Abstract
Sustainable and reproducible large animal models that closely replicate the clinical sequelae of myocardial infarction (MI) are important for the translation of basic science research into bedside medicine. Swine are well accepted by the scientific community for cardiovascular research, and they represent an established animal model for preclinical trials for US Food and Drug Administration (FDA) approval of novel therapies. Here we present a protocol for using porcine models of MI created with a closed-chest coronary artery occlusion-reperfusion technique. This creates a model of MI encompassing the anteroapical, lateral and septal walls of the left ventricle. This model infarction can be easily adapted to suit individual study design and enables the investigation of a variety of possible interventions. This model is therefore a useful tool for translational research into the pathophysiology of ventricular remodeling and is an ideal testing platform for novel biological approaches targeting regenerative medicine. This model can be created in approximately 8–10 h.
INTRODUCTION
Cardiovascular disease (CVD) currently affects 36.9% of the United States population with an estimated direct medical cost of $272.5 billion annually in 2010 (ref. 1). When considering indirect costs from lost productivity due to mortality and morbidity, the cost to society exceeds $444 billion annually1. Coronary heart disease, MI and heart failure account for a third of the total economic burden of CVD to the United States1. The direct medical costs of CVD are projected to double in the next 20 years, with the total cost of CVD exceeding one trillion dollars by 2030 (ref. 1). The 2011 American Heart Association statistics update revealed that there are 1,172,000 cases of acute coronary syndrome annually in the United States; of these cases, 730,000 are designated as acute MI2. Considering the personal and economic impact of CVD and the anticipated increase in prevalence in the next 20 years, the use of a sustainable and reproducible large animal model, as described below, that closely replicates the clinical sequelae of MI is required.
Swine are superior large animal models for studies of acute MI and ischemic cardiomyopathy (ICM)3. The blood vessel size and shape are comparable between swine and human vasculature3,4. In particular, the coronary artery anatomy, size, structure and distribution make them suitable for preclinical cardiovascular research4. The porcine anatomy allows for occlusion of the left anterior descending coronary artery (LAD), and this is commonly used as a model of human CVD5–9. As with human anatomy, the porcine LAD provides approximately half of the blood supply to the left ventricle (LV), and its occlusion creates an infarction of the anteroapical, lateral and septal walls of the LV that is similar in size and distribution to that occurring in humans with MI as a result of LAD occlusion3. This type of ischemia produces considerably more impairment of LV global function than left circumflex artery (LCX) occlusions. The higher level of global impairment leads to markedly different compensatory changes in infarct border zone function compared with LCX occlusions. Therefore, the location of MI is an important predictor of depressed global cardiac function after MI10. Although the LCX occlusion technique has its benefits, such as lower procedural mortality, LAD occlusion is the preferred model for preclinical studies, considering that this is where the majority of human MIs occur11. Moreover, the pattern of remodeling as a result of the MI closely resembles that occurring in humans10. Consequently, the greater change in global cardiac parameters and location of infarction zone produces the most clinically relevant results for translational research.
Given the similarities in human and swine anatomy, many of the same imaging techniques used in humans can be used on swine. Echocardiography and cardiac magnetic resonance imaging (CMR) allow for noninvasive cardiac measurements such as myocardial mass, scar mass, left ventricular volumes, geometric remodeling, scar reduction, contractility, myocardial perfusion and viable myocardial mass regeneration to be quantified. Invasive studies such as pressure-volume loop analysis can give a comprehensive evaluation of cardiovascular performance, including indices of preload, afterload, lusitropy, inotropy and measures of integrated cardiac function12. These similarities make the swine model highly appropriate for translational research, as the equipment and procedural techniques used to quantify these variables are analogous to those used in humans.
Experimental MI design
The model of ischemia-reperfusion MI described here is a closed-chest approach using clinical cardiac catheterization techniques. We attempted a percutaneous method of intravascular access to further minimize the invasiveness of the procedure, but porcine arteries are prone to vasospams and surgical access was often required after unsuccessful arterial puncture. The common carotid artery is too deep to palpate, thus hindering safe and reliable access percutaneously. In addition, the femoral vasculature is not ideal for percutaneous access, because of its smaller vessel size and the difficulty of palpating the femoral pulse. Surgical dissection of the vasculature at either location provides more dependable access with fewer complications. Surgical dissection of the neck further optimizes access, as it provides a direct route to the heart without having to navigate across the aortic arch. The vasculature of the neck has fewer branches than the femoral vasculature and is larger, further easing vascular control and access.
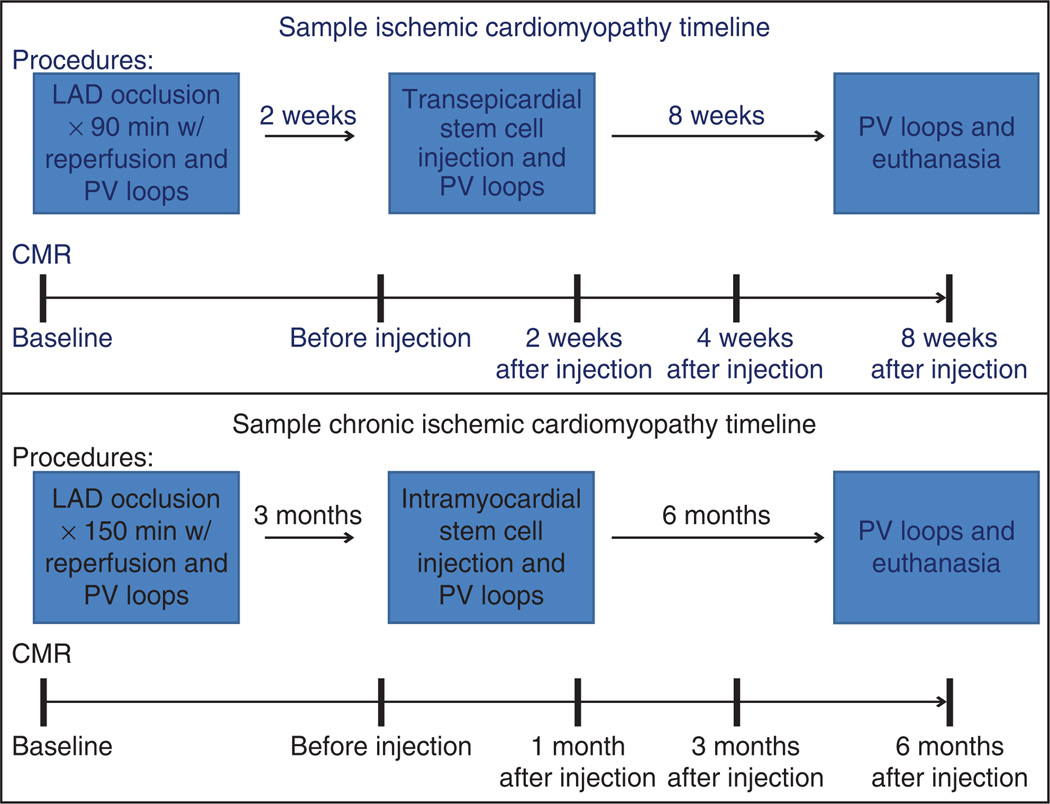
To create a reproducible model of MI, a percutaneous transluminal coronary angioplasty (PTCA) dilation catheter is inflated to occlude all flow distal to the first diagonal branch of the LAD. By maintaining the patency of the first diagonal branch, a portion of the LV continues to receive normal blood perfusion. Having some healthy LV muscle provides well-defined remote and border zones to the infarcted LV for comparative evaluation in preclinical studies. Consequently, this reduces the fatalities during the procedure and also creates fewer complications of congestive heart failure after MI. LAD occlusion is advantageous for research studies, as the infarct and border zones are easily accessible via both percutaneous transcatheter delivery techniques and thoracotomy, thus allowing for direct surgical interventions in study protocols. By using different breeds of swine, the timescale of the model can be varied. Figure 1 shows sample timelines for acute MI studies and chronic ICM studies. Farm pigs, like the Yorkshire, are particularly suited for acute MI studies, whereas the Göttingen (a trademark of Ellegaard) miniature swine are preferred for long-term ICM studies9. Yorkshire swine are excellent models for acute studies as they reach size and weights suitable for research within 3 months of birth. In addition, there is greater accessibility and less initial cost than miniature swine. However, the fast initial growth rate limits study length to around 3 months, given the age-dependence of cardiovascular compensatory mechanisms10. Göttingen swine are an ideal chronic model as they reach mature weights at around 1 year of age and the growth curve plateaus13. In addition, Göttingen swine also see closure of growth lines at 18–22 months, thereby allowing for long-term research without substantial growth.
Figure 1.
Flowchart depicting representative experimental timelines for acute chronic ICM. Typical experimental timeline showing surgical procedures and serial CMR analysis through 8 weeks and 6 months follow-up after intervention for acute and chronic ICM studies, respectively.
During the first month after an MI, the swine heart, similarly to the human heart, undergoes drastic left ventricular remodeling. After 3 months, the geometric and cellular remodeling process has stabilized and allows for chronic studies. Consequently, the acute model is useful for studying interventions to limit or prevent remodeling, whereas the chronic model is more applicable to focusing on the reversal and restoration of cardiac muscle tissue and the geometric configuration of the LV, bearing in mind the particular advantages of Yorkshire or Göttingen swine9,14.
Occlusion times of 90 min for Yorkshire swine or 150 min for Göttingen swine provide predictable and reproducible MI of the anteroapical, lateral and septal walls of the LV. After occlusion, the myocardium is fully reperfused to mimic the clinical MI disease model with relevant interventions in humans. Furthermore, reperfusion of the heart after coronary artery occlusion minimizes additional myocardial cell death and decreases swine mortality. Despite reperfusion of the ischemic areas, remodeling of the heart occurs over the next several months and continues to damage the heart and deteriorate cardiac performance15. Post-MI parameters, such as scar size, location and ventricular impairment, are easily titrated by varying occlusion time or location in order to vary LV remodeling and suit the needs of the research project. This provides an ideal model for investigation of potential interventions for acute, subacute and chronic studies after MI.
Implications for the swine MI model in cellular and regenerative therapy
It has long been thought that the mammalian heart lacks the capacity for regeneration, but recent breakthroughs in cardiovascular stem cell therapies are starting to decipher the mysteries of activating the dormant intrinsic regenerative capacity16. Stem cells are an emerging therapy for heart failure, and research shows many viable options for delivery of biological products to the heart (Boxes 1 and 2; Table 1). Whereas intravenous (i.v.) or intracoronary delivery allow for less invasive stem cell infusions, intramyocardial injections have the highest engraftment and retention rates with less systemic organ engraftment17–19. The disadvantage of direct transepicardial injections is the need to surgically expose the heart (Box 1). Concurrent delivery with coronary artery bypass graft, ventricular assist device placement or other open-chest procedures allows treatment with minimal additional surgical risk, but limits patients on the basis of the need for the aforementioned surgeries. Steerable percutaneous catheters allow for minimally invasive transendocardial stem cell injections (TESI; Box 2) to directed areas of interest from within the heart18,19.
Box 1 | Surgical transepicardial injection of stem cells ● TIMING 3–4 h.
Follow Steps 1–14 of the main PROCEDURE to prepare the animal and obtain CMR measurements.
-
Prepare the angiography table by placing a V-shaped trough tabletop adapter draped with a Bair hugger blanket and sand bags to position the animal properly and maintain euthermia. Place the swine in a dorsal recumbent position and secure it using sand bags. Retract the front hooves and hold them posteriorly to facilitate access to the vasculature of the neck (Fig. 4). Retract the rear hooves caudolaterally with slight flexion of the hip joint and restrain them in place to facilitate access to the femoral canal.
▲ CRITICAL STEP At a minimum, pulse oximetry and heart rate must be monitored during surgical preparation. Vital parameters such as pulse oximetry, heart rate, blood pressure, temperature and capnography should be monitored throughout the procedure to ensure proper anesthesia depth and swine health.
Obtain vascular access to the swine as detailed in Step 17 of the main PROCEDURE.
Obtain preinjection pressure-volume measurements, as detailed in Steps 18–23 of the main PROCEDURE.
Palpate the left chest to localize the fourth through sixth intercostal spaces.
Make a 6–8 cm incision immediately above the fifth rib, beginning ~3 cm lateral to the sternum and extending posteriorly. Transect the latissimus dorsi, external abdominal oblique, serratus ventralis and pectoralis ascendons, carefully ligating the veins and arteries encountered.
-
Once above the fifth rib, advance a mosquito forceps over the fifth rib and through the fourth intercostal space into the left thoracic cavity. Open the mosquito forceps and retract to enlarge the hole. Extend the incision along the intercostal space by using Metzenbaum scissors to create an adequate surgical window.
▲ CRITICAL STEP Care should be taken to protect the delicate lung tissue while entering the thoracic cavity. Pausing mechanical ventilation at peak exhalation can minimize the risk of pulmonary injury.
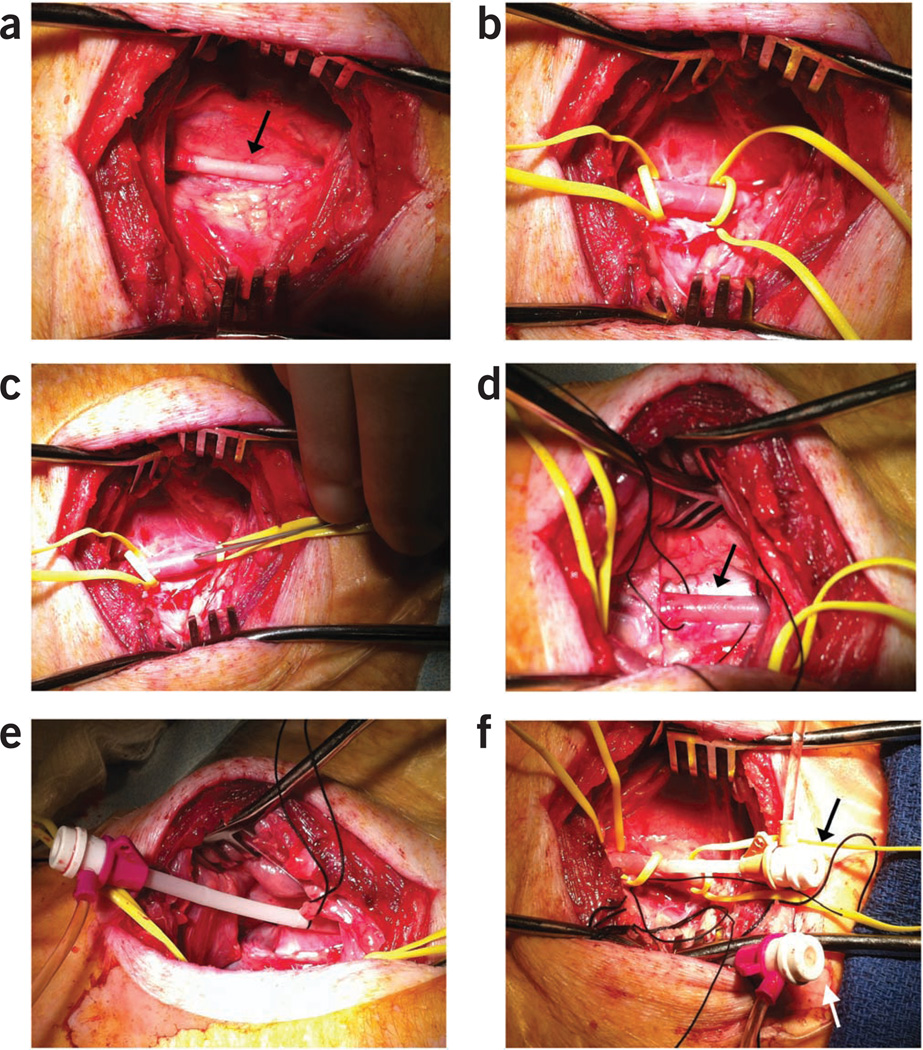
Place a Weitlaner within this incision to retract the ribs and provide a working window to the left thoracic cavity (Fig. 10a). Swine have small intercostal spaces and may require enlargement of the surgical window by transecting the anterior aspect of the fifth rib.
-
Place a 5-mm port in the 6th intercostal space, and insert a 5-mm endoscope into the left thoracic cavity to improve visualization of the heart and aid in placement of the transepicardial injections.
▲ CRITICAL STEP Purkinje cells in the swine myocardium are very abundant and very reactant to physical stimulus. Administration of antiarrhythmic drugs is suggested (lidocaine, 2.0–4.0 mg kg−1).
-
Under thoracoscopic guidance, retract the pericardium away from the myocardium and incise the pericardium using Metzenbaum scissors to provide access and visualization of the infarcted and border zones of the heart (Fig. 10b).
▲ CRITICAL STEP Care should be taken to open the pericardium to avoid injuring the phrenic nerve, which runs along the posterior aspect of the pericardium. Pausing mechanical ventilation at peak exhalation can minimize the risk of pulmonary injury and enhance visualization of the myocardium.
-
Directly visualize the heart by using the endoscope to map and plan the distribution of the product to be injected to the area(s) of interest.
▲ CRITICAL STEP Preoperative review of the previously acquired LGE-CMR scar images (Fig. 3), left ventriculograms and coronary angiograms obtained during induction of MI will help in mapping the infarct and border zones. Injection volumes should not exceed 0.5 ml per injection site and should be equally distributed throughout the targeted area(s).
-
Insert a curved 27-gauge needle perpendicularly to the epicardium and advance into the myocardium (Fig. 10c,d). Direct intramyocardial injections into the infarct and border zone, taking care to avoid intracoronary injection. Lack of blood return with slight negative pressure on the syringe will confirm proper placement.
▲ CRITICAL STEP Administer injections slowly and carefully. Watch injection sites for bleeding or product washout after needle withdrawal. Bleeding can be controlled with direct digital pressure if needed.
? TROUBLESHOOTING
Repeat step 12 for each injection.
After completion of myocardial injections, check the thoracic cavity for bleeding and control as necessary.
Advance a 12F chest tube through the 5-mm port into the thoracic cavity in a dorsocranial position and connect it to a chest drainage system providing −20 cm of water suction.
Close the minithoracotomy incision site in layers providing a watertight seal.
Remove the vascular introducer(s) and repair or ligate the artery as needed. Ligate the vein.
Close femoral incision site in layers.
-
Clean the incision site(s) in a sterile manner; place triple antibiotic ointment over the incision sites. Cover incision sites with sterile nonadherent pads and enclose with waterproof dressings.
? TROUBLESHOOTING
-
A water seal should exist in the chest drainage system without bubbling, indicating complete evacuation of the pneumothorax. Confirm complete lung re-expansion by fluoroscopy before removing the chest tube.
▲ CRITICAL STEP Do not remove the chest tube if there is ≥10 ml h−1 drainage or an air leak present, or if the animal has an unstable respiratory status or oxygenation status. Positive pressure ventilations after removing the chest tube can increase the risk of tension pneumothorax.
Place a purse-string suture around the chest tube. On peak inspiration, gently but swiftly remove the chest tube while simultaneously tying the purse-string suture.
Clean the chest tube site in a sterile manner; place triple antibiotic ointment over the site and cover incision sites with sterile nonadherent pads and enclose with waterproof dressings.
Once the chest tube has been removed, use fluoroscopy to confirm no large residual pneumothorax. Extubate the animal and allow it to recover from anesthesia.
Box 2 | Percutaneous transendocardial stem cell injections ● TIMING 45–70 min.
See Table 1 for a list of transendocardial injection devices and their characteristics.
Follow Steps 1–14 of the main PROCEDURE to prepare the animal and obtain CMR measurements.
-
Prepare the angiography table by placing a V-shaped trough tabletop adapter draped with a Bair hugger blanket and sand bags to position the animal properly and maintain euthermia. Secure the swine’s caudal end in a dorsal recumbent position and restrain the hind limbs caudolaterally with slight flexion of the hip joint in order to properly palpate the femoral canal. Rotate the cranial end in a right lateral decubitus position with the front limbs secured. This positioning allows access to the left or right femoral vasculature and exposes the left lateral chest wall for surgical access to the heart via the left pleural cavity.
▲ CRITICAL STEP At a minimum, pulse oximetry and heart rate must be monitored during surgical preparation. Vital parameters such as pulse oximetry, heart rate, blood pressure, temperature and capnography should be monitored throughout the procedure to ensure proper anesthesia depth and swine health.
Obtain vascular access to the swine, as detailed in Step 17 of the main PROCEDURE.
Obtain preinjection pressure-volume measurements, as detailed in Steps 18–23 of the main PROCEDURE.
By using cardiac catheterization techniques under fluoroscopy guidance, advance a 0.038-inch J-tip guidewire through the arterial introducer into the LV retrograde across the aortic valve and advance a 6F pigtail catheter over the wire into the LV and retract the wire.
-
Under biplane fluoroscopy (Fig. 8a; 30° left anterior oblique and 30° right anterior oblique) obtain left ventriculograms. Pause the ventriculogram images at end diastole and trace endocardial borders on transparencies overlaying the on-screen images.
▲ CRITICAL STEP Do not move or adjust the swine, image intensifies or C-arms once the ventriculogram is obtained, in order to ensure proper guiding of the injections.
Demarcate infarction and border zones on the transparencies on the basis of wall motion scoring and prior evaluation of post-MI CMR. Akinetic territories indicate infarct zones and hypokinetic territories identify border zones.
Advance a 0.038-inch J-tip exchange length guidewire through the pigtail into the LV, remove the pigtail over the wire, advance a steerable catheter (Table 3) over the wire into the LV chamber and retract the wire. Advance a delivery catheter, if needed, through the steerable catheter into the LV.
-
Direct and advance the injection needle at the tip of the catheter to the targeted injection site under fluoroscopic guidance. Engage the delivery catheter into the myocardium at the targeted location (Fig. 10e). Watch for needle stability during cardiac cycle to ensure proper engagement. Premature ventricular contractions also may indicate needle engagement with viable myocardium, but may not be present if engaged with scar tissue.
▲ CRITICAL STEP Myocardial wall thickness of the injection site, determined by at least one imaging modality, must be greater than nine millimeters.
Mark the catheter location on the LV tracings to record injection location and order. The tracing also provides a map of injections for later histopathological analysis if desired.
-
Administer the infusion of cells slowly to ensure safe delivery and minimize washout. After injection, leave the needle to dwell for a short time and then disengage it from the myocardium.
▲ CRITICAL STEP Proper mapping with CMR and left ventriculograms allows the investigator to plan and properly distribute the injections to the area(s) of interest, ensuring myocardial thickness of greater than 9 mm. Injection volumes should be planned not to exceed 0.5 ml per injection site and should be equally distributed throughout the targeted area(s).
? TROUBLESHOOTING
Repeat steps 9–11 for the desired number of injections (typically 10–15).
Close the incision site in layers.
-
Clean the surgical site in a sterile manner; place triple antibiotic ointment over the incision site and cover incision sites with sterile nonadherent pads and enclose with waterproof dressings.
? TROUBLESHOOTING
■ PAUSE POINT Follow-up should be coordinated with the sample timelines shown in Figure 1, or adjusted to the researchers’ goals and anticipated timelines.
TABLE 1.
Catheter systems for transendocardial delivery.
| Catheter system |
Guidance | Core |
Support |
Studies |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outer diameter (mm) |
Length (cm) |
Needle type and configuration |
Size (F) |
Length (cm) |
Configuration | Preclinical | Clinical | |||
| Helix (BioCardia) | Fluoroscopy | 0.51 | 124 | Stainless steel helical tip, 25 gauge | 8 | 110 | Deflectable catheter, guidewire lumen | Amado5 | TAC-HFT24, De la Fuente30, Tomkowiak31 | |
| Myostar (Biologies Delivery Systems) | NOGA electroanatomical mapping; inte-grated with MRI imaging | 0.41 | 123 | Nititol straight, 27 gauge | 8 | 115 | Integrated; multiple curves, no guidewire lumen | Perin18, Baklanov32, Dib35 | Fuchs33, Perin34, Losordo36, van Ramshort37, Krause38, Smits39, CAuSMIC40 | |
The swine MI model was crucial to demonstrating that steerable catheters are able to safely deliver cell therapies to target areas of the heart. Swine models also provide important engraftment and safety data for TESI using steerable catheters while demonstrating their enhanced ability to target specific areas in the heart6,20–22. Additionally, interventional cardiologists used the swine model to acquire the technical proficiencies required before conducting the first TESI for clinical trials.
At present, translational research focusing on regenerative medicine aims to establish the safety and efficacy of cell-based therapy (and other biological strategies), with the goal of offering patients a new therapeutic intervention. Stem cell–based research aims to not only restore cardiac function, but also prevent or reverse the negative effects seen from post-MI remodeling, thereby fulfilling a major unmet medical need. New and exciting stem cell therapies are already suited for clinical application, as seen in the SCIPIO (Stem Cell Infusion in Patients with Ischemic Cardiomyopathy), PROMETHEUS (Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery), POSEIDON (the PercutaneOus StEm Cell Injection Delivery Effects on Neomyogenesis pilot study), POSEIDON-DCM (dilated cardiomyopathy), and TAC-HFT (Transendocardial Autologous Cells in Ischemic Heart Failure Trial) trials16,21–24. The use of a sustainable and reproducible swine MI model was a crucial step in order for these various research endeavors to obtain FDA Investigational New Drug (IND) approval.
Experimental intramyocardial stem cell injections design
The injection model is highly adaptable to the individual researcher, study design and expected endpoints. In addition to the ability to titrate scar size and ventricular impairment by altering occlusion time or location, interventions can be planned for time points from immediately after MI to days or weeks after MI. Interventions can easily be administered through catheter-based or direct surgical techniques, and both allow high levels of specificity in delivery locations and dosages within the myocardium. Although this protocol was developed for studying stem cell therapy in models of acute and chronic MI, this model can easily be adapted for other investigational studies, such as targeted pharmacological or gene therapies, and it provides an important step between in vivo small animal model research and the regulatory approval required for clinical trials25–27. Considering the reproducibility of this model, research is easily conducted and compared against existing published data or placebo animals (i.e., those treated under the same experimental conditions, but given an inert agent or delivery vehicle only).
Previous studies comparing intramyocardial injections through surgical or percutaneous methods have indicated that both methods have similar delivery capabilities, resulting in equivalent engraftment and cellular retention18,19,26. With this in mind, and with proper pre-IND coordination with the FDA, investigational studies using a surgical approach for product delivery can be translated to catheter-based delivery for clinical trials to reduce costs and data sharing requirements of some steerable catheter manufacturers.
Early preclinical stem cell studies used the 60-min infarct protocol, whereas the 120-min protocol has been used in previous chronic studies of ICM6,9. These studies focused more on ejection fraction (EF) as an endpoint for efficacy of treatment and used a shorter duration of infarct than is currently used. As factors such as LV remodeling and scar reduction became more important endpoints of clinical studies, larger scar mass and greater LV dilation were needed for greater sensitivity in the results. To achieve this, the occlusion time was prolonged by 30 min in both the acute and chronic study protocols to create larger transmural infarcts.
MATERIALS
REAGENTS
Göttingen swine, female, 10–14 months old (Marshall BioResources, http://www.marshallbio.com/)
Yorkshire swine, female, 20–30 kg (Looper Farms) ! CAUTION All animal requisitions, housing, treatment and procedures must conform to all federal, state and institutional laws, guidelines and regulations. The protocol described here was reviewed by the University of Miami Institutional Animal Care and Use Committee and followed all federal and state guidelines concerning the use of animals in research and teaching, as denned by the Guide for the Care and Use of Laboratory Animals.
Ketamine HC1, 100 mg ml−1 (Webster Veterinary, cat. no. 07-881-9413, http://www.webstervet.com/; a DEA Schedule 3N Controlled drug)
Buprenorphine, 0.3 mg vial (Webster Veterinary, cat. no. 07-836-8775, http://www.webstervet.com/; a DEA Schedule III controlled drug)
Isoflurane (Webster Veterinary, cat. no. 07-836-6544, http://www.webstervet.com/) ! CAUTION Anesthetic agent, causes central nervous system depression. Avoid exposure to vapors.
Fentanyl transdermal patch, 25 µg h−1(Webster Veterinary, cat. no. 07-859-4067, http://www.webstervet.com/; a DEA Schedule II controlled drug) ! CAUTION It is very hazardous in case of ingestion or inhalation. It is hazardous in case of skin contact (irritant, permeator) and slightly hazardous in case of eye contact (irritant). Severe overexposure can result in death.
Cefazolin, 1 g (Webster Veterinary, cat. no. 07-802-5851, http://www.webstervet.com/)
Epinephrine, 1 mg ml−1 (Webster Veterinary, cat. no. 07-834-4348, http://www.webstervet.com/)
Atropine 1/120 grain, 100 ml (Webster Veterinary, cat. no. 07-806-9535, http://www.webstervet.com/) ! CAUTION It is a skin and eye irritant and is toxic to the kidneys, the nervous system, the liver, the mucous membranes, the gastrointestinal tract and the cardiovascular system. Repeated or prolonged exposure can produce organ damage.
Lidocaine (2% wt/vol), 50 ml (Webster Veterinary, cat. no. 07-865-8420, http://www.webstervet.com/)
Heparin, 1,000 U ml−1,10 ml (Webster Veterinary, cat. no. 07-883-4916, http://www.webstervet.com/)
Potassium chloride, 20 mEq/10 ml (Webster Veterinary, cat. no. 07-841-6527, http://www.webstervet.com/)
Omnipaque 350 (GE Healthcare, cat. no. Y-546, http://www.gehealthcare.com/)
Magnevist (Bayer Imaging, NDC no. 50419-188-01, http://www.bayerimaging.com/)
Sodium chloride (0.9% (wt/vol)), 1 liter (Abbott Labs, cat. no. 04925-10, http://www.abbottanimalhealth.com/)
Lactated Ringer’s solution, 1 liter (Abbott Labs, cat. no. 04860-10, http://www.abbottanimalhealth.com/)
Compressed oxygen cylinder, USP grade (Airgas, cat. no. OX USP200, http://www.airgas.com/)
EQUIPMENT
Siemens 3 Tesla Trio MRI scanner (Siemens, http://www.siemens.com/)
Siemens Artis zee biplane angiography system (Siemens, http://www.siemens.com/)
Angiographic procedural pack 1 (Merit Medical, cat. no. K09T-08878, http://www.merit.com/)
Weitlaner retractor, 3 × 4, 8 inch blunt (Roboz, cat. no. RS-8616, http://www.roboz.com/)
Weitlaner retractor, 3×4, 6.5 inch blunt (Roboz, cat. no. RS-8614, http://www.roboz.com/)
Adson forceps (Roboz, cat. no. RS-5233, http://www.roboz.com/)
Crile-Wood needle holder, 7 inch (Roboz, cat. no. RS-7862, http://www.roboz.com/)
Mayo scissors, 6.75 inch curved (Roboz, cat. no. RS-6873, http://www.roboz.com/)
Metzenbaum scissors, 7 inch curved tungsten carbide, extra delicate (Roboz, cat. no. RS-6956, http://www.roboz.com/)
Scalpel handle no. 3, solid 4 inch (Roboz, cat. no. RS-9843, http://www.roboz.com/)
Gemini-Mixter forceps, 7 inch full curve delicate (Roboz, cat. no. RS-7233, http://www.roboz.com/)
Mixter forceps, 5.25 inch curved extra delicate (Roboz, cat. no. RS-7291, http://www.roboz.com/)
Liston bone cutter, straight, 8.5 inch length (Roboz, cat. no. RS-8544, http://www.roboz.com/)
Debakey tissue forceps, 6 inch, 1.5 mm (Roboz, cat. no. RS-7561, http://www.roboz.com/)
Long straight serrated Gerals forceps, 7 inch (Roboz, cat. no. RS-5270, http://www.roboz.com/)
Metzenbaum (Lahey) scissors, 7 inch curved, carbide blades (Roboz, cat. no. RS-6955, http://www.roboz.com/)
Senn retractor, 6.5 inch blunt double end (Roboz, cat. no. RS-6612, http://www.roboz.com/)
Debakey Bulldog clamp, 4 inch, 90° (Roboz, cat. no. RS-7587, http://www.roboz.com/)
Debakey Bulldog clamp, 4.75 inch straight (Roboz, cat. no. RS-7580, http://www.roboz.com/)
Halstead mosquito forceps, curved, 5 inch (Roboz, cat. no. RS-7111, http://www.roboz.com/)
Debakey tissue forceps, 7.75 inch, 2.5 mm (Roboz, cat. no. RS-7562, http://www.roboz.com/)
Debakey tissue forceps, 8 inch, 1.5 mm (Roboz, cat. no. RS-7566, http://www.roboz.com/)
Intracardiac needle holder, 7 inch extra delicate tungsten carbide (Roboz, cat. no. RS-7804, http://www.roboz.com/)
Precut silk sutures, black braided, 18 inches, 12 per pack, 5-0 (Covidien, cat. no. S-182, http://www.covidien.com/)
Precut sutures, black braided, 18 inches, six per pack, 0 (Covidien, cat. no. S-196, http://www.covidien.com/)
Polysorb suture, braided absorbable, 30 inches, 3-0, C-13 cutting needle (Covidien, cat. no. SL-694, http://www.covidien.com/)
Polysorb suture, braided absorbable, 30 inches, 4-0, C-13 cutting needle (Covidien, cat. no. SL-635, http://www.covidien.com/)
6 French (F) Vista Brite tip Judkins right 3.5 (Cordis, cat. no. 670-080-00, http://www.cordis.com/)
6F INFINITI ventricular pigtail 6sh (Cordis, cat. no. 534-650S, http://www.cordis.com/)
7F AVANTI + catheter sheath introducers with miniwire (Cordis, cat. no.504-607X, http://www.cordis.com/)
9F AVANTI + catheter sheath introducers with miniwire (Cordis, cat. no. 504-609X, http://www.cordis.com/)
Emerald fixed core PTFE-coated J-tip wire, 175 cm length, 0.038 inches (Cordis, cat. no. 502-584, http://www.cordis.com/)
All track guidewire floppy, straight tip, 0.014 inch, 195 cm (Cordis, cat. no. 595-014, http://www.cordis.com/)
Fire Star PTCA dilation catheter, 3.00 × 20 mm balloon (Cordis, cat. no. 801-20300, http://www.cordis.com/)
Fire Star PTCA dilation catheter, 2.50 × 20 mm balloon (Cordis, cat. no. 801-20250, http://www.cordis.com/)
Fire Star PTCA dilation catheter, 2.00 × 20 mm balloon (Cordis, cat. no. 801-2020D, http://www.cordis.com/)
BasixCOMPAK inflation device (Merit Medical, cat. no. IN-4130, http://www.merit.com/)
Fogarty balloon occlusion catheter, 8F, 28 mm (Edwards Lifesciences, cat. no. 62080814F, http://www.edwards.com/)
Macrobore, 15 gtt, 78 inch i.v. set (Abbott Labs, cat. no. 32084-80, http://www.abbottanimalhealth.com/)
Peripheral i.v. catheter, 22 G, 1 inch (Abbott Labs, cat. no. 32055-22, http://www.abbottanimalhealth.com/)
C/S 10 blade (Cincinnati Surgical, cat. no. 0010, http://www.cincinnatisurgical.com/)
Access Plus hemostasis valve (Merit Medical, cat. no. MAP150, http://www.merit.com/)
Fixed male-to-male pressure monitoring tubing, 36 inch (Merit Medical, cat. no. PM6036, http://www.merit.com/)
Fixed male-to-female pressure monitoring tubing, 24 inch (Merit Medical, cat. no. PM6124, http://www.merit.com/)
Pressure infuser bags, 1 liter (Merit Medical, cat. no. PIB1000, http://www.merit.com/)
Delta-Flow II, 3 ml h−1, T configuration with Snap-Tab (Utah Medical, cat. no. 100-202, http://www.utahmed.com/)
Meritrans pressure transducer MER100 (Merit Medical, cat. no. Kl 1-00100AP, http://www.merit.com/)
Zoll M Series CCT defibrillator (Zoll, http://www.zoll.com/)
Bair hugger 750 temperature management unit with blankets (Arizant, model 750, http://www.arizant.com/)
MATRX VMS plus anesthesia cart (Midmark, cat. no. 91800081, http://www.midmark.com/)
MATRX isoflurane VIP 3000 vaporizer (Midmark, cat. no. 91305430, http://www.midmark.com/)
MATRX model 3000 veterinary anesthesia ventilator (Midmark, cat. no. 91806001, http://www.midmark.com/)
Oxygen regulator (Midmark, cat. no. 93305327, http://www.midmark.com/)
Karl Storz Telecam SL (Karl Storz, cat. no. 20212120, http://wwwkarlstorzcom/)
Karl Storz Xenon 300 (Karl Storz, cat. no. 20133120, http://www.karlstorz.com/)
Karl Storz Hopkins II, 30° rigid, 5 mm endoscope (Karl Storz, cat. no. 26046 BA, http://www.karlstorz.com/)
Karl Storz telecam (Karl Storz, cat. no. 20212130, http://www.karlstorz.com/)
Karl Storz fiber-optic light cable (Karl Storz, cat. no. 495 NA, http://www. karlstorz.com/)
Ethicon ENDOPATH XCEL, 5 mm Trocar and port, B5XT (Ethicon Endo-Surgery, http://www.ees.com/)
Triple antibiotic ointment (Fougera, cat. no. 0168-0012-09, http://www.fougera.com/)
Tegaderm film dressing with nonadherent pad (3M, http://www.shop3m.com/)
PROCEDURE
Preparation (preanesthesia, anesthesia, cleaning, i.v. access and intubation) ● TIMING 30–60 min
-
1|
Fast the swine for 12 h prior to surgery but allow continued access to water up to the time of surgery to avoid dehydration.
-
2|
Induce anesthesia with intramuscular (i.m.) or subcutaneous (s.c.) anesthetic agents (ketamine (33 mg kg−1) is recommended because of its minimal cardiovascular side effects). Once the animal is immobilized by anesthesia, transfer it to the preparation area and initiate maintenance anesthesia. (We recommend 3–4% isoflurane and 22–44 ml kg−1 100% oxygen via simple nose cone and partial rebreather anesthesia circuit until the animal is intubated.)
▲ CRITICAL STEP At a minimum, pulse oximetry and heart rate must be monitored through anesthesia induction, maintenance and recovery. Induction and maintenance anesthesia regimens should be tailored to the preference and the proficiency of the team and to the animals used.
-
3|
Clean the swine as needed, obtain i.v. access via the posterior auricular vein with a 22-gauge angiocatheter, and place a secure airway (endotracheal intubation with a 6.5- to 7.5-mm endotracheal tube is preferred for superior airway control and protection). Shave the surgical sites as necessary and move the animal from the preparation area to the procedure suite.
? TROUBLESHOOTING
-
4|
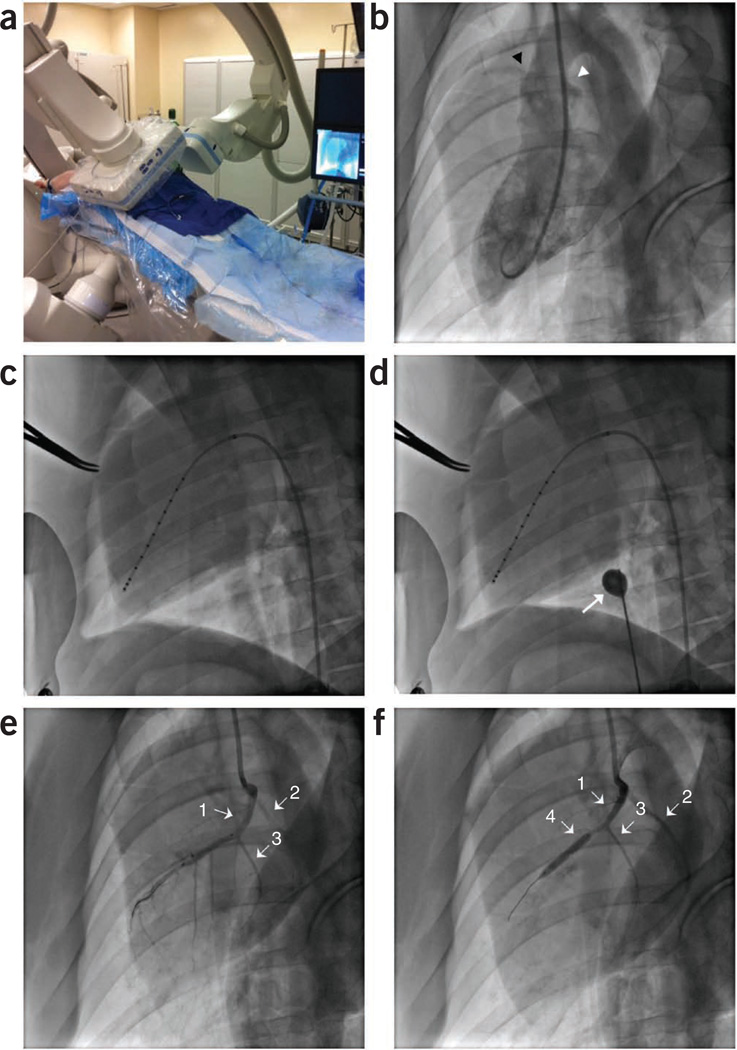
Deliver ongoing maintenance anesthesia with 1–2% isoflurane and 0.5–1.5 liters per min oxygen through a mechanically ventilated closed-loop anesthesia machine (Fig. 2).
▲ CRITICAL STEP The pulmonary tissues of swine are very fragile, so care should be taken to avoid pulmonary barotrauma. Standard tidal volumes for swine are 5–15 ml kg−1 and peak inspiratory pressure should not exceed 18–20 cmH2O.
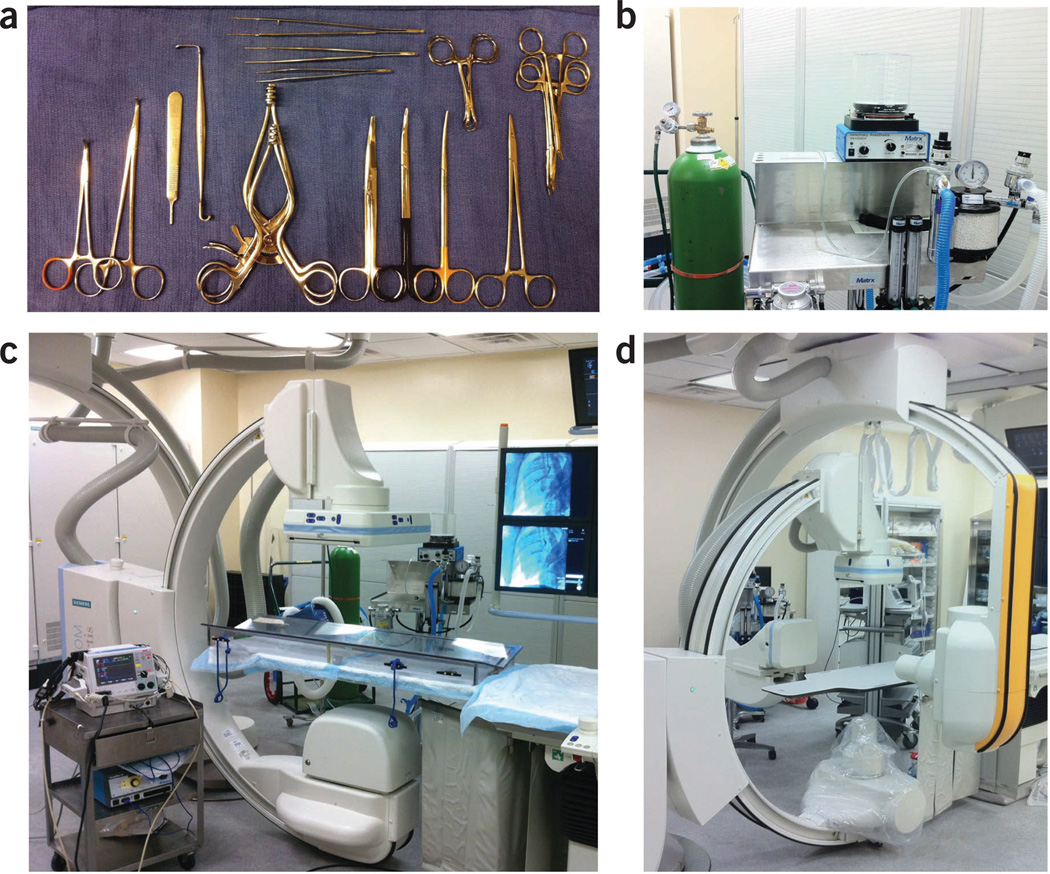
Figure 2.
Surgery, anesthesia and angiography equipment required for models of ischemic heart disease. (a) Typical surgical tray setup with Gemini-Mixter forceps, no. 3 scalpel handle, Senn retractor, Weitlaner retractors, Mayo scissors, Metzenbaum scissors, needle holder, Kelly and mosquito forceps, Backhaus towel clamps, Gerald forceps, Debakey forceps and Adson forceps. (b) Closed-circuit anesthesia system with isoflurane vaporizer, veterinarian anesthesia ventilator and oxygen cylinder. (c) Biplane system, anesthesia cart, swine positioning table adapter. (d) Siemens Artis zee biplane system.
CMR ● TIMING 45–60 min
-
5|
Place the animal on the table head first and in the supine position. Place sand bags on either side of the animal in order to maintain the position.
▲ CRITICAL STEP Baseline noninvasive studies (CMR and late gadolinium enhancement (LGE)-CMR are recommended) of the heart should be performed to ensure normal cardiac structure and function before MI model creation.
-
6|
By using the table coil elements posteriorly, place a dedicated phased array cardiac coil (body array coil) over the anterior part of the chest.
-
7|
Acquire multiplanar gradient echo scout images for optimal short axis, two-chamber, three-chamber and four-chamber long axis views of the heart.
-
8|
Obtain suspended respiration images with the use of a veterinary anesthesia delivery system. Obtain retrospective ECG-gated steady-state gradient echo–tagged (grid) and fast imaging steady-state free precession (TrueFisp) cine images in the short axis plane from the base of the LV to the apex of the heart. Field of view (280–300 mm) should be optimized according to the size of the animal. Take slice thickness at 4 mm for the TrueFisp images and 8 mm for the tagged images with a slice gap of 25% between slices. The tagged images require one average using a resolution matrix size of 224 × 167, whereas the TrueFisp images require two averages using a resolution matrix size of 2562 (interpolated). The average acquisition time should be 15–20 s per slice.
-
9|
Obtain TrueFisp cine images in the two-chamber, three-chamber and four-chamber long axis view of the heart.
-
10|
Obtain perfusion and delayed enhancement images using a double-dose (0.2 mmol kg−1) contrast injection of Magnevist (gadopentetate dimeglumine). Inject contrast at a dose of 0.1 mmol kg−1, with the power injector (Medrad) set at 5 ml s−1, followed by 10 ml of saline flush for first-pass perfusion imaging. Deliver the second contrast dose of 0.1 mmol kg−1 after the perfusion scan has been completed.
-
11|
Eight minutes after the first injection, obtain a TI scout (inversion time scout; for optimal inversion time for the delayed enhanced images) to null the myocardium.
-
12|
Obtain prospectively gated single–shot, phase-sensitive inversion-recovery (PSIR) delayed enhancement images in the short axis, two-chamber, three-chamber and four-chamber long axis view (Fig. 3a–c).
-
13|
Analyze images with offline software (Qmass) to determine end diastolic volume (EDV), end systolic volume (ESV), stroke volume, EF, LV mass and scar mass (Fig. 3d).
■ PAUSE POINT CMR images can be acquired up to 48 h before the procedure to allow time for offline analysis and review by the surgical team. The animal would then be recovered from anesthesia and Steps 1–4 would need to be followed again to prepare the animal for surgery.
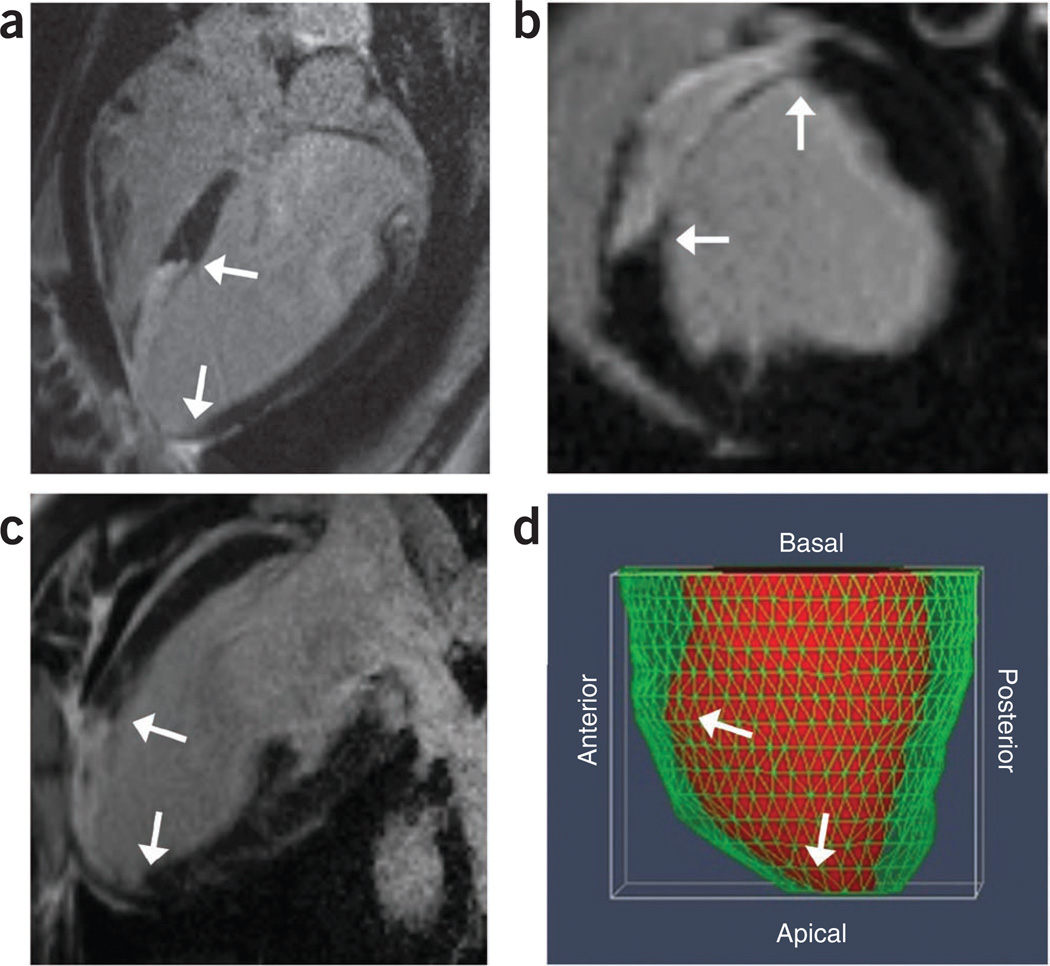
Figure 3.
Cardiac MRI imaging techniques showing the extent of MI. (a–d) Myocardial wall thinning, LV chamber dilation and scar, highlighted by white arrows, 2 weeks after MI as shown in LGE-MRI (a); four-chamber long axis showing apical septal scar (b); LGE-MRI short axis images showing anteroseptal scar (c); and LGE-MRI two-chamber long axis showing anterior scar (d). Three-dimensional reconstruction of the heart. Red fill shows the endocardial wall of the LV chamber and the green mesh indicates the epicardial wall. The reconstruction demonstrates the dilation and thinning of the anterior-apical walls.
Surgical preparation ● TIMING 15–20 min
-
14|
Give prophylactic antibiotics 30 min to 1 h before the procedure to reduce the risk of surgical site infection.
(i.m. administration of Cefazolin 1 g is recommended).
▲ CRITICAL STEP Antibiotic treatment is not a substitute for adhering to aseptic techniques for survival surgeries.
-
15|
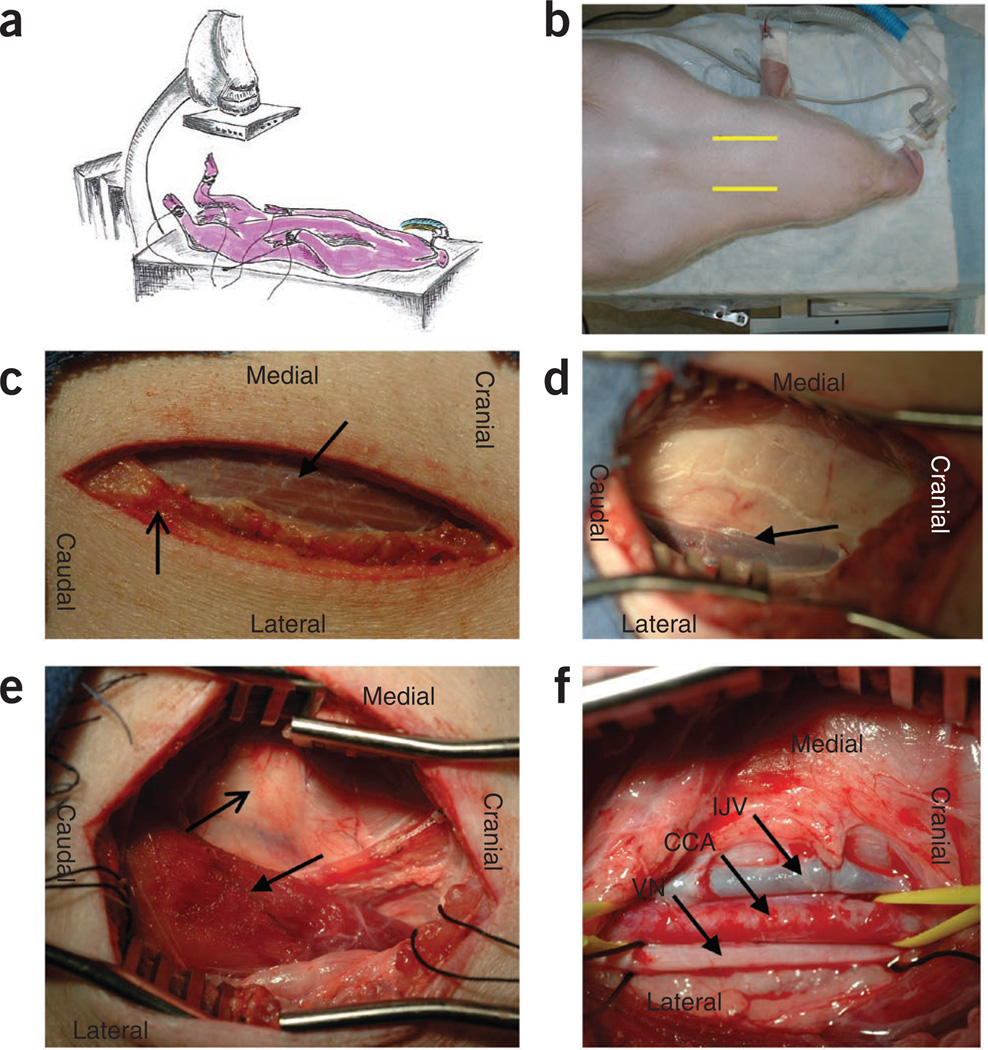
Prepare the angiography table by placing a V-shaped trough tabletop adapter draped with a Bair hugger blanket and sand bags to position the animal properly and maintain euthermia. Place the swine in a dorsal recumbent position and secure it using sand bags. Retract the front hooves and hold them posterior to facilitate access to the vasculature of the neck (Fig. 4). Retract the rear hooves caudolaterally with slight flexion of the hip joint and restrain them in place to facilitate access to the femoral canal.
▲ CRITICAL STEP At a minimum, pulse oximetry and heart rate must be monitored during surgical preparation. Vital parameters such as pulse oximetry, heart rate, blood pressure, temperature and capnography should be monitored throughout the procedure to ensure proper anesthesia depth and swine health.
-
16|
Prepare and drape the surgical site(s) in an aseptic manner.
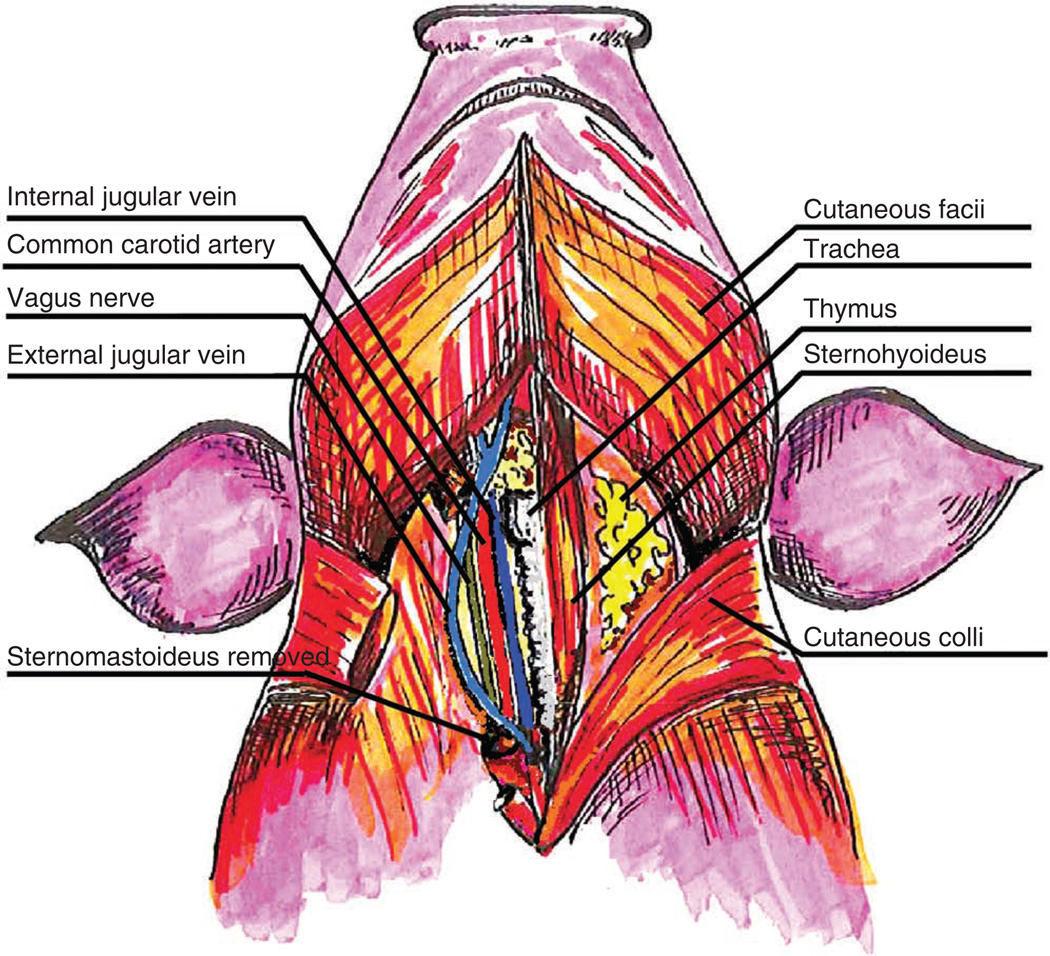
Figure 4.
Neck vasculature and musculature of the swine. Superficial and deep dissection illustrating the landmarks and vessels used for surgical dissection and cannulation of the vasculature in the neck. Note that the sternomastoideus has been removed on the swine’s right to show the underlying anatomical structures in the carotid sheath.
Surgical dissection and vasculature access ● TIMING 30–45 min
-
17|
Place intra-arterial and i.v. introducers to access the central vasculature through either the carotid or femoral arteries and external jugular (EJ) or femoral veins. Follow option A for carotid artery and EJ vein access. Follow option B for femoral artery and vein access.
▲ CRITICAL STEP The carotid dissection provides easier central arterial and venous access because of the shorter and more direct route to the heart. The femoral artery and vein also have considerably more branches requiring ligation.
The femoral vessels are typically smaller in size than the neck vessels.
(A) Carotid artery and EJ vein access
-
Make a 6–8-cm incision perpendicular and slightly medial to the line from the point of the jaw to the point of the shoulder (Fig. 5). Dissect the s.c. tissue and the muscular plane between the cutaneous facii and cutaneous colli. Dissect the facial plane between the trachea and the sternomastoideus to palpate the carotid pulse and localize the carotid sheath (Fig. 5e). Surgical dissection and cannulation of the right carotid artery provides the most direct, timely and reliable access to the left coronary sinus.
▲ CRITICAL STEP Care should be taken to avoid damaging the vagus nerve (Fig. 5f) and associated ganglion. Damage to the vagus nerve can lead to denervation of the recurrent laryngeal nerve and paralysis of the ipsilateral vocal cord with resulting loss of airway control. Damage to the cranial cervical sympathetic ganglion, overlaying the carotid artery, can lead to iatrogenic Horner syndrome.
? TROUBLESHOOTING
Isolate the common carotid artery using blunt dissection in order to prevent damage to the vagus nerve that lies within the carotid sheath (Fig. 6). Ligate arterial branches as needed to reduce blood loss and minimize risk of dissection of the artery. Gain vessel control with vessel loops or 0 surgical silk sutures (Fig. 6b).
-
Access the carotid artery using a Seldinger guidewire technique and secure a 7 or 9 French (F) introducer to the artery (Fig. 6c). Bathe the artery in 2% lidocaine to dilate the vessel and facilitate access.
▲ CRITICAL STEP The smallest introducers that allow passage of all required catheters should be used in order to minimize trauma to all vessels that are not ligated upon removal of introducers.
-
Bluntly dissect over the dorsal surface of the stemocephalic muscle to expose the EJ vein. Gain vessel control with 0 surgical silk sutures (Fig. 6d). The EJ vein lies posterior to the planes of the stemocephalic and brachiocephalic muscles and anterior to the stemomastoideus.
▲ CRITICAL STEP The internal jugular vein is considerably smaller in caliber than the EJ vein, and therefore the EJ vein is isolated to obtain central venous access.
-
Access the EJ vein with the Seldinger technique and secure a 9F introducer in the vein (Fig. 6e). Tie the proximal silk suture around the vein and introducer to prevent hemorrhage, and tie the distal silk suture to ligate the vein distal to the catheterization site.
▲ CRITICAL STEP All introducers must be secured to prevent uncontrolled hemorrhage.
Figure 5.
Surgical dissection of the vasculature of the neck in the swine. (a) Pig positioned in dorsal recumbent position and secured with legs retracted caudolaterally. (b) Pig with i.v. and ET tube properly secured. Yellow lines highlight jugular furrow for incision site. (c) Incision site over jugular furrow with cutaneous colli highlighted by the open-tip arrow and the sternohyoideus highlighted by the closed-tip arrow. (d) External jugular vein (EJV) highlighted by the arrow. The sternohyoideus is retracted in the medial aspect of the picture and the brachiocephalic muscle is retracted in the lateral aspect of the picture. The sternomastoideus muscle is located beneath the white fascia in the center of the photo, (e) The carotid sheath is located medial to the sternomastoideus (closed tip arrow) and dorsally along the lateral plane of the trachea (open tip arrow). (f) The internal jugular vein (IJV), common carotid artery (CCA) and the vagus nerve (VN) are exposed after blunt dissection of the carotid sheath.
Figure 6.
Vasculature access via a modified Seldinger technique. (a) Right carotid artery exposed and bluntly dissected (arrow). (b) Distal and proximal control of the artery is obtained using vessel loops. Note the dilation of the artery after bathing with 2% lidocaine. (c) 18-gauge, 2 3/4-inch Seldinger needle passing into the arterial lumen to advance an 0.038-inch guidewire before the introduction of a 7F introducer. (d) External jugular vein with proximal and distal 0 silk ties in place for vessel control. (e) 10F introducer in the vein with proximal tie and distal ligation of the vessel. (f) 7F and 10F sheaths in the carotid artery (black arrow) and the external jugular vein (white arrow), respectively, for vascular access.
(B) Femoral artery and vein access
-
Localize the femoral artery by palpating the femoral canal between the sartorius and gracilis muscles. The femoral canal houses the femoral artery, vein and nerve. The vessels lie along the medial edge of the sartorius muscle.
▲ CRITICAL STEP Palpating the femoral pulse is not easy and the anatomical landmarks offer a more dependable guide to locating the vessels.
Make a 5–6-cm incision along the femoral canal and the s.c. tissue, and then dissect the muscular plane between the gracilis and the sartorius muscles to expose the femoral artery and vein.
-
Isolate the femoral artery and vein using blunt dissection in order to prevent damage to the femoral nerve. Gain vessel control with vessel loops or 0 surgical silk sutures.
▲ CRITICAL STEP The femoral vessels may have many branches that require ligation to minimize bleeding and aid in vessel control and access.
-
Enter the femoral vessels with the Seldinger guidewire technique and place a 7F or 9F introducer in the artery and vein. Bathe the artery in 2% lidocaine to dilate the vessel, thereby aiding in access.
▲ CRITICAL STEP The smallest introducers that allow passage of all required catheters should be used to minimize trauma to all vessels that are not ligated upon removal of introducers. All introducers placed must be secured to prevent uncontrolled hemorrhage.
Invasive pressure-volume measurements ● TIMING 30–45 min
-
18|
Open recording software on a PC and connect the catheter (Fig. 7). Perform catheter setup and pressure calibration according to the manufacturer’s recommendations.
-
19|
Under fluoroscopy guidance, advance a 6F Judkins right catheter over the wire into the LV through the carotid/femoral artery.
-
20|
Remove the wire while retaining the 6F Judkins right catheter in place. Advance a 5F straight conductance catheter (under fluoroscopy guidance) to the level of the LV. When the latter reaches the apex of the LV, start retracting the 6F Judkins right catheter over the conductance catheter to the level of the aorta, thereby exposing all of the conductance catheter electrodes (Fig. 8).
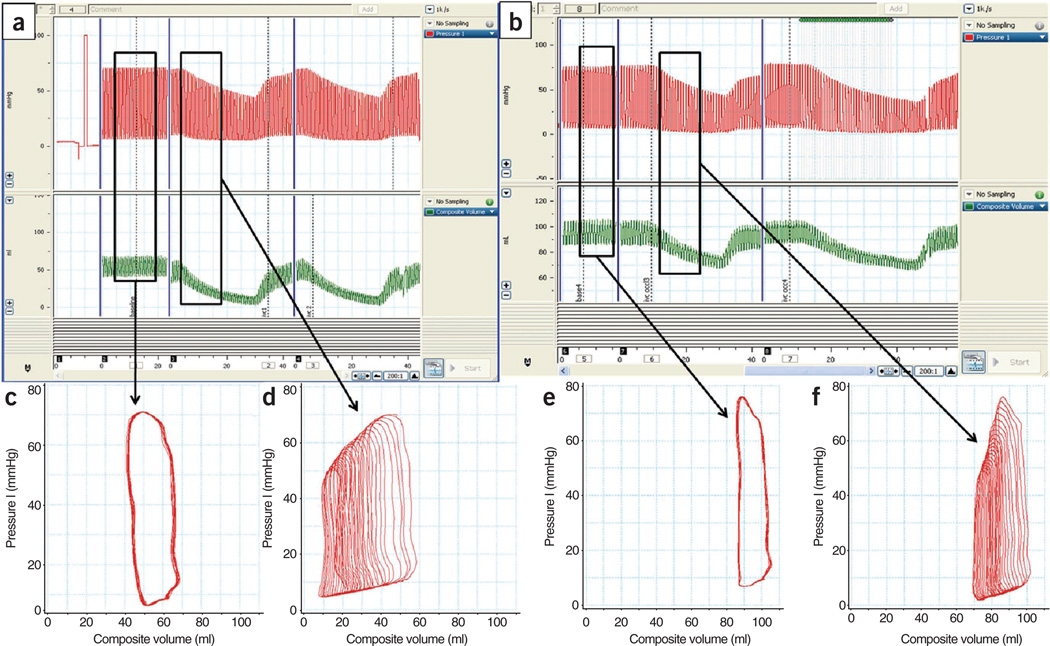
▲ CRITICAL STEP Proximal segments having a figure eight loop indicate aortic measurements and must be excluded; alternatively, the catheter must be advanced further into the LV. Ensure that all the electrodes of the conductance catheter are inside the left ventricular chamber and that the tip of the catheter is facing the apex (Fig. 8c). Ensure that the conductance catheter retains a stable and straight position approximately in the middle of the ventricular chamber, avoiding wall contact. If needed, adjust the position of the catheter to obtain rectangular-shaped pressure-volume loops (in diseased animals the shape may not be rectangular).
-
21|
After stabilization of the heart rhythm, stop mechanical ventilation and record baseline pressure-volume loops during steady state. When enough loops are recorded, stop recording and restore mechanical ventilation.
-
22|
Under fluoroscopy guidance, advance a 22F Fogarty balloon-occlusion catheter through the venous introducer into the inferior vena cava to the level of the diaphragm. When proper position is reached, stop mechanical ventilation and record pressure-volume loops during decreasing preload. The latter is achieved by inflating the balloon, leading to total inferior vena cava occlusion (Fig. 8d). At this point, stop recording, restore ventilation and deflate the balloon. Repeat this process at least three times and average the indices.
▲ CRITICAL STEP In case of premature ventricular contractions during the recording of baseline or occlusion, exclude the corresponding loops (plus both pre- and post-incident loops). In case of multiple incidents, all the occlusion recordings should be discarded and repeated.
-
23|
Perform offline analysis of the recorded data by calibrating the volume with the point and difference method using the EDV and stroke volume derived from the CMR studies (performed within 48 h of recording the pressure-volume loops, Fig. 7). Start the dynamic state analysis at the point at which both systolic pressure and volume decline simultaneously and end at the point at which their decline reaches a plateau (Fig. 7d,f).
? TROUBLESHOOTING
Figure 7.
I Pressure-volume data obtained using a micromanometer conductance catheter. (a–f) Characteristic changes (a,b) in porcine LV pressure (red trace) and volume (green trace) from steady state (c,e) to various changes in preloads (d,f). Panels a,c and d show pressure-volume relationships at the baseline, whereas panels b,e and f show the corresponding relationships 2 weeks after MI.
Figure 8.
Cardiac fluoroscopic and angiographic imaging depicting placement of diagnostic catheters and induction of MI. MI model. (a) Swine placed dorsally recumbent under biplane angiography. Vascular access is shown through the right carotid artery and the right external jugular vein. (b) Left ventriculogram (right anterior oblique 30° view) showing the right and left coronary ostia, as highlighted by the black and white arrowheads, respectively. (c) Pressure-volume conductance catheter properly placed along the long axis of the left ventricle. (d) Balloon occlusion catheter (white arrow) inflated to decrease preload on heart during pressure-volume loop data collection. (e) Left coronary angiogram showing the LAD (arrow 1), LCX (arrow 2) and the first diagonal branch of the LAD (arrow 3). (f) Left coronary angiogram showing the LAD (arrow 1), LCX (arrow 2), and the first diagonal branch of the LAD (arrow 3) and the PTCA balloon obstructing all distal flow of the LAD past the first diagonal (arrow 4).
LAD occlusion ● TIMING 100–300 min
-
24|
Administer heparin (150 IU kg−1) intravenously before catheterization of the heart with a goal-activated clotting time of greater than 200 s.
-
25|
By using cardiac catheterization techniques under fluoroscopy guidance, advance a 0.038-inch J-tip exchange wire through the arterial introducer into the LV retrograde across the aortic valve.
-
26|
Advance a 6F pigtail catheter over the wire into the LV and retract the wire. Obtain a left ventriculogram (30° left anterior oblique view with 20 ml of Omnipaque 350). Visualization of the coronary sinuses and left main coronary anatomy will allow faster placement of the PTCA balloon (Fig. 8b).
-
27|
Advance the J-tip wire through the pigtail and remove the catheter over the wire.
-
28|
For carotid access, advance a 6F Judkins right catheter over the wire to the level of the left coronary sinus and place at the coronary ostium without full engagement. For femoral access, use a 6F Judkins left catheter. Remove the J-tip wire from the catheter.
▲ CRITICAL STEP Placement is confirmed with a contrast angiogram of the left main coronary artery, ensuring reflux of contrast into the aorta (Fig. 8e). Complete engagement of catheter with left coronary ostia can cause ventricular arrhythmias.
-
29|
Advance a 0.014-inch guidewire into the LAD artery and advance a 3.0 × 20 mm (for Yorkshires) or 2.0 × 20 mm (for Göttingens) PTCA dilatation catheter over the wire until it is distal to the first diagonal branch of the LAD.
? TROUBLESHOOTING
-
30|
Inflate PTCA balloon to the minimum pressure needed (3–9 atm) to ensure occlusion of the LAD distal to the balloon. Confirm occlusion with coronary angiogram while ensuring flow down the first diagonal (Fig. 8f). Leave the PTCA balloon inflated, occluding all blood flow distal to the balloon for 90 min (for Yorkshires) or 150 min (for Göttingens). Scar size can be titrated to the desired MI size by varying occlusion times on the basis of the research design and the needs of the investigator. See also Figure 9 in the event of ECG changes.
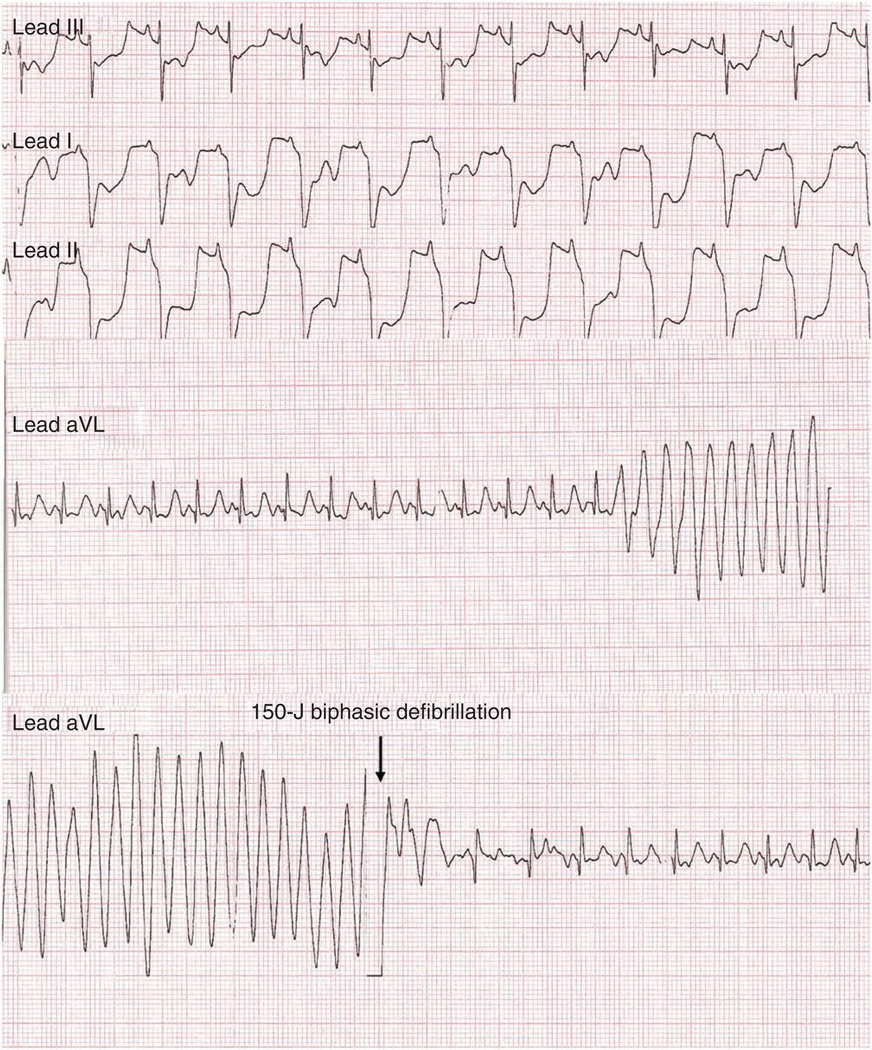
▲ CRITICAL STEP Watch for ECG changes (Fig. 9). Prepare to administer cardiopulmonary resuscitation if necessary. Have epinephrine, atropine, lidocaine and a defibrillator available. Early identification of dysrhythmias and rapid intervention markedly increases animal survival.
? TROUBLESHOOTING
Figure 9.
Representative electrocardiographic changes during MI. Leads I, II and III show morphological ST segment depression indicative of cardiac ischemia. aVL leads show entry of ventricular fibrillation and return of normal sinus rhythm after 150-J biphasic defibrillation.
Reperfusion and confirmation ● TIMING 5–10 min
-
31|
Slowly deflate the PTCA balloon and demonstrate reperfusion with coronary angiography. Perform a repeat left ventriculogram to demonstrate the area of dyskinesis.
? TROUBLESHOOTING
-
32|
Remove the PTCA balloon and guide catheter. The swine may be kept under anesthesia for post-MI CMR (Repeat Steps 5–13) and post-MI experimental interventions (Boxes 1 and 2; Fig. 10). If the study design calls for intervention days, weeks or months after MI, continue on to Step 33.
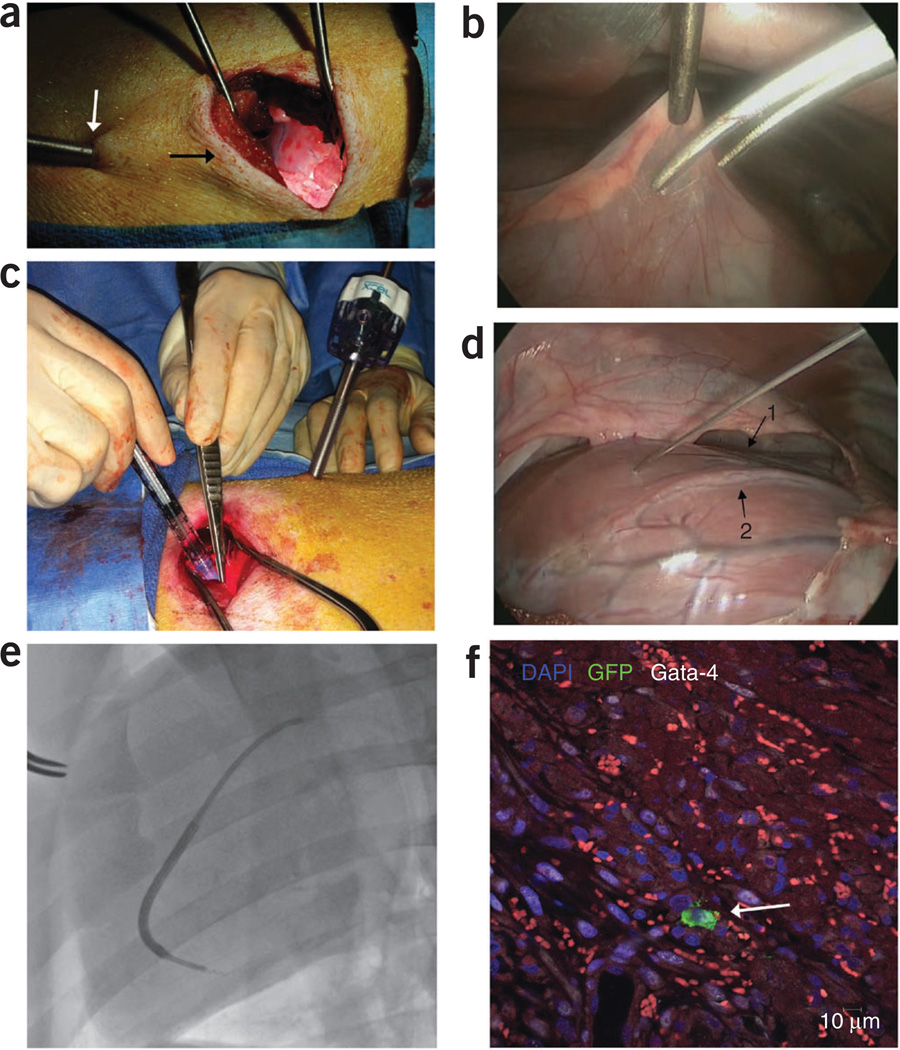
Figure 10.
Surgical- and catheter-based injections of stem cells. Intramyocardial injection model (a) minithoracotomy (black arrow) with thoracoscope (white arrow) shown illuminating the heart. (b) Thoracoscopic view of the pericardiotomy. (c) Intramyocardial injections through the minithoracotomy sight. (d) Thoracoscopic view of the intramyocardial injections. Note the LAD running in the intraventricular groove (arrow 1) and the first diagonal branch of the LAD (arrow 2). (e) BioCardia Morph with deflectable tip directing the Helix biotherapeutic delivery catheter system into the targeted area of the myocardium adjacent to the infarction. (f) GFP+ stem cell identified after intramyocardial injection through postmortem histopathology analysis (white arrow). DAPI, 4’,6-diamidino-2-phenylindole; Gata-4, Transcription factor Gata-4.
Surgical closure and recovery ● TIMING 60–120 min
-
33|
Remove introducer(s) and repair or ligate the artery and ligate the vein.
-
34|
Close surgical incision site(s) in layers.
-
35|
Clean the incision site(s) in a sterile manner; place triple antibiotic ointment over the incision site(s); cover incisions with a nonadherent pad and enclose them with a waterproof dressing.
-
36|
Remove the swine and allow it to recover from anesthesia. Extubate it, give it postsurgical pain medications (a 72-h transdermal Fentanyl patch is suggested (1 µg kg−1 h−1) and i.m. buprenorphine (0.03 mg kg−1) and monitor it postoperatively. Tables 2 and 3 show anticipated left ventricular parameters in Yorkshire and Göttingen swine with 2-week and 3-month follow-ups, respectively. The appropriate time periods and animals to be used should be considered on the basis of the experimental design and expected endpoints.
▲ CRITICAL STEP Extubation should not be performed until the swine is swallowing and can breathe without ventilator support.
? TROUBLESHOOTING
■ PAUSE POINT After MI creation, the animal may be held until the appropriate post-MI time point for intervention.
For an acute ICM model, interventions may begin days or weeks after MI up until 3 months after MI, when the left ventricular remodeling process has ended. For a chronic ICM model, interventions may begin at 3 months after MI, when remodeling is completed.
-
37|
For follow-up imaging after injection, use noninvasive techniques such as CMR to assess the safety and efficacy of the injections. Conduct serial imaging thereafter until the termination of the study.
-
38|
Upon completion of the study time points or endpoints, follow Steps 1–23 to obtain final measurements. As a nonsurvival surgery, aseptic or sterile techniques and equipment are not needed.
-
39|
Humanely euthanize the animal under deep anesthesia with KCl (≥2 mEq kg−1). Surgically excise the heart, fix and store it for histopathological studies as needed.
TABLE 2.
LV CMR parameters before and 2 weeks after MI in Yorkshire swine.
| Baseline (n = 50) | 2 weeks after MI (n= 50) | Percentage change (%) | |
|---|---|---|---|
| Swine mass (kg) | 35.09 ± 0.76 | 39.19 ±0.71 | 11.85 ±1.86 |
| Heart rate (b.p.m.) | 94.60 ± 2.23 | 91.76 ±1.99 | −1.21 ±2.70 |
| LV mass (g) | 62.69 ± 1.52 | 77.86 ±1.73 | 25.12 ±2.36 |
| EDV (ml) | 72.10 ±1.71 | 96.17 ±2.90 | 34.66 ±4.06 |
| ESV (ml) | 44.92 ±1.13 | 68.69 ±2.56 | 54.32 ±5.46 |
| SV (ml) | 27.83 ±0.93 | 27.45 ±0.88 | 1.72 ±3.58 |
| EF (%) | 37.55 ±0.75 | 29.07 ±0.84 | −22.24 ±1.95 |
| Scar mass (g) | 0 | 13.4 ±0.59 | NA |
| LV percent scar mass (%) | 0 | 17.2 ±0.66 | NA |
SV, stroke volume; NA, not applicable. ALL results are shown as average ± s.e.m.
TABLE 3.
LV CMR parameters before and 90 d after MI in Göttingen swine.
| Baseline (n = 18) | 90 d after MI (n = 18) | Percentage change (%) | |
|---|---|---|---|
| Swine mass (kg) | 24.18 ± 0.45 | 28.83 ± 0.50 | 19.79 ± 2.64 |
| Heart rate (b.p.m.) | 97.75 ± 4.12 | 98.11 ± 4.58 | 0.69 ± 3.11 |
| LV mass (g) | 33.42 ±0.81 | 36.81 ± 0.93 | 10.70 ± 2.72 |
| EDV (ml) | 31.37 ± 1.75 | 46.64 ± 1.95 | 52.57 ± 6.93 |
| ESV (ml) | 16.08 ± 0.97 | 31.64 ± 1.62 | 102.20 ± 10.24 |
| SV (ml) | 15.30 ± 0.90 | 14.98 ± 0.78 | 0.63 ± 5.19 |
| EF (%) | 48.79 ± 1.12 | 32.42 ± 1.40 | −33.61 ± 2.30 |
| Scar mass (g) | 0 | 6.8 ± 0.31 | NA |
| LV percent scar mass (%) | 0 | 17.69 ± 0.65 | NA |
SV, stroke volume; NA, not applicable. All results are shown as average ± s.e.m.
? TROUBLESHOOTING
Troubleshooting advice can be found in Table 4.
TABLE 4.
Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 3 | Difficult intubation | Laryngospasm and fluid accumulation in the pharyngeal region are common when too large of an endotracheal tube (ET) is selected, when too much force is used when passing the ET tube through the vocal cords, or when improper technique is used | Consider anticholinergics, such as atropine 0.02–0.05 mg kg−1, to reduce secretions. Xylocaine spray or lidocaine given topically can alleviate laryngospasm. Smaller ET tubes and proper placement of the laryngoscope can alleviate further complications |
| 17 | Difficulty accessing the artery | The arteries will spasm with manipulation. If the artery is not properly exposed, the fascia surrounding it can cause the needle to miss the vessel lumen | Bath the artery in 2% (wt/vol) lidocaine to dilate it. Be sure to expose the adventia by dissecting away the fascia covering the artery to ensure proper placement of the needle into the vessel lumen. Proper proximal control of the vessel with vessel loops will allow light tension to be placed on the artery to help the needlepoint pierce the vessel (Fig. 6c) |
| 17A(i) | Swine has ipsilateral eyelid ptosis, pupil miosis and facial anhy-drosis after surgery | Cervical ganglia in the swine are located within the carotid sheath and can be easily damaged during carotid sheath surgery | Carefully dissect the carotid artery within the carotid sheath and be mindful of the cranial cervical sympathetic ganglion that are located within the carotid sheath41 |
| 23 | Problems obtaining quality invasive pressure-volume loops | Improper setup or calibration, improper place-ment; anesthesia too deep; animal too cold or hot; animal dehydrated | Follow the manufacturer’s recommendations for proper setup and calibration. Ensure the proper depth of anesthesia and maintain the animal’s hydration and body temperature. Refer to Pacher et al.12 for further information on pressure-volume loop collection and analysis in laboratory animals |
| 29 | Problems identify-ing the first diagonal branch for proper bal-loon placement | Ramus intermedius can be confused with the first diagonal of LAD; the right coronary artery or septal branches can obscure or compli-cate diagonal identification in right anterior oblique 30° projection | Swine have highly variable coronary distribution and structure, but overall they follow standard human anatomy. Start with a right anterior oblique 30° projection of the heart to identify the LAD and associated branches. Other projec-tions can be used to properly identify the origin and orientation of all coronary anatomy and ensure proper PTCA balloon placement distal to the first diagonal of the LAD. Refer to Scanlon et al.42 as well as Alderman and Stadius43 for further information on standardized coronary anatomy descriptions and naming conventions for humans that can be translated into the swine model |
| 30 | Cardiac dysrhythmia | Cardiac dysrhythmia is an expected side effect of LAD occlusion causing myocardial ischemia; however, irreversible arrhythmia can be caused by partial or full occlusion of the first diago-nal, complete occlusion of the left main ostia, too large a PTCA balloon or an overinflated PTCA balloon | Prophylactic lidocaine can be administered by a 2.0–4.0 mg kg−1 bolus followed by a 50 µg kg−1 min−1 infusion to lower the incidence of prema-ture ventricular contractions (PVCs), ventricular tachycardia (VT) and ventricular fibrillation (VF). High-quality chest compressions and rapid defi-brillation should return heart to a sinus rhythm for VT and VF. In cases of VF or VT resistant to defibrillation, consider higher voltage defibrillation and epinephrine, 30 µg kg−1. For bradycardia or asystole, administer chest compressions and epinephrine and/or atropine, 0.05 mg kg−1. If arrhythmia is reversible, check for correct place-ment and ensure that the appropriate-sized PTCA balloon was selected and not overinflated |
| 29, 31 | Coronary vasospasm | The coronary arteries can spasm from excessive manipulation of catheters inside the vessels or during reperfusion | Intracoronary nitroglycerin (200 µg) given via slow intracoronary infusion (diluted in 2 ml of saline) can be given to dilate the artery |
| 36 and Box 2 | Difficult recovery | If the swine has become hypothermic, hypo-tensive or hypovolemic during the procedure, it can lead to prolonged recovery | Maintain swine homeostasis and monitor vital signs closely throughout procedure. Forced warm air blankets are easy and safe to use to help maintain proper temperature. Isotonic fluids should be administered at 10–15 ml kg−1 h−1 to maintain proper hydration throughout the proce-dure. Weaning anesthesia slowly while closing the surgical access site can reduce recovery times, and slowly weaning from ventilator support can quicken the return to spontaneous respiration |
| Boxes 1 and 2 | Ventricular arrhythmia during injections | PVCs during needle engagement or injections are an indication of viable muscle at injec-tion site. However, VT or VF can be caused by excessive delivery volume, too-rapid delivery or improper needle placement and engagement | Ensure adequate volume and rate for injection sites. Avoid coronary vasculature, conduction pathways or the basal aspect of the heart dur-ing injections. Properly plan injection sites to adequately target desired areas of interest |
● TIMING
Steps 1–4, preparation (preanesthesia, anesthesia, cleaning, i.v. access and intubation): 30–60 min
Steps 5–13, CMR: 45–60 min
Steps 14–16, surgical preparation: 15–20 min
Step 17, surgical dissection and vasculature access: 30–45 min
Steps 18–23, invasive pressure-volume measurements: 30–45 min
Steps 24–30, LAD occlusion: 100–300 min
Steps 31 and 32, reperfusion and confirmation: 5–10 min
Steps 33–39, surgical closure and recovery: 60–120 min
Box 1, surgical transepicardial injection of stem cells: 3–4 h
Box 2, percutaneous transendocardial stem cell injections: 45–70 min
ANTICIPATED RESULTS
The protocol described here provides a consistent and reliable model of MI along with techniques for performing intramyocardial injection of stem cells. Notably, the stem cell injection techniques reported can be easily adapted to other preclinical studies of targeted therapies after ML The ischemia-reperfusion model of MI results in similar changes in cardiac structure and function to those seen in humans and closely replicates the clinical interventions for MI.
Fifty female Yorkshire swine weighing 35.0 ± 0.8 kg (weight-based inclusion criteria) and 18 Göttingen swine 45.8 ± 1.64 weeks old (age-based inclusion criteria) underwent the experimental protocol described to create our MI model. In the Yorkshire swine, the LV EF at baseline was 37.55 ± 0.75% (mean ± s.e.m.) and decreased to 29.07 ± 0.84% after MI, resulting in an average 22.24 ± 1.95% mean percent change decrease in EF (Tables 2 and 3). Similarly, the LV EF in Göttingen swine at baseline was 48.79 ± 1.12% and decreased to 32.42 ± 1.40%, resulting in a 33.6 ± 2.30 mean percent change decrease in EF. However, EF may not be the most reliable measure of cardiac performance because there are many hemodynamic factors that influence EF, such as contractility, preload, afterload and lusitropy28. Figure 11 shows characteristic changes in lusitropy as seen after MI creation by pressure-volume loop analysis. Because of this, remodeling has been suggested as a more sensitive noninvasive measurement than EF for endpoints in stem cell clinical trials and other advanced heart failure therapies28,29.
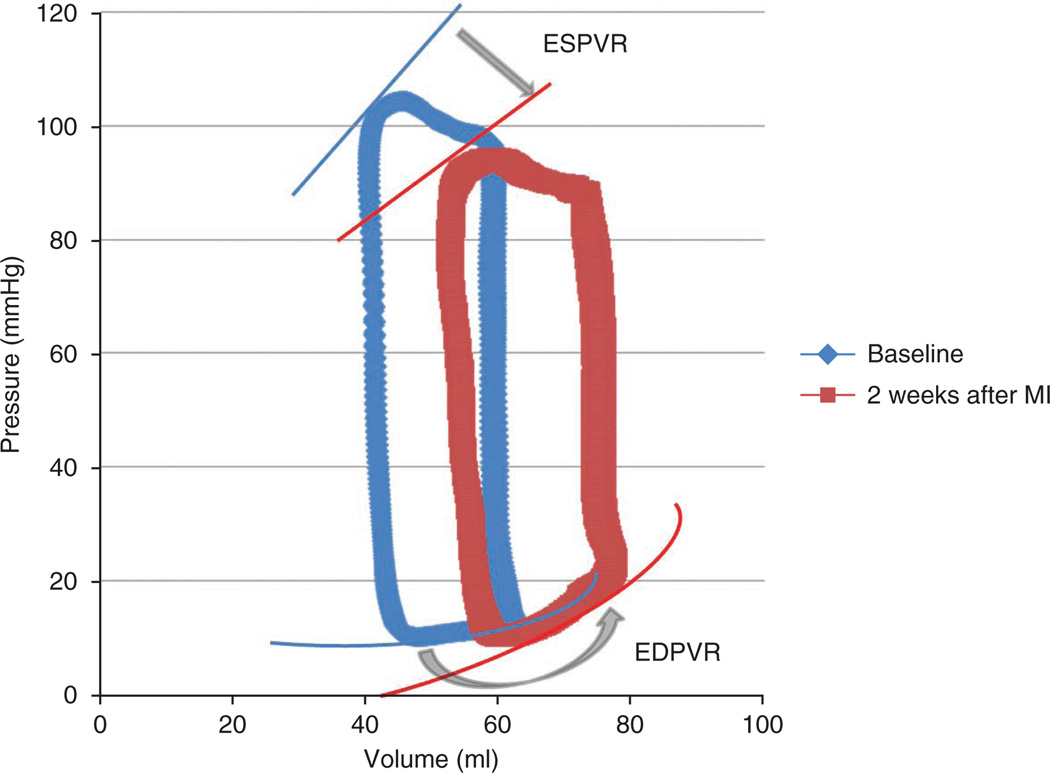
Figure 11.
Representative pressure-volume loops before and after MI model creation. Representative pressure-volume loops demonstrating pathological changes from baseline to 2 weeks after MI. The decrease in the slope of the end systolic pressure-volume relationship (ESPVR) curve (straight arrow) indicates considerable contractility impairment, and the steeper slope of the end diastolic pressure-volume relationship (EDPVR) curve (curved arrow) reflects the increase in ventricular stiffness.
In addition to measuring EF, CMR is a robust modality for analyzing a variety of cardiac performance measurements, such as EDV, ESV, myocardial mass and scar size quantification. EDV before MI in Yorkshire swine was 72.10 ± 1.71 ml and increased to 96.17 ± 2.90 ml, resulting in an average increase of 34.66 ± 4.06% with a 2-week follow-up (Table 2). Similarly, EDV in Göttingen swine was 31.37 ± 1.75 ml at baseline and increased to 46.64 ± 1.95 ml, resulting in a 52.57 ± 6.93% increase with 3-month follow-up (Table 3). Marked LV dilation is evident in serial CMR follow-up studies, as seen from aneurysmal dilations forming in the apical third of the LV from post-MI remodeling (Figs. 3d and 12). The scar mass and scar percentage of LV mass are also two useful parameters for demonstrating the reproducibility of the size of the infarct. In the Yorkshire swine, the scar mass is 13.4 ± 0.59 g and the scar percentage of LV mass was calculated as 17.2 ± 0.66%. In the Göttingen swine, the scar mass was 6.8 ± 0.31 g and the scar percentage of LV mass was calculated as 17.69 ± 0.65%. This model can then be used to inject stem cells using minimally invasive surgical techniques or catheter-based delivery systems. The protocol described should allow investigators to use a highly reproducible model with excellent animal survival.
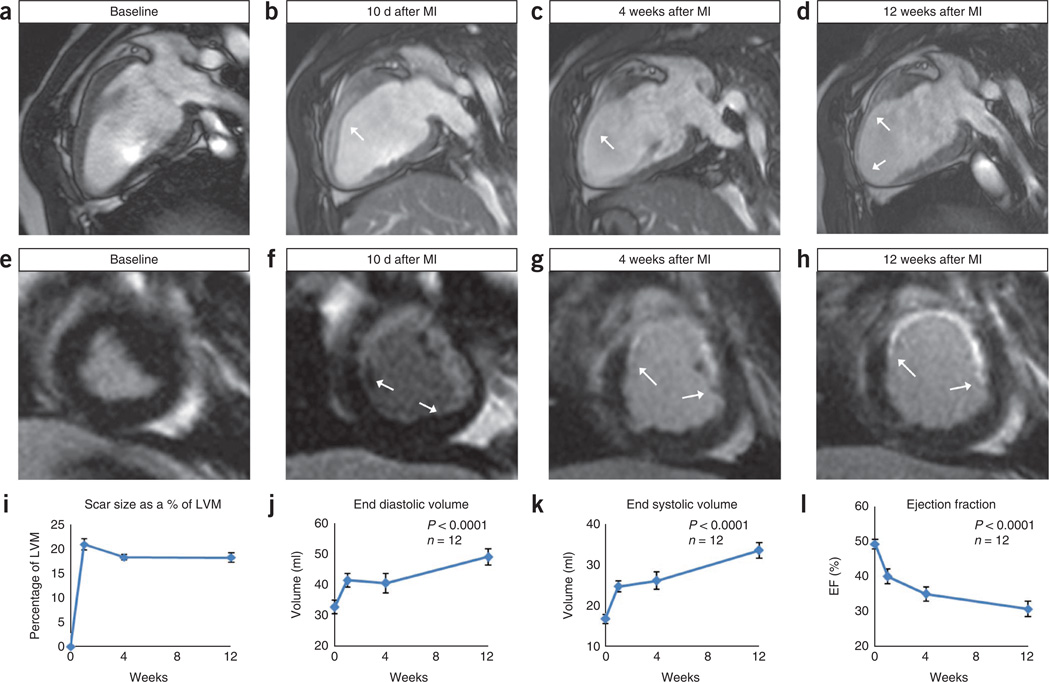
Figure 12.
Ventricular remodeling in the chronic MI model. MRI data for Göttingen swine MI. (a–d) Two-chamber long axis MRI views that illustrate the chamber dilation and wall thinning after MI. (e–h) Short axis LGE-MRI images that show the scar progression and stabilization. Images a and e are baseline images taken before MI. Images b and f are 10 d after MI, images c and g are 4 weeks after MI and images d and h represent 12 weeks after MI. (i–l) Graphic representations in the changes seen in measures of cardiovascular function after MI. Image i demonstrates the initial increase in scar size as a result of the infarction and subsequent stabilization. Images j and k show the steady rise in EDV and ESV over the 12-week duration. Image l shows the decreasing EF over the 12-week duration.
ACKNOWLEDGMENTS
This research is supported by US National Institutes of Health grants R01 HL094849, P20 HL101443, R01 HL084275, R01 HL107110, R01 HL110737 and U54 HL081028 (J.M.H.); and by the University of Miami Miller School of Medicine, Interdisciplinary Stem Cell Institute. The researchers would like to thank R. Gonzalez, D. Valdes, J. Rodriguez and M. Rosado for their technical contributions, and C. Sanina for her artistic contributions.
Footnotes
AUTHOR CONTRIBUTIONS F.C.M. was involved in manuscript conception and design, creation/collection/assembly of data and manuscript writing; K.S.T. contributed to collection/assembly of data and manuscript writing; V.K. and V.Y.S. contributed equally to manuscript writing and editing, and to the creation and collection of data; A.W.H. and A.R.W. contributed to surgical and experimental conception and design, and to manuscript editing; M.M. was involved in manuscript writing; J.M.H., team leader, provided project and manuscript conception and design, financial support, manuscript writing and final approval of the manuscript.
COMPETING FINANCIAL INTERESTS The authors declare competing financial interests: details are available in the online version of the paper.
References
- 1.Heidenreich PA, et al. Forecasting the future of cardiovascular disease in the United States. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, et al. Heart Disease and Stroke Statistics—2011 Update. Circulation. 2011;123:el8–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swindle M. Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques. Taylor & Francis Group; 2007. [Google Scholar]
- 4.Dondelinger RF, et al. Relevant radiological anatomy of the pig as a training model in interventional radiology. Eur. Radiol. 1998;8:1254–1273. doi: 10.1007/s003300050545. [DOI] [PubMed] [Google Scholar]
- 5.Amado LC, et al. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc. Natl. Acad. Sci. USA. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hatzistergos KE, et al. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation/novelty and significance. Circ. Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishikawa K, et al. Development of a preclinical model of ischemic cardiomyopathy in swine. Am. J. Physiol. Heart Circ. Physiol. 2011;301:H530–H537. doi: 10.1152/ajpheart.01103.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mewton N, et al. Determination of the myocardial area at risk with pre-versus post-reperfusion imaging techniques in the pig model. Basic Res. Cardiol. 2011;106:1247–1257. doi: 10.1007/s00395-011-0214-8. [DOI] [PubMed] [Google Scholar]
- 9.Schuleri KH, et al. The adult Göttingen minipig as a model for chronic heart failure after myocardial infarction: focus on cardiovascular imaging and regenerative therapies. Comp. Med. 2008;58:568–579. [PMC free article] [PubMed] [Google Scholar]
- 10.Verdouw PD, van den Doel MA, de ZS, Duncker DJ. Animal models in the study of myocardial ischaemia and ischaemic syndromes. Cardiovasc. Res. 1998;39:121–135. doi: 10.1016/s0008-6363(98)00069-8. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, et al. Functional and bioenergetic consequences of postinfarction left ventricular remodeling in a new porcine model: MRI and 31P–MRS Study. Circulation. 1996;94:1089–1100. doi: 10.1161/01.cir.94.5.1089. [DOI] [PubMed] [Google Scholar]
- 12.Pacher P, Nagayama T, Mukhopadhyay P, Batkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat. Protoc. 2008;3:1422–1434. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohn F, Sharifi AR, Simianer H. Modeling the growth of the Goettingen minipig. J. Animal Sci. 2007;85:84–92. doi: 10.2527/jas.2006-271. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki Y, Lyons JK, Yeung AC, Ikeno F. In vivo porcine model of reperfused myocardial infarction: in situ double staining to measure precise infarct area/area at risk. Cathet. Cardiovasc. Intervent. 2008;71:100–107. doi: 10.1002/ccd.21329. [DOI] [PubMed] [Google Scholar]
- 15.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 16.Williams AR, Hare JM. Mesenchymal stem cells; biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ. Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hou D, et al. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery. Circulation. 2005;112:150–156. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- 18.Perin EC, et al. Comparison of intracoronary and transendocardial delivery of allogeneic mesenchymal cells in a canine model of acute myocardial infarction. J. Mol. Cellular Cardiol. 2008;44:486–495. doi: 10.1016/j.yjmcc.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Freyman T, et al. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur. Heart J. 2006;27:1114–1122. doi: 10.1093/eurheartj/ehi818. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, et al. Stem cells for myocardial repair with use of a transarterial catheter. Circulation. 2009;120:S238–S246. doi: 10.1161/CIRCULATIONAHA.109.885236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trachtenberg B, et al. Rationale and design of the Transendocardial Injection of Autologous Human Cells (bone marrow or mesenchymal) in Chronic Ischemic Left Ventricular Dysfunction and Heart Failure Secondary to Myocardial Infarction (TAC-HFT) trial: a randomized, double-blind, placebo-controlled study of safety and efficacy. Am. Heart J. 2011;161:487–493. doi: 10.1016/j.ahj.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 22.Quevedo HC, et al. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proc. Natl. Acad. Sci. USA. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolli R, et al. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPI0): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Williams AR, et al. Intramyocardial stem cell injection in patients with ischemic cardiomyopathy/novelty and significance. Circ. Res. 2011;108:792–796. doi: 10.1161/CIRCRESAHA.111.242610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang NF, et al. A rodent model of myocardial infarction for testing the efficacy of cells and polymers for myocardial reconstruction. Nat. Protoc. 2006;1:1596–1609. doi: 10.1038/nprot.2006.188. [DOI] [PubMed] [Google Scholar]
- 26.Kornowski R, et al. Electromagnetic guidance for catheter-based transendocardial injection: a platform for intramyocardial angiogenesis therapy: results in normal and ischemic porcine models. J. Am. Coll. Cardiol. 2000;35:1031–1039. doi: 10.1016/s0735-1097(99)00642-7. [DOI] [PubMed] [Google Scholar]
- 27.van Laake LW, et al. Monitoring of cell therapy and assessment of cardiac function using magnetic resonance imaging in a mouse model of myocardial infarction. Nat. Protoc. 2007;2:2551–2567. doi: 10.1038/nprot.2007.371. [DOI] [PubMed] [Google Scholar]
- 28.Suncion VY, Schulman IH, Hare JM. Concise review: the role of clinical trials in deciphering mechanisms of action of cardiac cell-based therapy. Stem Cells Trans. Med. 2012;1:29–35. doi: 10.5966/sctm.2011-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vilahur G, et al. Molecular and cellular mechanisms involved in cardiac remodeling after acute myocardial infarction. J. Mol. Cell. Cardiol. 2011;50:522–533. doi: 10.1016/j.yjmcc.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 30.de la Fuente LM, et al. Transendocardial autologous bone marrow in chronic myocardial infarction using a helical needle catheter: 1-year follow-up in an open-label, nonrandomized, single-center pilot study (the TABMMI study) Am. Heart J. 2007;154:79. doi: 10.1016/j.ahj.2007.04.051. [DOI] [PubMed] [Google Scholar]
- 31.Tomkowiak MT, et al. Targeted transendocardial therapeutic delivery guided by MRI-X-ray image fusion. Catheter. Cardiovasc. Interv. 2011;78:468–478. doi: 10.1002/ccd.22901. [DOI] [PubMed] [Google Scholar]
- 32.Baklanov DV, et al. Live 3D echo guidance of catheter-based endomyocardial injection. Catheter. Cardiovasc. Interv. 2005;65:340–345. doi: 10.1002/ccd.20379. [DOI] [PubMed] [Google Scholar]
- 33.Fuchs S, et al. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J. Am. Coll. Cardiol. 2001;37:1726–1732. doi: 10.1016/s0735-1097(01)01200-1. [DOI] [PubMed] [Google Scholar]
- 34.Perin EC, et al. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107:2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- 35.Dib N, et al. Endoventricular transplantation of allogenic skeletal myoblasts in a porcine model of myocardial infarction. J. Endovasc. Ther. 2002;9:313–319. doi: 10.1177/152660280200900310. [DOI] [PubMed] [Google Scholar]
- 36.Losordo DW, et al. Intramyocardial transplantation of autologous CD34+ stem cells for intractable angina. Circulation. 2007;115:3165–3172. doi: 10.1161/CIRCULATIONAHA.106.687376. [DOI] [PubMed] [Google Scholar]
- 37.van Ramshorst J, et al. Intramyocardial bone marrow cell injection for chronic myocardial ischemia. JAMA. 2009;301:1997–2004. doi: 10.1001/jama.2009.685. [DOI] [PubMed] [Google Scholar]
- 38.Krause K, et al. Percutaneous intramyocardial stem cell injection in patients with acute myocardial infarction: first-in-man study. Heart. 2009;95:1145–1152. doi: 10.1136/hrt.2008.155077. [DOI] [PubMed] [Google Scholar]
- 39.Smits PC, et al. Catheter-based intramyocardial injection of autologous skeletal myoblasts as a primary treatment of ischemic heart failure: clinical experience with six-month follow-up. J. Am. Coll. Cardiol. 2003;42:2063–2069. doi: 10.1016/j.jacc.2003.06.017. [DOI] [PubMed] [Google Scholar]
- 40.Dib N, et al. Safety and feasibility of autologous myoblast transplantation in patients With ischemic cardiomyopathy. Circulation. 2005;112:1748–1755. doi: 10.1161/CIRCULATIONAHA.105.547810. [DOI] [PubMed] [Google Scholar]
- 41.Ding P, et al. Horner syndrome after carotid sheath surgery in a pig: anatomic study of cervical sympathetic chain. Comp. Med. 2011;61:453–456. [PMC free article] [PubMed] [Google Scholar]
- 42.Scanlon PJ, et al. ACC/AHA guidelines for coronary angiography: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Coronary Angiography) developed in collaboration with the Society for Cardiac Angiography and Interventions. J. Am. Coll. Cardiol. 1999;33:1756–1824. doi: 10.1016/s0735-1097(99)00126-6. [DOI] [PubMed] [Google Scholar]
- 43.Alderman EL, Stadius M. The angiographic definitions of the bypass angioplasty revascularization investigation. Coron. Artery Dis. 1992;3:1189–1207. [Google Scholar]