Abstract
In this chapter, we will briefly discuss recent literature on the role of MET receptor tyrosine kinase (RTK) in brain development and how perturbation of MET signaling may alter normal neurodevelopmental outcomes. Recent human genetic studies have established MET as a risk factor for autism, and the molecular and cellular underpinnings of this genetic risk are only beginning to emerge from obscurity. Unlike many autism risk genes that encode synaptic proteins, the spatial and temporal expression pattern of MET RTK indicates this signaling system is ideally situated to regulate neuronal growth, functional maturation, and establishment of functional brain circuits, particularly in those brain structures involved in higher levels of cognition, social skills, and executive functions.
1. INTRODUCTION
Autism spectrum disorders (ASD), which include autistic disorder, Asperger’s syndrome, and pervasive developmental disorder (PDD)-not otherwise specified, are a group of neurodevelopmental syndromes that share a disease onset during early brain development and maturation (Abrahams & Geschwind, 2008; Geschwind & Levitt, 2007; Walsh, Morrow, & Rubenstein, 2008). There have been no unifying neuropathologic or neurobiological features that define ASDs. The diagnosis is based on clinical assessment of some core behavioral features, including impaired communicative skills, atypical social behavior, and restricted interests and repetitive behaviors. Two cardinal features of ASD are heritability and heterogeneity. Heritability refers to the fact that autism has evidently the strongest genetic components of all the developmental neuropsychiatric disorders. This is exemplified by the 82–92% concordance rate for autism among monozygotic twins as compared with ~10% concordance rate for dizygotic twins (Abrahams & Geschwind, 2008; Bailey et al., 1995; Constantino et al., 2013). Heterogeneity is reflected by the enormous number (>200) of gene loci (Aldinger, Plummer, Qiu, & Levitt, 2011; Ebert & Greenberg, 2013; Piggot, hirinyan, Shemmassian, Vazirian, & Alarcon, 2009) that contribute to the risk of developing ASD, hence imposing a major challenge for the identification of causative genes. While this genetic heterogeneity can manifest as noncoding variations, de novo mutations that produce syndromic disorders with autistic traits, copy number variations, and chromosome abnormalities (Marshall et al., 2008; Nakatani et al., 2009; Piggot et al., 2009; Sebat et al., 2007; Walsh et al., 2008), their functional implication spans even wider, from neuronal growth, projection and motility, GTPase/Ras-mediated signaling and cytoskeletal organization, proteolysis, to activity-dependent synaptic remodeling (Levitt & Campbell, 2009; Pinto et al., 2010). Thus, to gain insights into the underlying mechanisms of ASD will require a multidisciplinary approach focusing on brain regions, neural networks, and cellular substrates.
ASD is a complex disorder and, as such, identification of causative genes has been hampered by many inherent problems, such as multiple gene effects/interactions, environmental factors, gene–environment interactions, variable penetrance for each individual gene, and genetic heterogeneity. Many well-established autism risk genes encode proteins that are involved in the molecular networks controlling formation and function of the glutamatergic synapse, the submicron-scale structure that connects individual neurons into functional networks capable of computational outputs. These well-established genes include, but are not limited to, NRXN1, PTEN, SHANK3, UBE3a, NF1, NLGN3/4, CNTNAP2, SYNGAP1, and FMR1 (Alarcon et al., 2008; Bourgeron, 2009; Clement et al., 2012; Durand et al., 2007; Penagarikano et al., 2011; Piggot et al., 2009; Tabuchi et al., 2007; Yashiro et al., 2009). These molecules function by mediating pre- and postsynaptic assembly, scaffolding the synaptic structure, controlling neurotransmitter release, and affecting the activity-dependent structural changes, processes critical to sculpting our experience into neuronal circuits to guide future behavior. Not surprisingly, pathogenic mutations of the previously mentioned ASD genes during development have been shown to lead to synaptic dysfunction, impact the brain circuit, and disrupt the balanced excitatory/inhibitory brain networks (Ebert & Greenberg, 2013; Rubenstein & Merzenich, 2003; Tabuchi et al., 2007).
It is important to note, however, that synaptogenesis and neural circuit dynamics are relatively late events during the neurodevelopmental timeline. Prior to these events, the production and positioning of neurons in a correct cellular and network context must take place in order for synaptogenesis and circuit remodeling to occur. These early histogenic events are determined by genetic programs encoding neurogenesis, migration, neurite outgrowth and polarization, and axon guidance at critical developmental stages. At the cellular level, once a neuron is born, it migrates a long distance before arriving at its destination and differentiating. Neurons extend two classes of processes: a single axon to carry its output and several dendrites to collect information input. Once this neuronal polarity is established, the axon navigates through a complex environment to find its target, and dendrites undergo extensive growth and branching. The last step in forming functional circuitry is the establishment of synaptic connections between different neurons (Bradke & Dotti, 2000; Craig & Banker, 1994; Mueller, 1999; Tessier- Lavigne & Goodman, 1996). Two major types of synapses, excitatory and inhibitory, coexist within any functional circuitry, and their balanced action on the postsynaptic neurons shapes their functional output (Rubenstein & Merzenich, 2003). Therefore, aberrant genetic programs during this early extended timeline (as compared to impaired synaptic function at later stages) may profoundly affect brain function as well. Consistently, autism risk genes have been shown to control wide aspects of developmental events including neurogenesis, synaptogenesis, glutamatergic transmission, endosomal trafficking, and protein turnover (Ebert & Greenberg, 2013; Qiu, Aldinger, & Levitt, 2012; Walsh et al., 2008). As diverse as these risk genes appear, they may converge on a final common molecular pathway to disrupt developmental outcomes that perturb circuit formation and maturation.
The development of the central nervous system (CNS) is a complex process driven by a myriad of factors including a large family of growth factors and their receptors. Protein receptor tyrosine kinases (RTKs), which are cell-surface receptors for many polypeptide growth factors, hormones, and cytokines (Robinson, Wu, & Lin, 2000), regulate many aspects of neuronal physiology, including neurogenesis and survival, differentiation and migration, patterned connectivity, and plasticity. The human gene MET, which encodes MET RTK (Cooper et al., 1984), has emerged as a prominent risk factor for ASD (Campbell et al., 2006, 2009; Jackson et al., 2009; Sousa et al., 2009; Thanseem et al., 2010). MET plays a pleiotropic role in cell proliferation, motogenesis, differentiation, and survival in many tissue types (Birchmeier, Birchmeier, Gherardi, & Vande Woude, 2003; Maina et al., 1998). The ligand for MET receptor, hepatocyte growth factor (HGF), is a polypeptide growth factor that activates MET (Naldini, Weidner, et al., 1991). Both MET and HGF are expressed in the developing brain, with distinct spatial and temporal profiles (Judson, Amaral, & Levitt, 2011; Judson, Bergman, Campbell, Eagleson, & Levitt, 2009; Jung et al., 1994). Genetic studies from multiple laboratories have found that functional MET promoter variants are associated with differential risks for ASD. Consistently, clinical imaging and animal studies have provided evidence that disrupted MET signaling levels produce both morphological and functional alterations in neurons in those brain regions implicated in producing the ASD endophenotypes. In this chapter, we will briefly discuss how MET signaling might be ideally situated to regulate circuits and modify neuronal function. We review recent literature and hypothesize that MET signaling plays a critical role in balancing neuronal growth, functional maturation, and establishing functional circuits.
2. MET RECEPTOR TYROSINE KINASE-MEDIATED SIGNALING HAS A PLEIOTROPIC ROLE IN MULTIPLE ORGAN ONTOGENESIS
The MET RTK and its sole polypeptide growth factor ligand, HGF, exemplify a versatile signaling system that has effects not only on neurons but also on multiple target tissues during embryogenesis. HGF, also known as “scatter factor,” was originally identified as a molecule capable of triggering proliferation, motility, and morphogenesis in many epithelial cell types and is also involved in organ regeneration, angiogenesis, and tumor invasion (Naldini, Vigna, et al., 1991). The MET receptor was first identified as a proto-oncogene and later as a receptor for HGF (Bottaro et al., 1991; Cooper et al., 1984; Naldini, Vigna, et al., 1991). Soon after, MET/HGF-mediated signaling was found to be involved in a number of normal physiological processes. The signaling system appears important in mesenchymal–epithelial interactions during fetal development: genetic inactivation of Met or Hgf in mice leads to embryonic lethality, resulting from impaired liver development, loss of parenchymal cells, and failed development of placenta trophoblast cells and muscles (Bladt, Riethmacher, Isenmann, Aguzzi, & Birchmeier, 1995; Huh et al., 2004; Schmidt et al., 1995; Uehara et al., 1995). The context in which MET function is best understood is in cancer biology. HGF signaling through MET is said to be morphogenic, motogenic, and mitogenic. The function of this signaling extends to early steps of cell proliferation, survival, branching morphogenesis, neuronal induction, organ regeneration, angiogenesis, and tumor metastasis (Furge, Zhang, & Vande Woude, 2000; Maina et al., 1998).
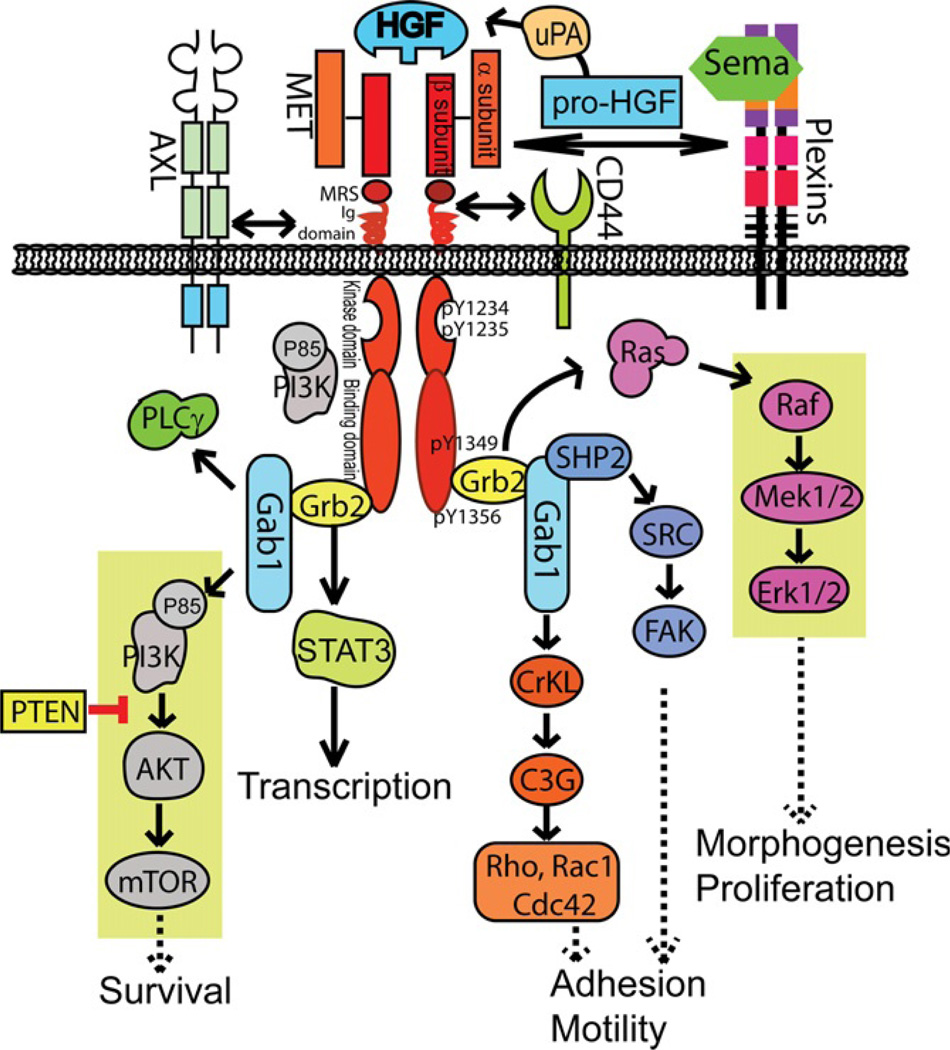
This pleiotropic role suggests that the molecular basis for MET signaling is of broad significance. Human MET protein is produced as a ~170 kD single-chain precursor (Cooper et al., 1984; Faletto et al., 1992). The precursor is proteolytically processed, resulting in a highly glycosylated extracellular α-subunit (50 kD) and a transmembrane β-subunit (145 kD) (Furge et al., 2000; Tempest, Stratton, & Cooper, 1988) (Fig. 5.1). The two subunits are linked together by a disulfide bond. The β-subunit has extracellular, transmembrane, and intracellular domains. The extracellular domain of both α- and β-subunits contains homology to semaphorins (Sema domain); the β chain has cysteine-rich MET-related sequences, glycine-proline-rich repeats, and four immunoglobulin-like domains (Ig domain). The intracellular β-subunits contain motifs of tyrosine kinase domain and a multisubstrate docking site. The function of both domains is dependent on several critical tyrosine residues. Upon HGF activation, MET dimerizes and transphosphorylation occurs on Tyr1234 and Tyr1235 within the activation loop of the tyrosine kinase domain, and this activates the intrinsic kinase activity of the receptor (Naldini, Weidner, et al., 1991). Close to the C-terminal region, two tyrosine residues (1349 and 1356), residing in the multisubstrate docking site, are capable of recruiting downstream Src homology-2 (SH2) domain-containing adaptor proteins (Ponzetto et al., 1994). Some adaptor proteins, such as Grb2, Src, SHC, and PI3K, interact with the multisubstrate docking site directly, whereas many other effects are mediated through the large scaffolding protein Gab1, which is sequentially tyrosine-phosphorylated and recruits a number of downstream effector proteins such as PI3K, SHP2, and PLC-γ (Faletto et al., 1992; Gual et al., 2000).
Figure 5.1.
Potential molecular signaling pathways mediated by MET receptor tyrosine kinase in neurons. The activation of the MET signaling pathway is initiated by hepatocyte growth factor (HGF) binding, which induces MET dimerization and transphosphorylation of two critical tyrosine residues (Tyr1234 and Tyr1235) in the tyrosine kinase domain to activate the intrinsic kinase activity of MET. The ensuing phosphorylation of two additional tyrosine residuals (Tyr1349 and Tyr1356) in the multisubstrate docking sites recruits downstream adaptor proteins including Grb2, Gab1, and SHC to activate cascades of downstream pathways that involve major signal transducers such as PLCγ, AKT, MAPK/Erk1/2, STAT3, focal adhesion kinase (FAK), and Rho family of small GTPases (Rho, Rac1, and Cdc42). Note that PI3 kinase can be directly activated by binding either to the multisubstrate docking site or downstream to Gab1 activation. Although most of these signaling events are established in nonneuronal cells, it is possible that these molecular pathways cooperate in developing neurons to mediate the outcome of neuronal survival, morphogenesis and proliferation, projection and motility, and activity-dependent gene transcription. Indeed, there has been some experimental evidence that MET signaling in neurons activates PI3K–AKT pathway and MAP kinase pathway (indicated by shaded boxes). MET has been shown to directly interact with other membrane-bound proteins in neurons, such as AXL, CD44, and plexins (which bind to semaphorins). The extent to which these membrane protein interactions and the intracellular signaling pathways in mediating the functional developmental outcomes in neurons has yet to be ascertained.
During peripheral tissue ontogenesis, the majority of the MET signaling outcomes are mediated by the adaptor proteins Grb2 and Gab1 (Maina et al., 1996, 2001; Ponzetto et al., 1994, 1996), which activate downstream pathways involving Ras, Rho family GTPases (such as Rho, Rac1, and Cdc42), ERK/MAPKs, guanine nucleotide exchange factors, Src family kinases, PI3K, and PKB/AKT (Fig. 5.1). The signaling mediates a diversity of events, including cell polarity, actin cytoskeleton reorganization, proliferation and cell-cycle progression, cell motility and migration, angiogenesis, organ regeneration (Arthur, Schwartz, Kuenzler, & Birbe, 2004; Ido, Numata, Kodama, & Tsubouchi, 2005; Royal, Lamarche-Vane, Lamorte, Kaibuchi, & Park, 2000; Tahara et al., 2003; Takaishi et al., 1994), immune and hormone responses (Beilmann, Vande Woude, Dienes, & Schirmacher, 2000; Okunishi et al., 2005; Roccisana et al., 2005), and tumor invasion (Birchmeier et al., 2003). MET signaling is initiated through HGF binding and the ensuing dimerization and tyrosine phosphorylation at its intracellular multisubstrate docking site (Naldini, Weidner, et al., 1991; Ponzetto et al., 1994). In nonneuronal cells, MET can activate multiple signaling cascades, including the Ras/MAP kinase and JNK/SAP kinase pathways, phospholipid pathways through binding of PI3K, PLC-γ, SHP2 tyrosine phosphatase, and Src tyrosine kinase.
MET activation recruits adaptor proteins to engage various molecular signaling pathways leading to different development outcomes (Maina et al., 1998, 2001). Generally, these multiple pathways are connected to cell growth and invasion following MET–HGF signaling. For example, activating RAS pathways serves as a cellular scatter and proliferation signal (O’Brien et al., 2004). The sustained RAS activation also leads to a protracted MAPK activity (Marshall, 1995). Additionally, PI3K can be activated by RAS or through binding to the multifunctional docking site. Activation of PI3K activates cell motility through remodeling of cell adhesion and localized cytoskeletal reorganization, processes which involve recruitment of transducers such as small GTPase Rac1- and p21-activated kinase. By activating PKB/AKT, PI3K is conferring a survival signal to the cells (Fan et al., 2001; Moumen et al., 2007). MET is also capable of activating the STAT3 transcription factor through binding to its SH2 domain (Boccaccio et al., 1998), which is necessary for the HGF-induced branching morphogenesis. In cancer cells, MET can be associated with β-catenin, which forms a complex with MET intracellular kinase domain. Upon HGF activation, β-catenin translocates to the nucleus to guide gene expression, an effect not seen in cells overexpressing a dominant-negative form of MET (only contains extracellular and transmembrane regions of MET and is therefore signaling-incompetent) (Monga et al., 2002).
3. MET SIGNALING PLAYS A ROLE IN A LARGE NUMBER OF NEURODEVELOPMENT EVENTS
The molecular signaling events discussed earlier are mostly ascertained in human epithelial or cancer cell lines, and, collectively, they mediate cell growth and invasive programs. The recognition of MET serving as a key signaling component in specific neurodevelopmental events is relatively new compared with the well-established roles in cancer biology (Judson, Eagleson, & Levitt, 2011; Maina et al., 1998). It is currently unclear to what extent these signaling events are operating in neurons during brain development. Nonetheless, accumulating evidence suggests that MET signaling is also required for multiple neurodevelopmental events. For example, MET is required for neuronal lineage commitment. Streit et al. (1995) showed that grafts of Hensen’s node into chick embryos enhanced the expression of neuronal markers in neighboring epiblast cells. In the presence of HGF, epiblast explant cultures prepared from chick embryos can differentiate into cells with neuronal morphology and express neuronal markers. This suggests that HGF plays a role during the early steps of neural induction, perhaps by inducing or maintaining the competence of the epiblast to respond to neural-inducing signals.
It has also been shown that postnatal proliferation of cerebellar granule neurons requires the full level of HGF/MET signaling. Cerebellum development occurs mainly postnatally and implies cell proliferation and migration during this period. HGF and MET are coexpressed in the developing cerebellum (Ieraci, Forni, & Ponzetto, 2002). MET is localized in granule cell precursors, and cultures of these cells respond to HGF with proliferation. HGF and MET are involved inmediating these responses, and a hypomorphicMET mutant (Grb2-binding incompetent) results in reduced size of the cerebellum, foliation defects, and reduced granule cell proliferation (Ieraci et al., 2002). HGF/MET signaling has been shown to modulate migration of specialized neuron types (Garzotto, Giacobini, Crepaldi, Fasolo, & De Marchis, 2008; Giacobini et al., 2007; Krasnoselsky et al., 1994; Powell, Mars, & Levitt, 2001; Segarra, Balenci, Drenth, Maina, & Lamballe, 2006). For example, HGF/MET signaling can elicit transtelencephalic migration of interneurons during forebrain development (Powell et al., 2001). The activation of HGF requires urokinase-type plasminogen activator receptor (uPAR). uPAR-deficient mice showed impaired scatter activity of forebrain neurons and reduced number of inter-neurons in the frontal and parietal cortex, likely due to impaired interneuron migration from the ganglionic eminence (Eagleson, Bonnin, & Levitt, 2005; Powell, Campbell, et al., 2003). HGF has also been shown to act as a motogen and guidance signal for gonadotropin hormone-releasing hormone-1 neuronal migration (Giacobini et al., 2007), an effect that is mediated by molecular cross talk between MET and the AXL receptor tyrosine kinase (Salian-Mehta, Xu, & Wierman, 2013). MET also has been shown to regulate the migration of olfactory interneuron precursors in the rostral migratory stream (Garzotto et al., 2008), thus potentially contributing to olfactory sensory processing. MET-triggered cortical neuron migratory effects seem to depend on combined MET–Grb2 coupling and signaling through ERK, PI3K/AKT, andRAC1/p38 (Segarra et al., 2006).
MET signaling has a profound effect on neuronal growth and morphology. In cortical organotypic slice culture, exogenous HGF increases dendritic growth and branching of pyramidal neurons, whereas applying function-blocking HGF antibody or transfection of neurons with a MET dominant-negative mutant receptor reduced the size and complexity of the dendritic arbors (Gutierrez, Dolcet, Tolcos, & Davies, 2004), suggesting that HGF plays a role in regulating dendritic morphology in the developing cerebral cortex. This is in agreement with studies showing in vivo manipulation of MET in dorsal pallial-specific knockout mice (Judson et al., 2009). A recent study has shown that exogenous HGF treatment of cultured hippocampal neurons enhanced the phosphorylation and activation of MET, increased the number of dendrites, and increased the total dendritic length. These effects are mediated by AKT activation, subsequent phosphorylation of glycogen synthase kinase-3 beta, and ultimately impinging upon cytoskeletal proteins (Lim &Walikonis, 2008). This study suggests that the PI3K pathway is involved in mediating HGF-induced neuronal growth effect. It would be interesting to examine whether axonal outgrowth involves other molecular mechanisms such as peripheral tissue development or tumor cell metastasis. MET signaling also seems to have a role in sensorimotor gating. HGF promotes development of sensory neuron target innervations (Maina, Hilton, Ponzetto, Davies, & Klein, 1997), cooperates with nerve growth factor to enhance sympathetic neuron axonal outgrowth, and increases the numbers of neurites of sensory neurons (Maina et al., 1998). HGF is also growth-promoting a chemoattractant for cranial motor axons during development (Caton et al., 2000), an axonal chemoattractant, and a neurotrophic factor for spinal motor neurons (Ebens et al., 1996). One potential mechanism for HGF to promote optimal axonal growth ganglion neurons is through an intrinsic, local dendritic autocrine mechanism (Yang et al., 1998).
In addition to mediating neuronal growth and morphological development in vivo, HGF/MET could affect neuronal function, likely through both cell-autonomous and cell nonautonomous mechanisms. In cultured hippocampal neurons, Tyndall and Walikonis (2006) reported that MET is clustered at excitatory synapses and colocalizes with NMDA receptor subunit NR2B and PSD-95 protein. This is revealed by immunocytochemistry and ultrastructural verification through immunoelectron microscopy. Additionally, MET protein is enriched at the postsynaptic density fraction, and HGF treatment can induce MET phosphorylation and enhance the expression and clustering of synaptic proteins including NR2B, calmodulin-dependent protein kinase II, and the AMPA receptor subunit GluA1. These findings suggest a direct functional connection with MET signaling and glutamatergic synapses.
Many studies have established HGF as a neurotrophic/neuroprotective factor. HGF promotes motor neuron survival and synergizes with ciliary neurotrophic factor to promote growth of sensory and parasympathetic neurons (Davey, Hilton, & Davies, 2000; Wong et al., 1997). HGF secreted by muscle fibers serves as a survival factor for certain populations of embryonic motoneurons (Yamamoto et al., 1997). Additionally, HGF signaling alleviates neuronal injury in a rat model for amyotrophic lateral sclerosis, and transgenic overexpression of HGF in a mouse model for amyotrophic lateral sclerosis delays the disease progression and prolongs life span of the mouse (Ishigaki et al., 2007; Sun, Funakoshi, & Nakamura, 2002). HGF also promotes endogenous repair and functional recovery after spinal cord injury (Kitamura et al., 2007). HGF is capable of protecting hippocampal neurons from injury induced by ischemia and preventing cultured rat cerebellar granule neurons from apoptosis (Miyazawa et al., 1998), an effect probably involving the activation of the Ras/MAPK and PI3K/AKT pathways. The fact that HGF has neuroprotective effects implies a therapeutic potential of MET signaling on the CNS. A recent study (Bai et al., 2012) showed that conditioned medium from cultured human mesenchymal stem cells reduces functional deficits in experimental autoimmune encephalomyelitis mouse model by promoting the development of oligodendrocytes and neurons. Functional tests from the same study identified HGF and MET as mediators of the conditioned medium-stimulated recovery. This protective effect is due to HGF and MET promotion of neural cell development and remyelination.
4. MET RECEPTOR TYROSINE KINASE EXPRESSION IN THE DEVELOPING BRAIN
To better understand the developmental capacity of MET/HGF signaling in the brain, it is important to ascertain the normal spatiotemporal patterns of MET/HGF expression levels. Several early studies have attempted to resolve this question. Di Renzo et al. (1993) showed that MET is expressed in the human CNS and MET protein is detectable in human brain tissues using Western blot. Immunohistochemical staining of MET revealed a rather extensive labeling of both gray and white matter, particularly in cells showing morphological and immunochemical markers for microglia cells, suggesting a potential role of HGF/MET in microglial reactions to CNS injuries. Another earlier study using in situ hybridization demonstrated that both Hgf and Met transcripts are expressed in developing and adult mouse brain (Jung et al., 1994). Specifically, Hgf mRNA is primarily localized in the neurons of hippocampus, cortex, and the granule cell layer of the cerebellum, while Met mRNA is more specifically restricted to the CA1 area of the hippocampus, the septum, and the cortex. Both Hgf and Met mRNA transcripts are detectable as early as embryonic days 12–13, respectively. Functionally, neurons respond to HGF/MET signaling by increased immediate early gene c-Fos transcription.
In light of recent human genetic studies implicating MET as an ASD risk gene (see discussion in the succeeding text), a detailed investigation of MET/HGF expression across the spatial and temporal domains (preferably in multiple species) during brain development would be informative to understanding the biological role of MET signaling at cellular and system levels. By examining specific brain structures, one can focus on the expression patterns of MET in defined brain circuits that are behaviorally relevant to autism. In a recent study, Judson et al. (2009) had systematically investigated the expression pattern of Met transcripts and protein levels using complementary Western blotting, in situ hybridization, and immunohistochemical approaches. The study was conducted in mice forebrain throughout late embryonic and postnatal development (embryonic day E17.5–P35). It was found that the expression of MET protein levels was tightly regulated across the time domain in the forebrain. MET protein levels are relatively low around the late embryonic stage in mouse (E16.5) but increase dramatically during perinatal development (postnatal day 0, P0) to reach a peak at P7. Thereafter, MET levels are relatively stable during the second postnatal week but decline drastically after P21 to very low levels at adult stage. The study has revealed that peak levels of MET expression coincide with principal periods of neurite outgrowth and synaptogenesis. High-resolution immunohistochemistry staining from the same study reveals that MET is expressed by discrete subtypes of long-projecting neurons of the forebrain, especially those of dorsal pallial origin. Interestingly, MET protein is found to be enriched in the developing axons of these projection neurons. In P7– P14 mouse, MET immunoreactivity is strongly distributed to axons and neuropil throughout the anteroposterior axis in the cortex. The corpus callosum has the highest level of MET expression. There is also a clear laminar patterning of Met transcript and protein expression in the neocortex in which the barrel cortex and layer IV of the cortex distinctly lack MET expression. Because layer IV is the synaptic input layer from subcortical structures, this is consistent with the observation that the majority of subcortical region shows minimum MET immunoreactivity in mouse. Another interesting finding from this study is the apparent discrepant patterns of expression for Met transcripts and proteins in the striatum. For instance, Western blot analysis showed that striatum tissue contains abundant MET proteins, whereas in situ hybridization failed to reveal Met transcripts in the striatum. Therefore, the presence of MET protein in the striatum is exclusively attributed to cortical projecting axons. Since the medium spiny neurons in the striatum do not express MET, changes to corticostriatal circuits following ablation of MET in dorsal pallium structures can be therefore attributed to a presynaptic mechanism.
The brainstem circuitry is implicated in ASD pathophysiology and autonomic function control, and a recent study (Wu & Levitt, 2013) has examined Met and Hgf mRNA expression in the developing rodent brainstem using in situ hybridization and immunohistochemistry to probe protein levels. This study revealed a highly selective expression pattern of MET in the brainstem in a subpopulation of neurons in cranial motor nuclei, the dorsal raphe, Barrington’s nucleus, and the nucleus of solitary tract. All of these brainstem structures show strong Met transcripts and immunoreactivity, which indicates that MET signaling may influence the development of brainstem circuits that control autonomic function, such as central regulation of respiration and circulation, gastrointestinal function, tongue movement, speech, stress, and mood.
Compared with the rodent brain, there has been limited information of MET protein expression levels in the brains of higher organisms. It has been shown that MET protein and mRNAs can be readily detected by quantitative Western blots and RT-PCR from postmortem temporal lobe gray matter samples (Brodmann areas 41/42, 52, or 22) in both ASD cases and their matched controls (Campbell et al., 2007). In fetal human brain, MET mRNA expression during midgestation (weeks 15–20) can be detected and is restricted almost exclusively to portions of the temporal and occipital lobes (Mukamel et al., 2011). However, little is known on the physiological MET protein expression patterns at later developmental stages in postnatal human or primate brain.
It is of interest to examine whether the orthologs of MET receptor function similarly in the developing primate forebrain because of the presumed circuit similarity of primate brain in producing social and communication phenotypes. Judson, Amaral, et al. (2011) had found that MET expression levels in the rhesus macaque forebrain are similar to mouse brain in that strong temporal conservation of expression exists during the time of rapid axon development and at the onset of robust synapse formation. The expression patterns of MET in axon fiber tracts (e.g., corpus callosum, anterior commissure, and cortiothalamic projections) and limbic structures (entorhinal cortical projections of the perforant pathway) were similar in both species. Most strikingly, the neocortex MET expression patterns showed highly divergent pattern: while the mouse neocortex shows a generally uniform distribution of MET, the macaque brain exhibited more restricted expression to the cingulate cortex, posterior parietal, inferior temporal, and visual cortices, including the putative face-processing temporal lobe cortex. This unique pattern in the primate brain may indicate a more prominent role for MET-expressing neurons in establishing circuits relevant to species-appropriate responses, such as vision-guided social behavior. Although extreme caution should be taken on how to interpret these findings in mice and make them relevant to understanding ASD, this study nonetheless suggests that, when evaluating expression pattern of ASD risk genes, it is important to consider the alterations in the spatial and temporal distributions of gene products rather than the absolute levels of proteins with regard to their role in the formation of brain circuits.
There has been some ultrastructural evidence on the subcellular distribution of MET in neurons. Using immunoelectron microscopy, Tyndall and Walikonis (2006) had found that MET protein is localized at the postsynaptic dendritic site, suggesting MET could be part of the postsynaptic signaling complex. In a recent study by Kawas, Benoist, Harding, Wayman, and Abu- Lail (2013), it was found that MET protein levels are especially enriched in brain regions that undergo extensive synaptic remodeling and plasticity, such as the hippocampus CA1 region. Additionally, MET activation increases the dendritic spine density and number of synapses. The authors then used atomic force microscopy combined with a specific MET antibody to address the question of subcellular localization of MET and found that the activated multimeric form of MET is concentrated in the dendritic spine compartment. In comparison, the inactivated monomeric form of MET is prominent on the soma of neurons. This ultrastructural study provides the first direct evidence of functional activation of MET in neurons. A comprehensive morphology study by Eagleson, Milner, Xie, and Levitt (2013) attempted to resolve the perisynaptic location of MET, that is, whether MET is expressed in the presynaptic, postsynaptic, or glial compartments. Combining immunoelectron microscopy and in situ proximity ligation assay (PLA), the authors found that MET localization is rather dynamic, depending on the postnatal age of mouse examined. In the striatum radiatum layer of CA1 region of P7 mouse (peak stage of rapid neuronal dendritic growth and morphogenesis), MET expression is equally located at both pre- and postsynaptic compartments. At a later stage when extensive synaptogenesis occurs, MET expression is predominantly presynaptic, with a small proportion of immunoreactivity arising from glial cells at this time. These morphological observations are consistent with their PLA analysis in cultured neurons and Western blot analysis of MET levels in the sub-synaptic compartments in brain tissues. This study provides conclusive evidence that MET is enriched at synapses during development, and its expression is dynamically regulated. The study also provides structural evidence that signaling of MET can potentially recruit both pre- and postsynaptic mechanisms.
A current important unanswered question is how MET receptor tyrosine kinase is regulated to allow its spatiotemporal specificity in the developing brain. Initial study by Campbell et al. (2006) has shown that the transcription factors SP1 and PC4 (encoded by SUB1) bind to the 5′-transcriptional regulatory region of the MET gene, but the functional significance of this binding is not clear. A recent study (Mukamel et al., 2011) has identified Forkhead box protein P2 (FOXP2) as a novel transcriptional repressor of the MET gene. FOXP2 has been established as a regulatory repressor protein and has been implicated in regulating higher cognitive functions, including language development (Lai, Fisher, Hurst, Vargha-Khadem, & Monaco, 2001). In the cortical plate of the developing human brain, the laminar pattern of MET expression is complementary to that of FOXP2, indicating that FOXP2 may be capable of repressing MET gene expression. Over-expression of FOXP2 in normal human neuronal progenitor cells leads to reduced levels of MET protein expression in vitro. Using an EMSA assay, these authors identified a direct FOXP2-binding site in the 5′-regulatory region of MET gene. Considering the role of FOXP2 in language development, it is possible downstream regulation by FOXP2 of key gene networks including MET ultimately impacts wiring of ASD at-risk circuits. Therefore, despite the fact that relatively little is known on the transcriptional regulation of MET, FOXP2 seems to be a functional repressor for MET expression. In the future, it would be interesting to examine whether genetic inactivation of FOXP2 expression, such as in the brain-specific conditional knockout mouse, will alter the patterned expression of MET across spatial and temporal domains. Nonetheless, although the detailed regulatory mechanisms for MET expression are yet to be determined, the functional significance of this regulation can be dramatic. We can predict that this intrinsic regulatory mechanism limiting MET signaling is important in that (1) since MET signaling plays a role in neurite outgrowth and synaptogenesis, once these major events pass their peak time, MET expression is downregulated so there is limited redundancy for this signaling system and, (2) alternatively, the reduced level of MET signaling following the peak of neurite outgrowth and synaptogenesis may be a prerequisite for functional maturation of the glutamatergic synapses.
5. THE HUMAN MET GENE EMERGES AS A PROMINENT AUTISM RISK FACTOR
The human MET gene (OMIM 164860; chromosome 7q31) was first reported by Campbell et al. as a risk factor for autism based on genome-wide association studies aimed to identify genetic variants that are overrepresented in individuals with autism compared to control populations (Campbell et al., 2006). MET was hypothesized as a candidate gene based on the following observations prior to this study: First, MET is located on human chromosome 7q31, under a linkage peak identified in multiple whole-genome scan studies of ASD (IMGSAC, 1998, 2001; Yonan et al., 2003). Second, MET signaling mediates invasive growth and neurite extension and contributes to the development of the brain (Beilmann et al., 2000; Gutierrez et al., 2004; Ido et al., 2005; Ieraci et al., 2002; Maina et al., 1997; Okunishi et al., 2005; Powell et al., 2001). Additionally, MET signaling plays a role in immune function and gastrointestinal repair (Arthur et al., 2004; Tahara et al., 2003), and both are impaired modalities seen in some ASD cases. Lastly, there have been converging developmental biological studies indicating hypomorphic MET signaling in the cortex results in abnormal interneuron migration and decreased proliferation of granule cells in the cerebellum (Eagleson et al., 2005; Ieraci et al., 2002; Powell, Campbell, et al., 2003).
The study by Campbell et al. (2006) analyzed the MET gene in a family-based study of ASD including >1200 cases. The study revealed strong genetic association of a common C allele (rs1858830 “C”) in the 5′- transcriptional regulatory region of the MET gene in >200 autism families. Additionally, in multiplex families with more than one autistic child, the rs1858830 “C” allelic association is even stronger. Overall, the relative risk for autism diagnosis was 2.27 (95% CI 1.41–3.65) for the CC genotype and 1.67 (95% CI 1.11–2.49) for the CG type compared with the GG type. The autism risk susceptibility is correlated with MET promoter activity and the promoter sequence’s binding for specific transcription factors SP1 and PC4 (encoded by SUB1) in a functional assay. A subsequent study following this initial report by the same group examined MET expression levels in the postmortem tissue from the temporal lobe of autism and control cases. The study found decreased MET transcript and protein expression in individuals with ASD compared to matched controls (Campbell et al., 2007), further supporting the notion that reduction or hypomorphic MET signaling is a risk factor for autism.
Additional genetic and pathophysiological evidence that dysfunctional MET signaling contributes to ASD risk has been complimentary to the original findings. Campbell, Li, Sutcliffe, Persico, and Levitt (2008) tested whether genes in the MET pathway (multiple genes encoding proteins that regulate MET expression and activity), such as HGF, and PLAUR transcripts are significantly altered in the ASD brain. The PLAUR gene encodes the urokinase plasminogen activator receptor, which is required for the urokinase plasminogen activator to process the HGF precursor into an active form. In addition, the SERPINE1 gene, which encodes plasminogen activator inhibitor-1, was also examined. Both PLAUR and SERPINE1 exhibited significant association with autism (Campbell et al., 2008). PLAUR promoter variant rs344781 T allele was associated with ASD by both family-based association test and case–control analyses. There is also significant gene–gene interaction contributing to ASD risk between MET and PLAUR. This study further supports that multiple components of the MET signaling pathway contribute to ASD genetic susceptibility.
Additional independent genetic studies in different populations have further confirmed the association of MET with ASD risk. A study by Sousa et al. (2009) found a positive correlation between rs38845 in the MET gene and autism in an additional two cohorts with ASD diagnoses. However, this study did not find a correlation between the rs1858830 “C” allele variants and autism. Shortly after this report, a third independent study screened two additional cohorts and found the rs1858830 “C” variant to be associated with the autism cohort but not the PDD cohort (Jackson et al., 2009). At a similar time, another group (Thanseem et al., 2010) performed a trio association study of MET with ASD in a Japanese population and revealed an additional SNP in intron 1, rs38841, that is associated with ASD risk. Therefore, these combined results including five unrelated cohorts all revealed a strong association between MET and ASD, irrespective of the source of genetic variation.
How do these functional MET variants confer risk for ASD, and how do they relate to the disrupted circuit connectivity seen in ASD cases? Impaired local and long-range cortical connectivity has been posited as a pathophysiological hallmark of ASD brain (Anderson et al., 2011; Courchesne & Pierce, 2005; Geschwind & Levitt, 2007). A recent functional imaging study by Rudie et al. (2012) has provided evidence on how MET impacts functional and structural networks in the human brain and offered a critical missing puzzle piece in the context of convergent genetic, clinical, and neurobiological findings regarding MET as a candidate for mediating ASD risk. Rudie et al. have examined the functional ASD risk variant (rs1858830 “CC”) on network functions in ASD and control subjects by examining the relationship between MET risk genotype and functional activation patterns to social stimuli (emotional faces). MET risk genotype (“CC” allele) is capable of predicting atypical fMRI activation and deactivation patterns to social stimuli and is correlated with reduced functional and structural connectivity in temporoparietal lobes, areas known to have high levels of MET expression. Additionally, the MET rs1858830 “CC” risk allele exhibits the largest alterations in structural and functional endophenotypes in individuals with ASD. This study is also important in that it shows that genetic stratification may reduce heterogeneity and helps clarify the biological basis of ASD and potentially other neuropsychiatric conditions.
6. IMPLICATION OF MET SIGNALING IN NEURAL DEVELOPMENT AND FUNCTIONAL CONNECTIVITY
ASD is considered a developmental disconnection syndrome, and the core pathophysiological basis can likely be attributed to disrupted ontogeny of neural connectivity (Courchesne & Pierce, 2005; Geschwind & Levitt, 2007). The specificity and the timing of brain circuits that are involved and the severity of disruption determine the presentation of clinical phenotypes. There is strong molecular and cellular basis for this hypothesized mis-wiring. MET signaling is required for multiple neurodevelopmental events, including neuronal lineage commitment and survival (Bronner-Fraser, 1995; Streit et al., 1995), proliferation (Ieraci et al., 2002), migration (Garzotto et al., 2008; Giacobini et al., 2007; Krasnoselsky et al., 1994; Powell et al., 2001; Segarra et al., 2006), neurite outgrowth (Gutierrez et al., 2004; Maina et al., 1997; Tyndall, Patel, & Walikonis, 2007), sensorimotor axon pathfinding (Caton et al., 2000; Ebens et al., 1996; Powell, Muhlfriedel, Bolz, & Levitt, 2003), and neuronal repair and survival (Maina et al., 1997, 1998; Miyazawa et al., 1998; Wong et al., 1997; Yamamoto et al., 1997). MET signaling in vitro enhances axon outgrowth, dendritogenesis, and synaptogenesis (Ebens et al., 1996; Gutierrez et al., 2004; Tyndall et al., 2007). It has been shown that MET is required not only for excitatory neuron development but also for migration of inhibitory interneurons. For instance, HGF stimulates migration of GABAergic inter-neurons from cultured ganglionic eminence explants (Powell et al., 2001). Hypomorphic MET signaling in uPAR−/− mouse leads to disruption of cortical interneuron development and atypical emotional and social behavior (Eagleson et al., 2005; Powell, Campbell, et al., 2003). All of these evidence suggests MET has an essential role in hardwiring circuits during early histogenetic events. It is important to note that these distinct physiological processes may involve differential intracellular pathways mediated by MET signaling. However, it is unclear which signaling cascades are responsible for each of these processes involving differentiation, axonal outgrowth, and synaptogenesis. Most likely, common intracellular mechanisms are shared among different tissue types, and these mechanisms converge on the regulation of adhesion molecules and cytoskeleton proteins that ultimately impinge upon cell growth and motility.
Studies have shown that MET signaling functions beyond early histogenic events. In relatively mature synapses, such as long-term in vitro cultures of hippocampal neurons, MET protein is concentrated at excitatory synapses and colocalizes with postsynaptic density proteins. Treatment of cultured neurons with HGF induces MET tyrosine phosphorylation and enhances clustering of synaptic proteins, such as NR2B, GluR1, and CaMKII (Tyndall & Walikonis, 2006). MET signaling seems capable of modifying and responding to activity-dependent neural plasticity as well. Enhancing neuronal activity in cultured developing hippocampal neurons, which produces growth effects, also increased HGF immunoreactivity and clustering (Tyndall et al., 2007), suggesting MET signaling can be a downstream player employed by activity-dependent mechanisms. In neuronal circuits closely resembling in vivo conditions (i.e., the hippocampal slice preparation), MET signaling by HGF application enhanced phosphorylation of NMDA receptor subunit GluN1 (Ser 896/897), augmented NMDA receptor-mediated currents, and increased the amplitude of long-term potentiation induced by elevated neuronal activity (Akimoto et al., 2004). The physiological role of this MET signaling in the adult brain is further supported by the finding that tissue plasminogen activator, which is required for HGF activation, is released in a neuronal activity-dependent manner (Thewke & Seeds, 1999). Therefore, although anatomical studies (Judson et al., 2009) indicate a dramatically reduced MET expression level in adult brain, the remaining levels of MET expression, at least in the hippocampus (Akimoto et al., 2004), could likely play a physiological role in regulating synaptic transmission and plasticity.
There is strong evidence that MET signaling may affect the assembly and function of neural circuits, and the substrates of neurological dysfunction have been studied at the synaptic and microcircuit levels in mouse models with disrupted MET signaling. Because genetic knockout of Hgf or Met results in embryonic lethality in mouse (Bladt et al., 1995; Huh et al., 2004; Schmidt et al., 1995; Uehara et al., 1995), Judson et al. have taken advantage of a conditional knockout mouse model by crossing two genetic modified mouse lines (Judson et al., 2009). In one of the mouse lines, the mouse Met was modified by conditional gene targeting (Metfx/fx) (Huh et al., 2004). These Metfx/fx floxed mutant mice contain loxP sites flanking exon 16 and, when crossed to mice that express Cre recombinase, the resulting offspring will have an exon 16 deletion. Exon 16 encodes a critical ATP-binding site (Lys1108), and this deletion inactivates the intracellular tyrosine kinase activity of MET, which is essential for its function. In the second mouse line, Cre recombinase is expressed from an Emx1 knockin site (Emx1-IRES-cre) (Gorski et al., 2002). Crossing these two lines will lead to MET inactivation in the Emx1-expressing dorsal pallial structures. The cellular elements with inactivated MET signaling will include radial glia, Cajal–Retzius cells, glutamatergic pyramidal neurons, astrocytes, and oligodendrocytes. In comparison, most of the pallial GABAergic neurons arising outside the Emx1-expressing lineage are not affected.
Anatomical studies from Met forebrain conditional knockout mice (Metfx/fx/Emx1cre) have provided important clues on MET signaling in the development of normal neuronal morphology. Judson et al. used lucifer yellow microinjection technique to reveal the detailed morphology of defined neuron types and compared that in wild-type and Metfx/fx/Emx1cre mice (Judson, Eagleson, Wang, & Levitt, 2010). The study revealed morphological deficits in cortical pyramidal neurons, specifically a reduction in apical dendritic arborization, and a decreased cortical volume that can be sampled by Metfx/fx/Emx1cre neurons. Interestingly, although the dendritic spine density in cortical pyramidal neurons is not altered, the spine head volume is significantly increased by ~20%. In comparison, medium spiny neurons in the striatum, which do not express MET but receive MET-containing presynaptic cortical input (see discussion earlier), exhibited significant increase in total dendritic arbor length and enlarged spine head volume. Considering that dendritic spine size and geometry is correlated with glutamate receptor content and synapse maturity (Matsuzaki et al., 2001), these findings suggest the effect of MET signaling on the dendritic structure appears to be circuit-selective, and developmental loss of presynaptic MET signaling can affect postsynaptic morphogenesis through cell nonautonomous mechanisms. Additionally, reduced MET signaling could impair both local and long-range connectivity within circuits relevant to ASD by altering the time course of glutamatergic synapse maturation.
Numerous functional and structural imaging studies in patients support connectivity-based etiology for ASD (Geschwind & Levitt, 2007; Hong et al., 2011; Just, Cherkassky, Keller, Kana, & Minshew, 2007; Just, Cherkassky, Keller, & Minshew, 2004; Kana, Keller, Cherkassky, Minshew, & Just, 2009; Sahyoun, Belliveau, Soulieres, Schwartz, & Mody, 2010; Shukla, Keehn, Smylie, & Muller, 2011). These imaging studies consistently indicate alterations in both local brain regions and long-range connectivity among different functional regions. For example, Just et al. (2007, 2004) had shown that there is less synchronous activity of language-processing areas in ASD patients in response to a semantic comprehension task, and the impaired synchrony is selectively seen in frontoparietal areas during the executive function testing. A similar conclusion on compromised synchrony was obtained during social processing tasks (Kana et al., 2009). Reduced functional connectivity with frontal cortical regions in individuals with ASD was also observed during face recognition task or visuospatial and linguistic reasoning (Sahyoun et al., 2010). This phenotype of hypofunctioning in long-range circuits is supported by the anatomical evidence. For instance, altered white matter structure, as evidenced by reduced corpus callosum volume, has been reported in some ASD patients (Hong et al., 2011; Just et al., 2007; Shukla, Keehn, & Muller, 2011; Shukla, Keehn, et al., 2011; Thomas, Humphreys, Jung, Minshew, & Behrmann, 2011). Diffusion tensor imaging of the ASD brain reveals reduced fractional anisotropy in most major long-range fiber tracts, indicating a possibility for global deficit in functional connectivity (Shukla, Keehn, et al., 2011) in ASD brains. In addition to these clinical imaging findings, convergent genetic and developmental neurobiology studies have supported the role of MET in influencing synapse development in circuits relevant to core behavioral domains of ASD. Based on these combined evidence, a basic mechanistic hypothesis accounting for MET-induced ASD genetic risk can be formulated: developmental dysregulation of the MET signaling pathway increases the risk of ASD-relevant brain circuit miswiring.
Complementary to neurogenetic and neuroanatomical approaches, functional microcircuit analysis is emerging as an important line of investigation due to the fact that this technique is capable of providing a direct readout of circuit function (Luo, Callaway, & Svoboda, 2008). It holds promise for resolving the underlying pathophysiology and also for designing potential novel therapeutic strategies targeting specific neurological pathways. A recent study has revealed functional circuit abnormality in Metfx/fx/Emx1cre mice model (Qiu, Anderson, Levitt, & Shepherd, 2011). We used laser scanning photostimulation (LSPS) combined with glutamate uncaging to investigate a major local synaptic pathway in mouse frontal cortical region and compared this circuit function in Metfx/fx/Emx1cre mice and their littermate controls. The study found that laminar synaptic input from layer 2/3 into layer 5 pyramidal neurons is increased. Specifically, the layer 2/3 to layer 5 corticostriatal neurons (which project to striatum, as identified by retrograde tracer injection) are selectively increased by twofold. In comparison, the layer 2/3 to layer 5 corticopontine neurons (which project to the brainstem) synaptic connectivity did not change. The enhanced connectivity from layer 2/3 to layer 5 is also seen at synaptically connected neuronal pairs, suggesting stronger unitary connections in local brain regions resulting from MET loss of function. Although this study did not reveal whether pre-or postsynaptic mechanisms contribute to increased synaptic drive, the enhanced synaptic connectivity seen in a major local synaptic circuit may be reminiscent of hyperconnectivity of local brain regions seen in ASD patients. It would be interesting to examine long-range circuit functionality in future studies. Due to the limitations that LSPS mapping can only be done in brain slices where intact long-range circuits cannot be preserved, adaptation of new optogenetic tools into this technique provides a feasible approach to map long-range circuits (Petreanu, Huber, Sobczyk, & Svoboda, 2007). Lastly, although neuroanatomical and functional mapping gained valuable insight into the static circuit property, neural circuits are very dynamic in that they exhibit a remarkable ability to scale their activity in response to changes of activity or experimental perturbations, a process known as homeostatic plasticity (Pozo & Goda, 2010; Turrigiano & Nelson, 2000). It would be interesting for future work to look at the principal substrates of synaptic homeostasis, that is, the compensatory adaptations in synaptic strength or intrinsic excitability, and how these components respond to disrupted MET signaling.
7. CONCLUDING REMARKS
Translating the genetic contributions to neurodevelopmental disorders, such as ASD, into pathophysiological mechanisms will bridge the current knowledge gap and facilitate developing novel interventions and treatments. Many of the most compelling candidate genes identified for rare/syndromic and idiopathic forms of ASD so far are involved in brain wiring and synaptic function by being an integral part of synaptic molecular machinery, by regulating gene transcriptions, or by contributing to the excitatory/inhibitory balances (Bourgeron, 2009). MET signaling may be a unique mechanism capable of regulating a multitude of neuron behavior, including differentiation, growth, neurite extension, and synapse maturation, all of which are prerequisite steps in establishing brain circuits. Therefore, efforts in deciphering the functional significance of MET at molecular, cellular, and system levels are central to understanding how MET contributes to ASD pathophysiology. A recent genetic study (Pinto et al., 2010) focusing on the functional impact of global rare copy number variations in ASD has reported “an enrichment of CNVs disrupting functional gene sets involved in cellular proliferation, projection and motility, and GTPase/Ras signaling.” Existing experimental evidence suggests that MET signaling plays a role in each of these functional domains. MET expression levels peak in the early postnatal phase of development, which coincides a period when synaptic microcircuits are undergoing development, refinement, and maturation (Fig. 5.2). During these protracted processes, the signaling is tightly regulated by developmentally encoded intrinsic mechanisms yet to be defined and can be influenced by multiple factors through possible cross talks through the intracellular molecular pathways. These pathways are fundamentally involved in neurodevelopmental, plasticity, and disorder processes and may be engaged by other autism risk genes. These processes, when disrupted, may lead to impaired final common molecular pathways and the most replicated ASD-related endophenotype— the disrupted synaptic connectivity.
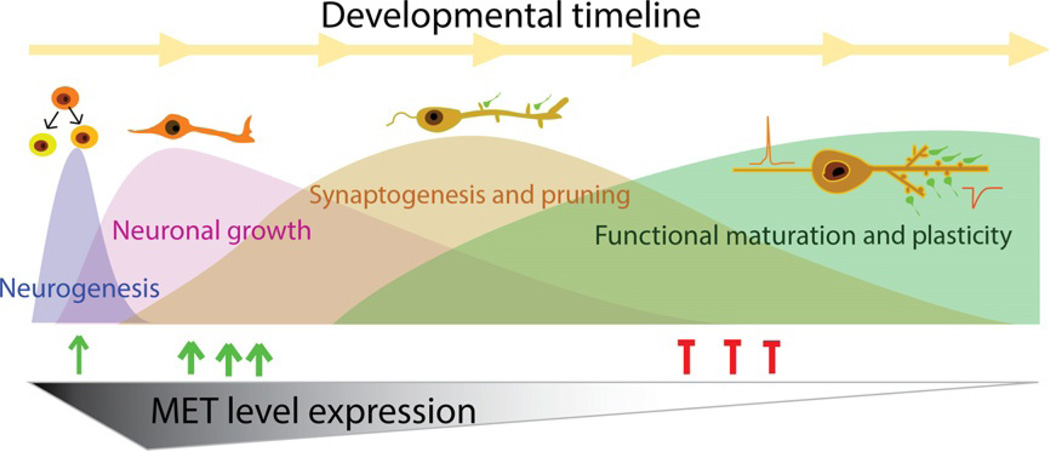
Figure 5.2.
MET receptor tyrosine kinase as a synaptic player that balances neuronal growth, synaptic plasticity, and functional maturation. The expression of MET protein in the developing brain is tightly regulated in both spatial and temporal domains. MET expression is turned on during the perinatal period in mouse and peaks during the period of extensive neurite growth and synaptogenesis. This suggests that MET-mediated signaling plays a role in these early processes of brain development (green arrows). MET protein is dramatically reduced as the brain circuits undergo functional maturation and synaptic plasticity (red ticks). Disturbances of MET signaling, such as carrying a hypofunctional MET allele, could have detrimental effects in the protracted neuronal developmental timeline and contribute to impaired circuit function in the adult brain.
Convergent genetic, clinical, and neurobiological findings from recent research studies have contributed to an accumulating body of evidence that MET is a critical signaling element in the developing brain. The biological basis of the genetic risk mediated by hypomorphic MET expression may occur through alterations in MET-mediated signaling pathways in neurons. MET activation by HGF induces a signaling cascade that involves many molecular components, such as TSC1, NF, PTEN, Ras/MAPK, and PI3K/AKT/mTOR (Fig. 5.1; also reviewed in Levitt & Campbell, 2009). These molecules either are known ASD risk factors themselves or interact with signaling pathways involving other ASD risk genes. The recent finding that MET expression levels are regulated by FOXP2 (Mukamel et al., 2011), a well-known risk factor for language dysfunction (Lai et al., 2001), further supports the view that MET is part of a complex molecular network implicated in ASD risk. The clinical relevance of MET signaling has been exemplified by 50% lower levels of MET protein in postmortem brain studies (Campbell et al., 2007). MET is integrated within a cell signaling network of synaptic proteins that regulates the early organization and function of synapses in MET-expressing circuits. The functional nature of the common risk allele in regulating levels of gene expression (Campbell et al., 2006, 2007), the patterns of connectivity and circuit activity in human brain (Rudie et al., 2012), the dramatic restriction of neocortical expression to regions that are implicated in ASD dysfunction in primates (Judson, Amaral, et al., 2011; Mukamel et al., 2011), and the circuit abnormality resulting from MET loss of function in animal models (Qiu et al., 2011) all support this conclusion. The literature discussed here further supports the view that ASD is a developmental “disconnection syndrome,” and MET signaling is critical for the normal synaptic connectivity established during development. We reason that the core pathophysiology of ASD brain lies not only in the impaired construction of circuit topography but also perhaps, more importantly, in the refinement and plasticity of these circuits in response to constant, adaptive behavior input of the individual processes of which can be all profoundly shaped by MET receptor tyrosine kinase.
ACKNOWLEDGMENTS
The authors thank Dr. Aaron McGee, Zhongming Lu, and Mariel Piechowicz for proofreading and their critiques of this chapter.
REFERENCES
- Abrahams BS, Geschwind DH. Advances in autism genetics: On the threshold of a new neurobiology. Nature Reviews Genetics. 2008;9(5):341–355. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akimoto M, Baba A, Ikeda-Matsuo Y, Yamada MK, Itamura R, Nishiyama N, et al. Hepatocyte growth factor as an enhancer of nmda currents and synaptic plasticity in the hippocampus. Neuroscience. 2004;128(1):155–162. doi: 10.1016/j.neuroscience.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Alarcon M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, et al. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. American Journal of Human Genetics. 2008;82(1):150–159. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldinger KA, Plummer JT, Qiu S, Levitt P. SnapShot: Genetics of autism. Neuron. 2011;72(2):418, e411. doi: 10.1016/j.neuron.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Anderson JS, Druzgal TJ, Froehlich A, DuBray MB, Lange N, Alexander AL, et al. Decreased interhemispheric functional connectivity in autism. Cerebral Cortex. 2011;21(5):1134–1146. doi: 10.1093/cercor/bhq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur LG, Schwartz MZ, Kuenzler KA, Birbe R. Hepatocyte growth factor treatment ameliorates diarrhea and bowel inflammation in a rat model of inflammatory bowel disease. Journal of Pediatric Surgery. 2004;39(2):139–143. doi: 10.1016/j.jpedsurg.2003.10.001. discussion 139–143. [DOI] [PubMed] [Google Scholar]
- Bai L, Lennon DP, Caplan AI, DeChant A, Hecker J, Kranso J, et al. Hepatocyte growth factor mediates mesenchymal stem cell-induced recovery in multiple sclerosis models. Nature Neuroscience. 2012;15(6):862–870. doi: 10.1038/nn.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, et al. Autism as a strongly genetic disorder: Evidence from a British twin study. Psychological Medicine. 1995;25(1):63–77. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- Beilmann M, Vande Woude GF, Dienes HP, Schirmacher P. Hepatocyte growth factor-stimulated invasiveness of monocytes. Blood. 2000;95(12):3964–3969. [PubMed] [Google Scholar]
- Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nature Reviews Molecular Cell Biology. 2003;4(12):915–925. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376(6543):768–771. doi: 10.1038/376768a0. [DOI] [PubMed] [Google Scholar]
- Boccaccio C, Ando M, Tamagnone L, Bardelli A, Michieli P, Battistini C, et al. Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature. 1998;391(6664):285–288. doi: 10.1038/34657. [DOI] [PubMed] [Google Scholar]
- Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251(4995):802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- Bourgeron T. A synaptic trek to autism. Current Opinion in Neurobiology. 2009;19(2):231–234. doi: 10.1016/j.conb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Bradke F, Dotti CG. Establishment of neuronal polarity: Lessons from cultured hippocampal neurons. Current Opinion in Neurobiology. 2000;10(5):574–581. doi: 10.1016/s0959-4388(00)00124-0. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M. Hepatocyte growth factor/scatter factor (HGF/SF) in early development: Evidence for a role in neural induction. Trends in Genetics. 1995;11(11):423–425. doi: 10.1016/s0168-9525(00)89136-2. [DOI] [PubMed] [Google Scholar]
- Campbell DB, Buie TM, Winter H, Bauman M, Sutcliffe JS, Perrin JM, et al. Distinct genetic risk based on association of MET in families with co-occurring autism and gastrointestinal conditions. Pediatrics. 2009;123(3):1018–1024. doi: 10.1542/peds.2008-0819. [DOI] [PubMed] [Google Scholar]
- Campbell DB, D’Oronzio R, Garbett K, Ebert PJ, Mirnics K, Levitt P, et al. Disruption of cerebral cortex MET signaling in autism spectrum disorder. Annals of Neurology. 2007;62(3):243–250. doi: 10.1002/ana.21180. [DOI] [PubMed] [Google Scholar]
- Campbell DB, Li C, Sutcliffe JS, Persico AM, Levitt P. Genetic evidence implicating multiple genes in the MET receptor tyrosine kinase pathway in autism spectrum disorder. Autism Research. 2008;1(3):159–168. doi: 10.1002/aur.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DB, Sutcliffe JS, Ebert PJ, Militerni R, Bravaccio C, Trillo S, et al. A genetic variant that disrupts MET transcription is associated with autism. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(45):16834–16839. doi: 10.1073/pnas.0605296103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caton A, Hacker A, Naeem A, Livet J, Maina F, Bladt F, et al. The branchial arches and HGF are growth-promoting and chemoattractant for cranial motor axons. Development. 2000;127(8):1751–1766. doi: 10.1242/dev.127.8.1751. [DOI] [PubMed] [Google Scholar]
- Clement JP, Aceti M, Creson TK, Ozkan ED, Shi Y, Reish NJ, et al. Pathogenic SYNGAP1 mutations impair cognitive development by disrupting maturation of dendritic spine synapses. Cell. 2012;151(4):709–723. doi: 10.1016/j.cell.2012.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Todorov A, Hilton C, Law P, Zhang Y, Molloy E, et al. Autism recurrence in half siblings: Strong support for genetic mechanisms of transmission in ASD. Molecular Psychiatry. 2013;18(2):137–138. doi: 10.1038/mp.2012.9. [DOI] [PubMed] [Google Scholar]
- Cooper CS, Park M, Blair DG, Tainsky MA, Huebner K, Croce CM, et al. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature. 1984;311(5981):29–33. doi: 10.1038/311029a0. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: Local over-connectivity but long-distance disconnection. Current Opinion in Neurobiology. 2005;15(2):225–230. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Craig AM, Banker G. Neuronal polarity. Annual Review of Neuroscience. 1994;17:267–310. doi: 10.1146/annurev.ne.17.030194.001411. [DOI] [PubMed] [Google Scholar]
- Davey F, Hilton M, Davies AM. Cooperation between HGF and CNTF in promoting the survival and growth of sensory and parasympathetic neurons. Molecular and Cellular Neurosciences. 2000;15(1):79–87. doi: 10.1006/mcne.1999.0803. [DOI] [PubMed] [Google Scholar]
- Di Renzo MF, Bertolotto A, Olivero M, Putzolu P, Crepaldi T, Schiffer D, et al. Selective expression of the Met/HGF receptor in human central nervous system microglia. Oncogene. 1993;8(1):219–222. [PubMed] [Google Scholar]
- Durand CM, Betancur C, Boeckers TM, Bockmann J, Chaste P, Fauchereau F, et al. Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nature Genetics. 2007;39(1):25–27. doi: 10.1038/ng1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagleson KL, Bonnin A, Levitt P. Region- and age-specific deficits in gamma-aminobutyric acidergic neuron development in the telencephalon of the uPAR(−/−) mouse. Journal of Comparative Neurology. 2005;489(4):449–466. doi: 10.1002/cne.20647. [DOI] [PubMed] [Google Scholar]
- Eagleson KL, Milner TA, Xie Z, Levitt P. Synaptic and extrasynaptic location of the receptor tyrosine kinase Met during postnatal development in the mouse neocortex and hippocampus. The Journal of Comparative Neurology. 2013;521(14):3241–3259. doi: 10.1002/cne.23343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebens A, Brose K, Leonardo ED, Hanson MG, Jr, Bladt F, Birchmeier C, et al. Hepatocyte growth factor/scatter factor is an axonal chemoattractant and a neurotrophic factor for spinal motor neurons. Neuron. 1996;17(6):1157–1172. doi: 10.1016/s0896-6273(00)80247-0. [DOI] [PubMed] [Google Scholar]
- Ebert DH, Greenberg ME. Activity-dependent neuronal signalling and autism spectrum disorder. Nature. 2013;493(7432):327–337. doi: 10.1038/nature11860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faletto DL, Tsarfaty I, Kmiecik TE, Gonzatti M, Suzuki T, Vande Woude GF. Evidence for non-covalent clusters of the c-met proto-oncogene product. Oncogene. 1992;7(6):1149–1157. [PubMed] [Google Scholar]
- Fan S, Ma YX, Gao M, Yuan RQ, Meng Q, Goldberg ID, et al. The multisubstrate adapter Gab1 regulates hepatocyte growth factor (scatter factor)-c-Met signaling for cell survival and DNA repair. Molecular and Cellular Biology. 2001;21(15):4968–4984. doi: 10.1128/MCB.21.15.4968-4984.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furge KA, Zhang YW, Vande Woude GF. Met receptor tyrosine kinase: Enhanced signaling through adapter proteins. Oncogene. 2000;19(49):5582–5589. doi: 10.1038/sj.onc.1203859. [DOI] [PubMed] [Google Scholar]
- Garzotto D, Giacobini P, Crepaldi T, Fasolo A, De Marchis S. Hepatocyte growth factor regulates migration of olfactory interneuron precursors in the rostral migratory stream through Met-Grb2 coupling. The Journal of Neuroscience. 2008;28(23):5901–5909. doi: 10.1523/JNEUROSCI.1083-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, Levitt P. Autism spectrum disorders: Developmental disconnection syndromes. Current Opinion in Neurobiology. 2007;17(1):103–111. doi: 10.1016/j.conb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Giacobini P, Messina A, Wray S, Giampietro C, Crepaldi T, Carmeliet P, et al. Hepatocyte growth factor acts as a motogen and guidance signal for gonadotropin hormone-releasing hormone-1 neuronal migration. The Journal of Neuroscience. 2007;27(2):431–445. doi: 10.1523/JNEUROSCI.4979-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, Jones KR. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. The Journal of Neuroscience. 2002;22(15):6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gual P, Giordano S, Williams TA, Rocchi S, Van Obberghen E, Comoglio PM. Sustained recruitment of phospholipase C-gamma to Gab1 is required for HGF-induced branching tubulogenesis. Oncogene. 2000;19(12):1509–1518. doi: 10.1038/sj.onc.1203514. [DOI] [PubMed] [Google Scholar]
- Gutierrez H, Dolcet X, Tolcos M, Davies A. HGF regulates the development of cortical pyramidal dendrites. Development. 2004;131(15):3717–3726. doi: 10.1242/dev.01209. [DOI] [PubMed] [Google Scholar]
- Hong S, Ke X, Tang T, Hang Y, Chu K, Huang H, et al. Detecting abnormalities of corpus callosum connectivity in autism using magnetic resonance imaging and diffusion tensor tractography. Psychiatry Research. 2011;194(3):333–339. doi: 10.1016/j.pscychresns.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Huh CG, Factor VM, Sanchez A, Uchida K, Conner EA, Thorgeirsson SS. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(13):4477–4482. doi: 10.1073/pnas.0306068101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ido A, Numata M, Kodama M, Tsubouchi H. Mucosal repair and growth factors: Recombinant human hepatocyte growth factor as an innovative therapy for inflammatory bowel disease. Journal of Gastroenterology. 2005;40(10):925–931. doi: 10.1007/s00535-005-1705-x. [DOI] [PubMed] [Google Scholar]
- Ieraci A, Forni PE, Ponzetto C. Viable hypomorphic signaling mutant of the Met receptor reveals a role for hepatocyte growth factor in postnatal cerebellar development. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(23):15200–15205. doi: 10.1073/pnas.222362099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IMGSAC. A full genome screen for autism with evidence for linkage to a region on chromosome 7q. International Molecular Genetic Study of Autism Consortium. Human Molecular Genetics. 1998;7(3):571–578. doi: 10.1093/hmg/7.3.571. [DOI] [PubMed] [Google Scholar]
- IMGSAC. Further characterization of the autism susceptibility locus AUTS1 on chromosome 7q. Human Molecular Genetics. 2001;10(9):973–982. doi: 10.1093/hmg/10.9.973. [DOI] [PubMed] [Google Scholar]
- Ishigaki A, Aoki M, Nagai M, Warita H, Kato S, Kato M, et al. Intrathecal delivery of hepatocyte growth factor from amyotrophic lateral sclerosis onset suppresses disease progression in rat amyotrophic lateral sclerosis model. Journal of Neuropathology and Experimental Neurology. 2007;66(11):1037–1044. doi: 10.1097/nen.0b013e318159886b. [DOI] [PubMed] [Google Scholar]
- Jackson PB, Boccuto L, Skinner C, Collins JS, Neri G, Gurrieri F, et al. Further evidence that the rs1858830 C variant in the promoter region of the MET gene is associated with autistic disorder. Autism Research. 2009;2(4):223–236. doi: 10.1002/aur.87. [DOI] [PubMed] [Google Scholar]
- Judson MC, Amaral DG, Levitt P. Conserved subcortical and divergent cortical expression of proteins encoded by orthologs of the autism risk gene MET. Cerebral Cortex. 2011;21(7):1613–1626. doi: 10.1093/cercor/bhq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson MC, Bergman MY, Campbell DB, Eagleson KL, Levitt P. Dynamic gene and protein expression patterns of the autism-associated met receptor tyrosine kinase in the developing mouse forebrain. Journal of Comparative Neurology. 2009;513(5):511–531. doi: 10.1002/cne.21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson MC, Eagleson KL, Levitt P. A new synaptic player leading to autism risk: Met receptor tyrosine kinase. Journal of Neurodevelopmental Disorders. 2011;3(3):282–292. doi: 10.1007/s11689-011-9081-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson MC, Eagleson KL, Wang L, Levitt P. Evidence of cell-nonautonomous changes in dendrite and dendritic spine morphology in the met-signaling-deficient mouse forebrain. Journal of Comparative Neurology. 2010;518(21):4463–4478. doi: 10.1002/cne.22467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung W, Castren E, Odenthal M, Vande Woude GF, Ishii T, Dienes HP, et al. Expression and functional interaction of hepatocyte growth factor-scatter factor and its receptor c-met in mammalian brain. The Journal of Cell Biology. 1994;126(2):485–494. doi: 10.1083/jcb.126.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Kana RK, Minshew NJ. Functional and anatomical cortical underconnectivity in autism: Evidence from an FMRI study of an executive function task and corpus callosum morphometry. Cerebral Cortex. 2007;17(4):951–961. doi: 10.1093/cercor/bhl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain. 2004;127(Pt. 8):1811–1821. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, Just MA. Atypical frontal-posterior synchronization of Theory of Mind regions in autism during mental state attribution. Social Neuroscience. 2009;4(2):135–152. doi: 10.1080/17470910802198510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawas LH, Benoist CC, Harding JW, Wayman GA, Abu-Lail NI. Nanoscale mapping of the Met receptor on hippocampal neurons by AFM and confocal microscopy. Nanomedicine. 2013;9(3):428–438. doi: 10.1016/j.nano.2012.08.008. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Iwanami A, Nakamura M, Yamane J, Watanabe K, Suzuki Y, et al. Hepatocyte growth factor promotes endogenous repair and functional recovery after spinal cord injury. Journal of Neuroscience Research. 2007;85(11):2332–2342. doi: 10.1002/jnr.21372. [DOI] [PubMed] [Google Scholar]
- Krasnoselsky A, Massay MJ, DeFrances MC, Michalopoulos G, Zarnegar R, Ratner N. Hepatocyte growth factor is a mitogen for Schwann cells and is present in neurofibromas. The Journal of Neuroscience. 1994;14(12):7284–7290. doi: 10.1523/JNEUROSCI.14-12-07284.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CS, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. 2001;413(6855):519–523. doi: 10.1038/35097076. [DOI] [PubMed] [Google Scholar]
- Levitt P, Campbell DB. The genetic and neurobiologic compass points toward common signaling dysfunctions in autism spectrum disorders. The Journal of Clinical Investigation. 2009;119(4):747–754. doi: 10.1172/JCI37934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CS, Walikonis RS. Hepatocyte growth factor and c-Met promote dendritic maturation during hippocampal neuron differentiation via the Akt pathway. Cellular Signalling. 2008;20(5):825–835. doi: 10.1016/j.cellsig.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Callaway EM, Svoboda K. Genetic dissection of neural circuits. Neuron. 2008;57(5):634–660. doi: 10.1016/j.neuron.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina F, Casagranda F, Audero E, Simeone A, Comoglio PM, Klein R, et al. Uncoupling of Grb2 from the Met receptor in vivo reveals complex roles in muscle development. Cell. 1996;87(3):531–542. doi: 10.1016/s0092-8674(00)81372-0. [DOI] [PubMed] [Google Scholar]
- Maina F, Hilton MC, Andres R, Wyatt S, Klein R, Davies AM. Multiple roles for hepatocyte growth factor in sympathetic neuron development. Neuron. 1998;20(5):835–846. doi: 10.1016/s0896-6273(00)80466-3. [DOI] [PubMed] [Google Scholar]
- Maina F, Hilton MC, Ponzetto C, Davies AM, Klein R. Met receptor signaling is required for sensory nerve development and HGF promotes axonal growth and survival of sensory neurons. Genes and Development. 1997;11(24):3341–3350. doi: 10.1101/gad.11.24.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina F, Pante G, Helmbacher F, Andres R, Porthin A, Davies AM, et al. Coupling Met to specific pathways results in distinct developmental outcomes. Molecular Cell. 2001;7(6):1293–1306. doi: 10.1016/s1097-2765(01)00261-1. [DOI] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: Transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80(2):179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Marshall CR, Noor A, Vincent JB, Lionel AC, Feuk L, Skaug J, et al. Structural variation of chromosomes in autism spectrum disorder. American Journal of Human Genetics. 2008;82(2):477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nature Neuroscience. 2001;4(11):1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa T, Matsumoto K, Ohmichi H, Katoh H, Yamashima T, Nakamura T. Protection of hippocampal neurons from ischemia-induced delayed neuronal death by hepatocyte growth factor: A novel neurotrophic factor. Journal of Cerebral Blood Flow and Metabolism. 1998;18(4):345–348. doi: 10.1097/00004647-199804000-00001. [DOI] [PubMed] [Google Scholar]
- Monga SP, Mars WM, Pediaditakis P, Bell A, Mule K, Bowen WC, et al. Hepatocyte growth factor induces Wnt-independent nuclear translocation of beta-catenin after Met-beta-catenin dissociation in hepatocytes. Cancer Research. 2002;62(7):2064–2071. [PubMed] [Google Scholar]
- Moumen A, Ieraci A, Patane S, Sole C, Comella JX, Dono R, et al. Met signals hepatocyte survival by preventing Fas-triggered FLIP degradation in a PI3k-Akt-dependent manner. Hepatology. 2007;45(5):1210–1217. doi: 10.1002/hep.21604. [DOI] [PubMed] [Google Scholar]
- Mueller BK. Growth cone guidance: First steps towards a deeper understanding. Annual Review of Neuroscience. 1999;22:351–388. doi: 10.1146/annurev.neuro.22.1.351. [DOI] [PubMed] [Google Scholar]
- Mukamel Z, Konopka G, Wexler E, Osborn GE, Dong H, Bergman MY, et al. Regulation of MET by FOXP2, genes implicated in higher cognitive dysfunction and autism risk. The Journal of Neuroscience. 2011;31(32):11437–11442. doi: 10.1523/JNEUROSCI.0181-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani J, Tamada K, Hatanaka F, Ise S, Ohta H, Inoue K, et al. Abnormal behavior in a chromosome-engineered mouse model for human 15q11-13 duplication seen in autism. Cell. 2009;137(7):1235–1246. doi: 10.1016/j.cell.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L, Vigna E, Ferracini R, Longati P, Gandino L, Prat M, et al. The tyrosine kinase encoded by the MET proto-oncogene is activated by autophosphorylation. Molecular and Cellular Biology. 1991;11(4):1793–1803. doi: 10.1128/mcb.11.4.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini L, Weidner KM, Vigna E, Gaudino G, Bardelli A, Ponzetto C, et al. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. The EMBO Journal. 1991;10(10):2867–2878. doi: 10.1002/j.1460-2075.1991.tb07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien LE, Tang K, Kats ES, Schutz-Geschwender A, Lipschutz JH, Mostov KE. ERK and MMPs sequentially regulate distinct stages of epithelial tubule development. Developmental Cell. 2004;7(1):21–32. doi: 10.1016/j.devcel.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Okunishi K, Dohi M, Nakagome K, Tanaka R, Mizuno S, Matsumoto K, et al. A novel role of hepatocyte growth factor as an immune regulator through suppressing dendritic cell function. Journal of Immunology. 2005;175(7):4745–4753. doi: 10.4049/jimmunol.175.7.4745. [DOI] [PubMed] [Google Scholar]
- Penagarikano O, Abrahams BS, Herman EI, Winden KD, Gdalyahu A, Dong H, et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147(1):235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nature Neuroscience. 2007;10(5):663–668. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- Piggot J, Shirinyan D, Shemmassian S, Vazirian S, Alarcon M. Neural systems approaches to the neurogenetics of autism spectrum disorders. Neuroscience. 2009;164(1):247–256. doi: 10.1016/j.neuroscience.2009.05.054. [DOI] [PubMed] [Google Scholar]
- Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, Regan R, et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466(7304):368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzetto C, Bardelli A, Zhen Z, Maina F, dalla Zonca P, Giordano S, et al. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77(2):261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- Ponzetto C, Zhen Z, Audero E, Maina F, Bardelli A, Basile ML, et al. Specific uncoupling of GRB2 from the Met receptor. Differential effects on transformation and motility. The Journal of Biological Chemistry. 1996;271(24):14119–14123. doi: 10.1074/jbc.271.24.14119. [DOI] [PubMed] [Google Scholar]
- Powell EM, Campbell DB, Stanwood GD, Davis C, Noebels JL, Levitt P. Genetic disruption of cortical interneuron development causes region- and GABA cell type-specific deficits, epilepsy, and behavioral dysfunction. The Journal of Neuroscience. 2003;23(2):622–631. doi: 10.1523/JNEUROSCI.23-02-00622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell EM, Mars WM, Levitt P. Hepatocyte growth factor/scatter factor is a motogen for interneurons migrating from the ventral to dorsal telencephalon. Neuron. 2001;30(1):79–89. doi: 10.1016/s0896-6273(01)00264-1. [DOI] [PubMed] [Google Scholar]
- Powell EM, Muhlfriedel S, Bolz J, Levitt P. Differential regulation of thalamic and cortical axonal growth by hepatocyte growth factor/scatter factor. Developmental Neuroscience. 2003;25(2–4):197–206. doi: 10.1159/000072268. [DOI] [PubMed] [Google Scholar]
- Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66(3):337–351. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qui S, Aldinger KA, Levitt P. Modeling of autism genetic variations in mice: Focusing on synaptic and microcircuit dysfunctions. Developmental Neuroscience. 2012;34(2–3):88–100. doi: 10.1159/000336644. [DOI] [PubMed] [Google Scholar]
- Qiu S, Anderson CT, Levitt P, Shepherd GM. Circuit-specific intracortical hyperconnectivity in mice with deletion of the autism-associated Met receptor tyrosine kinase. The Journal of Neuroscience. 2011;31(15):5855–5864. doi: 10.1523/JNEUROSCI.6569-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19(49):5548–5557. doi: 10.1038/sj.onc.1203957. [DOI] [PubMed] [Google Scholar]
- Roccisana J, Reddy V, Vasavada RC, Gonzalez-Pertusa JA, Magnuson MA, Garcia-Ocana A. Targeted inactivation of hepatocyte growth factor receptor c-met in beta-cells leads to defective insulin secretion and GLUT-2 downregulation without alteration of beta-cell mass. Diabetes. 2005;54(7):2090–2102. doi: 10.2337/diabetes.54.7.2090. [DOI] [PubMed] [Google Scholar]
- Royal I, Lamarche-Vane N, Lamorte L, Kaibuchi K, Park M. Activation of cdc42, rac, PAK, and rho-kinase in response to hepatocyte growth factor differentially regulates epithelial cell colony spreading and dissociation. Molecular Biology of the Cell. 2000;11(5):1709–1725. doi: 10.1091/mbc.11.5.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: Increased ratio of excitation/inhibition in key neural systems. Genes, Brain and Behavior. 2003;2(5):255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudie JD, Hernandez LM, Brown JA, Beck-Pancer D, Colich NL, Gorrindo P, et al. Autism-associated promoter variant in MET impacts functional and structural brain networks. Neuron. 2012;75(5):904–915. doi: 10.1016/j.neuron.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahyoun CP, Belliveau JW, Soulieres I, Schwartz S, Mody M. Neuroimaging of the functional and structural networks underlying visuospatial vs. linguistic reasoning in high-functioning autism. Neuropsychologia. 2010;48(1):86–95. doi: 10.1016/j.neuropsychologia.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salian-Mehta S, Xu M, Wierman ME. AXL and MET crosstalk to promote gonadotropin releasing hormone (GnRH) neuronal cell migration and survival. Molecular and Cellular Endocrinology. 2013;374(1–2):92–100. doi: 10.1016/j.mce.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Bladt F, Goedecke S, Brinkmann V, Zschiesche W, Sharpe M, et al. Scatter factor/hepatocyte growth factor is essential for liver development. Nature. 1995;373(6516):699–602. doi: 10.1038/373699a0. [DOI] [PubMed] [Google Scholar]
- Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316(5823):445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarra J, Balenci L, Drenth T, Maina F, Lamballe F. Combined signaling through ERK, PI3K/AKT, and RAC1/p38 is required for met-triggered cortical neuron migration. The Journal of Biological Chemistry. 2006;281(8):4771–4778. doi: 10.1074/jbc.M508298200. [DOI] [PubMed] [Google Scholar]
- Shukla DK, Keehn B, Muller RA. Tract-specific analyses of diffusion tensor imaging show widespread white matter compromise in autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2011;52(3):286–295. doi: 10.1111/j.1469-7610.2010.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla DK, Keehn B, Smylie DM, Muller RA. Microstructural abnormalities of short-distance white matter tracts in autism spectrum disorder. Neuropsychologia. 2011;49(5):1378–1382. doi: 10.1016/j.neuropsychologia.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa I, Clark TG, Toma C, Kobayashi K, Choma M, Holt R, et al. MET and autism susceptibility: Family and case-control studies. European Journal of Human Genetics. 2009;17(6):749–758. doi: 10.1038/ejhg.2008.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit A, Stern CD, Thery C, Ireland GW, Aparicio S, Sharpe MJ, et al. A role for HGF/SF in neural induction and its expression in Hensen’s node during gastrulation. Development. 1995;121(3):813–824. doi: 10.1242/dev.121.3.813. [DOI] [PubMed] [Google Scholar]
- Sun W, Funakoshi H, Nakamura T. Overexpression of HGF retards disease progression and prolongs life span in a transgenic mouse model of ALS. The Journal of Neuroscience. 2002;22(15):6537–6548. doi: 10.1523/JNEUROSCI.22-15-06537.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318(5847):71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahara Y, Ido A, Yamamoto S, Miyata Y, Uto H, Hori T, et al. Hepatocyte growth factor facilitates colonic mucosal repair in experimental ulcerative colitis in rats. The Journal of Pharmacology and Experimental Therapeutics. 2003;307(1):146–151. doi: 10.1124/jpet.103.054106. [DOI] [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kato M, Yamochi W, Kuroda S, Nakamura T, et al. Involvement of Rho p21 small GTP-binding protein and its regulator in the HGF-induced cell motility. Oncogene. 1994;9(1):273–279. [PubMed] [Google Scholar]
- Tempest PR, Stratton MR, Cooper CS. Structure of the met protein and variation of met protein kinase activity among human tumour cell lines. British Journal of Cancer. 1988;58(1):3–7. doi: 10.1038/bjc.1988.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274(5290):1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Thanseem I, Nakamura K, Miyachi T, Toyota T, Yamada S, Tsujii M, et al. Further evidence for the role of MET in autism susceptibility. Neuroscience Research. 2010;68(2):137–141. doi: 10.1016/j.neures.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Thewke DP, Seeds NW. The expression of mRNAs for hepatocyte growth factor/scatter factor, its receptor c-met, and one of its activators tissue-type plasminogen activator show a systematic relationship in the developing and adult cerebral cortex and hippocampus. Brain Research. 1999;821(2):356–367. doi: 10.1016/s0006-8993(99)01115-4. [DOI] [PubMed] [Google Scholar]
- Thomas C, Humphreys K, Jung KJ, Minshew N, Behrmann M. The anatomy of the callosal and visual-association pathways in high-functioning autism: A DTI tractography study. Cortex. 2011;47(7):863–873. doi: 10.1016/j.cortex.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Hebb and homeostasis in neuronal plasticity. Current Opinion in Neurobiology. 2000;10(3):358–364. doi: 10.1016/s0959-4388(00)00091-x. [DOI] [PubMed] [Google Scholar]
- Tyndall SJ, Patel SJ, Walikonis RS. Hepatocyte growth factor-induced enhancement of dendritic branching is blocked by inhibitors of N-methyl-D-aspartate receptors and calcium/calmodulin-dependent kinases. Journal of Neuroscience Research. 2007;85(11):2343–2351. doi: 10.1002/jnr.21390. [DOI] [PubMed] [Google Scholar]
- Tyndall SJ, Walikonis RS. The receptor tyrosine kinase Met and its ligand hepatocyte growth factor are clustered at excitatory synapses and can enhance clustering of synaptic proteins. Cell Cycle. 2006;5(14):1560–1568. doi: 10.4161/cc.5.14.2918. [DOI] [PubMed] [Google Scholar]
- Uehara Y, Minowa O, Mori C, Shiota K, Kuno J, Noda T, et al. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature. 1995;373(6516):702–705. doi: 10.1038/373702a0. [DOI] [PubMed] [Google Scholar]
- Walsh CA, Morrow EM, Rubenstein JL. Autism and brain development. Cell. 2008;135(3):396–400. doi: 10.1016/j.cell.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong V, Glass DJ, Arriaga R, Yancopoulos GD, Lindsay RM, Conn G. Hepatocyte growth factor promotes motor neuron survival and synergizes with ciliary neurotrophic factor. The Journal of Biological Chemistry. 1997;272(8):5187–5191. doi: 10.1074/jbc.272.8.5187. [DOI] [PubMed] [Google Scholar]
- Wu HH, Levitt P. Prenatal expression of MET receptor tyrosine kinase in the fetal mouse dorsal raphe nuclei and the visceral motor/sensory brainstem. Developmental Neuroscience. 2013;35(1):1–16. doi: 10.1159/000346367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Livet J, Pollock RA, Garces A, Arce V, deLapeyriere O, et al. Hepatocyte growth factor (HGF/SF) is a muscle-derived survival factor for a subpopulation of embryonic motoneurons. Development. 1997;124(15):2903–2913. doi: 10.1242/dev.124.15.2903. [DOI] [PubMed] [Google Scholar]
- Yang XM, Toma JG, Bamji SX, Belliveau DJ, Kohn J, Park M, et al. Autocrine hepatocyte growth factor provides a local mechanism for promoting axonal growth. The Journal of Neuroscience. 1998;18(20):8369–8381. doi: 10.1523/JNEUROSCI.18-20-08369.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro K, Riday TT, Condon KH, Roberts AC, Bernardo DR, Prakash R, et al. Ube3a is required for experience-dependent maturation of the neocortex. Nature Neuroscience. 2009;12(6):777–783. doi: 10.1038/nn.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonan AL, Alarcon M, Cheng R, Magnusson PK, Spence SJ, Palmer AA, et al. A genomewide screen of 345 families for autism-susceptibility loci. American Journal of Human Genetics. 2003;73(4):886–897. doi: 10.1086/378778. [DOI] [PMC free article] [PubMed] [Google Scholar]