Abstract
Background
Cholesterol exerts complex effects upon inflammation. There has been little investigation of whether serum cholesterol is associated with asthma, an inflammatory airways disease with great public health impact.
Objective
To determine relationships between levels of three serum cholesterol measures (total cholesterol [TC], high density lipoprotein-cholesterol [HDL-C], and non-HDL-C), and asthma/wheeze in a sample representative of the U.S. population.
Methods
Cross-sectional study of 7,005 participants aged ≥6 years from the 2005–2006 National Health and Nutrition Examination Survey.
Results
Serum TC and non-HDL-C were lower in current asthmatics than in subjects without current asthma in the overall population (TC: 188.5 vs. 192.2 mg/dL; non-HDL-C: 133.9 vs. 137.7 mg/dL; p<.05 for both), whereas HDL-C was not different. Adjusted odds ratios (ORs) from multivariate logistic regression per 1-standard deviation increase of TC and non-HDL-C for current asthma were 0.92 (95% confidence interval [CI], 0.86–0.98) and 0.91 (95% CI, 0.85–0.98), respectively. Upon racial/ethnic stratification, these relationships reflect marked reductions unique to Mexican Americans (MAs)(TC: 171.4 vs. 189.3 mg/dL [p<.001], OR 0.62 [95% CI, 0.48–0.80]; non-HDL-C: 119.8 vs. 137.9 mg/dL [p<.001], OR 0.62 [95% CI, 0.48–0.79]). Among MAs, the adjusted OR for wheeze requiring medical attention was 0.57 (95% CI, 0.43–0.75) for TC and 0.53 (95% CI, 0.33–0.85) for non-HDL-C. Relationships between cholesterol and asthma/wheeze were independent of body mass index and serum C-reactive protein, and similar between atopic and non-atopic participants.
Conclusion
Serum TC and non-HDL-C are inversely related to asthma in the U.S. population, chiefly reflecting a relationship among MAs.
Keywords: Lung, Asthma, Wheeze, Cholesterol, Atopy, Hispanic
INTRODUCTION
Complex interactions have been reported between cholesterol and inflammation. Both cholesterol loading and cholesterol depletion of leukocytes, including macrophages, neutrophils, and mast cells may trigger pro-inflammatory cellular responses.1–3 Dyslipidemia (i.e., elevated non-high density lipoprotein-cholesterol [HDL-C], reduced HDL-C) causes vascular inflammation through activation of innate immunity,4 though may also attenuate innate immunity and skew adaptive immunity toward a Th2 response in other settings,5 with differential effects depending on the genetic background of the host.6 Conversely, serum cholesterol declines during inflammation.7 Collectively, these reports suggest that bidirectional interactions between cholesterol and inflammation may impact disease differentially among human populations, and that cholesterol may influence inflammatory diseases other than atherosclerosis.
To date, relationships between cholesterol and inflammatory lung disease have been little studied, though emerging research indicates that cholesterol metabolism and inflammation are linked in the lung.8–10 Given the high prevalences in the U.S. of dyslipidemia and asthma, relationships between these disease entities may carry tremendous clinical implications. The few studies to date examining relationships between serum cholesterol and asthma have been limited in statistical power and demographic sampling. One group reported elevated serum total cholesterol (TC) to be associated with asthma,11 whereas two others found no association.12, 13 Significant inter-racial/ethnic differences exist not only in asthma prevalence,14–17 but also in the prevalence and molecular features of dyslipidemia, and in the relationship between dyslipidemia and disease, such as atherosclerotic cardiovascular disease (CVD).18–20 Mexican Americans (MAs), an understudied group, are distinguished by having an unexplained low prevalence of asthma,14–17 as well as a relatively low prevalence of CVD despite increased rates of dyslipidemia and inflammation.18–20 As with cholesterol and CVD, the relationship between cholesterol and asthma may be anticipated to differ among racial/ethnic backgrounds.
The National Health and Nutrition Examination Survey (NHANES) 2005–2006 measured serum cholesterol and characterized asthma and wheeze in a sample of subjects representative of the U.S. population. We hypothesized that serum TC and non-HDL-C would be directly associated with current asthma and wheeze requiring medical attention in the NHANES, and that these relationships would differ among racial/ethnic groups. Thus, stratification by race/ethnicity was prespecified. Our primary goal was to test for an independent relationship between serum cholesterol concentrations and asthma prevalence. Our secondary goal was to test for an independent relationship between serum cholesterol and wheeze requiring medical attention, a surrogate indicator of poorly controlled asthma and other obstructive lung disease. Given that cholesterol might have different relationships with allergic and non-allergic inflammation, we also tested for interactions of atopy with cholesterol in the outcomes.
METHODS
Study Population
Data were obtained from the NHANES 2005–2006, which was designed to assess the health and nutritional status of the civilian, noninstitutionalized U.S. population. Details may be found online: http://www.cdc.gov/nchs/. The NHANES classifies participants into 5 main racial/ethnic groups: non-Hispanic White (NHW), non-Hispanic Black (NHB), MA, Other Hispanic, Other/Multi-race. Due to the heterogeneity of the Other Hispanic and Other/Multi-race categories (non-Mexican Hispanics, Asians, Native Americans, Multiracial), we included them in analysis of the overall population, but did not analyze them separately. Low density lipoprotein cholesterol (LDL-C) was measured only in participants who were asked to fast for ≥8.5 hours (n=3,026), of whom n=250 did not meet the fasting requirements. By contrast, TC and HDL-C were measured in fasting and non-fasting participants (n=7,360). Fasting time (time since consumption of anything other than water) was recorded in this combined study population, permitting assignment of fasting duration to individual cholesterol measurements. Non-HDL-C (i.e., TC minus HDL-C) has comparable or better predictive value than LDL-C for CVD,21, 22 and both fasting and non-fasting non-HDL-C are predictive of CVD.23 Thus, all analyses were based upon TC and HDL-C measured in a combined fasting and non-fasting study population.
Clinical Outcomes
Doctor-diagnosed current asthma was assessed by questionnaire. Current asthmatics were defined as participants who answered in the affirmative to the questions, “Has a doctor ever told you that you had asthma?” and “Do you still have asthma?” For assessment of wheezing, participants were first asked “Have you had wheezing or whistling in your chest at any time in the past 12 months?” If answering ‘yes’, they were then asked “In the past 12 months, how many times have you gone to the doctor’s office or the hospital emergency room for attacks of wheezing or whistling?” Wheezing requiring medical attention in the past 12 months (hereafter referred to as ‘medical wheeze’) was defined as one or more episodes in response to the second question.
Serum cholesterol measurement
Serum TC and HDL-C were measured using a Roche Hitachi 717 or 912. For TC, coupled enzymatic reactions were used involving cholesteryl ester hydrolase, cholesterol oxidase, and peroxidase, followed by phenazone absorbance detection. HDL-C measurement was by the Roche/Boehringer-Mannheim Diagnostics direct HDL method. Non-HDL-C was derived by subtracting HDL-C from TC.
Covariates
Covariates were obtained from questionnaire, lab analyses (immunoglobulin E [IgE], serum cotinine, serum C-reactive protein [CRP]), and physical examination (height, weight). Atopy was defined as ≥1 detectable (≥0.35 kU/L) serum allergen-specific IgE to a panel of 19 allergens: Alternaria alternata, Apergillus fumigatus, Bermuda grass (Cynodon dactylon), birch (Betula verrucose), cat dander, cockroach (Blatella germanica), dog dander, dust mite (Dermatophagoides farinae, D. pteronyssinus), egg white, milk, mouse urine proteins, oak (Quercus alba), peanut (Arachis hypgaea), ragweed (Ambrosia elatior), rat urine proteins, Russian thistle (Salsola kali), rye grass (Lolium perenne), and shrimp (Pandalus borealis). IgE was measured with the Pharmacia Diagnostics ImmunoCAP 1000 System (Kalamazoo, Michigan). Cotinine was measured by isotope dilution-high performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry. CRP was measured by latex-enhanced nephelometry. Body mass index (BMI) is weight in kilograms divided by height in meters squared (kg/m2).
Statistical analyses
Relationships between serum cholesterol and asthma/wheeze were examined in stratified bivariate and logistic regression analyses. For each outcome, mean cholesterol values were compared for participants with and without that outcome for the total population and by race/ethnicity using the t-test. Odds ratios (ORs) for the associations between serum cholesterol concentrations and current asthma (dependent variable) and medical wheeze (dependent variable) were estimated with logistic regression per 1-standard deviation (SD) increase of each cholesterol subtype. The weighted SD for TC (39.1 mg/dL), non-HDL-C (39.3 mg/dL), and HDL-C (14.4 mg/dL) were determined for the overall NHANES 2005–2006 population for which these values were available (n=7,360). Covariates in fully adjusted models included age, sex, race/ethnicity (total population only), householder education, BMI, log-transformed cotinine, CRP, and number of hours fasting before blood draw. Associations in participants with and without atopy were compared using multiplicative interaction terms in logistic models. Separate models were run by race/ethnicity and for each cholesterol subtype. Interaction between serum cholesterol and race in the outcomes was tested. All analyses were adjusted for the NHANES complex sampling design using SAS statistical software (Version 9.1.3, Cary, NC) survey procedures (SURVEYFREQ, SURVEYMEAN, SURVEYREG) according to NHANES analysis specifications. Statistical significance was p≤.05 (main effects) or ≤.10 (interactions).
RESULTS
Table I shows the features of 7,005 subjects aged ≥6 years in the NHANES 2005–2006 for whom data were available for the respiratory questionnaire, serum TC and HDL-C, and other covariates as shown. The mean (SE) prevalences of current asthma and medical wheeze were 8.7 (0.5) % and 6.6 (0.5) %. Of those participants reporting current asthma, 36.5% reported medical wheeze, and of those reporting medical wheeze, 48.6% reported current asthma (see Table E1 in the Online Repository). Among racial/ethnic groups, the mean prevalence of self-reported physician-diagnosed current asthma was lowest in MAs (4.5% in MAs, 9.9% in NHBs [p<.001], 9.1% in NHWs [p<.001]). The prevalence of wheeze episodes over the preceding 12 months requiring medical attention was also lowest in MAs (2.8% in MAs, 5.3% in NHBs [p=.005], 7.2% in NHWs [p<.001]). Mean HDL-C was lowest among MAs, whereas MA non-HDL-C and TC were intermediate between that of NHBs and NHWs. Serum cotinine was lowest among MAs and highest among NHBs.
Table I.
Characteristics of study population by race/ethnicity.*
| Total Population‡ n=7005 (SE)† |
Mexican American n=1739 (SE)† |
Non-Hispanic Black n=1841 (SE)† |
Non-Hispanic White n=2897 (SE)† |
p-value § | |
|---|---|---|---|---|---|
| Age (Years) | 40.0 (0.8) | 31.5 (0.6) | 36.6 (0.9) | 42.3 (0.9) | < .001 |
| Age (%) | |||||
| 6–17 years | 17.1 (0.6) | 24.7 (0.2) | 19.8 (0.4) | 15.1 (0.7) | < .001 |
| 18–39 years | 32.5 (1.1) | 44.6 (0.5) | 36.8 (0.9) | 29.6 (0.9) | |
| >= 40 years | 50.4 (1.2) | 30.8 (0.4) | 43.4 (0.9) | 55.3 (2.0) | |
| Sex (%) | |||||
| Male | 49.0 (0.5) | 52.7 (0.5) | 46.4 (0.9) | 49.5 (1.6) | .07 |
| Female | 51.0 (0.6) | 47.3 (0.5) | 53.6 (1.1) | 50.5 (1.4) | |
| Education (%) | |||||
| < 9th grade | 6.3 (0.6) | 34.3 (0.5) | 4.4 (0.1) | 2.8 (0.4) | < .001 |
| 9th to < 12TH grade | 11.5 (1.1) | 19.1 (0.2) | 19.7 (0.5) | 9.2 (1.1) | |
| High School – GED | 25.2 (1.1) | 20.6 (0.3) | 24.7 (0.5) | 26.2 (1.5) | |
| Some college | 31.2 (1.0) | 19.7 (0.3) | 35.4 (0.7) | 32.2 (1.2) | |
| College Graduate | 25.8 (2.1) | 6.4 (0.1) | 15.8 (0.4) | 29.7 (2.1) | |
| Body Mass Index (kg/m2) | 27.2 (0.2) | 26.9 (0.2) | 28.8 (0.3) | 27.2 (0.3) | < .001 |
| Body Mass Index (%) | |||||
| Underweight or Normal | 50.0 (1.6) | 47.0 (0.5) | 41.2 (0.9) | 51.1 (1.3) | < .01 |
| Overweight (BMI 85% – 94%) | 22.6 (0.7) | 26.0 (0.3) | 22.4 (0.4) | 22.5 (1.2) | |
| Obese (BMI ≥95%) | 27.4 (1.3) | 27.0 (0.3) | 36.4 (0.8) | 26.5 (1.5) | |
| Serum Cotinine‖ (ng/mL) | 0.37 (0.04) | 0.12 (0.02) | 0.68 (0.17) | 0.40 (0.06) | < .001 |
| Smoking Status (%) | |||||
| Never Smoked | 57.3 (1.4) | 69.1 (0.7) | 67.1 (1.5) | 52.5 (2.1) | < .001 |
| Former Smoker | 21.4 (0.8) | 15.1 (0.2) | 13.2 (0.2) | 24.7 (0.9) | |
| Current Smoker | 21.3 (0.9) | 15.8 (0.2) | 19.8 (0.4) | 22.7 (1.1) | |
| C reactive protein (mg/dL) | 0.38 (0.01) | 0.42 (0.04) | 0.44 (0.02) | 0.38 (0.02) | .04 |
| Fasting Time (hours) | 7.0 (0.1) | 7.9 (0.2) | 7.5 (0.2) | 6.9 (0.1) | < .001 |
| Total Cholesterol (mg/dL) | 191.9 (0.7) | 188.5 (1.4) | 184.3 (0.7) | 193.4 (0.7) | < .001 |
| HDL Cholesterol (mg/dL) | 54.5 (0.3) | 51.4 (0.7) | 57.6 (0.3) | 54.4 (0.4) | < .001 |
| Non-HDL Cholesterol (mg/dL) | 137.4 (0.8) | 137.1 (1.6) | 126.7 (0.8) | 139.0 (0.9) | < .001 |
| Current Asthma (%) | 8.7 (0.5) | 4.5 (0.8) | 9.9 (0.7) | 9.1 (0.5) | < .001 |
| Medical Wheeze (%) | 6.6 (0.5) | 2.8 (0.5) | 5.3 (0.6) | 7.2 (0.7) | < .001 |
| Atopy (%) | 44.8 (1.2) | 46.7 (1.6) | 57.8 (1.6) | 41.7 (1.3) | < .001 |
| Total IgE (kU/L) | 139.7 (6.3) | 194.3 (18) | 260.1 (26) | 106.8 (6.9) | < .001 |
Mean values unless otherwise specified.
Standard error of the mean or percent.
Total Population also includes “Other/Multi-Race”.
P-value result from Chi-square test of homogeneity or ANOVA test of main effect for 3 race/ethnicity groups as appropriate.
Geometric mean reported since log(cotinine) is used as a covariate in the adjusted logistic regression models. Medical wheeze denotes wheeze episodes requiring medical attention. Atopy was defined as ≥1 detectable allergen-specific IgE value. HDL; high density lipoprotein.
To explore relationships between current asthma and serum cholesterol, we first compared mean serum cholesterol values between subjects with and without current asthma (Table II). TC and non-HDL-C were both significantly lower in asthmatics than in non-asthmatics in the overall population. Upon racial/ethnic stratification, the reduced TC and non-HDL-C was specific to MA asthmatics (p<.001 for both). By contrast, among NHBs and NHWs, TC and non-HDL-C were not significantly different between subjects with and without asthma. HDL-C was not significantly different between asthmatics and nonasthmatics in the overall population nor in any racial/ethnic stratum.
Table II.
Mean serum cholesterol among participants with and without current asthma by race/ethnicity.
| Total Population§ Mean (SE)* n overall n=7005 |
Mexican American Mean (SE)* n overall n=1739 |
Non-Hispanic Black Mean (SE)* n overall n=1841 |
Non-Hispanic White Mean (SE)* n overall n=2897 |
|
|---|---|---|---|---|
| Total Cholesterol (mg/dL) | ||||
| Current Asthma: Yes | 188.5 (1.4) 623 |
171.4 (4.5) 102 |
182.6 (3.2) 201 |
192.0 (1.7) 274 |
| Current Asthma: No | 192.2 (0.7)† 6382 |
189.3 (1.4)‡ 1637 |
184.5 (0.7) 1640 |
193.6 (0.7) 2623 |
| HDL Cholesterol (mg/dL) | ||||
| Current Asthma: Yes | 54.5 (0.9) 623 |
51.6 (1.1) 102 |
58.0 (1.5) 201 |
54.4 (1.2) 274 |
| Current Asthma: No | 54.5 (0.3) 6382 |
51.3 (0.7) 1637 |
57.5 (0.4) 1640 |
54.4 (0.3) 2623 |
| Non-HDL Cholesterol (mg/dL) | ||||
| Current Asthma: Yes | 133.9 (1.6) 623 |
119.8 (4.5) 102 |
124.6 (3.4) 201 |
137.6 (2.1) 274 |
| Current Asthma: No | 137.7 (0.8)† 6382 |
137.9 (1.5)‡ 1637 |
127.0 (0.8) 1640 |
139.2 (0.9) 2623 |
SE = Standard error of the mean.
P-value < .05 for t-test of null hypothesis that mean cholesterol levels are equal comparing participants with current asthma to those without current asthma.
P-value < .001 for t-test of null hypothesis that mean cholesterol levels are equal comparing participants with current asthma to those without current asthma.
Total Population also includes “Other/Multi-Race”. HDL; high density lipoprotein.
As with asthma, among MAs, mean TC and non-HDL-C were both lower in those subjects with than in those without medical wheeze (p<.001 for TC; p<.05 for non-HDL-C)(Table III). TC was also reduced among wheezing NHBs, albeit more modestly.
Table III.
Mean serum cholesterol among participants with and without wheeze requiring medical attention by race/ethnicity.
| Total Population§ Mean (SE)* n overall n=7005 |
Mexican American Mean (SE)* n overall n=1739 |
Non-Hispanic Black Mean (SE)* n overall n=1841 |
Non-Hispanic White Mean (SE)* n overall n=2897 |
|
|---|---|---|---|---|
| Total Cholesterol (mg/dL) | ||||
| Wheeze | 192.5 (2.5) 394 |
167.7 (4.8) 60 |
175.9 (3.6) 99 |
197.1 (3.4) 203 |
| No Wheeze | 191.8 (0.7) 6611 |
189.1 (1.4)‡ 1679 |
184.8 (0.7)† 1742 |
193.2 (0.7) 2694 |
| HDL Cholesterol (mg/dL) | ||||
| Wheeze | 53.6 (0.9) 394 |
52.8 (2.3) 60 |
53.6 (1.5) 99 |
53.8 (1.0) 203 |
| No Wheeze | 54.6 (0.3) 6611 |
51.3 (0.7) 1679 |
57.8 (0.3)† 1742 |
54.5 (0.4) 2694 |
| Non-HDL Cholesterol (mg/dL) | ||||
| Wheeze | 139.0 (2.3) 394 |
114.9 (6.7) 60 |
122.3 (3.5) 99 |
143.2 (3.2) 203 |
| No Wheeze | 137.3 (0.8) 6611 |
137.8 (1.6)† 1679 |
127.0 (0.8) 1742 |
138.7 (0.8) 2694 |
SE = Standard error of the mean.
P-value < .05 for t-test of null hypothesis that mean cholesterol levels are equal comparing participants with Wheeze to those without Wheeze.
P-value < .001 for t-test of null hypothesis that mean cholesterol levels are equal comparing participants with Wheeze to those without Wheeze.
Total Population also includes “Other/Multi-Race”. HDL; high density lipoprotein.
The relationships between serum cholesterol and current asthma were next analyzed by multivariate logistic regression (Table IV). In the overall population, after adjustment for covariates, including age, race/ethnicity, sex, householder education, BMI, serum CRP, log-transformed serum cotinine, and number of hours fasting, the odds ratio (OR) per 1-SD increase for current asthma diagnosis was 0.92 (95% confidence interval [CI], 0.86–0.98) for TC, and 0.91 (95% CI, 0.85–0.98) for non-HDL-C. Upon racial/ethnic stratification, these significant inverse relationships in the overall population between TC and asthma and non-HDL-C and asthma were determined largely to reflect striking inverse relationships specific to MAs (TC: adjusted OR 0.62 [95% CI, 0.48–0.80; race/ethnicity interaction p=.007]; non-HDL-C: adjusted OR 0.62 [95% CI, 0.48–0.79; race/ethnicity interaction p=.02]). Calculated ORs were essentially unchanged when CRP was left out of the model (data not shown). No significant relationship was found between any cholesterol variable and asthma in NHBs or NHWs, nor between HDL-C and asthma in MAs. Similar results for non-HDL-C were found, albeit with wider CIs, in an analysis limited to the fasting study subpopulation (n=2,776), in which the LDL-C-asthma relationship was also examined (see Table E2 in Online Repository). No statistically significant interaction by atopy was found for the three cholesterol measures in the relationship to asthma (see Table E3 in the the Online Repository). The adjusted relationships between TC, non-HDL-C, and HDL-C, and current asthma for the different racial/ethnic groups, as modeled by logistic regression using serum cholesterol values measured in the NHANES 2005–2006, are depicted in the Figure. In order to explore the cholesterol-asthma relationship in the overall population further, we performed analyses stratified by age, gender, smoking status, and BMI category (see Tables E4–E7 in the Online Repository). Of interest among these analyses was an interaction with gender observed in the HDL-C- and non-HDL-C-asthma relationships (Table E5). We also analyzed the cholesterol-asthma relationship using clinically relevant cutpoints for the three cholesterol measures (see Table E8 in the Online Repository).
Table IV.
Associations between current asthma and serum cholesterol from logistic regression analysis
| Total Cholesterol* | HDL Cholesterol* | Non-HDL Cholesterol* | ||||
|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) |
Adjusted OR§ (95% CI) |
Unadjusted OR (95% CI) |
Adjusted OR§ (95% CI) |
Unadjusted OR (95% CI) |
Adjusted OR§ (95% CI) |
|
| Total Population† | 0.92 (0.87–0.98) |
0.92 (0.86–0.98) |
1.00 (0.92–1.10) |
1.02 (0.91–1.15) |
0.92 (0.86–0.99) |
0.91 (0.85–0.98) |
| Mexican American‡ | 0.63 (0.49–0.82) |
0.62 (0.48–0.80) |
1.02 (0.85–1.21) |
0.95 (0.76–1.19) |
0.64 (0.50–0.81) |
0.62 (0.48–0.79) |
| Non-Hispanic Black‡ | 0.96 (0.82–1.12) |
0.98 (0.85–1.14) |
1.02 (0.88–1.20) |
1.06 (0.92–1.24) |
0.94 (0.80–1.11) |
0.95 (0.81–1.12) |
| Non-Hispanic White‡ | 0.97 (0.90–1.03) |
0.97 (0.91–1.04) |
1.00 (0.89–1.13) |
1.01 (0.88–1.17) |
0.97 (0.89–1.05) |
0.97 (0.90–1.05) |
Odds ratio (OR) and 95% confidence interval (CI) for each cholesterol-specific standard deviation (SD) change. SDs (TC = 39.1 mg/dL, non-HDL-C = 39.3 mg/dL, HDL-C = 14.4 mg/dL) were determined for the total NHANES 2005–2006 population for which serum cholesterol was measured (n=7,360).
From unadjusted model (current asthma = cholesterol) and fully adjusted model including age, race/ethnicity, sex, householder education, body mass index, serum C-reactive protein, log-transformed serum cotinine, and hours fasting.
Fully adjusted model does not include race/ethnicity.
Adjusted racial/ethnic interaction p=0.007 for TC, p=0.33 for HDL-C, and p=0.02 for non-HDL-C. HDL; high density lipoprotein.
Figure.
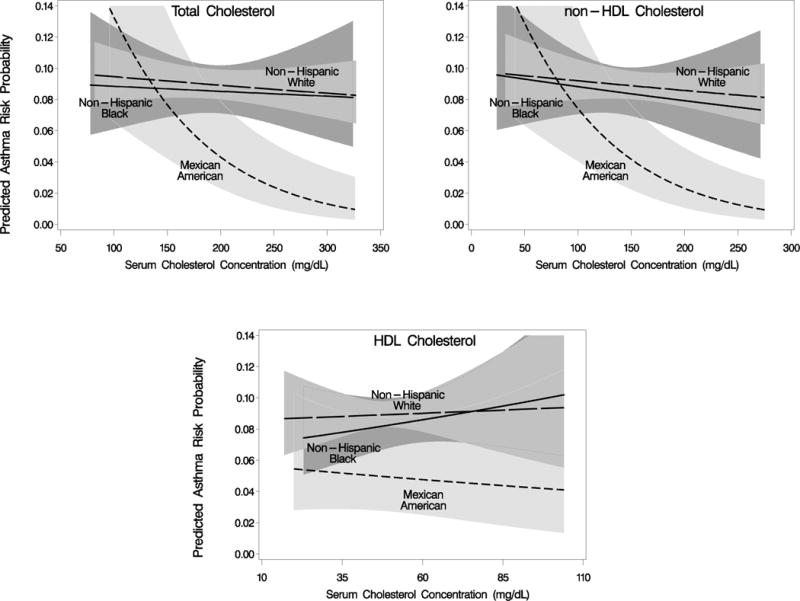
Predicted probability of current asthma for racial/ethnic categories in the NHANES 2005–2006 in relation to serum values of total-, non-high density lipoprotein (HDL)-, and HDL- cholesterol. Logistic regression curves are adjusted for age, sex, householder education, body mass index, serum C-reactive protein, serum cotinine, and hours fasting. Range for each figure is limited to overall mean +/− 3.5 standard deviations. 95% confidence intervals are shown with shading.
We next tested for an independent relationship between serum cholesterol and medical wheeze. As shown in Table V, among MAs, the adjusted OR for wheeze per 1-SD increase of TC was 0.57 (95% CI, 0.43–0.75), and that per 1-SD increase of non-HDL-C was 0.53 (95% CI, 0.33–0.85), indicating that TC and non-HDL-C are inversely related to medical wheeze. A similar, albeit more modest relationship was found between TC and wheeze and non-HDL-C and wheeze among NHBs, but not among NHWs (race/ethnicity interaction p<.001 for both TC and non-HDL-C). An inverse relationship between non-HDL-C and wheeze was also found for MAs and NHBs in an analysis limited to the fasting study subpopulation; similar findings were noted in an analysis using LDL-C (see Table E9 in the Online Repository). No statistically significant interaction by atopy was found for the three cholesterol measures in the wheeze outcome (see Table E10 in the the Online Repository). Analyses of the cholesterol-wheeze relationship stratified by age, gender, smoking status, and BMI category appear as Tables E11–E14 in the Online Repository. The cholesterol-wheeze relationship analyzed using clinically relevant cholesterol cutpoints appears as Table E15 in the Online Repository.
Table V.
Associations between wheeze requiring medical attention and serum cholesterol from logistic regression analysis
| Total Cholesterol* | HDL Cholesterol* | Non-HDL Cholesterol* | ||||
|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) |
Adjusted OR§ (95% CI) |
Unadjusted OR (95% CI) |
Adjusted OR§ (95% CI) |
Unadjusted OR (95% CI) |
Adjusted OR§ (95% CI) |
|
| Total Population† | 1.02 (0.92–1.12) |
0.99 (0.88–1.10) |
0.94 (0.84–1.06) |
0.97 (0.86–1.09) |
1.04 (0.94–1.14) |
1.00 (0.90–1.10) |
| Mexican American‡ | 0.57 (0.43–0.76) |
0.57 (0.43–0.75) |
1.10 (0.82–1.47) |
1.07 (0.75–1.53) |
0.55 (0.35–0.86) |
0.53 (0.33–0.85) |
| Non-Hispanic Black‡ | 0.79 (0.66–0.96) |
0.80 (0.70–0.93) |
0.78 (0.64–0.96) |
0.79 (0.62–1.02) |
0.89 (0.74–1.05) |
0.88 (0.78–0.99) |
| Non-Hispanic White‡ | 1.09 (0.95–1.24) |
1.08 (0.94–1.24) |
0.96 (0.85–1.08) |
1.00 (0.88–1.13) |
1.10 (0.98–1.24) |
1.08 (0.96–1.22) |
Odds ratio (OR) and 95% confidence interval (CI) for each cholesterol-specific standard deviation (SD) change. SDs (TC = 39.1 mg/dL, non-HDL-C = 39.3 mg/dL, HDL-C = 14.4 mg/dL) were determined for the total NHANES 2005–2006 population for which serum cholesterol was measured (n=7,360).
From unadjusted model (wheeze = cholesterol) and fully adjusted model including age, race/ethnicity, sex, householder education, body mass index, serum C-reactive protein, log-transformed serum cotinine, and hours fasting.
Fully adjusted model does not include race/ethnicity.
Adjusted racial/ethnic interaction p<.001 for TC, p=0.12 for HDL-C, and p<.001 for non-HDL-C. HDL; high density lipoprotein.
DISCUSSION
Dyslipidemia affects inflammation,1–4 and emerging studies suggest that cholesterol trafficking and inflammation are coupled in the lung.8–10 Given this, we tested for a relationship between serum cholesterol and asthma, an inflammatory lung disease, in the NHANES 2005–2006, a large, nationally representative survey. We report that, contrary to their risk factor status for CVD, TC and non-HDL-C are inversely related to current asthma in the overall U.S. population, and that this phenomenon chiefly reflects a relationship that is specific to MAs. TC and non-HDL-C were also inversely related to wheeze requiring medical attention in MAs and NHBs. These relationships are independent of both obesity (BMI), an established asthma risk factor,24 and CRP, an inflammatory biomarker, and are not modified by atopy.
To our knowledge, this is the first study to investigate a connection between cholesterol and asthma in a national survey, and the first to identify a factor that may possibly contribute to the reduced prevalence and morbidity of asthma in MAs. The few previous investigations of serum cholesterol and asthma have differed substantially in design and methodology. Al-Shawwa et al. reported that TC was positively associated with asthma, but this single-center study included only 189 NHW children.11 Picado et al. found no difference in serum cholesterol between 121 controls and 118 asthmatics at a single center, but did not stratify by race/ethnicity.12 In a nested case-control study of 1,537 adults in Germany, Schafer et al. found no significant relationship between serum cholesterol and asthma.13 In the present study, a very large sample size revealed significant inter-racial/ethnic differences in the cholesterol-asthma relationship in the U.S. population. While modest, statistically nonsignificant relationships were found in NHWs and NHBs, a striking relationship was observed in MAs.
Although Hispanics are the fastest growing minority in the U.S. and are projected to be 24.4% of the U.S. population by 2050,25 asthma in Hispanics has been understudied,26, 27 leading to its identification as an urgent research priority.28 Consistent with our findings, others have reported lower asthma prevalence in MAs than in NHWs and NHBs.14–17 This low prevalence remains unexplained, especially in the face of heightened asthma risk factors among MAs, such as obesity and low socioeconomic status.14, 15, 29 Of interest, among Hispanics, asthma prevalence and severity varies dramatically by subgroup. Whereas MAs have the lowest asthma prevalence in the U.S., Puerto Ricans have the highest prevalence30 and higher disease severity than MAs.31 Because of the complexity and extremity of the asthma phenotype among Hispanics, study of this population provides an opportunity to dissect novel determinants of asthma that may also have generalizable significance to other populations.
Several reports have indicated that MAs have increased dyslipidemia and inflammation, yet attenuated disease manifestations of cholesterol dysregulation, suggesting that a distinct dyslipidemia-inflammation-disease axis may characterize this population. Like us, other national surveys have found reduced HDL-C in MAs.32 Also reported are higher cardiovascular risk in MAs than NHWs,33 higher rates of metabolic syndrome in Hispanics than in NHWs and NHBs,34 and higher levels of circulating cytokines in MAs.6, 35 Despite increased dyslipidemia and inflammation, MAs have an attenuated risk relationship between metabolic syndrome and atherosclerosis,6, 34, 36 and, in fact, reduced CVD.18–20 Collectively, this literature suggests that MAs have a distinct lipid-inflammation setpoint and may have a distinct relationship between dyslipidemia and lipid-related inflammatory disease.
While our analysis was cross-sectional, there is some cellular and animal model evidence to suggest that non-HDL-C, and, in particular LDL, the major lipoprotein component of the non-HDL fractions, may reduce asthma risk. LDL is taken up by the lung37 and induces functional responses in lung-resident cells. LDL suppresses cellular responsiveness to TGF-β,38 a pivotal asthma mediator, inhibits histamine release from human lung mast cells,39 and inhibits histamine effect.40 In mice, dyslipidemia is associated with dampened airway inflammation (M. Fessler, unpublished observations). However, whether LDL was causal in the present study or was a marker of other causal mediators such as oxidized LDL is uncertain. Of interest, oxidized LDL, which is elevated in MAs compared to NHWs,41 is a Toll like Receptor (TLR)4 stimulus, and TLR4 stimulation dampens asthmatic phenotypes in rodent models.42 Moreover, oxidized LDL phospholipids impair, and HDL restores, dendritic cell differentiation and antigen presentation to T cells,43 immunological events critical to asthma.
Our study had limitations. As the NHANES is cross-sectional, the causality and directionality of the cholesterol-asthma relationship is uncertain. Despite the multiple adjustments we made, the possibility of unmeasured confounders remains. Given the multiple, non-prespecified comparisons made in our secondary, stratified analyses (Tables E2–E15 in the Online Repository), these findings are best viewed as exploratory and hypothesis-generating. We performed our analyses with combined ‘fasting’ and ‘non-fasting’ cholesterol values; this notwithstanding, the fasting time in our study population had tight variation, was very close to the 8 hour fast traditionally imposed for fasting serum lipid measurement, and was adjusted for in the regressions. Moreover, upon stratification by fasting status (8.5 hr cutpoint), we found no significant interactions of fasting with either TC or non-HDL-C in the asthma or wheeze outcomes (data not shown). We were unable to adjust for non-prescription medication usage due to non-availability of these data. Two trials have reported that statins do not affect clinical asthma outcomes.44, 45 Consistent with this, we found no significant interactions between statin use and any of the cholesterol measures in either the asthma or wheeze outcome (data not shown). The effect of inhaled and oral glucocorticoids on serum lipids is somewhat controversial, though a large analysis of NHANES III reported no differences in TC, LDL-C, or HDL-C between glucocorticoid users and non-users among participants <60 years old.46 Finally, we did not address diet. Multiple nutritional factors, including antioxidants, fruits, minerals, and fatty acids have been variably associated with altered risk for asthma and allergy.47 As non-HDL-C-raising westernized diets are generally low in antioxidants, fruit, and some minerals, these reportedly asthma-protective factors are not likely to confound the cholesterol-asthma relationship we report. Nevertheless, future studies may be warranted, especially given likely inter-racial/ethnic differences in dietary composition.
Several clinical questions arise. Do cholesterol-reducing therapies modify asthma risk differentially among MAs, NHWs, and NHBs? Do changes in asthma severity temporally affect serum cholesterol, and, if so, does cholesterol track with exacerbation and treatment response? Do other metabolic syndrome components interact with serum cholesterol to modify asthma risk? In MA subjects with respiratory symptoms, do serum cholesterol levels have good predictive value in diagnosis of asthma?
In conclusion, serum TC and non-HDL-C were negatively associated with asthma and wheeze in MAs in this cross-sectional analysis. To our knowledge, this is the first report of a putative ‘protective’ biological factor that may distinguish asthma in MAs from that in other racial/ethnic groups. If independently confirmed, we speculate that these findings may have implications for the treatment of dyslipidemia in MAs, and may also provide new insights into basic mechanisms of asthma pathogenesis and treatment relevant to additional populations.
Supplementary Material
Key Messages.
Asthma is inversely related to serum total and non-high density lipoprotein cholesterol in the U.S. population, chiefly reflecting a relationship among Mexican Americans.
The cholesterol-asthma relationship is independent of body mass index and serum C-reactive protein, and similar among atopic and non-atopic subjects.
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (Z01 ES102005).
Abbreviations
- BMI
Body Mass Index
- CI
Confidence Interval
- CRP
C-Reactive Protein
- CVD
Cardiovascular Disease
- HDL-C
high density lipoprotein-cholesterol
- IgE
immunoglobulin E
- LDL-C
low density lipoprotein-cholesterol
- MA
Mexican American
- NHANES
National Health and Nutrition Examination Survey
- NHB
non-Hispanic Black
- NHW
non-Hispanic White
- OR
Odds Ratio
- SD
Standard Deviation
- TC
total cholesterol
- TLR4
Toll Like Receptor 4
References
- 1.Fessler MB, Arndt PG, Frasch SC, Lieber JG, Johnson CA, Murphy RC, et al. Lipid rafts regulate lipopolysaccharide-induced activation of Cdc42 and inflammatory functions of the human neutrophil. J Biol Chem. 2004;279:39989–98. doi: 10.1074/jbc.M401080200. [DOI] [PubMed] [Google Scholar]
- 2.Li Y, Schwabe RF, DeVries-Seimon T, Yao PM, Gerbod-Giannone MC, Tall AR, et al. Free cholesterol-loaded macrophages are an abundant source of tumor necrosis factor-alpha and interleukin-6: model of NF-kappaB- and map kinase-dependent inflammation in advanced atherosclerosis. J Biol Chem. 2005;280:21763–72. doi: 10.1074/jbc.M501759200. [DOI] [PubMed] [Google Scholar]
- 3.Baumruker T, Csonga R, Pursch E, Pfeffer A, Urtz N, Sutton S, et al. Activation of mast cells by incorporation of cholesterol into rafts. Int Immunol. 2003;15:1207–18. doi: 10.1093/intimm/dxg120. [DOI] [PubMed] [Google Scholar]
- 4.Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, et al. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res. 2007;100:1589–96. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- 5.Robertson AK, Zhou X, Strandvik B, Hansson GK. Severe hypercholesterolaemia leads to strong Th2 responses to an exogenous antigen. Scand J Immunol. 2004;59:285–93. doi: 10.1111/j.0300-9475.2004.01403.x. [DOI] [PubMed] [Google Scholar]
- 6.Allison MA, Budoff MJ, Wong ND, Blumenthal RS, Schreiner PJ, Criqui MH. Prevalence of and risk factors for subclinical cardiovascular disease in selected US Hispanic ethnic groups: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2008;167:962–9. doi: 10.1093/aje/kwm402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabana VG, Siegel JN, Sabesin SM. Effects of the acute phase response on the concentration and density distribution of plasma lipids and apolipoproteins. J Lipid Res. 1989;30:39–49. [PubMed] [Google Scholar]
- 8.Baldan A, Gomes AV, Ping P, Edwards PA. Loss of ABCG1 results in chronic pulmonary inflammation. J Immunol. 2008;180:3560–8. doi: 10.4049/jimmunol.180.5.3560. [DOI] [PubMed] [Google Scholar]
- 9.Fessler MB, Young SK, Jeyaseelan S, Lieber JG, Arndt PG, Nick JA, et al. A role for hydroxy-methylglutaryl coenzyme a reductase in pulmonary inflammation and host defense. Am J Respir Crit Care Med. 2005;171:606–15. doi: 10.1164/rccm.200406-729OC. [DOI] [PubMed] [Google Scholar]
- 10.Wojcik AJ, Skaflen MD, Srinivasan S, Hedrick CC. A critical role for ABCG1 in macrophage inflammation and lung homeostasis. J Immunol. 2008;180:4273–82. doi: 10.4049/jimmunol.180.6.4273. [DOI] [PubMed] [Google Scholar]
- 11.Al-Shawwa B, Al-Huniti N, Titus G, Abu-Hasan M. Hypercholesterolemia is a potential risk factor for asthma. J Asthma. 2006;43:231–3. doi: 10.1080/02770900600567056. [DOI] [PubMed] [Google Scholar]
- 12.Picado C, Deulofeu R, Lleonart R, Agusti M, Casals E, Quinto L, et al. Lipid and protein metabolism in asthma. Effects of diet and corticosteroid therapy. Allergy. 1999;54:569–75. doi: 10.1034/j.1398-9995.1999.00024.x. [DOI] [PubMed] [Google Scholar]
- 13.Schafer T, Ruhdorfer S, Weigl L, Wessner D, Heinrich J, Doring A, et al. Intake of unsaturated fatty acids and HDL cholesterol levels are associated with manifestations of atopy in adults. Clin Exp Allergy. 2003;33:1360–7. doi: 10.1046/j.1365-2222.2003.01780.x. [DOI] [PubMed] [Google Scholar]
- 14.Arif AA, Borders TF, Patterson PJ, Rohrer JE, Xu KT. Prevalence and correlates of paediatric asthma and wheezing in a largely rural USA population. J Paediatr Child Health. 2004;40:189–94. doi: 10.1111/j.1440-1754.2004.00335.x. [DOI] [PubMed] [Google Scholar]
- 15.Arif AA, Delclos GL, Lee ES, Tortolero SR, Whitehead LW. Prevalence and risk factors of asthma and wheezing among US adults: an analysis of the NHANES III data. Eur Respir J. 2003;21:827–33. doi: 10.1183/09031936.03.00054103a. [DOI] [PubMed] [Google Scholar]
- 16.Eldeirawi K, McConnell R, Freels S, Persky VW. Associations of place of birth with asthma and wheezing in Mexican American children. J Allergy Clin Immunol. 2005;116:42–8. doi: 10.1016/j.jaci.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 17.Homa DM, Mannino DM, Lara M. Asthma mortality in U.S. Hispanics of Mexican, Puerto Rican, and Cuban heritage, 1990–1995. Am J Respir Crit Care Med. 2000;161:504–9. doi: 10.1164/ajrccm.161.2.9906025. [DOI] [PubMed] [Google Scholar]
- 18.Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2005;111:1313–20. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 19.Liao Y, Cooper RS, Cao G, Kaufman JS, Long AE, McGee DL. Mortality from coronary heart disease and cardiovascular disease among adult U.S. Hispanics: findings from the National Health Interview Survey (1986 to 1994) J Am Coll Cardiol. 1997;30:1200–5. doi: 10.1016/s0735-1097(97)00278-7. [DOI] [PubMed] [Google Scholar]
- 20.Reaven PD, Thurmond D, Domb A, Gerkin R, Budoff MJ, Goldman S. Comparison of frequency of coronary artery calcium in healthy Hispanic versus non-Hispanic white men by electron beam computed tomography. Am J Cardiol. 2003;92:1198–200. doi: 10.1016/j.amjcard.2003.07.030. [DOI] [PubMed] [Google Scholar]
- 21.Davidson MH. Is LDL-C passed its prime? The emerging role of non-HDL, LDL-P, and ApoB in CHD risk assessment. Arterioscler Thromb Vasc Biol. 2008;28:1582–3. doi: 10.1161/ATVBAHA.108.172718. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Sempos CT, Donahue RP, Dorn J, Trevisan M, Grundy SM. Non-high-density lipoprotein and very-low-density lipoprotein cholesterol and their risk predictive values in coronary heart disease. Am J Cardiol. 2006;98:1363–8. doi: 10.1016/j.amjcard.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 23.Mora S, Rifai N, Buring JE, Ridker PM. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118:993–1001. doi: 10.1161/CIRCULATIONAHA.108.777334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shore SA. Obesity and asthma: possible mechanisms. J Allergy Clin Immunol. 2008;121:1087–93. doi: 10.1016/j.jaci.2008.03.004. quiz 94–5. [DOI] [PubMed] [Google Scholar]
- 25.U.S. interim projections by age, sex, race, and Hispanic origin [Internet] U.S. Census Bureau; Available from: htt’://www.census.gov/ipc/www/usinterimproj/ [released March 18, 2004]; [Google Scholar]
- 26.Brahan D, Bauchner H. Changes in reporting of race/ethnicity, socioeconomic status, gender, and age over 10 years. Pediatrics. 2005;115:e163–6. doi: 10.1542/peds.2004-1437. [DOI] [PubMed] [Google Scholar]
- 27.Cohen RT, Celedon JC. Asthma in Hispanics in the United States. Clin Chest Med. 2006;27:401–12. v. doi: 10.1016/j.ccm.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 28.Flores G, Fuentes-Afflick E, Barbot O, Carter-Pokras O, Claudio L, Lara M, et al. The health of Latino children: urgent priorities, unanswered questions, and a research agenda. Jama. 2002;288:82–90. doi: 10.1001/jama.288.1.82. [DOI] [PubMed] [Google Scholar]
- 29.Update: prevalence of overweight among children, adolescents, and adults–United States, 1988–1994. MMWR Morb Mortal Wkly Rep. 1997;46:198–202. [PubMed] [Google Scholar]
- 30.Carter-Pokras OD, Gergen PJ. Reported asthma among Puerto Rican, Mexican-American, and Cuban children, 1982 through 1984. Am J Public Health. 1993;83:580–2. doi: 10.2105/ajph.83.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burchard EG, Avila PC, Nazario S, Casal J, Torres A, Rodriguez-Santana JR, et al. Lower bronchodilator responsiveness in Puerto Rican than in Mexican subjects with asthma. Am J Respir Crit Care Med. 2004;169:386–92. doi: 10.1164/rccm.200309-1293OC. [DOI] [PubMed] [Google Scholar]
- 32.Carroll MD, Lacher DA, Sorlie PD, Cleeman JI, Gordon DJ, Wolz M, et al. Trends in serum lipids and lipoproteins of adults, 1960–2002. Jama. 2005;294:1773–81. doi: 10.1001/jama.294.14.1773. [DOI] [PubMed] [Google Scholar]
- 33.Crimmins EM, Kim JK, Alley DE, Karlamangla A, Seeman T. Hispanic paradox in biological risk profiles. Am J Public Health. 2007;97:1305–10. doi: 10.2105/AJPH.2006.091892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bertoni AG, Wong ND, Shea S, Ma S, Liu K, Preethi S, et al. Insulin resistance, metabolic syndrome, and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2007;30:2951–6. doi: 10.2337/dc07-1042. [DOI] [PubMed] [Google Scholar]
- 35.Ho RC, Davy KP, Hickey MS, Melby CL. Circulating tumor necrosis factor alpha is higher in non-obese, non-diabetic Mexican Americans compared to non-Hispanic white adults. Cytokine. 2005;30:14–21. doi: 10.1016/j.cyto.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell BD, Stern MP, Haffner SM, Hazuda HP, Patterson JK. Risk factors for cardiovascular mortality in Mexican Americans and non-Hispanic whites. San Antonio Heart Study. Am J Epidemiol. 1990;131:423–33. doi: 10.1093/oxfordjournals.aje.a115517. [DOI] [PubMed] [Google Scholar]
- 37.Nistor A, Simionescu M. Uptake of low density lipoproteins by the hamster lung. Interactions with capillary endothelium. Am Rev Respir Dis. 1986;134:1266–72. doi: 10.1164/arrd.1986.134.6.1266. [DOI] [PubMed] [Google Scholar]
- 38.Chen CL, Liu IH, Fliesler SJ, Han X, Huang SS, Huang JS. Cholesterol suppresses cellular TGF-beta responsiveness: implications in atherogenesis. J Cell Sci. 2007;120:3509–21. doi: 10.1242/jcs.006916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schulman ES, Quinn TJ, Post TJ, O’Donnell P, Rodriguez A, Gonen B. Low density lipoprotein (LDL) inhibits histamine release from human mast cells. Biochem Biophys Res Commun. 1987;148:553–9. doi: 10.1016/0006-291x(87)90912-0. [DOI] [PubMed] [Google Scholar]
- 40.Harrison GJ, Jordan LR, Selley ML, Willis RJ. Low-density lipoproteins inhibit histamine and NaNO2 relaxations of the coronary vasculature and reduce contractile function in isolated rat hearts. Heart Vessels. 1995;10:249–57. doi: 10.1007/BF01744904. [DOI] [PubMed] [Google Scholar]
- 41.Holvoet P, Jenny NS, Schreiner PJ, Tracy RP, Jacobs DR. The relationship between oxidized LDL and other cardiovascular risk factors and subclinical CVD in different ethnic groups: the Multi-Ethnic Study of Atherosclerosis (MESA) Atherosclerosis. 2007;194:245–52. doi: 10.1016/j.atherosclerosis.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez D, Keller AC, Faquim-Mauro EL, de Macedo MS, Cunha FQ, Lefort J, et al. Bacterial lipopolysaccharide signaling through Toll-like receptor 4 suppresses asthma-like responses via nitric oxide synthase 2 activity. J Immunol. 2003;171:1001–8. doi: 10.4049/jimmunol.171.2.1001. [DOI] [PubMed] [Google Scholar]
- 43.Cruz D, Watson AD, Miller CS, Montoya D, Ochoa MT, Sieling PA, et al. Host-derived oxidized phospholipids and HDL regulate innate immunity in human leprosy. J Clin Invest. 2008 doi: 10.1172/JCI34189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menzies D, Nair A, Meldrum KT, Fleming D, Barnes M, Lipworth BJ. Simvastatin does not exhibit therapeutic anti-inflammatory effects in asthma. J Allergy Clin Immunol. 2007;119:328–35. doi: 10.1016/j.jaci.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 45.Hothersall EJ, Chaudhuri R, McSharry C, Donnelly I, Lafferty J, McMahon AD, et al. Effects of atorvastatin added to inhaled corticosteroids on lung function and sputum cell counts in atopic asthma. Thorax. 2008 doi: 10.1136/thx.2008.100198. [DOI] [PubMed] [Google Scholar]
- 46.Choi HK, Seeger JD. Glucocorticoid use and serum lipid levels in US adults: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2005;53:528–35. doi: 10.1002/art.21329. [DOI] [PubMed] [Google Scholar]
- 47.Litonjua AA. Dietary factors and the development of asthma. Immunol Allergy Clin North Am. 2008;28:603–29. ix. doi: 10.1016/j.iac.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


