Abstract
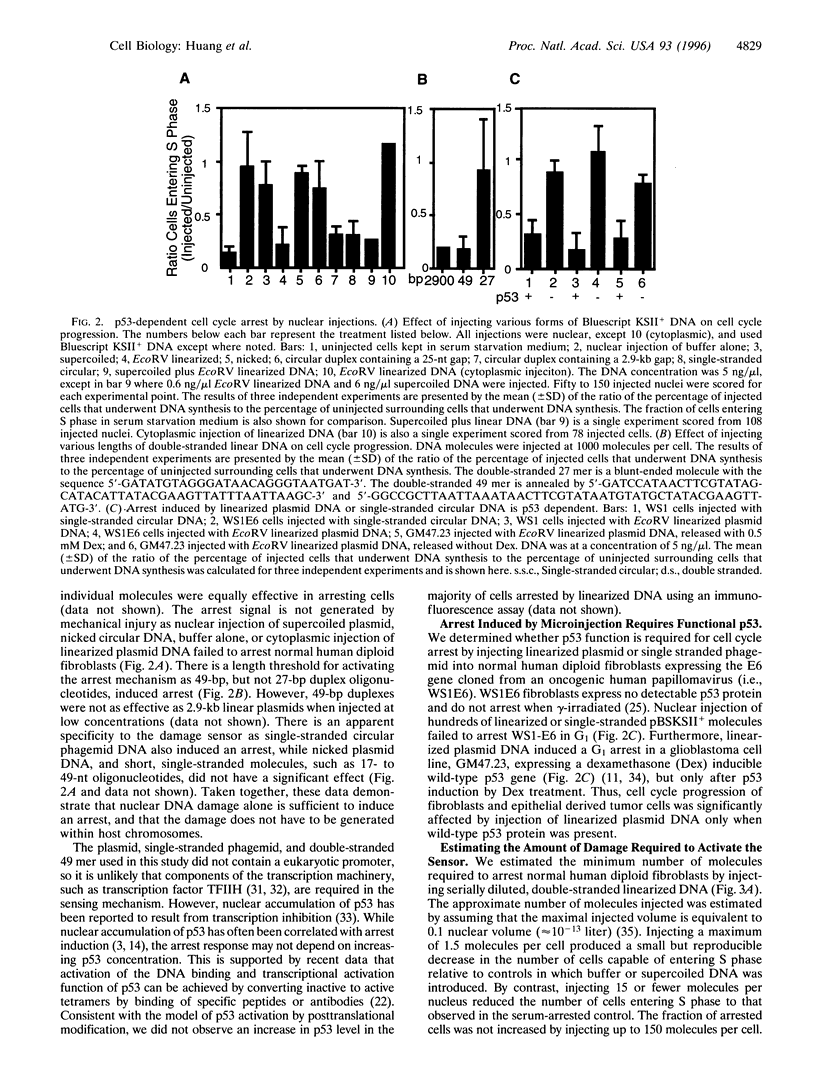
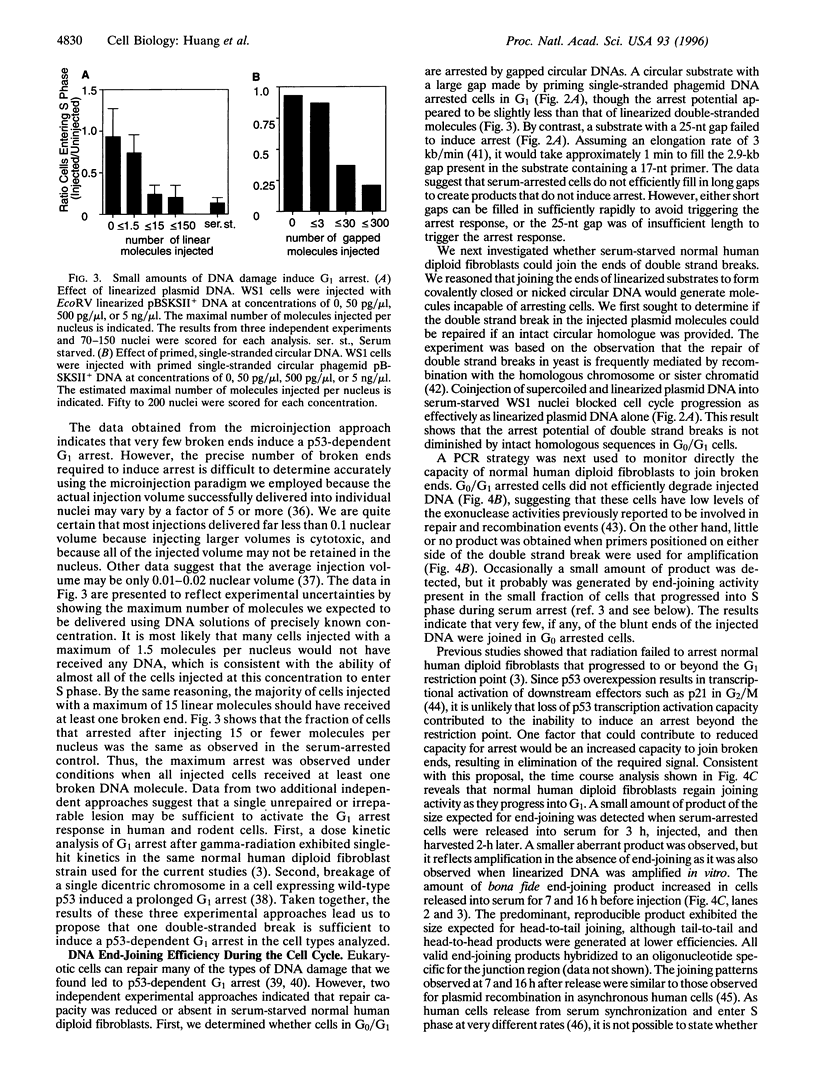
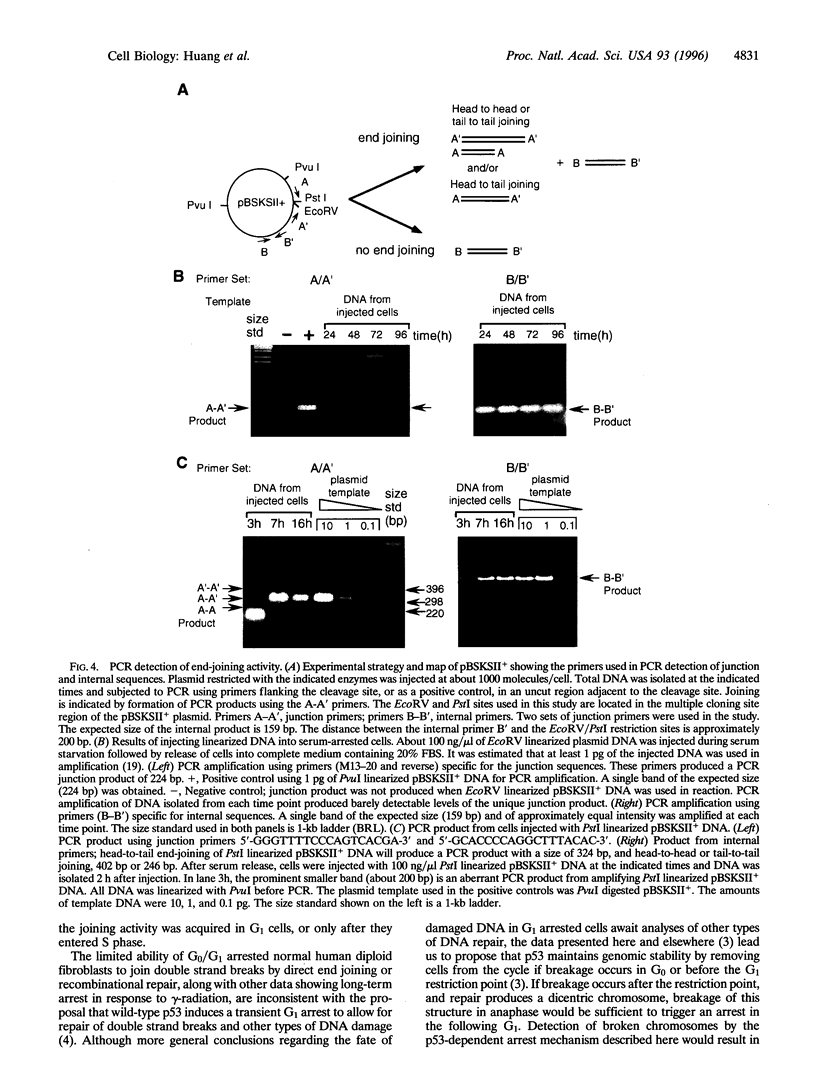
The tumor suppressor p53 contributes to maintaining genome stability by inducing a cell cycle arrest or apoptosis in response to conditions that generate DNA damage. Nuclear injection of linearized plasmid DNA, circular DNA with a large gap, or single-stranded circular phagemid is sufficient to induce a p53-dependent arrest. Supercoiled and nicked plasmid DNA, and circular DNA with a small gap were ineffective. Titration experiments indicate that the arrest mechanism in normal human fibroblasts can be activated by very few double strand breaks, and only one may be sufficient. Polymerase chain reaction assays showed that end-joining activity is low in serum-arrested human fibroblasts, and that higher joining activity occurs as cells proceed through G1 or into S phase. We propose that the exquisite sensitivity of the p53-dependent G1 arrest is partly due to inefficient repair of certain types of DNA damage in early G1.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal M. L., Agarwal A., Taylor W. R., Stark G. R. p53 controls both the G2/M and the G1 cell cycle checkpoints and mediates reversible growth arrest in human fibroblasts. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8493–8497. doi: 10.1073/pnas.92.18.8493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsopp R. C., Vaziri H., Patterson C., Goldstein S., Younglai E. V., Futcher A. B., Greider C. W., Harley C. B. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10114–10118. doi: 10.1073/pnas.89.21.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almasan A., Linke S. P., Paulson T. G., Huang L. C., Wahl G. M. Genetic instability as a consequence of inappropriate entry into and progression through S-phase. Cancer Metastasis Rev. 1995 Mar;14(1):59–73. doi: 10.1007/BF00690212. [DOI] [PubMed] [Google Scholar]
- Bennett C. B., Lewis A. L., Baldwin K. K., Resnick M. A. Lethality induced by a single site-specific double-strand break in a dispensable yeast plasmid. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5613–5617. doi: 10.1073/pnas.90.12.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R. High efficiency transformation by direct microinjection of DNA into cultured mammalian cells. Cell. 1980 Nov;22(2 Pt 2):479–488. doi: 10.1016/0092-8674(80)90358-x. [DOI] [PubMed] [Google Scholar]
- Clarke A. R., Purdie C. A., Harrison D. J., Morris R. G., Bird C. C., Hooper M. L., Wyllie A. H. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993 Apr 29;362(6423):849–852. doi: 10.1038/362849a0. [DOI] [PubMed] [Google Scholar]
- Desautels L., Brouillette S., Wallenburg J., Belmaaza A., Gusew N., Trudel P., Chartrand P. Characterization of nonconservative homologous junctions in mammalian cells. Mol Cell Biol. 1990 Dec;10(12):6613–6618. doi: 10.1128/mcb.10.12.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Leonardo A., Linke S. P., Clarkin K., Wahl G. M. DNA damage triggers a prolonged p53-dependent G1 arrest and long-term induction of Cip1 in normal human fibroblasts. Genes Dev. 1994 Nov 1;8(21):2540–2551. doi: 10.1101/gad.8.21.2540. [DOI] [PubMed] [Google Scholar]
- Di Leonardo A., Linke S. P., Yin Y., Wahl G. M. Cell cycle regulation of gene amplification. Cold Spring Harb Symp Quant Biol. 1993;58:655–667. doi: 10.1101/sqb.1993.058.01.073. [DOI] [PubMed] [Google Scholar]
- Frankenberg-Schwager M. Induction, repair and biological relevance of radiation-induced DNA lesions in eukaryotic cells. Radiat Environ Biophys. 1990;29(4):273–292. doi: 10.1007/BF01210408. [DOI] [PubMed] [Google Scholar]
- Gottlieb T. M., Jackson S. P. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell. 1993 Jan 15;72(1):131–142. doi: 10.1016/0092-8674(93)90057-w. [DOI] [PubMed] [Google Scholar]
- Greider C. W. Telomeres, telomerase and senescence. Bioessays. 1990 Aug;12(8):363–369. doi: 10.1002/bies.950120803. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Riggs A. D. On the mechanism of DNA replication in mammalian chromosomes. J Mol Biol. 1968 Mar 14;32(2):327–341. doi: 10.1016/0022-2836(68)90013-2. [DOI] [PubMed] [Google Scholar]
- Hupp T. R., Sparks A., Lane D. P. Small peptides activate the latent sequence-specific DNA binding function of p53. Cell. 1995 Oct 20;83(2):237–245. doi: 10.1016/0092-8674(95)90165-5. [DOI] [PubMed] [Google Scholar]
- Iliakis G. The role of DNA double strand breaks in ionizing radiation-induced killing of eukaryotic cells. Bioessays. 1991 Dec;13(12):641–648. doi: 10.1002/bies.950131204. [DOI] [PubMed] [Google Scholar]
- Ishizaka Y., Chernov M. V., Burns C. M., Stark G. R. p53-dependent growth arrest of REF52 cells containing newly amplified DNA. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3224–3228. doi: 10.1073/pnas.92.8.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S. K., Inman R. B., Cox M. M. Putative three-stranded DNA pairing intermediate in recA protein-mediated DNA strand exchange: no role for guanine N-7. J Biol Chem. 1992 Feb 25;267(6):4215–4222. [PubMed] [Google Scholar]
- Jayaraman J., Prives C. Activation of p53 sequence-specific DNA binding by short single strands of DNA requires the p53 C-terminus. Cell. 1995 Jun 30;81(7):1021–1029. doi: 10.1016/s0092-8674(05)80007-8. [DOI] [PubMed] [Google Scholar]
- Jones C. J., Wynford-Thomas D. Is TFIIH an activator of the p53-mediated G1/S checkpoint? Trends Genet. 1995 May;11(5):165–166. doi: 10.1016/S0168-9525(00)89033-2. [DOI] [PubMed] [Google Scholar]
- Kadyk L. C., Hartwell L. H. Sister chromatids are preferred over homologs as substrates for recombinational repair in Saccharomyces cerevisiae. Genetics. 1992 Oct;132(2):387–402. doi: 10.1093/genetics/132.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- LaMorte V. J., Goldsmith P. K., Spiegel A. M., Meinkoth J. L., Feramisco J. R. Inhibition of DNA synthesis in living cells by microinjection of Gi2 antibodies. J Biol Chem. 1992 Jan 15;267(2):691–694. [PubMed] [Google Scholar]
- Lane D. P. Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- Livingstone L. R., White A., Sprouse J., Livanos E., Jacks T., Tlsty T. D. Altered cell cycle arrest and gene amplification potential accompany loss of wild-type p53. Cell. 1992 Sep 18;70(6):923–935. doi: 10.1016/0092-8674(92)90243-6. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Ruley H. E., Jacks T., Housman D. E. p53-dependent apoptosis modulates the cytotoxicity of anticancer agents. Cell. 1993 Sep 24;74(6):957–967. doi: 10.1016/0092-8674(93)90719-7. [DOI] [PubMed] [Google Scholar]
- Lowe S. W., Schmitt E. M., Smith S. W., Osborne B. A., Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993 Apr 29;362(6423):847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- Lu X., Lane D. P. Differential induction of transcriptionally active p53 following UV or ionizing radiation: defects in chromosome instability syndromes? Cell. 1993 Nov 19;75(4):765–778. doi: 10.1016/0092-8674(93)90496-d. [DOI] [PubMed] [Google Scholar]
- Mercer W. E., Shields M. T., Amin M., Sauve G. J., Appella E., Romano J. W., Ullrich S. J. Negative growth regulation in a glioblastoma tumor cell line that conditionally expresses human wild-type p53. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6166–6170. doi: 10.1073/pnas.87.16.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger L., Iliakis G. Kinetics of DNA double-strand break repair throughout the cell cycle as assayed by pulsed field gel electrophoresis in CHO cells. Int J Radiat Biol. 1991 Jun;59(6):1325–1339. doi: 10.1080/09553009114551201. [DOI] [PubMed] [Google Scholar]
- Milne D. M., Campbell L. E., Campbell D. G., Meek D. W. p53 is phosphorylated in vitro and in vivo by an ultraviolet radiation-induced protein kinase characteristic of the c-Jun kinase, JNK1. J Biol Chem. 1995 Mar 10;270(10):5511–5518. doi: 10.1074/jbc.270.10.5511. [DOI] [PubMed] [Google Scholar]
- Minaschek G., Bereiter-Hahn J., Bertholdt G. Quantitation of the volume of liquid injected into cells by means of pressure. Exp Cell Res. 1989 Aug;183(2):434–442. doi: 10.1016/0014-4827(89)90402-3. [DOI] [PubMed] [Google Scholar]
- Nelson W. G., Kastan M. B. DNA strand breaks: the DNA template alterations that trigger p53-dependent DNA damage response pathways. Mol Cell Biol. 1994 Mar;14(3):1815–1823. doi: 10.1128/mcb.14.3.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberosler P., Hloch P., Ramsperger U., Stahl H. p53-catalyzed annealing of complementary single-stranded nucleic acids. EMBO J. 1993 Jun;12(6):2389–2396. doi: 10.1002/j.1460-2075.1993.tb05893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell W. K., Chu G. Involvement of the Ku autoantigen in the cellular response to DNA double-strand breaks. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7623–7627. doi: 10.1073/pnas.91.16.7623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzing J., Lane D. P. p53-dependent growth arrest following calcium phosphate-mediated transfection of murine fibroblasts. Oncogene. 1995 May 4;10(9):1865–1868. [PubMed] [Google Scholar]
- Roth D. B., Porter T. N., Wilson J. H. Mechanisms of nonhomologous recombination in mammalian cells. Mol Cell Biol. 1985 Oct;5(10):2599–2607. doi: 10.1128/mcb.5.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandell L. L., Zakian V. A. Loss of a yeast telomere: arrest, recovery, and chromosome loss. Cell. 1993 Nov 19;75(4):729–739. doi: 10.1016/0092-8674(93)90493-a. [DOI] [PubMed] [Google Scholar]
- Shibata T., Cunningham R. P., Radding C. M. Homologous pairing in genetic recombination. Purification and characterization of Escherichia coli recA protein. J Biol Chem. 1981 Jul 25;256(14):7557–7564. [PubMed] [Google Scholar]
- Smith M. L., Chen I. T., Zhan Q., Bae I., Chen C. Y., Gilmer T. M., Kastan M. B., O'Connor P. M., Fornace A. J., Jr Interaction of the p53-regulated protein Gadd45 with proliferating cell nuclear antigen. Science. 1994 Nov 25;266(5189):1376–1380. doi: 10.1126/science.7973727. [DOI] [PubMed] [Google Scholar]
- Smith M. L., Chen I. T., Zhan Q., O'Connor P. M., Fornace A. J., Jr Involvement of the p53 tumor suppressor in repair of u.v.-type DNA damage. Oncogene. 1995 Mar 16;10(6):1053–1059. [PubMed] [Google Scholar]
- Swanson J. A., Lee M., Knapp P. E. Cellular dimensions affecting the nucleocytoplasmic volume ratio. J Cell Biol. 1991 Nov;115(4):941–948. doi: 10.1083/jcb.115.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlsty T. D., Margolin B. H., Lum K. Differences in the rates of gene amplification in nontumorigenic and tumorigenic cell lines as measured by Luria-Delbrück fluctuation analysis. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9441–9445. doi: 10.1073/pnas.86.23.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlsty T. D. Normal diploid human and rodent cells lack a detectable frequency of gene amplification. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3132–3136. doi: 10.1073/pnas.87.8.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E. A., Capecchi M. R. Effect of cell cycle position on transformation by microinjection. Somat Cell Mol Genet. 1985 Jan;11(1):43–51. doi: 10.1007/BF01534733. [DOI] [PubMed] [Google Scholar]
- Wright J. A., Smith H. S., Watt F. M., Hancock M. C., Hudson D. L., Stark G. R. DNA amplification is rare in normal human cells. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1791–1795. doi: 10.1073/pnas.87.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Lozano G. NF-kappa B activation of p53. A potential mechanism for suppressing cell growth in response to stress. J Biol Chem. 1994 Aug 5;269(31):20067–20074. [PubMed] [Google Scholar]
- Xiao H., Pearson A., Coulombe B., Truant R., Zhang S., Regier J. L., Triezenberg S. J., Reinberg D., Flores O., Ingles C. J. Binding of basal transcription factor TFIIH to the acidic activation domains of VP16 and p53. Mol Cell Biol. 1994 Oct;14(10):7013–7024. doi: 10.1128/mcb.14.10.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaizumi M., Sugano T. U.v.-induced nuclear accumulation of p53 is evoked through DNA damage of actively transcribed genes independent of the cell cycle. Oncogene. 1994 Oct;9(10):2775–2784. [PubMed] [Google Scholar]
- Yin Y., Tainsky M. A., Bischoff F. Z., Strong L. C., Wahl G. M. Wild-type p53 restores cell cycle control and inhibits gene amplification in cells with mutant p53 alleles. Cell. 1992 Sep 18;70(6):937–948. doi: 10.1016/0092-8674(92)90244-7. [DOI] [PubMed] [Google Scholar]
- Zetterberg A., Larsson O. Kinetic analysis of regulatory events in G1 leading to proliferation or quiescence of Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5365–5369. doi: 10.1073/pnas.82.16.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- el-Deiry W. S., Harper J. W., O'Connor P. M., Velculescu V. E., Canman C. E., Jackman J., Pietenpol J. A., Burrell M., Hill D. E., Wang Y. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994 Mar 1;54(5):1169–1174. [PubMed] [Google Scholar]
- el-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993 Nov 19;75(4):817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]





