Abstract
Acute myeloid leukemia (AML) is a malignancy of stem cells with an unlimited capacity for self-renewal. MUC1 is a secreted, oncogenic mucin that is expressed aberrantly in AML blasts, but its potential uses to target AML stem cells have not been explored. Here we report that MUC1 is highly expressed on AML CD34+/lineage−/CD38− cells as compared to their normal stem cell counterparts. MUC1 expression was not restricted to AML CD34+ populations as similar results were obtained with leukemic cells from patients with CD34− disease. Engraftment of AML stem cell populations that highly express MUC1 (MUC1high) led to development of leukemia in NSG immunodeficient mice. In contrast, MUC1low cell populations established normal hematopoiesis in the NSG model. Functional blockade of the oncogenic MUC1-C subunit with the peptide inhibitor GO-203 depleted established AML in vivo, but did not affect engraftment of normal hematopoietic cells. Our results establish that MUC1 is highly expressed in AML stem cells and they define the MUC1-C subunit as a valid target for their therapeutic eradication.
Keywords: AML, LSCs, MUC1
Introduction
Acute myelogenous leukemia (AML) is a clonal disorder of hematopoietic stem cells that have an unrestrained proliferative capacity (1, 2). Patients with AML often achieve complete remissions with induction chemotherapy; however, the majority relapse and succumb to their disease (3). The leukemic stem cell (LSC) population is considered to be resistant to chemotherapy and responsible for disease relapse (2). LSCs have been characterized by a CD34+/CD38− phenotype and the capability of generating leukemia in immunodeficient mice (4, 5). Nonetheless, the leukemic CD34+/CD38− cell population can be heterogenous and include normal hematopoietic stem cells. LSCs can also exhibit varying levels of CD34 and CD38 expression (6, 7). Moreover, AML CD34− populations have been shown to contain leukemia-initiating cells (8). For these reasons, a functional definition of leukemic engraftment in immunocompromized mice has been adopted to further define the LSC population (7–9). Markers of LSCs, such as CD32, CD35, the IL-3 receptor alpha chain and CD47, have been identified based on their selective expression in LSCs compared to normal hematopoietic stem cells (10–12). In addition, CD32− and CD35-positive LSCs initiate AML in mice and exhibit chemoresistance in vivo (12). Intermediate levels of aldehyde dehydrogenase (ALDH) activity have also been incorporated to distinguish CD34+/CD38− LSCs from their normal counterparts that exhibit relatively higher levels of activity (13). These findings have collectively supported the delineation of LSC markers and have provided potential targets for selective LSC treatment.
Mucin 1 (MUC1) is a heterodimeric epithelial cell glycoprotein that is aberrantly expressed in AML cell lines and primary blasts from patients (14, 15). MUC1 is translated as a single polypeptide that undergoes autocleavage into two subunits which in turn form a stable noncovalent heterodimer (16). The MUC1 N-terminal subunit (MUC1-N) is the glycosylated mucin component of the heterodimer that resides at the cell surface in a complex with the C-terminal transmembrane subunit (MUC1-C) (16). MUC1-C includes a 58-amino acid (aa) extracellular domain, a 28-aa transmembrane domain and a 72-aa cytoplasmic tail. The MUC1-C subunit interacts with receptor tyrosine kinases (RTKs) at the cell membrane and localizes to the nucleus where it interacts with transcription factors, such as NF-κB and the β-catenin/TCF4 complex, that have been linked to transformation (17–19). Localization of MUC1-C to the nucleus is dependent on the formation of homodimers through a CQC motif in the MUC1-C cytoplasmic tail (20). Accordingly, the cell-penetrating peptide, designated GO-203, was developed that binds to the CQC motif and blocks MUC1-C homodimerization and function (21). Treatment of AML cell lines and primary blasts with GO-203 was associated with increases in reactive oxygen species (ROS), arrest of growth and induction of terminal differentiation (21). These findings provided support for the MUC1-C subunit as a target for inhibiting the self-renewal capacity of AML cells.
The present studies demonstrate that MUC1 is highly expressed by leukemic CD34+/lineage−/CD38− and CD34−/lineage− cells as compared to normal hematopoietic stem cells. We show that the AML MUC1high, but not MUC1low, cells initiate AML in the NSG mouse model and that treatment with the MUC1-C inhibitor depletes engrafted AML cells in vivo.
Materials and Methods
Isolation of AML cell populations
Bone marrow aspirates and peripheral blood samples were obtained from patients with AML as per an institutionally approved protocol (Table 1). Mononuclear cells were isolated by ficoll density centrifugation. For assessment of MUC1 expression, CD34+ cells were isolated using the MiniMacs CD34 cell isolation kit (Miltenyi Biotec). As controls, CD34+ populations were isolated from (i) mobilized peripheral blood stem cell products obtained from healthy donors and (ii) bone marrow aspirates from patients with lymphoid malignancies without evidence of marrow involvement. For in vivo experiments, CD34+/lineage− and CD34−/lineage− cells were isolated from bone marrow samples from patients with AML using flow cytometric sorting (FACSAria). Lineage− is defined as negative for CD3, CD14, CD16, CD19, CD20 and CD56.
Table 1.
AML Patient Characteristics
| CD34+ | |||||||
|---|---|---|---|---|---|---|---|
| Specimen | Source | Status | karyotype | CD34% | FLT3/ITD | NPM | MUC1%* |
| AML1 | BM | diagnosis | normal | 95% | neg | neg | 65% |
| AML2 | BM | relapse | normal | 20% | ND | ND | 73% |
| AML3 | BM | diagnosis | normal | 32% | ND | ND | 81% |
| AML4 | BM | diagnosis | complex | 90% | neg | neg | 85% |
| AML5 | BM | diagnosis | trisomy 8 | 79% | ND | ND | 51% |
| AML6 | PB | diagnosis | normal | 99% | ND | ND | 89% |
| AML7 | PB | diagnosis | normal | 99% | neg | neg | 68% |
| AML8 | BM | relapse | complex | 17% | ND | ND | 90% |
| AML9 | BM | diagnosis | normal | 70% | neg | neg | 98% |
| AML10 | PB | diagnosis | normal | 10% | ND | ND | 64% |
| AML11 | BM | relapse | 45,xx,inv (3)(q21q26.2),−7 |
57% | neg | neg | 65% |
| AML12 | BM | relapse | 8;21 9q deletion | 63% | neg | ND | 44% |
| AML13 | BM | diagnosis | trisomy 8 translocation:11; 17(q23;12–21) MLL |
99% | ND | ND | 90% |
| AML14 | BM | diagnosis | normal | 92% | neg | neg | 57% |
| AML15 | BM | diagnosis | normal | 42% | pos | pos | 47% |
| AML16 | BM | diagnosis | complex | 21% | neg | ND | 56% |
| AML17 | BM | diagnosis | complex | 26% | ND | ND | 97% |
| AML18 | PB | diagnosis | 2/20 trisomy 13(47 xy) |
94% | ND | ND | 32% |
| AML19 | BM | diagnosis | normal | 59% | pos | pos | 92% |
| AML20 | BM | diagnosis | trisomy 14 | 73% | neg | neg | 36% |
| CD34− | |||||||
| Specimen | Source | Status | karyotype | CD34% | FLT3/ITD | NPM | MUC1% |
| AML21 | BM | diagnosis | 45,X,−Y[20] | 0% | neg | pos | 50% |
| AML22 | BM | diagnosis | normal | 2% | neg | pos | 50% |
| AML23 | PB | diagnosis | normal | 0% | neg | pos | 70% |
| AML24 | BM | diagnosis | normal | 1% | neg | pos | 25% |
| AML25 | BM | diagnosis | MLL | 0.1% | neg | neg | 52% |
| AML26 | BM | diagnosis | normal | 0.1% | pos | pos | 92% |
Results represent the percentage of CD34+ cells that are MUC1+.
Detection of MUC1 expression by flow cytometry
AML CD34+/lineage−/CD38−, CD34+/lineage−/CD38+ and CD34−/lineage− cells were analyzed for MUC1 expression by multichannel flow cytometric analysis. Normal CD34+/lineage−/CD38− cells were used as controls. Cells were incubated with MAb DF3 (anti-MUC1-N) (22) or a contol mouse IgG1 for 30 min, followed by secondary labeling of the cells with PE-conjugated goat anti-mouse IgG for an additional 30 min. The cells were then incubated with APC-conjugated anti-CD34, PE-Cy7-conjugated anti-CD38 or anti-lineage MAbs (CD3, CD14, CD16, CD19, CD20 and CD56) and fixed in 2% paraformaldehyde. Stained cells were analyzed by flow cytometry using FACScan and CellQuest Pro software (BD Biosciences).
Immunohistochemistry
Cytospins of CD34+ cells were prepared after isolation using anti-CD34 magnetic beads. Cells were stained with anti-MUC1 (MAb DF3) or goat-anti-mouse IgG using the Vectastain ABC kit (Vector Laboratories). The cells were then fixed in 2% paraformaldehyde (Sigma-Aldrich) and visualized by phase contrast light microscopy (Olympus AX70 microscope) using an oil immersion objective lens (×100).
Reverse Transcription-Polymerase Chain Reaction (RT-PCR) analysis
Total cellular RNA was extracted in Trizol reagent (RNeasy Mini Kit) and dissolved in RNase free water. One microgram of total RNA was reverse transcribed into cDNA, and PCR amplifications were performed in a programmable thermal cycler. MUC1-specific primers (5'-TGTCAGTGCCGCCGAAAGAAC-3' and 5'-CAAGTTGGCAGAAGTGGCTGC-3') and GAPDH primers (5'-CCATGGAGAAGGCTGGGG-3'and 5'-CAAAGTTGTCATGGATGACC-3' and) were designed to yield PCR products of 203 and 195 bp, respectively. Amplified fragments were analyzed by electrophoresis in 1.5% agarose gels.
Assessment of donor/recipient chimerism following allogeneic transplantation
CD34+ cells isolated from a female AML patient following sex mismatched allogeneic transplantation were analyzed by immunohistochemical staining with MAb DF3, followed by FISH analysis with probes identifying the Y and X chromosomes. Cells were then analyzed using the BioView Duet™ automated scanning system. A total of 100 interphase nuclei were scored.
Fluorescence in situ hybridization (FISH) analysis
Cytospin cell preparations were fixed by immersion in 3:1 methanol:acetic acid for 1–2 hours and stored at −20°C until use. The cytospin slides were treated with pepsin (Digest-All 3; Invitrogen) for 5 min at 37°C, followed by rinsing for 5 min at room temperature and dehydration in ethanol. Appropriate commercially available FISH probes (Abbott Molecular) were selected to detect the chromosomal abnormalities reported in cytogenetic analyses of the patient AML samples (Table 1); for example, the MLL Dual Color Break Apart Probe was used to detect the MLL rearrangement. The remainder of the FISH procedure was performed as described in the protocol provided with each probe. Hybridized slides were examined on an Olympus BX-51 microscope equipped with appropriate filters, and images captured with CytoVysion (Leica) imaging software. One hundred nuclei were scored, except where indicated, for each specimen.
Leukemia engraftment by MUC1high and MUC1low AML progenitors
CD34+ or CD34− lineage−/MUC1high and lineage−/MUC1low cells were isolated from patients with AML by flow cytometric sorting. The cells were inoculated retro-orbitally (0.5–1×106 cells/0.2 ml/mouse) into sub-lethally irradiated (300 rads) NOD-SCID IL2Rgammanull (NSG; 6 week old female) mice (Jackson Laboratories). After sacrifice, bone marrow and spleen cells were harvested and the red blood cells (RBC) were removed using RBC lysis buffer (Sigma). Engraftment, as defined by >1% human hCD45+ cells in the bone marrow, was detected by staining cells with PE-conjugated anti-hCD45 and, as a control, FITC-conjugated anti-mouse mCD45. In certain experiments, the cells were also analyzed for hCD34, hCD11C, hCD19 or hCD20 by multichannel flow cytometry using CellQuest or Diva software. Cell populations were then isolated using flow cytometric sorting (FACSAria) and the preparation of cytospins for morphologic assessment and FISH analysis.
GO-203 treatment of NSG mice
In a prevention model, CD34+/lineage−/MUC1high and CD34/lineage−/MUC1low cells were inoculated (0.5–1×106 cells/0.2 ml/mouse) into sub-lethally irradiated (300 rads) NSG mice. After 24 h, the mice were injected subcutaneously every 24 h with PBS or 14 mg/kg GO-203 for 21 days. The mice were sacrificed at 8–9 weeks after completing treatment. Leukemia cells were detected by dual staining for hCD45 and mCD45 by flow cytometric analysis. In a treatment model, mice were inoculated with MUC1high and MUC1low AML progenitors. Treatment with GO-203 was initiated on day 60 after inoculation when circulating hCD45+ cells were detected by flow cytometric analysis. The mice were then injected subcutaneously every 24 h with PBS or 14 mg/kg GO-203 for 21 days. Bone marrow and spleen cells were analyzed as described above.
Results
Expression of MUC1 by AML CD34+ cells
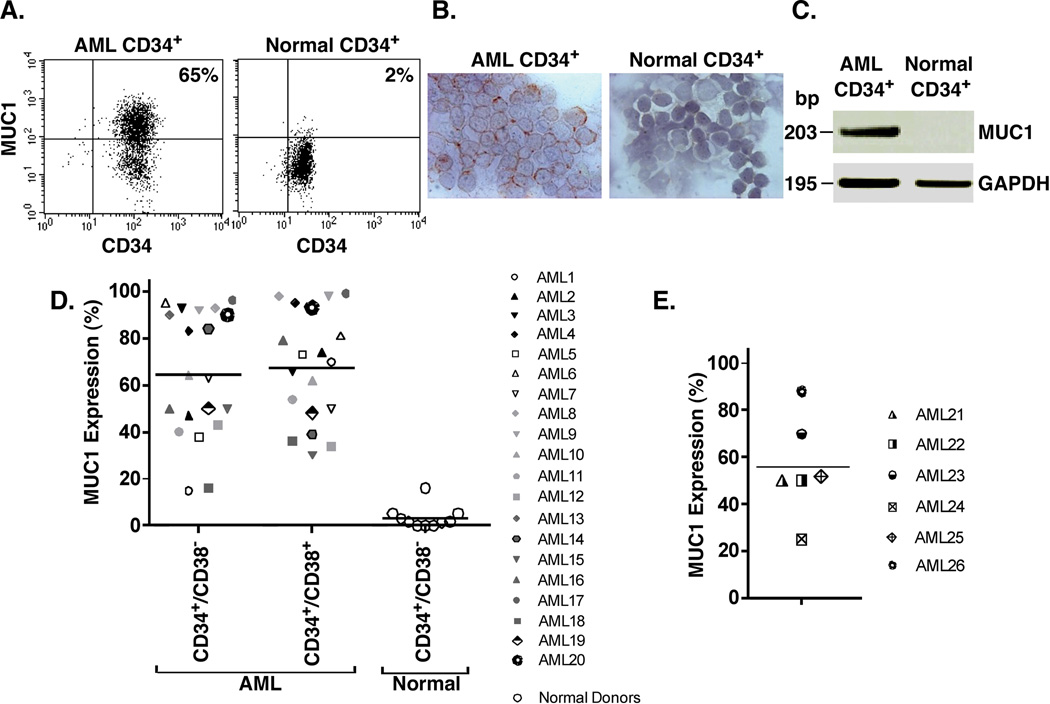
AML blasts obtained from a patient with ~90% involvement of the bone marrow were analyzed for MUC1 and CD34 expression. As determined by flow cytometry, MUC1 and CD34 were coexpressed in 65% of the blasts (Fig. 1A, left). In comparison, a similar analysis of peripheral blood hematopoietic stem cells from a normal donor demonstrated that 2% of cells coexpress MUC1 and CD34 (Fig. 1A, right). These findings were confirmed by immunohistochemical analysis of these cells for MUC1 expression, which demonstrated staining of the AML CD34+, and not the normal CD34+, cell populations (Fig. 1B). Further analysis by RT-PCR confirmed prominent expression of MUC1 in the AML CD34+ cells and a low to undetectable level in normal CD34+ cells (Fig. 1C). Based on these findings, we analyzed bone marrows from 20 patients with active AML for whom the clinical features, cytogenetic profiles and disease characteristics are summarized in Table 1. Analysis of CD34+/lineage−/CD38− cell populations demonstrated that MUC1 is expressed in all of the samples, ranging from 15% to 96% (mean 64%) for the individual patient cell preparations (Fig. 1D). Similar results were obtained for the CD34+/lineage−/CD38+ cell populations (Fig. 1D). These findings were in contrast to those obtained with CD34+/lineage−/CD38− stem cells from the peripheral blood of normal donors and bone marrows from patients with lymphoid malignancies without evidence of bone marrow involvement (Fig. 1D). Studies have shown that leukemia-initiating cells are not restricted to CD34+ population and have been identified in CD34− AML (8). Accordingly, we analyzed CD34−/lineage− populations from 6 patients with CD34− AML (Fig. 1E). Here, we also found that the CD34−/lineage− cells express MUC1 (range 20% to 92%; mean 51%). These findings thus demonstrate that MUC1 is selectively expressed by both CD34+ and CD34− AML cells as compared to normal hematopoietic stem cells.
Figure 1. Selective expression of MUC1 by CD34+ and CD34− AML cell populations.
A–C. Bone marrow mononuclear cells from a patient with CD34+ AML (left) and mobilized peripheral blood stem cells from a normal donor (right) were isolated with anti-CD34 magnetic beads and analyzed for MUC1 and CD34 expression by flow cytometry (A). The percentage of MUC1+ cells is highlighted in the upper right panel. The AML CD34+ and normal CD34+ cells were also analyzed by immunohistochemical staining for MUC1 (B) and by RT-PCR using primers for MUC1 and, as a control, GAPDH (C). D. AML cells from 20 patients with CD34+ active disease were analyzed by flow cytometry for MUC1 expression on CD34+/lineage−/CD38− and CD34+/lineage−/CD38+ populations. Each symbol represents the individual patients. Peripheral blood stem cells from 7 normal donors and bone marrows from 3 patients with lymphoid malignancies without evidence of tumor involvement were also analyzed for MUC1 expression as controls. The results are expressed as the percentage of cells that are MUC1+ with the horizontal bars representing the mean percentage of MUC1 expression for the different groups. E. CD34−/lineage− AML cells from 6 patients with CD34− active disease were analyzed for MUC1 expression. The results are expressed as the percentage of cells that are MUC1+ with the horizontal bar representing the mean percentage of MUC1 expression.
Assessment of MUC1-positive leukemic cells in a chimeric bone marrow population
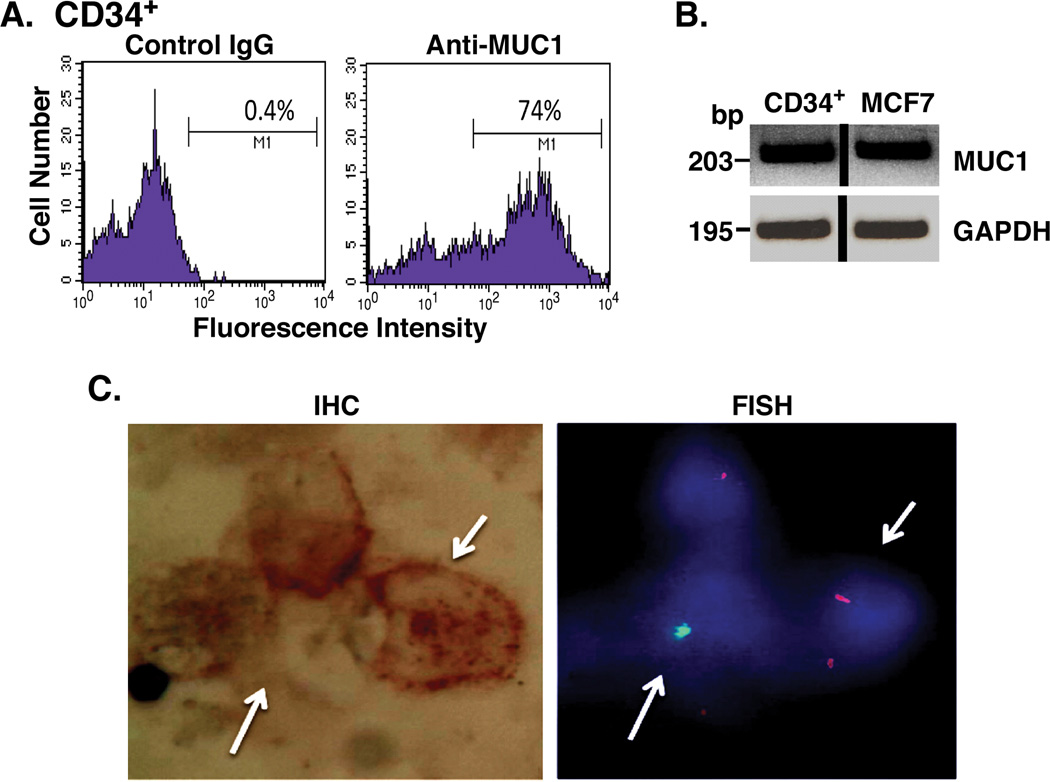
The selective expression of MUC1 by AML as compared to normal hematopoietic cells was further assessed in studies of a patient who had undergone an allogeneic transplant from her HLA-matched brother. In the post-transplant recovery period, CD34+ cells isolated from the patient’s bone marrow were analyzed for MUC1 expression. Flow cytometry of the CD34+ cell population clearly demonstrated the presence of MUC1+ cells (Fig. 2A). In addition, RT-PCR analysis of the isolated CD34+ population confirmed the detection of MUC1 expression (Fig. 2B). To define the derivation of the MUC1+ cells, we used the BioView Duet System in which isolated CD34+ cells were analyzed by concomitant immunohistochemical staining for MUC1 and cytogenetic detection of sex chromosomes (Fig. 2C). Of the 100 CD34+ cells that were analyzed, MUC1 expression was detectable in 41 cells, of which 39 were of female or recipient origin. By contrast, 11 of 13 CD34+ cells identified as of male or donor origin were negative for MUC1 expression. The correlation between MUC1 expression and recipient-derived cells was statistically significant (p=0.04). These findings supported the premise that in the setting of post-transplant persistent disease, MUC1 expression differentiated the recipient-derived leukemia cells (MUC1+) from normal donor hematopoietic stem cells (MUC1−). In concert with the presence of recipient-derived leukemia cells following transplantation, this patient relapsed 1 month after the above analysis.
Figure 2. MUC1 expression by recipient cells in a patient following allogeneic transplantation.
CD34+/lineage− cells were isolated from the bone marrow of a female patient with AML after a sex mismatched allogeneic bone marrow transplant. A. The CD34+ cell population was incubated with a control IgG (left) and anti-MUC1 (right), and analyzed by flow cytometry. B. CD34+ cells were analyzed for MUC1 and GAPDH mRNA levels by RT-PCR. RNA from MUC1+ MCF-7 breast cancer cells was used a positive control. C. CD34+ cells were analyzed for MUC1 expression by immunohistochemical staining (left) and sex chromosomes by FISH (right) using the BioView System. Representative female recipient cells (XX; red signals) and male donor cells (XY; green signals) are highlighted with arrows.
Engraftment of AML CD34+ MUC1high and MUC1low cells in NSG mice
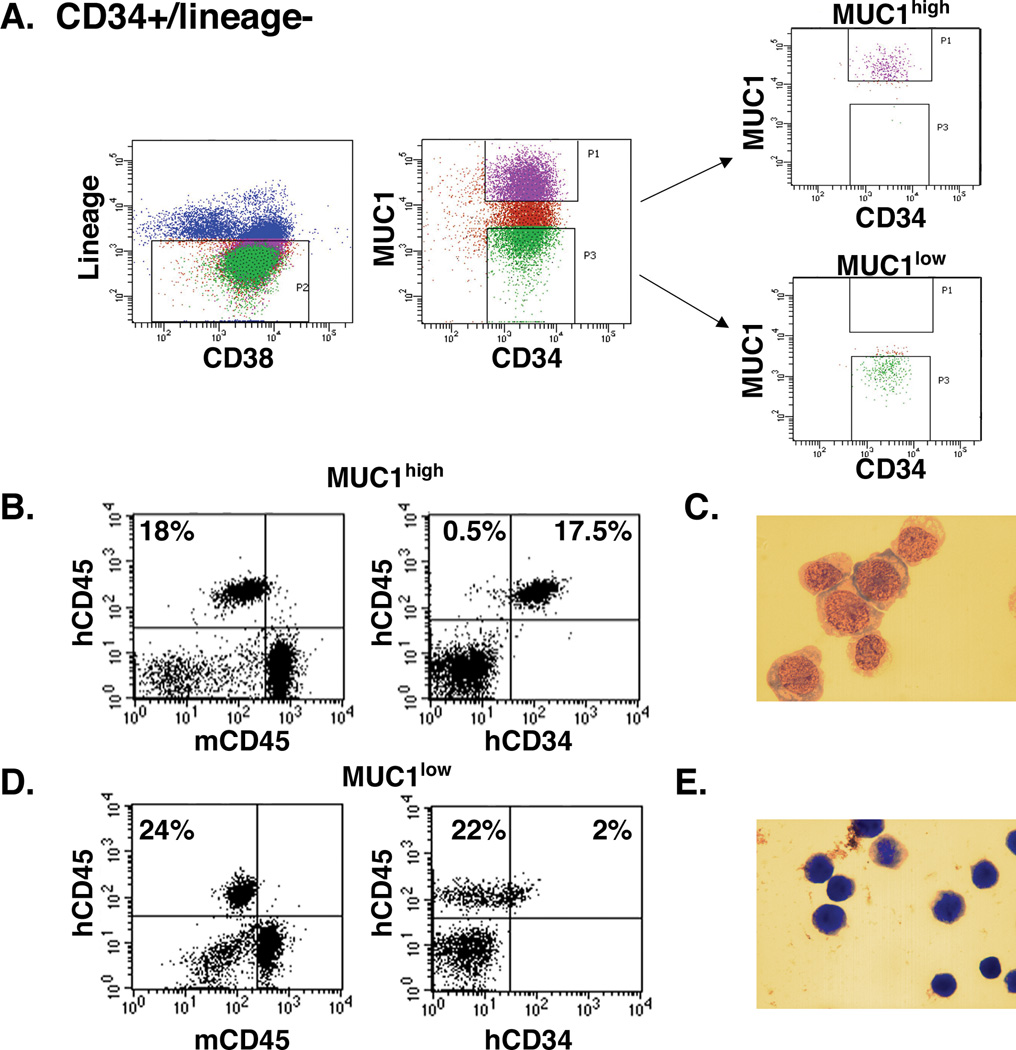
The LSC population is defined in part by the functional capability for engraftment of AML in immunocompromised mice (7–9). To assess the leukemia-initiating capacity of MUC1+ AML cells, we isolated the CD34+/lineage− population from the bone marrow of patient #1 with AML, who had blasts that uniformly expressed CD34, and sorted into MUC1high and MUC1low cells (Fig. 3A). The MUC1high cells were inoculated into NSG mice, which were then followed for 90 days. At that time, analysis of the bone marrow demonstrated the presence of human CD45+ cells (Fig. 3B, left). Further analysis confirmed that the human CD45+ population was also positive Article File with Changes in BOLD fontCD34 (Fig. 3B, right). Assessment of the isolated human CD45+/CD34+ cells demonstrated morphologic characteristics consistent with leukemic blasts (Fig. 3C). Similar findings were obtained in 4 of 5 mice inoculated with MUC1high cells and the one remaining mouse had no evidence for engraftment. Of the 4 mice with engraftment, human CD45+/CD34+ cells in the bone marrow reached a mean of 33% of the total mononuclear cell population. By contrast, of 5 mice inoculated with MUC1low cells, engraftment was observed in 2 mice. One of the 2 mice had engraftment of both human CD45+/CD34+ and CD45+/CD34− cells (Figs. 3D, left and right). The other mouse had engraftment of only human CD45+/CD34+ cells. For hCD45+/hCD34+ cells, mean involvement in the bone marrow of both mice was only 1.7% of the mononuclear cell population. Moreover, analysis of the hCD45+/hCD34− population demonstrated a morphology consistent with normal cells (Fig. 3E). To extend these observations, cells from AML patient #20 with trisomy 14 were sorted into CD34+/lineage− MUC1high and MUC1low cells and each of these two populations were inoculated into 6 NSG mice. Here, the MUC1high cells failed to engraft, consistent with the lack of engraftment encountered with certain AML samples (9, 11, 23). However, 5 of 6 mice inoculated with MUC1low cells engrafted with cytogenetically normal CD19+ lymphocytes (Supplemental Fig. S1A and B).
Figure 3. Engraftment of AML CD34+ MUC1high and MUC1low cells in NSG mice.
A. CD34+/lineage− cells were isolated from the bone marrow of patient #1 and sorted into MUC1high and MUC1low cells. B. Five mice were inoculated with CD34+/lineage−/MUC1high cells (1×106/mouse). After 90 days, bone marrows were harvested and analyzed for human hCD45 cells and, as a control, mouse mCD45 (left), and human hCD45+/hCD34+ cells (18% positive; right). C. Wright-Giemsa stain of the isolated hCD45+/hCD34+ cell population. D. Five mice were inoculated with CD34+/lineage−/MUC1low cells (1×106/mouse). Bone marrows were harvested after 90 days and analyzed for hCD45 cells (left) and hCD45+/hCD34− cells (22% positive; right). E. Wright-Giemsa stain of the isolated hCD45+/hCD34− cell population.
Engraftment of AML CD34− MUC1high and MUC1low cells
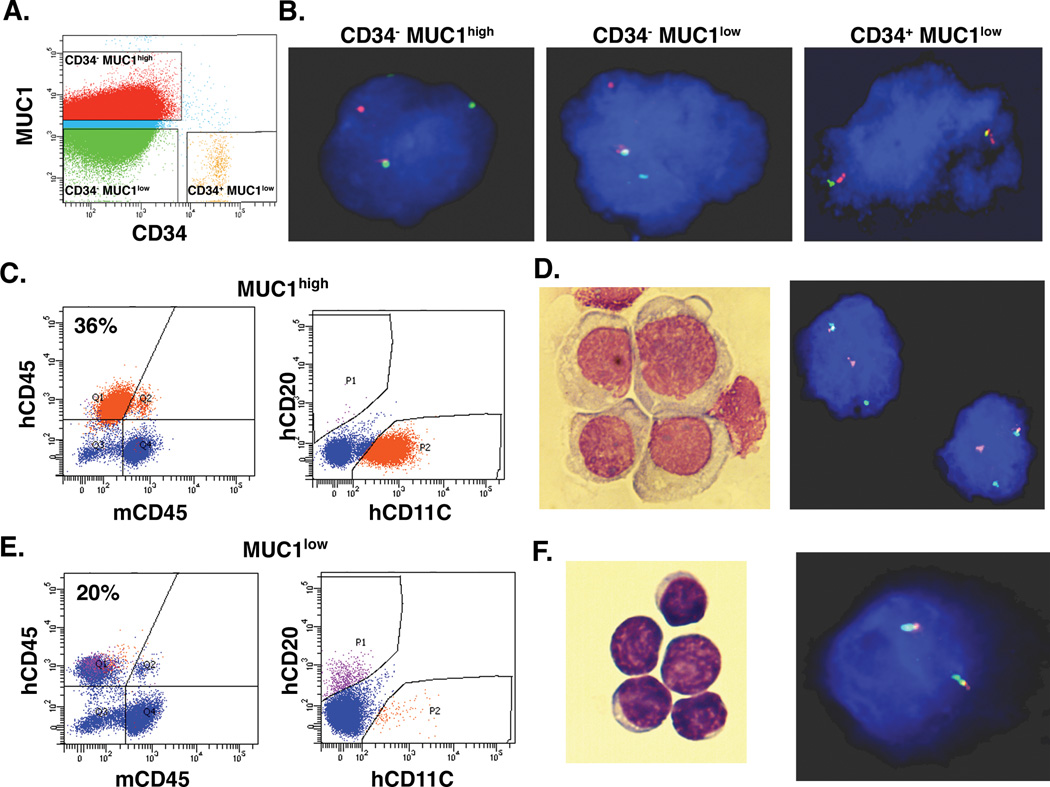
As noted above, blasts from patients with CD34− AML also express MUC1. In this context, we analyzed MUC1high and MUC1low populations from patient #25, who had CD34− AML blasts that harbored a rearrangement of the mixed-lineage leukemia (MLL) gene, making it possible to distinguish the leukemic cell population by detection of the abnormal karyotype. Flow cytometric sorting was performed to isolate the (i) CD34−/lineage− MUC1high, and (ii) CD34−/lineage− MUC1low cell populations (Fig. 4A). In addition, approximately 0.1% of the MUC1low cells were identified as having a CD34+/lineage− phenotype (Fig. 4A). FISH analysis demonstrated that 99% of the CD34−/lineage− MUC1high cells harbored the MLL gene rearrangement (Fig. 4B). Similar results were obtained with the CD34−/lineage− MUC1low cells, indicating that both MUC1high and MUC1low cells originate from the leukemic clone (Fig. 4B). Moreover, the few CD34+/lineage− MUC1low cells had a normal karyotype (Fig. 4B) and thereby represented residual normal hematopoietic stem cells. The isolated lineage− MUC1high cells were inoculated into 6 NSG mice and, after 90 days, the bone marrows were analyzed for AML engraftment. Analysis of a bone marrow from a representative mouse demonstrated the presence of 36% human CD45+ cells (Fig. 4C, left) that all expressed human CD11C and not CD20 (Fig. 4C, right), consistent with a myeloid phenotype. Morphology of the human CD45+/CD11C cells was also consistent with AML cells (Fig. 4D, left). Moreover, the engrafted hCD45+/hCD11C population had the MLL gene rearrangement, in concert with the leukemic genotype (Fig. 4D, right). Notably, all of the 6 inoculated mice exhibited leukemic engraftment with a mean of 66% involvement of blasts in the bone marrows. FISH analysis further demonstrated that these cells contained the MLL rearrangement, indicating the absence of normal hematopoietic cells. For comparison, the lineage− MUC1low cells were inoculated into 6 mice. Analysis of the bone marrows after 90 days demonstrated that all of these 6 mice also had engraftment of human CD45+ cells (Fig. 4E, left). However, in contrast to the MUC1high cells, engraftment with MUC1low cells consisted of predominantly human CD20+ and not CD11C+ cells, supporting a lymphoid population (Fig. 4E, right). Indeed, morphology of the CD45+/CD20+ cells was consistent with normal lymphocytes (Fig. 4F, left) that lacked the MLL gene rearrangement (Fig. 4F, right). Mean human normal cell involvement of the bone marrows from the 6 mice was 11%. These findings and those described above with CD34+/lineage− cells support the contention that MUC1high cells engraft with leukemia, whereas MUC1low cells predominantly engraft with normal hematopoietic cells.
Figure 4. Engraftment of AML CD34− MUC1high and MUC1low cells in NSG mice.
A and B. Blasts from AML patient #25 with a MLL gene rearrangement were sorted into CD34− MUC1high, CD34− MUC1low and CD34+ MUC1low cells (A). The indicated cell populations were analyzed for the MLL gene rearrangement by FISH (B). C. Six mice were inoculated with lineage−/MUC1high cells (1×106/mouse). After 90 days, bone marrows were harvested and analyzed for hCD45 cells (left) and hCD45+/hCD11C cells (36% positive; right). D. Wright-Giemsa stain (left) and FISH analysis for the MLL rearrangement (right) of the isolated hCD45+/hCD11C cell population. E. Six mice were inoculated with pooled lineage−/MUC1low cells (1×106/mouse). Bone marrows were harvested after 90 days and analyzed for hCD45 cells (left) and hCD45+/hCD20+ cells (right). F. Wright-Giemsa stain (left) and FISH analysis for the MLL rearrangement (right) of the isolated hCD45+ cell population.
Targeting MUC1 abrogates AML CD34+ cell engraftment in prevention and treatment models
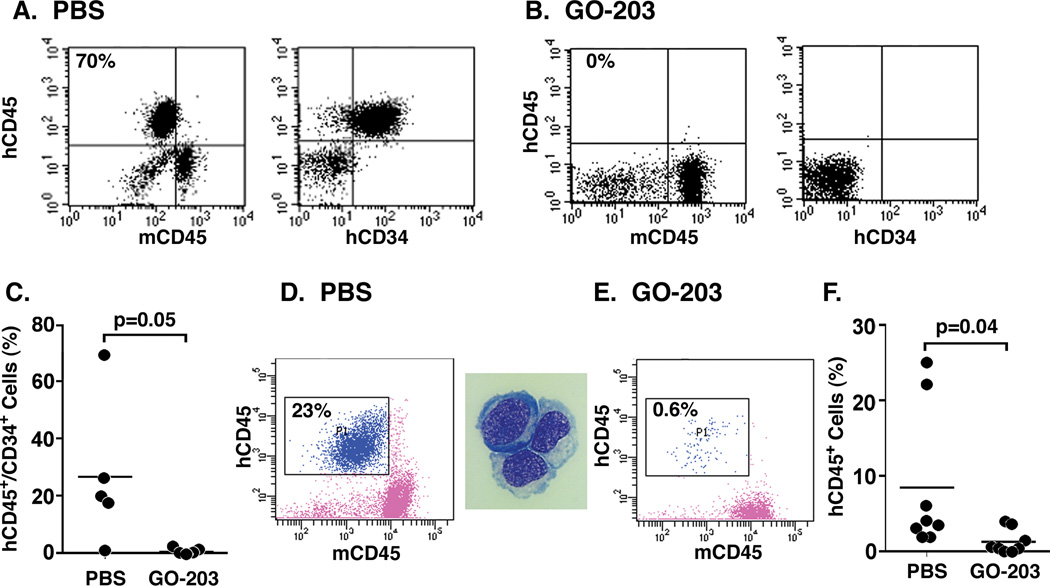
The demonstration that MUC1high cells confer the engraftment of leukemia in NSG mice invoked the possibility that targeting MUC1 could be effective in preventing the establishment of disease. To address the effects of targeting MUC1, we inoculated 10 mice with CD34+/lineage− MUC1high cells from patient #1. After 24 h, the mice were treated with PBS, the vehicle control, or with GO-203, a cell-penetrating peptide inhibitor of the oncogenic MUC1-C subunit. GO-203 consists of a poly-Arg cell transduction domain linked to the CQCRRKN sequence that binds to the MUC1-C cytoplasmic tail ([R]9-CQCRRKN; all D-amino acids) (24). Subcutaneous administration of GO-203 was continued for 21 days and then the mice were followed for an additional 70 days. Analysis of the bone marrow from a PBS-treated mouse demonstrated the presence of human CD45+ cells (Fig. 5A, left) that were also CD34+ (Fig. 5A, right). By contrast, analysis of the bone marrow from a GO-203-treated mouse demonstrated few if any detectable human CD45+/CD34+ cells (Fig. 5B, left and right). As compared to the control mice, treatment with GO-203 abrogated leukemic engraftment in 4 of the 5 GO-203-treated mice (Fig. 5C). The bone marrow from the 5th mouse treated with GO-203 had 1.9% involvement of the mononuclear population with human CD45+/CD34+ cells (Fig. 5C). Bone marrows of the GO-203-treated mice had a mean of 0.5% involvement with leukemic cells as compared to 27% for the 5 PBS-treated mice (Fig. 5C). To assess the effects of treating established leukemia, we isolated CD34+/lineage− cells from the bone marrow of patient #19. Over 90% of this CD34+/lineage− population expressed high levels of MUC1, which was inoculated into 16 mice. At 60 days post inoculation, the mice were treated with PBS or GO-203 for 21 days. Analysis of the bone marrow from a PBS-treated mouse demonstrated the presence of human CD45+ cells (Fig. 5D, left) that had morphologic characteristics consistent with leukemic blasts (Fig. 5D, right). By comparison, GO-203 treatment was associated with a significant decrease in human CD45+ leukemic cells (Figs. 5E and F), indicating that targeting MUC1 decreases the engrafted human leukemic cell population.
Figure 5. Effects of targeting MUC1 on AML CD34+ cell engraftment in prevention and treatment models.
A–C. In a prevention model, CD34+/lineage− MUC1high cells isolated from patient #1 were inoculated into 10 NSG mice (1×106/mouse). At 24 h after inoculation, the mice were treated with PBS or GO-203 administered subcutaneously daily for 21 days, and then followed for an additional 60 days. At that time, the mice were sacrificed and bone marrow cells from PBS- (A) and GO-203-treated (B) were analyzed for hCD45 and hCD34 expression. The results are expressed as the percentage of hCD45+/CD34+ leukemia cells in the bone marrows of the individual mice in the control and treated groups (C). The horizontal bar represents the mean percentage of hCD45+/CD34+ cells. D–F. In a treatment model, CD34+/lineage− cells from patient #19 that expressed high MUC1 levels in >90% of the population were inoculated into NSG mice (0.5×106/mouse). At 60 days after inoculation, the mice were treated with PBS or GO-203 administered subcutaneously daily for 21 days, and then sacrificed. Bone marrow cells from a representative PBS-treated mice were analyzed for hCD45 expression (D, left) and this population was isolated for assessment of a leukemic cell morphology by Wright-Giemsa staining (D, right). Bone marrow cells from GO-203-treated mice were also analyzed for hCD45 expression (E). The results are expressed as the percentage of hCD45+ leukemia cells in the bone marrows of the individual mice in the control and treated groups (F). The horizontal bar represents the mean percentage of hCD45+ cells.
Targeting MUC1 is effective in the treatment of established CD34− AML in NSG mice
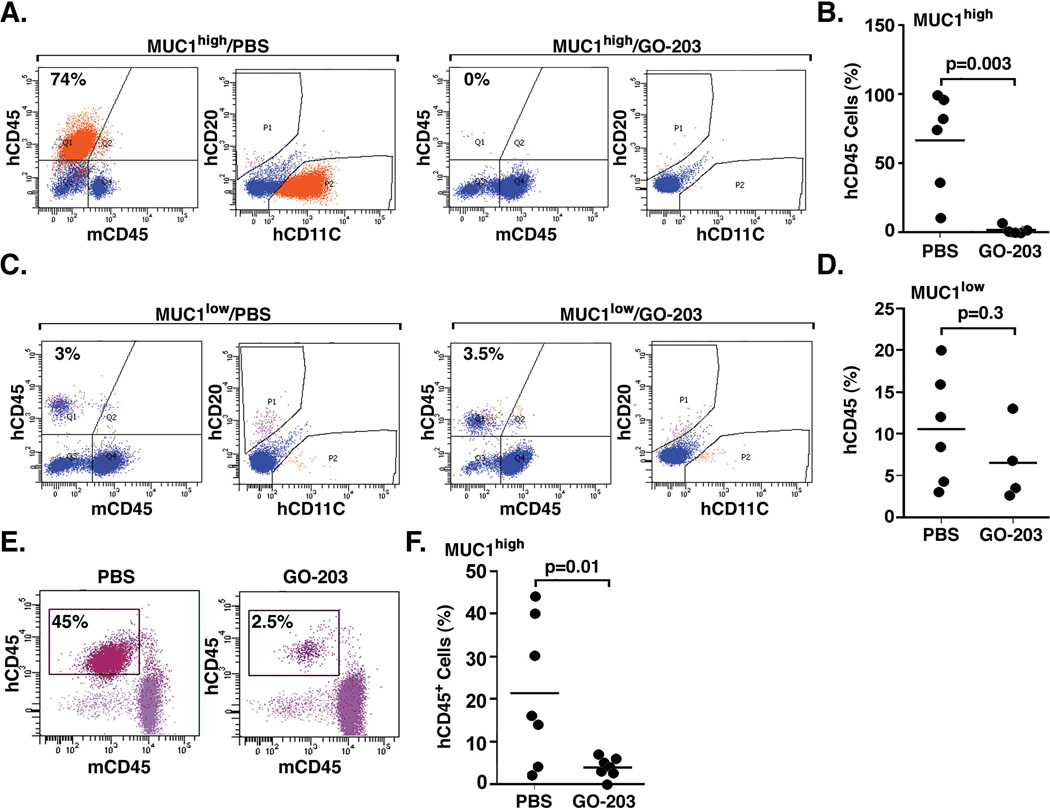
To extend the above findings, blasts from patient #25 with CD34− AML were sorted into lineage− MUC1high and MUC1low populations. The lineage− MUC1high cells were inoculated into NSG mice and, after 2 months, treatment was initiated based on the detection of hCD45+ cells in the peripheral blood. Treatment with PBS and GO-203 was continued for 21 days, at which time the mice were sacrificed for analysis. The bone marrow of a representative PBS-treated mouse had 74% involvement with hCD45+ cells that also expressed hCD11C and not hCD20 (Fig. 6A, left), and had a leukemic cell morphology. Similar findings were obtained for involvement of leukemic cells in the spleen (Supplemental Fig. S2A). In a representative GO-203-treated mouse, there were few if any hCD45+ cells in the bone marrow (Fig. 6A, right) and 0.4% involvement of hCD20+ cells in the spleen (Supplemental Fig. S2B). Comparison of the bone marrows from the GO-203-treated mice demonstrated a statistically significant decrease in hCD45+ cell involvement compared with that obtained for the PBS-treated mice (Fig. 6B). In contrast to the above results obtained with MUC1high cells, studies of mice inoculated with MUC1low cells showed engraftment of hCD45+ cells that expressed in part CD20 in both the bone marrow (Fig. 6C, left) and spleen (Supplemental Fig. S2C), and had a normal lymphoid morphology. Notably, treatment with GO-203 had no apparent effect on the engraftment of normal hematopoietic cells in the bone marrow (Fig. 6C, right) and spleen (Supplemental Fig. S2D). These results were confirmed with analysis of the bone marrows of the GO-203-treated mice, which demonstrated no significant difference when compared to that obtained with that in the PBS-treated mice (Fig. 6D). As further confirmation of the effects of GO-203, we isolated the lineage− population from patient #26, who had CD34− AML with over 90% of these cells expressing high levels of MUC1. Mice inoculated with these cells were treated with PBS and GO-203 as described above. Analysis of the bone marrow from a representative PBS-treated mouse demonstrated 45% involvement with hCD45+ cells (Fig. 6E, left) that had a leukemic phenotype and morphology. By contrast, treatment with GO-203 was associated with a marked decrease in leukemic cell involvement (Fig. 6E, right) that was significantly different from that found in the bone marrows of the PBS-treated mice (Fig. 6F).
Figure 6. Effects of targeting MUC1 on treatment of established CD34− AML in NSG mice.
CD34−/lineage− cells from patient #25 were sorted into MUC1high and MUC1low populations. A. The CD34−/lineage− MUC1high cells were inoculated into 11 NSG mice (1×106/mouse). At 60 days after inoculation, the mice were treated with PBS or GO-203 administered subcutaneously daily for 21 days, and then sacrificed. Bone marrow cells from a representative PBS-treated mouse were analyzed for hCD45, hCD11C and hCD20 expression (A, left). The orange-P2 gate represents the homogenous hCD45+/hCD11C+ leukemia population (A, left). Bone marrow cells from a representative GO-203-treated mouse were analyzed for hCD45, hCD11C and hCD20 expression (A, right). B. The results are expressed as the percentage of hCD45+ leukemia cells in the bone marrows of the individual mice in the control and treated groups. The horizontal bar represents the mean percentage of hCD45+ cells. C. The CD34−/lineage− MUC1low cells were inoculated into 10 NSG mice (1×106/mouse). At 60 days after inoculation, the mice were treated with PBS or GO-203 administered subcutaneously daily for 21 days, and then sacrificed. Bone marrow cells from representative PBS- and GO-203-treated mice were analyzed for hCD45, hCD11C and hCD20 expression (C, left and right). D. The results are expressed as the percentage of hCD45+ leukemia cells in the bone marrows of the individual mice in the control and treated groups. The horizontal bar represents the mean percentage of hCD45+ cells. E–F. CD34−/lineage− cells from patient #26 that expressed high MUC1 levels in >90% of the population were inoculated into NSG mice (0.5×106/mouse). At 60 days after inoculation, the mice were treated with PBS or GO-203 administered subcutaneously daily for 21 days, and then sacrificed. Bone marrow cells from representative PBS- and GO-203-treated mice were analyzed for hCD45 expression (E, left and right). The results are expressed as the percentage of hCD45+ leukemia cells in the bone marrows of the individual mice in the control and treated groups (F). The horizontal bar represents the mean percentage of hCD45+ cells.
Discussion
MUC1 is a heterodimeric glycoprotein that is expressed on the apical borders of normal epithelial cells (16). With progression to carcinomas and loss of polarity, MUC1 is aberrantly expressed at high levels over the entire cell surface and contributes to the malignant phenotype (16, 25). Somewhat surprisingly for this epithelial cell protein, expression of MUC1 was identified in blasts from AML patients (14). In addition, subsequent studies showed that MUC1 is detectable in certain CD34+ cells found in human cord blood and at higher levels in AML CD34+ populations (15). In the present work, AML CD34+/lineage−/CD38− cells from patients with active disease were studied to determine whether this population, which has been associated with leukemic stem cells (5, 26), also expresses MUC1. Our findings from the analysis of 20 AML patients demonstrated the consistent expression of MUC1 in the CD34+/lineage−/CD38− cell population. Specifically, the fraction of CD34+/lineage−/CD38− cells that expressed MUC1 ranged for 15 to 96% with a mean of 64%. AML CD34+/CD38− cells have characteristics of malignant stem cells in that they have the capacity to generate AML, to give rise to progenitor leukemic cells and to self-renew (27, 28). However, the CD34+/CD38− population can include normal hematopoietic stem cells, invoking the possibility that MUC1 expression as detected here could be also attributable to this population. In that sense, we found that CD34+/lineage−/CD38− cells from transplant donors express MUC1, but at low to undetectable levels. Indeed, the fraction of MUC1+ normal CD34+/lineage−/CD38− cells was less than 3%, indicating that MUC1 expression is substantially higher in the leukemic CD34+/lineage−/CD38− population.
The CD34+/CD38− cell phenotype has been associated with leukemia-initiating cells that give rise to CD34+/CD38+ progenitors and more differentiated blasts (5). In certain AML samples, the leukemic CD34+/CD38+ progenitor population also contains leukemia-initiating cell capacity (29). In this respect, we found that CD34+/lineage−/CD38+ progenitor cell populations from AML patients express MUC1 over a similar range of percentages as that obtained for the CD34+/lineage−/CD38− cells. Leukemia-initiating activity has also been identified in CD34− cell populations. For example, nucleophosmin (NPM) is a frequently mutated protein in AML and NPM-mutated leukemia is associated with attenuated expression of CD34 (30, 31). Moreover, NPM-mutated AML samples have leukemia-initiating cells in the CD34− population (8). Accordingly, we studied 5 patients with NPM-mutated CD34− AML and found MUC1 expression in each of the CD34−/lineage− cell populations. Another patient with AML cells harboring the MLL gene arrangement had blasts that were CD34−, and here MUC1 expression was also detectable in the CD34−/lineage− population. Based on these findings, we conclude that MUC1 is expressed in both AML CD34+ and CD34− cells.
Given the phenotypic heterogeneity of AML stem cells, the available evidence has supported their functional definition based on the capacity for engraftment in immunodeficient mice. For this reason, we performed studies to determine whether leukemic CD34+/lineage− cells that express MUC1 engraft in NSG mice. Inoculation of MUC1high cells was associated with the engraftment of leukemic cells. By comparison, MUC1low cells were ineffective in conferring AML in vivo or this population engrafted with normal hematopoietic cells of lymphoid origin. In support of these results, we studied engraftment of leukemic CD34−/lineage− cells with the MLL gene rearrangement. Here, the MUC1high population engrafted with leukemic cells that contained the genetic alteration. Notably, however, the MUC1low population engrafted with normal hematopoietic cells, based on absence of the MLL gene rearrangement and the detection of cells with a lymphoid phenotype and morphology. These findings indicate that the MUC1high, as compared to the MUC1low, leukemic population is functional in initiating leukemia in the NSG model. Engraftment of human leukemic cells in immunocompromised mice is variable and may be dependent on aggressiveness of disease (9, 11, 23). Given the limited number of leukemic samples analyzed for leukemia initiating activity and the selection of MUC1high cells, the present studies have not determined whether levels of MUC1 expression in patient samples can predict success of engraftment. As found in the present work for MUC1, other studies have demonstrated that CD32 and CD25 are highly expressed in leukemic CD34+/CD38− stem cells that are functional in engraftment in NSG mice (12). CD47 is also highly expressed on AML stem cells and targeting CD47 blocks engraftment in vivo (11). Thus, MUC1 represents another potential target on the AML stem cell population, and one that functions as an oncoprotein and contributes to the growth and survival of malignant cells. In this context, targeting the oncogenic MUC1-C subunit in AML cell lines and primary blasts with GO-203 was found to be associated with loss of self-renewal capacity (21).
Our findings that MUC1 is expressed in AML stem cell populations that engraft in NSG mice prompted further studies to determine whether targeting MUC1-C has therapeutic potential in this model. MUC1-C promotes growth and blocks death in the response to DNA damage, ROS and other forms of stress (32, 33). These MUC1-C functions are dependent on its homodimerization (16, 25). Thus, agents, such as GO-203, that block MUC1-C homodimer formation increase ROS levels in AML cells and thereby induce death (21). In the present studies, GO-203 treatment blocked engraftment of AML CD34+/lineage−/MUC1high cells when administered at 24 h after inoculation. In addition to preventing engraftment, GO-203 was effective in treating leukemia that was established in NSG mice inoculated with the AML CD34+/lineage−/MUC1high population. In this way, GO-203 treatment was associated with a marked decrease in AML cell involvement in the bone marrow as compared to that in the control mice. These findings were extended to studies of targeting MUC1-C in leukemia established in NSG mice after inoculation of CD34−/lineage−/MUC1high cells. Here, GO-203 treatment was also highly effective in decreasing leukemic cell involvement in bone marrows and spleens. Additionally, GO-203 had little if any effect on normal hematopoiesis established by inoculation of the CD34−/lineage−/MUC1low population, consistent with the low to undetectable levels of MUC1 expression in normal hematopoietic stem cells.
The precise mechanism by which MUC1-C inhibition results in loss of engraftment potential by leukemia initiating cells remains to be elucidated. Modulation of ROS has been shown to be a critical factor for supporting the long term repopulation capacity of leukemia stem cells (34). Exposure of primary AML cells to GO-203 induces an up-regulation of ROS, cell differentiation and death. In contrast, concurrent blockade of ROS with NAC diminishes the lethal effect of MUC1 inhibition on AML cells (21). The effect of GO-203 on other downstream signaling pathways linked with leukemia stem cell function such as β-catenin is currently being explored.
In summary, our findings provide evidence that MUC1 is highly expressed on AML stem cells as compared to their normal counterparts and that MUC1 is a selective target for the treatment of AML in the engrafted NSG mouse model. GO-203 is under clinical evaluation in a Phase I trial for patients with refractory solid tumors. The experimental results presented here provide the rationale for defining the effects of GO-203 in targeting the leukemic stem cell for the treatment of patients with AML.
Supplementary Material
Acknowledgements
This study was supported in part by research funding from Lady Tata Memorial Trust to D.S. and grants from the Leukemia Lymphoma Society (6074-09 and 6226-12) and the National Cancer Institute (CA42802, CA100707).
We thank the Cytogenetics Core of Dana Farber Harvard Cancer Center (P30 CA006516) for assistance with FISH analysis.
Footnotes
Disclosures: Donald Kufe: Genus Oncology: Ownership Interest, Consultant
References
- 1.Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341:1051–1062. doi: 10.1056/NEJM199909303411407. [DOI] [PubMed] [Google Scholar]
- 2.Huntly BJ, Gilliland DG. Cancer biology: summing up cancer stem cells. Nature. 2005;435:1169–1170. doi: 10.1038/4351169a. [DOI] [PubMed] [Google Scholar]
- 3.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 4.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 5.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 6.Eppert K, Takenaka K, Lechman ER, Waldron L, Nilsson B, van Galen P, et al. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat Med. 2011;17:1086–1093. doi: 10.1038/nm.2415. [DOI] [PubMed] [Google Scholar]
- 7.Sarry JE, Murphy K, Perry R, Sanchez PV, Secreto A, Keefer C, et al. Human acute myelogenous leukemia stem cells are rare and heterogeneous when assayed in NOD/SCID/IL2Rgammac-deficient mice. J Clin Invest. 2011;121:384–395. doi: 10.1172/JCI41495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taussig DC, Vargaftig J, Miraki-Moud F, Griessinger E, Sharrock K, Luke T, et al. Leukemia-initiating cells from some acute myeloid leukemia patients with mutated nucleophosmin reside in the CD34(−) fraction. Blood. 2010;115:1976–1984. doi: 10.1182/blood-2009-02-206565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearce DJ, Taussig D, Zibara K, Smith LL, Ridler CM, Preudhomme C, et al. AML engraftment in the NOD/SCID assay reflects the outcome of AML: implications for our understanding of the heterogeneity of AML. Blood. 2006;107:1166–1173. doi: 10.1182/blood-2005-06-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jordan CT, Upchurch D, Szilvassy SJ, Guzman ML, Howard DS, Pettigrew AL, et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000;14:1777–1784. doi: 10.1038/sj.leu.2401903. [DOI] [PubMed] [Google Scholar]
- 11.Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, Jr, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito Y, Kitamura H, Hijikata A, Tomizawa-Murasawa M, Tanaka S, Takagi S, et al. Identification of therapeutic targets for quiescent, chemotherapy-resistant human leukemia stem cells. Sci Transl Med. 2010;2:17ra9. doi: 10.1126/scitranslmed.3000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerber JM, Smith BD, Ngwang B, Zhang H, Vala MS, Morsberger L, et al. A clinically relevant population of leukemic CD34(+)CD38(−) cells in acute myeloid leukemia. Blood. 2012;119:3571–3577. doi: 10.1182/blood-2011-06-364182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brossart P, Schneider A, Dill P, Grunebach F, Wirths S, Kanz L, et al. The epithelial tumor antigen MUC1 is expressed in hematological malignancies and is recognized by MUC1-specific cytotoxic T-lymphocytes. Cancer Res. 2001;29:6846–6850. [PubMed] [Google Scholar]
- 15.Fatrai S, Schepers H, Tadema H, Vellenga E, Daenen SM, Schuringa JJ. Mucin1 expression is enriched in the human stem cell fraction of cord blood and is upregulated in majority of the AML cases. Exp Hematol. 2008;36:1254–1265. doi: 10.1016/j.exphem.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Kufe D. Mucins in cancer: function, prognosis and therapy. Nature Reviews Cancer. 2009;9:874–885. doi: 10.1038/nrc2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahmad R, Raina D, Joshi MD, Kawano T, Kharbanda S, Kufe D. MUC1-C oncoprotein functions as a direct activator of the NF-κB p65 transcription factor. Cancer Res. 2009;69:7013–7021. doi: 10.1158/0008-5472.CAN-09-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang L, Chen D, Liu D, Yin L, Kharbanda S, Kufe D. MUC1 oncoprotein blocks GSK3β-mediated phosphorylation and degradation of β-catenin. Cancer Res. 2005;65:10413–10422. doi: 10.1158/0008-5472.CAN-05-2474. [DOI] [PubMed] [Google Scholar]
- 19.Rajabi H, Ahmad R, Jin C, Kosugi M, Alam M, Joshi M, et al. MUC1-C oncoprotein induces TCF7L2 activation and promotes cyclin D1 expression in human breast cancer cells. J Biol Chem. 2012;287:10703–10713. doi: 10.1074/jbc.M111.323311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leng Y, Cao C, Ren J, Huang L, Chen D, Ito M, et al. Nuclear import of the MUC1-C oncoprotein is mediated by nucleoporin Nup62. J Biol Chem. 2007;282:19321–19330. doi: 10.1074/jbc.M703222200. [DOI] [PubMed] [Google Scholar]
- 21.Yin L, Wu Z, Avigan D, Rosenblatt J, Stone R, Kharbanda S, et al. MUC1-C oncoprotein suppresses reactive oxygen species-induced terminal differentiation of acute myelogenous leukemia cells. Blood. 2011;117:4863–4870. doi: 10.1182/blood-2010-10-296632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kufe D, Inghirami G, Abe M, Hayes D, Justi-Wheeler H, Schlom J. Differential reactivity of a novel monoclonal antibody (DF3) with human malignant versus benign breast tumors. Hybridoma. 1984;3:223–232. doi: 10.1089/hyb.1984.3.223. [DOI] [PubMed] [Google Scholar]
- 23.Ailles LE, Gerhard B, Hogge DE. Detection and characterization of primitive malignant and normal progenitors in patients with acute myelogenous leukemia using long-term coculture with supportive feeder layers and cytokines. Blood. 1997;90:2555–2564. [PubMed] [Google Scholar]
- 24.Raina D, Kosugi M, Ahmad R, Panchamoorthy G, Rajabi H, Alam M, et al. Dependence on the MUC1-C oncoprotein in non-small cell lung cancer cells. Mol Cancer Therapeutics. 2011;10:806–816. doi: 10.1158/1535-7163.MCT-10-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kufe D. MUC1-C oncoprotein as a target in breast cancer: activation of signaling pathways and therapeutic approaches. Oncogene. 2013;32:1073–1081. doi: 10.1038/onc.2012.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang JC, Dick JE. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 2005;15:494–501. doi: 10.1016/j.tcb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, et al. Cancer stem cells--perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 28.Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, Tanaka S, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25:1315–1321. doi: 10.1038/nbt1350. [DOI] [PubMed] [Google Scholar]
- 29.Taussig DC, Miraki-Moud F, Anjos-Afonso F, Pearce DJ, Allen K, Ridler C, et al. Anti-CD38 antibody-mediated clearance of human repopulating cells masks the heterogeneity of leukemia-initiating cells. Blood. 2008;112:568–575. doi: 10.1182/blood-2007-10-118331. [DOI] [PubMed] [Google Scholar]
- 30.Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352:254–266. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- 31.Haferlach C, Mecucci C, Schnittger S, Kohlmann A, Mancini M, Cuneo A, et al. AML with mutated NPM1 carrying a normal or aberrant karyotype show overlapping biologic, pathologic, immunophenotypic, and prognostic features. Blood. 2009;114:3024–3032. doi: 10.1182/blood-2009-01-197871. [DOI] [PubMed] [Google Scholar]
- 32.Ren J, Agata N, Chen D, Li Y, Yu W-H, Huang L, et al. Human MUC1 carcinoma-associated protein confers resistance to genotoxic anti-cancer agents. Cancer Cell. 2004;5:163–175. doi: 10.1016/s1535-6108(04)00020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yin L, Kufe D. Human MUC1 carcinoma antigen regulates intracellular oxidant levels and the apoptotic response to oxidative stress. J Biol Chem. 2003;278:35458–35464. doi: 10.1074/jbc.M301987200. [DOI] [PubMed] [Google Scholar]
- 34.Guzman ML, Rossi RM, Karnischky L, Li X, Peterson DR, Howard DS, et al. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005;105:4163–4169. doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.