Abstract
Curcumin is naturally occurring polyphenolic compound found in turmeric and has many pharmacological activities. The present study was undertaken to evaluate anti-allergic inflammatory activity of curcumin, and to investigate its inhibitory mechanisms in immunoglobulin E (IgE)/Ag-induced mouse bone marrow-derived mast cells (BMMCs) and in a mouse model of IgE/Ag-mediated passive systemic anaphylaxis (PSA). Curcumin inhibited cyclooxygenase-2 (COX-2) dependent prostaglandin D2 (PGD2) and 5-lipoxygenase (5-LO) dependent leukotriene C4 (LTC4) generation dose-dependently in BMMCs. To probe the mechanism involved, we assessed the effects of curcumin on the phosphorylation of Syk and its downstream signal molecules. Curcumin inhibited intracellular Ca2+ influx via phospholipase Cγ1 (PLCγ1) activation and the phosphorylation of mitogen-activated protein kinases (MAPKs) and the nuclear factor-κB (NF-κB) pathway. Furthermore, the oral administration of curcumin significantly attenuated IgE/Ag-induced PSA, as determined by serum LTC4, PGD2, and histamine levels. Taken together, this study shows that curcumin offers a basis for drug development for the treatment of allergic inflammatory diseases.
Keywords: Curcumin, Mast cell, Prostaglandin D2, Leukotriene C4, Mitogen activated protein kinase, Passive systemic anaphylaxis
INTRODUCTION
Anaphylaxis is an immediate, immunoglobulin E (IgE)-mediated hypersensitivity reaction resulting from the sudden release of mast cell- and basophil-derived chemical mediators such as histamine, serotonin, newly synthesized lipid-derived mediators, such as prostaglandin D2 (PGD2), leukotriene B4 (LTB4) cysteinyl leukotrienes LTC4, LTD4, and LTE4, platelet-activating factor (PAF), and various cytokines (Kemp and Lockey, 2002; Simons, 2008). Furthermore, the releases of these chemical mediators are a consequence of the binding of IgE to FcεRI on the surfaces of mast cells. This initial interaction results in the phosphorylations of tyrosine residues in the immune receptor tyrosine based activation motif (ITAM) of the β and γ subunits of FcεR1 by Lyn and recruits the tyrosine kinase Syk, which is required for phosphorylation of linker for activation of T cells (LAT). Phosphorylated LAT binds phospholipase Cγ (PLCγ) and the adaptors Gads and Grb2 and generates inostitol-1,4,5-triphospahte (IP3), which can increase intracellular calcium release from endoplasmic reticulum (ER) calcium stores (Siraganian, 2003). Elevated intracellular calcium then triggers the degranulation and the translocation of cytosolic phospholipase A2 (cPLA2) and 5-lipoxygenase (5-LO) to the nuclear membrane. The syntheses of cyclooxygenase-2 (COX-2)-dependent PGD2 and of 5-LO-dependent LTC4 in mast cells are initiated by the release of free arachidonic acid (AA) from membrane phospholipid by cPLA2 (Kudo and Murakami, 1999; Yamaguchi et al., 1999). Furthermore, it is well known that COX-2 dependent PGD2 is very important to the development of inflammation and allergic disease such as asthma (Ricciotti and FitzGerald, 2011). LTC4 plays important roles in inflammatory and allergic diseases (Werz and Steinhilber, 2006), and increasing evidence suggests it play a role in cancer (Avis et al., 2001) and cardiovascular disease (Mehrabian and Allayee, 2003). Therefore, the inhibition of eicosanoid production constitutes an important therapeutic strategy against various allergic inflammatory diseases.
Curcumin (diferuloyl methane) is a major constituent of the rhizome of Curcuma longa, and is used traditionally to treat inflammation, gastrointestinal disorders, hepatic disorders, diabetic wounds, skin wounds, rheumatism, sinusitis, and other disorders (Ammon and Wahl, 1991). Furthermore, scientific studies have shown that curcumin inhibits histamine release and the secretions of tumor necrosis factor-α (TNF-α) and interleukin-4 (IL-4) from mast cells triggered by IgE, calcium ionophore A23187, or compound 48/80 (Suzuki et al., 2005; Lee et al., 2008; Choi et al., 2010). Curcumin has also been reported to inhibit IgE-induced type I hypersensitivity and ovalbumin-induced airway hyperreactivity (Yano et al., 2000; Ram et al., 2003; Lee et al., 2008), and to inhibit COX-2 gene expression in phorbol ester-treated human gastrointestinal epithelial cells and mouse skin (Chun et al., 2003; Ricciotti and FitzGerald, 2011) and in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis (Huang et al., 1991). However, the effect of curcumin on IgE/Ag-induced COX-2 dependent PGD2 and 5-LO dependent LTC4 generation in mast cells and IgE-mediated systemic anaphylactic response have not been well investigated.
In this study, we evaluated the effects of curcumin on the generation of eicosanoid (PGD2 and LTC4) in FcεRI-induced mast cells and on passive systemic anaphylaxis (PSA) response in mice.
MATERIALS AND METHODS
Plant material
Curcumin was isolated from the ethyl acetate fraction of a methanol extract of the rhizome of Curcuma longa, as described previously (Kiuchi et al., 1993), and produced a single TLC spot and had a HPLC determined purity of >99.5%. Curcumin was prepared by dissolving it in dimethyl sulfoxide (DMSO). The final concentration of DMSO in culture media was adjusted to 0.1% (v/v). DMSO alone was run as a control in all cases.
Chemicals and reagents
Mouse anti-dinitrophenyl (DNP) IgE and DNP-human serum albumin (HSA) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Fexofenadine-HCl (Fexo), a histamine H1 receptor antagonist, was obtained from Korea Pharma (Seoul, Korea). The rabbit polyclonal antibodies specific for phospho-IκB, IKKα/β, ERK1/2, JNK, p38, Akt, β-actin, and total form for IκB, ERK1/2, JNK, p38, and Akt were purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA). Rabbit polyclonal antibodies for phospho-cPLA2α (Ser505), cPLA2α, 5-LO, PLCγ1, IKKα/β lamin B and NF-κB p65 as well as secondary goat anti-rabbit IgG-HRP and rabbit anti-goat IgG-HRP antibodies, total Syk, total LAT, and Bay 61-3606 were purchased from Santa Cruz Biotechnology (Dallas, Texas, USA) and antibodies for phosphotyrosine was purchased from Millipore (Millipore, Billerica, MA, USA). The antibody-reactive bands were visualized with an enhanced chemiluminescence (ECL) system (Pierce Biotechnology, Rockford, IL, USA). The enzyme immnoassay (EIA) kits for PGD2, LTC4, histamine and the antibody for COX-2 were purchased from Cayman Chemicals (Ann Arbor, MI, USA).
Culture and activation of bone marrow derived mast cells (BMMCs)
BMMCs were isolated from bone marrow of C57BL/6 mice and differentiated as described previously (Lu et al., 2011). Briefly, BMMCs were cultured in RPMI 1640 medium containing 10% fetal bovine serum, 100 U/ml penicillin, 10 mM HEPES, 100 μM MEM non-essential amino acid solution (Invitrogen, Grand Island, NY, USA) and 20% pokeweed mitogen-spleen cell conditioned medium as a source of IL-3. For stimulation, 106 cells/ml were sensitized overnight with 500 ng/ml anti-DNP IgE, pretreated with indicated concentration of curcumin or Bay 61-3606, and stimulated for appropriate periods with 100 ng/ml DNP-HSA. The reactions were terminated by centrifugation of the cells at 3,000 rpm for 5 min at 4°C.
Determination of LTC4 and PGD2
Concentration of LTC4 and PGD2 were determined as described previously (Lu et al., 2011). IgE sensitized BMMCs suspended in enriched medium at a cell density of 1×106 cells/ ml were pretreated with indicated concentration of curcumin or Bay 61-3606 for 1 h and stimulated with DNP-HSA for 15 min. Supernatants were isolated for further analysis by EIA kit. The concentration of LTC4 was determined using an EIA kit. To assess COX-2-dependent PGD2 synthesis, BMMCs were preincubated with 1 μg/ml of aspirin for 2 h to irreversibly inactivate preexisting COX-1. After washing, BMMCs were incubated with 100 ng/ml DNP-HSA at 37°C for 7 h in the presence of curcumin or Bay 61-3606. PGD2 in the supernatants were quantified using PGD2 EIA kit and cells were used for immunoblots analysis. Under the conditions employed, LTC4 reached 4.75 ng/106 cells and PGD2 generation reached 2.12 ng/106 cells. All data were the arithmetic mean of triplicate determinations.
Measurement of intracellular Ca2+ level
Intracellular Ca2+ levels were determined using FluoForte™ Calcium Assay Kit (Enzo Life Sciences, Ann Arbor, MI, USA), as described previously (Hwang et al., 2013). Briefly, BMMCs (1×106 cells) were sensitized overnight with 500 ng/ml anti-DNP IgE. Sensitized BMMCs were preincubated with FluoForte™ Dye-Loading Solution for 1 h at room temperature. After washing the dye from cell surface with HBSS, cells (5×104) were seeded into 96-well microplates and pretreated with curcumin or Bay 61-3606 for 1 h before adding DNP-HSA. Fluorescence was measured using a fluorometric imaging plated reader at an excitation wavelength of 485 nm and an emission wavelength of 520 nm on a BMG Labtechnologies FLUOStar OPITIMA platereader (Offenburg, Germany).
Preparation of nuclear and cytoplasmic extracts
The nuclear and cytoplasmic extracts were prepared as described previously (Lu et al., 2011). BMMCs were sensitized to DNP-specific IgE (500 ng/ml, overnight) and pretreated with curcumin or Bay 61-3606 for 1 h, and then stimulated with DNP-HSA (100 ng/ml) for 30 min. Cultured BMMCs were collected by centrifugation, washed with PBS, and lysed in a buffer containing 10 mM HEPES (pH 7.9), 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 1 mM PMSF and 0.1% NP40 on ice for 10 min. Supernatants (cytosolic fractions) were obtained by centrifugation at 1,000 g for 4 min. Nuclear pellets were washed and lysed in a buffer containing 20 mM HEPES (pH 7.9), 25% (v/v) glycerol, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, and protease inhibitor cocktail.
Immunoprecipitation (IP)
Immunoprecipitation was performed as described previously (Hwang et al., 2013). Briefly, cell lysates were obtained using modified lysis buffer [0.1% Nonidet P-40, 50 mM HEPES (pH 7.0), 250 mM NaCl, 5 mM EDTA, 1 mM PMSF, and 0.5 mM dithiothreitol]. Total cell lysates (1 mg protein equivalent) were incubated with anti-Syk or anti-LAT antibodies for 2 h at 4°C and immunocomplexes were precipitated with 20 μl of protein A-Sepharose and washed 3 times with ice-cold lysis buffer. Precipitates and total cell lysates were subjected to SDS-PAGE and immunoblotted with appropriate antibodies.
Immunoblotting
Immunoblotting was performed as described previously (Lu et al., 2011). Cells were lysed in RIPA lysis buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 5 mM EDTA, 1% Nonidet P-40, 1mM phenylmethanesulfonylfluoride (PMSF), 1M dithiothreitol (DTT), 200 mM NaF, 200 mM Na3VO4, and protease inhibitor cocktail). Cell debris was removed by centrifugation at 14,000 g for 15 min at 4°C, and the resulting supernatant was western blotted. Protein concentration was measured using a Qubit Fluorometer (Invitrogen, USA). Samples were separated by 8% SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to nitrocellulose membranes (Millipore, Billerica, MA, USA). Immunoreactive proteins were incubated with HRP-coupled secondary antibodies diluted at 1:3,000 for 1 h at room temperature, and then developed using an enhanced chemiluminescence (ECL) detection kit.
Passive systemic anaphylaxis (PSA) reaction in mice
PSA was induced as described previously (Lu et al., 2011). Six ICR mice (Hyochang Science, Daegu, Korea) were placed per cage within a laminar airflow cabinet and fed standard laboratory chow (Purina, Seoul, Korea) and provided with water ad libitum. The animals were maintained in 22 ± 1°C, 55 ± 10% RH environment under a 12 h/12 h (light/dark) cycle for at least 7 days prior to experiments. Mice were injected with 2 μg of DNP-specific IgE in 100 μl of saline or with 100 μl of saline. 24 h later mice were orally administered curcumin (20 and 50 mg/kg) or fexofenadine-HCl (Fexo; 50 mg/kg) and 1 h later challenged with 4 mg i.v. of DNP-HSA in 200 μl of saline. Blood was collected by cardiac puncture 5 min after the Ag challenge. Serum LTC4, PGD2 and histamine concentrations were determined using appropriate EIA kits. All experiments using animals were approved beforehand by the Institutional Animal Care and Use Committee of Yeungnam University.
Statistical analysis
All experiments were performed three or more times. Average values are expressed as means ± S.D. Statistical analyses were performed using SPSS 19.0 (SPSS, Chicago, IL, USA). The Student’s t-test was used to compare pairs of independent groups. Statistical significance was accepted for p values <0.05.
RESULTS
Curcumin suppressed passive systemic anaphylaxis (PSA) in mice
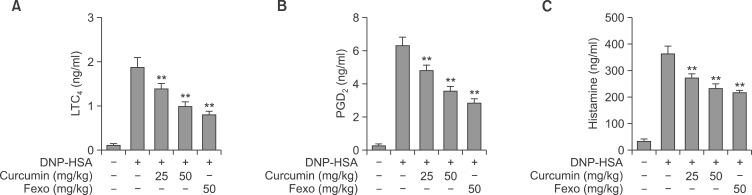
Anaphylaxis is an IgE-mediated hypersensitivity reaction caused by the release of various inflammatory mediators due to binding of specific IgE to FcεRI on the surfaces of mast cells or basophils (Siraganian, 2003). Furthermore, it has been reported that curcumin suppresses IgE or compound 48/80 induced passive cutaneous anaphylaxis (Suzuki et al., 2005; Lee et al., 2008; Choi et al., 2010). However, no report has been issued on the effect of curcumin on PSA in mice. Therefore, we assessed the anti-allergic-inflammatory effects of curcumin using a mouse PSA model. As shown in Fig. 1, PSA was induced using an i.v. injection of DNP-HSA in ICR mice after the oral administration of 25 or 50 mg/kg of curcumin or 50 mg/kg of Fexo (Ciprandi et al., 2003). One hour later, curcumin reduced serum LTC4 (Fig. 1A), PGD2 (Fig. 1B) and histamine (Fig. 1C) levels in a dose-dependent manner (n=6). The suppressive effect of 50 mg/kg of curcumin was similar to that of 50 mg/kg of Fexo, a histamine H1 receptor antagonist.
Fig. 1.
Curcumin inhibited IgE/Ag-induced PSA reaction. In the PSA test, ICR mice were sensitized by injecting 2 μg of IgE i.v. in 100 μl saline or injected with saline alone. 24 h later mice were administered 25 or 50 mg/kg of curcumin or 50 mg/kg fexofenadine-HCl (Fexo) and 1 h later were challenged with 4 mg of DNP-HSA i.v in 200 μl saline; blood was collected by cardiac puncture 5 min after the Ag challenge. The concentration of serum LTC4 (A), PGD2 (B), and histamine (C) were determined using appropriate enzyme immunoassay kits (Cayman Chemicals). The values indicate the mean ± S.D. from three independent experiments, **p<0.01 versus IgE/Ag sensitized mice). Fexo (50 mg/kg) was used as an anti-histamine control drug.
Curcumin inhibited LTC4 generation and Ca2+ influx in IgE/Ag-induced BMMCs
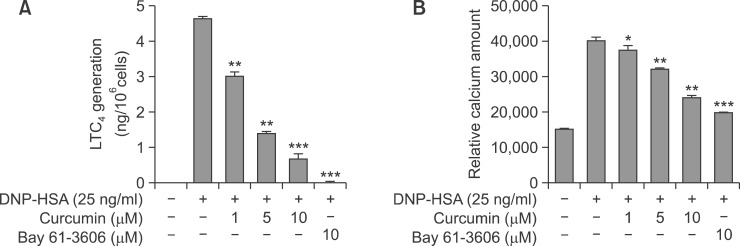
In vivo results let us to investigate the action mechanism responsible for the anti-allergic inflammatory activities of curcumin using mast cells, major players in PSA. LTC4 is metabolites of arachidonic acid derived from action of 5-LO/LTC4 synthase in mast cells, and LTC4 have been implicated in inflammation, proliferation and allergic conditions like asthma (Murphy and Gijon, 2007). Thus, we investigated the effects of curcumin on 5-LO dependent LTC4 generation in BMMCs. As shown in Fig. 2A, curcumin strongly inhibited LTC4 generation in a dose-dependent manner. It is well known that Ca2+ is essential for arachidonic acid (AA) release from phospholipid and degranulation in IgE/Ag-induced mast cells (Kudo and Murakami, 1999; Yamaguchi et al., 1999), thus we examined the effect of curcumin on intracellular Ca2+ influx in IgE/Ag-induced BMMCs. As shown in Fig. 2B, intracellular Ca2+ level in activated BMMCs was about three fold higher than in resting cells, and this increase was dose-dependently inhibited by curcumin. Bay 61-3606 (a Syk inhibitor) also strongly decreased intracellular LTC4 generation as well as Ca2+ influx. Consistent with a previous report (Lee et al., 2008), the release of β-Hex (a degranulation marker enzyme) was dose-dependently inhibited by curcumin (data not shown).
Fig. 2.
Effect of Curcumin on LTC4 generation and on Ca2+ mobilization in IgE/Ag-activated BMMCs. IgE-sensitized BMMCs were pre-incubated with the indicated concentration of curcumin or Bay61-3606 for 1 h and then stimulated with DNP-HSA for 15 min. LTC4 released into the supernatant was quantified using an enzyme immunoassay kit (A). Relative intracellular Ca2+ levels were determined (at 5 min) (B). *p<0.05, **p<0.01 and ***p<0.001 versus the IgE/Ag-treated group. Results are the averages of three independent experiments.
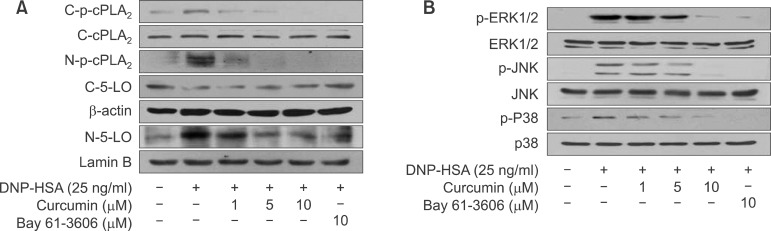
Curcumin inhibited cPLA2 phosphorylation, translocation of phospho-cPLA2α and 5-LO and activation of MAPKs
Recently, we and other group have reported that the release of free AA from membrane phospholipid in activated mast cells requires the phosphorylation of cPLA2α (p-cPLA2α) by mitogen activated protein kinases (MAPKs) (Lin et al., 1993; Lu et al., 2011), and the translocation of p-cPLA2α is dependent on intracellular Ca2+ influx (Gijon and Leslie, 1999; Lu et al., 2011). To determine whether curcumin inhibits the phosphorylation and translocation of cPLA2α, we pretreated BMMCs with different concentrations of curcumin or Bay 61-3606. As show in Fig. 3A, curcumin dose-dependently inhibited the phosphorylation (C-p-cPLA2α) and translocation of C-p-cPLA2α to the nuclear envelope (N-p-cPLA2α). The synthesis of 5-LO dependent LTC4 in IgE/Ag-induced mast cells is known to mediate the translocations of both p-cPLA2α and 5-LO to the nuclear envelope (Werz, 2002; Lu et al., 2011; Lu et al., 2012). Thus, we investigated the effect of curcumin on the translocation of 5-LO to the nuclear envelope. As was expected, curcumin or Bay 61-3606 inhibited the translocation of cytosolic 5-LO (C-5-LO) to nuclear envelope (N-5-LO). Next, to confirm that the inhibition of cPLA2α phosphorylation by curcumin occurred via the inhibition of MAPKs phosphorylation including extracellular regulated kinase1/2 (ERK1/2), cjun N-terminal kinase (JNK), and p38 MAP kinase, therefore we examined the effect of curcumin or Bay 61-3606 on the phosphorylation of MAPKs. As shown in Fig. 3B, curcumin or Bay 61-3606 inhibited the phosphorylations of three MAPKs in a dose dependent manner.
Fig. 3.
Effects of curcumin and Bay 61-3606 on cPLA2α and 5-LO translocation and MAPKs activation. IgE-sensitized BMMCs were pre-incubated for 1 h with the indicated concentrations of curcumin or Bay 61-3606 and then stimulated with DNP-HSA for 15 min. Cytosolic and nuclear fractions were immunoblotted with antibodies for phospho-cPLA2α (Ser505) and 5-LO (A), and cell lysates were immunoblotted for the total and phosphorylated forms of ERK1/2, JNK and p38 (B). Immunoblots of β-actin and lamin B were used as controls for cytosol and nuclear fractions, respectively.
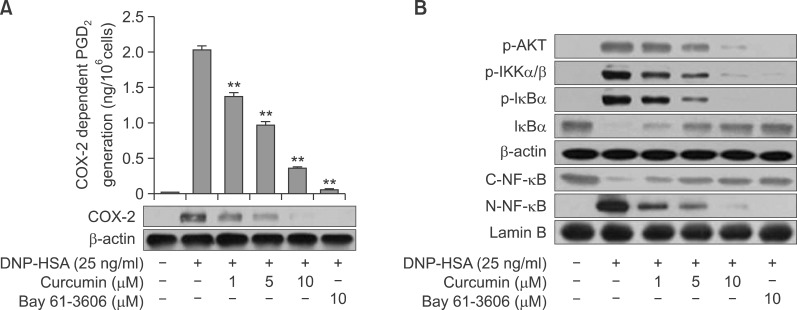
Curcumin inhibits COX-2 dependent PGD2 generation and NF-κB activation
In mast cells, unlike 5-LO dependent LTC4 generation, PGD2 generation occurs in a biphasic manner. Immediate PGD2 generation (occurring within 2 h), is associated with COX-1, and delayed PGD2 generation (during 2–10 h) is occurred by inducible COX-2 protein (Ashraf et al., 1996; Moon et al., 1998). To assess COX-2-dependent delayed PGD2 generation, BMMCs were pre-treated with aspirin to abolish preexisting COX-1 activity, followed by a brief wash, and then stimulated with Ag for 7 h with or without curcumin. As shown in Fig 4A, delayed PGD2 generation was dose-dependently inhibited by curcumin, with a concomitant suppression of COX-2 protein expression. Yu et al., reported that the Syk downstream molecules PI3K/Akt pathway affects transcription factor NF-κB activation in gastric cancer cells and mast cells (Yu et al., 2010; Lu et al., 2011) and NF-κB has been identified as an essential transcription factor for the induction of several inflammatory mediators including, TNF-α, COX-2, and inducible NO synthase (Reddy et al., 2000; Tak and Firestein, 2000; Lu et al., 2011). Thus, we examined the effect of curcumin on Akt/NF-κB axis activation. When IgE-sensitized BMMCs were pretreated with curcumin for 1 h and then stimulated with Ag for 15 min, phosphorylation of the Akt, IKK complex (p-IKKα/β) and IκBα (p-IκBα) was increased, with a concomitant decrease of total IκBα and nuclear translocation of NF-κB (C-NF-κB). As shown in Fig. 4B, both curcumin and Bay 61-3606 inhibited the phosphorylations of Akt, IKKα/β, IκBα, IκBα degradation and the translocation of cytosolic p65 to nuclear (N-NF-κB), suggesting that Syk mediated Akt/NF-κB pathway regulate the reduction of COX-2 dependent PGD2 by curcumin.
Fig. 4.
Effect of Curcumin on COX-2 dependent PGD2 generation and on Akt-NF-κB activation. IgE-sensitized BMMCs were pre-incubated with the indicated concentration of curcumin or Bay61-3606 for 1 h and then stimulated with DNP-HSA for 7 h. PGD2 released into the supernatant was quantified using an enzyme immunoassay kit and the cells were used for immunoblotting of COX-2 protein (A). IgE-sensitized BMMCs were pre-incubated with curcumin or Bay 61-3606 for 1 h and stimulated with DNP-HSA for 15 min. Then cells were taken for the immunoblot detection of Akt, IKK, IκBα cytosolic NF-κB (p65), nuclear NF-κB (p65), β-actin and lamin B. Nuclear extracts were used for the NF-κB (p65) immunoblot. Results are presented as mean ± S.D. of three independent experiments. **p<0.01 versus the IgE/Ag-treated group.
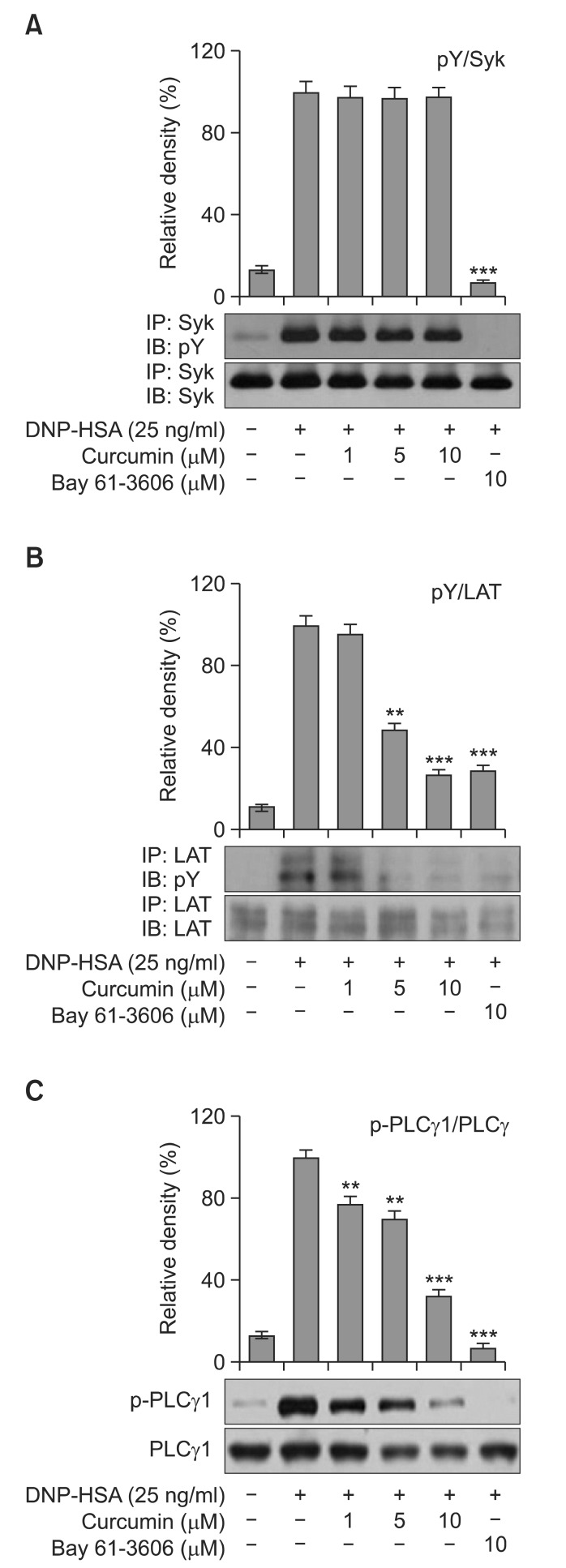
Curcumin inhibited the Syk pathway in IgE/Ag-induced BMMCs
Previously, we and others reported that Syk plays an essential role in the initiation of FcεRI-induced mast cells activation and mediates LAT, resulting in the activation of downstream signaling, including MAPKs, phosphatidylinositide 3-kinase (PI3K), and PLCγ (Lin et al., 1993; Lu et al., 2011). Furthermore, it has been reported that curcumin inhibited the releases of TNF-α and IL-4 from mast cells via a Syk dependent pathway, and that the inhibitions of these secretions by curcumin is dependent on its direct inhibition of Syk kinase activity rather than Syk phosphorylation (Lee et al., 2008). To determine whether curcumin affects Syk phosphorylation in IgE/Ag-induced BMMCs, we examined the effects of curcumin on the phosphorylation of Syk and its downstream signal molecules LAT and PLCγ1. As shown in Fig. 5A, curcumin did not affect Syk phosphorylation, but significantly and dose-dependently inhibited the phosphorylations of LAT and PLCγ1 (Fig. 5B, C), as previously reported (Lee et al., 2008). Bay 61-3606 (the Syk inhibitor used as a positive control) completely inhibited the phosphorylations of Syk, LAT and PLCγ1. These results suggest that curcumin inhibits PGD2, LTC4, and degranulation by regulating the Syk signal pathway.
Fig. 5.
Effect of curcumin on the Syk pathway. IgE-sensitized BMMCs were preincubated with curcumin or Bay 61-3606 for 1 h, and then stimulated with DNP-HSA for 5 min. Cell lysates were subjected to immunoprecipitation and immunoblot analysis for the phosphorylated forms of Syk, LAT and PLCγ1. Bay 61-3606 was used as a positive control with respect to the suppression of the Syk-mediated pathway. The relative ratios of p-Syk/Syk, p-LAT/LAT and p-PLCγ1/PLCγ1 protein levels were determined by measuring immunoblot band intensities by scanning densitometry (**p<0.01 and ***p<0.001). The results shown are representative of three independent experiments.
DISCUSSION
Curcumin has been shown to have diverse biological activities, such as, antioxidant, anti-allergic, anti-inflammatory, antiviral, antibacterial, antifungal, and anticancer activities (Ammon and Wahl, 1991). Several groups have reported that curcumin shows anti-allergic activity through the inhibition of histamine release, TNF-α and IL-4 from activated mast cells and in vivo type I hypersensitivity animal model (Yano et al., 2000; Ram et al., 2003; Lee et al., 2008). In addition, curcumin also suppresses arachidonic acid metabolizing enzymes such as cPLA2 phsophorylation, COX-2 expression and recombinant 5-LO activity in LPS-stimulated RAW 264.7 cells and A23187-stimulated HT human colon cancer cells (Hong et al., 2004) and inhibits in vitro LOX and COX activities in TPA-and arachidonic acid-induced inflammation in mouse epidermis (Huang et al., 1991). Furthermore, Curcumin modulates the inflammatory response by down-regulating the activity of COX-2 and inducible nitric oxide synthase (iNOS) enzymes through suppression of NF-κB activation (Surh et al., 2001). Even though they reported that curcumin inhibited generation of COX-dependent PGs and LOX-dependent hydroxyeicosatetraenoic acids (HETEs), previous reports were mainly examined as a part of anticancer activity of curcumin. However, the effect of curcumin on generation of COX-2 dependent PGD2 and 5-LO dependent LTC4 generation in IgE/Ag-induced mast cells and IgE-mediated PSA reaction have not been studied to date. Therefore, we investigated the effects of curcumin on the generation of eicosanoid (PGD2 and LTC4) in BMMCs and on PSA reaction in mice. It has been well established that lipid mediators, like LTC4 and PGD2, are closely associated with various allergic and inflammatory diseases (Werz and Steinhilber, 2006; Ricciotti and FitzGerald, 2011). Thus, the inhibition of LTC4 and PGD2 generation by mast cells is an important therapeutic strategy in the context of allergic-inflammatory disease. The aim of this study was to investigate the effect of curcumin on IgE/Ag-induced COX-dependent PGD2 and 5-LO dependent LTC4 generation in BMMCs and on IgE-induced passive systemic anaphylaxis (PSA) in mice. IgE binds to FcεRI on mast cells, the Syk-LAT axis activates PLCγ, which increases intracellular Ca2+ influx and leads to degranulation and eicosanoid production (Siraganian, 2003). As described above, IgE binds to FcεRI on BMMCs, it promptly elicits 5-LO dependent LTC4 generation, which is inhibited dose-dependently by curcumin (Fig. 2A). It has been reported that the synthesis of LTC4 in mast cells is regulated by two steps, namely, AA release from phospholipid by cPLA2α, MAPKs-mediated phosphorylation of cPLA2α and the conversion of free AA to LTC4 by 5-LO (Fischer et al., 2005). Translocation of both 5-LO and phospho-cPLA2α (C-p-cPLA2α) to the nuclear envelope are depend on the increase of cytosolic Ca2+ level (Werz, 2002; Lu et al., 2011; Lu et al., 2012). The present study showed that curcumin inhibited the translocations of both enzymes, which concurred with its observed inhibitory effect on intracellular Ca2+ influx (Fig. 2B). Next, to elucidate the effect of curcumin on COX-2 dependent delayed PGD2 generation, BMMCs were pre-treated with aspirin to abolish preexisting COX-1 activity, and then stimulated with Ag for 7 h with or without curcumin. We found that curcumin also suppressed COX-2 expression and attendant PGD2 generation (Fig. 4A). It has been previously reported that curcumin inhibited the TPA-induced up-regulation of COX-2 and MMP-9 by suppressing ERK1/2 phosphorylation and NF-κB transactivation in epithelial cells (Lee et al., 2005), and that it exerts anti-inflammatory effects by inhibiting the NF-κB and MAPKs pathways (Cho et al., 2007). Recently, we also reported that COX-2-dependent PGD2 generation and COX-2 expression in BMMCs occurs via the activation of the NF-κB and MAPKs pathways (Lu et al., 2011; Lu et al., 2012), thus we investigated the effect of curcumin on the MAPKs and NF-κB pathways in IgE/Ag-induced BMMCs. As shown in Fig. 3B, curcumin suppressed the phosphorylations of ERK1/2, JNK, and p38 MAP kinase in a dose dependent manner, which implied that it affects the phosphorylation and translocation of cPLA2. Since curcumin or Bay 61-3606 suppressed intracellular Ca2+ influx (Fig. 2B), MAPKs phosphorylation (Fig. 3B) and Akt/ NF-κB axis activation, these results suggest that Syk plays an important role in the generation of PGD2 and LTC4 in IgE/Ag-induced BMMCs. Therefore, we examined whether curcumin affects the phosphorylation of Syk in IgE/Ag-induced BMMCs. In agreement with a previous report (Lee et al., 2008), curcumin was found not to directly inhibit the phosphorylation of Syk, but to inhibit the phosphorylations of LAT and PLCγ1 which lie downstream of Syk. In view of the effect of phosphorylated PLCγ1 on inositol phospholipid turnover and the consequent increase in Ca2+ influx, it is likely that the observed inhibition of Ca2+ influx by curcumin (Fig. 2B) depends on its inhibitory effect on Syk-dependent PLCγ1 phosphorylation. In addition, to our in vitro results, curcumin also suppressed the IgE-dependent PSA reaction in a mast cell-dependent in vivo model of systemic allergic reaction (Wershil et al., 1987) with a potency equivalent to that of the H1 histamine antagonist, Fexo (Fig. 1A–C). We already reported that PGD2 and LTC4 play important roles in mast cell-mediated anaphylactic reaction (Lu et al., 2011; Hwang et al., 2013). Recently, several reports have demonstrated that Syk kinase inhibitors promised for the treatment of allergic and antibody-mediated autoimmune diseases (Ruzza et al., 2009) and clinical implications of Syk inhibitor showed antiallergic properties when administered orally (Mazuc et al., 2008).Taken together with previous results (Lee et al., 2008), the anti-allergic inflammatory activity of curcumin appear to be due to the suppressions of the secretions of TNF-α and IL-4 and histamine release and eicosanoid generation through the inhibition of Syk kinase pathway in IgE/Ag-induced mast cells.
Acknowledgments
This work was supported by Yeungnam University 2013 grant.
REFERENCES
- Ammon HP, Wahl MA. Pharmacology of Curcuma longa. Planta Med. 1991;57:1–7. doi: 10.1055/s-2006-960004. [DOI] [PubMed] [Google Scholar]
- Ashraf M, Murakami M, Kudo I. Cross-linking of the high-affinity IgE receptor induces the expression of cyclo-oxygenase 2 and attendant prostaglandin generation requiring interleukin 10 and interleukin 1 beta in mouse cultured mast cells. Biochem J. 1996;320:965–973. doi: 10.1042/bj3200965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avis I, Hong SH, Martinez A, Moody T, Choi YH, Trepel J, Das R, Jett M, Mulshine JL. Five-lipoxygenase inhibitors can mediate apoptosis in human breast cancer cell lines through complex eicosanoid interactions. FASEB J. 2001;15:2007–2009. doi: 10.1096/fj.00-0866fje. [DOI] [PubMed] [Google Scholar]
- Cho JW, Lee KS, Kim CW. Curcumin attenuates the expression of IL-1β, IL-6, and TNF-α as well as cyclin E in TNF-α-treated HaCaT cells; NF-κB and MAPKs as potential upstream targets. Int J Mol Med. 2007;19:469–474. [PubMed] [Google Scholar]
- Choi YH, Yan GH, Chai OH, Song CH. Inhibitory effects of curcumin on passive cutaneous anaphylactoid response and compound 48/80-induced mast cell activation. Anat Cell Biol. 2010;43:36–43. doi: 10.5115/acb.2010.43.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun KS, Keum YS, Han SS, Song YS, Kim SH, Surh YJ. Curcumin inhibits phorbol ester-induced expression of cyclooxygenase-2 in mouse skin through suppression of extracellular signal regulated kinase activity and NF-κB activation. Carcinogenesis. 2003;24:1515–1524. doi: 10.1093/carcin/bgg107. [DOI] [PubMed] [Google Scholar]
- Ciprandi G, Tosca MA, Cosentino C, Riccio AM, Passalacqua G, Canonica GW. Effects of fexofenadine and other antihistamines on components of the allergic response: adhesion molecules. J Allergy Clin Immunol. 2003;112:S78–82. doi: 10.1016/s0091-6749(03)01880-3. [DOI] [PubMed] [Google Scholar]
- Fischer L, Poeckel D, Buerker tE, Steinhilber D, A D Werz O. Inhibitors of actin polymerization stimulate arachidonic acid release and 5-lipoxygenase activation by upregulation of Ca2+ mobilisation in polymorphonuclear leukocytes involving Src family kinases. Biochim. Biophys. Acta. 2005;1736:109–119. doi: 10.1016/j.bbalip.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Gijon MA, Leslie CC. Regulation of arachidonic acid release and cytosolic phospholipase A2 activation. J Leukoc Biol. 1999;65:330–336. doi: 10.1002/jlb.65.3.330. [DOI] [PubMed] [Google Scholar]
- Hong J, Bose M, Ju J, Ryu JH, Chen X, Sang S, Lee MJ, Yang CS. Modulation of arachidonic acid metabolism by curcumin and related β-diketone derivatives: effects on cytosolic phospholipase A2, cyclooxygenases and 5-lipoxygenase. Carcinogenesis. 2004;25:1671–1679. doi: 10.1093/carcin/bgh165. [DOI] [PubMed] [Google Scholar]
- Huang MT, Lysz T, Ferraro T, Abidi TF, Laskin JD, Conney AH. Inhibitory effects of curcumin on in vitro lipoxygenase and cyclooxygenase activities in mouse epidermis. Cancer Res. 1991;51:813–819. [PubMed] [Google Scholar]
- Hwang SL, Li X, Lu Y, Jin Y, Jeong YT, Kim YD, Lee IK, Taketomi Y, Sato H, Cho YS, Murakami M, Chang HW. AMP-activated protein kinase negatively regulates FcεRI-mediated mast cell signaling and anaphylaxis in mice. J Allergy Clin Immunol. 2013;132:729–736. doi: 10.1016/j.jaci.2013.02.018. [DOI] [PubMed] [Google Scholar]
- Kemp SF, Lockey RF. Anaphylaxis: a review of causes and mechanisms. J Allergy Clin Immunol. 2002;110:341–348. doi: 10.1067/mai.2002.126811. [DOI] [PubMed] [Google Scholar]
- Kiuchi F, Goto Y, Sugimoto N, Akao N, Kondo K, Tsuda Y. Nematocidal activity of turmeric: synergistic action of curcuminoids. Chem Pharm Bull. 1993;41:1640–1643. doi: 10.1248/cpb.41.1640. [DOI] [PubMed] [Google Scholar]
- Kudo I, Murakami M. Diverse functional coupling of prostanoid biosynthetic enzymes in various cell types. Adv Exp Med Biol. 1999;469:29–35. doi: 10.1007/978-1-4615-4793-8_5. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kim JW, Ko NY, Mun SH, Her E, Kim BK, Han JW, Lee HY, Beaven MA, Kim YM, Choi WS. Curcumin, a constituent of curry, suppresses IgE-mediated allergic response and mast cell activation at the level of Syk. J Allergy Clin Immunol. 2008;121:1225–1231. doi: 10.1016/j.jaci.2007.12.1160. [DOI] [PubMed] [Google Scholar]
- Lee KW, Kim JH, Lee HJ, Surh YJ. Curcumin inhibits phorbol ester-induced up-regulation of cyclooxygenase-2 and matrix metalloproteinase-9 by blocking ERK1/2 phosphorylation and NF-kappaB transcriptional activity in MCF10A human breast epithelial cells. Antioxid Redox Signal. 2005;7:1612–1620. doi: 10.1089/ars.2005.7.1612. [DOI] [PubMed] [Google Scholar]
- Lin LL, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell. 1993;72:269–278. doi: 10.1016/0092-8674(93)90666-e. [DOI] [PubMed] [Google Scholar]
- Lu Y, Suh SJ, Li X, Hwang SL, Li Y, Hwangbo K, Park SJ, Murakami M, Lee SH, Jahng Y, Son JK, Kim CH, Chang HW. Citreorosein, a naturally occurring anthraquinone derivative isolated from Polygoni cuspidati radix, attenuates cyclooxygenase-2-dependent prostaglandin D2 generation by blocking Akt and JNK pathways in mouse bone marrow-derived mast cells. Food Chem Toxicol. 2012;50:913–919. doi: 10.1016/j.fct.2011.11.046. [DOI] [PubMed] [Google Scholar]
- Lu Y, Yang JH, Li X, Hwangbo K, Hwang SL, Taketomi Y, Murakami M, Chang YC, Kim CH, Son JK, Chang HW. Emodin, a naturally occurring anthraquinone derivative, suppresses IgE-mediated anaphylactic reaction and mast cell activation. Biochem Pharmacol. 2011;82:1700–1708. doi: 10.1016/j.bcp.2011.08.022. [DOI] [PubMed] [Google Scholar]
- Mazuc E, Villoutreix BO, Malbec O, Roumier T, Fleury S, Leonetti JP, Dombrowicz D, Daëron M, Martineau P, Dariavach P. A novel druglike spleen tyrosine kinase binder prevents anaphylactic shock when administered orally. J Allergy Clin Immunol. 2008;122:188–194. doi: 10.1016/j.jaci.2008.04.026. [DOI] [PubMed] [Google Scholar]
- Mehrabian M, Allayee H. 5-lipoxygenase and atherosclerosis. Curr Opin Lipidol. 2003;14:447–457. doi: 10.1097/00041433-200310000-00005. [DOI] [PubMed] [Google Scholar]
- Moon TC, Murakami M, Ashraf MD, Kudo I, Chang HW. Regulation of cyclooxygenase-2 and endogenous cytokine expression by bacterial lipopolysaccharide that acts in synergy with c-kit ligand and Fc epsilon receptor I crosslinking in cultured mast cells. Cell Immunol. 1998;185:146–152. doi: 10.1006/cimm.1998.1284. [DOI] [PubMed] [Google Scholar]
- Murphy RC, Gijon MA. Biosynthesis and metabolism of leukotrienes. Biochem J. 2007;405:379–395. doi: 10.1042/BJ20070289. [DOI] [PubMed] [Google Scholar]
- Ram A, Das M, Ghosh B. Curcumin attenuates allergen-induced airway hyperresponsiveness in sensitized guinea pigs. Biol Pharm Bull. 2003;26:1021–1024. doi: 10.1248/bpb.26.1021. [DOI] [PubMed] [Google Scholar]
- Reddy ST, Wadleigh DJ, Herschman HR. Transcriptional regulation of the cyclooxygenase-2 gene in activated mast cells. J Biol Chem. 2000;275:3107–3113. doi: 10.1074/jbc.275.5.3107. [DOI] [PubMed] [Google Scholar]
- Ricciotti E, FitzGerald GA. Prostaglandins and Inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzza P, Biondi B, Calderan A. Therapeutic prospect of Syk inhibitors. Expert Opin Ther Pat. 2009;19:1361–1376. doi: 10.1517/13543770903207039. [DOI] [PubMed] [Google Scholar]
- Simons FE. Anaphylaxis. J Allergy Clin Immunol. 2008;121:S402–407. doi: 10.1016/j.jaci.2007.08.061. [DOI] [PubMed] [Google Scholar]
- Siraganian RP. Mast cell signal transduction from the high-affinity IgE receptor. Curr Opin Immunol. 2003;15:639–646. doi: 10.1016/j.coi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflamatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat. Res. 2001;480–481:243–268. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Nakamura T, Iyoki S, Fujiwara A, Watanabe Y, Mohri K, Isobe K, Ono K, Yano S. Elucidation of anti-allergic activities of curcumin-related compounds with a special reference to their anti-oxidative activities. Biol Pharm Bull. 2005;28:1438–1443. doi: 10.1248/bpb.28.1438. [DOI] [PubMed] [Google Scholar]
- Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. J Clin Invest. 2000;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wershil BK, Mekori YA, Murakami T, Galli SJ. 125I-fibrin deposition in IgE-dependent immediate hypersensitivity reactions in mouse skin. Demonstration of the role of mast cells using genetically mast cell-deficient mice locally reconstituted with cultured mast cells. J Immunol. 1987;139:2605–2614. [PubMed] [Google Scholar]
- Werz O. 5-lipoxygenase: cellular biology and molecular pharmacology. Curr. Drug Targets Inflamm. Allergy. 2002;1:23–44. doi: 10.2174/1568010023344959. [DOI] [PubMed] [Google Scholar]
- Werz O, Steinhilber D. Therapeutic options for 5-lipoxygenase inhibitors. Pharmacol Ther. 2006;112:701–718. doi: 10.1016/j.pharmthera.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Yano S, Terai M, Shimizu KL, Futagami Y, Sekine T, Takamoto K, Saito K, Ueno K, Watanabe K. Antiallergic activity of Curcuma longa. (II). Features of inhibitory actions on histamine release from mast cells. Nat Med. 2000;54:325–329. [Google Scholar]
- Yamaguchi M, Sayama K, Yano K, Lantz CS, Noben-Trauth N, Ra C, Costa JJ, Galli SJ. IgE enhances Fc epsilon receptor I expression and IgE-dependent release of histamine and lipid mediators from human umbilical cord blood-derived mast cells: synergistic effect of IL-4 and IgE on human mast cell Fc epsilon receptor I expression and mediator release. J Immunol. 1999;162:5455–5465. [PubMed] [Google Scholar]
- Yu LL, Dai N, Yu HG, Sun LM, Si JM. Akt associates with nuclear factor kappaB and plays an important role in chemoresistance of gastric cancer cells. Oncol Rep. 2010;24:113–119. doi: 10.3892/or_00000835. [DOI] [PubMed] [Google Scholar]