Abstract
The isolation of cold-stable microtubules in high yields, described previously only from rodents, was extended to the brain of higher animals. Under optimal conditions, yields of 30 mg of cold-stable microtubles per 100 g of sheep brain could be obtained routinely. Material purified by two polymerization cycles displayed the same stability to cold temperature or to millimolar concentrations of calcium and the same lability to calmodulin and to ATP as did the purified material obtained from the rat [Job, D., Rauch, C.T., Fischer, E.H. & Margolis, R.L. (1982) Biochemistry 21, 509]. Furthermore, DE-52 chromatography of this material yielded a fraction that restored cold stability when added to cold-labile microtubules. Known to bind to calmodulin and to enhance microtubule assembly, tau proteins had no cold-stabilizing activity. Protein profiles of the cold-stabilizing fraction from sheep and rat brain were similar to one another but showed no protein bands corresponding to the tau proteins.
Full text
PDF
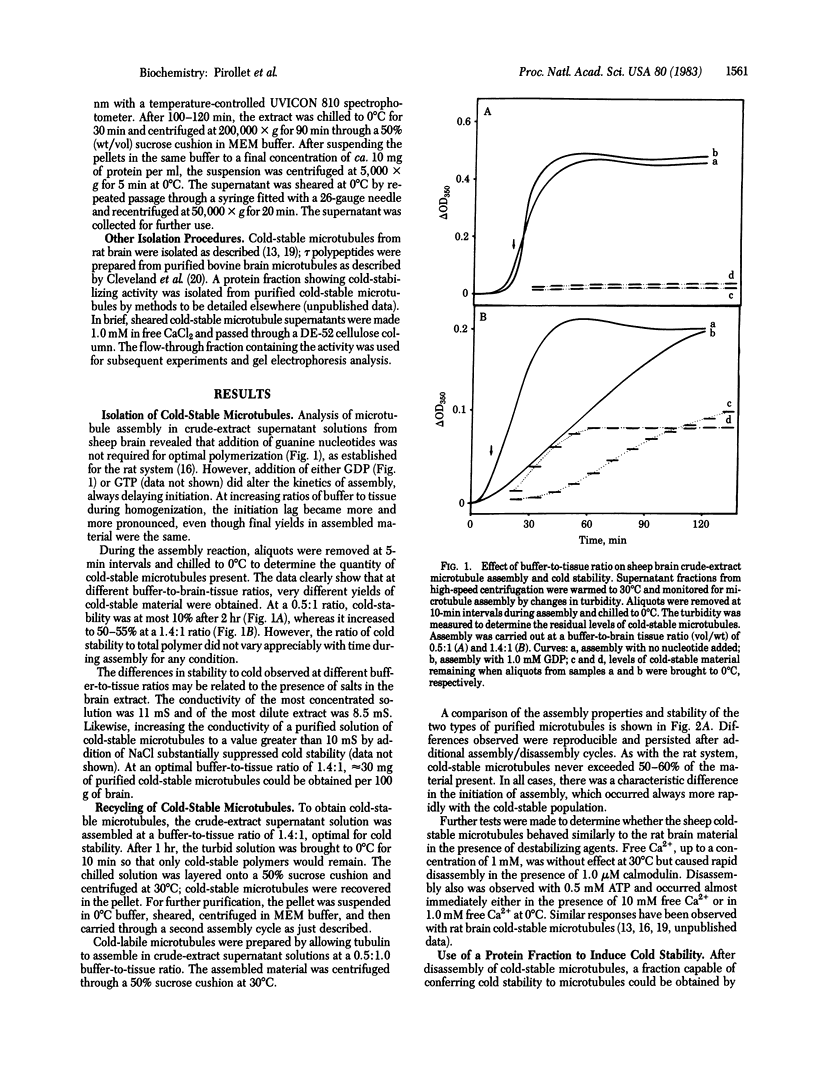
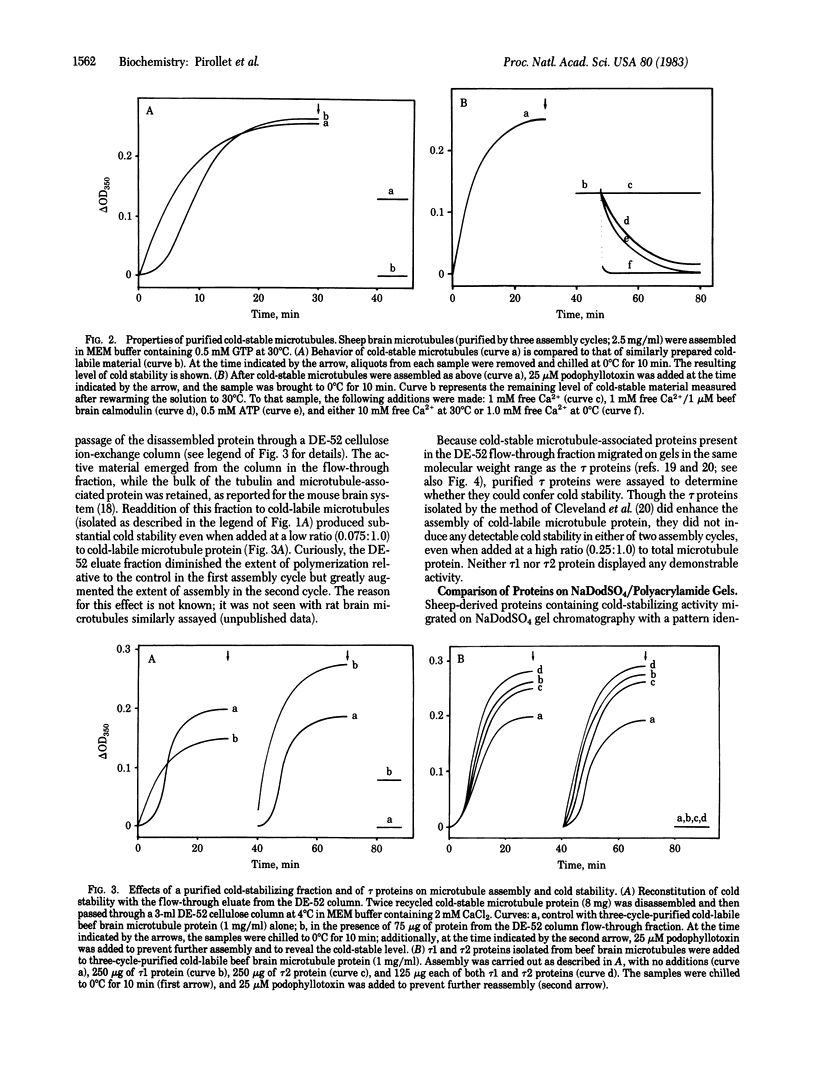
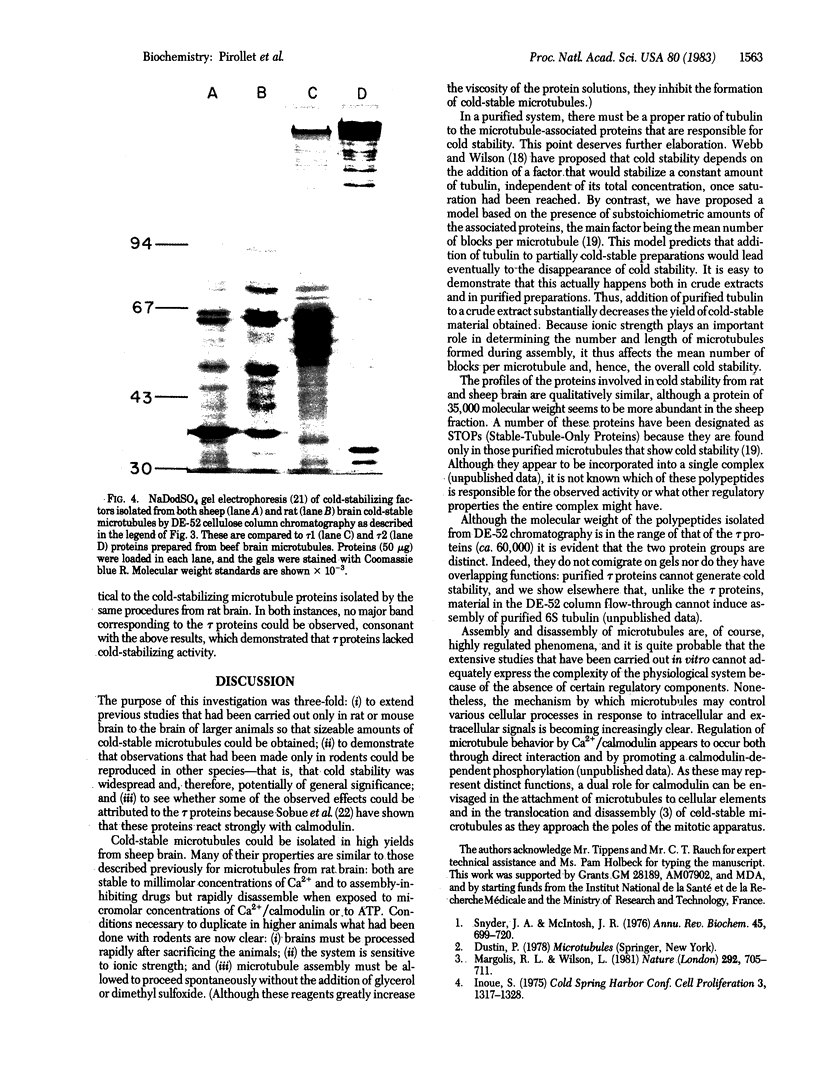

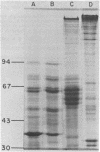
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cleveland D. W., Hwo S. Y., Kirschner M. W. Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J Mol Biol. 1977 Oct 25;116(2):207–225. doi: 10.1016/0022-2836(77)90213-3. [DOI] [PubMed] [Google Scholar]
- Jameson L., Caplow M. Modification of microtubule steady-state dynamics by phosphorylation of the microtubule-associated proteins. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3413–3417. doi: 10.1073/pnas.78.6.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job D., Fischer E. H., Margolis R. L. Rapid disassembly of cold-stable microtubules by calmodulin. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4679–4682. doi: 10.1073/pnas.78.8.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Job D., Rauch C. T., Fischer E. H., Margolis R. L. Recycling of cold-stable microtubules: evidence that cold stability is due to substoichiometric polymer blocks. Biochemistry. 1982 Feb 2;21(3):509–515. doi: 10.1021/bi00532a015. [DOI] [PubMed] [Google Scholar]
- Marcum J. M., Dedman J. R., Brinkley B. R., Means A. R. Control of microtubule assembly-disassembly by calcium-dependent regulator protein. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3771–3775. doi: 10.1073/pnas.75.8.3771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis R. L., Rauch C. T. Characterization of rat brain crude extract microtubule assembly: correlation of cold stability with the phosphorylation state of a microtubule-associated 64K protein. Biochemistry. 1981 Jul 21;20(15):4451–4458. doi: 10.1021/bi00518a033. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L., Keifer B. I. Mitotic mechanism based on intrinsic microtubule behaviour. Nature. 1978 Mar 30;272(5652):450–452. doi: 10.1038/272450a0. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Microtubule treadmills--possible molecular machinery. Nature. 1981 Oct 29;293(5835):705–711. doi: 10.1038/293705a0. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Opposite end assembly and disassembly of microtubules at steady state in vitro. Cell. 1978 Jan;13(1):1–8. doi: 10.1016/0092-8674(78)90132-0. [DOI] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Regulation of the microtubule steady state in vitro by ATP. Cell. 1979 Nov;18(3):673–679. doi: 10.1016/0092-8674(79)90122-3. [DOI] [PubMed] [Google Scholar]
- Nishida E., Kumagai H., Ohtsuki I., Sakai H. The interactions between calcium-dependent regulator protein of cyclic nucleotide phosphodiesterase and microtubule proteins. I. Effect of calcium-dependent regulator protein on the calcium sensitivity of microtubule assembly. J Biochem. 1979 May;85(5):1257–1266. [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Characterization of microtubule assembly in porcine brain extracts by viscometry. Biochemistry. 1973 Oct 9;12(21):4282–4289. doi: 10.1021/bi00745a037. [DOI] [PubMed] [Google Scholar]
- Regula C. S., Pfeiffer J. R., Berlin R. D. Microtubule assembly and disassembly at alkaline pH. J Cell Biol. 1981 Apr;89(1):45–53. doi: 10.1083/jcb.89.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff P. B., Horwitz S. B. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheir-Neiss G., Lai M. H., Morris N. R. Identification of a gene for beta-tubulin in Aspergillus nidulans. Cell. 1978 Oct;15(2):639–647. doi: 10.1016/0092-8674(78)90032-6. [DOI] [PubMed] [Google Scholar]
- Snyder J. A., McIntosh J. R. Biochemistry and physiology of microtubules. Annu Rev Biochem. 1976;45:699–720. doi: 10.1146/annurev.bi.45.070176.003411. [DOI] [PubMed] [Google Scholar]
- Sobue K., Fujita M., Muramoto Y., Kakiuchi S. The calmodulin-binding protein in microtubules is tau factor. FEBS Lett. 1981 Sep 14;132(1):137–140. doi: 10.1016/0014-5793(81)80447-4. [DOI] [PubMed] [Google Scholar]
- Webb B. C., Wilson L. Cold-stable microtubules from brain. Biochemistry. 1980 Apr 29;19(9):1993–2001. doi: 10.1021/bi00550a041. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]