Abstract
Wild pollinators have been shown to enhance the pollination of Brassica napus (oilseed rape) and thus increase its market value. Several studies have previously shown that pollination services are greater in crops adjoining forest patches or other seminatural habitats than in crops completely surrounded by other crops. In this study, we investigated the specific importance of forest edges in providing potential pollinators in B. napus fields in two areas in France. Bees were caught with yellow pan traps at increasing distances from both warm and cold forest edges into B. napus fields during the blooming period. A total of 4594 individual bees, representing six families and 83 taxa, were collected. We found that both bee abundance and taxa richness were negatively affected by the distance from forest edge. However, responses varied between bee groups and edge orientations. The ITD (Inter-Tegular distance) of the species, a good proxy for bee foraging range, seems to limit how far the bees can travel from the forest edge. We found a greater abundance of cuckoo bees (Nomada spp.) of Andrena spp. and Andrena spp. males at forest edges, which we assume indicate suitable nesting sites, or at least mating sites, for some abundant Andrena species and their parasites (Fig. 1). Synthesis and Applications. This study provides one of the first examples in temperate ecosystems of how forest edges may actually act as a reservoir of potential pollinators and directly benefit agricultural crops by providing nesting or mating sites for important early spring pollinators. Policy-makers and land managers should take forest edges into account and encourage their protection in the agricultural matrix to promote wild bees and their pollination services.
Keywords: Andrena, bee dispersal, ecosystem service, foraging range, Nomada, partial habitats, wild bees
Introduction
Pollinators play an important functional role in most terrestrial ecosystems and provide a key ecosystem service (Ashman et al. 2004). Insects, particularly bees, are the primary pollinators for the majority of the world's angiosperms (Ollerton et al. 2012). Without this service, many interconnected species and processes functioning within both wild and agricultural ecosystems could collapse (Kearns et al. 1998). Brassica napus (oilseed rape, OSR) represents the most widespread entomophilous crop in France with almost 1.5 Mha in 2010 (FAOSTAT August 10th, 2012). Results differ between varieties, but even though it seems that OSR produces 70% of its fruits through self-pollination (Downey et al. 1970 in Mesquida and Renard 1981), native bees are also known to contribute to its pollination (Morandin and Winston 2005; Jauker et al. 2012). Bee pollination leads to improved yields (Steffan-Dewenter 2003b; Sabbahi et al. 2005) and to a shorter blooming period (Sabbahi et al. 2006), thus increasing the crop's market value (Bommarco et al. 2012). The most widely used species in crop pollination is the honeybee (Apis mellifera L) which is sometimes assumed to be sufficient for worldwide crop pollination (Aebi and Neumann 2011). However, this assertion has been questioned by different authors (Ollerton et al. 2012), and several studies show that many wild bees are also efficient pollinators of crops (Klein et al. 2007; Winfree et al. 2008; Breeze et al. 2011). Recently, Garibaldi et al. (2013) found positive associations of fruit set with wild-insect visits to flowers in 41 crop systems worldwide. They demonstrate that honeybees do not maximize pollination, nor can they fully replace the contributions of diverse, wild-insect assemblages to fruit set for a broad range of crops and agricultural practices on all continents with farmland. Unfortunately, not only are honey bees declining due to a variety of different causes (vanEngelsdorp et al. 2009), wild bee populations are also dwindling (Potts et al. 2010). Their decline has been documented in two Western European countries (Britain and the Netherlands) by comparing data obtained before and after 1980 (Biesmeijer et al. 2006). These losses have mostly been attributed to the use of agrochemicals, the increase in monocultures, the loss of seminatural habitat and deforestation (Steffan-Dewenter et al. 2002; Steffan-Dewenter and Westphal 2008; Brittain and Potts 2011).
Figure 1.

Left, a Nomada sp male; right, an Andrena sp male.
Several studies have shown the importance of natural or seminatural habitats in sustaining pollinator populations or pollination services close to fruit crops (Steffan-Dewenter 2003a; Kremen et al. 2004; Greenleaf and Kremen 2006a; Carvalheiro et al. 2010). Morandin and Winston (2006) presented a cost–benefit model that estimates profit in OSR agroecosystems with different proportions of uncultivated land. They calculated that yield and profit could be maximized with 30% of the land left uncultivated within 750 m of field edges. Other studies have demonstrated a negative impact of the distance from forests on pollination services or bee abundance and richness both in tropical ecosystems (De Marco and Coelho 2004; Blanche et al. 2006; Chacoff and Aizen 2006) and in temperate ecosystems (Hawkins 1965; Taki et al. 2007; Arthur et al. 2010; Watson et al. 2011).
These studies all suggest that natural or seminatural habitats are important sources of pollinators, probably because they provide “partial habitats” (Westrich 1996) such as complementary mating, foraging, nesting, and nesting materials sites that bees need to complete their life cycle. In this study, we focused on the effect of distance to forest edge on bee assemblages in OSR ecosystems. Forest edges could provide one or more important partial habitats for different bee species in agricultural landscapes, in particular when associated with a mass-flowering crop such as OSR (Le Feon et al. 2011). For example, the availability of untilled soil and dead branches might provide ground-nesting and cavity-nesting bee species with numerous nesting sites. Moreover, during spring at least, the understory and the forest edge can provide cover containing flowering plants and wild trees such as Prunus spp, Castanea sativa, or Salix spp and thereby allow bees to find alternative floral resources.
During spring 2010 and 2011, in two areas in France, we examined wild bee abundance and taxa richness both along forest edges and inside OSR fields at different distances from the forest. Like other taxa, bees respond to environmental variables according to their biologic traits that determine access and requirements for nesting, mating, and forage resources, species mobility or physiological tolerance. Specifically, we hypothesized that (1) bee abundance, species richness, and composition of bee communities within the crop field are dependent on the distance from the forest edge (where complementary floral resources, nesting sites, shelters, etc. can be found) and on the orientation of the forest edge; (2) the identity of bees in the crop is related to their foraging range which we measured with the ITD (Inter-Tegular distance); (3) the forest edge may be the nesting or mating sites for cavity-nesting or ground-nesting bees such as Osmia spp or Andrena spp which are important groups of potential early spring pollinators for OSR.
Materials and Methods
Study sites
The field work was conducted in 2010 near Orleans, France (latitude 47.8537191, longitude 2.7499075), and in 2011 in the same area and in addition, near Toulouse, France (latitude 43.3030938, longitude 0.9914780). These two study areas are 700 km from each other. In 2010, we selected eight fields sown with B. napus and in 2011, a total of ten fields in both areas (Fig. 2). The 28 fields were selected with at least one of their sides directly adjacent to a forest with indigenous deciduous tree species (mainly Quercus, Carpinus and Populus spp.). We classified 11 forest edges as “cold orientation” (northern and eastern exposure) and 17 forest edges as “warm orientation” (southern and western exposure) according to the amount of Celsius degree they received during the day. The fields we selected in 2010 and 2011 had forest edges of at least 100 m in length. In 2010, we had two study point distances from forest edge, 50 m and 200 m. Our 200-m study points were distant from other edges by at least 200 m. In 2011, we also had two study point distances from forest edge, 10 m and the further one varied from 30 to 230 m (Fig. 3).
Figure 2.

Location of study areas and spatial arrangement of our sampling design.
Figure 3.

Design used to survey bees in oilseed rape crops at different distances from the forest edge. Circles represent yellow pan traps.
Bee sampling
We used yellow pan traps to sample bees, while the OSR was in bloom; this is a common passive sampling method (Dafni et al. 2005 in Westphal et al. 2008). The traps were plastic bowls (approximately 30 cm in diameter and 23 cm in height) with an UV-reflecting paint (S.P.R.L, Spray-color 18 133UK, Brussels, Belgium) sprayed on the inside. They were mounted on wooden poles at vegetation height (Westphal et al. 2008) and filled with approximately 2.4 L of water, 0.6 L of monopropylene glycol for conservation, and a few drops of liquid soap to lower surface tension, and then were exposed for 15 days during the blooming period. In 2010, we placed two pan traps at each distance from forest edge: 0 m (forest edge), 50 m, and 200 m into the crop. In 2011, we placed one pan trap at each distance: 0 m (forest edge), 10 m, and a third location varying between 30 and 230 m into the crop. Collected specimens were stored in a freezer, then dried, mounted, and identified to the species level when possible. Some specimens could only be determined to the genus (Nomada, Sphecodes) or subgenus (Micrandrena) level. The specimens were also separated into males and females.
Data analysis
Hypothesis 1: bee abundance, species richness, and composition of bee communities within the crop field are dependent on the distance from the forest edge and forest edge orientation
We constructed generalized additive mixed models (R; mgcv package) to test our hypotheses about total bee abundance and bee species richness as a function of distance and orientation (2-level categorical variable specifying a cold or warm orientation). In addition to the interaction between distance and orientation, we included year (n = 2) and field area (n = 2) as additional fixed effects and the field identity (n = 28) as a random effect. Residuals analyses motivated us to use a Poisson distribution for the abundance and a normal distribution for the species richness (Table 1). In our analysis of species richness, we also included total abundance as a covariate.
Table 1.
Estimates (± SE) of ecological effects from generalized additive mixed-effect models for bee abundance, species richness, mean female ITD, Andrena females and males, and Nomada.
 |
To examine how the composition of the bee community varied with distance and orientation, we used canonical analysis of principal coordinates (CAP) (R package: vegan, function: capscale; R Core Team 2012). This method allowed us to quantify and test the individual contribution of qualitative variables (year, geographic area, field, and orientation) and the quantitative variable (distance) to variations in total assemblage composition. We used the Jaccard similarity index and carried out an inertia partitioning to estimate the total variance in assemblage composition, total constrained inertia (i.e., explained by all the variables included in the model), and the relative individual contribution of each variable to the constrained inertia (Anderson and Willis 2003).
Hypothesis 2: the identity of bees in the crop is related to their foraging range
To examine how bee identity varied with distance and orientation of forest edge, we examined how the mean female ITD (Inter-Tegular distance: the distance between the bases of the two wings) varied with distance and orientation. As above, we modeled the mean female ITD using a generalized additive mixed model. In addition to the interaction between distance and orientation, we included year (n = 2) and field area (n = 2) as additional fixed effects and the field identity (n = 28) as a random effect. We used a normal distribution and we also included total abundance as a covariate. Only females and traps with at least two specimens were included in this analysis (2 traps were therefore excluded). Males were not included in the analysis of mean ITD because they do not take care of brood so they do not collect pollen; their principal requirement is finding females with which to mate. On the contrary, females take care of the brood so they must find appropriate nesting sites and supply the larvae with food. Moreover, females exhibit central-place foraging, so they actively travel from crop to nest. They are the actual OSR pollinators. The parasites Bombus (Psithyrus), Nomada, and Sphecodes were not included in the analysis of the mean ITD because they also do not take care of their broods; their presence or movements may be more linked to their nest host (Williams et al. 2010).
Hypothesis 3: the forest edge may be the nesting or mating sites for cavity-nesting or ground-nesting bees
To estimate the importance of forest edge for ground-or cavity-nesting bees, we constructed a generalized additive mixed model as above. We focused only on the Andrena responses because (1) other groups such as the cavity-nesting bees (Osmia spp) were probably underestimated because of the sampling method used (Westphal et al. 2008; Sobek et al. 2009); (2) Andrena were the only taxa whose males and parasites had already emerged and could be used as indirect indicators of nesting or mating sites; and (3) other studies in similar areas had already shown that Andrena are important visitors to B. napus (Delbrassine and Rasmont 1988; Le Feon et al. 2011).
We investigated the response of Andrena females and males separately. For Andrena females, our model contained the interaction between distance and orientation, and we included year (n = 2) and field area (n = 2) as additional fixed effects and the field identity (n = 28) as a random effect. Residuals analysis suggested a Poisson distribution. For the analysis of Andrena males, we further included a factor structuring the variance of error using the “weights” distribution function (varpower). In this case, we used a Gaussian distribution (Table 1).
Finally, for this hypothesis, we also examined the response of the Andrena cleptoparasites, Nomada, using the same model structure as for Andrena males.
Results
A total of 4594 individuals representing 83 taxa from 6 families, and 12 genera were recorded. The most abundant families were Halictidae (49.1% of total abundance, 31 species) and Andrenidae (39.5% of total abundance, 36 species). Their parasites, Sphecodes (12 specimens) and Nomada (191 specimens, 101 females, 90 males), respectively, represented 4.4% of the total abundance. The Apidae (Apis and Bombus spp.) family represented only 5.7% of total abundance. Furthermore, all Bombus spp. were queens indicating that colonies had not yet been established at the time of the study. The Bombus parasites (Bombus (Psithyrus) rupestris, Bombus (Psithyrus) sylvestris, and Bombus (Psithyrus) vestalis) with a total of 27 specimens accounted for 0.6% of total abundance. Females for all taxa combined represented 89.1% of total abundance with 4095 specimens, and males only 10.9% (499 specimens) with Andrena and Nomada males making up, respectively, 74.5% and 18% of male abundance (Fig. 4). Halictidae and Apidae males emerge later and were therefore absent in our samples.
Figure 4.

Abundance as a function of distance from the forest edge for different bee groups. We show absence and presence values and use different scales on Y-axes for clarity. Multiple points are plotted as “sunflowers” with multiple leaves (“petals”) such that over-plotting is visualized.
Hypothesis 1: bee abundance, species richness and composition of bee communities within the crop field are dependent on the distance from the forest edge and forest edge orientation
Distance had a significant negative effect on total abundance and richness (Table 1). The orientation of forest edge and its interaction with distance had no significant effect on total abundance, richness, abundance of Andrena males and females mean ITD (Table 1). We observed a positive effect of the interaction between cold orientation and distance on Andrena females and Nomada abundance. In other words, we observed a decrease in Andrena females and Nomada abundance with increasing distance from warm edges. Conversely, we observed an increase in Andrena females and Nomada abundance with increasing distance from cold edges (Data S1). However, for the Nomada, the model did not describe the data very well.
Inertia partitioning by CAP (canonical analysis of principal coordinates) showed that distance provided the second largest contribution to the variance in bee assemblages (29.4%), the first explanatory variable being the field ID (48.8%). Distance and field ID were the only significant variables with an independent contribution; the others had only joint contributions (Table 2).
Table 2.
Results of the canonical analysis of principal coordinates on the bee assemblage for the five factors.
| Total inertia | Pr (>F) | % constraint inertia | % own contribution | % joint contribution | |
|---|---|---|---|---|---|
| Field ID | 70.54 | 0.005 | 48.8 | 13.1 | 86.9 |
| Distance | 42.55 | 0.005 | 29.4 | 15.7 | 84.3 |
| Area | 20.29 | 0.005 | 14.0 | 0.0 | 100.0 |
| Year | 8.69 | 0.005 | 6.0 | 0.0 | 100.0 |
| Orientation | 2.56 | 0.067 | 1.8 | 0.0 | 100.0 |
| Residuals | 8.64 |
Hypothesis 2: the identity of bees in the crop is related to their foraging range
Distance had a significant positive effect on mean female ITD (Fig. 5). In other words, the further away collected bees were from the edge, the larger they were (Table 1).
Figure 5.
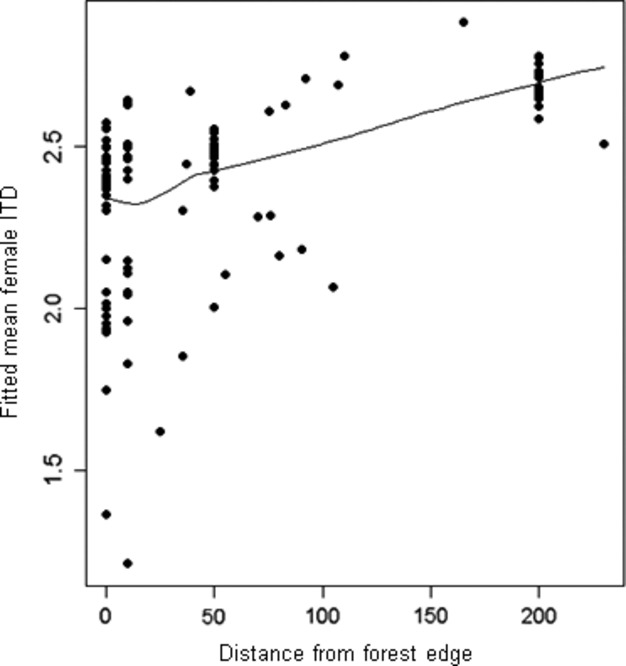
Fitted GAMM model of the response of female mean ITD including distance, year, female abundance, and geographic coordinates as fixed factors and geographic area and field as random factors.
Hypothesis 3: forest edge as nesting or mating sites for Andrena
For the Andrena, both females and males were negatively affected by longer distances. Nomada were also apparently negatively affected by longer distances; however, the model did not describe the data very well (Table 1). Even so, we decided to retain this model because of the high proportion of Nomada present at forest edges (81.7% of their total abundance).
Discussion
Hypothesis 1: bee abundance, species richness, and composition of bee communities within the crop field are dependent on the distance from the forest edge and forest edge orientation
In our study, we found a negative effect of distance from forest edge on bee abundance and richness. Distance also greatly affected assemblage composition. Our results provide evidence that distance strongly determines the spatial distribution of bees in the OSR field. In a meta-analysis, Ricketts et al. (2008) showed that native pollinator visitation rate drops to 50% of the maximum at a location 668 m away from natural habitats. Some other studies focusing on the effect of forest on bee visits or pollination services are consistent with these results (e.g., Hawkins 1965; De Marco and Coelho 2004; Chacoff and Aizen 2006). Together with ours, these studies highlight that forest edges are likely to be a pollinator source for different crops. Indeed, forest edges present a complex vertical structure and undisturbed soil offering shelter for all bees and a wide range of nesting sites for both cavity-and ground-nesting bee. In addition, they provide a diversity of floral resources throughout the bees' activity period. Finally, these studies also suggest that the pollination of the mass-flowering crop, OSR, could be negatively affected by too great distance from the forest (Morandin and Winston 2005), unless the few species that venture farther afield can provide on their own the supplementary pollination necessary for the crop.
We also observed a positive effect of the interaction between cold orientation and distance on Andrena females and Nomada abundance. This is consistent with the ecological requirements of solitary bees; they are thermophilous insects so they prefer warm exposed sites for foraging. They may therefore travel further into the field to forage in well-exposed areas. Moreover, rapeseed flowering could be sparse and occurs later along cold forest edges. In that case, bees would probably forage further into the crop where better exposure has encouraged more abundant floral resources.
Hypothesis 2: the identity of bees in the crop is related to their foraging range
In contrast to Lentini et al. (2012), we found that larger female bees were found in the fields further from the forest edge. However, all the fields in Lentini et al.'s study contained small untilled areas that could have provided alternative nectar sources or nesting sites and acted as local population sources within the otherwise homogeneous fields. Arthur et al. (2010) also presumed that the absence of an edge effect on solitary bees in OSR might indicate that some bees were nesting inside the crop fields, with minimum tillage technique ground nesting may be possible. In our study, we assumed that: (i) the recorded taxa could not nest in the field itself as mechanical tillage was carried out at least once a year and (ii) some taxa must have covered distances of up to 230 m to reach the OSR field from their nesting sites on the forest edge. We hypothesized that females would be distributed according to their foraging range, calculated by measuring their ITD (Greenleaf et al. 2007). In our study, we found that mean ITD increased with distance from forest edge. Overall, we found that distance was the second most important explanatory factor for the variance in bee communities. For large taxa, the higher energy consumption required to fly further may well be compensated for by less competition for forage resources. The social taxa, Bombus spp. and A. mellifera, may benefit even more than solitary taxa from the lower competition in the center of the plot because they need to store large amounts of resources to start colonies (Herrmann et al. 2007; Westphal et al. 2009). Additionally, the decline in total bee abundance with increasing distance into the OSR field may reflect a dilution effect: pollinators in the middle of the field have more flowers to choose from away from the forest edge (Arthur et al. 2010). Indeed, even though several species of solitary bees have been found to be able to return from distances of up to 400 meters (Zurbuchen et al. 2010), the smaller species' foraging ranges probably remain rather limited if resources are abundant nearby. This could result in a negative impact on pollination efficiency far from the forest edge with a decrease in interspecific interactions (Greenleaf and Kremen 2006b).
Hypothesis 3: forest edge as nesting or mating sites for Andrena
Wild bee nests are difficult to locate in the field (Waters et al. 2011), unless a very limited area is intensively studied. Therefore, we decided to use the distribution patterns of males and nest parasites as general indicators of the areas likely to be used by Andrena for nesting or mating; indeed, these two groups' activity is mostly, although not exclusively, focused around nesting or mating sites rather than forage sites (Eickwort and Ginsberg 1980). Andrena males patrol areas, marking vegetation with mandibular gland secretions around the nesting sites of females or their food plants (Tengo 1979; Ayasse et al. 2001) or actively search for receptive females at emergence sites (Butler 1965; Tengo 1979). The reproductive success of Nomada depends on the capacity of females to find host nests and gain entry into them (Tengo and Bergstrom 1977; Cane 1983). In our study, the preference shown by both Andrena males and their cleptoparasites for forest edges indicates suitable nesting, or at least mating sites, for some abundant Andrena species and their parasites. This is consistent with Calabuig (2000) who found that the abundance of males and inquilines was significantly higher along forest edges than along several of the other linear habitats tested. Moreover, we observed that several abundant females (A haemorrhoa, A nitida, A nigroaenea, and A cineraria) occurred at different distances, while their males were most abundant along forest edges. Therefore, forest edges may not just be “partial habitats”; they could be a population sources for potential pollinators to OSR fields.
Implications for bee conservation and agricultural landscape management
The main objective of this study was to assess whether forest edges are an important partial habitat for potential OSR pollinators. Our results clearly support this assumption. We found that the forest edge is likely to be a nesting site and/or mating site for an important group of pollinating bees, the Andrenidae. Furthermore, the forest edge is a potential foraging site for all bees because of the early spring-flowering trees or forbs it contains. Therefore, taking into account, the proportion of forest edges around a field could be an indirect way to measure direct factors such as food availability or the presence of suitable pollinator nesting sites and/or mating sites in a landscape (Roulston and Goodell 2011), at least during spring. We also show that forest edge value may vary depending on microclimatic conditions such as the amount of sunlight it receives. We therefore recommend that future studies include forest edges and seasonality as explanatory variables to explain bee abundance or richness in a given landscape.
The decrease in pollinators with distance seems to be caused by flight costs as indicated by the mean increase in bee size with distance. Therefore, preserving untilled conservation land inside crop fields may be a way to offset the absence of bees in large fields with distant forest edges (Lentini et al. 2012). Brosi et al. (2008) proposed a model farm configuration that would maximize crop yield; the highest-yield farm designs were those with a relatively small area of pollination reservoirs, suggesting a conservation strategy of small parcels of service-providing habitat interspersed throughout working landscapes. However, small pollination reservoirs are probably not complete habitats in themselves, so this farm design is likely to be dependent on bee flight range and their ability to disperse throughout the crop matrix (Bommarco et al. 2010). All these results suggest that forest edges are important sources of pollinators because they provide different “partial habitats” for bees (Westrich 1996). However, forest edges need to be spatially well integrated into the agricultural matrix: (i) to promote bee populations, (ii) to ensure pollination services, and (iii) to enhance opportunities for colonization via connecting habitats (Steffan-Dewenter et al. 2002). Unfortunately, trees are often negatively perceived by farmers because they compete with crops for sunlight, nutrients, or water (Huth et al. 2010). Yet, studies show that forest edges or trees may provide several additional ecosystems services such as pest control (Bianchi et al. 2005; Stutz et al. 2011), soil quality improvement, water regulation (Tsonkova et al. 2012), and wind breaks (Brandle et al. 2004). The loss in crop yield induced by forest edges should therefore be weighed up against the potential ecological benefits gained. We recommend that forest edges should be included in agro-environmental schemes and “green belt networks” (http://www.legrenelle-environnement.fr/spip.php, MEEDDM 2010) to promote bee populations, bee biodiversity, and diverse ecosystem services. We also recommend that forest edges should be associated with other agro-environmental schemes, such as fallow land or hedgerows, to supply partial habitats for different bee species throughout the bees' seasonal activity (Hannon and Sisk 2009; Lye et al. 2009).
Acknowledgments
We greatly thank Eric Dufrêne, David Genoud, Sébastien Patiny, Alain Pauly, Pierre Rasmont, Mickaël Terzö, and Erwin Scheuchl for bee identification, Julien Fleury, Nicolas Lagarde, and Carl Moliard who took part in data collection and in specimen preparation before identification and Annie Ouin, Marc Deconchat, Jérôme Willm, Phillippe Caniot, Bruno Dumora, and Martin Vigan for their data collection in the South of France. We also thank Philip Roche, Mickaël Terzö, Mickaël Henry, Serge Gadoum, Eric Dufrêne, and Emilie Andrieu for discussions on the project. We are also grateful to the landowners who granted us access to their land and Vicki Moore for checking the English language. Andrew Bates, Andrew Beckerman, and an anonymous reviewer significantly helped us to improve the manuscript. The contributions by S.G.P and S.P.M.R were supported by the STEP project (Status and Trends of European Pollinators, Grant no. 244090 – STEP – CP – FP). This research was funded by the French Ministry in Charge of the Ecology through the BGF program (convention Bilisse 10-MBGD-BGF-4-CVS-084, no CHORUS 2100215042). Part of this work was also funded by two grants from Irstea and the “Conseil Regional du Centre”.
Conflict of Interest
None declared.
Funding Information
This research was funded by the French Ministry in Charge of the Ecology through the BGF program (convention Bilisse 10-MBGD-BGF-4-CVS-084, no CHORUS 2100215042). Part of this work was also funded by two grants from Irstea and the “Conseil Regional du Centre”.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Fitted GAMM model of the response of total abundance, richness, mean female ITD, Andrena female abundance, Andrena male abundance and total Nomada abundance as a function of distance from the forest edge for the two qualitative variables “cold” and “warm” orientation.
References
- Aebi A, Neumann P. Endosymbionts and honey bee colony losses? Trends Ecol. Evol. 2011;26:494. doi: 10.1016/j.tree.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Anderson MJ, Willis TJ. Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology. 2003;84:511–525. [Google Scholar]
- Arthur AD, Li J, Henry S, Cunningham SA. Influence of woody vegetation on pollinator densities in oilseed Brassica fields in an Australian temperate landscape. Basic Appl. Ecol. 2010;11:406–414. [Google Scholar]
- Ashman TL, Knight TM, Steets JA, Amarasekare P, Burd M, Campbell DR, et al. Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology. 2004;85:2408–2421. [Google Scholar]
- Ayasse M, Paxton RJ, Tengo J. Mating behavior and chemical communication in the order Hymenoptera. Annu. Rev. Entomol. 2001;46:31–78. doi: 10.1146/annurev.ento.46.1.31. [DOI] [PubMed] [Google Scholar]
- Bianchi F, van Wingerden W, Griffioen AJ, van der Veen M, van der Straten M, Wegman R, et al. Landscape factors affecting the control of Mamestra brassicae by natural enemies in Brussels sprout. Agric. Ecosyst. Environ. 2005;107:145–150. [Google Scholar]
- Biesmeijer JC, Roberts SPM, Reemer M, Ohlemuller R, Edwards M, Peeters T, et al. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science. 2006;313:351–354. doi: 10.1126/science.1127863. [DOI] [PubMed] [Google Scholar]
- Blanche KR, Ludwig JA, Cunningham SA. Proximity to rainforest enhances pollination and fruit set in orchards. J. Appl. Ecol. 2006;43:1182–1187. [Google Scholar]
- Bommarco R, Biesmeijer JC, Meyer B, Potts SG, Pöyry J, Roberts SP, et al. Dispersal capacity and diet breadth modify the response of wild bees to habitat loss. Proc. R. Soc. B: Biol. Sci. 2010;277:2075–2082. doi: 10.1098/rspb.2009.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bommarco R, Marini L, Vaissiere BE. Insect pollination enhances seed yield, quality, and market value in oilseed rape. Oecologia. 2012;169:1025–1032. doi: 10.1007/s00442-012-2271-6. [DOI] [PubMed] [Google Scholar]
- Brandle JR, Hodges L, Zhou XH. Windbreaks in North American Agricultural Systems. Agrofor. Syst. 2004;61:65–78. [Google Scholar]
- Breeze TD, Bailey AP, Balcombe KG, Potts SG. Pollination services in the UK: how important are honeybees? Agric. Ecosyst. Environ. 2011;142:137–143. [Google Scholar]
- Brittain C, Potts SG. The potential impacts of insecticides on the life-history traits of bees and the consequences for pollination. Basic Appl. Ecol. 2011;12:321–331. [Google Scholar]
- Brosi BJ, Armsworth PR, Daily GC. Optimal design of agricultural landscapes for pollination services. Conserv. Lett. 2008;1:27–36. [Google Scholar]
- Butler CG. Sex attraction in Andrena flavipes Panzer (Hymenoptera: Apidae), with some observations on nest-site restriction. Proc. R. Entomol. Soc. Lond. A, General Entomol. 1965;40:77–80. [Google Scholar]
- Calabuig I. Solitary bees and bumble bees in a Danish agricultural landscape. Denmark: University of Copenhagen, Zoological Institute; 2000. PhD Thesis. [Google Scholar]
- Cane JH. Olfactory evaluation of Andrena host nest suitability by kleptoparasitic Nomada bees (Hymenoptera, Apoidea) Anim. Behav. 1983;31:138–144. [Google Scholar]
- Carvalheiro LG, Seymour CL, Veldtman R, Nicolson SW. Pollination services decline with distance from natural habitat even in biodiversity-rich areas. J. Appl. Ecol. 2010;47:810–820. [Google Scholar]
- Chacoff NP, Aizen MA. Edge effects on flower-visiting insects in grapefruit plantations bordering premontane subtropical forest. J. Appl. Ecol. 2006;43:18–27. [Google Scholar]
- De Marco P, Coelho FM. Services performed by the ecosystem: forest remnants influence agricultural cultures' pollination and production. Biodivers. Conserv. 2004;13:1245–1255. [Google Scholar]
- Delbrassine S, Rasmont P. Contribution à l'étude de la pollinisation du colza Brassica napus L; var oleifera (Moench) Delile en Belgique. Bull. Rech. Agron. Gembloux. 1988;23:123–152. [Google Scholar]
- Eickwort GC, Ginsberg HS. Foraging and mating – behavior in Apoidea. Annu. Rev. Entomol. 1980;25:421–446. [Google Scholar]
- vanEngelsdorp D, Evans JD, Saegerman C, Mullin C, Haubruge E, Nguyen BK, et al. Colony collapse disorder: a descriptive study. PLoS One. 2009;4:e6481. doi: 10.1371/journal.pone.0006481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garibaldi LA, Steffan-Dewenter I, Winfree R, Aizen MA, Bommarco R, Cunningham SA, et al. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science. 2013;339:1608–1611. doi: 10.1126/science.1230200. [DOI] [PubMed] [Google Scholar]
- Greenleaf SS, Kremen C. Wild bee species increase tomato production and respond differently to surrounding land use in Northern California. Biol. Conserv. 2006a;133:81–87. [Google Scholar]
- Greenleaf SS, Kremen C. Wild bees enhance honey bees'' pollination of hybrid sunflower. Proc. Natl Acad. Sci. USA. 2006b;103:13890–13895. doi: 10.1073/pnas.0600929103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenleaf SS, Williams NM, Winfree R, Kremen C. Bee foraging ranges and their relationship to body size. Oecologia. 2007;153:589–596. doi: 10.1007/s00442-007-0752-9. [DOI] [PubMed] [Google Scholar]
- Hannon LE, Sisk TD. Hedgerows in an agri-natural landscape: potential habitat value for native bees. Biol. Conserv. 2009;142:2140–2154. [Google Scholar]
- Hawkins RP. Factors affecting the yield of seed produced by different varieties of red clover. J. Agric. Sci. 1965;65:245–253. [Google Scholar]
- Herrmann F, Westphal C, Moritz RFA, Steffan-Dewenter I. Genetic diversity and mass resources promote colony size and forager densities of a social bee (Bombus pascuorum) in agricultural landscapes. Mol. Ecol. 2007;16:1167–1178. doi: 10.1111/j.1365-294X.2007.03226.x. [DOI] [PubMed] [Google Scholar]
- Huth NI, Robertson MJ, Poulton PL, Perry L. Regional differences in tree-crop competition due to soil, climate and management. Crop Pasture Sci. 2010;61:763–770. [Google Scholar]
- Jauker F, Bondarenko B, Becker HC, Steffan-Dewenter I. Pollination efficiency of wild bees and hoverflies provided to oilseed rape. Agric. For. Entomol. 2012;14:81–87. [Google Scholar]
- Kearns CA, Inouye D, Waser NM. Endangered mutualisms: the conservation of plant-pollinator interactions. Annu. Rev. Ecol. Evol. Syst. 1998;29:83–112. [Google Scholar]
- Klein AM, Vaissiere BE, Cane JH, Steffan-Dewenter I, Cunningham SA, Kremen C, et al. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B-Biol. Sci. 2007;274:303–313. doi: 10.1098/rspb.2006.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen C, Williams NM, Bugg RL, Fay JP, Thorp RW. The area requirements of an ecosystem service: crop pollination by native bee communities in California. Ecol. Lett. 2004;7:1109–1119. [Google Scholar]
- Le Feon V, Burel F, Chifflet R, Henry M, Ricroch A, Vaissière BE, et al. Solitary bee abundance and species richness in dynamic agricultural landscapes. Agric. Ecosyst. Environ. 2011;166:94–101. [Google Scholar]
- Lentini PE, Martin TG, Gibbons P, Fischer J, Cunningham SA. Supporting wild pollinators in a temperate agricultural landscape: maintaining mosaics of natural features and production. Biol. Conserv. 2012;149:84–92. [Google Scholar]
- Lye GC, Park K, Osborne J, Holland J, Goulson D. Assessing the value of Rural Stewardship schemes for providing foraging resources and nesting habitat for bumblebee queens (Hymenoptera: Apidae) Biol. Conserv. 2009;142:2023–2032. [Google Scholar]
- MEEDDM. 2010. (Ministère de l'Ecologie, de l'Energie, du Développement Durable et de la Mer). The Green and Blue Infrastucture in mainland France, Challenges and experiences.
- Mesquida J, Renard M. Pollination of male-fertile and male-sterile winter rapeseed (Brassica-napus L var Oleifera Metzger) by the honey bee (Apis-mellifica L) – Effects on phenology and yield. Apidologie. 1981;12:345–362. [Google Scholar]
- Morandin LA, Winston ML. Wild bee abundance and seed production in conventional, organic, and genetically modified canola. Ecol. Appl. 2005;15:871–881. [Google Scholar]
- Morandin LA, Winston ML. Pollinators provide economic incentive to preserve natural land in agroecosystems. Agric. Ecosyst. Environ. 2006;116:289–292. [Google Scholar]
- Ollerton J, Price V, Armbruster WS, Memmott J, Watts S, Waser NM, et al. Overplaying the role of honey bees as pollinators: a comment on Aebi and Neumann (2011) Trends Ecol. Evol. 2012;27:141–142. doi: 10.1016/j.tree.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 2010;25:345–353. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. ISBN 3-900051-07-0, URL http://www.R-project.org/ [Google Scholar]
- Ricketts T, Regetz J, Steffan-Dewenter I, Cunningham S, Kremen C, Bogdanski A, et al. Landscape effects on crop pollination services: are there general patterns? Ecol. Lett. 2008;11:499–515. doi: 10.1111/j.1461-0248.2008.01157.x. [DOI] [PubMed] [Google Scholar]
- Roulston TH, Goodell K. The role of resources and risks in regulating wild bee populations. Annu. Rev. Entomol. 2011;56:293–312. doi: 10.1146/annurev-ento-120709-144802. [DOI] [PubMed] [Google Scholar]
- Sabbahi R, De Oliveira D, Marceau J. Influence of honey bee (Hymenoptera: Apidae) density on the production of canola (Crucifera: Brassicacae) J. Econ. Entomol. 2005;98:367–372. [PubMed] [Google Scholar]
- Sabbahi R, De Oliveira D, Marceau J. Does the honeybee (Hymenoptera: Apidae) reduce the blooming period of canola? J. Agron. Crop Sci. 2006;192:233–237. [Google Scholar]
- Sobek S, Tscharntke T, Scherber C, Schiele S, Steffan-Dewenter I. Canopy vs. understory: does tree diversity affect bee and wasp communities and their natural enemies across forest strata? For. Ecol. Manage. 2009;258:609–615. [Google Scholar]
- Steffan-Dewenter I. Importance of habitat area and landscape context for species richness of bees and wasps in fragmented orchard meadows. Conserv. Biol. 2003a;17:1036–1044. [Google Scholar]
- Steffan-Dewenter I. Seed set of male-sterile and male-fertile oilseed rape (Brassica napus) in relation to pollinator density. Apidologie. 2003b;34:225–235. [Google Scholar]
- Steffan-Dewenter I, Westphal C. The interplay of pollinator diversity, pollination services and landscape change. J. Appl. Ecol. 2008;45:737–741. [Google Scholar]
- Steffan-Dewenter I, Munzenberg U, Burger C, Thies C, Tscharntke T. Scale-dependent effects of landscape context on three pollinator guilds. Ecology. 2002;83:1421–1432. [Google Scholar]
- Stutz S, Entling MH, Martin H. Effects of the landscape context on aphid-ant-predator interactions on cherry trees. Biol. Control. 2011;57:37–43. [Google Scholar]
- Taki H, Kevan PG, Ascher JS. Landscape effects of forest loss in a pollination system. Landscape Ecol. 2007;22:1575–1587. [Google Scholar]
- Tengo J. Odor-released behavior in Andrena male bees (Apoidea, Hymenoptera) Scandinavian Univ. Press. 1979;7:15–48. [Google Scholar]
- Tengo J, Bergstrom G. Cleptoparasitism and odor mimetism in bees – do Nomada males imitate odor of Andrena females? Science. 1977;196:1117–1119. doi: 10.1126/science.196.4294.1117. [DOI] [PubMed] [Google Scholar]
- Tsonkova P, Boehm C, Quinkenstein A, Freese D. Ecological benefits provided by alley cropping systems for production of woody biomass in the temperate region: a review. Agrofor. Syst. 2012;85:133–152. [Google Scholar]
- Waters J, O'Connor S, Park KJ, Goulson D. Testing a detection dog to locate bumblebee colonies and estimate nest density. Apidologie. 2011;2:200–205. [Google Scholar]
- Watson JC, Wolf AT, Ascher JS. Forested landscapes promote richness and abundance of native bees (Hymenoptera: Apoidea: Anthophila) in Wisconsin apple orchards. Environ. Entomol. 2011;40:621–632. doi: 10.1603/EN10231. [DOI] [PubMed] [Google Scholar]
- Westphal C, Bommarco R, Carre G, Lamborn E, Morison N, Petanidou T, et al. Measuring bee diversity in different European habitats and biogeographical regions. Ecol. Monogr. 2008;78:653–671. [Google Scholar]
- Westphal C, Steffan-Dewenter I, Tscharntke T. Mass flowering oilseed rape improves early colony growth but not sexual reproduction of bumblebees. J. Appl. Ecol. 2009;46:187–193. [Google Scholar]
- Westrich P, Matheson A, Buchmann SL, O'Toole C, Westrich P, Williams IH. The Conservation of Bees. London, U.K: Academic Press; 1996. Habitat requirements of central European bees and the problems of partial habitats; pp. 1–16. [Google Scholar]
- Williams NM, Crone EE, Roulston TH, Minckley RL, Packer L, Potts SG. Ecological and life-history traits predict bee species responses to environmental disturbances. Biol. Conserv. 2010;143:2280–2291. [Google Scholar]
- Winfree R, Williams NM, Gaines H, Ascher JS, Kremen C. Wild bee pollinators provide the majority of crop visitation across land-use gradients in New Jersey and Pennsylvania, USA. J. Appl. Ecol. 2008;45:793–802. [Google Scholar]
- Zurbuchen A, Landert L, Klaiber J, Muller A, Hein S, Dorn S. Maximum foraging ranges in solitary bees: only few individuals have the capability to cover long foraging distances. Biol. Conserv. 2010;143:669–676. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fitted GAMM model of the response of total abundance, richness, mean female ITD, Andrena female abundance, Andrena male abundance and total Nomada abundance as a function of distance from the forest edge for the two qualitative variables “cold” and “warm” orientation.


