While the fibronectin type 3-domain containing protein 5 (FNDC5) gene was first discovered over a decade ago (Ferrer-Martínez et al. 2002), new claims were recently attributed to the physiological function of FNDC5. In a paper published in Nature, Boström et al. (2012) reported that peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α)-mediated induction of muscle FNDC5 expression triggers a sequence of events leading to its trans-membrane cleavage and secretion of a polypeptide hormone causing the ‘browning’ of fat. This led the researchers to name the secreted polypeptide irisin, after Iris, the Greek messenger goddess. The phenotype of animals in which FDNC5 was over-expressed was resilience to obesity and insulin resistance. Finally, the authors reported increased circulating irisin in mice and humans exposed to endurance exercise, thereby implicating irisin in regulating some of the well-defined health benefits of exercise (in addition to its potential as a therapeutic target to treat metabolic disorders).
Any effect of FNDC5/irisin in regulating the health benefits of exercise is likely to be contingent upon its induction by exercise. Nonetheless, a comprehensive study capturing the effects of exercise
(including different modes) on facets of FNDC5 gene expression and serum concentrations of irisin had not yet been carried out. In the current issue of The Journal of Physiology, Pekkala et al. (2013) carried out investigations to address this gap (see Fig. 1 for a summary). Firstly they studied the effects of acute and chronic aerobic or resistance exercise and found that despite the anticipated robust upregulation of PGC-1α mRNA expression this was not paralleled by FNDC5 mRNA upregulation, the expression of which increased only moderately in response to resistance (but not endurance) exercise. These data point to a poor relationship between PGC-1α, the supposed driving force behind FNDC5 induction, and FNDC5 expression. Secondly, Pekkala et al. reported the abundance of muscle PGC-1α and FNDC5 mRNA, as well as circulating irisin, to be unaltered by chronic exercise, whether endurance, resistance or a combination of endurance and resistance-type exercise training. Therefore, allied to acute expression of FNDC5, this precludes any major impact of chronic exercise on FDNC5 mRNA or any sustained increase in serum irisin. The final ‘nail in the coffin’ was delivered with the authors finding no relationships between indices of metabolic health (obesity, fasting glucose/insulin) and FDNC5 mRNA.
Figure 1.
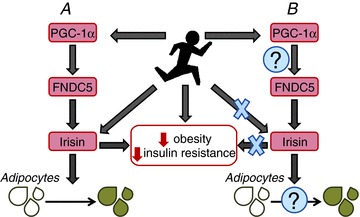
Purported actions of FDCN5/irisin (A) and the findings of Pekkala et al. (B)
Although the field of FNDC5/irisin is in its infancy, as with this study from Pekkala et al., on balance its function as a key player in regulating adaptation/health benefits of exercise has shaky foundations. For instance, large-scale genomics datasets from human muscle questioned induction of muscle FNDC5 mRNA as a common response to exercise (Timmons et al. 2012). In this latter study, upregulation of FNDC5 mRNA by exercise was at best the exception, rather than the rule. In terms of serum irisin, while moderate increases have been reported in some individuals, these were found acutely rather than chronically (Huh et al. 2012), in contrast to the original study. Outside the realm of exercise, on balance the results may be only moderately more encouraging. For example, while muscle FNDC5 mRNA and circulating irisin were shown to be down-regulated in obesity and diabetes (Moreno-Navarrete et al. 2013), these findings are balanced by studies showing the opposite, e.g. down-regulation of circulating irisin after bariatric surgery (Huh et al. 2012). Finally it is important to highlight the possible notion of ‘irisin resistance’ (as with leptin) to explain positive relationships between irisin and metabolic dysregulation.
This leaves us with a conundrum: why are there such conflicting results and where does this leave FNDC5/irisin research? First of all, it is important that methodological issues are ironed out. As pointed out in the current article by Pekkala et al., serum analysis of irisin may be affected by differences in FNDC5/irisin glycosylation. In a further demonstration of these issues, Erickson (2013) pointed out several problems, centring on a general lack of proper validation of available antibodies to detect the secreted fraction of irisin. Methodological matters such as this are not trivial and until these aspects are resolved, such studies relying on these measures should be subject to careful scrutiny. So where do we go next? It is important at this stage to distinguish the physiological role of FNDC5/irisin from its potential as a therapeutic target. The physiological role of FNDC5/irisin in mediating responses to exercise is without question in doubt, as perhaps is any strong evidence of altered FNDC5/irisin regulation in clinical scenarios of metabolic dysfunction (e.g. obesity and diabetes). Nonetheless, it is important to state that, to date, there are no published studies (beyond the original study by Bostrom et al. 2012) aimed at determining the potential therapeutic effects of targeting irisin in human valid models of pathophysiology. Until these are established, it is perhaps a little early to ‘shoot the messenger…’.
References
- Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson H. Adipocyte. 2013;2:1–5. doi: 10.4161/adip.26082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Martínez A, Ruiz-Lozano P, Chien KR. Dev Dyn. 2002;224:154–167. doi: 10.1002/dvdy.10099. [DOI] [PubMed] [Google Scholar]
- Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, Mantzoros CS. Metabolism. 2012;61:1725–1738. doi: 10.1016/j.metabol.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Navarrete JM, Ortega F, Serrano M, Guerra E, Pardo G, Tinahones F, Ricart W, Fernández-Real JM. J Clin Endocrinol Metab. 2013;98:E769–E778. doi: 10.1210/jc.2012-2749. [DOI] [PubMed] [Google Scholar]
- Pekkala S, Wiklund P, Hulmi JJ, Ahtiainen JP, Horttanainen M, Pöllänen E, Mäkelä KA, Kainulainen H, Häkkinen K, Nyman K, Alen M, Herzig K-H, Cheng S. J Physiol. 2013;591:5393–5400. doi: 10.1113/jphysiol.2013.263707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons JA, Baar K, Davidsen PK, Atherton PJ. Nature. 2012;488:E9–E10. doi: 10.1038/nature11364. [DOI] [PubMed] [Google Scholar]


