same age (Harkness 1989). Reinforcement was therefore exclusively induced by hedonic mechanisms without the influence of alterations in energy homeostasis.
The modified adjusting delay task was designed in such a way that the two reinforcers had equal caloric density. Therefore, independent from the choice of the immediate or delayed lever, every solution delivery was associated with a fixed caloric consumption (0.006 kcal). This aspect of the task is particularly relevant in the attempt to exclude the potential alternative explanation that lever choice may result from caloric needs rather than from specific effects on impulsive choice. This expedient would be helpful in several experimental settings where additional control studies may be required to exclude energy-homeostasis-related alternative hypotheses; for instance, when evaluating the effects on impulsive choice from drugs that affect food intake or metabolism or when evaluating differences in impulsivity of different groups of subjects in different metabolic states. The use of isocaloric reinforcers would eliminate these inconveniences.
Moreover, the two solutions used as reinforcers in this modified adjusting delay task have very low caloric density, which minimizes the influence of potential satiety mechanisms on the subjects’ performance throughout the session. The total energy intake upon completion of the entire task is indeed only 0.36 kcal, a value which represents <0.5% of the daily caloric intake of rats of the same age (Harkness 1989) and is significantly lower than the energy intake obtained within existing models assessing impulsive behavior. For instance, when using the delay discounting task (Mar et al. 2011; Stanis et al. 2008) upon completion of the entire session, a rat’s energy intake can be as high as ~36 kcal. In a session of five-choice serial reaction time task (Carli and Samanin 2000; Koskinen et al. 2000), energy intake can reach ~16 kcal, and in the adjusting delay task (Perry et al. 2007), a rat can eat pellets up to ~22 kcal.
To confirm the convergent validity of the modified adjusting delay task, the effects of two serotonergic drugs on impulsive choice were evaluated. Convergent validity is the degree to which a test correlates with other tests that attempt to measure the same construct (Markou 2000). Both the 5-HT2A/C receptor agonist DOI and the 5-HT1A receptor agonist 8-OH-DPAT were found to dose-dependently increase impulsive choice at the 0.1 and 0.3 mg/kg doses and 0.3 and 1 mg/kg doses, respectively. These results confirm previous observations: Evenden and Ryan (1999) found that DOI increased the preference for the small, immediate reinforcer in the delay of reinforcement and reduced chain lengths, a measure of impulsive choice in the fixed consecutive number schedule task (Evenden 1998b); in addition, DOI increased impulsivity in the five-choice serial reaction time task, by increasing the number of premature responses (Koskinen et al. 2003). Furthermore, 8-OH-DPAT was found to increase impulsive choice, reducing the preference for the large reinforcer in the delay discounting task (Winstanley et al. 2005).While 8-OH-DPAT was found to consistently increase impulsive choice, conflicting results were obtained on impulsive action; in the five-choice serial reaction time task, 8-OHDPAT has been found to either increase (Carli and Samanin 2000) or decrease (Winstanley et al. 2003) impulsive action. Neither the latency to respond nor the number of completed trials was affected by DOI treatment, suggesting that the observed effects did not result from effects in motor activity or in rats’ performances. While 8-OH-DPAT did not affect the number of completed trials, it did decrease the latency to respond at the highest dose. Therefore, while the drug treatment did not affect the subjects’ performances, a possible influence on motor activity in lever responding at the highest dose cannot be excluded. Several studies evaluated the effects of 8-OH-DPAT on locomotor activity with inconsistent findings, as the drug has been reported to either increase (Tricklebank et al. 1984) or decrease (Hillegaart and Hjorth 1989) locomotor activity. However, the effect of 8-OH-DPAT treatment on locomotor activity was observed only at the highest dose (1 mg/kg), whereas the effect on impulsive behavior was observed at a lower dose (0.3 mg/kg), suggesting a dissociation of the two effects. The mechanisms underlying the effects of these two compounds are still unknown. A putative mechanism of DOI’s effects was proposed by Koskinen and colleagues, who hypothesized that DOI increases impulsivity by interacting with α-adrenoreceptors (Koskinen et al. 2003), while the mechanism underlying the effects of 8-OH-DPAT has been proposed to relate to its capability to modulate dopamine release in the nucleus accumbens and the dorsal striatum (Ahlenius et al. 1989; Arborelius et al. 1993; Ichikawa et al. 1995), as well as dopamine and serotonin release in the ventral tegmental area (Chen and Reith 1995).
In summary, the modified adjusting delay task proposed here exhibits robust face and convergent validities. Subjects showed stable individual differences, as the primary variable of the task, MAD (or IS) showed a very high within-subject consistency, while varying heterogeneously between-subjects. In addition, reinforcement was exclusively induced by hedonic mechanisms without the influence of energy homeostatic mechanisms. The lack of food restriction improves the practicality of the experimental procedure, minimizing the impact of potential confounds, and therefore reducing the number of control experiments needed to exclude alternative interpretations associated with the metabolic and feeding state of the subjects. The use of isocaloric reinforcers eliminates the alternative interpretation that shifts in preference may reflect the metabolic or feeding state of the subjects.
Acknowledgments
We thank Frank Gibbs for technical assistance and Jina Kwak for the technical and editorial assistance. This publication was made possible by grant numbers DA023680, DA030425, MH091945, and AA016731 from the National Institute on Drug Abuse (NIDA), the National Institute of Mental Health (NIMH) and the National Institute on Alcohol Abuse and Alcoholism (NIAAA), and by the Peter Paul Career Development Professorship.
Footnotes
The authors declare no conflict of interest. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Angelo Blasio, Email: blasio@bu.edu, Laboratory of Addictive Disorders, Department of Pharmacology and Experimental Therapeutics, Boston University School of Medicine, 72 E Concord St, R-618, Boston, MA 02118, USA; Department of Human Physiology and Pharmacology, Sapienza, University of Rome, Rome, Italy.
Aditi R. Narayan, Laboratory of Addictive Disorders, Department of Pharmacology and Experimental Therapeutics, Boston University School of Medicine, 72 E Concord St, R-618, Boston, MA 02118, USA
Barbara J. Kaminski, Division of Behavioral Biology, Department of Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Luca Steardo, Department of Human Physiology and Pharmacology, Sapienza, University of Rome, Rome, Italy.
Valentina Sabino, Laboratory of Addictive Disorders, Department of Pharmacology and Experimental Therapeutics, Boston University School of Medicine, 72 E Concord St, R-618, Boston, MA 02118, USA.
Pietro Cottone, Email: cottone@bu.edu, Laboratory of Addictive Disorders, Department of Pharmacology and Experimental Therapeutics, Boston University School of Medicine, 72 E Concord St, R-618, Boston, MA 02118, USA.
References
- Ahlenius S, Hillegaart V, Wijkstrom A. Evidence for selective inhibition of limbic forebrain dopamine synthesis by 8-OH-DPAT in the rat. Naunyn Schmiedebergs Arch Pharmacol. 1989;339:551–556. doi: 10.1007/BF00167260. [DOI] [PubMed] [Google Scholar]
- Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82:463–496. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Arborelius L, Chergui K, Murase S, Nomikos GG, Hook BB, Chouvet G, Hacksell U, Svensson TH. The 5-HT1A receptor selective ligands, (R)-8-OH-DPAT and (S)-UH-301, differentially affect the activity of midbrain dopamine neurons. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:353–362. doi: 10.1007/BF00165384. [DOI] [PubMed] [Google Scholar]
- Bard JA, Zgombick J, Adham N, Vaysse P, Branchek TA, Weinshank RL. Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J Biol Chem. 1993;268:23422–23426. [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beshel J, Kopell N, Kay LM. Olfactory bulb gamma oscillations are enhanced with task demands. J Neurosci. 2007;27:8358–8365. doi: 10.1523/JNEUROSCI.1199-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi S, Robinson BM, Moran TH. Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1030–R1036. doi: 10.1152/ajpregu.00734.2002. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- Carli M, Samanin R. The 5-HT(1A) receptor agonist 8-OH-DPAT reduces rats’ accuracy of attentional performance and enhances impulsive responding in a five-choice serial reaction time task: role of presynaptic 5-HT(1A) receptors. Psychopharmacology (Berl) 2000;149:259–268. doi: 10.1007/s002139900368. [DOI] [PubMed] [Google Scholar]
- Carr KD, Kim G, Cabeza de Vaca S. Hypoinsulinemia may mediate the lowering of self-stimulation thresholds by food restriction and streptozotocin-induced diabetes. Brain Res. 2000;863:160–168. doi: 10.1016/s0006-8993(00)02143-0. [DOI] [PubMed] [Google Scholar]
- Carr KD, Tsimberg Y, Berman Y, Yamamoto N. Evidence of increased dopamine receptor signaling in food-restricted rats. Neuroscience. 2003;119:1157–1167. doi: 10.1016/s0306-4522(03)00227-6. [DOI] [PubMed] [Google Scholar]
- Cassin SE, von Ranson KM. Personality and eating disorders: a decade in review. Clin Psychol Rev. 2005;25:895–916. doi: 10.1016/j.cpr.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Chamberlain SR, Sahakian BJ. The neuropsychiatry of impulsivity. Curr Opin Psychiatry. 2007;20:255–261. doi: 10.1097/YCO.0b013e3280ba4989. [DOI] [PubMed] [Google Scholar]
- Chen NH, Reith ME. Monoamine interactions measured by microdialysis in the ventral tegmental area of rats treated systemically with (+/−)-8-hydroxy-2-(di-n-propylamino)tetralin. J Neurochem. 1995;64:1585–1597. doi: 10.1046/j.1471-4159.1995.64041585.x. [DOI] [PubMed] [Google Scholar]
- Cheng CM, Hicks K, Wang J, Eagles DA, Bondy CA. Caloric restriction augments brain glutamic acid decarboxylase-65 and - 67 expression. J Neurosci Res. 2004;77:270–276. doi: 10.1002/jnr.20144. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. FG 7142 specifically reduces meal size and the rate and regularity of sustained feeding in female rats: evidence that benzodiazepine inverse agonists reduce food palatability. Neuropsychopharmacology. 2007;32:1069–1081. doi: 10.1038/sj.npp.1301229. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. Opioid-dependent anticipatory negative contrast and binge-like eating in rats with limited access to highly preferred food. Neuropsychopharmacology. 2008;33:524–535. doi: 10.1038/sj.npp.1301430. [DOI] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Roberto M, Bajo M, Pockros L, Frihauf JB, Fekete EM, Steardo L, Rice KC, Grigoriadis DE, Conti B, Koob GF, Zorrilla EP. CRF system recruitment mediates dark side of compulsive eating. Proc Natl Acad Sci USA. 2009a;106:20016–20020. doi: 10.1073/pnas.0908789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottone P, Sabino V, Steardo L, Zorrilla EP. Consummatory, anxiety-related and metabolic adaptations in female rats with alternating access to preferred food. Psychoneuroendocrinology. 2009b;34:38–49. doi: 10.1016/j.psyneuen.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Boettiger CA. Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav. 2009;93:237–247. doi: 10.1016/j.pbb.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Mar AC, Economidou D, Robbins TW. Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav. 2008;90:250–260. doi: 10.1016/j.pbb.2007.12.021. [DOI] [PubMed] [Google Scholar]
- Davis C. Psychobiological traits in the risk profile for overeating and weight gain. Int J Obes (Lond) 2009;33(Suppl 2):S49–S53. doi: 10.1038/ijo.2009.72. [DOI] [PubMed] [Google Scholar]
- Davis C, Levitan RD, Carter J, Kaplan AS, Reid C, Curtis C, Patte K, Kennedy JL. Personality and eating behaviors: a case-control study of binge eating disorder. Int J Eat Disord. 2008;41:243–250. doi: 10.1002/eat.20499. [DOI] [PubMed] [Google Scholar]
- De Vry J, Schohe-Loop R, Heine HG, Greuel JM, Mauler F, Schmidt B, Sommermeyer H, Glaser T. Characterization of the aminomethylchroman derivative BAY × 3702 as a highly potent 5-hydroxytryptamine1A receptor agonist. J Pharmacol Exp Ther. 1998;284:1082–1094. [PubMed] [Google Scholar]
- Di Marzo V, Matias I. Endocannabinoid control of food intake and energy balance. Nat Neurosci. 2005;8:585–589. doi: 10.1038/nn1457. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Robbins TW. Inhibitory control in rats performing a stop-signal reaction-time task: effects of lesions of the medial striatum and d-amphetamine. Behav Neurosci. 2003a;117:1302–1317. doi: 10.1037/0735-7044.117.6.1302. [DOI] [PubMed] [Google Scholar]
- Eagle DM, Robbins TW. Lesions of the medial prefrontal cortex or nucleus accumbens core do not impair inhibitory control in rats performing a stop-signal reaction time task. Behav Brain Res. 2003b;146:131–144. doi: 10.1016/j.bbr.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Evenden JL. The pharmacology of impulsive behaviour in rats II: the effects of amphetamine, haloperidol, imipramine, chlordiazepoxide and other drugs on fixed consecutive number schedules (FCN 8 and FCN 32) Psychopharmacology (Berl) 1998a;138:283–294. doi: 10.1007/s002130050673. [DOI] [PubMed] [Google Scholar]
- Evenden JL. The pharmacology of impulsive behaviour in rats IV: the effects of selective serotonergic agents on a paced fixed consecutive number schedule. Psychopharmacology (Berl) 1998b;140:319–330. doi: 10.1007/s002130050773. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats VI: the effects of ethanol and selective serotonergic drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1999;146:413–421. doi: 10.1007/pl00005486. [DOI] [PubMed] [Google Scholar]
- Fulton S, Woodside B, Shizgal P. Modulation of brain reward circuitry by leptin. Science. 2000;287:125–128. doi: 10.1126/science.287.5450.125. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Titeler M, Lyon RA, Slusher RM. N, N-di-npropylserotonin: binding at serotonin binding sites and a comparison with 8-hydroxy-2-(di-n-propylamino)tetralin. J Med Chem. 1988;31:867–870. doi: 10.1021/jm00399a031. [DOI] [PubMed] [Google Scholar]
- Haleem DJ, Haider S. Food restriction decreases serotonin and its synthesis rate in the hypothalamus. Neuroreport. 1996;7:1153–1156. doi: 10.1097/00001756-199604260-00011. [DOI] [PubMed] [Google Scholar]
- Harkness JEWJ. The biology and medicine of rabbits and rodents. 3rd edition. Philadelphia: Lea and Febiger; 1989. [Google Scholar]
- Helton DR, Colbert WE. Alterations of in-vitro 5-HT receptor pharmacology as a function of multiple treatment with 5-hydroxytryptamine or 8-hydroxy-2-(di-N-propylamino) tetralin in rat isolated aorta, uterus and fundus, and guinea-pig isolated trachea. J Pharm Pharmacol. 1994;46:902–905. doi: 10.1111/j.2042-7158.1994.tb05711.x. [DOI] [PubMed] [Google Scholar]
- Hillegaart V, Hjorth S. Median raphe, but not dorsal raphe, application of the 5-HT1A agonist 8-OH-DPAT stimulates rat motor activity. Eur J Pharmacol. 1989;160:303–307. doi: 10.1016/0014-2999(89)90505-0. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Kuroki T, Kitchen MT, Meltzer HY. R(+)-8-OH-DPAT, a 5-HT1A receptor agonist, inhibits amphetamine-induced dopamine release in rat striatum and nucleus accumbens. Eur J Pharmacol. 1995;287:179–184. doi: 10.1016/0014-2999(95)00624-9. [DOI] [PubMed] [Google Scholar]
- Johansson A, Fredriksson R, Winnergren S, Hulting AL, Schioth HB, Lindblom J. The relative impact of chronic food restriction and acute food deprivation on plasma hormone levels and hypothalamic neuropeptide expression. Peptides. 2008;29:1588–1595. doi: 10.1016/j.peptides.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Knott PJ, Curzon G. Effect of increased rat brain tryptophan on 5-hydroxytryptamine and 5-hydroxyindolyl acetic acid in the hypothalamus and other brain regions. J Neurochem. 1974;22:1065–1071. doi: 10.1111/j.1471-4159.1974.tb04338.x. [DOI] [PubMed] [Google Scholar]
- Koskinen T, Ruotsalainen S, Puumala T, Lappalainen R, Koivisto E, Mannisto PT, Sirvio J. Activation of 5-HT2A receptors impairs response control of rats in a five-choice serial reaction time task. Neuropharmacology. 2000;39:471–481. doi: 10.1016/s0028-3908(99)00159-8. [DOI] [PubMed] [Google Scholar]
- Koskinen T, Haapalinna A, Sirvio J. Alpha-adrenoceptor-mediated modulation of 5-HT2 receptor agonist induced impulsive responding in a 5-choice serial reaction time task. Pharmacol Toxicol. 2003;92:214–225. doi: 10.1034/j.1600-0773.2003.920504.x. [DOI] [PubMed] [Google Scholar]
- Lindblom J, Johansson A, Holmgren A, Grandin E, Nedergard C, Fredriksson R, Schioth HB. Increased mRNA levels of tyrosine hydroxylase and dopamine transporter in the VTA of male rats after chronic food restriction. Eur J Neurosci. 2006;23:180–186. doi: 10.1111/j.1460-9568.2005.04531.x. [DOI] [PubMed] [Google Scholar]
- Mar AC, Walker AL, Theobald DE, Eagle DM, Robbins TW. Dissociable effects of lesions to orbitofrontal cortex subregions on impulsive choice in the rat. J Neurosci. 2011;31:6398–6404. doi: 10.1523/JNEUROSCI.6620-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou MAGaA. Neuropsychopharmacology: the fifth generation of progress. Philadelphia: LWW; 2000. Animal models of psychiatric disorders. [Google Scholar]
- Mazur JE. Fixed and variable ratios and delays: further tests of an equivalence rule. J Exp Psychol Anim Behav Process. 1986;12:116–124. [PubMed] [Google Scholar]
- Mazur JE. Estimation of indifference points with an adjusting-delay procedure. J Exp Anal Behav. 1988;49:37–47. doi: 10.1901/jeab.1988.49-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DL, Lucaites VL, Wainscott DB, Glennon RA. Comparisons of hallucinogenic phenylisopropylamine binding affinities at cloned human 5-HT2A, -HT(2B) and 5-HT2C receptors. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:1–6. doi: 10.1007/pl00005315. [DOI] [PubMed] [Google Scholar]
- O’Donnell JM, Seiden LS. Effects of monoamine oxidase inhibitors on performance during differential reinforcement of low response rate. Psychopharmacology (Berl) 1982;78:214–218. doi: 10.1007/BF00428153. [DOI] [PubMed] [Google Scholar]
- O’Donnell JM, Seiden LS. Altered effects of desipramine on operant performance after 6-hydroxydopamine-induced depletion of brain dopamine or norepinephrine. J Pharmacol Exp Ther. 1984;229:629–635. [PubMed] [Google Scholar]
- Pan Y, Berman Y, Haberny S, Meller E, Carr KD. Synthesis, protein levels, activity, and phosphorylation state of tyrosine hydroxylase in mesoaccumbens and nigrostriatal dopamine pathways of chronically food-restricted rats. Brain Res. 2006;1122:135–142. doi: 10.1016/j.brainres.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Anderson MM, Morgan AD, Carroll ME. Impulsivity (delay discounting) for food and cocaine in male and female rats selectively bred for high and low saccharin intake. Pharmacol Biochem Behav. 2007;86:822–837. doi: 10.1016/j.pbb.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN. To do or not to do? The complexities of addiction, motivation, self-control, and impulsivity. Am J Psychiatry. 2007;164:4–6. doi: 10.1176/ajp.2007.164.1.4. [DOI] [PubMed] [Google Scholar]
- Puder JJ, Munsch S. Psychological correlates of childhood obesity. Int J Obes (Lond) 2010;34(Suppl 2):S37–S43. doi: 10.1038/ijo.2010.238. [DOI] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, Cottone P, Madamba SG, Stouffer DG, Zorrilla EP, Koob GF, Siggins GR, Parsons LH. Corticotropin releasing factor-induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010;67:831–839. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruat M, Traiffort E, Leurs R, Tardivel-Lacombe J, Diaz J, Arrang JM, Schwartz JC. Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc Natl Acad Sci USA. 1993;90:8547–8551. doi: 10.1073/pnas.90.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Koob GF, Steardo L, Lee MJ, Rice KC, Zorrilla EP. Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 2006;189:175–186. doi: 10.1007/s00213-006-0546-5. [DOI] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Zhao Y, Iyer MR, Steardo L, Jr, Steardo L, Rice KC, Conti B, Koob GF, Zorrilla EP. The sigma-receptor antagonist BD-1063 decreases ethanol intake and reinforcement in animal models of excessive drinking. Neuropsychopharmacology. 2009;34:1482–1493. doi: 10.1038/npp.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabino V, Cottone P, Blasio A, Iyer MR, Steardo L, Rice KC, Conti B, Koob GF, Zorrilla EP. Activation of sigma-receptors induces binge-like drinking in Sardinian alcohol-preferring rats. Neuropsychopharmacology. 2011;36:1207–1218. doi: 10.1038/npp.2011.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Orbitofrontal cortex and basolateral amygdala encode expected outcomes during learning. Nat Neurosci. 1998;1:155–159. doi: 10.1038/407. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Changes in functional connectivity in orbitofrontal cortex and basolateral amygdala during learning and reversal training. J Neurosci. 2000;20:5179–5189. doi: 10.1523/JNEUROSCI.20-13-05179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelmeer AN, Drukarch B, De Vries TJ, Hogenboom F, Schetters D, Pattij T. Insulin modulates cocaine-sensitive monoamine transporter function and impulsive behavior. J Neurosci. 2011;31:1284–1291. doi: 10.1523/JNEUROSCI.3779-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrout PE, Fleiss JL. Intraclass correlations: Uses in assessing rater reliability. Psychol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- Skibicka KP, Hansson C, Alvarez-Crespo M, Friberg PA, Dickson SL. Ghrelin directly targets the ventral tegmental area to increase food motivation. Neuroscience. 2011;180:129–137. doi: 10.1016/j.neuroscience.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Stanis JJ, Burns RM, Sherrill LK, Gulley JM. Disparate cocaine-induced locomotion as a predictor of choice behavior in rats trained in a delay-discounting task. Drug Alcohol Depend. 2008;98:54–62. doi: 10.1016/j.drugalcdep.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torregrossa MM, Quinn JJ, Taylor JR. Impulsivity, compulsivity, and habit: the role of orbitofrontal cortex revisited. Biol Psychiatry. 2008;63:253–255. doi: 10.1016/j.biopsych.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricklebank MD, Forler C, Fozard JR. The involvement of subtypes of the 5-HT1 receptor and of catecholaminergic systems in the behavioural response to 8-hydroxy-2-(di-n-propylamino) tetralin in the rat. Eur J Pharmacol. 1984;106:271–282. doi: 10.1016/0014-2999(84)90714-3. [DOI] [PubMed] [Google Scholar]
- Valenstein ES, Cox VC, Kakolewski JW. Polydipsia elicited by the synergistic action of a saccharin and glucose solution. Science. 1967;157:552–554. doi: 10.1126/science.157.3788.552. [DOI] [PubMed] [Google Scholar]
- Waxman SE. A systematic review of impulsivity in eating disorders. Eur Eat Disord Rev. 2009;17:408–425. doi: 10.1002/erv.952. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Chudasama Y, Dalley JW, Theobald DE, Glennon JC, Robbins TW. Intra-prefrontal 8-OH-DPAT and M100907 improve visuospatial attention and decrease impulsivity on the five-choice serial reaction time task in rats. Psychopharmacology (Berl) 2003;167:304–314. doi: 10.1007/s00213-003-1398-x. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Robbins TW. Interactions between serotonin and dopamine in the control of impulsive choice in rats: therapeutic implications for impulse control disorders. Neuropsychopharmacology. 2005;30:669–682. doi: 10.1038/sj.npp.1300610. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Eagle DM, Robbins TW. Behavioral models of impulsivity in relation to ADHD: translation between clinical and preclinical studies. Clin Psychol Rev. 2006;26:379–395. doi: 10.1016/j.cpr.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky TD, Carr KD, Hiller JM, Simon EJ. Chronic food restriction alters mu and kappa opioid receptor binding in the parabrachial nucleus of the rat: a quantitative autoradiographic study. Brain Res. 1996;706:333–336. doi: 10.1016/0006-8993(95)01337-7. [DOI] [PubMed] [Google Scholar]