Abstract
Proopiomelanocortin (POMC) is a polypeptide hormone precursor that is expressed in the brain and in peripheral tissues such as in the pituitary gland, immune system, and skin. In the brain, POMC is processed to form several peptides including alpha-melanocyte stimulating hormone (α-MSH). alpha-MSH is expressed in the hypothalamic arcuate nucleus and in the nucleus tractus solitarius of the brainstem where it has a crucial role in the regulation of metabolic functions. Specifically, α-MSH is an anorexigenic peptide. Its production and maturation processes have been shown to be regulated according to the metabolic condition of the organism. This review summarizes our current knowledge on α-MSH processing including its maturation and degradation processes and pharmacological aspects of its manipulation.
Keywords: Melanocortin system, Hypothalamus, alpha-Melanocyte stimulating hormone, Propylcarboxypeptidase
Introduction
Proopiomelanocortin (POMC) neurons in the hypothalamus are considered major players in the regulation of energy metabolism. The synthesis and the activity of hypothalamic POMC neurons are increased in fed conditions. On the other hand, fasting inhibits and thus silences these neurons [1–3]. POMC neurons are regulated by several circulating signals including hormones such as leptin and insulin and nutrients such as glucose. The anatomical localization of POMC neurons has a crucial role in allowing these cells to rapidly respond to circulating nutrients and hormones. The critical role that the arcuate nucleus melanocortin system has in regulating energy metabolism has been the object of extensive studies. Activation of POMC neurons by leptin has been shown to trigger alpha-melanocyte stimulating hormone (α-MSH) production and release from POMC axon terminals, which in turn activates melanocortin receptor 3 (MC3R) and 4 (MC4R) leading to suppressed food intake and increased energy expenditure [4, 5]. POMC neurons activation is also affected by neighboring neurons producing neuropeptide Y (NPY), agouti-related protein (AgRP), and GABA (Fig. 1) [1–3, 6]. Simultaneously, leptin suppresses the activity of arcuate nucleus NPY/AgRP neurons [4, 5], which otherwise, through the release of AgRP, antagonize the effect of alpha-MSH on MC4R [7]. The NPY/AgRP system not only antagonizes anorexigenic melanocortin cells at their target sites where MC4Rs are located but it also very robustly and directly inhibits POMC perikarya. Both NPY as well as the small inhibitory amino acid neurotransmitter GABA [5] are involved in this inhibition [6, 8]. Thus, the interaction between the NPY/AgRP and POMC perikarya is unidirectional since there is no direct feedback mechanism from the POMC to the NPY/AgRP neurons [6]. As consequence of this, it appears that the baseline blueprint of this feeding circuit is likely to promote feeding, and it may be consider an advantage from an evolutionary point of view. On the other hand, in humans, it could be also consider as a contributor to the etiology of metabolic disorders such as obesity.
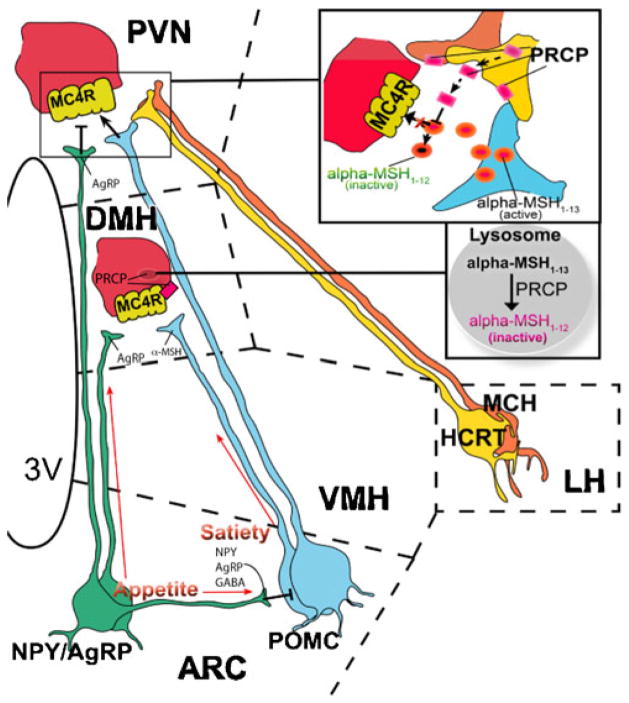
Fig. 1.
Schematic illustration showing the site of action of PRCP on α-MSH. PRCP is mainly expressed in the dorsomedial nucleus of the hypothalamus (DMH) where MC4R-expressing neurons are located and in the lateral hypothalamic (LH) hypocretin/orexin (Hcrt) and melanin-concentrating hormone (MCH) neurons. Thus, it is hypothesized that, in the DMH, PRCP degrades α-MSH at the membrane and/or intracellularly, terminating the effect of α-MSH on MC4R. From the lateral hypothalamus, Hcrt and MCH neurons project to several areas of the hypothalamus, such as the paraventricular nucleus (PVN), where α-MSH terminals strongly innervate MC4R-expressing neurons. Thus, it is hypothesized that PRCP is released from the axon terminals of Hcrt and/or MCH terminals in the PVN, degrading α-MSH extracellularly and increasing the antagonistic effect of AgRP. VMH ventromedial hypothalamic nucleus. Squares are magnifications of the corresponding zone in the figure. Dotted lines delimitate hypothalamic nuclei
Transcriptional regulation of POMC
The intracellular machinery leading to POMC transcription includes several molecular pathways, and it is currently object of intensive studies. Different molecular pathways in POMC neurons are activated or inhibited in response to metabolic signals including hormones, such as insulin and leptin.
Insulin, by binding to its receptors expressed on POMC neurons, induces the activation of phosphatidylinositol-3 kinase (PI3K) [9, 10]. PI3K, when activated, phosphorylates the membrane lipid phosphatidylinositol-4,5-bisphosphate to phosphatidylinositol-3,4,5-trisphosphate (PIP3). PIP3 binds phosphoinositide-dependent protein kinase 1 that phosphorylates AKT protein kinase. Phosphorylated AKT enters the nucleus to regulate neuropeptide expression through the phosphorylation and thus nuclear exclusion of the fork-head box-containing transcription factor of the O subfamily type 1 (FoxO1) [11, 12]. Concomitantly, PI3K activation also induces the opening of few ATP-sensitive potassium channels (KATP) [13] enabling increased firing of POMC neurons [14]. In addition to PI3K cascade regulation, FoxO1 has been recently shown to be regulated by a NAD+-dependent deacetylase, Sirt1 [15]. Sirt1 is expressed in POMC neurons and fasting has been shown to increase its activity levels [16]. In addition, it has been recently shown that fasting-induced decrease of POMC expression is Sirt1 dependent [15].
Leptin binding to its receptors on POMC neurons enables the activation of Janus kinase/signal transducers and activators of transcription as well as mitogen-activated protein kinase signal transduction pathways [17]. Stat 3 increases the POMC expression by recruiting histone acetylases to the POMC promoter [11]. Leptin can also induce the activation of the PI3K pathway, which has been shown to be crucial for the regulation of glucose metabolism and peripheral insulin sensitivity [18–20]. In addition, leptin regulates the expression of suppressor of cytokine signaling-3 mRNA, which seems to act as an inducible negative regulator of leptin-induced signal transduction [21]. Finally, leptin acts on fuel sensors including the mammalian target of rapamycin (mTOR) kinase and AMP-activated protein kinase (AMPK). While leptin inhibits AMPK an energy sensor which is activated by an increase in AMP/ATP ratio, leptin increases the phosphorylation of ribosomal S6 kinase, a major physiological substrate of the mTOR kinase in the hypothalamus. Rapamycin inhibits hypothalamic mTOR and attenuates leptin’s anorexigenic effects (for review, see [22]).
alpha-MSH maturation
The biosynthesis of POMC-derived peptides, as the majority of mammalian neuropeptide hormones, meets the criteria of the “prohormone theory”, which begins with mRNA translation process into a large, inactive precursor polypeptide, followed by posttranslational proteolysis to release different products [23]. In the case of POMC, this is achieved through differential processing by the action of different prohormone convertases (PCs), which results in biological and functional diversity within the central nervous system. Once encoded from the corresponding mRNA, POMC follows the intracellular trafficking of a secreted protein through the Golgi complex, ultimately reaching secretory granules in which the end products of processing are stored before being secreted by exocytosis. During this trafficking, POMC is proteolytically processed into a number of physiologically important peptides, including endorphins, adrenocorticotropic hormone (ACTH), and α-MSH (Fig. 2). The POMC polypeptide precursor contains eight pairs and one quadruplet of basic amino acids, which are the cleavage sites for PC1/3 and PC2 [24].
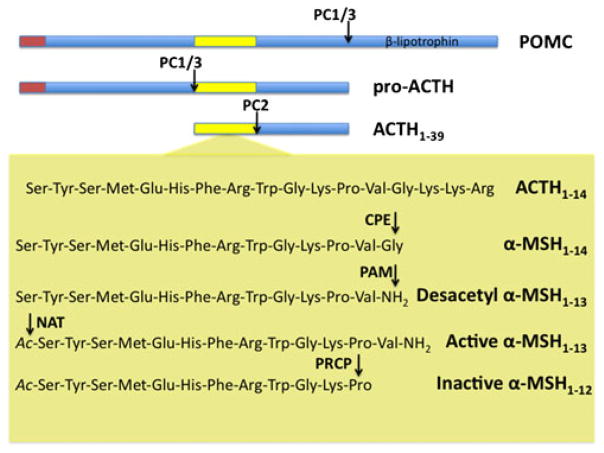
Fig. 2.
Schematic illustration showing hypothalamic POMC processing. The polypeptide prohormone precursor POMC is cleaved by prohormone convertase 1 (PC1/3) in pro-ACTH and β-lipotrophin. PC1/3 will further cleave pro-ACTH to form ACTH1–39 which will be cleaved by prohormone convertase 2 (PC2) to form ACTH1–17. Carboxypeptidase E (CPE) and then α-amidating monooxygenase (PAM) will further process ACTH1–17 to form desacetyl α-MSH1–13. The acetylation of α-MSH1–13 by a n-acetyltransferase (NAT) not yet identified will then produce acetyl-α-MSH1–13. The action of acetyl-α-MSH1–13 will be finally ended by prolylcarboxypeptidase (PRCP) which will convert acetyl-α-MSH1–13 in the inactive product acetyl-α-MSH1–12
In the arcuate neurons, POMC precursor is initially cleaved by PC1/3 to generate pro-ACTH and β-lipotrophin. Pro-ACTH is further cleaved by PC1/3 to generate a 16-kDa N-terminal peptide and ACTH. ACTH is further cleaved to generate ACTH (1–17) and CLIP (Fig. 2). Then carboxypeptidase E enzyme removes C-terminal basic amino acids from ACTH (1–17), and the peptidyl α-amidating monooxygenase enzyme amidates the peptide to generate desacetyl α-MSH, a 13 amino acids peptide (Fig. 2). Finally, the acetylation of desacetyl α-MSH to acetyl α-MSH (the most active form of α-MSH) by an unknown N-acetyl-transferase enzyme could occur immediately after the amino terminal side of these peptides becomes available subsequent to the PCs cleavage (Fig. 2) [25, 26]. Fasting-induced changes in the arcuate nucleus neurons cause a significant decrease in ACTH and desacetyl α-MSH consistent with a decrease in POMC biosynthesis during fasting [27]. A similar conclusion can be drawn from the fact that POMC and ACTH levels are reduced in cerebrospinal fluid during fasting [28]. This decrease in POMC is associated with a decrease in PC1/3 [28]. Leptin administration in fasted rats prevented the fasting-induced decrease in the content of all POMC-related peptides, demonstrating that leptin potently regulates the biosynthesis of POMC in the arcuate nucleus. Whereas a previous report suggested an increase in acetyl α-MSH due to leptin action on the yet-undefined N-acetyl-transferase activity, studies from different laboratories concluded that leptin does not regulate the N-acetylation of hypothalamic α-MSH (for a review, see [29]).
In conclusion, it is reasonable to suggest that this biochemical machinery is of crucial physiopathological significance, and deficits in any of these processing enzymes can lead to obesity, at least in part, due to an inadequate α-MSH production [30, 31]. Indeed alterations of several of the enzymes involved in α-MSH maturation have been shown to affect metabolism regulation in both mouse and human [32].
alpha-MSH degradation
Once produced, the active α-MSH acts on the postsynaptic melanocortin receptors to establish its known metabolic effects. Five melanocortin receptors have been cloned so far, MC1-5R. MC1R, MC2R, and MC5R have important peripheral functions, such as skin pigmentation, adrenal steroidogenesis, and thermoregulation, respectively [33–35]. On the other hand, MC3R and MC4R have been shown to have a major role in the regulation of food intake and energy balance [2, 36]. For example, knockout mice for either MC3R or MC4R show an obese phenotype. However, MC4R-deficient mice are hyperphagic [37], while MC3R mice are not [38, 39]. In addition, the differential distribution of these two receptors within the hypothalamus also supports their differentiated functions. MC3R is highly expressed in the arcuate nucleus where POMC neuron cell bodies are located. Thus, MC3R seems to have a role in the feedback regulation of POMC neuron activity [5, 40]. Contrary, MC4Rs are not expressed in the arcuate nucleus but in other areas of the hypothalamus where POMC fibers project, including PVN, DMH, and with a lesser extend LH [40–42]. The important role of MC4R in the control of energy metabolism is further strengthened by the fact that mutations in the MC4R gene in humans are associated with obesity. MC4R gene mutations indeed represent the most common monogenetic form of obesity in humans [43–45].
The extent of MC4R activation and hence the extent of appetite loss should be a consequence of the rate of α-MSH release from POMC neurons and the rate at which it is degraded or otherwise cleared from the synaptic space.
Whereas events leading to MC4R activation by α-MSH are known, virtually nothing was known about how α-MSH action is terminated until recently, when a new processing enzyme was identified as the responsible enzyme for α-MSH inactivation (Figs. 1 and 2). Prolylcarboxypeptidase (PRCP) catalyzes the inactivation of α-MSH by removal of the C-terminal valine residue, producing the inactive 12 amino acids peptide α-MSH1–12 (Figs. 1 and 2) [46]. Injection of α-MSH into the brain of wild-type mice inhibits feeding, whereas injection of the same amount of the truncated α-MSH1–12 is ineffective in reducing food consumption. This discovery begins with a C57BL/6 mouse strain that carries a small region of chromosome 7 from a BALB/c strain. Those mice are significantly leaner compared with C57BL/6 mice without the BALB/c chromosomal segment [47]. The relevant BALB/c region on chromosome 7 includes only four protein-encoding genes; of those genes, Prcp is the likely candidate, given previous reports of a link between PRCP and metabolic syndrome [48]. Sequencing studies have revealed a single-base polymorphism at –718 in the presumptive promoter/enhancer region of the Prcp gene that is associated with a significant reduction in the level of Prcp mRNA in the brain of the mice carrying the BALB/c allele. Knockout mice for Prcp gene (Prcp−/−) produces mice with a leaner phenotype and a decrease in feeding compared to the wild-type controls. As predicted, the Prcp−/− mice have elevated levels of hypothalamic α-MSH1–13. Furthermore, these animals are resistant to diet-induced obesity [46].
PRCP is an enzyme belonging to the family of carboxypeptidases, containing a serine, histidine, and asparagine residues at its active center, which are essential for its catalytic activity. PRCP gene is located on chromosomes 11 and 7 in human and mouse, respectively, and the encoded product is a single chain protein of approximately 58 kDa [49, 50]. The cDNA for human PRCP was cloned from a human kidney library, and the deduced protein sequence contains 496 amino acids, including a 30 residue signal peptide and a 15 amino acid propeptide [51]. PRCP specifically cleaves peptides containing a penultimate proline residue. PRCP was discovered from studies of bradykinin metabolism in kidney, when it was found that after removal of the C-terminal Arg of bradykinin, the Pro–Phe–OH bond, which is usually resistant to carboxypeptidases, was cleaved by a kidney extract [49]. The enzyme was initially named angiotensinase C since angiotensin II has the same C terminus and it was found to be a substrate. The extension of its cleaving properties to a variety of Pro-X bond-containing peptides prompted to rename this enzyme as PRCP.
PRCP is widely distributed in mice and humans and expressed in peripheral tissues such as kidney, liver, heart, and spleen and lung [51]. PRCP is also present in white blood cells and fibroblasts and is highly expressed in endothelial cells [52–54]. In the endothelial cells, PRCP is localized on the cellular membrane [54].
In addition, PRCP is also expressed in the central nervous system (CNS) in the hypothalamus and brainstem. Specifically, within the hypothalamus, higher expression of PRCP mRNA has been detected in the perifornical lateral hypothalamic region (LH) and dorso-medial nucleus (DMH; Fig. 1) [46], accordingly with its newly suggested role in melanocortin signaling regulation by α-MSH inactivation. Thus, PRCP mRNA is highly expressed in the DMH where MC4R expressing neurons are located and where α-MSH containing fibers project from ARC nucleus. PRCP mRNA has also high expression in the LH where MC4Rs are poorly expressed but, from this region, neurons such as the ones expressing hypocretin/orexin (Hcrt) and MCH project to the hypothalamic paraventricular nucleus (PVN), where MC4Rs are highly expressed.
At least two hypotheses can be made about the mechanism underlying PRCP action. First, in the DMH, as membrane protein, PRCP could be exposed onto the cell surface so directly cleaving α-MSH in the synaptic space (Fig. 1). However, since its activity has been reported to be present also in lysosomal fractions, in the DMH, it could also be part of intracellular peptide degradation pathway after cellular uptake and/or receptor recycling (Fig. 1). Second, it has been reported that PRCP can be released from cells to the extracellular medium or biological fluids in response to stimulation [50, 55]. Thus, PRCP could be released in the PVN from the Hcrt and MCH axon terminals into the synaptic space where it would then degrade extracellularly α-MSH (Fig. 1) and shortening its agonistic action on MC4R. This could explain the high expression of PRCP mRNA levels found in LH [46], a hypothalamic area poor in MC4R expressing neurons, but where Hcrt and MCH neurons originate from. So, PRCP may be poised in the synaptic space to degrade α-MSH1–13 thereby reducing its action and promoting satiety. Further studies need to confirm the mechanistic aspect of this hypothesis and to understand the nature of the stimuli triggering PRCP secretion from these neurons.
Pharmacological aspects
Obesity and related metabolic dysfunctions, such as type 2 diabetes, have emerged as a leading cause of morbidity and mortality in developed societies. The development of obesity and type 2 diabetes may be triggered or abrogated by CNS mechanisms. The hypothalamic melanocortin system and its major anorexigenic peptide α-MSH have an important role in such disorder, as revealed by the dramatic development of obesity after selective inactivation of the neuronal Pomc gene in mice and the presence of POMC mutations in humans with severe early-onset obesity [56, 57]. Thus, the α-MSH target MC4 receptor is an attractive candidate drug target to treat obesity, as it not only affects several aspects of feeding behavior [58, 59] but activation of the MC system also increases insulin sensitivity and energy expenditure [60, 61].
Pharmaceutical development of MC4 receptor-specific drugs has been the object of many studies [62–64]. However, it has been challenging to design selective MC4 receptor agonists that would completely lack affinity for the MC3 receptor [65, 66]. Nevertheless, mixed MC3/4 agonists might provide a therapeutic advantage since, as shown in MC3R knockout mice, reduction in MC3R activity is associated with an increased adipogenesis. However, studies in rodents and humans have revealed some side effects of the use of MC receptor agonists. For example, administration of MC4 receptor agonists is associated with penile erections as well as flushing, which has resulted in a new application area for these drugs in erectile dysfunction [67, 68]. Also, preclinical studies have identified roles for the melanocortin system in blood pressure regulation (MC receptor agonists have a depressor effect most probably via the nucleus tractus solitarius in brainstem) [69], in the inflammatory responses (where agonists limit thermogenic responses to pyrogens), and in pain processing (where antagonists suppress pain sensation) [70, 71]. Thus, the development of MC4 receptor agonists in the treatment of obesity should be carefully monitored to avoid undesired side effects due to melanocortin function in other systems.
Because of these side effects, PRCP becomes a very promising drug target. It has been already reported that inhibition of PRCP activity by small molecule protease inhibitors administered peripherally or centrally decrease food intake in wild type as well as obese animals [46]. The pharmacological manipulation of the melanocortin system accomplished by inhibiting α-MSH catabolism represents a highly innovative approach that may help to combat metabolic disorders such as obesity.
The local metabolism of neurotransmitter and neuro-modulator is generally considered an optimum drug target with a lower incidence of side/non-specific effects compared to direct agonists. This is the case, among others, of the actual pharmacotherapy for many pathologies of the CNS such as depression and Alzheimer disease. The discovery of PRCP as pharmacological target may allow, indeed, to selectively focus on the benefits deriving from the enhancement of melanocortin activation in discrete brain areas by its endogenous ligand, avoiding the whole set of possible central and systemic effects due to the use of MC receptor agonists.
In conclusion, further studies on PRCP will shed light on a novel central regulatory element in the regulation of energy metabolism. Differential activity levels of PRCP in specific brain areas in different metabolic condition by affecting α-MSH levels will, in turn, affect the efficacy of melanocortin signaling. Thus, understanding PRCP role in metabolism regulation is important to reveal whether PRCP can be used as new drug targets for metabolic disorders, including obesity and type 2 diabetes.
Conclusion
The studies reviewed here highlight the new mechanism of degradation of α-MSH. The recent discovery of PRCP as an important component of melanocortin signaling downstream of the melanocortin cells determining the efficacy of the melanocortin signaling is of great importance as further studies to understand its role in metabolic regulation will shed light on a novel target in the regulation of energy metabolism for the discovery of therapeutic drugs in the treatment and prevention of obesity and life-style related disorders.
Acknowledgments
This work was supported by NIDDK/NIH (Grant no. R01 DK084065).
Footnotes
Disclosure statement The authors have nothing to disclose.
Contributor Information
Giuseppe D’Agostino, Program in Integrative Cell Signaling and Neurobiology of Metabolism, Yale University School of Medicine, New Haven, CT 06520, USA. Department of Ob/Gyn & Reproductive Sciences, Yale University School of Medicine, New Haven, CT 06520, USA. Department of Experimental Pharmacology, University of Naples Federico II, Naples, Italy.
Sabrina Diano, Email: Sabrina.diano@yale.edu, Program in Integrative Cell Signaling and Neurobiology of Metabolism, Yale University School of Medicine, New Haven, CT 06520, USA. Department of Ob/Gyn & Reproductive Sciences, Yale University School of Medicine, New Haven, CT 06520, USA. Department of Neurobiology, Yale University School of Medicine, New Haven, CT 06520, USA. Yale School of Medicine, 330 Cedar Street LSOG 204B, New Haven, CT 06510, USA.
References
- 1.Saper CB, Chou TC, Elmquist JK. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 2.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 3.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 4.Elias CF, Kelly JF, Lee CE, Ahima RS, Drucker DJ, Saper CB, Elmquist JK. Chemical characterization of leptin-activated neurons in the rat brain. J Comp Neurol. 2000;423:261–281. [PubMed] [Google Scholar]
- 5.Cowley MA, Smart JL, Rubinstein M, Cerdar MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 6.Horvath TL, Naftolin F, Kalra SP, Leranth C. Neuropeptide-Y innervation of beta-endorphin-containing cells in the rat mediobasal hypothalamus: a light and electron microscopic double immunostaining analysis. Endocrinology. 1992;131:2461–2467. doi: 10.1210/endo.131.5.1425443. [DOI] [PubMed] [Google Scholar]
- 7.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 8.Csiffáry A, Görcs TJ, Palkovits M. Neuropeptide Y innervation of ACTH-immunoreactive neurons in the arcuate nucleus of rats: a correlated light and electron microscopic double immunolabeling study. Brain Res. 1990;506:215–222. doi: 10.1016/0006-8993(90)91253-d. [DOI] [PubMed] [Google Scholar]
- 9.Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 10.Niswender KD, Morton GJ, Stearns WH, Rhodes CJ, Myers MG, Jr, Schwartz MW. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature. 2001;413:794–795. doi: 10.1038/35101657. [DOI] [PubMed] [Google Scholar]
- 11.Kitamura T, Feng Y, Kitamura YI, Chua SC, Jr, Xu AW, Barsh GS, Rossetti L, Accili D. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- 12.Belgardt BF, Husch A, Rother E, Ernst MB, Wunderlich FT, Hampel B, Klöckener T, Alessi D, Kloppenburg P, Brüning JC. PDK1 deficiency in POMC-expressing cells reveals FOXO1-dependent and -independent pathways in control of energy homeostasis and stress response. Cell Metab. 2008;7:291–301. doi: 10.1016/j.cmet.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Shyng SL, Nichols CG. Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science. 1998;282:1138–1141. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- 14.Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 15.Cakir I, Perello M, Lansari O, Messier NJ, Vaslet CA, Nillni EA. Hypothalamic Sirt1 regulates food intake in a rodent model system. PLoS ONE. 2009;4:e8322. doi: 10.1371/journal.pone.0008322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramadori G, Lee CE, Bookout AL, Lee S, Williams KW, Anderson J, Elmquist JK, Coppari R. Brain SIRT1: anatomical distribution and regulation by energy availability. J Neurosci. 2008;28:9989–9996. doi: 10.1523/JNEUROSCI.3257-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vaisse C, Halaas JL, Horvath CM, Darnell JE, Jr, Stoffel M, Friedman JM. Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet. 1996;14:95–97. doi: 10.1038/ng0996-95. [DOI] [PubMed] [Google Scholar]
- 18.Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signaling in hypothalamic proopio-melanocortin neurons in mice. J Clin Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plum L, Ma X, Hampel B, Balthasar N, Coppari R, Munzberg H, Shanabrough M, Burdakov D, Rother E, Janoschek R, et al. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J Clin Invest. 2006;116:1886–1901. doi: 10.1172/JCI27123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plum L, Belgardt BF, Bruning JC. Central insulin action in energy and glucose homeostasis. J Clin Invest. 2006;116:1761–1766. doi: 10.1172/JCI29063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 22.Morris DL, Rui L. Recent advances in understanding leptin signaling and leptin resistance. Am J Physiol Endocrinol Metab. 2009;297:E1247–E1259. doi: 10.1152/ajpendo.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steiner DF. The proprotein convertases. Curr Opin Chem Biol. 1998;2:31–39. doi: 10.1016/s1367-5931(98)80033-1. [DOI] [PubMed] [Google Scholar]
- 24.Benjannet S, Rondeau N, Day R, Chretien M, Seidah NG. PC1 and PC2 are proprotein convertases capable of cleaving proopiomelanocortin at distinct pairs of basic residues. Proc Natl Acad Sci USA. 1991;88:3564–3568. doi: 10.1073/pnas.88.9.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbott CR, Rossi M, Kim MS, AlAhmed SH, Ghatei TGM, Smith DMMA, Bloom SR. Investigation of the melanocyte stimulating hormones on food intake. Lack of evidence to support a role for the melanocortin-3-receptor. Brain Res. 2000;869:203–210. doi: 10.1016/s0006-8993(00)02386-6. [DOI] [PubMed] [Google Scholar]
- 26.Guo L, Münzberg H, Stuart RC, Nillni EA, Bjørbaek C. N-Acetylation of hypothalamic alpha-melanocyte-stimulating hormone and regulation by leptin. Proc Natl Acad Sci USA. 2004;101:11797–11802. doi: 10.1073/pnas.0403165101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perello M, Stuart RC, Nillni EA. Differential effects of fasting and leptin on proopiomelanocortin peptides in the arcuate nucleus and in the nucleus of the solitary tract. Am J Physiol Endocrinol Metab. 2007;292:E1348–E1357. doi: 10.1152/ajpendo.00466.2006. [DOI] [PubMed] [Google Scholar]
- 28.Pritchard LE, Oliver RL, McLoughlin JD, Birtles S, Lawrence CB, Turnbull AV, White A. Proopiomelanocortin-derived peptides in rat cerebrospinal fluid and hypothalamic extracts: evidence that secretion is regulated with respect to energy balance. Endocrinology. 2003;144:760–766. doi: 10.1210/en.2002-220866. [DOI] [PubMed] [Google Scholar]
- 29.Wilkinson CW. Roles of acetylation and other post-translational modifications in melanocortin function and interactions with endorphins. Peptides. 2006;27:453–471. doi: 10.1016/j.peptides.2005.05.029. [DOI] [PubMed] [Google Scholar]
- 30.Naggert JK, Fricker LD, Varlamov O, Nishina PM, Rouille Y, Steiner DF, Carroll RJ, Paigen BJ, Leiter EH. Hyper-proinsulinaemia in obese fat/fat mice associated with a carboxy-peptidase E mutation which reduces enzyme activity. Nat Genet. 1995;10:135–142. doi: 10.1038/ng0695-135. [DOI] [PubMed] [Google Scholar]
- 31.Jackson RS, Creemers JW, Ohagi S, Raffin-Sanson ML, Sanders L, Montague CT, Hutton JC, O’Rahilly S. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet. 1997;16:303–306. doi: 10.1038/ng0797-303. [DOI] [PubMed] [Google Scholar]
- 32.Coll AP, Farooqi IS, Challis BG, Yeo GS, O’Rahilly S. Proopiomelanocortin and energy balance: insights from human and murine genetics. J Clin Endocrinol Metab. 2004;89:2557–2562. doi: 10.1210/jc.2004-0428. [DOI] [PubMed] [Google Scholar]
- 33.Chen W, Kelly MA, Opitz-Araya X, Thomas RE, Low MJ, Cone RD. Exocrine gland dysfunction in MC5-R-deficient mice: evidence for coordinated regulation of exocrine function by melanocortin peptides. Cell. 1997;91:789–798. doi: 10.1016/s0092-8674(00)80467-5. [DOI] [PubMed] [Google Scholar]
- 34.Clark AJ, McLoughlin L, Grossman A. Familial glucocorticoid deficiency associated with point mutation in the adrenocorticotropin receptor. Lancet. 1993;341:461–462. doi: 10.1016/0140-6736(93)90208-x. [DOI] [PubMed] [Google Scholar]
- 35.Robbins LS, Nadeau JH, Johnson KR, Kelly MA, Roselli-Rehfuss L, Baack E, Mountjoy KG, Cone RD. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell. 1993;72:827–834. doi: 10.1016/0092-8674(93)90572-8. [DOI] [PubMed] [Google Scholar]
- 36.Seeley RJ, Yagaloff KA, Fisher SL, Burn P, Thiele TE, van Dijk G, Baskin DG, Schwartz MW. Melanocortin receptors in leptin effects. Nature. 1997;390:349–363. doi: 10.1038/37016. [DOI] [PubMed] [Google Scholar]
- 37.Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- 38.Butler AA, Kesterson RA, Khong K, Cullen MJ, Pelleymounter MA, Dekoning J, Baetscher M, Cone RD. A unique metabolic syndrome causes obesity in the melanocortin-3 receptor-deficient mouse. Endocrinology. 2000;141:3518–3521. doi: 10.1210/endo.141.9.7791. [DOI] [PubMed] [Google Scholar]
- 39.Chen AS, Marsh DJ, Trumbauer ME, Frazier EG, Guan XM, Yu H, Rosenblum CI, Vongs A, Feng Y, Cao L, et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 40.Jegou S, Boutelet I, Vaudry H. Melanocortin-3 receptor mRNA expression in proopiomelanocortin neurones of the rat arcuate nucleus. J Neuroendocrinol. 2000;12:501–505. doi: 10.1046/j.1365-2826.2000.00477.x. [DOI] [PubMed] [Google Scholar]
- 41.Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J Neurosci. 2003;23:7143–7154. doi: 10.1523/JNEUROSCI.23-18-07143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- 43.Vaisse C, Clement K, Guy-Grand B, Froguel P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nat Genet. 1998;20:113–114. doi: 10.1038/2407. [DOI] [PubMed] [Google Scholar]
- 44.Yeo GS, Farooqi IS, Challis BG, Jackson RS, O’Rahilly S. The role of melanocortin signalling in the control of body weight: evidence from human and murine genetic models. Q J Med. 2000;93:7–14. doi: 10.1093/qjmed/93.1.7. [DOI] [PubMed] [Google Scholar]
- 45.Chiesi M, Huppertz C, Hofbauer KG. Pharmacotherapy of obesity: targets and perspectives. Trends Pharmacol Sci. 2001;22:247–254. doi: 10.1016/s0165-6147(00)01664-3. [DOI] [PubMed] [Google Scholar]
- 46.Wallingford N, Perroud B, Gao Q, Coppola A, Gyengesi E, Liu ZW, Gao XB, Diament A, Haus KA, Shariat-Madar Z, Mahdi F, Wardlaw SL, Schmaier AH, Warden CH, Diano S. Prolylcarboxypeptidase regulates food intake by inactivating α-MSH in rodents. J Clin Invest. 2009;119:2291–2303. doi: 10.1172/JCI37209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diament AL, Warden CH. Multiple linked mouse chromosome 7 loci influence body fat mass. Int J Obes Relat Metab Disord. 2004;28:199–210. doi: 10.1038/sj.ijo.0802516. [DOI] [PubMed] [Google Scholar]
- 48.McCarthy JJ, Meyer J, Moliterno DJ, Newby LK, Rogers WJ, Topol EJ. GenQuest multicenter study. Evidence for substantial effect modification by gender in a large-scale genetic association study of the metabolic syndrome among coronary heart disease patients. Hum Genet. 2003;114:87–98. doi: 10.1007/s00439-003-1026-1. [DOI] [PubMed] [Google Scholar]
- 49.Yang HYT, Erdos EG, Chiang TS. New enzymatic route for the inactivation of angiotensin. Nature. 1968;218:1224–1226. doi: 10.1038/2181224a0. [DOI] [PubMed] [Google Scholar]
- 50.Yang HYT, Erdos EG, Chiang TS, Jenssen TA, Rodgers JG. Characteristics of an enzyme that inactivates angiotensin II (angiotensinase C) Biochem Pharmacol. 1970;19:1201–1211. [Google Scholar]
- 51.Tan F, Morris PW, Skidgel RA, Erdos EG. Sequencing and cloning of human prolylcarboxypeptidase (angiotensinase C). Similarity to both serine carboxypeptidase and prolylendopeptidase families. J Biol Chem. 1993;268:16631–16638. [PubMed] [Google Scholar]
- 52.Kumamoto K, Stewart TA, Johnson AR, Erdos EG. Prolylcarboxypeptidase (angiotensinase C) in human cultured cells. J Clin Invest. 1981;67:210–215. doi: 10.1172/JCI110015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skidgel RA, Erdos EG. Cellular carboxypeptidases. Immunol Rev. 1998;161:129–141. doi: 10.1111/j.1600-065x.1998.tb01577.x. [DOI] [PubMed] [Google Scholar]
- 54.Shariat-Madar Z, Rahimy E, Mahdi F, Schmaier AH. Overexpression of prolylcarboxypeptidase enhances plasma pre-kallikrein activation on Chinese hamster ovary cells. Am J Physiol Heart Circ Physiol. 2005;289(6):H2697–H2703. doi: 10.1152/ajpheart.00715.2005. [DOI] [PubMed] [Google Scholar]
- 55.Smart JL, Tolle V, Low MJ. Glucocorticoids exacerbate obesity and insulin resistance in neuron-specific proopiomelanocortin-deficient mice. J Clin Invest. 2006;116:495–505. doi: 10.1172/JCI25243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Grüters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19:155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 57.Adan RA, Tiesjema B, Hillebrand JJ, la Fleur SE, Kas MJ, de Krom M. The MC4 receptor and control of appetite. Br J Pharmacol. 2006;149:815–827. doi: 10.1038/sj.bjp.0706929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adan RA, Vanderschuren LJ, la Fleur SE. Anti-obesity drugs and neural circuits of feeding. Trends Pharmacol Sci. 2008;29:208–217. doi: 10.1016/j.tips.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 59.Banno R, Arima H, Sato I, Hayashi M, Goto M, Sugimura Y, Murase T, Oiso Y. The melanocortin agonist melanotan II increases insulin sensitivity in OLETF rats. Peptides. 2004;25:1279–1286. doi: 10.1016/j.peptides.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 60.Heijboer AC, van den Hoek AM, Pijl H, Voshol PJ, Havekes LM, Romijn JA, Corssmit EP. Intracerebroventricular administration of melanotan II increases insulin sensitivity of glucose disposal in mice. Diabetologia. 2005;48:1621–1626. doi: 10.1007/s00125-005-1838-8. [DOI] [PubMed] [Google Scholar]
- 61.Tian X, Field T, Mazur AW, Ebetino FH, Wos JA, Crossdoersen D, Pinney BB, Sheldon RJ. Design, synthesis, and evaluation of proline based melanocortin receptor ligands. Bioorg Med Chem Lett. 2005;15:2819–2823. doi: 10.1016/j.bmcl.2005.03.120. [DOI] [PubMed] [Google Scholar]
- 62.Bakshi RK, Hong Q, Tang R, Kalyani RN, Macneil T, Weinberg DH, Van der Ploeg LH, Patchett AA, Nargund RP. Optimization of a privileged structure leading to potent and selective human melanocortin subtype-4 receptor ligands. Bioorg Med Chem Lett. 2006;16:1130–1133. doi: 10.1016/j.bmcl.2005.11.095. [DOI] [PubMed] [Google Scholar]
- 63.Nicholson JR, Kohler G, Schaerer F, Senn C, Weyermann P, Hofbauer KG. Peripheral administration of a melanocortin 4-receptor inverse agonist prevents loss of lean body mass in tumor-bearing mice. J Pharmacol Exp Ther. 2006;317:771–777. doi: 10.1124/jpet.105.097725. [DOI] [PubMed] [Google Scholar]
- 64.Holder JR, Haskell-Luevano C. Melanocortin ligands: 30 years of structure–activity relationship (SAR) studies. Med Res Rev. 2004;24:325–356. doi: 10.1002/med.10064. [DOI] [PubMed] [Google Scholar]
- 65.Todorovic A, Haskell-Luevano C. A review of melanocortin receptor small molecule ligands. Peptides. 2005;26:2026–2036. doi: 10.1016/j.peptides.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 66.Van der Ploeg LH, Martin WJ, Howard AD, Nargund RP, Austin CP, Guan X, Drisko J, Cashen D, Sebhat I, Patchett AA, et al. A role for the melanocortin 4 receptor in sexual function. Proc Natl Acad Sci USA. 2002;99:11381–11386. doi: 10.1073/pnas.172378699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Diamond LE, Earle DC, Rosen RC, Willett MS, Molinoff PB. Double-blind, placebo-controlled evaluation of the safety, pharmacokinetic properties and pharmacodynamic effects of intranasal PT-141, a melanocortin receptor agonist, in healthy males and patients with mild-to-moderate erectile dysfunction. Int J Impot Res. 2004;16:51–59. doi: 10.1038/sj.ijir.3901139. [DOI] [PubMed] [Google Scholar]
- 68.Wessells H, Blevins JE, Vanderah TW. Melanocortinergic control of penile erection. Peptides. 2005;26:1972–1977. doi: 10.1016/j.peptides.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Versteeg DH, Van Bergen P, Adan RA, De Wildt DJ. Melanocortins and cardiovascular regulation. Eur J Pharmacol. 1998;360:1–14. doi: 10.1016/s0014-2999(98)00615-3. [DOI] [PubMed] [Google Scholar]
- 70.Catania A, Gatti S, Colombo G, Lipton JM. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev. 2004;56:1–29. doi: 10.1124/pr.56.1.1. [DOI] [PubMed] [Google Scholar]
- 71.Vrinten DH, Kalkman CJ, Adan RA, Gispen WH. Neuropathic pain: a possible role for the melanocortin system? Eur J Pharmacol. 2001;429:61–69. doi: 10.1016/s0014-2999(01)01306-1. [DOI] [PubMed] [Google Scholar]