Abstract
To evaluate the impact of fallout from the Fukushima Daiichi Nuclear Power Plant accident on organisms, this study compared the morphology and viability of gall-forming aphids between the Fukushima population and control populations from noncontaminated areas. This study, in particular, focused on the morphology of first-instar gall formers derived from the first sexual reproduction after the accident. Of 164 first instars from Tetraneura sorini galls collected 32 km from Fukushima Daiichi in spring 2012, 13.2% exhibited morphological abnormalities, including four conspicuously malformed individuals (2.4%). In contrast, in seven control areas, first instars with abnormal morphology accounted for 0.0–5.1% (on average, 3.8%). The proportions of abnormalities and mortality were significantly higher in Fukushima than in the control areas. Similarly, of 134 first instars from T. nigriabdominalis galls, 5.9% exhibited morphological abnormalities, with one highly malformed individual. However, of 543 second-generation larvae produced in T. sorini galls, only 0.37% had abnormalities, suggesting that abnormalities found in the first generation were not inherited by the next generation. Although investigation is limited to one study site, this result suggests that radioactive contamination had deleterious effects on embryogenesis in eggs deposited on the bark surface, but a negligible influence on the second generation produced in closed galls. Furthermore, analysis of both species samples collected in spring 2013 indicated that the viability and healthiness of the aphids were significantly improved compared to those in the 2012 samples. Thus, the results of this study suggest the possibility that a reduced level of radiation and/or selection for radiation tolerance may have led to the improved viability and healthiness of the Fukushima population.
Keywords: malformation, morphology, radiation, Tetraneura, viability
Introduction
There is increasing interest in the effects of radionuclides released from nuclear power plant accidents on the biodiversity and genetics of wildlife (Møller and Mousseau 2006; Yablokov et al. 2009; Mousseau and Møller 2012a; Møller et al. 2013). Even 20 years after the 1986 Chernobyl nuclear disaster, bird and arthropod species abundances are negatively correlated with the level of radioactivity at ground level (Møller and Mousseau 2007, 2009). It is reasonable to suppose that the largest impact on biodiversity occurs immediately after an accident through the large amount of fallout. Alternatively, it is also possible that mutations accumulate over time and express deleterious effects several years after the accident in contaminated areas (Møller and Mousseau 2006). At present, we have limited knowledge about the effects of radiation on the wildlife. In the case of the Chernobyl nuclear disaster, the initial impacts on the abundance, phenotypes, and genetics of animal and plant species were not fully assessed (Yablokov et al. 2009; Møller et al. 2013). The Fukushima Daiichi Nuclear Power Plant exploded in mid-March 2011 and released 11,347 PBq of radionuclides, including 160 PBq of iodine-131, 15 PBq of cesium-137, and 0.14 PBq of strontium-90 into the atmosphere (Nuclear Emergency Response Headquarters, Government of Japan. 2011). An area northwest of the power plant was covered with fallout and highly contaminated by radioactive substances (Nuclear Regulation Authority, 2011a). For the Fukushima Daiichi accident, a vast area of contamination, including several landscapes, can be surveyed without legal restrictions, except within a 20-km radius of the power plant, and thus, there are opportunities to detect initial impacts of radionuclides on individual species and the ecosystem. Therefore, ecological and genetic investigations in the Fukushima area are an urgent issue.
Several researchers and governmental agencies have started evaluating the levels of radionuclides accumulated in wild animals and plants in the Fukushima area (e.g., Nakanishi and Tanoi 2013). Ishida (2013) started a long-term survey on the abundance of birds and mammals and confirmed a high level of radioactive contamination in bird feathers in the first year. Møller et al. (2012, 2013) conducted field censuses of birds and several insects and reported a significant negative correlation between densities and background radiation level. Hiyama et al. (2012) reported that the lycaenid butterfly Zizeeria maha that emerged in 2011 in contaminated areas exhibited morphological abnormalities and that these abnormalities were inherited by the next generation, which exhibited a higher proportion of abnormalities than the first generation. Hiyama et al.'s report is the first example of phenotypic abnormalities in the Fukushima area, so it is difficult to understand whether the occurrence of phenotypic abnormalities is common to the populations of other animals and plants in the Fukushima area or not. Thus, phenotypic and genetic studies of other systems might be encouraged.
In attempts to clarify the effects of radiation on mammals, birds, and arthropods, phenotypic abnormalities have been evaluated based on adults (Møller 1993, 2002; Møller and Mousseau 2006; Møller et al. 2007; Yablokov et al. 2009) except in a few studies (Williams et al. 2001). However, the effects of radiation emerge most strongly in hatchlings or newborns, resulting in mortality and phenotypic abnormalities, as shown in irradiation experiments (Elbadry 1965; Tilton et al. 1966; McGregor and Newcombe 1968; Vereecke and Pelerents 1969; Matranga et al. 2010) as well as field studies in Chernobyl (Krivolutzkii and Pokarzhevskii 1992; Krivolutsky 1995). When exposed to high radiation, hatchlings or newborns often fail to grow, with few individuals reaching adulthood. Therefore, it is possible to overlook the emergence of abnormalities if the research focus is limited to adult phenotypes only (Møller 1997). The present study focuses on gall-forming aphids and reports the extent to which morphological abnormalities emerge in first-instar aphids derived from the first sexual reproduction after the Fukushima Daiichi nuclear accident.
Aphids are tiny, soft-bodied insects that reproduce asexually from spring to autumn, with many consecutive generations (Dixon 1998). The use of aphids for a bioassay of radiation appears to have three merits. First, aphids always maintain high reproduction rates, with numerous developing embryos in their abdomens (Dixon 1998). Reports indicate that rapidly developing embryos are readily affected by radiation (Russell and Russell 1952; Vereecke and Pelerents 1969; Cerutti 1974). After the fallout that occurred in mid-March 2011, aphids continued to reproduce asexually under radioactive contamination until autumn, so that aphid embryos likely were damaged by radiation. Genetic damages, if any, would be inherited and accumulated through clonal lines. Through sexual reproduction in autumn 2011, damaged genes may have been genetically recombined to the next generation, leading to a release of a large phenotypic variation (Lynch and Gabriel 1983). Second, as hemimetabolous insects, aphid first instars possess several metrical characters, which would facilitate the detection of morphological abnormalities. So far, aphid first instars have been reported to exhibit phenological and morphological traits that well reflect genetic and environmental variation (Akimoto 1988, 1990, 1998, 2006; Komatsu and Akimoto 1995; Akimoto and Yamaguchi 1997; Komazaki 1998). Third, in gall-forming aphids, first-instar larvae induce leaf galls, in which they develop and produce offspring (Blackman and Eastop 1994). Thus, using aphid species that induce closed galls, the performance and mortality of gall formers can be evaluated without the disturbance of predators (Whitham 1978; Akimoto and Yamaguchi 1994; Wool 2004; Aoyama et al. 2012). If gall formers die in the gall, we can examine the morphology, and if they show some deformation, we can exclude the possibility of predation as a cause (Ballengée and Sessions 2009; Bowerman et al. 2010). The presence of malformed first instars in gall-forming aphids has been reported previously (Akimoto 1985a).
In the present study, I collected closed galls of Tetraneura aphid species from leaves of Ulmus davidiana var. japonica at a point 32 km from the Fukushima Daiichi Nuclear Power Plant and inspected the morphology of the gall formers at the first stadium. I report frequent occurrences of morphological abnormalities and mortalities in two gall-forming aphids in the Fukushima area by comparing them with other populations in noncontaminated areas. Although investigation is limited to one site in Fukushima, the results from two species corroborate the possibility of an initial, detrimental impact of fallout.
Materials and Methods
Life cycle of Tetraneura aphids
Tetraneura species are host-alternating and associated with two kinds of plants: elm trees Ulmus spp. as the primary host and gramineous plants as the secondary host (Blackman and Eastop 1994; Wool 2004). In spring, after hatching from overwintered eggs on the trunks of elm trees, first instars move to developing new leaves and start gall formation. The first instar (gall former) settles on the underside of a new leaf and, using its stylet, stimulates one point of the leaf. The stimulated plant tissues rapidly proliferate upward, encasing the gall former inside, to form a closed, hollow gall. During gall formation, the gall formers continuously stimulate the tissues without moving. The gall former develops inside the gall and parthenogenetically produces second-generation larvae (Fig. S1). The second generations develop into winged adults, which emerge from the cracked galls in mid-June and migrate to gramineous plants to produce third-generation larvae. These larvae move to the roots, where they develop into wingless adults. On the roots, several generations of wingless adults repeat reproduction from early summer until autumn, when winged adults emerge and return to elm trees. Winged adults produce wingless males and sexual females on the trunks of elm trees. After copulation, the sexual female oviposits a single egg in a crevice of the bark. Eggs overwinter until the next spring.
Gall collection in Fukushima
I collected a total of 284 galls of Tetraneura aphid species from 1 U. davidiana var. japonica tree at Kawamata Town, Fukushima Prefecture (37o 35′ N, 140 o 42′ E) on 3 June 2012. Leaves with galls were haphazardly collected from the tree, and all galls on the collected leaves were preserved in 80% ethanol. Galls of Tetraneura species are bean or horn shaped, a maximum of 25 mm in height, and formed in various positions on a leaf. On one leaf, 10 or more galls of two or more species are sometimes formed. The sample aphids were collected on roadsides that are not included in special reservation areas, so that no specific permissions were required for the collections. The aphid species collected are not designated as endangered or protected species.
After fixation, all galls were dissected under a binocular microscope. When the gall former gave birth to larvae, the adult and the cast-off skin of the first-instar gall former were collected from the gall and mounted on a glass slide. In the Eriosomatinae, the cast-off skin of the first-instar gall former is strongly sclerotized and characterized by species-specific features (Aoki 1975; Akimoto 1983, 1985b). The second-generation larvae produced in a gall were preserved in a vial of 80% ethanol, and all the larvae were later inspected for morphological abnormality. When the gall former was immature, only the cast-off skin of the first instar was mounted on a glass slide. When the gall former died in the gall for some reason, it was mounted for morphological examination.
Before mounting, first-instars' cast-off skins and adult gall formers were kept in 10% KOH for 1 or 2 days. The exoskeleton of the adult or first instar was rinsed in 80% ethanol with a small amount of acetic acid and dehydrated in carboxylol (phenol:xylol = 4:1) for 15 min and then in xylol for 10 min. The dehydrated samples were mounted on a glass slide with Canada balsam. All of the mounted specimens are preserved in the Laboratory of Systematic Entomology, Graduate School of Agriculture, Hokkaido University. No permits were required for the described study, which complied with all relevant regulations.
Gall collection in other areas
Prior to the Fukushima Daiichi nuclear accident, no specimens had been collected in or around the Fukushima area. However, numerous specimens of Tetraneura species were collected before the accident in several localities of Japan and are preserved in the Laboratory of Systematic Entomology, Graduate School of Agriculture, Hokkaido University (Table 1). These specimens were used for comparison with the Fukushima specimens. In 2012, I collected Tetraneura aphids in Kashiwa, Chiba Prefecture (35°53′N, 139°56′E), Sapporo, Hokkaido (43°4′N, 141°20′E), and Iwamizawa, Hokkaido (43°11′N, 141°46′E), as control specimens. The sample aphids were collected on public parks and a university campus that are not included in special reservation areas, so that no specific permissions were required for the collections.
Table 1.
Collection data of Tetraneura samples used for the inspection of morphological abnormalities. Galls or larval gall formers of Tetraneura sorini or T. nigriabdominalis were collected from Ulmus davidiana or U. parvifolia
| Species | Locality (Japan) | Year | Host tree | Stage | No. |
|---|---|---|---|---|---|
| T. sorini | Bibai, Hokkaido | 5 June 1981 | U. davidiana | Larva | 334 |
| T. sorini | Bibai, Hokkaido | 22 May 1987 | U. davidiana | Larva | 385 |
| T. sorini | Iwamizawa, Hokkaido | 8 June 1997 | U. davidiana | Gall | 108 |
| T. sorini | Iwamizawa, Hokkaido | 9 June 2012 | U. davidiana | Gall | 290 |
| T. sorini | Sapporo, Hokkaido | 22 May 1989 | U. davidiana | Larva | 99 |
| T. sorini | Kawamata, Fukushima | 3 June 2012 | U. davidiana | Gall | 167 |
| T. sorini | Kawamata, Fukushima | 21 May 2013 | U. davidiana | Gall | 50 |
| T. sorini | Kashiwa, Chiba | 30 April 2012 | U. davidiana | Larva | 243 |
| T. sorini | Ukiha, Fukuoka | 8 April 1985 | U. parvifolia | Larva | 100 |
| T. nigriabdominalis | Bibai, Hokkaido | 5 June 1981 | U. davidiana | Larva | 112 |
| T. nigriabdominalis | Sapporo, Hokkaido | 5 June 2012 | U. davidiana | Gall | 224 |
| T. nigriabdominalis | Kawamata, Fukushima | 3 June 2012 | U. davidiana | Gall | 136 |
| T. nigriabdominalis | Kawamata, Fukushima | 21 May 2013 | U. davidiana | Gall | 347 |
| T. nigriabdominalis | Kashiwa, Chiba | 30 April 2012 | U. davidiana | Larva | 564 |
| T. nigriabdominalis | Kyoto, Kyoto | 25 April 1981 | U. davidiana | Larva | 454 |
| T. nigriabdominalis | Tsu, Mie | 16 May 1991 | U. davidiana | Gall | 95 |
| U. parvifolia | Gall | 47 | |||
| T. nigriabdominalis | Ukiha, Fukuoka | 8 April 1985 | U. parvifolia | Larva | 181 |
Gall collection, the examination of the gall contents, and the preparation of slide-mounted specimens were conducted as in the Fukushima samples. Where first-instar larvae were sampled, I haphazardly collected developing host buds infested with the first instars and preserved them in vials of 80% ethanol. Later, first instars were removed from the buds and mounted on glass slides.
Morphological abnormalities and mortality
All mounted specimens of two common species, T. sorini and T. nigriabdominalis, were examined under a microscope (Axiophoto, Carl Zeiss, Oberkochen, Germany) for the morphological abnormalities and mortality. Because first-instar gall formers from all the samples were available for the microscopic inspection, the present study focused specifically on comparisons of first-instar morphology; 167 T. sorini first instars and 136 T. nigriabdominalis first instars from the Fukushima area were compared with 1559 T. sorini first instars from seven control areas and 1677 T. nigriabdominalis first instars from six control areas, respectively. For all the mounted specimens, including dead individuals, I inspected the morphology of legs, antennae, rostra, and tergites. The first instars of T. sorini and T. nigriabdominalis were approximately 0.90 ± 0.099 (SD) mm and 0.72 ± 0.028 (SD) mm in body length, respectively.
Several morphological abnormalities, ranging from slight variants to serious deformations, were found among first-instar gall formers. The abnormalities were classified into three categories, and the proportion of each category was calculated for samples in each area. The levels of abnormalities were determined irrespective of mortality; that is, dead first instars may be considered normal morphologically, while first instars in any of three abnormality categories may molt to the next larval stadium. Categorization of abnormal first instars was conducted for demonstrating the peculiarity of the Fukushima population, but in statistical tests, first instars with any abnormal morphology were pooled. Mortality was assessed for each gall former at the time galls were collected.
Comparison of growth performance
To examine whether growth performance in the Fukushima samples was reduced or not, the growth rate of gall formers was compared between the Fukushima and Iwamizawa samples collected in early June 2012. Based on the cast-off skin of the first instar and the adult collected from each gall, I measured the lengths of larval and adult hind femurs of the same individual using an eyepiece micrometer installed on the microscope. The regression line of adult femur length on larval femur length demonstrates the mode of growth in each population. A steeper slope of the regression line indicates higher growth performance. Thus, ANCOVA (Sokal and Rohlf 1995) was applied to detect the difference in the regression slope or the intercept between the two populations. Galls from Fukushima were collected from one host tree; galls from Iwamizawa were collected from three host trees and pooled in the analysis.
Comparison between the 2012 and 2013 samples from Fukushima
To evaluate temporal changes in the viability and healthiness of the Fukushima population, Tetraneura galls were again collected from the same elm tree from which the 2012 sample was collected, on 21 May 2013. For the 2013 samples, 50 and 347 gall formers of T. sorini and T. nigriabdominalis, respectively, were mounted on glass slides. The proportions of morphological abnormality and mortality were evaluated based on the gall formers at the first to the last stadium. By sampling galls from the same tree in both years, it is possible to remove the effects of plant factors on the morphology and viability of gall formers.
Logistic regression was used to test whether the proportions of morphological abnormality and mortality changed or not between the 2 years. In the model, the proportions of healthy, abnormal, and dead gall formers were treated as the dependent variable, while species (T. sorini or T. nigriabdominalis) and years (2012 or 2013) were specified as independent variables. The interaction between years and species was also included in the model.
Measurements of radioactivity
Radioactivity levels were measured at the time of gall collection using Geiger counters (SOEKS-01M [SOEKS, Russia] and PA-1000 Radi [Horiba, Kyoto]). Measurement was conducted at a height of 1 m and at the ground level near the tree from which galls were collected. For the other areas in 2012 (Kashiwa, Sapporo, and Iwamizawa), I used the published data from the document “Monitoring information of environmental radioactivity level” by the Nuclear Regulation Authority, Government of Japan (http://radioactivity.nsr.go.jp/map/ja/).
Results
Categorization of abnormalities
From the 284 galls collected in Fukushima, four species of gall-forming Tetraneura were found, including 162 T. sorini, 118 T. nigriabdominalis, 3 T. radicicola, and 1 undescribed species. Galls occasionally contained two or more gall formers inside (of the T. sorini and T. nigriabdominalis galls, 3.7% and 9.3%, respectively). From the T. sorini galls, 167 gall formers were found, and 76 (71.7% of gall formers that attained at least the second instar) gave birth to larvae. From the T. nigriabdominalis galls, 136 gall formers were found, and only 1 (2.2%) gave birth to larvae.
I detected several morphological abnormalities in the first-instar gall formers of the two species both from Fukushima and from the other areas. Slight abnormalities (level 1) included the atrophy or bending of 1 leg (Fig. S2A,B), small ganglia on the ventral surface (Fig. S2C), partial fusion of adjacent abdominal tergites (Fig. S2D), and tissue necrosis in 1 leg or antenna (Fig. S3A,B). Tissue necrosis that occurred inside an appendage resulted in the partial or complete loss of that appendage after molting. Level 2 abnormalities included the atrophy or bending of 2 legs and tissue necrosis in two or more appendages. I categorized the complete or partial loss of one appendage of first instars as level 2 (Fig. S3C,D). Such first instars appear to have already lost an appendage at their hatching. Intense level 3 abnormalities included the loss of two or more appendages, the loss of 1 leg and atrophy of another leg, the appearance of new features, and conspicuous asymmetry in bilateral characters. These abnormalities were common in the two species.
At the time of gall collection in Fukushima on 3 June 2012, the radiation dose was 4.0 μSv/h at 1 m and 6.0 μSv/h at ground level. In contrast, the radiation doses in Kashiwa, Sapporo, and Iwamizawa were 0.117, 0.038, and 0.040 μSv/h, respectively.
Abnormalities and mortality in T. sorini
Of the 167 T. sorini first instars collected in Fukushima, 13.2% exhibited any of the abnormalities (Fig. 1). Of the seven control areas, the samples from Ukiha, Fukuoka Prefecture, exhibited no abnormalities. Of the samples from Sapporo in 1990, 5.1% exhibited level-1 abnormalities, and this value was the maximum found in the control areas (Fig. 1). The incidence of morphological abnormalities in Fukushima was significantly higher than that in other areas (Table 2; on average, 3.8% for other areas). The Fukushima samples were peculiar because they contained four level-3 malformed individuals (Fig. 1). One of the individuals had a bifurcated abdomen with 2 caudae (Fig. 2). Despite this intense malformation, this individual molted successfully four times and attained adulthood with two caudae. Although larviposition was not confirmed, this individual contained mature embryos in its abdomen. The second example (Fig. 3A) was found dead in an incipient gall. This individual had an empty, distended abdomen and a projection on the joint of the mid-femur and tibia; this projection bore several setae, suggesting that the projection was a homologous leg structure. The third example (Fig. 3B) was also found dead in a gall and bore a large protuberance on the abdomen. In addition, it had another solid protuberance at the base of the mid-femur. The last example (Fig. 4A), which was also found dead in a gall, had 1 hind leg with apical segments missing due to necrosis and another hind leg that was atrophied.
Figure 1.
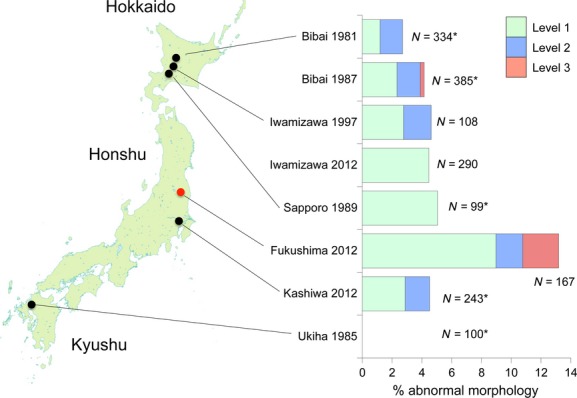
Percentage abnormal morphologies in Tetraneura sorini first-instar gall formers from eight populations. Asterisks in sample sizes indicate that first instars were collected from buds, whereas nonasterisked figures indicate that first-instars' cast-off skins were collected from galls. Level 1, slight abnormalities included the atrophy or bending of one leg (Fig. S2A,B), small ganglia on the ventral surface (Fig. S2C), partial fusion of adjacent abdominal tergites (Fig. S2D), and tissue necrosis in one leg or antenna (Fig. S3A,B). Level 2, abnormalities included the atrophy or bending of two legs and tissue necrosis in two or more appendages. I categorized the complete or partial loss of one appendage of first instars as level 2 (Fig. S3C,D). Level 3, intense abnormalities included the loss of two or more appendages, the loss of one leg and atrophy of another leg, the appearance of new features, and conspicuous asymmetry in bilateral characters.
Table 2.
Comparison of the proportion of morphological abnormalities between the Fukushima population and control populations. P-values from log-likelihood ratio tests were adjusted for multiple comparisons using the sequential Bonferroni method
| Comparison of populations | χ2 | P | |
|---|---|---|---|
| Fukushima 2012 | Bibai 1981 | 19.61 | <0.0001* |
| Fukushima 2012 | Bibai 1987 | 13.44 | 0.0002* |
| Fukushima 2012 | Iwamizawa 1979 | 5.41 | 0.0148* |
| Fukushima 2012 | Iwamizawa 2012 | 10.81 | 0.001* |
| Fukushima 2012 | Sapporo 1989 | 4.94 | 0.0262* |
| Fukushima 2012 | Kashiwa 2012 | 9.83 | 0.0017* |
| Fukushima 2012 | Ukiha 1985 | 21.82 | <0.0001* |
Significant difference at P = 0.05 after the application of the sequential Bonferroni method.
Figure 2.
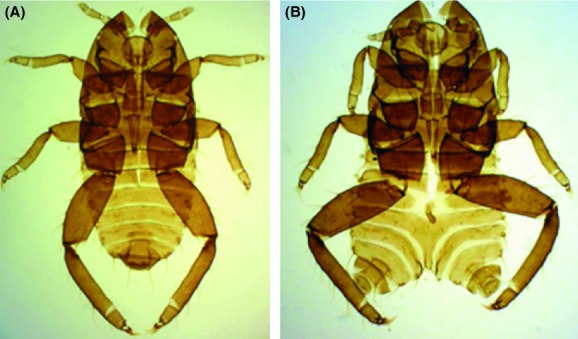
Cast-off skins of first-instar gall formers of T. sorini from Fukushima. (A) normal morphology, (B) level-3 malformation with a bifurcated abdomen.
Figure 3.

Two level-3 malformed T. sorini first instars from Fukushima. (A) a dead first instar having a distended abdomen (arrowed) and a projection on the joint of the mid-femur and tibia (arrowed). (B) projection having a seta (arrowed), (C) a dead first instar having protuberances on the abdomen (arrowed) and the base of a mid-leg (arrowed). The mid-leg was transposed from the original position. (D) protuberance on the base of the trochanter.
Figure 4.
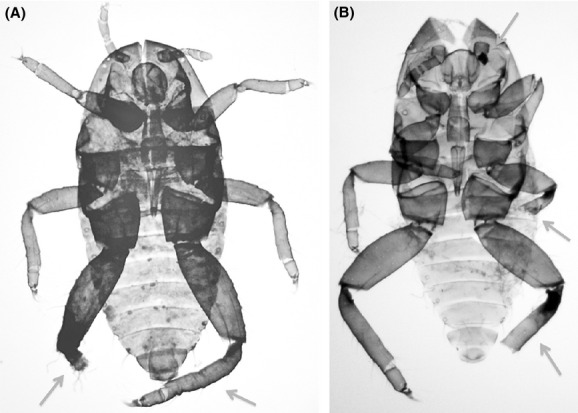
Two level-3 malformed first instars from Fukushima. (A) dead T. sorini first instar with a hind tibia missing due to tissue necrosis (arrowed) and the other hind leg atrophied (arrowed). (B) cast-off skin of a T. nigriabdominalis first instar with a hind leg, a mid-leg, and an antenna missing (arrowed).
In the areas other than Fukushima, only 1 T. sorini first instar exhibited a conspicuous level-3 malformation. This first instar, collected in Bibai City in 1987, had a trifurcated tarsus on 1 hind leg that was thicker than the other (Fig. 5). Therefore, except for Fukushima, a level-3 malformation was found only once in 1559 individuals (0.064%).
Figure 5.
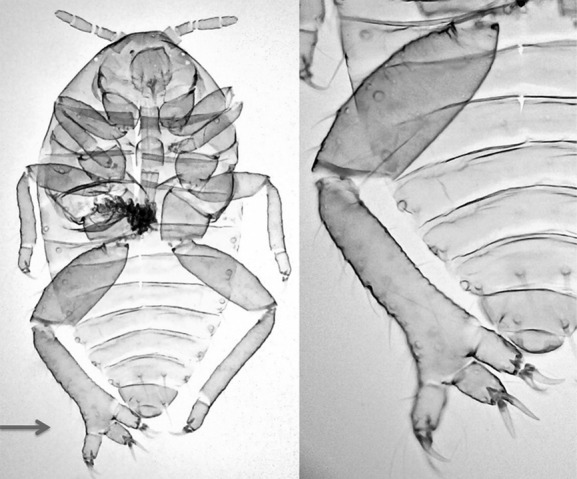
T. sorini first-instar gall former with a trifurcated tarsus (arrowed) collected from Bibai, Hokkaido in 1987.
Mortality in the gall formers in the Fukushima population was compared with that in the Iwamizawa populations collected in 1997 and 2012 (Fig. S4A). Mortality in Fukushima was 14.4%, which was significantly higher than the mortality in Iwamizawa (1.9%–3.2%) (log-likelihood ratio test, for 1997 Iwamizawa, χ2 = 14.7, P = 0.0001; for 2012 Iwamizawa, χ2 = 19.0, P < 0.0001). To examine whether this difference was due to host suitability or not, the growth rate of gall formers was compared between the Fukushima and Iwamizawa samples collected in 2012. ANCOVA for the two populations indicated that there were no significant differences in the slopes or intercepts of the regression lines (Fig. S5; for area*larval femur length, t = −1.45, P = 0.150; for area, t = 0.78, P = 0.436; for larval femur length, t = 11.91, P < 0.0001). This suggests that despite the difference in mortality, the growth performance of the gall formers that survived the larval stages did not vary between the two populations.
I inspected a total of 543 second-generation larvae deposited in 76 galls collected in Fukushima. Only 2 larvae (0.37%) from 1 gall (that is, they are clonal) were found to have aberrant morphology; they both lacked 1 antenna completely, whereas the other larvae in the same gall were normal. The gall former in that gall did not exhibit abnormality at the first stadium or adulthood.
Abnormalities in T. nigriabdominalis and T. radicicola
The first instars of T. nigriabdominalis collected in Fukushima exhibited a higher proportion of abnormalities (5.9%) than those in the six control areas (Fig. 6). However, in T. nigriabdominalis, the absolute number of abnormal individuals was small, so a significant difference was limited among the populations after the sequential Bonferroni correction (for Fukushima vs. Ukiha, log-likelihood ratio test, df = 1, χ2 = 8.6, P < 0.05; for Fukushima vs. Kyoto, df = 1, χ2 = 7.7, P < 0.05; for the other combinations, P > 0.05). Nevertheless, a level-3 abnormality was found in only 1 first instar from Fukushima. This first instar lost 1 hind tibia, 1 mid-tibia, and 1 antenna because of necrosis during embryogenesis, but successfully molted into the second stadium (Fig. 4B).
Figure 6.
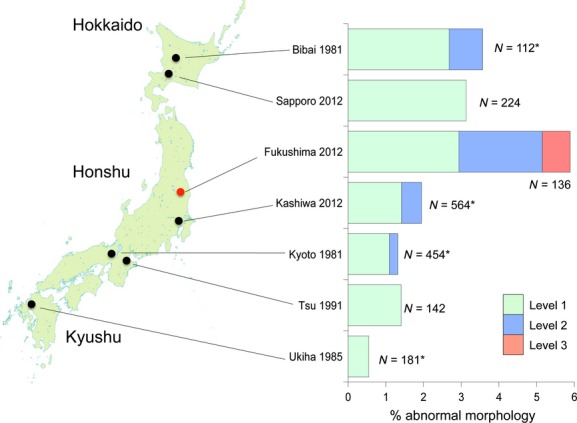
Percentage abnormal morphologies in T. nigriabdominalis first-instar gall formers from seven populations. Asterisks in sample sizes indicate that first instars were collected from buds, whereas nonasterisked figures indicate that first-instars' cast-off skins were collected from galls. The definitions of three levels of abnormality are the same as in Fig. 1.
The larval mortality of T. nigriabdominalis in Fukushima was compared with that in Sapporo, Hokkaido (Fig. S4B). Galls from Fukushima were collected from 1 elm tree in 3 June 2012, while those from Sapporo were collected from about 20 trees in 5 June 2012. In T. nigriabdominalis as well, mortality in the Fukushima population (16.9%) was significantly higher than that in the Sapporo population (1.3%) (log-likelihood ratio test, χ2 = 31.3, P < 0.0001).
In T. radicicola, for which only three galls were collected, the gall formers exhibited abnormalities in all of the galls. One gall former was found dead at the first stadium in an incipient gall, one exhibited necrosis in three legs at the third stadium, and one lost a mid-tibia at the second stadium.
Comparison between the 2012 and 2013 samples from Fukushima
Of 50 T. sorini and 347 T. nigriabdominalis gall formers collected in 2013, first instars with abnormal morphology accounted for 6.0% and 2.6%, respectively, and all of these first instars were categorized in level 1, except 1 T. sorini that was at level 2. In the comparison between the years, gall formers of T. sorini and T. nigriabdominalis were categorized into three groups; healthy, morphologically abnormal, and dead aphids. In this categorization, a gall former with abnormal morphology at the first stadium was considered dead if it finally failed to grow in the gall. Thus, the abnormal category includes living aphids only, and in this category, aphids that had lost appendages accounted for 85.7% and 88.2% in T. sorini and T. nigriabdominalis, respectively. When the galls were collected in the Fukushima area on 21 May 2013, the radiation dose was 2.4 μSv/h at 1 m and 4.0 μSv/h at ground level.
The proportions of dead and abnormal aphids decreased, and the proportion of healthy aphids increased in 2013 compared to the 2012 samples in both species (Fig. 7). The mortality of T. sorini decreased from 14.4% to 0.0%, and similarly, the mortality of T. nigriabdominalis declined from 16.9% to 4.3%. Logistic regression indicated that the fates of gall formers significantly changed between the years (df = 2, χ2 = 33.1, P < 0.0001) and between the species (df = 2, χ2 = 8.9, P < 0.011). However, there was no significant interaction between years and species (df = 2, χ2 = 4.4, P = 0.111). These results suggest that the healthiness of the two Tetraneura species was much improved from 2012 to 2013 in the Fukushima population.
Figure 7.
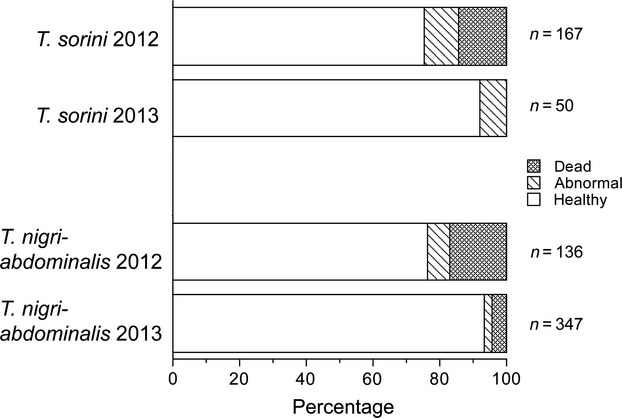
Comparison of the fates of gall formers collected from one elm tree in the Fukushima area between 2012 and 2013. Gall formers in the galls were categorized into three groups: healthy, morphological abnormal, and dead aphids (fundatrices).
Discussion
Because the present study is based on aphid samples collected from only one host tree near Fukushima Daiichi, there is as yet no proof that morphological abnormalities in aphids are increasing or prevailing in the Fukushima area. However, investigations on organisms just after the Nuclear Power Plant accident are almost completely lacking, so that the results from two aphid species may provide useful information for future studies including genetic one.
Morphological comparisons of T. soniri first instars reveal that the Fukushima population contains a higher percentage of morphological abnormalities than do control populations. Some kinds of abnormalities are not specific to Fukushima but are common to the control populations. Possibly, morphological abnormalities recorded in this study result from environmental stresses as well as genetic factors. For example, embryogenesis under poor nutritional conditions, or in low or high temperatures, or pathogens may be responsible for aberrant morphogenesis in aphids (Shingleton et al. 2003; Kobayashi and Murai 2012). In laboratory experiments, the application of precocene derivatives reportedly can induce abnormal phenotypes in several species of aphids (Hardie 1986). Furthermore, as a genetic factor, inbreeding may contribute to the emergence of abnormalities. Eriosomatine aphids, including Tetraneura species, have a breeding system conducive to inbreeding (Dixon 1998; Akimoto 2006), so that homozygosity of deleterious recessive genes due to inbreeding may be causally linked to the high incidence of morphological abnormalities. Nevertheless, the Fukushima samples notably contained not only many aberrant first instars but also five highly deformed first instars of T. sorini and T. nigriabdominalis. To my knowledge, such deformations have not previously been reported in aphids. Furthermore, the finding that three Tetraneura species on the same tree commonly exhibit abnormalities at high percentages suggests that in Fukushima, some extrinsic factors are responsible for the induction of morphological abnormalities.
Akimoto (2006) examined the phenotypic effect of intense inbreeding on first-instar aphids using the eriosomatine aphid Prociphilus oriens. Enforced intraclonal mating (genetically identical to selfing) resulted in lowered hatchability and delayed timing of egg hatch compared to first instars derived from outbreeding. Morphologically, selfing often led to enlarged appendages relative to body size in the first instars, thus resulting in alterations in the allometric relationships among body parts. However, selfing did not lead to malformations in terms of the asymmetry of bilateral characters, appearance of novel characters, or extreme dwarfism. The involvement of inbreeding in the emergence of malformations is evident in some cases (Olsson et al. 1996; McCune et al. 2002), but not in others (Williams et al. 2008). This discrepancy may reflect a different extent of inbreeding in wild populations. Where an organism has a regular system of partial inbreeding, deleterious genes with a serious phenotypic effect would be readily purged from the population. As a result, inbreeding would not lead to morphological abnormalities but to a fitness reduction (i.e., inbreeding depression) due to slightly deleterious mutations (DeRose and Roff 1999; Hedric and Kalinowski 2000; Frankham et al. 2007). Therefore, it is reasonable to suppose that frequent occurrences of abnormalities in Fukushima are affected by some extrinsic factors rather than inbreeding. Except for the Fukushima area, the only example of malformation was detected in Bibai, Hokkaido in 1987. The egg of this individual was produced in the autumn of 1986, when Hokkaido was slightly affected by fallout from the Chernobyl nuclear accident (Fukuda 2007). However, it is not clear whether this accident is linked to the emergence of the malformation.
Comparison with a Hokkaido population suggests that gall formers of T. sorini and T. nigriabdominalis in Fukushima more often fail to develop in the galls. Such high mortality during development might be due to the resistance of the host plant to gall induction rather than extrinsic factors such as mutagens. A number of studies have documented that the performance or viability of gall-forming insects is reduced when they are associated with host plants of the resistant type, which coexists with the susceptible type in a population (Anderson et al. 1989; Simms and Fritz 1990; Paige and Capman 1993). However, the fact that the growth performance of gall formers did not vary between the Fukushima and Iwamizawa populations (Fig. S5) implies that the quality of the host plant in Fukushima was not inferior to that in Iwamizawa. Thus, the results of this analysis suggest that a high mortality in Fukushima cannot be attributed to host unsuitability, but may possibly be caused by external factors.
Environmental contaminants or other extrinsic factors may lead to genomic alterations that ultimately cause malformations (Gilbert and Epel 2008). Mutagens that impede the developmental process of Tetraneura aphids might include chemical substances such as ethyl methane sulfate (EMS) and N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) (Singer and Kusmierek 1982) or radioactive substances (Møller and Mousseau 2006). Because the present study focuses on morphology, it is not possible to clarify which mutagen is linked to the morphological abnormalities. Nevertheless, it is difficult to imagine the occurrence of chemical mutagens around the study area in Fukushima. The study area is surrounded by rice and other crop fields and natural forests, and there is no industrial waste disposal plant in the vicinity. This collection locality is in a planned evacuation area from which local people are obliged to evacuate, and thus, cultivations in 2011 and 2012 were completely abandoned. As a result, no chemical substances including insecticides and herbicides have been applied. An increasing number of studies have documented morphological abnormalities from wild anurans (Stocum 2000; Lannoo 2008), and several alternative hypotheses are proposed for the cause, with difficulty determining the critical factor. In the case of Tetraneura aphids as well, several factors may be associated with the incidence of abnormality and mortality. However, because abnormalities in Tetraneura are detected in the first-instar gall formers, causal factors should be one that affected embryogenesis in eggs. In this respect, predators and parasites are safely ignored as the causal factor.
Analysis of the 2013 Fukushima samples corroborated the view that morphological abnormality and high mortality in the 2012 samples cannot be attributed to chemical mutagens, plant factors, nor inbreeding. This is because the healthiness of gall formers of the two species is improved on the same host tree in 2013 despite the lack of environmental changes. This result implies that some extrinsic factors that appeared specifically from 2011 to the spring 2012 may have led to frequent morphological abnormality and mortality.
The study area in Fukushima was intensively contaminated with radioactive substances emitted from the Fukushima Daiichi Nuclear Power Plant. At the time of the nuclear accident, Tetraneura aphids were overwintering in the egg stage on the bark surface of elm trunks, and thus, the eggs were exposed directly to the fallout. A report from the Nuclear Regulation Authority, Government of Japan (2011b) indicates that weeds collected in the same place as the study area (Yamakiya, Kawamata Town) on 16 March 2011 were contaminated with iodine-131 at 727,000 Bq/kg, cesium-134 at 157,000 Bq/kg, and cesium-137 at 158,000 Bq/kg. The eggs were probably exposed to the same level of radiation. In early May, first-instar gall formers hatched and started gall formation under intense contamination. In mid-June, second-generation winged adults migrated from the cracked galls to gramineous plants, and subsequent generations continued to reproduce asexually on the roots. The soil surface was also contaminated with radionuclides (Tanaka et al. 2013a), so that Tetraneura aphids infesting the host roots, near the soil surface or 5 cm deep at the most, may also have been exposed to external radiation. Thus, if radiation damaged the genes of the developing embryos, such damaged genes may have been inherited through clonal lines to the autumnal sexual generation.
In the autumn of 2011, winged adults returned to elm trees from gramineous plants and produced the sexual generation, whose females produced eggs after copulation. Thus, damaged genes, if any, might have been genetically recombined during sexual reproduction, resulting in a large variation in the accumulation of damaged genes among first instars hatching from the eggs in 2012. In addition to the accumulation effects of deleterious genes, eggs deposited in the autumn of 2011 may have been subjected to radiation on the elm bark for approximately 4 months. The bark surface of trees reportedly was intensively contaminated with radionuclides (Kuroda et al. 2013; Tanaka 2013). Therefore, if radiation was the main factor causing abnormal morphologies, then two mechanisms can be assumed: (1) the accumulation of damaged genes through clonal reproduction (Muller's ratchet) and the release of diverse phenotypes due to the first recombination after the accident, and (2) short-term physiological effects due to continuous exposure to radiation on the host bark during embryogenesis (Møller et al. 2005; Mousseau and Møller 2012b). In some insect species, released radioactive substances have been attributed to malformations (Hesse-Honegger and Wallimann 2008; Hiyama et al. 2012). The proportion of abnormalities detected in Fukushima was probably underestimated because seriously deformed first instars were selected out before gall formation due to low viability (Møller 1997) or intraspecific competition (Akimoto and Yamaguchi 1997).
In gamma irradiation experiments conducted so far, study insects were subjected to various doses of radiation ranging from dozens to hundreds of Gy to examine the impact on insect survival, development, and reproduction (Cole et al. 1959; Elbadry 1965; Elvin et al. 1966; Burgess and Bennett 1971; Burditt et al. 1989). Under such experimental conditions, most dividing cells in the insects were possibly adversely affected over the entire body. By contrast, in the Fukushima area, the environmental radioactivity level (4–6 μGy/h) was much lower than that used in the irradiation experiments. However, in the radiation-contaminated area, radioactive particles were scattered over the soil surface or the bark surface of host trees (Kuroda et al. 2013; Tanaka 2013; Tanaka et al. 2013a). Thus, we have to consider the effects of radioactive particles on the surface of oviposition substrates. My main hypothesis is that if a radioactive particle was on the egg surface or in the vicinity on the bark surface, radiation from the particle possibly affected a restricted number of cells during embryogenesis. Thus, depending on the distance from contamination spots on the bark surface, eggs may have been subjected to varying levels of radiation. In addition, I speculate that as the eggs of Tetraneura aphids are smaller than 1 mm in the major axis, not only gamma radiation but also alpha and beta radiation from hot particles may have had strong effects on embryogenesis. On the other hand, contaminated food cannot be considered a cause of malformations because the overwintering egg does not ingest food or water.
The malformed individual with a bifurcated abdomen (Fig. 2) successfully reached adulthood and developed embryos in her abdomen. This result implies that even if external radiation was responsible for this malformation, some cells were detrimentally affected during embryogenesis, but other cells were free from the adverse effects of radiation. Similarly, the low level of abnormality in the second generations indicates that radionuclides may not have penetrated a closed gall, which develops from one point of the leaf tissue through the rapid proliferation of cells. In fact, translocation of radiocesium from a contaminated branch to new leaves growing after the accident was not clearly observed (Tanaka et al. 2013b). There may be other reasons for the lack of abnormalities in the second generation. Galls were induced on leaves, on average, a few meters from the trunk, and the embryogenesis of the second generation is temporally separated from that of the first generation. These spatial and temporal differences may partly be responsible for the lack of abnormalities in the second generation. Gamma radiation may easily penetrate gall wall and affect the second-generation aphids inside. However, the gall wall may prevent alpha and beta radiation, which, in contrast, probably affects embryogenesis directly on the bark. Otherwise, the difference in the incidence of abnormalities between the first and second generations may be explained by differences in the penetrance of damaged genes between generations.
I assume two possibilities for the improvements in viability and healthiness in 2013. First, a decrease in radiation dose around the study tree may be responsible for the improvements, although a level of 4 μGy/h is still observed at the ground. The second possibility is the action of natural selection for radiation tolerance in the aphid species. Through 2011 and 2012, I suppose that aphid clones were subjected to radiation on the host plants and that many first instars were selected out before and during gall formation. Thus, natural selection may have favored aphid clones tolerant to radiation. If this is true, the populations of the two Tetraneura species may have survived the first impact of fallout by acquiring radiation tolerance. High reproductive rates in aphids may contribute to this improved viability. On the other hand, it is reported that radiation may interact with other environmental stressors such as drought or high temperature and create negative effects in some years (Mousseau et al. 2013). However, there is no record of special climatic events, for example, drought and extreme low or high temperatures, during the period of gall formation in May 2011 and 2012 in the Fukushima area (Japan Metrological Agency; Fukushima WMO Station http://www.data.jma.go.jp/obd/stats/etrn/view/monthly_s3_en.php?block_no=47595%view=1). Therefore, it appears to me that morphological abnormality and mortality are temporary phenomena just after nuclear fallout and disappear in later generations.
Conclusion
Several factors are probably responsible for morphological abnormality and mortality in insects, and at present, there is no decisive evidence that any single factor is causally related to the observed abnormalities. However, comparisons between the research results in 2012 and 2013 suggest that a specific environmental factor from 2011 until the spring 2012 was involved in the incidence of abnormalities and mortalities. Evidence from this study suggests that of several potential factors, radioactive fallout from Fukushima Daiichi in the spring 2011 is most likely to have had the strongest effect, because other factors can be readily ignored as the causal factor.
Provided that radioactive fallout is the critical factor, I further postulate that morphological abnormalities found in first-instar gall formers result from the localized effect of radioactive particles on developing embryos in eggs. At present, it is difficult to determine which of the two hypotheses (the accumulation of damaged genes and short-term physiological effects) adequately accounts for the occurrence of morphological abnormalities in the gall formers. However, because most of the abnormalities were not inherited by the next generation or by the next year's generations, the possible effect of radioactive particles appears to have been confined to short-term physiological effects arising from, for example, oxidative stress (Mousseau and Møller 2012b). However, because damaged genes possibly just start to accumulate and because contamination continues long, the adverse effects of damaged genes may appear some years later. Thus, it is necessary to survey the effects of radiation on the morphology and genetics of Tetraneura aphids continuously over several years.
This study possibly demonstrates the operation of selective pressures on radiation tolerance just after nuclear fallout. This kind of selective death has not been reported in the studies of the wildlife in Chernobyl. Through natural selection for aphid clones tolerant to radiation, coupled with a lowered level of environmental radiation, Tetraneura populations in Fukushima are apparently recovering. However, it is not clear whether this tendency continues hereafter or not. Therefore, in the future study, it is critical to explore whether the aphid populations overcome the nuclear contamination through acquiring radiation tolerance or confront abnormalities again several years later due to the accumulation of damaged genes.
Acknowledgments
I am very grateful to Dr. Yoshitomo Kikuchi for his help in the field and discussion. This research was partly supported by Grants-in-Aid (no. 23370037) for Scientific Research from the Japan Society for the Promotion of Science.
Conflict of Interest
None declared.
Funding information
This research was partly supported by Grants-in-Aid (no. 23370037) for Scientific Research from the Japan Society for the Promotion of Science.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Gall formation by Tetraneura aphids.
Level-1 morphological abnormalities in Tetraneura first-instar gall formers.
Level-1 tissue necrosis and level-2 appendage loss.
Percentage mortality in T. sorini and T. nigriabdominalis gall formers in their gall.
Relationship between first-instar hind femur length and adult hind femur length in the same individual.
References
- Akimoto S. A revision of the genus Eriosoma and its allied genera in Japan (Homoptera: Aphidoidea) Insec. Matsum. N. S. 1983;27:37–106. [Google Scholar]
- Akimoto S. Occurrence of abnormal phenotypes in a host-alternating aphid and their implications for genome organization and evolution. Evol. Theor. 1985a;7:179–193. [Google Scholar]
- Akimoto S. Taxonomic study on gall aphids, Colopha Paracolopha and Kaltenbachiella (Aphidoidea: Pemphigidae) in East Asia, with special reference to their origins and distributional patterns. Insec. Matsum. N. S. 1985b;31:1–79. [Google Scholar]
- Akimoto S. The evolution of gall parasitism accompanied by a host shift in the gall aphid, Eriosoma yangi (Homoptera, Aphidoidea) Biol. J. Linn. Soc. Lond. 1988;35:297–312. [Google Scholar]
- Akimoto S. Local adaptation and host race formation of a gall-forming aphid in relation to environmental heterogeneity. Oecologia. 1990;83:162–170. doi: 10.1007/BF00317747. [DOI] [PubMed] [Google Scholar]
- Akimoto S. Heterogeneous selective pressures on egg-hatching time and the maintenance of its genetic variance in a Tetraneura gall-forming aphid. Ecol. Entomol. 1998;23:229–237. [Google Scholar]
- Akimoto S. Inbreeding depression, increased phenotypic variance, and a trade-off between gonads and appendages in selfed progeny of the aphid Prociphilus oriens. Evolution. 2006;60:77–86. [PubMed] [Google Scholar]
- Akimoto S, Yamaguchi Y. Phenotypic selection on the process of gall formation of a Tetraneura aphid (Pemphigidae) J. Anim. Ecol. 1994;63:727–738. [Google Scholar]
- Akimoto S, Yamaguchi Y. Gall usurpation by the gall-forming aphid, Tetraneura sorini (Insecta Homoptera) Ethol. Ecol. Evol. 1997;9:159–168. [Google Scholar]
- Anderson SS, McCrea KD, Abrahamson WG, Hartzel LM. Host genotype choice by the ball gallmaker Eurosta solidaginis (Diptera: Tephritidae) Ecology. 1989;70:1048–1054. [Google Scholar]
- Aoki S. Descriptions of the Japanese species of Pemphigus and its allied genera (Homoptera: Aphidoidea) Insec. Matsum. N. S. 1975;5:1–63. [Google Scholar]
- Aoyama T, Akimoto S, Hasegawa E. Gall distribution as a compromise between the optimal gall-site selection and the synchrony to host-plant phenology in the aphid Kaltenbachiella japonica. Arthr. Plant Interact. 2012;6:461–469. [Google Scholar]
- Ballengée B, Sessions SK. Explanation for missing limbs in deformed amphibians. J. Exp. Zool. 2009;312B:770–779. doi: 10.1002/jez.b.21296. [DOI] [PubMed] [Google Scholar]
- Blackman R, Eastop VF. Aphids on the world's trees: an identification and information guide. Wallingford: CAB International; 1994. p. 987. [Google Scholar]
- Bowerman J, Johnson PTJ, Bowerman T. Sublethal predators and their injured prey: linking aquatic predators and severe limb abnormalities in amphibians. Ecology. 2010;91:242–251. doi: 10.1890/08-1687.1. http://dx.doi.org/10.1890/08-1687.1. [DOI] [PubMed] [Google Scholar]
- Burditt AK, Jr, Hungate FP, Toba HH. Gamma irradiation: effect of dose and dose rate on development of mature codling moth larvae and adult eclosion. Int. J. Rad. Appl. Instrum. C. 1989;34:979–984. [Google Scholar]
- Burgess EE, Bennett SE. Mortality and abnormalities caused by gamma irradiation of alfalfa weevil larvae. Ann. Entomol. Soc. Amer. 1971;64:88–90. [Google Scholar]
- Cerutti PA. Effects of ionizing radiation on mammalian cells. Naturwissenschaften. 1974;61:51–59. doi: 10.1007/BF00596195. [DOI] [PubMed] [Google Scholar]
- Cole MM, Labrecque GC, Burden GS. Effects of gamma radiation on some insects affecting man. J. Econ. Entomol. 1959;52:448–450. [Google Scholar]
- DeRose MA, Roff DA. A comparison of inbreeding depression in life-history and morphological traits in animals. Evolution. 1999;53:1288–1292. doi: 10.1111/j.1558-5646.1999.tb04541.x. [DOI] [PubMed] [Google Scholar]
- Dixon AFG. Aphid ecology: an optimization approach. 2nd ed. London: Chapman & Hall; 1998. p. 300. p. [Google Scholar]
- Elbadry E. Some effects of gamma radiation on the potato tuberworm, Gnorimoschema operculella (Lepidoptera: Gelechiidae) Ann. Entomol. Soc. Am. 1965;58:206–209. doi: 10.1093/aesa/58.2.206. [DOI] [PubMed] [Google Scholar]
- Elvin T, Wendell BE, Robert CR. Effects of gamma radiation on Rhyzopertha dominica, Sitophilus oryzae, Tribolium confusum, and Lasioderma serricorne. J. Econ. Entomol. 1966;59:1363–1368. doi: 10.1093/jee/59.6.1363. [DOI] [PubMed] [Google Scholar]
- Frankham R, Ballou JD, Briscoe DA. Introduction to conservation genetics. Cambridge: Cambridge Univ. Press; 2007. p. 617. p. [Google Scholar]
- Fukuda K. A review of environmental radioactivity survey in the Hokkaido Institute of Public Health for 50 years. Rep. Hokkaido Instit. Pub. Health. 2007;57:1–14. (in Japanese) [Google Scholar]
- Gilbert SF, Epel D. Ecological Developmental Biology: Integrating Epigenetics, Medicine, and Evolution. Sunderland, MA: Sinauer Associates; 2008. p. 459. p. [Google Scholar]
- Hardie J. Morphogenetic effects of precocenes on three aphid species. J. Insec. Physiol. 1986;32:813–818. [Google Scholar]
- Hedric PW, Kalinowski ST. Inbreeding depression in conservation biology. Ann. Rev. Ecol. Syst. 2000;31:139–162. [Google Scholar]
- Hesse-Honegger C, Wallimann P. Malformation of true bug (Heteroptera): a phenotype field study on the possible influence of artificial low-level radioactivity. Chem. Biodivers. 2008;5:499–539. doi: 10.1002/cbdv.200800001. [DOI] [PubMed] [Google Scholar]
- Hiyama A, Nohara C, Kinjo S, Taira W, Gima S, Tanahara A, Otaki JM. The biological impacts of the Fukushima nuclear accident on the pale grass blue butterfly. Sci. Rep. 2012;2:570. doi: 10.1038/srep00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida K, Nakanishi TM, Tanoi K. Agricultural implications of the Fukushima nuclear accident. Tokyo: Springer; 2013. Contamination of wild animals: Effects on wildlife in high radioactivity areas of the agricultural and forest landscape; pp. 119–129. [Google Scholar]
- Kobayashi M, Murai T. Development and reproduction of five populations of the foxglove aphid, Aulacorthum solani (Kaltenbach) (Hemiptera: Aphididae), on nine soybean cultivars. Jpn. J. Appl. Entomol. Zool. 2012;56:49–55. (in Japanese) [Google Scholar]
- Komatsu T, Akimoto S. Genetic differentiation as a result of adaptation to the phenologies of individual host trees in the galling aphid Kaltenbachiella japonica. Ecol. Entomol. 1995;20:33–42. [Google Scholar]
- Komazaki S. Difference of egg diapause in two host races of the spirea aphid, Aphis spiraecola. Entomol. Exp. Appl. 1998;89:201–205. [Google Scholar]
- Krivolutsky DA. Chernobyl nuclear disaster and long-term changes in biodiversity induced by the radiation pollution. Ecol. Indust. Reg. 1995;1:37–43. [Google Scholar]
- Krivolutzkii DA, Pokarzhevskii AD. Effects of radioactive fallout on soil animal populations in the 30 km zone of the Chernobyl atomic power station. Sci. Total Environ. 1992;112:69–77. doi: 10.1016/0048-9697(92)90239-o. [DOI] [PubMed] [Google Scholar]
- Kuroda K, Kagawa A, Tonosaki M. Radiocesium concentrations in the bark, sapwood and heartwood of three tree species collected at Fukushima forests half a year after the Fukushima Dai-ichi nuclear accident. J. Environ. Radioact. 2013;122:37–42. doi: 10.1016/j.jenvrad.2013.02.019. [DOI] [PubMed] [Google Scholar]
- Lannoo M. Malformed frogs: the collapse of aquatic ecosystems. Berkeley: Univ. of California Press; 2008. p. 288. p. [Google Scholar]
- Lynch M, Gabriel M. Phenotypic evolution and parthenogenesis. Am. Nat. 1983;122:745–764. [Google Scholar]
- Matranga V, Zito F, Costa C, Bonaventura R, Giarrusso S, Celi F. Embryonic development and skeletogenic gene expression affected by X-rays in the Mediterranean sea urchin Paracentrotus lividus. Ecotoxicology. 2010;19:530–537. doi: 10.1007/s10646-009-0444-9. [DOI] [PubMed] [Google Scholar]
- McCune AR, Fuller RC, Aquilina AA, Dawley RM, Fadoo JM, Houle D, et al. A low genomic number of recessive lethals in natural populations of bluefin killifish and zebrafish. Science. 2002;296:2398–2401. doi: 10.1126/science.1071757. [DOI] [PubMed] [Google Scholar]
- McGregor JF, Newcombe HB. Malformations in trout embryos irradiated prior to active organogenesis. Radiat. Res. 1968;35:282–300. [PubMed] [Google Scholar]
- Møller AP. Morphology and sexual selection in the barn swallow Hirundo rustica in Chernobyl, Ukraine. Proc. R. Soc. Lond. B. 1993;252:51–57. [Google Scholar]
- Møller AP. Developmental selection against developmentally unstable offspring and sexual selection. J. Theor. Biol. 1997;185:415–422. [Google Scholar]
- Møller AP. Developmental instability and sexual selection in stag beetles from Chernobyl and a control area. Ethology. 2002;108:193–204. [Google Scholar]
- Møller AP, Mousseau TA. Biological consequences of Chernobyl: 20 years after the disaster. Trends Ecol. Evol. 2006;21:200–207. doi: 10.1016/j.tree.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Møller AP, Mousseau TA. Species richness and abundance of birds in relation to radiation at Chernobyl. Biol. Lett. 2007;3:483–486. doi: 10.1098/rsbl.2007.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller AP, Mousseau TA. Reduced abundance of insects and spiders linked to radiation at Chernobyl 20 years after the accident. Biol. Lett. 2009;5:356–359. doi: 10.1098/rsbl.2008.0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller AP, Surai PF, Mousseau TA. Antioxidants, radiation and mutation as revealed by sperm abnormality in barn swallows from Chernobyl. Proc. R. Soc. B. 2005;272:247–252. doi: 10.1098/rspb.2004.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller AP, Mousseau TA, de Lope F, Saino N. Elevated frequency of abnormalities in barn swallows from Chernobyl. Biol. Lett. 2007;3:414–417. doi: 10.1098/rsbl.2007.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller AP, Hagiwara A, Matsui S, Kasahara S, Kawatsu K, Nishiumi I, et al. Abundance of birds in Fukushima as judged from Chernobyl. Environ. Pollut. 2012;164:36–39. doi: 10.1016/j.envpol.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Møller AP, Nishiumi I, Suzuki H, Ueda K, Mousseau TA. Differences in effects of radiation on abundance of animals in Fukushima and Chernobyl. Ecol. Indic. 2013;24:75–81. http://dx.doi.org/10.1016/j.ecolind.2012.06.001. [Google Scholar]
- Mousseau TA, Møller AP. Chernobyl and Fukushima: differences and similarities, a biological perspective. Trans. Am. Nucl. Soc. 2012a;107:200–203. http://cricket.biol.sc.edu/chernobyl/papers/Mousseau-Moller-ANS-2012.pdf. [Google Scholar]
- Mousseau TA, Møller AP. Entomological studies in Chernobyl and Fukushima. Am. Entomol. 2012b;58:148–150. [Google Scholar]
- Mousseau TA, Welch SM, Chizhevsky I, Bondarenko O, Milinevsky G, Tedeschi DJ, et al. Tree rings reveal extent of exposure to ionizing radiation in Scots pine Pinus sylvestris. Trees. 2013;27:1443–1453. [Google Scholar]
- Nakanishi TM, Tanoi K. Agricultural implications of the Fukushima nuclear accident. Osaka: Springer; 2013. p. 204. p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuclear Emergency Response Headquarters, Government of Japan. 2011. Report of the Japanese Government to the IAEA Ministerial Conference on Nuclear Safety—The Accident at TEPCO's Fukushima Nuclear Power Stations. Attachment IV-2. Available at http://www.kantei.go.jp/foreign/kan/topics/201106/iaea_houkokusho_e.html.
- Nuclear Regulation Authority, Government of Japan. Results of airborne monitoring by the Ministry of Education, Culture, Sports, Science and Technology and the U.S. Department of Energy; 2011a. (May 6, 2011). Available at http://radioactivity.nsr.go.jp/en/contents/4000/3180/24/1304797_0506.pdf. [Google Scholar]
- Nuclear Regulation Authority, Government of Japan. 2011b. Monitoring information of environmental radioactivity level. Available at http://radioactivity.nsr.go.jp/en/contents/4000/3563/24/1307583_0622.pdf.
- Olsson M, Gullberg A, Tegelström H. Malformed offspring, sibling matings, and selection against inbreeding in the sand lizard (Lacerta agilis. J. Evol. Biol. 1996;9:229–242. [Google Scholar]
- Paige KN, Capman WC. The effects of host-plant genotype, hybridization, and environment on gall-aphid attack and survival in cottonwood: the importance of genetic studies and the utility of RFLPS. Evolution. 1993;47:36–45. doi: 10.1111/j.1558-5646.1993.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Russell LB, Russell WL. Radiation hazards to the embryo and fetus. Radiology. 1952;58:369–377. doi: 10.1148/58.3.369. [DOI] [PubMed] [Google Scholar]
- Shingleton AW, Sisk GC, Stern DL. Diapause in the pea aphid (Acyrthosiphon pisum) is a slowing but not a cessation of development. BMC Dev. Biol. 2003;3:7. doi: 10.1186/1471-213X-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms EL, Fritz RS. The ecology and evolution of host-plant resistance to insects. Trends Ecol. Evol. 1990;5:356–360. doi: 10.1016/0169-5347(90)90094-T. [DOI] [PubMed] [Google Scholar]
- Singer B, Kusmierek JT. Chemical mutagenesis. Annu. Rev. Biochem. 1982;52:655–693. doi: 10.1146/annurev.bi.51.070182.003255. [DOI] [PubMed] [Google Scholar]
- Sokal RR, Rohlf JF. Biometry. 3rd ed. New York: WH Freeman and Company; 1995. p. 887. p. [Google Scholar]
- Stocum D. Frog limb deformities: an “eco-devo” riddle wrapped in multiple hypotheses surrounded by insufficient data. Teratology. 2000;62:147–150. doi: 10.1002/1096-9926(200009)62:3<147::AID-TERA2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Tanaka D, Nakanishi TM, Tanoi K. Agricultural implications of the Fukushima nuclear accident. Tokyo: Springer; 2013. Distribution of radiocesium from the radioactive fallout in fruit trees; pp. 143–162. [Google Scholar]
- Tanaka K, Sakaguchi A, Kanai Y, Tsuruta H, Shinohara A, Takahashi Y. Heterogeneous distribution of radiocesium in aerosols, soil and particulate matters emitted by the Fukushima Daiichi Nuclear Power Plant accident: retention of micro-scale heterogeneity during the migration of radiocesium from the air into ground and river systems. J. Radioanal. Nucl. Chem. 2013a;295:1927–1937. [Google Scholar]
- Tanaka K, Iwatani H, Sakaguchi A, Takahashi Y, Onda Y. Local distribution of radioactivity in tree leaves contaminated by fallout of the radionuclides emitted from the Fukushima Daiichi Nuclear Power Plant. J. Radioanal. Nucl. Chem. 2013b;295:2007–2014. [Google Scholar]
- Tilton E, Burkholder WE, Cogburn RR. Effects of gamma radiation on Rhyzopertha dominica, Sitophilus oryzae, Tribolium confusum, and Lasioderma serricorne. J. Econ. Entomol. 1966;59:1363–1368. doi: 10.1093/jee/59.6.1363. [DOI] [PubMed] [Google Scholar]
- Vereecke A, Pelerents C. Sensitivity to gamma radiation of Tribolium confusum eggs at various developmental stages. Entomol. Exp. Appl. 1969;12:62–66. [Google Scholar]
- Whitham TG. Habitat selection by Pemphigus aphids in response to resource limitation and competition. Ecology. 1978;59:1164–1176. [Google Scholar]
- Williams DD, Nesterovitch AI, Tavares AF, Muzzatti EG. Morphological deformities occurring in Belarusian chironomids (Diptera: Chironomidae) subsequent to the Chernobyl nuclear disaster. Freshw. Biol. 2001;46:503–512. [Google Scholar]
- Williams RN, Bos DH, Gopurenko D, DeWoody JA. Amphibian malformations and inbreeding. Biol. Lett. 2008;4:5549–5552. doi: 10.1098/rsbl.2008.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wool D. Galling aphids: specialization, biological complexity, and variation. Annu. Rev. Entomol. 2004;49:175–192. doi: 10.1146/annurev.ento.49.061802.123236. [DOI] [PubMed] [Google Scholar]
- Yablokov AV, Nesterenko VB, Nesterenko AV. Ann. N Y. Acad. Sci. Vol. 1181. Boston, MA: Blackwell Publishing; 2009. Chernobyl: consequences of the catastrophe for people and the environment; p. 327. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gall formation by Tetraneura aphids.
Level-1 morphological abnormalities in Tetraneura first-instar gall formers.
Level-1 tissue necrosis and level-2 appendage loss.
Percentage mortality in T. sorini and T. nigriabdominalis gall formers in their gall.
Relationship between first-instar hind femur length and adult hind femur length in the same individual.


