Abstract
Enterovirus 71 (EV71) is the predominant cause of hand, foot and mouth disease (HFMD). The antiviral activity of hederasaponin B from Hedera helix against EV71 subgenotypes C3 and C4a was evaluated in vero cells. In the current study, the antiviral activity of hederasaponin B against EV71 C3 and C4a was determined by cytopathic effect (CPE) reduction method and western blot assay. Our results demonstrated that hederasaponin B and 30% ethanol extract of Hedera helix containing hederasaponin B showed significant antiviral activity against EV71 subgenotypes C3 and C4a by reducing the formation of a visible CPE. Hederasaponin B also inhibited the viral VP2 protein expression, suggesting the inhibition of viral capsid protein synthesis.These results suggest that hederasaponin B and Hedera helix extract containing hederasaponin B can be novel drug candidates with broad-spectrum antiviral activity against various subgenotypes of EV71.
Keywords: Enterovirus 71, Antiviral activity, Hederasaponin B, Hedera helix, Hand foot and mouth disease
INTRODUCTION
Enterovirus 71 (EV71) is a positive-stranded RNA virus that belongs to the enterovirus (EV) genus of the Picornaviridae family (McMinn, 2002). EV71 is classified in the genus enterovirus along with other polioviruses, coxsackievirus A (CVA) and coxsackievirus B (CVB), and echoviruses (E) (Poyry et al., 1996; Hyypia et al., 1997; Laine et al., 2005). At species level, EV71 together with coxsackievirus (CV) A2–A8, A10, A12, A14 and A16 are members of HEV-A. The EV71 genome encodes a long polyprotein with a single open reading frame and includes three genomic regions designated P1, P2 and P3. The P1 region encodes four structural capsid proteins (VP4, VP2, VP3, and VP1), while the P2 and P3 regions encode seven nonstructural proteins (2A, 2B, 2C, 3A, 3B, 3C, and 3D) (Chan et al., 2010; Huang et al., 2010). EV71 genotypes are genetically classified into genogroups A, B and C on the basis of the VP1 sequence analyses (Brown et al., 1999).
EV71 is a causative agent of hand, foot and mouth disease (HFMD) and herpangina; and it can also cause severe neurological diseases, such as brainstem encephalitis and poliomyelitis-like paralysis (Chumakov et al., 1979; McMinn, 2002; Wang et al., 2003). HFMD, primarily caused by EV71 and CVA16, commonly occurs in young children, particularly those less than 5 years of age (Zhang et al., 2011). CVA16-associated HFMD is milder than that caused by EV71, and has a much lower incidence of severe complications, including death (Chang et al., 1999). In recent years, numerous large outbreaks of EV71-associated HFMD with high morbidity and mortality have occurred in eastern and southeastern Asian countries and regions. The large HFMD outbreaks with fatal neurological complications that have occurred since 2007 are mainly due to the subgenotype C4a of EV71 (Zhang et al., 2011). Also, the subgenotype C3 was detected in Korea in 2000 (Cardosa et al., 2003). However, until currently, neither a vaccine nor a therapeutic treatment is available.
Hedera helix (English ivy, Common ivy) is an evergreen dioecious woody liana, one of the 15 species of the genus Hedera, Araliaceae family. The dry extract of Hedera helix is currently known to act as an anti-inflammatory (Suleyman et al., 2003; Gepdiremen et al., 2005), anti-bacterial, mucolytic and anti-spasmodic agent (Trute et al., 1997; Sieben et al., 2009). Also, Hedera helix extract has been claimed to exhibit in vitro bronchodilatatory effect on cell cultures (Trute et al., 1997; Sieben et al., 2009), and the pharmaceutical manufacturers declare the beneficial effect of ivy-based remedies in the treatment of cough symptoms during the course of acute and chronic bronchitis. However, to date, no detailed study has been carried out to assess the antiviral activity of Hedera helix against EV71.
In the current study, we reported a novel antiviral activity of 30% EtOH extract of Hedera helix against EV71 C3 and EV71 C4a, and conducted a bioassay-guided isolation and identification of an active compound from Hedera helix against EV71 C3 and EV71 C4a. The antiviral activity of hederasaponin B and the extract and fractionates of Hedera helix against EV71 C3 and EV71 C4a is promising and urgently need to be evaluated in vivo for its potential capacity as the therapeutics of HFMD, since the 30% EtOH extract of Hedera helix is a very safe medicine currently used for the treatment of bronchitis in children.
MATERIALS AND METHODS
Viruses and cell lines
EV71 C3 and EV71 C4a were obtained from the division of vaccine research in Korea Centers for Disease Control and Prevention (KCDC), and propagated in African green monkey kidney (vero) cells at 37°C. Vero cells were maintained in minimal essential medium (MEM) supplemented with 10% fetal bovine serum (FBS) and 0.01% antibiotic-antimycotic solution. Antibiotic-antimycotic solution, trypsin-EDTA, FBS, and MEM were supplied by Gibco BRL (Invitrogen Life Technologies, Karlsruhe, Germany). The tissue culture plates were purchased from Falcon (BD Biosciences, San Jose, CA, USA). Sulforhodamine B (SRB) was purchased from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals were of reagent grade.
Fractionation and isolation
For preparation of materials, 30% EtOH extract of Hedera helix was obtained from the Sampoong Corporation (Korea) in May 2011. 30% EtOH extract (500 g) was suspended in water and then partitioned with EtOAc and n-BuOH, successively. Each soluble fraction was evaporated in vacuo to yield the residues of EtOAc (23 g), and n-BuOH (150 g) extracts, respectively. n-BuOH soluble fraction (100 g) was column chromatographed on a Diaion HP-20 (500 g, 10×50 cm) using stepwise-gradient with MeOH:H2O (0:100, 20:80, 40:60, 60:40, 80:20, 100:0; each 1,000 ml) to afford 6 fractions. Each extract and fraction was tested for SRB-based cytotoxicity and antiviral activity against EV71 C3 and C4a. For further purification, the active fraction was subjected to ODS column chromatography (300g, YMC-Gel ODS-A, 150 μm, 5×50 cm) using isocratic elution with MeOH:H2O (70:30) to give a pure compound. The purified compound was identified as hederasaponin B by direct comparison with the authentic compound.
Antiviral activity assay
Assays of antiviral activity was evaluated by the SRB method using CPE reduction, recently reported (Choi et al., 2009). One day before infection, vero cells were seeded onto a 96-well culture plate at a concentration of 2×104 cells/ well. Next day, medium was removed and then washed with 1×PBS. Subsequently, 0.09 ml of the diluted virus suspension containing 50% cell culture infective dose (CCID50) of the virus stock was added to produce appropriate CPE within 48h after infection, followed by the addition of 0.01 ml of medium supplemented with FBS containing an appropriate concentration of the compounds were added. The antiviral activity of each test material was determined with a 5-fold diluted concentration ranging from 0.4 to 50 μg/ml. Four wells were used as virus controls (virus-infected non-compound-treated cells) while four wells were used as cell controls (non-infected non-compound-treated cells). The culture plates were incubated at 37°C in 5% CO2 for 2 days until appropriate CPE was achieved. Subsequently, the 96-well plates were washed once with 1× PBS, and 100 μL of cold (−20°C) 70% acetone was added on each well and left standing for 30 min at −20°C. After the removal of 70% acetone, the plates were dried in a dry oven for 30 min, followed by the addition of 100 μl of 0.4% (w/v) SRB in 1% acetic acid solution to each well, and left standing at room temperature for 30 min. SRB was then removed, and the plates were washed 5 times with 1% acetic acid before oven-drying. The plates were dried in a dry oven. After drying for 1 day, the morphology of the cells to observation the effect of compounds on EV71 C3 and EV71 C4a-induced CPE were observed under microscope at 0.4×10 magnification (Axiovert 10; Zeiss, Wetzlar, Germany), and images were recorded. Bound SRB was then solubilized with 100 μl of 10 mM unbuffered Tris-base solution, and the plates were left on a table for 30 min. The absorbance was then read at 540 nm by using a VERSAmax microplate reader (Molecular Devices, Palo Alto, CA, USA) with a reference absorbance at 620 nm. The results were then transformed into percentage of the controls, and the percent protection achieved by the test CFS in the EV71-infected cells was calculated using the following formula:{(ODt)EV71 −(ODc)EV71}÷{(ODc)mock− (ODc)EV71}×100 (expressed in %), where (ODt)EV71 is the optical density measured with a given CFS in EV71-infected cells; (ODc)EV71 is the optical density measured for the control untreated EV71-infected cells; and (ODc)mock is the optical density measured for control untreated mock-infected cells. Antiviral activity was presented as % of control. Ribavirin and DMSO were used for positive and negative control, respectively.
Cytotoxicity assay
We seeded that vero cells onto a 96-well culture plate at a concentration of 2×104 cells/well. Next day, medium was removed and then washed with phosphate buffered saline (PBS). The 96-well plates were treated to compounds in maintenance medium for 48 h at 37°C, in parallel with the virus-infected cell cultures. For each compounds, 3 wells were used as controls (non-compound-treated cells). After 48 h of incubation, cytotoxicity was evaluated by SRB assay as previously described (Lin et al., 1999). Cytotoxicity was presented as % of control.
Western blot analysis
Vero cells were plated onto 6-well culture plates at a density of 5×105 cells/well 24 h before infection with EV71 C3 and EV71 C4a. EV71 C3 and EV71 C4a infected cells were treated with hederasaponin B and ribavirin at a concentration 50 μg/ml for 48 h for detection of viral VP2 protein. Mock-infected cells treated with 0.1% DMSO and EV71 infected cells treated with 0.1% DMSO was used as controls. Cells were lysed in ice-cold RIPA lysis buffer containing 50 mM Tris-Hcl pH7.4, 150 mM NaCl, 1% Nonidet P-40, 1% sodium deoxycholate, 0.1% SDS, 1 mM EDTA, 1% SDS, 5 μg/ml aprotinin, 5 μg/ml leupeptin, 1 mM PMSF, 5 mM sodium fluoride and 5 mM sodium orthovanadate. The preparation of sample protein (30 μg) boiled for 10 min at 100°C and separated in 12% acrylamide gels run at 100 V for 1 h (for detection of VP1). The SeeBlue®Plus2 prestained protein ladder (Invitrogen) was used as a molecular weight standard. The gels were transferred to a nitrocellulose membrane using the Invitrogen iBlot®Gel Transfer Device (Invitrogen, Carlsbad, CA) at 20 V for 7 min.
For detection of VP2, membranes were blocked with 5% skim milk (Difco) dissolved in phosphate buffered saline-Tween 20 (PBST) overnight at 4°C on a shaker. The blots were washed three times with PBST before being incubated with primary mouse anti-enterovirus 71 monoclonal antibody (Millipore) dissolved in 5% skim milk at a dilution of 1:1,000. For the loading control, separate blots containing the same samples were incubated with primary α-Tubulin mouse monoclonal IgG1 (Santacruz Biotechnology) dissolved in 5% skim milk at a dilution of 1:1,000. The blots were incubated with primary antibodies at room temperature on a shaker. The blots were then washed three times with PBST for 10 min each time. This was followed by incubation with the secondary antibodies polyclonal goat anti-mouse IgG (H+L)-HRP (GenDEPOT) for 1 h at room temperature on shaker. Dilution of secondary antibody was done in 5% skim milk at a ratio of 1:5,000. Membranes were then rinsed three times with PBST for 10 min each time. Membranes were developed by the enhanced chemiluminescence (ECL) method using West-Q chemiluminescent substrate (GenDEPOT).
RESULTS
Antiviral activity of 30% EtOH extract of Hedera helix against EV71 C3 and EV71 C4a
During the screening of antiviral activity of several medical plant extracts against EV71, we found that 30% EtOH extract of Hedera helix, which was widely used in clinic for the treatment of cough symptoms, also possessed significant antiviral activity against EV71 C3 (Table 1) and C4a (Table 2). To find the active antiviral compounds in 30% EtOH extract of Hedera helix, the extract which was further fractionated into EtOAc, n-BuOH, CHCl3, and Hexane fraction, and we found that the antiviral activity was highly retained in n-BuOH fraction (data not shown). Thus, we decided to separate the n-BuOH soluble fraction using a Diaion HP-20 column and obtained 6 fractions after stepwise-gradient with MeOH:H2O. Each fraction was tested for SRB-based cytotoxicity and antiviral activity against EV71 C3 (Table 1) and C4a (Table 2), and we found that the 40% and 60% of MeOH fractions showed significant antiviral activity. After further purification, we identified hederasaponin B as a major compound in the 40% and 60% of MeOH fractions (data not shown).
Table 1.
Antiviral activity of the extract and fractions of Hedera helix against EV71 C3
| Test material | CC50a | EC50b | TIc |
|---|---|---|---|
| Hedera helix 30% EtOH extract | >50 | 6.58 ± 0.11 | 7.59 |
| 100% H2O | >50 | NDd | - |
| 20% MeOH | >50 | 25.23 ± 4.93 | 1.98 |
| 40% MeOH | >50 | 7.92 ± 1.44 | 6.31 |
| 60% MeOH | >50 | 2.75 ± 1.05 | 18.18 |
| 80% MeOH | >50 | 38.22 ± 4.13 | 1.31 |
| 100% MeOH | 31.03 | NDd | - |
Results are presented as the mean EC50 values ± SD obtained from three independent experiments carried out in triplicate.
Concentration required to reduce cell growth by 50% (μg/ml).
Concentration required to inhibit virus-induced CPE by 50% (μg/ml).
Therapeutic index=CC50/EC50.
Not determined.
Table 2.
Antiviral activity of the extract and fractions of Hedera helix against EV71 C4a
| Test material | CC50a | EC50b | TIc |
|---|---|---|---|
| Hedera helix 30% EtOH extract | >50 | 22.00 ± 2.06 | 4.55 |
| 100% H2O | >50 | NDd | - |
| 20% MeOH | >50 | NDd | - |
| 40% MeOH | >50 | 43.12 ± 1.91 | 1.16 |
| 60% MeOH | >50 | 47.10 ± 5.16 | 1.06 |
| 80% MeOH | >50 | NDd | - |
| 100% MeOH | 31.03 | NDd | - |
Results are presented as the mean EC50 values ± SD obtained from three independent experiments carried out in triplicate.
Concentration required to reduce cell growth by 50% (μg/ml).
Concentration required to inhibit virus-induced CPE by 50% (μg/ml).
Therapeutic index=CC50/EC50.
Not determined.
Antiviral activity and cytotoxicity of hederasaponin B against EV71 C3 and EV71 C4a
Next, hederasaponin B from Hedera helix was further investigated for its antiviral activity against EV71 C3 and EV71 C4a. The antiviral assay demonstrated that hederasaponin B possessed antiviral activity against EV71 C3 with an EC50 value of 24.77 μg/ml and against EV71 C4a with an EC50 value of 41.77 μg/ml (Table 3). In addition, it was not toxic to vero cells with a cell viability of about 100% at a concentration of 50 μg/ml.
Table 3.
Antiviral activity of hederasaponin B isolated from Hedera helix against EV71 C3 and C4a
| Compound | EV71 C3
|
EV71 C4a
|
||||
|---|---|---|---|---|---|---|
| CC50a | EC50b | TIc | CC50a | EC50b | TIc | |
| Hederasaponin B | >50 | 24.77 ± 12.56 | 2.02 | >50 | 41.77 ± 0.76 | 1.18 |
| Ribavirin | >50 | NDd | - | >50 | NDd | - |
Results are presented as the mean EC50 values ± S.D obtained from three independent experiments carried out in triplicate.
Concentration required to reduce cell growth by 50% (μg/ml).
Concentration required to inhibit virus-induced CPE by 50% (μg/ml).
Therapeutic index=CC50/EC50.
Not determined.
The effect of hederasaponin B on EV71 C3 and EV71 C4a induced CPE
The effect of hederasaponin B on EV71 C3- and EV71 C4a-induced CPE was recorded by capturing images using a microscope (Axiovert 10; Zeiss, Wetzlar, Germany). In the absence of infection of vero cells with EV71 C3 and EV71 C4a, cells treated with vehicle (Fig. 1A, 2A), 50 μg/ml hederasaponin B (Fig. 1B, 2B), or ribavirin (Fig. 1C, 2C) showed typical spread-out shapes with normal morphology. At this concentration, especially, no signs of cytotoxicity of hederasaponin B were observed. Infection with EV71 C3 and EV71 C4a in the absence of hederasaponin B resulted in a severe CPE (Fig. 1D, 2D). Addition of hederasaponin B to the infected vero cells inhibited the formation of a visible CPE (Fig. 1E, 2E). However, the addition of ribavirin in vero cells infected with EV71 C3 or EV71 C4a could not prevent the CPE (Fig. 1F, 2F). This indicates that the CPE of the viral infection is prevented by the presence of hederasaponin B.
Fig. 1.
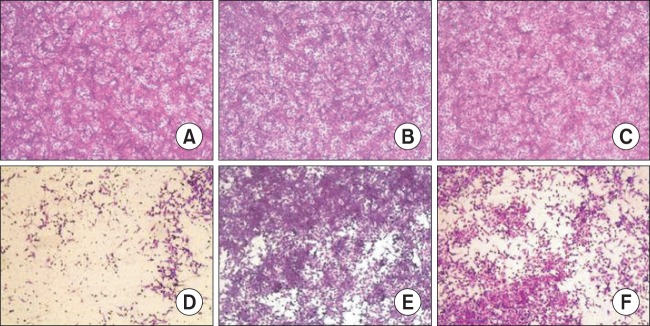
The effect of hederasaponin B on Enterovirus 71 (EV71) C3-induced cytopathic effect. The virus-infected vero cells were treated with ribavirin or 50 μg/ml hederasaponin B. After, cell viability was evaluated by sulforhodamine B (SRB) assay, and the morphology of cells was photographed using a microscope. (A) Non-infected cells, (B) Non-infected cells treated with hederasaponin B, (C) Non-infected cells treated with ribavirin, (D) EV71 C3-infected cells, (E) EV71 C3-infected cells treated with hederasaponin B, (F) EV71 C3-infected cells treated with ribavirin.
Fig. 2.
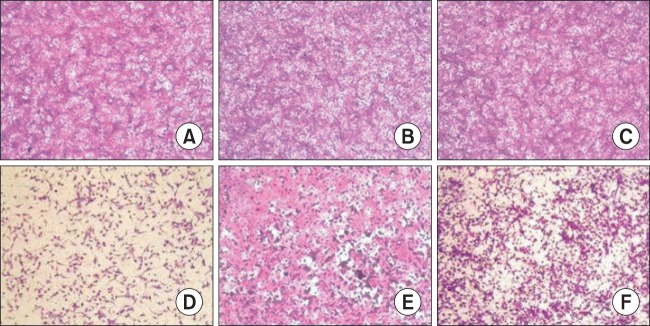
The effect of hederasaponin B on Enterovirus 71 (EV71) C4a-induced cytopathic effect. The virus-infected vero cells were treated with ribavirin or 50 μg/ml hederasaponin B. After, cell viability was evaluated by sulforhodamine B (SRB) assay, and the morphology of cells was photographed using a microscope. (A) Non-infected cells, (B) Non-infected cells treated with hederasaponin B, (C) Non-infected cells treated with ribavirin, (D) EV71 C4a-infected cells, (E) EV71 C4a -infected cells treated with hederasaponin B, (F) EV71 C4a-infected cells treated with ribavirin.
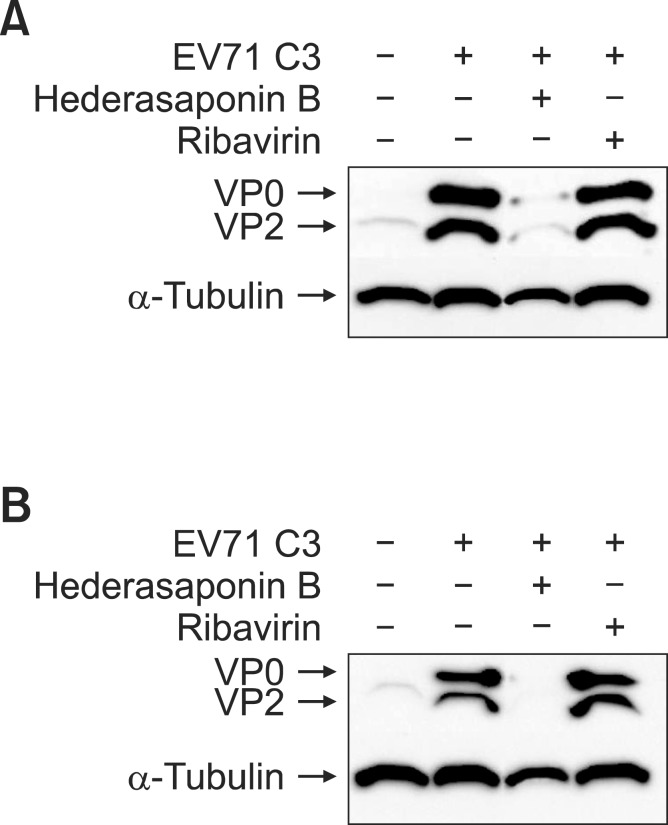
Hederasaponin B affects viral VP2 protein synthesis
Viral VP2 proteins syntheses were compared between drug-treated and untreated infected cells. As shown in Fig. 3, when cells were infected with virus and cultured in the absence of drugs until processed for western blot, virus VP2 protein could be detected in the untreated cells. The size of EV71 VP2 protein has been determined to be 34 kDa and α-Tubulin was used as a loading control in the experiment as well as to ensure that hederasaponin B used in this study did not affect the synthesis and expression of host cellular proteins. The western blot analysis also showed that the viral VP2 protein expression was decreased dramatically by hederasaponin B (50 μg/ml) at 48 h after infection by EV71 C3 (Fig. 3A) and EV71 C4a (Fig. 3B). However, ribavirin used as a positive control did not show cytotoxicity as well as antiviral activity in vitro and in western blot analysis, which is consistent with the previous reportsby Choi et al. (2009). Collectively, these results suggested that hederasaponin B possessed antiviral activity against EV71 C3 and C4a by inhibiting viral protein expression, and thus could be considered as an antiviral drug candidate for the treatment of HFMD.
Fig. 3.
The effect of hederasaponin B on the VP2 expression. Western blot analyses were performed to determine the effect of hederasaponin B and ribavirin on the production of EV71 C3 and EV71 C4a VP2 proteins. The reduction in protein expression of EV71 C3 VP2 (A) and EV71 C4a VP2 (B) was identified after treatment with 50 μg/ml concentration of hederasaponin B or ribavirin for 48 h. α-tubulin was used as a loading control for each set of samples.
DISSCUSION
The current antiviral drug armamentarium comprises of almost 40 compounds that have been officially approved for clinical use including 19 drugs for treating human immunodeficiency virus (HIV) infection, 3 drugs for treating hepatitis B virus (HBV) infection, 7 drugs for treating herpes simplex virus (HSV) infection and varicella-zoster virus (VZV) infection, 2 drugs for treating respiratory syncytial virus (RSV) infection and hepatitis C virus (HCV) infection, 5 drugs for treating cytomegalovirus (CMV) infection and 4 drugs for treating influenza virus infection. However, to date, there is no approved antiviral drug for the treatment of enterovirus infections (Park et al., 2012).
Pleconaril is a potent anti-viral inhibitor of enteroviruses that is under evaluation for the treatment of diseases associated with picornavirus infections (Pevear et al., 1999). Pleconaril exerts its activity on capsid function by integrating with high affinity and specificity in the hydrophobic pocket of the virion. In 2007, Schering-Plough completed a phase II double-blind, placebo-controlled trial to study the effects of pleconaril nasal spray on common cold symptoms (De Palma et al., 2008), but the US Food and Drug Administration has not approved pleconaril because of concerns of emergence of viral resistance and side effects in patients (Fleischer and Laessig, 2003). The relevance of pleconaril resistance was demonstrated in a study by Pevear et al. (1999). Other candidates having an antiviral effect against EV71 include WIN54954, SCH48973, rupintrivir, raoulic acid, and punicalagin. In addition, antiviral activity of betulin, betulinic acid, betulonic acid, chebulagic acid isolated from the fruits of Terminalia chebula, and that of matrine isolated from the root of Chinese Sophora herb plants has been studied against enterovirus. Ribavirin has also been used to treat various DNA and RNA virus infections, although acquired resistance to it has been demonstrated in various virus populations and in some patients (Graci and Cameron, 2006).
Further, we and others could not observe significant anti-viral activity of ribavirin against EV71 in vero cells. Therefore, broad-spectrum antiviral compounds should be developed against various genogroups of enteroviruses in the future.
The present study describes the cytotoxicity and antiviral activity of hederasaponin B. Hederasaponin B was shown to exhibit anti-viral activity against EV71 C3 and EV71 C4a by reducing the formation of a visible CPE. In addition, the inhibitory effects of hederasaponin B and ribavirin against EV71 were analyzed by western blot assay. The expression of EV71 C3 and C4a VP proteins was inhibited in the presence of 50 μg/ ml of hederasaponin B. However, ribavirin did not show any inhibitory effect against EV71 infection. These results suggest that hederasaponin B could be abroad-spectrum antiviral compound that is effective against various EV71 subgenotypes. In addition, 30% EtOH extract of Hedera helix, which has been widely used for the treatment of acute and chronic obstructive pulmonary bronchitis due to its secretolytic and bronchiolytic effects in adults and children, also has a significant anti-viral activity against EV71 C3 and C4a, thereby suggesting that it can be developed as an anti-viral drug for EV71 infection. We also found that 40% and 60% MeOH fractions from 30% EtOH extract of Hedera helix also have significant anti-viral effects against EV71 C3 and C4a; and especially, hederasaponin B, one of the major compounds of the 40% and 60% MeOH fractions showed a significant anti-EV71 activity.
In conclusion, hederasaponin B was shown to be effective against EV71. Also, anti-EV71 activity analysis with EV71 subgenotypes C3 and C4a did not reveal a subgenotype-specific activity pattern. Further studies will be required to explore the detailed antiviral mechanism of action of hederasaponin B. We will carry out research focusing on suppression of enterovirus replication by hederasaponin B because hederasaponin B is well known to be belonged to triterpenoid saponins (Han et al., 2013) and it has been demonstrated that triterpenoid saponins inhibit viral nucleotide synthesis against herpes simplex virus type-1 in previous studies (Simoes et al., 1999).
Acknowledgments
This work was supported by a grant from R&D project (2013-NG48001-00) at Korea National Institute of Health.
REFERENCES
- Brown BA, Oberste MS, Alexander JP, Jr, Kennett ML, Pallansch MA. Molecular epidemiology and evolution of enterovirus 71 strains isolated from 1970 to 1998. J Virol. 1999;73:9969–9975. doi: 10.1128/jvi.73.12.9969-9975.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardosa MJ, Perera D, Brown BA, Cheon D, Chan HM, Chan KP, Cho H, McMinn P. Molecular epidemiology of human enterovirus 71 strains and recent outbreaks in the Asia-Pacific region: comparative analysis of the VP1 and VP4 genes. Emerg Infect Dis. 2003;9:461–468. doi: 10.3201/eid0904.020395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YF, Sam IC, AbuBakar S. Phylogenetic designation of enterovirus 71 genotypes and subgenotypes using complete genome sequences. Infect Genet Evol. 2010;10:404–412. doi: 10.1016/j.meegid.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Chang LY, Lin TY, Huang YC, Tsao KC, Shih SR, Kuo ML, Ning HC, Chung PW, Kang CM. Comparison of enterovirus 71 and coxsackie-virus A16 clinical illnesses during the Taiwan enterovirus epidemic, 1998. Pediatr Infect Dis J. 1999;18:1092–1096. doi: 10.1097/00006454-199912000-00013. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Kim JH, Lee CH, Ahn YJ, Song JH, Baek SH, Kwon DH. Antiviral activity of quercetin 7-rhamnoside against porcine epidemic diarrhea virus. Antiviral Res. 2009;81:77–81. doi: 10.1016/j.antiviral.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chumakov M, Voroshilova M, Shindarov L, Lavrova I, Gracheva L, Koroleva G, Vasilenko S, Brodvarova I, Nikolova M, Gyurova S, Gacheva M, Mitov G, Ninov N, Tsylka E, Robinson I, Frolova M, Bashkirtsev V, Martiyanova L, Rodin V. Enterovirus 71 isolated from cases of epidemic poliomyelitis-like disease in Bulgaria. Arch Virol. 1979;60:329–340. doi: 10.1007/BF01317504. [DOI] [PubMed] [Google Scholar]
- De Palma AM, Vliegen I, De Clercq E, Neyts J. Selective inhibitors of picornavirus replication. Med Res Rev. 2008;28:823–884. doi: 10.1002/med.20125. [DOI] [PubMed] [Google Scholar]
- Fleischer R, Laessig K. Safety and efficacy evaluation of pleconaril for treatment of the common cold. Clin Infect Dis. 2003;37:1722. doi: 10.1086/379830. [DOI] [PubMed] [Google Scholar]
- Gepdiremen A, Mshvildadze V, Suleyman H, Elias R. Acute anti-inflammatory activity of four saponins isolated from ivy: alpha-hederin, hederasaponin-C, hederacolchiside-E and hederacolchiside-F in carrageenan-induced rat paw edema. Phytomedicine. 2005;12:440–444. doi: 10.1016/j.phymed.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Graci JD, Cameron CE. Mechanisms of action of ribavirin against distinct viruses. Rev Med Virol. 2006;16:37–48. doi: 10.1002/rmv.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han LT, Fang Y, Li MM, Yang HB, Huang F. The antitumor effects of triterpenoid saponins from the anemone flaccida and the underlying mechanism. Evid Based Complement Alternat Med. 2013;2013:517931. doi: 10.1155/2013/517931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YP, Lin TL, Hsu LC, Chen YJ, Tseng YH, Hsu CC, Fan WB, Yang JY, Chang FY, Wu HS. Genetic diversity and C2-like subgenogroup strains of enterovirus 71, Taiwan, 2008. Virol J. 2010;7:277. doi: 10.1186/1743-422X-7-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyypia T, Hovi T, Knowles NJ, Stanway G. Classification of enteroviruses based on molecular and biological properties. J Gen Virol. 1997;78:1–11. doi: 10.1099/0022-1317-78-1-1. [DOI] [PubMed] [Google Scholar]
- Laine P, Savolainen C, Blomqvist S, Hovi T. Phylogenetic analysis of human rhinovirus capsid protein VP1 and 2A protease coding sequences confirms shared genus-like relationships with human enteroviruses. J Gen Virol. 2005;86:697–706. doi: 10.1099/vir.0.80445-0. [DOI] [PubMed] [Google Scholar]
- Lin ZX, Hoult JR, Raman A. Sulphorhodamine B assay for measuring proliferation of a pigmented melanocyte cell line and its application to the evaluation of crude drugs used in the treatment of vitiligo. J Ethnopharmacol. 1999;66:141–150. doi: 10.1016/s0378-8741(98)00199-8. [DOI] [PubMed] [Google Scholar]
- McMinn PC. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol Rev. 2002;26:91–107. doi: 10.1111/j.1574-6976.2002.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Park KS, Choi YJ, Park JS. Enterovirus infection in Korean children and anti-enteroviral potential candidate agents. Korean J Pediatr. 2012;55:359–366. doi: 10.3345/kjp.2012.55.10.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevear DC, Tull TM, Seipel ME, Groarke JM. Activity of pleconaril against enteroviruses. Antimicrob Agents Chemother. 1999;43:2109–2115. doi: 10.1128/aac.43.9.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyry T, Kinnunen L, Hyypia T, Brown B, Horsnell C, Hovi T, Stanway G. Genetic and phylogenetic clustering of enteroviruses. J Gen Virol. 1996;77:1699–1717. doi: 10.1099/0022-1317-77-8-1699. [DOI] [PubMed] [Google Scholar]
- Sieben A, Prenner L, Sorkalla T, Wolf A, Jakobs D, Runkel F, Haberlein H. Alpha-hederin, but not hederacoside C and hederagenin from Hedera helix, affects the binding behavior, dynamics, and regulation of beta 2-adrenergic receptors. Biochemistry. 2009;48:3477–3482. doi: 10.1021/bi802036b. [DOI] [PubMed] [Google Scholar]
- Simoes CM, Amoros M, Girre L. Mechanism of antiviral activity of triterpenoid saponins. Pytother Res. 1999;13:323–328. doi: 10.1002/(SICI)1099-1573(199906)13:4<323::AID-PTR448>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Suleyman H, Mshvildadze V, Gepdiremen A, Elias R. Acute and chronic antiinflammatory profile of the ivy plant, Hedera helix, in rats. Phytomedicine. 2003;10:370–374. doi: 10.1078/0944-7113-00260. [DOI] [PubMed] [Google Scholar]
- Trute A, Gross J, Mutschler E, Nahrstedt A. In vitro antispasmodic compounds of the dry extract obtained from. Hedera helix Planta Med. 1997;63:125–129. doi: 10.1055/s-2006-957627. [DOI] [PubMed] [Google Scholar]
- Wang SM, Lei HY, Huang KJ, Wu JM, Wang JR, Yu CK, Su IJ, Liu CC. Pathogenesis of enterovirus 71 brainstem encephalitis in pediatric patients: roles of cytokines and cellular immune activation in patients with pulmonary edema. J Infect Dis. 2003;188:564–570. doi: 10.1086/376998. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang J, Guo W, Wang H, Zhu S, Wang D, Bai R, Li X, Yan D, Wang H, Zhang Y, Zhu Z, Tan X, An H, Xu A, Xu W. Emergence and transmission pathways of rapidly evolving evolutionary branch C4a strains of human enterovirus 71 in the Central Plain of China. PloS One. 2011;6:e27895. doi: 10.1371/journal.pone.0027895. [DOI] [PMC free article] [PubMed] [Google Scholar]