Abstract
α-Asarone exhibits a number of pharmacological actions including neuroprotective, anti-oxidative, anticonvulsive, and cognitive enhancing action. The present study investigated the effects of α-asarone on pro-inflammatory cytokines mRNA, microglial activation, and neuronal damage in the hippocampus and on learning and memory deficits in systemic lipopolysaccharide (LPS)-treated C57BL/6 mice. Varying doses of α-asarone was orally administered (7.5, 15, or 30 mg/kg) once a day for 3 days before the LPS (3 mg/kg) injection. α-Asarone significantly reduced TNF-α and IL-1β mRNA at 4 and 24 hours after the LPS injection at dose of 30 mg/kg. At 24 hours after the LPS injection, the loss of CA1 neurons, the increase of TUNEL-labeled cells, and the up-regulation of BACE1 expression in the hippocampus were attenuated by 30 mg/kg of α-asarone treatment. α-Asarone significantly reduced Iba1 protein expression in the hippocampal tissue at a dose of 30 mg/kg. α-Asarone did not reduce the number of Iba1-expressing microglia on immunohistochemistry but the average cell size and percentage areas of Iba1-expressing microglia in the hippocampus were significantly decreased by 30 mg/kg of α-asarone treatment. In the Morris water maze test, α-asarone significantly prolonged the swimming time spent in the target and peri-target zones. α-Asarone also significantly increased the number of target heading and memory score in the Morris water maze. The results suggest that inhibition of pro-inflammatory cytokines and microglial activation in the hippocampus by α-asarone may be one of the mechanisms for the α-asarone-mediated ameliorating effect on memory deficits.
Keywords: α-Asarone, Memory deficit, Microglial activation, TNF-α, IL-1β, Neuroinflammation
INTRODUCTION
α-Asarone is a principal oil component in Acori graminei rhizoma which is one of the best-known herbal medicines frequently used for central nervous system disorders and cognitive dysfunction (Zhang et al., 2007). α-Asarone is shown to exhibit a number of pharmacological actions including sedative, neuroprotective, anti-oxidative, and anticonvulsive action (Cho et al., 2002; Manikandan and Devi, 2005; Limon et al., 2009; Pages et al., 2010; Chen et al., 2013; Huang et al., 2013). α-Asarone exhibits neuroprotective action through the blockade of N-methyl-D-aspartate (NMDA) receptor function action (Cho et al., 2002) and by inhibiting the nitric oxide (NO) overproduction in the brain tissue (Limon et al., 2009). α-Asarone also exhibits anti-oxidative properties, reduces levels of superoxide dismutase (SOD), catalase, and glutathione peroxidase in the hippocampus against noise-stress (Manikandan and Devi, 2005) and in various seizure models (Pages et al., 2010). Recently, the anticonvulsant activity of α-asarone has been reported using epileptic seizure animal models (Chen et al., 2013; Huang et al., 2013). In addition, α-asarone displays antidepressant-like and anxiolytic-like effects in in vivo behavior studies (Liu et al., 2012b; Han et al., 2013). These previous studies demonstrated that α-asarone can affect brain functions and behaviors.
There is also increasing evidence to support that α-asarone has cognitive enhancing effects. α-Asarone improved spatial memory impairment and protected the hippocampal neurons from damage in β-amyloid (Aβ)-treated rats through the inhibition of NO overproduction (Limon et al., 2009). α-Asarone attenuated the increased activity of acetylcholinesterase (AChE), and normalized the malondialdehyde and SOD levels in the hippocampus of scopolamine-induced amnesic mice (Kumar et al., 2012). However, the mechanisms involved in the ameliorating effect of α-asarone on cognitive impairment still remains uncertain.
Neuroinflammation, which is characterized by the activation of microglia and expression of major inflammatory mediators in the brain tissue, contributes to progression of neurodegenerative diseases including Alzheimer’s disease (AD) and Parkinson’s disease (PD) (Harry and Kraft, 2008). Microglia are key cellular elements in acute neuroinflammatory response and the primary source of pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α) and interleukin (IL)-1β, detected in the brain (Graeber and Streit, 2010). Pro-inflammatory mediators disrupt hippocampal neuronal functions, including long-term potentiation (LTP) and working memory consolidation (Thomson and Sutherland, 2005; Liu et al., 2012c). Neuroinflammation causes cognitive impairment, even if it is acutely stimulated by immunostimulatory component such as lipopolysaccharide (LPS) (Shaw et al., 2001). To better understand the ameliorating effects of α-asarone on cognitive deficits, the present study investigated its effects on pro-inflammatory cytokine expression, microglial activation, and neuronal damage in the hippocampus and on learning and memory deficits induced by systemic LPS treatment in mice.
MATERIALS AND METHODS
Animals
Male C57BL/6 mice (25–28 g, Nara Biotechnology, Korea) were used for this study. All animal protocols were approved by the Ethics Committee for the Care and Use of Laboratory Animals at Kyung Hee University. The animals were housed in plastic cages at constant temperature (22 ± 2°C) and humidity (55 ± 10%) with 12 h–12 h light-dark conditions. The animals were allowed free access to food and water before the experiment.
Materials
α-Asarone (trans-1-Propenyl-2,4,5-trimethoxybenzene, Fig. 1) and lipopolysaccharide (from Escherichia coli O55:B5) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Mouse anti-TNF-α antibody, goat anti-rabbit and goat anti-mouse IgG horseradish peroxidase (HRP) conjugated secondary antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse anti-amyloid precursor protein (APP) antibody was purchased from Millipore (Billerica, MA, USA). Rabbit anti-IL-1β and rabbit anti-β-site APP-converting enzyme 1 (BACE1) antibodies were purchased from Abcam (Cambridge, UK). Rabbit anti-ionized calcium binding adaptor molecule 1 (Iba1) antibodies (#016-20001, #019-19741) were purchased from Wako Pure Chemical Industries (Osaka, Japan). Mouse anti-actin antibody was purchased from Chemicon International (Temecula, CA, USA). The other chemicals and reagents used were of high quality and obtained from various commercial sources.
Fig. 1.
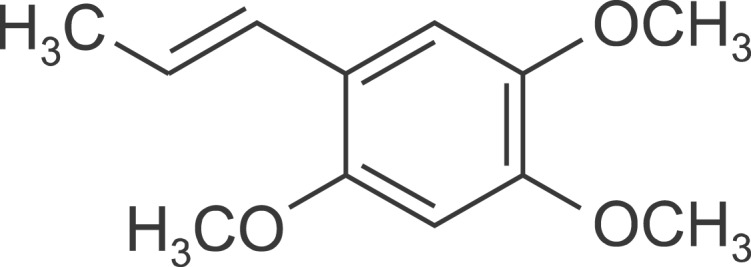
Chemical structure of α-asarone.
Experimental groups
For the quantitative real-time polymerase chain reaction (PCR) study, mice were randomly divided into five groups. The normal group (Normal) was allowed free access to food and water without any treatment. The control group (LPS) was intraperitoneally (i.p.) injected with a single dose of LPS (3 mg/kg) and received vehicle (normal saline) orally. The α-asarone treatment groups [LPS+ASR(7.5), LPS+ASR(15), LPS+ASR(30)] were administered ASR (7.5, 15, or 30 mg/kg, dissolved in normal saline, orally) respectively, once a day for 3 days before the LPS injection. For the Morris water maze study, mice were randomly divided into three groups. The normal group (Normal) was allowed free access to food and water without any treatment. The control group (LPS) was i.p. injected with a single dose of LPS (3 mg/kg) on the 3rd day of the experiment. The α-asarone treatment group (LPS+ASR) was administered 30 mg/kg of α-asarone, a reliable dose from the PCR study, once a day for 3 days prior to the LPS injection and administered one additional dose 2 hours before the retention test on the 4th day of the experiment. A total of 84 mice, 48 mice for the PCR study and 36 mice for the Morris water maze study were used.
Real-time PCR measurement
Pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) in the hippocampal tissue were measured by the real-time PCR method at 4 and 24 hours after the LPS injection. The mice were sacrificed by decapitation and the hippocampus was rapidly dissected from the brain on ice. Total RNA was extracted from the samples with Trizol (Qiagen, Germany) according to the manufacturer’s protocol. One microgram of total RNA was transcribed into DNA using iScript cDNA synthesis Kit (Bio-Rad, USA). After reverse transcription, quantitative real-time PCR was performed using preoptimized primer/probe mixture with iQ SYBR Green Supermix kit (Bio-Rad, USA) and the CFX 96 RT-PCR Detection System (Bio-Rad, USA). Primer sequences for the analyzed genes were as follows: (1) TNF-α; forward, 5′-TGA GAA GTT CCC AAA TGG C-3′; reverse, 5′-GCT ACA GGC TTG TCA CTC-3′; (2) IL-1β; forward, 5′-TGA GCA CCT TCT TTT CCT TCA-3′; reverse, 5′-TTG TCT AAT GGG AAC GTC ACA C-3′; (3) IL-6; forward, 5′-AGA CTT CAC AGA GGA TAC CA-3′; reverse, 5′-GCA TCA TCG TTG TTC ATA CA-3′; (4) β-actin; forward, 5′-TTT CCA GCC TTC CTT GGG TAT G-3′; reverse, 5′-CAC TGT GTT GGC ATA GAG GTC TTT AC-3′. The relative difference in expression between samples is represented by cycle time values normalized to the measurement of the housekeeping gene β-actin as a reference. The sample values represent x-fold differences from a normal sample (given as a designated value of 1) within the same experiment.
Morris water maze test
The Morris water maze test was performed for 4 days. The acquisition training was performed for 3 days prior to the LPS injection and the retention test on the 4th day. The apparatus consisted of a circular water pool 100 cm in diameter and 40 cm in height. It was filled with 23 ± 1°C water with a depth of 28 cm and covered a black platform (10 cm in diameter). The platform was submerged approximately 0.5 cm below the surface of water. The pool was divided into four quadrants: northeast (NE), northwest (NW), southeast (SE), and southwest (SW) at equal distances on the rim. The platform was located in the center of the southwest quadrant. During the first 3 days acquisition test, mice were given 8 trials per day to find the hidden platform. Each mouse (12 mice per group) was gently placed into the water facing the wall in the direction of north (N), east (E), south (S), and west (W) in two series of order. The mouse was allowed to swim until they reached the hidden platform (maximum swim time was 60 seconds). The escape latency to reach the platform was recorded and they remained on the platform for 20 seconds before being removed. The mouse which failed to find the platform within 60 seconds was guided to the hidden platform and then was placed on the platform for 20 seconds for reinforcement before being removed. On the 3rd day, LPS was injected into the mice 1 hour before the acquisition test.
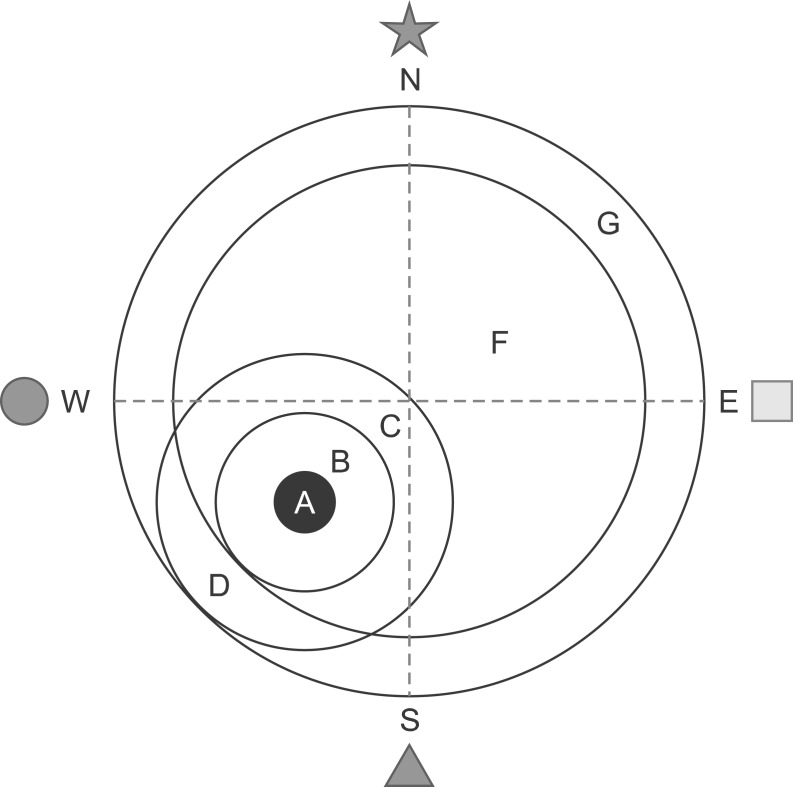
One trial of the retention test without the platform was performed on the 4th day, ∼24 hours after the LPS injection, to assess the memory of the correct platform location. The mice were placed into the pool and swam freely for 60 seconds. The swimming paths were recorded by a video camera linked to a computer-based image analyzer (SMART 2.5 video-tracking system, Panlab, Spain). The number of target heading and the swimming time in each zone was analyzed with a grid design of 6 zones (Fig. 2). This grid design, constructed with a computer-based image analyzer, was superimposed over the maze and viewed on a monitor. Memory scores were calculated using the formula (time in zone A×10)+(time in zone B ×8)+(time in zone C×6)+(time in zone D×3)+(time in zone F ×2)+(time in zone G×1)=memory score. The grid design and the formula for calculating the memory score were based on and modified from a previous behavior study (Smith et al., 1993). The mice were sacrificed after the retention test trial and the brains were randomly used either for western blotting (6 mice) or immunohistochemistry (6 mice).
Fig. 2.
Computerized grid design which used in the retention test. Discrete zones are labeled with letters, zone A representing the platform site. Memory scores derived from the formula (time in zone A×10)+(time in zone B×8)+(time in zone C×6)+(time in zone D×3)+(time in zone F×2)+(time in zone G×1)=memory score.
Western blotting
At 24 hours after the LPS injection, just after the retention test trial on the 4th day, the mice were sacrificed by decapitation and the hippocampal tissue was rapidly dissected. The hippocampal tissue was homogenized and sonicated on ice in lysis buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1 mM EDTA, 1% protease inhibitor cocktail; Sigma). After centrifugation, the supernatant was collected and assayed for protein concentration using the Bradford method. Lysate samples containing 50 μg of protein were fractionated by SDS-10% polyacrylamide gel electrophoresis, and then subjected to Western blot analysis. The primary antibodies used in this study were mouse anti-APP antibody (MAB34, Millipore, USA), rabbit anti-BACE1 antibody (ab2077, Abcam, UK), rabbit anti-Iba1 antibody (#016-20001, Wako, Japan), and mouse anti-β-actin antibody (Chemicon, USA).
Immunohistochemistry
After the retention test trial, the mice were deeply anesthetized and perfused transcardially with 0.05 M phosphate-buffered saline (PBS) containing 4% paraformaldehyde. The brain was removed and was postfixed in the same perfusing solution overnight at 4°C. Thirty μm thick coronal sections of brain tissue were made using a freezing microtome (Leica, 2800N, Germany). The brain sections were stained by the free-floating DAB reaction. The sections were rinsed with 0.05 M PBS and incubated for 15 min in 1% hydrogen peroxide PBS at room temperature. The sections were incubated overnight at 4°C with primary antibody against TNF-α (1:100, sc-1349, Santa Cruz, USA), IL-1β (1:200, ab9787, Abcam UK) and Iba1 (1:500, #019-19741, Wako, Japan), then incubated with biotinylated anti-mouse secondary antibody (1:200, Millipore, USA) for 1 hour at room temperature, after which the avidin-biotin complex (Vector Laboratories, USA) method was carried out with peroxidase coupling in a mixture containing 0.05% DAB (Sigma-Aldrich, USA) and 0.03% H2O2 for 2–5 minutes. Images of the DAB-colorized brain sections were captured using a light microscope (BX51, Olympus, Japan) equipped with CCD camera (DP70, Olympus, Japan).
Cresyl violet staining and TUNEL labeling
Neuronal damage and apoptosis in the CA1 of the hippo-campus were observed using cresyl violet staining and terminal transferase dUTP nick-end labeling (TUNEL) assay. The hippocampal tissue was came from the mice which performed the Morris water maze test. TUNEL labeling was carried out using TACS 2 TdT-DAB in situ Apoptosis Detection kit (Trevigen, #4810-30-K, Gaithersburg, MD, USA) according to the manufacturer’s protocol.
Image analysis
Relative optical densities of TNF-α and IL-1β expressions, counting of the CA1 neurons and TUNEL-labeled cells, and the Iba1 immuno-positive microglia were analyzed using the ImageJ software (Ver. 1.44p, NIH, USA). The relative optical densities were measured in the pyramidal cell layer of the CA1 by the mean gray value on an inverted black-white binary image and were normalized to the value of the normal group. The numbers of the CA1 neurons and TUNEL-labeled cells were counted in the 500 μm length of the pyramidal cell layer of the CA1. The number, the average size and the percentage area of microglia in the CA1 and dentate gyrus (DG) of the hippocampus were measured on an inverted black-white binary image by determination of threshold gray value and pixels definition using the ImageJ software. Data were normalized with the same area (105 μm2). The mean values from the four sections analyzed in each mouse were used for statistical analysis.
Statistical analysis
All data in this study are presented as means ± standard errors and evaluated using Student’s t-test. A probability value of less than 0.05 was used to indicate a significant difference. Additionally, differences between groups in the acquisition test were evaluated using one-way ANOVA.
RESULTS
α-Asarone reduces pro-inflammatory cytokines in the hippocampal tissue of LPS-treated mice
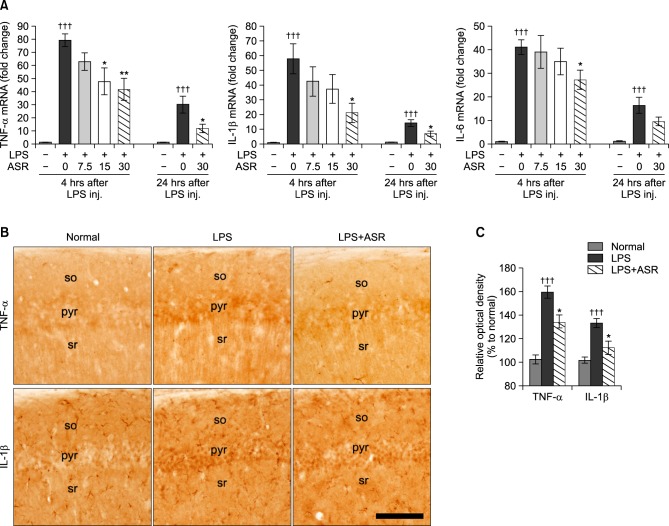
Levels of TNF-α, IL-1β, and IL-6 mRNA were measured at 4 hours after the LPS injection using quantitative real-time PCR method. Systemic LPS treatment induced robust increases of TNF-α (79.3 ± 4.9 fold), IL-1β (57.8 ± 10.2 fold), and IL-6 (41.0 ± 3.1 fold) mRNA in the hippocampal tissue compared to the normal group. α-Asarone treatment significantly reduced TNF-α mRNA at doses of 15 and 30 mg/kg (47.7 ± 10.1 fold, p<0.05; 41.6 ± 8.7 fold, p<0.01; respectively) compared to the LPS group (Fig. 3A). α-Asarone treatment reduced IL-1β and IL-6 mRNA in the hippocampal tissue only at a dose of 30 mg/ kg (21.1 ± 6.6 fold, p<0.05; 27.2 ± 4.1 fold, p<0.05; respectively) compared to the LPS group (Fig. 3A). Levels of TNF-α, IL-1beta;, and IL-6 mRNA at 24 hours after the LPS injection were still significantly higher than that of the normal group, while α-asarone significantly attenuated the up-regulation of TNF-α mRNA (11.7 ± 3.3 vs. 30.1 ± 6.4 fold, p<0.05) and IL-1β mRNA (6.9 ± 1.8 vs. 14.2 ± 2.2 fold, p<0.05) at a dose of 30 mg/kg (Fig. 3A). In addition, TNF-α and IL-1β expressions in the hippocampus of the mice which performed the Morris water maze test were attenuated by α-asarone treatment (Fig. 3B). The relative optical density of TNF-α expression in the LPS+ASR group was 134.0 ± 6.4 (p<0.05) compared to 159.6 ± 5.3 of the LPS group (Fig. 3C). The relative optical density of IL-1β expression in the LPS+ASR group was 112.4 ± 5.6 (p<0.05) compared to 133.4 ± 3.9 of the LPS group (Fig. 3C). These results indicate that α-asarone reduced the over-expression of pro-inflammatory cytokines in the hippocampus induced by systemic treatment with LPS.
Fig. 3.
Effects of α-asarone on pro-inflammatory cytokine levels in hippocampal tissue. (A) Changes of TNF-α, IL-1β, and IL-6 mRNA levels in the hippocampus at 4 and 24 hours after the LPS injection. α-Asarone significantly attenuated the up-regulation of hippocampal TNF-α and IL-1β mRNA levels at 30 mg/kg administration (†††p<0.001 compared to the normal group; *p<0.05; **p<0.01 compared to the LPS group). Data are represented by mean ± SEM (n=6 in each group). (B) Representative photographs show immunohistochemistry against TNF-α and IL-1β in the CA1 of the hippocampus (so, stratum oriens; pyr, pyramidal cell layer; sr, stratum radiatum). Scale bar is 100 μm, applicable to all sections. The immunohistochemistry against TNF-α and IL-1β was processed using the hippocampal tissue of the mice which performed the Morris water maze test. (C) Relative optical densities of TNF-α and IL-1β expressions in the CA1 of the hippocampus. α-Asarone significantly attenuated the up-regulation of TNF-α and IL-1β expressions (†††p<0.001 compared to the normal group; *p<0.05 compared to the LPS group). Data are represented by mean ± SEM (n=6 in each group).
α-Asarone attenuates neuronal damage and BACE1 expression in the hippocampus of LPS-treated mice
Systemic LPS treatment significantly reduced the CA1 neurons and significantly increased the TUNEL-labeled cells in the hippocampus compared to the normal group (Fig. 4). α-Asarone significantly attenuated the loss of CA1 neurons (110.4 ± 3.8 vs. 98.6 ± 3.1 cells/500 μm, p<0.05) and the increase of TUNEL-labeled cells (27.9 ± 3.6 vs. 40.3 ± 3.4 cells/500 μm, p<0.05) in the hippocampus induced by the LPS injection (Fig. 4). Using western blotting, APP and BACE1 protein expressions were evaluated in the hippocampal tissue of the mice performed the Morris water maze test. α-Asarone did not affect to the APP expression (112.6 ± 5.1 vs. 125.5 ± 6.3%, Fig. 5A, B), while the up-regulation of BACE1 expression was significantly attenuated by 30 mg/kg of α-asarone treatment (147.7 ± 6.1 vs. 172.4 ± 8.2%, p<0.05, Fig. 5A, C). These results suggest that α-asarone might reduce the neuronal apoptosis and Ab generation in the hippocampus induced by the LPS treatment.
Fig. 4.

Effects of α-asarone on neuronal damage and TUNEL-labeled cells in the CA1 of the hippocampus. (A) Representative photographs show CA1 neurons and TUNEL-labeled cells in the hippocampus of the mice which performed the Morris water maze test (so, stratum oriens; pyr, pyramidal cell layer; sr, stratum radiatum). Scale bar is 100 μm, applicable to all sections. (B) α-Asarone significantly attenuated the loss of CA1 neurons and the increase of TUNEL-labeled cells in the hippocampus induced by the LPS injection (†††p<0.001 compared to the normal group; *p<0.05 compared to the LPS group). Data are represented by mean ± SEM (n=6 in each group).
Fig. 5.
Effects of α-asarone on APP, BACE1 and Iba1 protein expressions in hippocampal tissue. (A) Representative western blots illustrating differences in the bands of APP, BACE1, and Iba1. APP expression was not different between the LPS and the LPS+ASR groups (B), while α-asarone significantly attenuated the up-regulation of BACE1 expression (C) and Iba1 expression (D) at 30 mg/kg administration (††p<0.01; †††p<0.001 compared to the normal group; *p<0.05 compared to the LPS group). Data are represented by mean ± SEM (n=6 in each group).
α-Asarone attenuates microglial activation in the hippo-campus of LPS-treated mice
Iba1 protein expression, a marker of microglial activation, in the hippocampal tissue was measured at 24 hours after the LPS injection using western blotting method. Systemic LPS treatment induced a ∼235% increase of Iba1 expression in the hippocampus compared to the normal group. α-Asarone treatment significantly reduced Iba1 expression at a dose of 30 mg/kg (188.0 ± 12.6% vs. 235.3 ± 13.0%, p<0.05), a reliable dose obtained from the pro-inflammatory cytokine study, compared to the LPS group (Fig. 5A, D).
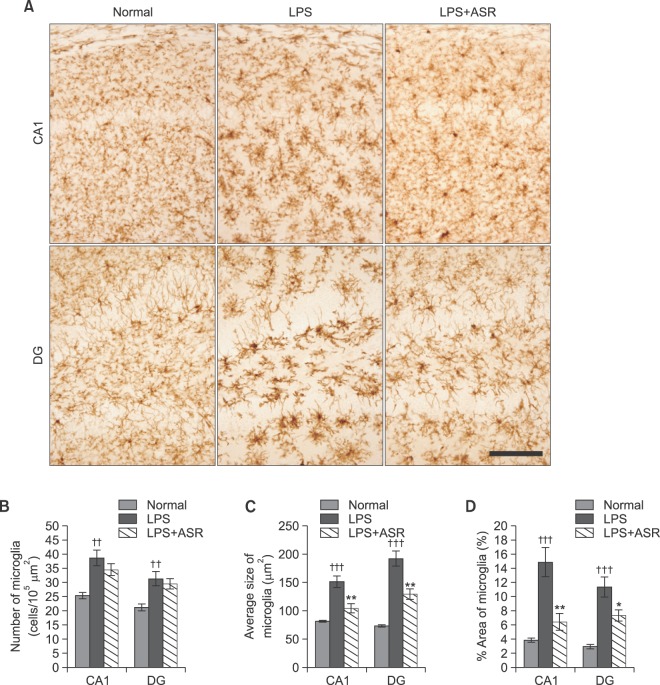
Immunohistochemistry against Iba1 showed that systemic LPS treatment activated microglia, as demonstrated by the significant increase of cell number, cell size and % area of Iba1-expressing microglia, both in the CA1 and DG region of the hippocampus compared to the normal group. Although α-asarone treatment did not attenuate the number of Iba1-expressing microglia both in the CA1 (34.5 ± 2.1 vs. 38.7 ± 2.8 cells/105 μm2) and DG region (29.5 ± 1.9 vs. 31.3 ± 2.5 cells/105 μm2) of the hippocampus compared to that of the LPS group (Fig. 6A, B), the average cell size of the Iba1-expressing microglia was significantly decreased by 30 mg/kg of α-asarone treatment both in the CA1 (103.9 ± 8.5 vs. 150.9 ± 10.3 μm2, p<0.01) and DG region (128.9 ± 9.1 vs. 191.7 ± 13.6 μm2, p<0.01) of the hippocampus compared to that of the LPS group (Fig. 6A, C). α-Asarone treatment also decreased the % area of Iba1-expressing microglia both in the CA1 (6.4 ± 1.2 vs. 14.8 ± 2.1%, p<0.01) and DG region (7.3 ± 0.8 vs. 11.3 ± 1.4%, p<0.05) of the hippocampus compared to that of the LPS group (Fig. 6A, D). The results indicate that α-asarone attenuated the microglial activation related over-expression of pro-inflammatory cytokines in the hippocampus induced by systemic LPS treatment.
Fig. 6.
Effects of α-asarone on microglial activation in hippocampal tissue. (A) Representative photographs show Iba1 immuno-stained microglia in the hippocampus. Scale bar is 100 μm, applicable to all sections. (B) α-Asarone did not attenuate numbers of Iba1-expressing microglia in the hippocampus, while α-asarone significantly reduced average sizes (C) and percentage areas (D) of Iba1-expressing microglia in the hippocampus at 30 mg/kg administration (††p<0.01; †††p<0.001 compared to the normal group; *p<0.05; **p<0.01 compared to the LPS group). Data are represented by mean ± SEM (n=6 in each group).
α-Asarone can affect spatial learning deficit of LPS-treated mice
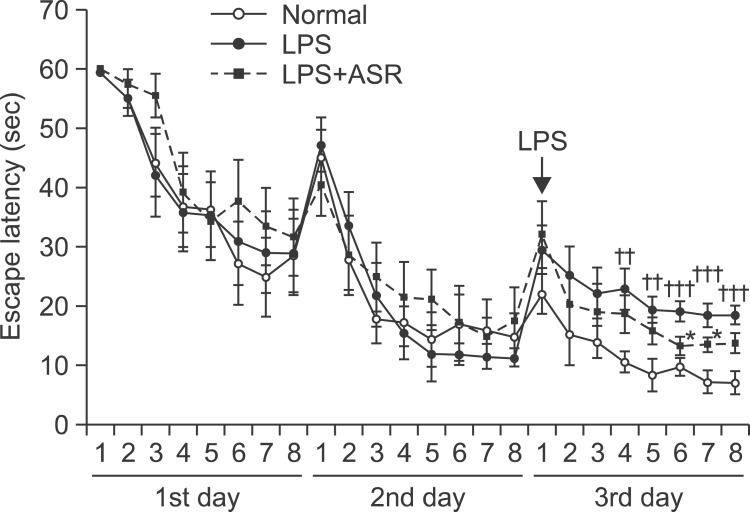
In the acquisition trials before the LPS treatment, all study groups showed relatively comparable results in the escape latency on the 1st (F2,21=0.33, p=0.723) and 2nd day (F2,21=0.13, p=0.882). After the LPS treatment on the 3rd day, the escape latency of the LPS group was significantly longer than that of the normal group (F1,14=19.81, p<0.001), while the escape latencies of the 8 trials were significantly different between the three groups (normal, LPS, and LPS+ASR) (F2,21=7.90, p<0.01). The LPS+ASR group showed significantly shorter escape latency at the 6th and 7th trials on the 3rd day (p<0.05, respectively), but the overall escape latencies for all the trials on the 3rd day were not different from that of the LPS group (F1,14=1.98, p=0.181) (Fig. 7). The results demonstrated that α-asarone treatment was not fully effective, but showed a potential effect in improving spatial learning of the LPS-treated mice.
Fig. 7.
Effect of α-asarone on the acquisition training trials. The escape latencies on the 1st and 2nd day were not different among the normal, LPS, and LPS+ASR (30 mg/kg of α-asarone administration) groups (F2,21=0.33, p=0.723; F2,21=0.13, p=0.882; respectively). After the LPS treatment on the 3rd day, the escape latency of the LPS group was significantly longer than that of the normal group (F1,14=19.81, p<0.001). The LPS+ASR group showed significantly shorter escape latency at the 6th and 7th trial on the 3rd day (††p<0.01; †††p<0.001 compared to the normal group; *p<0.05 compared to the LPS group), but the escape latency of total trials on the 3rd day was not different from that of the LPS group (F1,14=1.98, p=0.181). Data are represented by mean ± SEM (n=12 in each group).
α-Asarone ameliorates memory deficit of LPS-treated mice
In the retention trial done on the 4th day using the Morris water maze test, the swimming time spent in the various zones, number of the target heading, and memory score were used to estimate spatial memory. The LPS group spent significantly less time in zone A (the target platform site; p<0.001) and zones B and C (p<0.001, p<0.05, respectively), while they spent significantly longer time in zones D and F (p<0.05, p<0.001, respectively) compared to those of the normal group. α-Asarone treatment significantly prolonged the swimming time spent in zones A (p<0.05) and B (p<0.01) compared to that of the LPS group (Fig. 8A, B). The number of target heading in the LPS group was significantly reduced (p<0.01) compared to the normal group, while α-asarone treatment significantly increased the number of target heading (p<0.05) compared to the LPS group (Fig. 8A, C). The LPS group revealed significantly lower memory score (p<0.001) than that of the normal group, while the memory score of the LPS+ASR group was significantly higher (p<0.05) than that of the LPS group (Fig. 8D). The results indicate that pre-treatment with α-asarone was effective in ameliorating spatial memory deficit in LPS-treated mice.
Fig. 8.
Effects of α-Asarone on the retention memory test. (A) Representative swimming tracts of the normal, LPS, and LPS+ASR (30 mg/kg of α-asarone administration) groups. (B) Effects of α-asarone on the swimming time spent in discrete zones. α-Asarone significantly prolonged the swimming time spent in zone A and B at 30 mg/kg administration (†p<0.05; †††p<0.001 compared to the normal group; *p<0.05; **p<0.01 compared to the LPS group). α-Asarone significantly increased the number of target heading on platform site (C) and memory score (D) in the retention test at 30 mg/kg administration (††p<0.01; †††p<0.001 compared to the normal group; *p<0.05 compared to the LPS group). Data are represented by mean ± SEM (n=12 in each group).
DISCUSSION
Accumulating evidence indicates that Acori graminei rhizoma and its active components, α- and β-asarone have cognitive enhancing effects that can be used to remedy learning and memory impairment (Kim et al., 2009; Limon et al., 2009; Geng et al., 2010; Kumar et al., 2012). The present study showed that α-asarone improved systemic LPS-induced memory deficit. This in vivo study showed that α-asarone ameliorated the over-production of pro-inflammatory cytokines and microglial activation in the hippocampus, a region in the brain that is affected in learning and memory impairment.
LPS is a component of the outer membrane of Gram negative bacteria, and is commonly used to induce inflammation in the periphery and in the central nervous system. It stimulates the inflammatory responses in the brain through the toll-like receptor-4 mediated signaling pathway (Lien et al., 2000). Upon exposure to LPS, microglia are activated and produce pro-inflammatory mediators such as cytokines, chemokines, prostanoids, and reactive oxygen species (Block et al., 2007). Systemic or intraventricular LPS injection into rodents is popularly used as a model for studying the interaction between inflammation, brain functions, and memory deficits. In an acute systemic LPS treatment study, mRNA/protein expression of inflammatory mediators in the brain appeared within 4–8 hours and subsided in 1–3 days (Jeong et al., 2010). In our previous study, pro-inflammatory cytokines (TNF-α, IL-1β, and IL-6) mRNA in the brain tissue was robustly increased at 4 hours after a single LPS (3 mg/kg) i.p. injection (Park et al., 2012). In the present study, this dose of systemic LPS injection also induced robust increase of TNF-α, IL-1β, and IL-6 mRNA in the hippocampal tissue. While 30 mg/kg of α-asarone treatment significantly reduced the LPS induced over-expression of TNF-α and IL-1β mRNA in the hippocampal tissue at 4 and 24 hours after the LPS injection. The result of this in vivo study showed that α-asarone had a modulatory effect on pro-inflammatory cytokines using.
TNF-α and IL-1β are the most potent pro-inflammatory cytokines to induce behavioral alterations. TNF-α plays a central role in initiating and sustaining the inflammatory response. Elevated levels of TNF-α have been demonstrated in AD patients (Alvarez et al., 2007). While TNF-α protein synthesis inhibitor reversed cognitive deficits induced by chronic LPS-infusion into the ventricle of rats (Belarbi et al., 2012). IL-1β has been shown to reduce in adult hippocampal neurogenesis (Wu et al., 2013), induce synaptic loss of hippocampal neurons (Mishra et al., 2012), and to regulate long-term potentiation (LTP) and synaptic plasticity deficiency in the hippo-campus (Imamura et al., 2011). Direct central administration of IL-1β impaired hippocampal-dependent learning and memory (Hein et al., 2007). In particular, pathologically increased IL-6 in the brain resulted in impairments in memory performance (Baier et al., 2009). Moreover, systemic LPS injection induced cognitive impairment and elevated amyloidogenesis (Lee et al., 2008). A single injection of LPS impaired learning while a daily injection did not (Shaw et al., 2001).
Based on previous reports and results from the present study, the Morris water maze test was performed at 30 mg/ kg dose of α-asarone treatment against a single 3 mg/kg i.p. injection of LPS for 4 days. The escape latencies on the 1st and 2nd day in the acquisition training period before the LPS treatment were relatively similar in all groups. The LPS injection given on the 3rd day resulted in significant extension of the escape latency and the escape latencies for the 8 trials were significantly different among the groups (normal, LPS, and LPS+ASR). In the retention memory test done on the 4th day, α-asarone treatment significantly prolonged the swimming time spent in the target of platform site and the peri-target area. The number of target heading and the memory score were also significantly higher among the α-asarone treated group than those of the LPS group. The results from the Morris water maze test showed α-asarone’s ameliorating effect on spatial learning and memory deficits induced by acute systemic LPS treatment. The cognitive enhancing effects of α-asarone have been reported previously. Limon et al. (2009) performed intrahippocampal injection of Ab (25–35) to induce memory impairment and spatial memory was tested in the eight-arm maze. They showed that α-asarone decreased the Aβ induced memory impairment and Aβ-neurotoxicity; and suggested the antioxidant effects of α-asarone and the same being potentially used as a novel therapy for Aβ-induced neurotoxicity. Kumar et al. (2012) did intraperitoneal injection of scopolamine which is a muscarinic acetylcholine receptor blocker, and the animals were tested with the step-through passive avoidance test and the Y-maze test. They also suggested that the antioxidant effects of α-asarone may be beneficial in cognitive impairment induced by cholinergic system dysfunction through inhibition of AChE activity. The present study demonstrated that the anti-neuroinflammatory effect of α-asarone exerted an ameliorating effect on memory deficit through inhibition of pro-inflammatory cytokines in the hippo-campus after systemic LPS treatment using the Morris water maze.
For better understanding of α-asarone’s anti-neuroinflammatory effect in the hippocampus, observation of microglial activation in the hippocampal tissue of mice challenged with the Morris water maze was made. Microglial activation is involved in learning and memory impairment through the release of TNF-α and IL-1β and the negative impact on hippocampal LTP (Griffin et al., 2006; Liu et al., 2012a). α-Asarone treatment significantly reduced Iba1 protein expression and morphological indicators of microglial activation, such as the significantly decreased average cell size and percentage areas of Iba1-expressing microglia in the hippocampal tissue. In line with the reducing effects of α-asarone upon pro-inflammatory cytokines in the hippocampal tissue, the microglial activation attenuating effects of α-asarone supports that α-asarone exerts an anti-neuroinflammatory action against systemic LPS treatment.
Systemic LPS treatment induces neuronal damage in the CA1 of the hippocampus (Lee et al., 2008). In the present study, α-asarone reduced the neuronal damage and TUNEL-labeled cells in the CA1 of the hippocampus induced by the LPS injection. The results implies that α-asarone exerts an anti-apoptotic effect against neuronal damage. Recent studies demonstrated that β-asarone, cis type of asarone, attenuates neuronal apoptosis in Aβ- and NMDA-induced excitotoxicity (Geng et al., 2010; Li et al, 2010; Zou et al., 2011; Wei et al., 2013). The anti-apoptotic effect of α-asarone against neuronal damage is still unclear. Recently, we reported that effects of β-asarone on pro-inflammatory cytokines and learning and memory impairment in LPS-treated mice (Choi et al., 2013). Although it was not possible to compare directly, the α-asarone was found to exhibit more potent anti-amnesic effects than the β-asarone. A line of previous reports on α-and β-asarone suggests that α-asarone exhibits more potent anti-oxidative effects (Limon et al., 2009; Kumar et al., 2012) and β-saraone exhibits more potent neuroprotective effects (Geng et al., 2010; Li et al., 2012).
A single intraperitoneal injection of LPS increased Aβ generation, β-secretase activity and expression of APP and BACE (Lee et al., 2008). Aβ generation and BACE1 activity are highly associated with inflammatory mediators (Sastre et al., 2008). TNF-α and IL-1β augmented APP expression, Ab formation, and β-secretase activity (Buxbaum et al., 1992; Blasko et al., 1999; Sastre et al., 2003). In the present study, α-asarone attenuated the up-regulation of BACE1 expression in the hippo-campus induced by the LPS injection. The result is inadequate to explain the effect of α-asarone on Ab generation. However, it is clear that pro-inflammatory cytokines and microglial activation are implicated in BACE1 expression (Heneka et al., 2005; Gallagher et al., 2013). According to the results from the present study, the inhibitory effects of α-asarone on pro-inflammatory cytokines and microglial activation might also affect to the activation of BACE1 expression.
In conclusion, α-asarone effectively ameliorated the memory deficits induced by systemic LPS treatment. The effects of α-asarone were found to be mediated through the inhibition of pro-inflammatory cytokines and microglial activation in the hippocampus. α-Asarone also showed a potential capacity in anti-apoptotic and anti-amyloidogenic effects. Further studies, such as long-term behavioral test and extensive observation on Aβ accumulation, are required to clarify the exact mechanism related to the therapeutic efficacy of α-asarone. This study suggests that α-asarone may be a potential therapeutic drug in neurodegenerative diseases associated with neuroinflammation.
Acknowledgments
This work was supported by a grant (KHU-20120347) from the Kyung Hee University in 2012.
REFERENCES
- Alvarez A, Cacabelos R, Sanpedro C, Garcia-Fantini M, Aleix-andre M. Serum TNF-alpha levels are increased and correlate negatively with free IGF-I in Alzheimer disease. Neurobiol Aging. 2007;28:533–536. doi: 10.1016/j.neurobiolaging.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Baier PC, May U, Scheller J, Rose-John S, Schiffelholz T. Impaired hippocampus-dependent and -independent learning in IL-6 deficient mice. Behav Brain Res. 2009;200:192–196. doi: 10.1016/j.bbr.2009.01.013. [DOI] [PubMed] [Google Scholar]
- Belarbi K, Jopson T, Tweedie D, Arellano C, Luo W, Greig NH, Rosi S. TNF-alpha protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. J Neuroinflammation. 2012;9:23. doi: 10.1186/1742-2094-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasko I, Marx F, Steiner E, Hartmann T, Grubeck-Loebenstein B. TNFalpha plus IFNgamma induce the production of Alzheimer beta-amyloid peptides and decrease the secretion of APPs. FASEB J. 1999;13:63–68. doi: 10.1096/fasebj.13.1.63. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Oishi M, Chen HI, Pinkas-Kramarski R, Jaffe EA, Gandy SE, Greengard P. Cholinergic agonists and interleukin 1 regulate processing and secretion of the Alzheimer beta/A4 amyloid protein precursor. Proc Natl Acad Sci USA. 1992;89:10075–10078. doi: 10.1073/pnas.89.21.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen QX, Miao JK, Li C, Li XW, Wu XM, Zhang XP. Anticonvulsant activity of acute and chronic treatment with α-asarone from Acorus gramineus in seizure models. Biol Pharm Bull. 2013;36:23–30. doi: 10.1248/bpb.b12-00376. [DOI] [PubMed] [Google Scholar]
- Cho J, Kim YH, Kong JY, Yang CH, Park CG. Protection of cultured rat cortical neurons from excitotoxicity by asarone, a major essential oil component in the rhizomes of Acorus gramineus. Life Sci. 2002;71:591–599. doi: 10.1016/s0024-3205(02)01729-0. [DOI] [PubMed] [Google Scholar]
- Choi M, Kwak H, Kweon K, Hwang J, Shin J, Sohn N. Effects of β-asarone on pro-inflammatory cytokines and learning and memory impairment in lipopolysaccharide-treated mice. Kor J Herbology. 2013;28:119–127. [Google Scholar]
- Gallagher JJ, Minogue AM, Lynch MA. Impaired performance of female APP/PS1 mice in the Morris water maze is coupled with increased Abeta accumulation and microglial activation. Neurodegener Dis. 2013;11:33–41. doi: 10.1159/000337458. [DOI] [PubMed] [Google Scholar]
- Geng Y, Li C, Liu J, Xing G, Zhou L, Dong M, Li X, Niu Y. Beta-asarone improves cognitive function by suppressing neuronal apoptosis in the beta-amyloid hippocampus injection rats. Biol Pharm Bull. 2010;33:836–843. doi: 10.1248/bpb.33.836. [DOI] [PubMed] [Google Scholar]
- Graeber MB, Streit WJ. Microglia: biology and pathology. Acta Neuropathol. 2010;119:89–105. doi: 10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]
- Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch MA. The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem. 2006;99:1263–1272. doi: 10.1111/j.1471-4159.2006.04165.x. [DOI] [PubMed] [Google Scholar]
- Han P, Han T, Peng W, Wang XR. Antidepressant-like effects of essential oil and asarone, a major essential oil component from the rhizome of Acorus tatarinowii. Pharm Biol. 2013;51:589–594. doi: 10.3109/13880209.2012.751616. [DOI] [PubMed] [Google Scholar]
- Harry GJ, Kraft AD. Neuroinflammation and microglia: considerations and approaches for neurotoxicity assessment. Expert Opin Drug Metab Toxicol. 2008;4:1265–1277. doi: 10.1517/17425255.4.10.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein AM, Stutzman DL, Bland ST, Barrientos RM, Watkins LR, Rudy JW, Maier SF. Prostaglandins are necessary and sufficient to induce contextual fear learning impairments after interleukin-1 beta injections into the dorsal hippocampus. Neuroscience. 2007;150:754–763. doi: 10.1016/j.neuroscience.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Sastre M, Dumitrescu-Ozimek L, Dewachter I, Walter J, Klockgether T, Van Leuven F. Focal glial activation coincides with increased BACE1 activation and precedes amyloid plaque deposition in APP[V717I] transgenic mice. J Neuroinflammation. 2005;2:22. doi: 10.1186/1742-2094-2-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Li WG, Zhang XB, Wang L, Xu TL, Wu D, Li Y. α-asarone from Acorus gramineus alleviates epilepsy by modulating A-type GABA receptors. Neuropharmacology. 2013;65:1–11. doi: 10.1016/j.neuropharm.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Imamura Y, Wang H, Matsumoto N, Muroya T, Shimazaki J, Ogura H, Shimazu T. Interleukin-1beta causes long-term potentiation deficiency in a mouse model of septic encephalopathy. Neuroscience. 2011;187:63–69. doi: 10.1016/j.neuroscience.2011.04.063. [DOI] [PubMed] [Google Scholar]
- Jeong HK, Jou I, Joe EH. Systemic LPS administration induces brain inflammation but not dopaminergic neuronal death in the substantia nigra. Exp Mol Med. 2010;42:823–832. doi: 10.3858/emm.2010.42.12.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Hahm DH, Lee HJ, Pyun KH, Shim I. Acori graminei rhizoma ameliorated ibotenic acid-induced amnesia in rats. Evid Based Complement Alternat Med. 2009;6:457–464. doi: 10.1093/ecam/nem158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H, Kim BW, Song SY, Kim JS, Kim IS, Kwon YS, Koppula S, Choi DK. Cognitive enhancing effects of alpha asarone in amnesic mice by influencing cholinergic and antioxidant defense mechanisms. Biosci Biotechnol Biochem. 2012;76:1518–1522. doi: 10.1271/bbb.120247. [DOI] [PubMed] [Google Scholar]
- Lee JW, Lee YK, Yuk DY, Choi DY, Ban SB, Oh KW, Hong JT. Neuro-inflammation induced by lipopolysaccharide causes cognitive impairment through enhancement of beta-amyloid generation. J Neuroinflammation. 2008;5:37. doi: 10.1186/1742-2094-5-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Xing G, Dong M, Zhou L, Li J, Wang G, Zou D, Wang R, Liu J, Niu Y. Beta-asarone protection against beta-amyloid-induced neurotoxicity in PC12 cells via JNK signaling and modulation of Bcl-2 family proteins. Eur J Pharmacol. 2010;635:96–102. doi: 10.1016/j.ejphar.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Li Z, Zhao G, Qian S, Yang Z, Chen X, Chen J, Cai C, Liang X, Guo J. Cerebrovascular protection of beta-asarone in Alzheimer’s disease rats: a behavioral, cerebral blood flow, biochemical and genic study. J Ethnopharmacol. 2012;144:305–312. doi: 10.1016/j.jep.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Lien E, Means TK, Heine H, Yoshimura A, Kusumoto S, Fukase K, Fenton MJ, Oikawa M, Qureshi N, Monks B, Finberg RW, Ingalls RR, Golenbock DT. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Invest. 2000;105:497–504. doi: 10.1172/JCI8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limon ID, Mendieta L, Diaz A, Chamorro G, Espinosa B, Zenteno E, Guevara J. Neuroprotective effect of alpha-asarone on spatial memory and nitric oxide levels in rats injected with amyloid-beta (25–35) Neurosci Lett. 2009;453:98–103. doi: 10.1016/j.neulet.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Liu MC, Liu XQ, Wang W, Shen XF, Che HL, Guo YY, Zhao MG, Chen JY, Luo WJ. Involvement of microglia activation in the lead induced long-term potentiation impairment. PLoS One. 2012a;7:e43924. doi: 10.1371/journal.pone.0043924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Chen SW, Xu N, Liu XH, Zhang H, Wang YZ, Xu XD. Anxiolytic-like effect of alpha-asarone in mice. Phytother Res. 2012b;26:1476–1481. doi: 10.1002/ptr.4596. [DOI] [PubMed] [Google Scholar]
- Liu X, Wu Z, Hayashi Y, Nakanishi H. Age-dependent neuroinflammatory responses and deficits in long-term potentiation in the hippocampus during systemic inflammation. Neuroscience. 2012c;216:133–142. doi: 10.1016/j.neuroscience.2012.04.050. [DOI] [PubMed] [Google Scholar]
- Manikandan S, Devi RS. Antioxidant property of alpha-asarone against noise-stress-induced changes in different regions of rat brain. Pharmacol Res. 2005;52:467–474. doi: 10.1016/j.phrs.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Mishra A, Kim HJ, Shin AH, Thayer SA. Synapse loss induced by interleukin-1beta requires pre- and post-synaptic mechanisms. J Neuroimmune Pharmacol. 2012;7:571–578. doi: 10.1007/s11481-012-9342-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pages N, Maurois P, Delplanque B, Bac P, Stables JP, Tamariz J, Chamorro G, Vamecq J. Activities of alpha-asarone in various animal seizure models and in biochemical assays might be essentially accounted for by antioxidant properties. Neurosci Res. 2010;68:337–344. doi: 10.1016/j.neures.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Park SM, Choi MS, Sohn NW, Shin JW. Ginsenoside Rg3 attenuates microglia activation following systemic lipopolysaccharide treatment in mice. Biol Pharm Bull. 2012;35:1546–1552. doi: 10.1248/bpb.b12-00393. [DOI] [PubMed] [Google Scholar]
- Sastre M, Dewachter I, Landreth GE, Willson TM, Klockgether T, van Leuven F, Heneka MT. Nonsteroidal anti-inflammatory drugs and peroxisome proliferator-activated receptor-gamma agonists modulate immunostimulated processing of amyloid precursor protein through regulation of beta-secretase. J Neurosci. 2003;23:9796–9804. doi: 10.1523/JNEUROSCI.23-30-09796.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastre M, Walter J, Gentleman SM. Interactions between APP secretases and inflammatory mediators. J Neuroinflammation. 2008;5:25. doi: 10.1186/1742-2094-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw KN, Commins S, O’Mara SM. Lipopolysaccha-ride causes deficits in spatial learning in the watermaze but not in BDNF expression in the rat dentate gyrus. Behav Brain Res. 2001;124:47–54. doi: 10.1016/s0166-4328(01)00232-7. [DOI] [PubMed] [Google Scholar]
- Smith DH, Okiyama K, Thomas MJ, McIntosh TK. Effects of the excitatory amino acid receptor antagonists kynure-nate and indole-2-carboxylic acid on behavioral and neurochemical outcome following experimental brain injury. J Neurosci. 1993;13:5383–5392. doi: 10.1523/JNEUROSCI.13-12-05383.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson LM, Sutherland RJ. Systemic administration of lipopolysaccharide and interleukin-1beta have different effects on memory consolidation. Brain Res Bull. 2005;67:24–29. doi: 10.1016/j.brainresbull.2005.05.024. [DOI] [PubMed] [Google Scholar]
- Wei G, Chen YB, Chen DF, Lai XP, Liu DH, Deng RD, Zhou JH, Zhang SX, Li YW, Lii H, Liu LF, Wang Q, Nie H. beta-Asarone inhibits neuronal apoptosis via the CaMKII/CREB/Bcl-2 signaling pathway in an in vitro model and AbetaPP/PS1 mice. J Alzheimers Dis. 2013;33:863–880. doi: 10.3233/JAD-2012-120865. [DOI] [PubMed] [Google Scholar]
- Wu MD, Montgomery SL, Rivera-Escalera F, Olschowka JA, O’Banion MK. Sustained IL-1beta expression impairs adult hippocampal neurogenesis independent of IL-1 signaling in nestin+ neural precursor cells. Brain Behav Immun. 2013;32:9–18. doi: 10.1016/j.bbi.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Han T, Yu CH, Rahman K, Qin LP, Peng C. Ameliorating effects of essential oil from Acori graminei rhizoma on learning and memory in aged rats and mice. J Pharm Pharmacol. 2007;59:301–309. doi: 10.1211/jpp.59.2.0016. [DOI] [PubMed] [Google Scholar]
- Zou DJ, Wang G, Liu JC, Dong MX, Li XM, Zhang C, Zhou L, Wang R, Niu YC. Beta-asarone attenuates beta-amyloid-induced apoptosis through the inhibition of the activation of apoptosis signal-regulating kinase 1 in SH-SY5Y cells. Pharmazie. 2011;66:44–51. [PubMed] [Google Scholar]