Abstract
Increasing heart rate enhances cardiac contractility (force frequency relationship, FFR) and accelerates cardiac relaxation (frequency-dependent acceleration of relaxation, FDAR). The positive FFR together with FDAR promotes rapid filling and ejection of blood from the left ventricle (LV) at higher heart rates. Recent studies indicate that the multifunctional Ca2+/calmodulin-dependent protein kinase II (CaMKII) is involved in regulating FFR and FDAR. We used isolated perfused mouse hearts to study the mechanisms of FFR and FDAR in different genetic models, including transgenic myocardial CaMKII inhibition (AC3-I) and phosphalamban knockout (PLN−/−). When the rate was increased from 360 beats/min to 630 beats/min in wild type mouse hearts, the LV developed pressure (LVDP) and the maximum rate of increase in pressure (dP/dt max) increased by 37.6 ± 4.7% and 77.0 ± 8.1%, respectively. However, hearts from AC3-I littermates showed no increase of LVDP and a relatively modest (20.4 ± 3.9 %) increase in dP/dt max. PLN−/− hearts had a negative FFR, and myocardial AC3-I expression did not change the FFR in PLN−/− mice. PLN−/− mouse hearts did not exhibit FDAR, while PLN−/−mice with myocardial AC3-I expression showed further frequency dependent reductions in cardiac relaxation, suggesting CaMKII targets in addition to PLN were critical to myocardial relaxation. We incubated a constitutively active form of CaMKII with chemically-skinned myocardium and found that several myofilament proteins were phosphorylated by CaMKII. However, CaMKII did not affect myofilament calcium sensitivity. Our study shows that CaMKII plays an important role in modulating FFR and FDAR in murine hearts and suggest that PLN is a critical target for CaMKII effects on FFR, while CaMKII effects on FDAR partially require PLN-alternative targets.
Keywords: CaM kinase II, force-frequency relation, frequency-dependent acceleration of relaxation, phospholamban
1. Introduction
The positive force frequency relationship (FFR) is an important intrinsic regulatory mechanism that allows the heart to increase cardiac output at higher rates, as part of the ‘fight or flight’ physiological response. Although, this phenomenon was first described by Bowditch in 1871, its underlying mechanisms are still not well understood.[1-4] Another important physiological change in response to increasing heart rate is the frequency-dependent acceleration of relaxation (FDAR) which makes rapid left ventricular (LV) filling at higher heart rates feasible. Together FFR and FADR coordinate cardiac output, but it remains unclear if common signals and targets coordinate FFR and FDAR.
Relatively recently, the multifunctional Ca2+ and calmodulin (CaM)-dependent protein kinase II (CaMKII) has emerged as a candidate for transducing rate-dependent intracellular Ca2+ changes to enhance performance of target proteins that coordinate the FFR and FDAR.[5-6] CaMKII is abundant in myocardium, and under resting conditions CaMKII is largely inactive by virtue of a tonic negative regulatory interaction between the catalytic domain and the autoinhibitory region. Increases in intracellular Ca2+ are sensed by CaM and calcified CaM (Ca2+/CaM) binds to the CaM-binding region of CaMKII, disrupting catalytic domain restraint by the autoinhibitory region and activating CaMKII.[7-8] CaMKII is a serine-threonine kinase that catalyzes the phosphorylation of proteins essential for excitation-contraction coupling and FFR.[8] CaMKII phosphorylation of the SR membrane protein phospholamban (PLN) reduces the inhibitory potency of PLN for the sarcoplasmic-endoplasmic reticulum Ca2+ ATPase (SERCA),[3, 9-11] leading to increased SR Ca2+ uptake and enhanced relaxation.[10, 12] . PLN[13-15] is thought to be an important target for CaMKII potentiation of FFR.[1, 16-18] However, the effects of PLN and CaMKII on FDAR are controversial.[19-20] CaMKII also catalyzes the phosphorylation of myofilament proteins,[21-22] but little is known about whether CaMKII affects the relationship between Ca2+ and developed tension.
We studied the FFR and FDAR in Langendorff-perfused mouse hearts isolated from models where CaMKII activity was genetically controlled by transgenic expression of AC3-I,[23] or in mice lacking PLN (PLN−/−) [24] to test the role of CaMKII on specific molecular targets. We also isolated small myocardial strips after detergent-mediated destruction of cellular membranes in the presence and absence of a constitutively active form of CaMKII to test the potential effects of CaMKII on the tension-pCa (-Log[Ca2+]) relationship. Here we show that CaMKII inhibition flattens the FFR, preserving mechanical functions at low stimulation frequencies. We found that PLN−/− eliminated FFR and increased the maximum rate of relaxation (-dP/dt min). CaMKII was effective at phosphorylating myofilament proteins, but did not affect the tension-pCa relationship. PLN−/− hearts with CaMKII inhibition showed that the absolute value of -dP/dt min was less than in PLN−/− hearts, suggesting that CaMKII has additional, as yet unidentified, target(s) for enhancing cardiac relaxation.
2. Materials and methods
2.1. Animal models
Mice with CaMKII inhibition due to transgenic, myocardial-delimited expression of a CaMKII inhibitory peptide (AC3-I) were described previously.[23] Wild type control mice (WT) were AC3-I littermates. PLN−/− mice were interbred with AC3-I and used for experiments.[25-26] All animal use was approved by the IACUC at the University of Iowa.
2.2 Isolated perfused heart experiments
Four month old mice of either sex were anesthetized using 2.5% Avertin (tribomoethanol 10g + Tert-amyl alcohol 10ml) and injected with 66μg/10g heparin. Hearts were surgically removed, cannulated on the tip of a modified 22-gauge needle and perfused retrogradely at constant pressure of 80 mmHg (37.5 °C) with a modified Krebs solution (in mM): 118 NaCl, 25 NaHCO3, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 2.5 CaCl2, 0.5 EDTA, 10 glucose, 5 Pyruvate and 5 U/L insulin. A very thin balloon was inserted into the LV through the mitral valve and connected to an APT-300 pressure transducer, which was connected to a computer. The LV balloon was filled with degassed water to adjust LV end diastolic pressure (LVEDP) to 4~8 mmHg. We measured the following parameters: the LV contractility parameters - left ventricular developed pressure (LVDP, the difference between the peak LV systolic pressure and LVEDP) and the maximum LV pressure change rate during systole (dP/dt max), the relaxation parameter- minimum LV pressure change rate during diastole (-dP/dt min). All data were recorded on a personal computer running Isoheart software (HSE Harvard apparatus). The atrioventricular node was mechanically ablated to slow the heart rate for stable over-drive ventricular pacing, which is necessary to test FFR between a wide range of heart rates in mice. The ventricles were paced at 6 Hz (or 7 Hz in PLN−/− mice) using a field stimulator at amplitude of 20% above threshold and were allowed to equilibrate for 30 min before collecting data. The hearts were subjected to repeated rapid pacing frequency changes: from 6 Hz to 7.5 Hz, 6 Hz to 9 Hz and 6 Hz to 10.5 Hz in WT, and AC3-I mice, or from 7 Hz to 8 Hz, 7 Hz to 9 Hz, 7 Hz to 10 Hz and 7 Hz to 10.5 Hz in PLN−/− and PLN−/− x AC3-I mice, because the PLN−/− hearts needed slightly higher stimulation rates to obtain stable baseline data. The LVDP, dP/dt max and –dP/dt min were measured at the steady state while pacing at 6 or 7 Hz when the beat-by-beat LVDP variability was ≤ 2% over 15 seconds. In WT, and AC3-I mice, the LV pressure increased after switching to the higher pacing rates, the parameters were measured at the highest pressure after switching the pacing rate, usually at 5-12 seconds after switch. For PLN−/− and PLN−/− x AC3-I mice, the LV pressure decreased after increasing the pacing rates, the parameters were obtained at the lowest steady-state LV pressure (change less than 1% between beats), usually within15 seconds after pacing frequency switch. In the isoproterenol (ISO) treatment experiments, 5 nM ISO was added to the perfusate after stable baseline data (~30 minutes from start) was obtained. Data were obtained again 10 minutes after ISO treatment.
2.3 In vitro contractile protein phosphorylation assay
LV papillary muscle from four month old WT mice were dissected in oxygenated modified Kreb solution and were skinned in relaxing solution (RS) with 1% Triton X-100 overnight at 3 °C.[27] Muscles were then washed thoroughly with RS, then incubated in a kinase buffer (50 mM HEPES, pH 7.5, 10 mM magnesium acetate, 0.5 mM calcium chloride, 1 mg/ml BSA, 0.4 mM ATP32) with a constitutively active form of CaMKII (T287D) purified from insect cells [28] and 32P-γ-ATP for 30 minutes at 22°C. The myocardium was homogenized in solubilization buffer, and the lysates run on SDS-PAGE 12% gel. The gel was Coomassie blue stained, dried and exposed to film. Protein bands were identified by molecular weight. We performed triple experiments and the MyBP-C phosphorylation was quantified by measuring optic density.
2.4 Myocardial calcium sensitivity experiments
Papillary muscles from the LV of four month old WT mice were dissected, chemically-skinned, washed thoroughly with RS then stored at − 20 °C in RS containing 50% (v/v) glycerol to be used within two weeks.[27] Skinned papillary muscles were dissected into small strips and attached to a force transducer and to a length controller, which were mounted on top of an inverted microscope stage. The stage contained 8 wells with different pCa solutions in which the muscles could be placed. The muscle sarcomere length (SL) was measured online from the striation image. The wells were temperature controlled at 15 °C. We measured the thickness and width of the preparation and calculated the cross-sectional area (CSA).[27, 29] The CSA was used to convert measured forces into tension (in mN/mm2) to allow comparison between different muscle strips.
We used RS (pCa 9.0), pre-activating solution (Pre-A), and maximal activating solution (AS, pCa 4.5). For solution compositions see reference 27.[30] Different pCa solutions were obtained by mixing RS and AS with the free [Ca2+] calculated according to Fabiato and Fabiato.[31] Relaxed fibers were set at a SL of ~2.00 μm. The fibers were activated in the following sequence: pre-A, AS, RS, pre-A, pCa 6.30, 6.15, 6.00, 5.85, 5.70, and 4.5, RS. The pCa 4.5 activation at the beginning and end of each experiment was used to calculate the rundown. The protocol was then repeated after the muscles were incubated for 30 min at room temperature (22 °C) with RS containing 12.0 μg/ml constitutively active CaMKII (active without calcium or/and CaM). The measured tensions at each submaximal activation were normalized by the maximal activation tension (Fmax), and the normalized tensions were plotted against the pCa to determine the tension–pCa curve. The tension–pCa curves were fit to the Hill equation: T/Tmax (relative tension) = [Ca2+]nH / (K + [Ca2+]nH), where nH is the Hill coefficient, and pCa50 = (− logK)/nH, pCa for half-maximal activation was calculated. This pCa50 was used as an indicator of calcium sensitivity.
2.5. Statistical analysis
Data are expressed as mean values and standard errors of measurement. Student's paired or unpaired t-test, ANOVA and Fisher test were used as appropriate, with statistical significance at P < 0.05 (significance symbols on figures: * or # P <0.05, ** or ## P < 0.01).
3. Results
3.1 CaMKII inhibition blunts the FFR and enhances performance at low stimulation frequencies
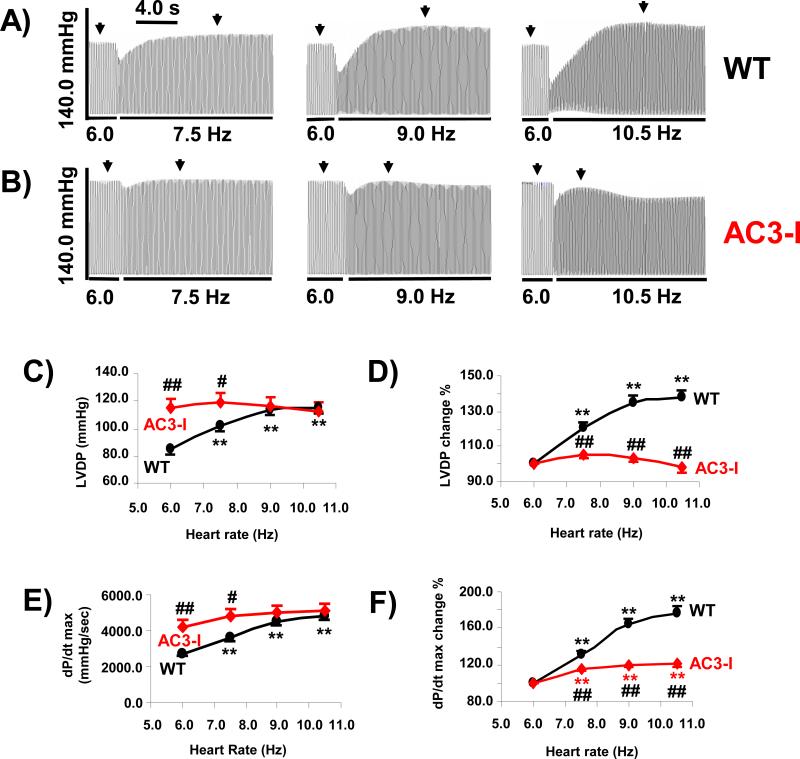
We measured the FFR in WT and AC3-I mouse hearts. The example tracings show that LVDP was more sensitive to increasing stimulation frequency in WT (Fig 1A) compared to AC3-I (Fig 1B). In WT mice the LVDP increased at pacing rates between 6 Hz (360 beats/min [bpm]) to 10.5 Hz (630 bpm) (Fig 1 C). The LVDP increased by 37.6 ± 4.7% from 6 Hz to 10.5 Hz (Fig 1D), dP/dt max increased by 77.0 ± 8.1% in response to an increase in pacing rate from 6 to 10.5 Hz (Fig 1 E and F). In contrast, the positive LVDP-frequency relation was diminished in AC3-I mice (Fig 1 C and D), with no significant increase of LVDP between 6 – 10.5 Hz. The LVDP and dP/dt max from AC3-I mice at lower pacing rates (6 and 7.5 Hz) are significantly higher than that from WT mice (Fig 1C and 1E). Furthermore the positive dP/dt max-frequency relation was blunted in AC3-I hearts with only a 20.4 ± 3.9% increase in the dP/dt max from 6 Hz to 10.5 Hz (Fig 1E and F). These findings suggest that CaMKII inhibition increases LVDP and dP/dt max at low stimulation rates and flattens the FFR at higher stimulation frequencies.
Fig 1. Effects of chronic CaMKII inhibition on frequency dependent LV contractility.
A. An example of LV pressure-frequency relation in wild type (WT) shows LV pressure increases after increase in pacing rate. B. An example of LV pressure-frequency relation in heart of Ca2+/calmodulin-dependent protein kinase II (CaMKII) inhibition (AC3-I) shows no significant increase of LV pressure after increase in pacing rate. C. Summary of left ventricular developed pressure (LVDP) at different pacing rates. D. LVDP at different pacing rates normalized by the LVDP at 6 Hz. E. Summary of maximum LV pressure change rate (dP/dt max) at different pacing rates. F. dP/dt max at different pacing rates normalized by the dP/dt max at 6 Hz. Data from 10 WT and 11 AC3-I isolated heart experiments. ## P<0.01, # P<0.05 vs WT; **P<0.01vs data at 6 Hz. ↓ Indicates where data was measured.
3.2 CaMKII phosphorylates myofilament proteins but does not change myocardial Ca2+ sensitivity
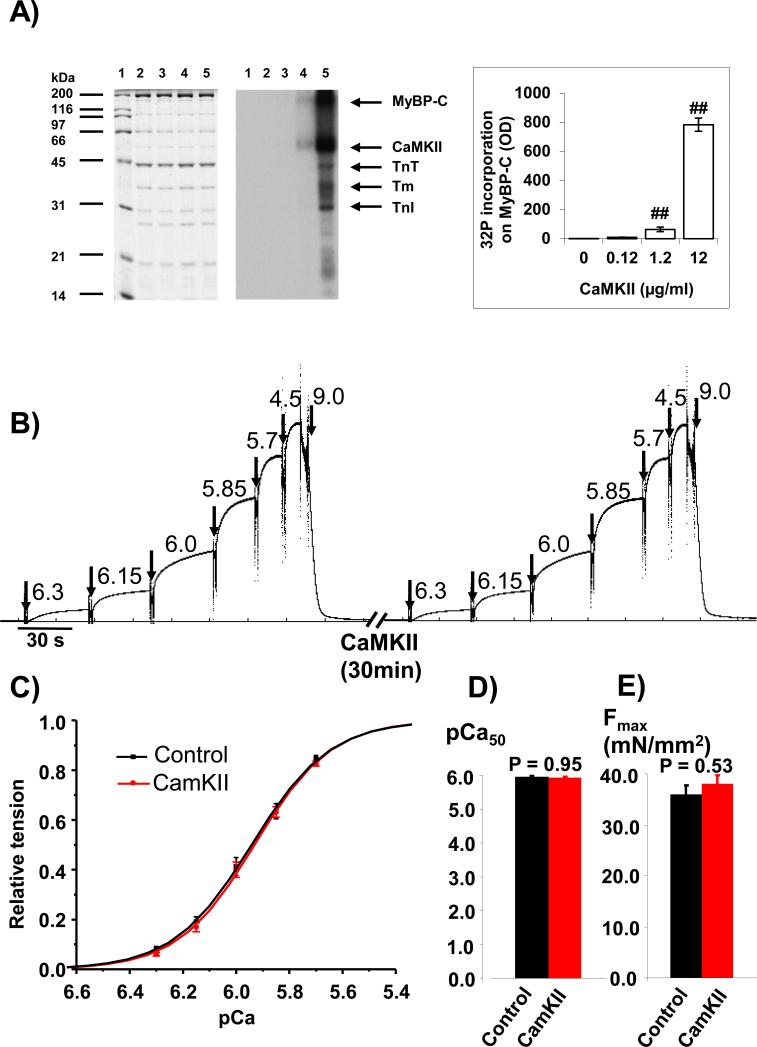
To test whether CaMKII catalyzed phosphorylation of myofilament proteins impacts the Ca2+ sensitivity of force development, we introduced constitutively active CaMKII (T287D) at different concentrations (0.0, 0.12, 1.2 and 12.0 μg/ml) to chemically-skinned WT myocardium at room temperature for 30 minutes. We found that several myofilament proteins including myosin binding protein C (MyBP-C),troponin T (TnT), tropomysin (Tm), and troponin I (TnI) show increased phosphorylation in the presence of CaMKII (12.0 μg/ml) under these experimental conditions (Fig 2A). We next measured the tension-pCa relationship in the myocardium before and after CaMKII incubation (Fig 2B). There was no noticeable rundown of the maximum active tension over a one hour period. The average tension-pCa curves before and after CaMKII treatment did not reveal any differences (Fig 2C). The pCa50 value (Fig 2D) and the maximum active tension (Fig 2E) were not affected by addition of CaMKII. These findings show that myofilament proteins are substrates for CaMKII-mediated phosphorylation, but they do not support that CaMKII phosphorylation affects the myofilament Ca2+ sensitivity measured under steady state condition.
Fig 2. Effects of CaMKII on myocardium calcium sensitivity.
A: CaMKII phosphorylated myofilamental proteins including myosin binding protein C (MyBP-C), troponin T (TnT), tropomysin (Tm), and troponin I (TnI) in chemical-skinned WT myocardium. Left panel is the gel with Coomassie blue stain, the middle panel is the autoradiograpgy of the gel, and the right panel is the summary of the quantification of MyBP-C from three experiments. Lane 1 is the molecular weight markers; lanes 2 treated with 0.00 μg/ml CaMKII; lanes 3 treated with 0.12 μg/ml CaMKII; lanes 4 treated with 1.20 μg/ml CaMKII; lanes 5 treated with 12.00 μg/ml CaMKII. B: An example of calcium sensitivity measurements before and after CaMKII incubation by switching the skinned myocardium strip to different pCa solutions and finally into relaxation solution (pCa 9.0). Maximum activation force (Fmax) was measured at pCa 4.5. C: Average tension-pCa curves in control (before CaMKII treatment) and after CaMKII incubation (CaMKII). D and E: summary of pCa50 and Fmax. There is no statistically significant difference between these two groups. (n = 11). ## P<0.01, vs control (no CaMKII treatment).
3.3 Loss of PLN eliminates positive FFR
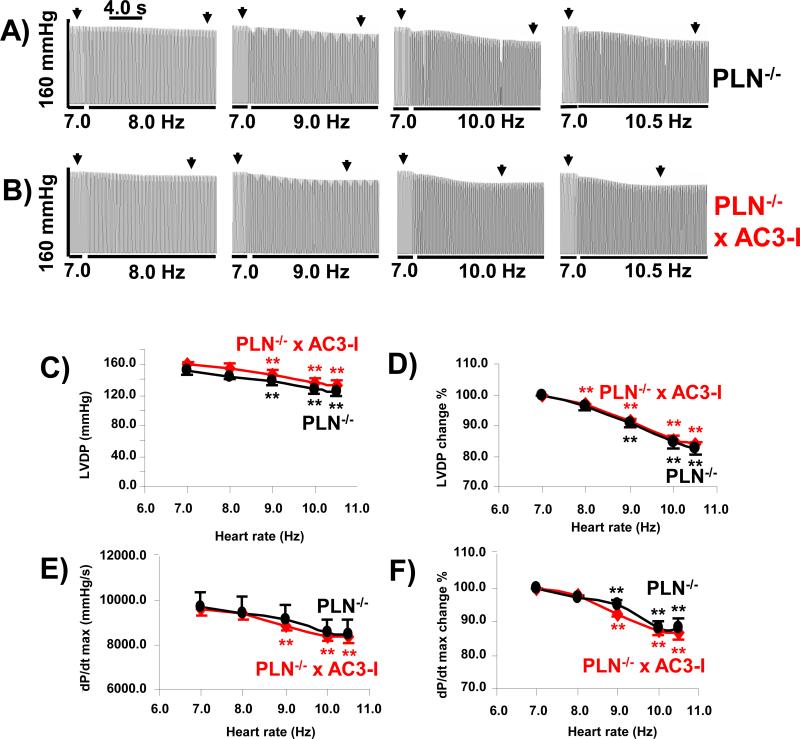
Given our findings that myofilament proteins were unlikely to explain the effects of CaMKII on FFR, we next examined the effect of PLN knock out on FFR. The example tracings show a negative LV pressure–frequency relationship in PLN−/− (Fig 3A) and PLN−/− x AC3-I hearts (Fig 3B). The LVDP and dP/dt max decreased between 7 Hz (420 bpm) and 10.5 Hz (630 bpm) (Fig 3C). The LVDP decreased by 17.4 ± 2.3% from 7 Hz to 10.5 Hz (Fig 3D), and similar changes were seen in dP/dt max (Fig 3E and 3F). PLN−/− hearts exhibited a negative FFR between 7-10.5 Hz, a finding that is consistent with the lack of a dynamic intracellular Ca2+ response to pacing in ventricular myocytes isolated from PLN−/− hearts. [25] Given the resemblance of FFR phenotypes in PLN−/− and AC3-I hearts, we asked if AC3-I effects would be eliminated by loss of PLN. We interbred PLN−/− with AC3-I transgenic mice (PLN−/−xAC3-I). The PLN−/−xAC3-I hearts also showed a negative FFR (Fig 3B). The LVDP and dP/dt max were both decreased at stimulation frequencies between 7 Hz (420 bpm) to 10.5 Hz (630 bpm) (Fig 3 C to 3F). The PLN−/− x AC3-I hearts showed a similar FFR as observed in PLN−/− hearts, suggesting that the effects of CaMKII on FFR require PLN.
Fig 3. Effects of chronic CaMKII inhibition on frequency dependence of LV contractility in isolated mouse hearts with phospholamban knockout (PLN−/−).
A. An example of LV pressure–frequency relation in PLN−/− mouse heart shows LV pressure decreases with increasing pacing rates. B. An example of LV pressure–frequency relation in PLN−/− x AC3-I mouse heart shows LV pressure decreases with increasing pacing rates. C. Summary of LVDP at different pacing frequencies. D. LVDP at different pacing rates normalized by the LVDP at 7 Hz. E. Summary of dP/dt max at different pacing rates. F. dP/dtmax at different pacing rates normalized by the dP/dt max at7 Hz. Data from 8 PLN−/− and 9 PLN−/− x AC3-I isolated heart experiments. **P<0.01 vs data at 6Hz. Indicates where data was measured.
3.4 Contractility capacity is not maximized at low pacing rate
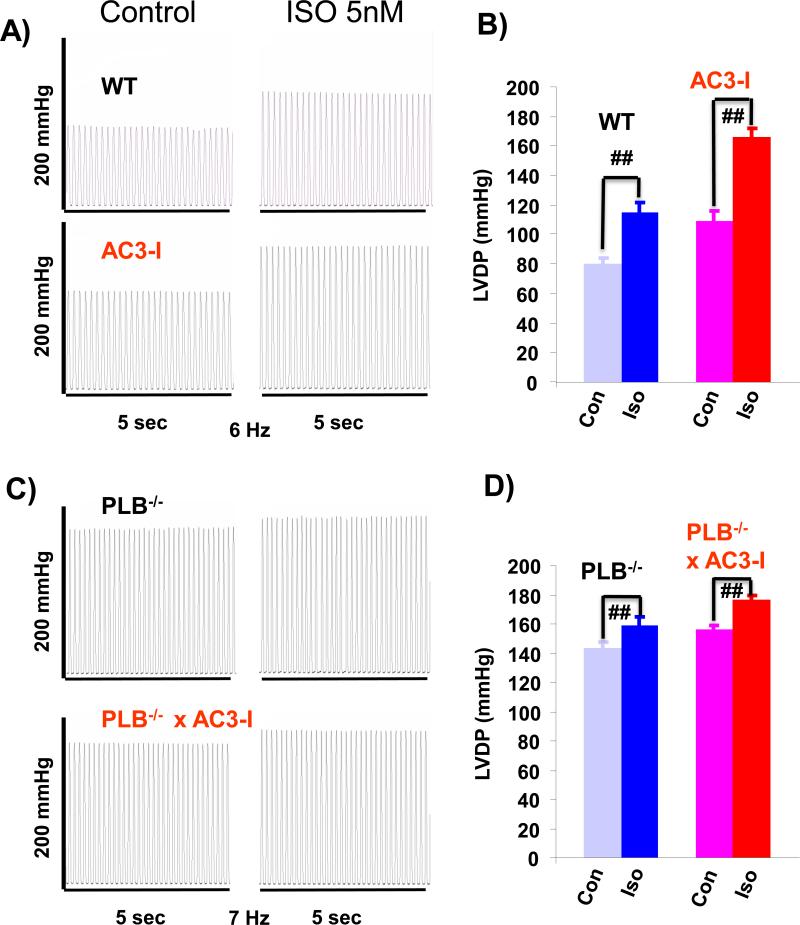
Given the flat or negative FFR in AC3-I and PLN−/− or PLN−/− X AC3-I mouse hearts, we added a low concentration of ISO to the perfusate to test whether the isolated hearts had reached their maximum pressure development at low pacing rates which would prevent a further increase in pressure generation at higher pacing rates. Fig 4 shows the examples and summary of the LVDP change after 10 minutes of 5 nM ISO treatment (Fig 4 A-D). All WT, AC3-I, PLN−/− and PLN−/− X AC3-I mouse hearts had significant increases in LVDP after ISO, although the amplitude was much smaller in PLN−/− or PLN−/− X AC3-I mouse hearts(Fig 4 B and D).
Fig 4. Effects of isoproterenol on LV pressure in the mouse hearts paced at low rates.
A. Examples of LV pressure change after 10 minutes of 5nM isoproterenol treatment in WT and AC3-I mice at pacing rate of 6Hz. B. Summary of LVDP change from 9 WT mouse hearts and 9 AC3-I mouse hearts before (con) and after isoproterenol (Iso) treatment. C. Examples of LV pressure change after 10 minutes of 5nM isoproterenol treatment in PLB−/− and PLB−/− X AC3-I mice heart at pacing rate of 7Hz. D. Summary of LVDP change from 7 PLB−/− mouse hearts and 6 PLB−/− X AC3-I mouse hearts before (con) and after isoproterenol (Iso) treatment. ## P<0.01.
3.5 CaMKII inhibition impairs cardiac relaxation
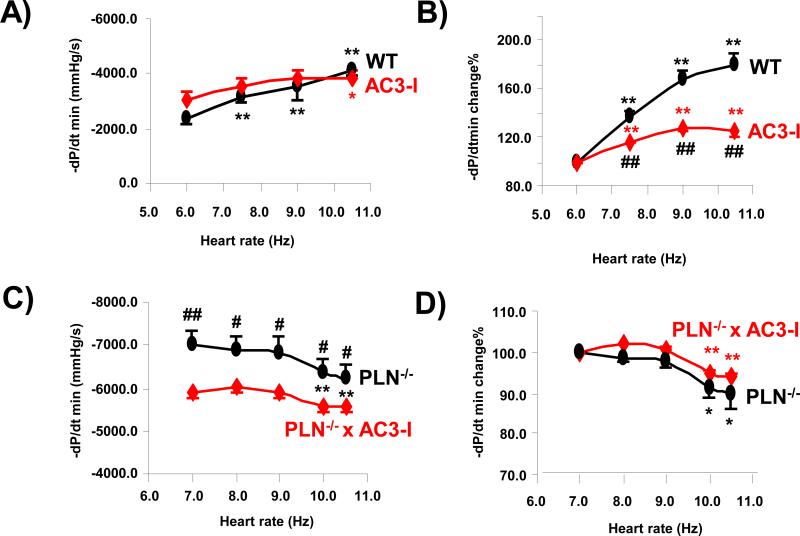
To test the effects of CaMKII and its interaction with PLN on FDAR, we measured the -dP/dt min-frequency relation in WT, AC3I, PLN−/− and PLN−/− x AC3-I mice. We found that in WT mice the increase of -dP/dt min was significantly greater than in AC3-I at pacing rates between 6 Hz (360 bpm) to 10.5 Hz (630 bpm) (Fig 5A and 5B). The –dP/dt min increased by 86.4 ±9.1% between 6 Hz to 10.5 Hz in WT mice but only 24.0 ± 3.3%in AC3-I mice (Fig 5 B). A negative -dP/dt min-frequency relation was present in PLN−/− hearts (Fig 5 C). The -dP/dt min decreased by 10.4 ± 3.3% from 7 Hz to 10.5 Hz (Fig 5D). The -dP/dt min – frequency relation was similar in PLN−/− and PLN−/− x AC3-I mice, but at any given pacing rate, the -dP/dt min was significantly higher in PLN−/− than in PLN−/− x AC3-I mice (Fig 5C). Thus the FDAR is blunted in AC3-I mice. PLN and CaMKII both participate in FADR, but CaMKII inhibition appears to suppress relaxation by affecting targets in addition to PLN.
Fig 5. Effects of chronic CaMKII inhibition on frequency dependence of LV relaxation in isolated mouse heart with or without phospholamban.
A. Summary of -dP/dt min from 10 WT and 11 AC3-I mouse hearts. B. –dP/dt min at different pacing rates normalized by the -dP/dt min at 6 Hz in WT and AC3-I mouse hearts. C. Summary of -dP/dt min at different pacing rates in 8 PLN−/− and 9 PLN−/− x AC3-I mouse hearts. D. –dP/dt min at different pacing rate normalized by the -dP/dt min at 7 Hz in PLN−/− and PLN−/− x AC3-I mouse hearts. **P<0.01, *P<0.05 vs data at 6 Hz or 7 Hz; ##P<0.01, #P<0.05 vs AC3-I or PLN−/− x AC3-I.
1. Discussion
We used a cohort of mouse models to probe the role of CaMKII in the FFR and FDAR. We found that chronic inhibition of CaMKII flattens the FFR but enhances the cardiac performance at low stimulation rates. Our results also indicate that CaMKII effects on FFR may not be due to direct actions on myofilament proteins. Furthermore, we found that results obtained under our experimental conditions reinforced earlier work showing that PLN is a critical player in FFR[13] and an important target for CaMKII effects on FFR. Lack of PLN also abolishes FDAR and inhibition of CaMKII reduces the maximum rate of cardiac relaxation in the absence of PLN, suggesting that both PLN and CaMKII have important roles in regulating cardiac relaxation, However, CaMKII affects relaxation, at least in part, by a PLN-independent pathway.
We used constitutively active CaMKII (T287D) to phosphorylate chemically-skinned myocardium and found that CaMKII was able to phosphorylate several myofilament proteins including MyBP-C, TnT, Tm, and TnI, but did not change calcium sensitivity (similar pCa50) nor the maximum active force at a working sarcomere length (2.0 μm). This result is consistent with another study that showed increasing myocardial pacing rate did not affect myofilament calcium sensitivity in normal rat trabeculae. [32] In contrast, other research demonstrates that CaMKII inhibition reduced both MyBP-C and TnI phosphorylation and decreased myofilament calcium sensitivity in intact mouse papillary muscles. However increasing the stimulation frequency caused increased force production per unit calcium concentration and decreased frequency-dependent calcium sensitivity during relaxation in both control and CaMKII inhibition. [33] These findings are consistent with other studies that show that FDAR involves decreased myofilament calcium sensitivity.[4] It is possible that because several myofilament proteins were phosphorylated by CaMKII in our experiments –that might have opposing effects and that the net result is an unchanged calcium sensitivity. Thus our results do not rule out a physiological role for CaMKII phosphorylation of myofilament proteins, but show that CaMKII does not affect the steady state maximal tension development and calcium sensitivity of skinned muscle.
Transgenic myocardial expression of AC3-I did not alter the amount of SERCA, RyR2, and calsequestrin, but reduced CaMKII activity by 40% and PLN phosphorylation by 90% [23]which blunted the FFR, a finding that is similar to a study where isolated ventricular myocytes expressing AC3-I had reduced dynamic intracellular Ca2+ responses to pacing. In this earlier study, we found that the intracellular Ca2+ at the 10th beat had a reduced increase over baseline (~10%) in AC3-I expressing ventricular myocytes compared to the increase observed in WT ventricular myocytes (~40%).[25] In rabbit papillary muscle experiments, CaMKII inhibitors, W-7 and trifluoperazine, blunted the increase in intracellular Ca2+ transient amplitudes and force generation at higher stimulation rates.[34] In contrast, a recent study showed that 2 μmol KN-93 did not affect the FFR in sheep cardiac trabeculae. [6] We do not know the reason for these disparate results, but we speculate that because the peak systolic force and the ratio of peak systolic to diastolic force in that study were low compared to other studies,[35-36] that these may have been unhealthy preparations or that experimental conditions were suboptimal.
An important finding of our study was that PLN is a critical target for CaMKII effects on FFR. We found a negative FFR and no FDAR in both PLN−/− and PLN−/− x AC3-I mice. The slopes of negative relations of LVDP-frequency, dP/dt max-frequency and -dP/dt min-frequency were similar in both groups indicating inhibition of CaMKII does not further affect the FFR and FDAR in PLN−/− mice. These findings support the idea that PLN plays a critical role in FFR and that CaMKII affects FFR mainly via PLN. These results are consistent with other studies.[13, 37] Negative FFR has also been shown in both the isolated heart and cardiac muscle preparations of PLN−/− mice.[13, 38] Taken together, these results provide strong support for the concept that PLN is a major determinant of cardiac force-frequency relationship. The flat FFR in AC3-I and negative FFR in PLN KO mouse hearts are not due to the maximization of LV pressure development at low pacing rates since low dose of ISO enhanced the LV performance. PLN also can be phosphorylated by PKA which can be activated by ISO. It is controversial whether serine16 of PLN phosphorylated by PKA has effects on FFR [14-15, 39], however it is well accepted that PKA phosphorylation of PLN contributes to FDAR. [1, 4, 40] We used isolated heart preparation avoiding the effects of autonomic nervous system at the cost of not knowing the net effects of increasing heart rate on the cardiac performance in the AC3-I or PLN KO mouse in the in vivo situation. The activity of cardiac SERCA is regulated not only by PLN but also by sarcolipin (SLN ). A recent report revealed that threonine-5 of SLN phosphorylated by CaMKII relieves the suppression of SERCA which might be involving in FFR.[41] In addition, studies also showed that the PLN-SERCA ratio might control the FFR.[12] Thus our study does not exclude a role for CaMKII to mediate mechanical responses by actions on SLN.
Mouse of a validated CaMKII phosphorylation site (serine 2814) replaced with a nonphosphorylatable amino acid (S2814A) showed that lacking RyR2 phosphorylation by CaMKII blunted the FFR. [5] Since our results showed that similar negative FFR in the PLN−/− and PLN−/− X AC3-I mouse hearts. It seems that RyR2 phosphorylation by CaMKII may be not so important in FFR when PLN is absent. However, the exact role of RyR2 phosphorylation by CaMKII on FFR in PLN absent mouse needs further investigation.
The -dP/dt min increase at higher stimulation rates was much less in AC3-I mice than in WT mice, which confirmed a previous study that the frequency-dependent acceleration of the decline in intracellular calcium is diminished by selective CaMKII inhibitors. [3, 19, 42-43] CaMKII phosphorylates PLN and reduces the inhibition of SERCA2,[10, 37, 44-45] thereby accelerating the SR uptake of calcium during diastole. The effect of PLN on FDAR is controversial.[15, 46] One study found a good correlation between the increase of PLN threonine 17 phosphorylation and FDAR in rat myocytes,[14] and diminished FDAR in mice with PLN-threonine17 mutated to alanine.[38] However, others found that FDAR persisted in PLN−/− myocardium.[19] A CaMKII inhibitor, KN-93, reduced the FDAR in both WT and PLN−/− intact cardiac muscle and in isolated myocytes suggesting CaMKII plays a key role in FDAR, even in the absence of PLN. [19] With increasing pacing rates, the negative slopes of –dP/dt min are similar in the PLN−/− and PLN−/− X AC3-I mouse hearts indicating similar FDAR in these two groups. But the -dP/dt min was higher in PLN−/− compared to PLN−/− x AC3-I mice at any given stimulation rate indicating that CaMKII plays an important role in regulating relaxation partially independent of PLN. We did not see any FDAR in PLN−/− or PLN−/− x AC3-I mice; the -dP/dt min minimally decreased at higher stimulation rates in both mice. Thus, our findings show that CaMKII is critical for the lusitropic aspects of the FFR and that PLN participates in these processes as well.
5. Summary
This study supports the view that CaMKII plays an important role in both the FFR and the FDAR. Although CaMKII was found to phosphorylate myofilament proteins, the force-pCa studies do not support a role of CaMKII regulating calcium sensitivity of the myofilaments. Instead our results suggest that the effects of CaMKII on FFR are solely governed through PLN, but the effects on cardiac relaxation are in part through unidentified proteins in addition to PLN.
Acknowledgements
This work was funded by National Institutes of Health (NIH) Grants HL 079031, HL 096652, HL 113001 and HL 070250 (MEA), HL062881 (HG), the University of Iowa Research Foundation and the Foundation Leducq Alliance for CaMKII Signaling.
Footnotes
Disclosures: none declared.
References
- 1.Janssen PM. Myocardial contraction-relaxation coupling. Am J Physiol Heart Circ Physiol. 2010;299:H1741–9. doi: 10.1152/ajpheart.00759.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Endoh M. Force-frequency relationship in intact mammalian ventricular myocardium: physiological and pathophysiological relevance. Eur J Pharmacol. 2004;500:73–86. doi: 10.1016/j.ejphar.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Janssen PM, Periasamy M. Determinants of frequency-dependent contraction and relaxation of mammalian myocardium. J Mol Cell Cardiol. 2007;43:523–31. doi: 10.1016/j.yjmcc.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Varian KD, Janssen PM. Frequency-dependent acceleration of relaxation involves decreased myofilament calcium sensitivity. Am J Physiol Heart Circ Physiol. 2007;292:H2212–9. doi: 10.1152/ajpheart.00778.2006. [DOI] [PubMed] [Google Scholar]
- 5.Kushnir A, Shan J, Betzenhauser MJ, Reiken S, Marks AR. Role of CaMKIIdelta phosphorylation of the cardiac ryanodine receptor in the force frequency relationship and heart failure. Proc Natl Acad Sci U S A. 2010;107:10274–9. doi: 10.1073/pnas.1005843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sossalla S, Fluschnik N, Schotola H, Ort KR, Neef S, Schulte T, et al. Inhibition of elevated Ca2+/calmodulin-dependent protein kinase II improves contractility in human failing myocardium. Circ Res. 2010;107:1150–61. doi: 10.1161/CIRCRESAHA.110.220418. [DOI] [PubMed] [Google Scholar]
- 7.Anderson ME. Calmodulin kinase signaling in heart: an intriguing candidate target for therapy of myocardial dysfunction and arrhythmias. Pharmacol Ther. 2005;106:39–55. doi: 10.1016/j.pharmthera.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Couchonnal LF, Anderson ME. The role of calmodulin kinase II in myocardial physiology and disease. Physiology (Bethesda) 2008;23:151–9. doi: 10.1152/physiol.00043.2007. [DOI] [PubMed] [Google Scholar]
- 9.MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4:566–77. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 10.Maier LS. Role of CaMKII for signaling and regulation in the heart. Front Biosci. 2009;14:486–96. doi: 10.2741/3257. [DOI] [PubMed] [Google Scholar]
- 11.Maier LS, Bers DM. Role of Ca2+/calmodulin-dependent protein kinase (CaMK) in excitation-contraction coupling in the heart. Cardiovasc Res. 2007;73:631–40. doi: 10.1016/j.cardiores.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Meyer M, Bluhm WF, He H, Post SR, Giordano FJ, Lew WY, et al. Phospholamban-to-SERCA2 ratio controls the force-frequency relationship. Am J Physiol. 1999;276:H779–85. doi: 10.1152/ajpheart.1999.276.3.H779. [DOI] [PubMed] [Google Scholar]
- 13.Bluhm WF, Kranias EG, Dillmann WH, Meyer M. Phospholamban: a major determinant of the cardiac force-frequency relationship. Am J Physiol Heart Circ Physiol. 2000;278:H249–55. doi: 10.1152/ajpheart.2000.278.1.H249. [DOI] [PubMed] [Google Scholar]
- 14.Hagemann D, Kuschel M, Kuramochi T, Zhu W, Cheng H, Xiao RP. Frequency-encoding Thr17 phospholamban phosphorylation is independent of Ser16 phosphorylation in cardiac myocytes. J Biol Chem. 2000;275:22532–6. doi: 10.1074/jbc.C000253200. [DOI] [PubMed] [Google Scholar]
- 15.Mattiazzi A, Mundina-Weilenmann C, Guoxiang C, Vittone L, Kranias E. Role of phospholamban phosphorylation on Thr17 in cardiac physiological and pathological conditions. Cardiovasc Res. 2005;68:366–75. doi: 10.1016/j.cardiores.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Antoons G, Mubagwa K, Nevelsteen I, Sipido KR. Mechanisms underlying the frequency dependence of contraction and [Ca(2+)](i) transients in mouse ventricular myocytes. J Physiol. 2002;543:889–98. doi: 10.1113/jphysiol.2002.025619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bombardini T. Myocardial contractility in the echo lab: molecular, cellular and pathophysiological basis. Cardiovasc Ultrasound. 2005;3:27. doi: 10.1186/1476-7120-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–51. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeSantiago J, Maier LS, Bers DM. Frequency-dependent acceleration of relaxation in the heart depends on CaMKII, but not phospholamban. J Mol Cell Cardiol. 2002;34:975–84. doi: 10.1006/jmcc.2002.2034. [DOI] [PubMed] [Google Scholar]
- 20.Valverde CA, Mundina-Weilenmann C, Said M, Ferrero P, Vittone L, Salas M, et al. Frequency-dependent acceleration of relaxation in mammalian heart: a property not relying on phospholamban and SERCA2a phosphorylation. J Physiol. 2005;562:801–13. doi: 10.1113/jphysiol.2004.075432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boontje NM, Merkus D, Zaremba R, Versteilen A, de Waard MC, Mearini G, et al. Enhanced myofilament responsiveness upon beta-adrenergic stimulation in post-infarct remodeled myocardium. J Mol Cell Cardiol. 2011;50:487–99. doi: 10.1016/j.yjmcc.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 22.Cazorla O, Lucas A, Poirier F, Lacampagne A, Lezoualc'h F. The cAMP binding protein Epac regulates cardiac myofilament function. Proc Natl Acad Sci U S A. 2009;106:14144–9. doi: 10.1073/pnas.0812536106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, et al. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–17. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 24.Kadambi VJ, Ball N, Kranias EG, Walsh RA, Hoit BD. Modulation of force-frequency relation by phospholamban in genetically engineered mice. Am J Physiol. 1999;276:H2245–50. doi: 10.1152/ajpheart.1999.276.6.H2245. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y, Shintani A, Grueter C, Zhang R, Hou Y, Yang J, et al. Suppression of dynamic Ca(2+) transient responses to pacing in ventricular myocytes from mice with genetic calmodulin kinase II inhibition. J Mol Cell Cardiol. 2006;40:213–23. doi: 10.1016/j.yjmcc.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Werdich AA, Lima EA, Dzhura I, Singh MV, Li J, Anderson ME, et al. Differential effects of phospholamban and Ca2+/calmodulin-dependent kinase II on [Ca2+]i transients in cardiac myocytes at physiological stimulation frequencies. Am J Physiol Heart Circ Physiol. 2008;294:H2352–62. doi: 10.1152/ajpheart.01398.2006. [DOI] [PubMed] [Google Scholar]
- 27.Lee EJ, Peng J, Radke M, Gotthardt M, Granzier HL. Calcium sensitivity and the Frank-Starling mechanism of the heart are increased in titin N2B region-deficient mice. J Mol Cell Cardiol. 2010;49:449–58. doi: 10.1016/j.yjmcc.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–74. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Y, Tobias AH, Bell K, Barry W, Helmes M, Trombitas K, et al. Cellular and molecular mechanisms of systolic and diastolic dysfunction in an avian model of dilated cardiomyopathy. J Mol Cell Cardiol. 2004;37:111–9. doi: 10.1016/j.yjmcc.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 30.Cazorla O, Wu Y, Irving TC, Granzier H. Titin-based modulation of calcium sensitivity of active tension in mouse skinned cardiac myocytes. Circ Res. 2001;88:1028–35. doi: 10.1161/hh1001.090876. [DOI] [PubMed] [Google Scholar]
- 31.Fabiato A, Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75:463–505. [PubMed] [Google Scholar]
- 32.Lamberts RR, Hamdani N, Soekhoe TW, Boontje NM, Zaremba R, Walker LA, et al. Frequency-dependent myofilament Ca2+ desensitization in failing rat myocardium. J Physiol. 2007;582:695–709. doi: 10.1113/jphysiol.2007.134486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tong CW, Gaffin RD, Zawieja DC, Muthuchamy M. Roles of phosphorylation of myosin binding protein-C and troponin I in mouse cardiac muscle twitch dynamics. J Physiol. 2004;558:927–41. doi: 10.1113/jphysiol.2004.062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Endoh M. Frequency-dependent inhibition of the intracellular calcium transients by calmodulin antagonists in the aequorin-injected rabbit papillary muscle. Adv Exp Med Biol. 1989;255:461–70. doi: 10.1007/978-1-4684-5679-0_49. [DOI] [PubMed] [Google Scholar]
- 35.Gao WD, Perez NG, Marban E. Calcium cycling and contractile activation in intact mouse cardiac muscle. J Physiol. 1998;507(Pt 1):175–84. doi: 10.1111/j.1469-7793.1998.175bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stuyvers BD, McCulloch AD, Guo J, Duff HJ, ter Keurs HE. Effect of stimulation rate, sarcomere length and Ca(2+) on force generation by mouse cardiac muscle. J Physiol. 2002;544:817–30. doi: 10.1113/jphysiol.2002.024430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94:e61–70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 38.Zhao W, Uehara Y, Chu G, Song Q, Qian J, Young K, et al. Threonine-17 phosphorylation of phospholamban: a key determinant of frequency-dependent increase of cardiac contractility. J Mol Cell Cardiol. 2004;37:607–12. doi: 10.1016/j.yjmcc.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 39.Brixius K, Wollmer A, Bolck B, Mehlhorn U, Schwinger RH. Ser16-, but not Thr17-phosphorylation of phospholamban influences frequency-dependent force generation in human myocardium. Pflugers Arch. 2003;447:150–7. doi: 10.1007/s00424-003-1163-3. [DOI] [PubMed] [Google Scholar]
- 40.Janssen PM. Kinetics of cardiac muscle contraction and relaxation are linked and determined by properties of the cardiac sarcomere. Am J Physiol Heart Circ Physiol. 2010;299:H1092–9. doi: 10.1152/ajpheart.00417.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhupathy P, Babu GJ, Ito M, Periasamy M. Threonine-5 at the N-terminus can modulate sarcolipin function in cardiac myocytes. J Mol Cell Cardiol. 2009;47:723–9. doi: 10.1016/j.yjmcc.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bassani RA, Mattiazzi A, Bers DM. CaMKII is responsible for activity-dependent acceleration of relaxation in rat ventricular myocytes. Am J Physiol. 1995;268:H703–12. doi: 10.1152/ajpheart.1995.268.2.H703. [DOI] [PubMed] [Google Scholar]
- 43.Li L, Chu G, Kranias EG, Bers DM. Cardiac myocyte calcium transport in phospholamban knockout mouse: relaxation and endogenous CaMKII effects. Am J Physiol. 1998;274:H1335–47. doi: 10.1152/ajpheart.1998.274.4.H1335. [DOI] [PubMed] [Google Scholar]
- 44.Vittone L, Mundina-Weilenmann C, Mattiazzi A. Phospholamban phosphorylation by CaMKII under pathophysiological conditions. Front Biosci. 2008;13:5988–6005. doi: 10.2741/3131. [DOI] [PubMed] [Google Scholar]
- 45.Mattiazzi A, Kranias EG. CaMKII regulation of phospholamban and SR Ca2+ load. Heart Rhythm. 2011;8:784–7. doi: 10.1016/j.hrthm.2010.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mattiazzi A, Mundina-Weilenmann C, Vittone L, Said M, Kranias EG. The importance of the Thr17 residue of phospholamban as a phosphorylation site under physiological and pathological conditions. Braz J Med Biol Res. 2006;39:563–72. doi: 10.1590/s0100-879x2006000500001. [DOI] [PubMed] [Google Scholar]