Abstract
The antiprogestin mifepristone has been used for more than 20 years as a medical alternative for early pregnancy termination. After mifepristone administration, significant changes have been observed in the endometrial vessels, with cell injury and cell death in capillary endothelial cells. In this study, the effect of mifepristone on human endometrial endothelial cells (HEECs) in vitro was evaluated using proliferation and viability assays, quantitative polymerase chain reaction of markers important for the regulation of angiogenesis, and by tube formation assay. There were no detectable effects of mifepristone on HEECs messenger RNA expression of the studied markers. Exposure to mifepristone did not alter tube formation. However, mifepristone exposure to HEECs cocultured with endometrial stromal cells significantly reduced the activity in the tube formation assay compared with mifepristone exposure of HEECs in monoculture. This implies that mifepristone causes changes in HEEC-associated angiogenic activity and that this effect is mediated through stromal cells via paracrine mechanisms.
Keywords: mifepristone, human endometrial endothelial cells, endometrium, angiogenesis
Introduction
The human endometrium is a dynamic tissue that undergoes cyclic changes in preparation for embryo implantation. These include changes in endometrial angiogenic activity and endometrial vasculature. These processes are probably under the control of sex steroid hormones. Besides endometrial angiogenesis and angiogenesis during corpus luteum formation, there is rarely any physiological angiogenesis in adulthood. Endometrial angiogenesis is initiated during menstruation to repair the vascular bed and continues during the proliferative phase with endometrial regrowth and during the secretory phase when spiral arteries grow and coil and the subepithelial capillary network is formed.1 Endometrial angiogenesis mainly occurs through vessel elongation, intussusceptions, and expansion of preexisting blood vessels, while vascular sprouting is less common.1 Endothelial cells cover the luminal surface of all blood vessels. These cells control vascular morphology through angiogenesis and vascular remodeling and they control other functional changes such as the regulation of vascular tone. Human endometrial endothelial cells (HEECs) differ to some extent from other endothelial cells, since their activities are regulated by changes in estrogen and progesterone levels during the menstrual cycle. It has also been shown that HEEC has a higher vascular endothelial growth factor (VEGF)-binding capacity than a number of other endothelial cell types such as human coronary endothelial cells, human umbilical vein endothelial cells, and human dermal endothelial cells, suggesting that HEECs are more sensitive to VEGF compared to other endothelial cells.2 There are several studies in which the expression of sex steroid receptors in HEEC has been investigated,2–8 but up to now estrogen receptor β (ER-β) is the only one proven to be present. Results regarding the expression of other steroid receptors are conflicting, but there are some reports that progesterone receptor (PR) is probably present.2,6 Although the regulation of endometrial growth and regression by estrogen and progesterone has been well studied, the direct and indirect roles of these steroids in angiogenesis and in HEECs are not clear. It has been suggested that ovarian steroids are capable of inducing genes involved in endometrial differentiation of human uterine microvascular endothelial cells.9 The progesterone and the PR antagonist mifepristone (RU-486) have been shown to affect the expression of PR-A in HUVECs.10 The PR antagonists or antiprogestins, such as mifepristone, can be used in fertility regulation by blocking the action of progesterone. In the presence of progesterone, mifepristone acts as an antagonist, but mifepristone alone exhibits endometrial progestational effects at low doses and antiproliferative (antiestrogenic) effects at higher doses.11 Being the first available antiprogestin, mifepristone has been successfully used for early pregnancy termination for many years. At low doses, it is also suitable for emergency contraception through inhibition of ovulation and alteration of endometrial function.12 In women and nonhuman primates, administration of mifepristone is associated with reduction in menstrual bleeding, or even amenorrhea, possibly due to effects on the endometrial vasculature.13–15
Johannisson et al observed significant changes in the endometrial vessels after mifepristone administration, seen as cell injury and cell death in capillary endothelial cells.16 This vascular damage has been proposed to compromise the embryo survival.17 Mifepristone has also been previously reported to affect the endometrial receptivity markers in a coculture system of human endometrial epithelial and stromal cells.18 Levonorgestrel is a progestin used in hormonal contraceptives; it is mainly a PR agonist and was therefore included in this study for comparisons with the PR antagonist mifepristone. Levonorgestrel binds to PR and also to several other human steroid hormone receptors such as the androgen receptor (AR), the glucocorticoid receptor (GR), and to a very limited extent also ER.19
In this study, the effects of the PR antagonist mifepristone and 2 PR agonists progesterone and levonorgestrel on HEECs in vitro were investigated using coculture and monoculture models. This was done to provide in vitro insights into the effects of the PR antagonist on human endometrial angiogenesis.
Materials and Methods
The study was approved by the Regional Ethics Committee, Uppsala, Sweden. Informed consent was obtained from all women included in the study.
Cell Cultures
Endometrial biopsy samples from 4 premenopausal women undergoing hysterectomy for benign medical conditions at Uppsala University Hospital were obtained for quantitative polymerase chain reaction (qPCR). Biopsies from 5 additional women were collected to be used in tube formation assay. None of the women had endometriosis or submucous fibroids, and all were nonsmokers. According to several previous in vitro studies, cultured endometrial endothelial cells behave the same in vitro regardless of the phase of the menstrual cycle in which they were collected.20–22
The endometrial biopsies were immediately put into cold sterile phosphate-buffered saline (PBS; Invitrogen, Carlsbad, California) and transferred to the laboratory. The samples were washed in sterile PBS, followed by a series of brief washings in iodine solution (Apoteket AB, Stockholm, Sweden), and finally 3 times in PBS. They were then cut into 1-mm pieces and thereafter exposed to a sterile-filtered solution of digesting enzymes (2.5 mg/mL collagenase type II [Invitrogen], 50 µg/mL deoxyribonuclease II [Sigma-Aldrich, St Louis, Missouri], 200 µg/mL hyaluronidase [Sigma-Aldrich], and gentamicin 4 mg/mL [Invitrogen]) in PBS for 1 hour in an atmosphere of 5% CO2 in humidified air at 37°C in a Forma Scientific CO2 incubator (AB Nino Lab, Upplands Väsby, Sweden). After 1 hour, the cell suspension was poured through a 40-µm nylon cell strainer (BD Sciences, Franklin Lakes, New Jersey) into a sterile centrifugation tube containing PBS, and the undigested tissue was reincubated in fresh enzyme solution for an additional hour. The remaining cell suspension was thereafter poured through the cell strainer into the centrifugation tube, and the cell suspension was centrifuged at 400g for 10 minutes. The supernatant was discarded, and the cell pellet was dissolved in 1 to 2 mL of sterile PBS containing 0.1% bovine serum albumin (BSA; Sigma-Aldrich). The dissolved cell pellet was transferred into a smaller sterile tube, and 25 µL of Dynabeads CD31 Endothelial Cell (Dynal Biotech ASA, Oslo, Norway) was added to per mL of the cell suspension. The tube was placed on a rocker for 30 minutes at 2°C to 8°C after which the suspension was split into 2 tubes, and 2 to 3 times the suspension volume of sterile 0.1% BSA-PBS was added. By placing the tubes in a magnetic holder for 2 minutes, the Dynabead-attached endothelial cells were attracted by the magnet and stuck to the test tube wall, while the stromal cells in the supernatant were poured into a separate tube. The tube containing endothelial cells was removed from the magnetic holder, endothelial cells were resuspended in fresh 0.1% BSA-PBS, and the procedure with the magnetic holder was repeated 2 to 3 times. Both stromal and endothelial cells were suspended and seeded in endothelial cell medium (ECM) containing 5% fetal bovine serum and endothelial cell growth supplement (ScienCell Research Laboratories Carlsbad, California). Endothelial cells were seeded into 25-cm2 flasks and stromal cells into75-cm2 flasks (Fischer Scientific GTF, Västra Frölunda, Sweden) and incubated in an atmosphere of 5% CO2 in humidified air at 37°C. The culture medium was changed 2 to 3 times per week, and the cells were regularly checked using an inverted phase-contrast microscope (Nikon Diaphot 300; TeknoOptik AB, Skärholmen, Sweden). At subconfluency in passages 0 and 1, subcultivation of cells was performed by trypsination with trypsin-EDTA (Gibco; Invitrogen) according to the standard procedures.
The method of isolation is a standard procedure at our laboratory, and we have previously characterized the isolated endothelial cells.23 Each biopsy sample gave rise to 1 individual monoculture and 1 coculture; cells from different women were not pooled. The HEECs in passages 1 to 3 were grown as monocultures or as cocultures with stromal cells and exposed to mifepristone, progesterone, levonorgestrel, or vehicle (ethanol). In cocultures, the HEECs were grown together with stromal cells growing in inserts, allowing communication but no direct contact between the 2 cell types.
Exposure to Test Substances
The HEEC were exposed to the test substance mifepristone, progesterone, levonorgestrel (Sigma-Aldrich), or vehicle for 48 hours. Stock solutions were prepared in ethanol. Mifepristone stock solution was diluted in ECM (ScienCell Research Laboratories) to final concentrations of 0.01, 0.1, 1, 10, and 100 µmol/L. Progesterone and levonorgestrel were diluted in cell culture medium to final concentrations of 0.01 and 1 µmol/L, respectively. The final ethanol concentration was 0.1% (v/v) in all the treatment groups and the vehicle-only groups. A common clinically used dose of mifepristone is a single dose of 600 mg orally. This means a plasma concentration of approximately 5 µmol/L after about 1.3 hours.
Proliferation and Viability Assays
Cell proliferation (BrdU) enzyme-linked immunosorbent assays and cell viability WST-1 assays (Roche Diagnostics Scandinavia AB, Bromma, Sweden) were carried out using 6 replicates from 4 individual women according to the manufacturer’s instructions, as described previously.7 Briefly in proliferation assays, the treated HEECs were exposed to BrdU-labeling solution for the last 24 hours of the 48-hour incubation. Labeling was visualized using a peroxidase reagent, and absorbance was measured at 450 nm. Viability was assessed using a WST-1 kit, where 10 µL of WST-1 solution was added to the test substance-treated HEECs for the last hour of a 48-hour incubation. After 1 hour of incubation at 37°C, the absorbance was measured at 450 nm.
Tube Formation Assay
Endothelial tube formation assays were performed as described previously.24 The HEECs from 5 women were cultured in 6-well plates either as monocultures or as cocultures with stromal cells and treated with 0.01, 0.1, 1, or 10 µmol/L mifepristone, 0.01 µmol/L progesterone, 1 µmol/L levonorgestrel, or vehicle for 48 hours. The wells of a 96-well cell culture plate were loaded with 70 μL Geltrex-reduced growth factor extracellular matrix membrane (Invitrogen Life Sciences, Paisley, United Kingdom) and incubated for 30 minutes at 37°C until it gelled. Thereafter, the HEECs were detached by trypsinization and held as single-cell suspensions in the cell culture medium containing mifepristone, progesterone, levonorgestrel, or vehicle. A Bürker chamber was used to determine cell number and to assure cell viability. The HEECs were placed on top of the extracellular matrix membrane (9000 cells in 100 μL cell culture medium per well) and incubated for 6 hours at 37°C. In total, 4 replicates from 5 individual women were used for each treatment, and VEGF (CAS: 127464-60-2; Sigma-Aldrich) was added to 2 of the 4 replicates for each treatment to a final concentration of 100 ng/mL. Tube formation was analyzed using a Zeiss Axiovert S100 and A 5×/0.16 objective (Carl Zeiss AG, Germany). The settings for the microscope, camera, and software were the same for all images. The images were analyzed using the Wimtube formation module in WIMASIS Image Analysis (Munich, Germany). This online software module identifies cellular tubes on a multiparametric basis. Wimtube was compared with other methods for quantification of tube formation, and the conclusion was that it was easy to use and accurately quantified angiogenesis.25
The following parameters were determined: covered area (%), total numbers of tubes, total tube length, total branching points, total loops (loops are defined as the closed areas inside the tubular structure), and total nets (are the different tubular structures on the image that are not connected to each other.).
RNA Isolation, Complementary DNA Synthesis, and qPCR
RNA from mono- and cocultured HEECs from 4 women was isolated for messenger RNA (mRNA) expression analysis of some angiogenesis-associated genes.
At subconfluency in passage 2, HEECs seeded in sterile 6-well cell culture plates were exposed to 0.01, 0.1, 1, or 10 µmol/L mifepristone, 0.01 µmol/L progesterone, 1 µmol/L levonorgestrel, or vehicle for 48 hours. Stromal cells were seeded into inserts in the same well as the HEECs (Nunc CC Inserts—Anopore membrane; Thermo Fisher Scientific Inc, Waltham, Massachusetts). After exposure, the HEECs were washed with PBS before being harvested in the lysis buffer. Total RNA was isolated according to the RNeasy Mini Kit spin protocol (Qiagen AB, Solna, Sweden). The concentration of the isolated RNA was determined with the Qubit fluorometer (Invitrogen), and the quality was determined with the Agilent RNA Pico Kit in the Agilent 2100 Bioanalyzer (Agilent Technologies, Sweden AB, Kista, Sweden). Only high-quality RNA with no signs of degradation was used for further experiments. Isolated RNA was frozen in aliquots at −70°C until used.
The analyzed mRNA expressions of interest were PR-A + B NM_000926.4 forward: 5′AGCCCTAAGCCAGAGATTCA 3′, reverse: 5′ AGGATCTCCATCCTAGACC 3′; GR NM_001204258.1 forward: 5′ GCGATGGTCTCAGAAACCAAAC 3′, reverse: 5′ CAGAGGATAACTTCCTCTGTAATCTC 3′; and AR NM_001011645.2 forward: 5′ CCAGGAGACCTGCCTGATCTG 3′, reverse: 5′ CCTCCTGTAGTTTCAGATTACC 3′, and ERα and β, hypoxia-inducible factor (HIF), von Willebrand factor (VWF), VEGF, placental growth factor (PLGF), and VEGF receptors 1 and 2 (VEGFR1 and VEGFR2) primer sequences.7
Statistical Analysis
Significance within and between the groups regarding proliferation, viability, qPCR, and tube formation assays were analyzed using the Mann-Whitney U test. Values of P ≤ .05 were considered to denote a statistically significant difference. The means of 6 replicates from each individual were used in the statistical analysis of data on proliferation and viability of HEEC exposed to the chemicals or the vehicle. The means of 2 replicates from each individual were used in the analysis of tube formation of HEECs. The 2 replicates that were exposed to VEGF were analyzed separately in the same way. Mean normalized expression, based on the ratio between mean of triplicate Ct-values of target and reference genes and the efficiency of the PCR reactions, was calculated as a measure of target gene transcription as described by Muller and coworkers.26 Differences between mono- and cocultures concerning proliferation, viability, qPCR, and tube formation were analyzed using Kruskal-Wallis 1-way analysis of variance. All statistical analyses were carried out using the Statistical Package for the Social Sciences (SPSS (version 20).
Results
Proliferation and Viability
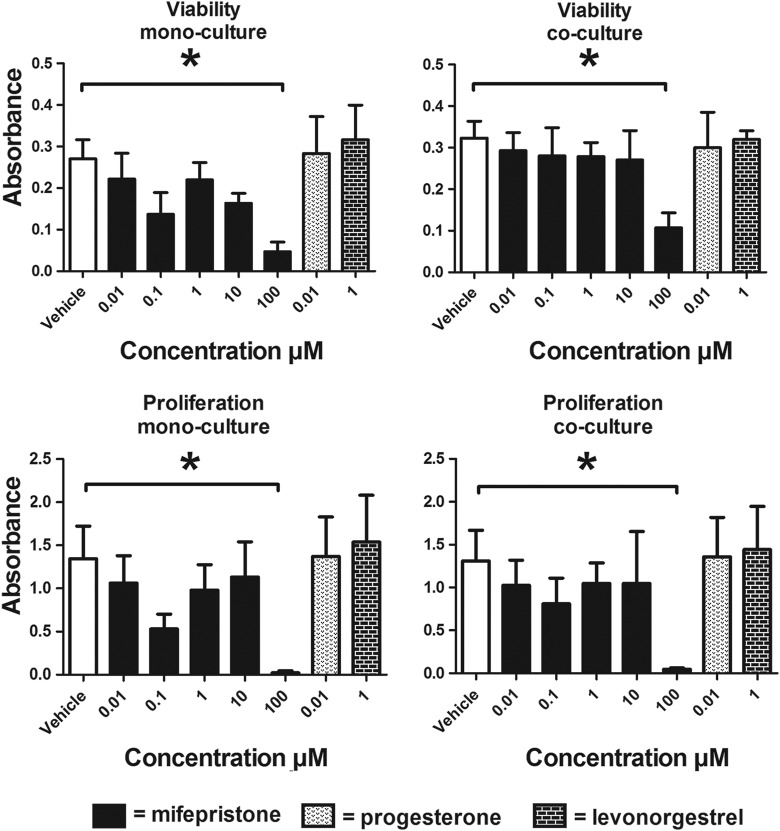
Proliferation and viability were not altered by any treatment, with the exception of 100 µmol/L mifepristone, which significantly decreased these parameters (Figure 1).
Figure 1.
Effects of mifepristone, progesterone, and levonorgestrel on monocultured and cocultured HEECs. Mifepristone of 100 µmol/L significantly decreased both viability and proliferation of monocultured and cocultured HEECs from 4 patients. * P < .05. Data presented as mean ± SEM. HEECs indicates human endometrial endothelial cells; SEM, standard error of the mean.
Tube Formation
There was no change in tube formation when monocultures of HEEC were exposed to mifepristone, progesterone, or levonorgestrel. But, at a group level when HEEC were grown in coculture with stromal cells and exposed to mifepristone, they displayed significantly lower “total covered areas” (P = .049) and fewer “total loops” (P = .023) compared to HEECs grown in monocultures (Figures 2 and 3). The HEECs grown in cocultures and exposed to mifepristone also showed tendencies toward reduced “total branching points” (P = .068), “total tube length” (P = .072), and “total nets” (P = .063) compared to monocultured HEECs. The addition of VEGF at 100 ng/mL did not affect HEECs tube formation. The HEECs exposed to mifepristone in combination with VEGF showed no significant differences between mono- and cocultures. There were also no differences between mono- and cocultured HEECs after exposure to progesterone or levonorgestrel.
Figure 2.
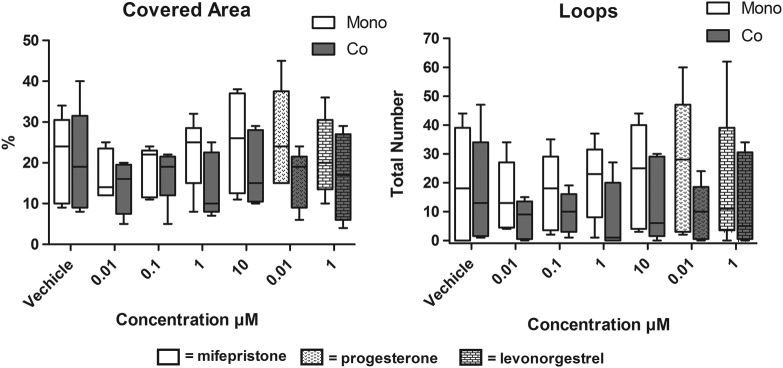
HEECs from 5 patients exposed to mifepristone and grown in cocultures displayed significantly lower covered areas and total numbers of loops compared to mifepristone exposed HEEC in monoculture. Whiskers represent maximum and minimum values. HEEC indicates human endometrial endothelial cells.
Figure 3.
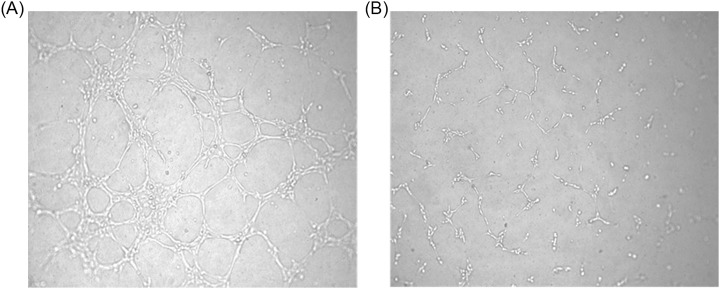
Representative images of tube formation of HEECs after exposure to 1 µmol/L mifepristone. A, Tube formation assay of monocultured HEECs and (B) tube formation assay of HEECs cocultured with stromal cells. HEECs indicates human endometrial endothelial cell.
Quantitative PCR of Angiogenesis-Related Genes in HEECs
The HEECs exposed to 0.01, 0.1, 1, or 10 µmol/L mifepristone, 0.01 µmol/L progesterone, or 1 µmol/L levonorgestrel did not show any significant changes in the gene expression of any of the investigated mRNAs (ER-β, HIF, VWF, GR, AR, VEGF, PLGF, VEGFR1, and VEGFR2). The ER-α and PR-A + B expression were too low to be further analyzed. There were no differences between HEECs grown in monocultures and cocultures.
Discussion
The HEECs exposed to mifepristone showed significantly reduced tube formation activity when grown in cocultures with stromal cells compared to HEECs grown in monocultures,without any changes in the expression of the evaluated angiogenesis-related genes. The presence of stromal cells thus seems to have influenced the effect of mifepristone on HEECs’ angiogenic activity. It is known that mifepristone, progesterone, and levonorgestrel influence the endometrial bleeding pattern,13,16 probably by inducing changes in angiogenic activity and causing functional and morphological changes in the endometrial vasculature.13 In a previous study, in which the effects of ovarian steroids on HEEC monocultures were investigated, it was shown that estrogen and progesterone increased cell proliferation.5 In the same study, when HEECs were grown on extracellular matrix and exposed to estrogen and/or progesterone, there was no significant difference in tube formation compared to controls,5 which is in line with our results. Endometrial stromal cells express ER-α, ER-β, PR-A, and PR-B,27,28 while the expression in HEECs is uncertain except for ER-β.5 It has been proposed that downregulation of paracrine/autocrine stromal growth factors are related to blockade of progesterone action on the spiral arteries.14 In women, progesterone acts on the estradiol-primed endometrium to initiate the transformation of stromal cells to decidual cells around the spiral arterioles. Decline in serum progesterone contributes to menstrual shedding, which is initiated by vasoconstriction. In primates, the only cells expressing the PR at that time is the stromal and myometrial cells, which indicates that those cells secrete factors that affect spiral arteries.15 In this study, HEECs’ tube formation was negatively affected when HEECs had grown together with stromal cells and exposed to mifepristone, indicating similar occurrence as Brenner and Slayden saw in nonhuman primates.29
The HEECs exposed to 100 µmol/L mifepristone showed heavily reduced proliferation and viability rates. This concentration was therefore considered as cytotoxic and was not further investigated.
Mifepristone has been shown to cause vascular changes in the endometrium.16 Low-dose mifepristone administered as an alternative to other estrogen-free contraceptive pills is associated with amenorrhea, which could be related to the effects on the endometrial vasculature.13 Amenorrhea seen after mifepristone treatment may however also be a consequence of effects on ovulation.12,30 Narvekar and coworkers also showed that stromal VEGF expression was significantly reduced following mifepristone treatment,13 a finding that might be in line with our results showing reduced angiogenic activity of HEECs cocultured with stromal cells. Whether our finding is a result of downregulation of stromal expression or release of VEGF remains to be shown. The VEGF may not necessarily be the major regulator of HEECs proliferation in the complex interaction between angiogenesis promoters and inhibitors in the human endometrium.31 In addition, most studies have failed to reveal a clear link between the expression of endometrial angiogenic factors and angiogenesis.1 Our results show that HEECs tube formation did not increase after adding VEGF at 100 ng/mL in spite of the fact that VEGF is generally a known inducer of endothelial cell proliferation. This finding is probably explained by the fact that tube formation does not depend primarily on endothelial cell proliferation.32
Mifepristone may also bind to ARs and GR, which are expressed by human endometrial stromal cells.33, 34 This implies that the effects on HEEC caused by mifepristone via stromal cells might be a result of binding of mifepristone to glucocorticoid and/or ARs. In this study, AR and GR mRNA expression was detected in HEECs, but it remained unaffected by exposure to mifepristone, progesterone, or levonorgestrel. When mifepristone is used for early pregnancy termination, there are both morphological and functional changes in the endometrium, myometrium, decidua, and cervix, changes that might be induced partly by interaction between mifepristone and the AR and GR.33–35 It must however be emphasized that these mifepristone-related changes are most probably induced by mifepristone interacting with the PRs on stromal cells, and that effects on HEECs probably are mediated through stromal cells via paracrine mechanisms. It is also known that androgens play a role in the regulation of both VEGF expression and VEGF-induced cell signaling in normal tissues and hormone-responsive cancers,36 which further supports their possible involvement in the control of endometrial angiogenesis.
Working with primary cells is a great advantage compared to cell lines, since they mimic the in vivo situation better than cell lines. However, there are disadvantages such as interindividual variations, demanding culture procedures, and small population size. Despite these difficulties, we find it important to use primary HEECs to study the angiogenic effects of mifepristone to gain knowledge on events in the in vivo situation.
In conclusion, it was found that exposure of HEECs to mifepristone when cocultured with endometrial stromal cells resulted in significantly reduced angiogenic activity compared with HEEC in monoculture. It thus seems as if mifepristone induces changes in angiogenic activity and that these effects are probably mediated through stromal cells via paracrine mechanisms.
Acknowledgment
The authors would like to thank Eva Davey for technical assistance.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported financially by the Swedish Fund for Research without Animal Experiments and the Family Planning Fund, Uppsala.
References
- 1. Gargett CE, Rogers PA. Human endometrial angiogenesis. Reproduction. 2001;121(2):181–186 [DOI] [PubMed] [Google Scholar]
- 2. Iruela-Arispe ML, Rodriguez-Manzaneque JC, Abu-Jawdeh G. Endometrial endothelial cells express estrogen and progesterone receptors and exhibit a tissue specific response to angiogenic growth factors. Microcirculation. 1999;6(2):127–140 [PubMed] [Google Scholar]
- 3. Critchley HOD, Brenner RM, Henderson TA, et al. Estrogen receptor β, but not estrogen receptor α, is present in the vascular endothelium of the human and nonhuman primate endometrium. J Clin Endocrinol Metab. 2001;86(3):1370–1378 [DOI] [PubMed] [Google Scholar]
- 4. Lecce G, Meduri G, Ancelin M, Bergeron C, Perrot-Applanat M. Presence of estrogen receptor β in the human endometrium through the cycle: expression in glandular, stromal, and vascular Cells. J Clin Endocrinol Metab. 2001;86(3):1379–1386 [DOI] [PubMed] [Google Scholar]
- 5. Kayisli UA, Luk J, Guzeloglu-Kayisli O, Seval Y, Demir R, Arici A. Regulation of angiogenic activity of human endometrial endothelial cells in culture by ovarian steroids. J Clin Endocrinol Metab. 2004;89(11):5794–5802 [DOI] [PubMed] [Google Scholar]
- 6. Krikun G, Schatz F, Taylor R, et al. Endometrial endothelial cell steroid receptor expression and steroid effects on gene expression. J Clin Endocrinol Metab. 2005;90(3):1812–1818 [DOI] [PubMed] [Google Scholar]
- 7. Helmestam M, Stavreus-Evers A, Olovsson M. Cadmium chloride alters mRNA levels of angiogenesis related genes in primary human endometrial endothelial cells grown in vitro. Reprod Toxicol. 2010;30(3):370–376 [DOI] [PubMed] [Google Scholar]
- 8. Helmestam M, Andersson H, Stavreus-Evers A, Brittebo E, Olovsson M. Tamoxifen modulates cell migration and expression of angiogenesis-related genes in human endometrial endothelial Cells. Am J Pathol. 2012;180(6):2527–2535 [DOI] [PubMed] [Google Scholar]
- 9. Yasuo T, Kitaya K. Effect of ovarian steroids on gene expression profile in human uterine microvascular endothelial cells. Fertili Sterili. 2009;92(2):709–721 [DOI] [PubMed] [Google Scholar]
- 10. Toth B, Scholz C, Ochsenkühn R, et al. Effects of progesterone and Its antagonist mifepristone on progesterone receptor a expression in human umbilical vein endothelial cells. Gynecol Obstet Invest. 2009;67(4):269–274 [DOI] [PubMed] [Google Scholar]
- 11. Wolf JP, Hsiu JG, Anderson TL, Ulmann A, Baulieu EE, GD H. Noncompetitive antiestrogenic effect of RU 486 in blocking the estrogen-stimulated luteinizing hormone surge and the proliferative action of estradiol on endometrium in castrate monkeys. Fertili Sterili. 1989;52(6):1055–1060 [DOI] [PubMed] [Google Scholar]
- 12. Baird DT, Brown A, Critchley HOD, Williams AR, Lin S, Cheng L. Effect of long-term treatment with low-dose mifepristone on the endometrium. Hum Reprod. 2003;18(1):61–68 [DOI] [PubMed] [Google Scholar]
- 13. Narvekar N, Critchley HOD, Cheng L, Baird DT. Mifepristone-induced amenorrhoea is associated with an increase in microvessel density and glucocorticoid receptor and a decrease in stromal vascular endothelial growth factor. Hum Reprod. 2006;21(9):2312–2318 [DOI] [PubMed] [Google Scholar]
- 14. Chwalisz K, Brenner RM, Fuhrmann UU, Hess-Stumpp H, Elger W. Antiproliferative effects of progesterone antagonists and progesterone receptor modulators on the endometrium. Steroids. 2000;65 (10–11):741–751 [DOI] [PubMed] [Google Scholar]
- 15. Slayden OD, Brenner RM. Hormonal regulation and localization of estrogen, progestin and androgen receptors in the endometrium of nonhuman primates: effects of progesterone receptor antagonists. Arch Histol Cytol. 2004;67(5):393–409 [DOI] [PubMed] [Google Scholar]
- 16. Johannisson E, Oberholzer M, Swahn M-L, Bygdeman M. Vascular changes in the human endometrium following the administration of the progesterone antagonist RU 486. Contraception. 1989;39(1):103–117 [DOI] [PubMed] [Google Scholar]
- 17. Spitz IM. Mifepristone: where do we come from and where are we going?: Clinical development over a quarter of a century. Contraception. 2009;82(5):442–452 [DOI] [PubMed] [Google Scholar]
- 18. Meng C-X, Andersson KL, Bentin-Ley U, Gemzell-Danielsson K, Lalitkumar PGL. Effect of levonorgestrel and mifepristone on endometrial receptivity markers in a three-dimensional human endometrial cell culture model. Fertili Sterili. 2009;91(1):256–264 [DOI] [PubMed] [Google Scholar]
- 19. Sitruk-Ware R. New progestagens for contraceptive use. Hum Reprod Update. 2006;12(2):169–178 [DOI] [PubMed] [Google Scholar]
- 20. Bredhult C, Bäcklin B-M, Olovsson M. Effects of some endocrine disruptors on the proliferation and viability of human endometrial endothelial cells in vitro. Reprod Toxicol. 2007;23(4):550–559 [DOI] [PubMed] [Google Scholar]
- 21. Bredhult C, Sahlin L, Olovsson M. Gene expression analysis of human endometrial endothelial cells exposed to op'-DDT. Mol Hum Reprod. 2008;14(2):97–106 [DOI] [PubMed] [Google Scholar]
- 22. Bredhult C, Sahlin L, Olovsson M. Gene expression analysis of human endometrial endothelial cells exposed to bisphenol A. Reprod Toxicol. 2009;28(1):18–25 [DOI] [PubMed] [Google Scholar]
- 23. Moberg C, Catalano RD, Charnock-Jones DS, Olovsson M. VEGF-A and serum withdrawal induced changes in the transcript profile in human endometrial endothelial cells. Reprod Sci. 2010;17(6):590–611 [DOI] [PubMed] [Google Scholar]
- 24. Arnaoutova I, Kleinman HK. In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat Protoc. 2010;5(4):628–635 [DOI] [PubMed] [Google Scholar]
- 25. Khoo CP, Micklem K, Watt SM. A comparison of methods for quantifying angiogenesis in the matrigel assay in vitro. Tissue Eng Part C Methods. 2011;17(9):895–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muller PY, Janovjak H, Miserez AR, Dobbie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. Biotechniques. 2002;32(6):1372-1374, 1376, 1378–1379 [PubMed] [Google Scholar]
- 27. Uhlén M, Björling E, Agaton C, et al. A Human protein atlas for normal and cancer tissues based on antibody proteomics. Mol Cell Proteomics. 2005;4(12):1920–1932 [DOI] [PubMed] [Google Scholar]
- 28. Yuhki M, Kajitani T, Mizuno T, Aoki Y, Maruyama T. Establishment of an immortalized human endometrial stromal cell line with functional responses to ovarian stimuli. Reprod Biol Endocrinol. 2011;9(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brenner RM, Slayden OD. Steroid receptors in blood vessels of the rhesus macaque endometrium: a review. Arch Histol Cytol. 2004;67(5):411–416 [DOI] [PubMed] [Google Scholar]
- 30. Chen Y, Wang W, Zhuang Y, Chen X, Huang L. Effects of low-dose mifepristone administration in two different 14-day regimens on the menstrual cycle and endometrial development: a randomized controlled trial. Contraception. 2011;84(1):64–70 [DOI] [PubMed] [Google Scholar]
- 31. Gargett CE, Lederman FL, Lau TM, Taylor NH, Rogers PAW. Lack of correlation between vascular endothelial growth factor production and endothelial cell proliferation in the human endometrium. Hum Reprod. 1999;14(8):2080–2088 [DOI] [PubMed] [Google Scholar]
- 32. Arnaoutova I, George J, Kleinman H, Benton G. The endothelial cell tube formation assay on basement membrane turns 20: state of the science and the art. Angiogenesis. 2009;12(3):267–274 [DOI] [PubMed] [Google Scholar]
- 33. Bamberger A-M, Milde-Langosch K, Loning T, Bamberger CM. The glucocorticoid receptor is specifically expressed in the stromal compartment of the human endometrium. J Clin Endocrinol Metab. 2001;86(10):5071–5074 [DOI] [PubMed] [Google Scholar]
- 34. Brenner RM, Slayden OD, Nayak NR, Baird DT, Critchley HOD. A role for the androgen receptor in the endometrial antiproliferative effects of progesterone antagonists. Steroids. 2003;68 (10–13):1033–1039 [DOI] [PubMed] [Google Scholar]
- 35. Schatz F, Papp C, Aigner S, Krikun G, Hausknecht V, Lockwood CJ. Biological mechanisms underlying the clinical effects of RU 486: modulation of cultured endometrial stromal cell stromelysin-1 and prolactin expression. J Clin Endocrinol Metab. 1997;82(1):188–193 [DOI] [PubMed] [Google Scholar]
- 36. Dietrich W, Gaba A, Zhegu Z, et al. Testosterone dependent androgen receptor stabilization and activation of cell proliferation in primary human myometrial microvascular endothelial cells. Fertili Sterili. 2011;95(4):1247–1255. e1–2 [DOI] [PubMed] [Google Scholar]