Abstract
Panax ginseng is a medicinal herb that is used worldwide. Its medicinal effects are primarily attributable to ginsenosides located in the root, leaf, seed, and flower. The flower buds of Panax ginseng (FBPG) are rich in various bioactive ginsenosides, which exert immunomodulatory and anti-inflammatory activities. The aim of the present study was to assess the effect of 18 ginsenosides isolated from steamed FBPG on the transcriptional activity of NF-κB and the expression of tumor necrosis factor-α (TNF-α)-stimulated target genes in liver-derived cell lines. Noticeably, the ginsenosides Rk3 and Rs4 exerted the strongest activity, inhibiting NF-κB in a dose-dependent manner. SF and Rg6 also showed moderately inhibitory effects. Furthermore, these four compounds inhibited the TNF-α-induced expression of IL8, CXCL1, iNOS, and ICAM1 genes. Consequently, ginsenosides purified from steamed FBPG have therapeutic potential in TNF-α-mediated diseases such as chronic hepatic inflammation.
Keywords: NF-κB inhibitory activity, Panax ginseng flower buds, Tumor necrosis factor-α, Hepatocyte derived cells
INTRODUCTION
Nuclear factor-κB (NF-κB) plays an important role in immune and inflammatory responses by regulating genes encoding pro-inflammatory cytokines, adhesion molecules, chemokines, growth factors, and inducible enzymes (Li and Verma, 2002; Gasparini and Feldmann, 2012). Therefore, NF-κB pathway inhibitors act as anti-inflammatory compounds (Nam, 2006; Luqman and Pezzuto, 2010). Although inflammation is a basic response to injury or infection, the harmful effects of inflammation can cause a variety of chronic diseases including arthritis, fibrosis, and cancer. Inflammatory responses also play important roles in the development of liver disease in both humans and animals. Hepatitis, which can be either acute or chronic, results from inflammation of the liver, and is characterized by the presence of hepatic inflammatory cells. Chronic hepatitis is associated with a high risk of hepatic carcinoma (Berasain et al., 2009). At the molecular level, free radicals and aldehydes produced during chronic hepatitis can induce deleterious gene mutations and posttranslational modifications in cancer-associated genes (Kawanishi et al., 2006). Other inflammatory products, including cytokines, growth factors, and transcription factors such as nuclear factor-κB (NF-κB), regulate the expression of cancer-related (both tumor suppressor genes and oncogenes) and key inflammatory genes, such as interleukin-8 (IL-8), chemokine (C-X-C motif) ligand-1 (CXCL-1), inducible nitric oxide synthase (iNOS), and intercellular adhesion molecule-1 (ICAM-1) (Lentsch and Ward, 2000; Elsharkawy and Mann, 2007; Holt et al., 2008; Farinati et al., 2010).
Panax ginseng (PG) Meyer (Araliaceae), a traditional herbal drug in Oriental medicine, is used to treat a variety of diseases (Hofseth and Wargovich, 2007; Ernst, 2010; Vuksan et al., 2010). Ginsenosides are the major active components of PG. Although roots are considered to be the best source of PG, leaves also contain high concentrations of ginsenosides (Tung et al., 2009; Liu et al., 2010; Tung et al., 2010a). Therefore, ginseng leaves could function as a supplementary source of pharmacologically active ginsenosides (Christensen, 2009; Wang et al., 2009).
Traditionally, PG root is air-dried, yielding white ginseng, or steamed at 100°C to produce red ginseng. Steamed ginseng is believed to be more pharmacologically effective than air-dried ginseng. The differences in the biological effects of air-dried and steamed ginseng are attributed to significant changes to ginsenosides during steaming (Baek et al., 1996). However, the anti-inflammatory effects of steamed PG from flowers have not been assessed.
Our previous studies focused on identifying the bioactive constituents in steamed FBPG, leading to the identification of one dammarane-type saponin, ginsenoside SF, and 17 known saponins, including ginsenoside Rh4, ginsenoside Rk3, ginsenoside F1, (20E)-ginsenoside F4, ginsenoside Rg2, pseudoginsenoside RC1, ginsenoside Rg6, ginsenoside Rs4, ginsenoside Rg1, 6’-acetyl-ginsenoside Rg1, ginsenoside Rb1, ginsenoside Rc, ginsenoside Rb2, ginsenoside Re, vinaginsenoside R4, ginsenoside Mb, and ginsenoside F4 (Tung et al., 2010b). Some of these compounds possess antioxidant and anticancer activities, and inhibit LPS-stimulated IL-12 production (Tung et al., 2010c, 2010d, 2011).
In this study, the effects of 18 ginsenosides, isolated from steamed FBPG, on TNF-α-induced NF-κB transcriptional activity in human hepatocyte-derived cells (HepG2 and SKHep1) were evaluated using an NF-κB-luciferase assay. Their effects on iNOS promoter activity, and the expression of NF-κB target genes, including interleukin-8 (IL-8), chemokine (CX-C motif) ligand-1 (CX-CL-1), inducible nitric oxide synthase (iNOS), and intercellular adhesion molecule-1 (ICAM-1), were evaluated by RT-PCR in TNF-α-stimulated cells.
MATERIALS AND METHODS
Chemical and sample preparation
Ginsenosides were isolated from the steamed flower buds of Panax ginseng (FBPG) as identified in our previous reports (Tung et al., 2010b). Apigenin, potently inhibited the transcriptional activity of NF-κB (Funakoshi-Tago et al., 2011), was the product of Sigma-Aldrich. All other chemicals and reagents were of analytical grade. The tested ginsenosides and apigenin (Sigma-Aldrich) were dissolved in DMSO.
Cell lines and culture
HepG2 and SK-Hep1 cells were maintained in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA, USA) containing 10% heat-inactivated fetal bovine serum, 100 units/ml penicillin, and 10 μg/ml streptomycin, at 37°C and 5% CO2. Human TNF-α was purchased from ATgen (Seoul, Korea).
Cell toxicity assay
Cell-Counting Kit (CCK)-8 (Dojindo, Kumamoto, Japan) was used to analyze the effect of compounds on cell toxicity according to the manufacturer’s instructions. Cells were cultured overnight in 96-well plate (∼1×104 cells/well). Cell toxicity was assessed after the addition of compounds on dose-dependent manner. After 24 h of treatment, 10 μl of the CCK-8 solution was added to triplicate wells, and incubated for 1 h. Absorbance was measured at 450 nm to determine viable cell numbers in wells.
NF-κB and iNOS-luciferase assay
Human hepatocarcinoma HepG2 and SK-Hep1 cells were maintained in Dulbecco’s modified Eagles’ medium (DMEM) (Invitrogen, Carlsbad, CA, USA) containing 10% heat-inactivated fetal bovine serum (FBS), 100 units/ml penicillin, and 10 μg/ml streptomycin at 37°C and 5% CO2. The luciferase vector was first transfected into cells. After a limited amount of time, the cells were lysed, and luciferin, the substrate of luciferase, was introduced into the cellular extract along with Mg2+ and an excess of ATP. Under these conditions, luciferase enzymes expressed by the reporter vector could catalyze the oxidative carboxylation of luciferin. Cells were seeded at 2×105 cells per well in a 12-well plate and grown. After 24 h, cells were transfected with inducible NF-κB or iNOS firefly luciferase reporter and constitutively expressing Renilla reporter. After 24 h of transfection, medium was changed to assay medium (Opti-MEM+0.5% FBS+0.1 mM NEAA+1 mM sodium pyruvate+100 units/ml penicillin+10 μg/ml streptomycin) and cells were pretreated for 1 h with either vehicle (DMSO) and compounds, followed by 1 h of treatment with 10 ng/ml TNF-α for 20 h. Unstimulated cells were used as a negative control (–), apigenin was used as a positive control. Dual Luciferase assay was performed 48 h after transfection, and promoter activity values are expressed as arbitrary units using a Renilla reporter for internal normalization.
RNA preparation and reverse transcriptase polymerase chain reaction (RT-PCR)
RNA Preparation and Reverse Transcriptase Polymerase Chain Reaction (RT-PCR): Total RNA was extracted using Easy-blue reagent (Intron Biotechnology, Seoul, Korea). Approximately 2 μg total RNA was subjected to reverse transcription using Moloney murine leukemia virus (MMLV) reverse transcriptase and oligo-dT primers (Promega, Madi-son, WI, USA) for 1 h at 42°C. PCR for synthetic cDNA was performed using a Taq polymerase pre-mixture (TaKaRa, Japan). The PCR products were separated by electrophoresis on 1% agarose gels and stained with EtBr. PCR was conducted with the following primer pairs: iNOS sense 5′-TCATCCGCTATGCTGGCTAC-3′, iNOS antisense 5′-CTCAGGGTCACGGCCATTG-3′, ICAM-1 sense 5′-CTGCAGACAGTGACCATC-3′, ICAM-1 antisense 5′-GTCCAGTTTCCCGGACAA-3′, IL-8 sense 5′-GGGTCTGTTGTAG-GGTTGCC-3′, IL-8 antisense 5′-TCTGGATCCTGGCTAGCA-GA-3′, CXCL-1 sense 5′-AGGGAATTCACCCCAAGAAC-3′, CXCL-1 antisense 5′-5′-TAACTATGGGGGATGCAGGA-3′, β-actin sense 5′-TCACCCACACTGTGCCCATCTACG-3′, and β-actin antisense 5′-CAGCGGAACCGCTCATTGCCAATG-3′. HepG2 and SK-Hep1 cells were pretreated in the absence and presence of compounds for 1 hr, then exposed to 10 ng/ml TNF-α for 6 h. Total mRNA was prepared from the cell pellets using Easy-blue. The levels of mRNA were assessed by RT-PCR.
Statistical analysis
Unless otherwise stated, all experiments were performed with triplicate samples and repeated at least three times. All results are expressed as the mean ± S.E.M. Data was analyzed by one-factor analysis of variance (ANOVA). Upon observation of a statistically significant effect, the Newman-Keuls test was performed to determine the difference between the groups. A p value *(<0.05) and **(<0.01) were considered to be significant.
RESULTS
Ginsenosides inhibit NF-κB activity in hepatocyte-derived cell lines
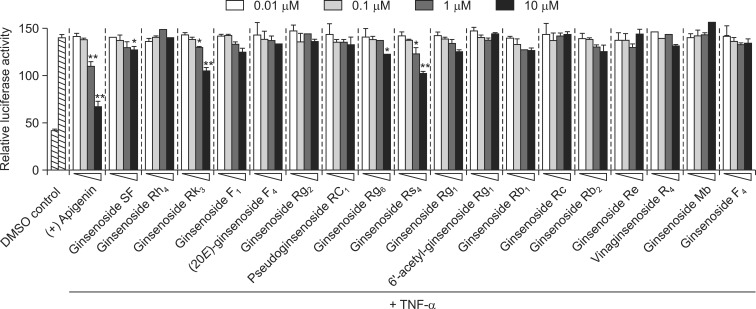
To identify novel NF-κB inhibitors from the steamed flower buds of Panax ginseng (FBPG), 18 dammarane-type ginsenosides were evaluated using the NF-κB reporter system. To determine non-toxic concentrations, HepG2 cells were treated with 0.1, 1, and 10 μM of each compound, and cell viability was assessed by MTS assay. No compounds were significantly cytotoxic at up to 10 μM, suggesting that NF-κB inhibition was not toxic (data not shown). HepG2 cells were then pre-treated with different ginsenosides at concentrations ranging from 0.01 to 10 μM for 1 h, and induced with TNF-α for 20 h. Rk3 and Rs4 significantly inhibited TNF-α-induced NF-κB transcriptional activity, with IC50 values of 14.24 ± 1.30 and 12.44 ± 2.01 μM, respectively (Table 1, Fig. 1, 2). SF and Rg6 also reduced NF-κB transcriptional activity, with IC50 values of 33.86 ± 4.14 and 29.34 ± 2.22 μM, respectively (Table 1, Fig. 1, 2). Six additional ginsenosides F1, Rg1, Rb1, and Rb2, (20E)-ginsenoside F4, and pseudoginsenoside RC1 moderately inhibited the transcriptional activity of NF-κB with IC50 values of 42.51 ± 1.97, 45.47 ± 3.64, 61.22 ± 3.69, 37.46 ± 5.01, 89.62 ± 10.64, and 98.24 ± 7.61 μM, respectively (Fig. 2). Finally, ginsenosides Rh4, Rg2, Rc, Re, Mb, F4, 6’-acetyl-ginsenoside Rg1, and vinaginsenoside R4 had no effect (Fig. 2). Apigenin, the positive control, potently inhibited the transcriptional activity of NF-κB, with an IC50 of 1.64 ± 0.19 μM. These results suggest that the ginsenosides SF, Rk3, Rg6, and Rs4 inhibited TNF-α-induced NF-κB transcriptional activity in HepG2 cells.
Table 1.
Inhibitory effects of ginsenosides SF, Rk3, Rg6, and Rs4 on the TNF-α-induced NF-κB transcriptional activity and iNOS promoter activity in HepG2 and SK-Hep1 hepatocyte-derived cells
| Ginsenoside or Compound | IC50 (μM)*
|
|||
|---|---|---|---|---|
| HepG2
|
SK-Hep1
|
|||
| NF-κB | iNOS | NF-κB | iNOS | |
| Ginsenoside SF | 33.86 ± 4.14 | 13.07 ± 0.64 | 18.17 ± 0.69 | 11.26 ± 0.87 |
| Ginsenoside Rk3 | 14.24 ± 1.30 | 9.83 ± 0.06 | 15.32 ± 0.29 | 6.02 ± 0.37 |
| Ginsenoside Rg6 | 29.34 ± 2.22 | 19.45 ± 0.55 | 25.12 ± 1.04 | 10.36 ± 0.69 |
| Ginsenoside Rs4 | 12.44 ± 2.01 | 10.01 ± 1.21 | 11.98 ± 0.85 | 6.92 ± 0.65 |
| Apigenin** | 1.64 ± 0.19 | 4.45 ± 0.24 | 3.60 ± 0.21 | 3.63 ± 0.17 |
Results are the means ± SEM of three independent experiments performed in triplicate.
Positive control. apigenin
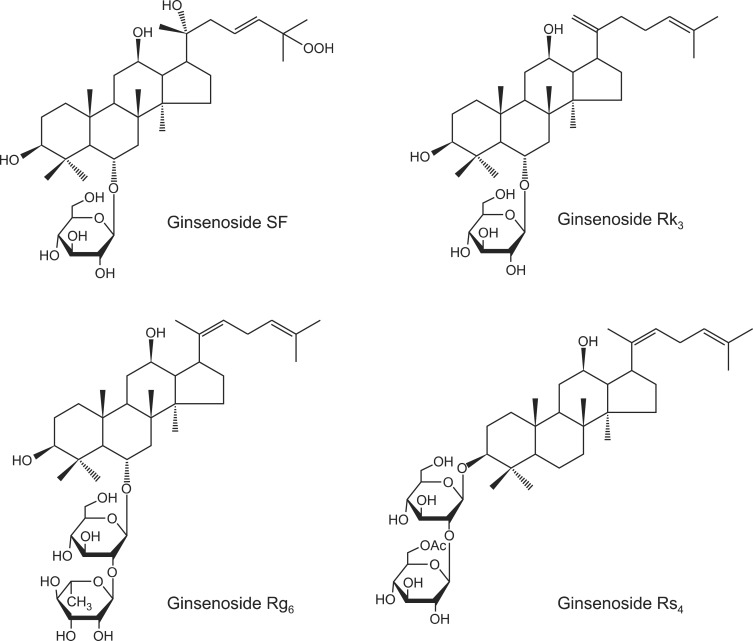
Fig. 1.
Chemical structures of the ginsenosides SF, Rk3, Rg6, and Rs4.
Fig. 2.
Effect of 18 ginsenosides on TNF-α-stimulated NF-κB transcriptional activity in HepG2 cells. Cells transiently transfected with pNF-κB-Luc were pretreated for 1 h with either vehicle (DMSO) or compounds, then treated with TNF-α (10 ng/ml). Unstimulated cells acted as a negative control. Cells were harvested and assayed for luciferase activity. Apigenin was used as a positive control (+). Results are expressed as the mean luciferase activity ± S.E.M. (n=3). Statistical significance is indicated as *p<0.05, **p<0.01.
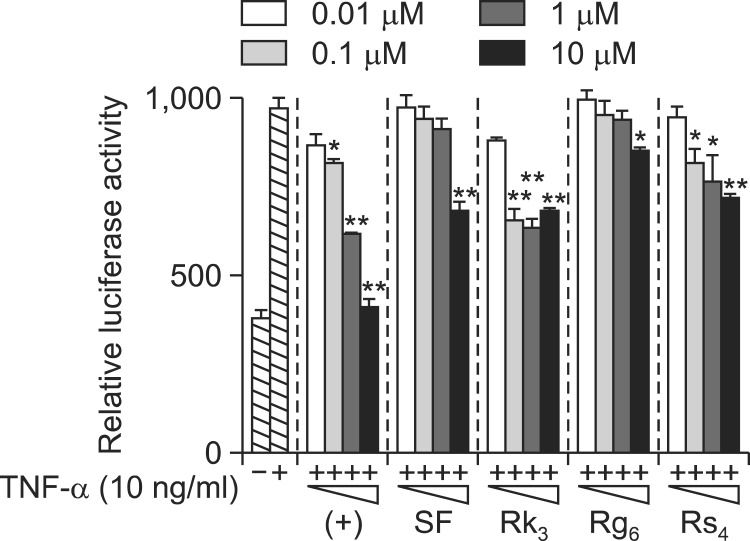
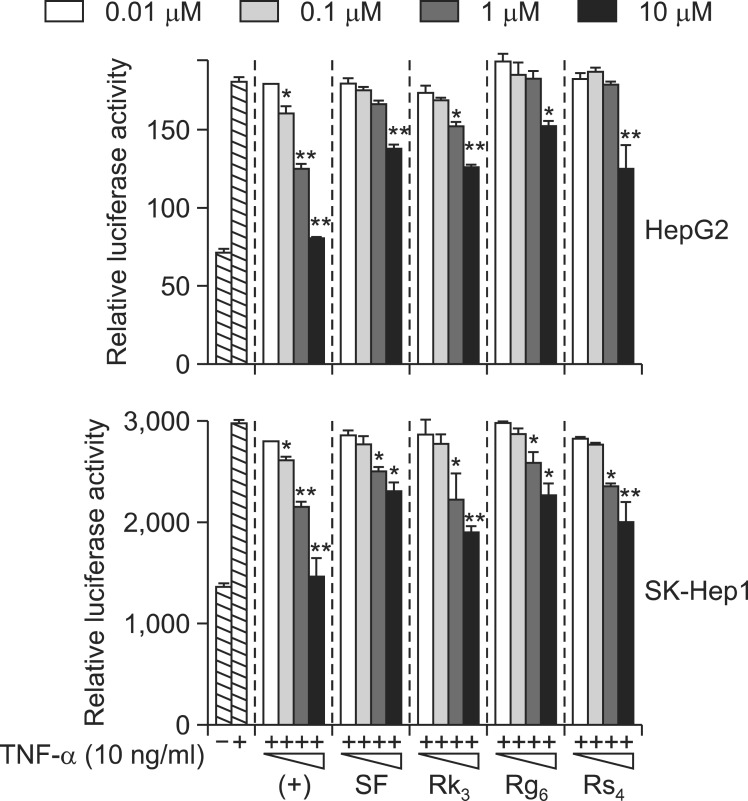
To confirm that these compounds inhibited NF-κB activation in HepG2 cells, their effects on TNF-α-induced NF-κB transcriptional activity in SK-Hep1 cells were evaluated using the NF-κB reporter system. Cells were pretreated with the four ginsenosides at concentrations ranging from 0.01 to 10 μM for 1 h, and then induced with TNF-α for 20 h. SF, Rk3, Rg6, and Rs4 significantly inhibited TNF-α-induced NF-κB transcriptional activity, with IC50 values of 10.17 ± 0.69, 15.32 ± 0.29, 25.12 ± 1.04, and 11.98 ± 0.85 μM, respectively, consistent with the data from HepG2 cells (Table 1, Fig. 3). As expected, apigenin potently inhibited NF-κB transcriptional activity, with an IC50 of 3.60 ± 0.21 μM. These data suggest that the ginsenosides SF, Rk3, Rg6, and Rs4 inhibited TNF-α-induced NF-κB transcriptional activity in HepG2 and SK-Hep1 cells.
Fig. 3.
Effect of ginsenosides SF, Rk3, Rg6, and Rs4 on TNF-α stimulated NF-κB transcriptional activity in SK-Hep1 cells. Cells transiently transfected with pNF-κB-Luc were pretreated for 1 h with either vehicle (DMSO) or compounds, then treated with TNF-α (10 ng/ml). Unstimulated cells acted as a negative control. Cells were harvested and assayed for luciferase activity. Results are expressed as the mean luciferase activity ± SEM (n=3). Statistical significance is indicated as *p<0.05, **p<0.01.
Effect of ginsenosides on NF-κB target genes expression
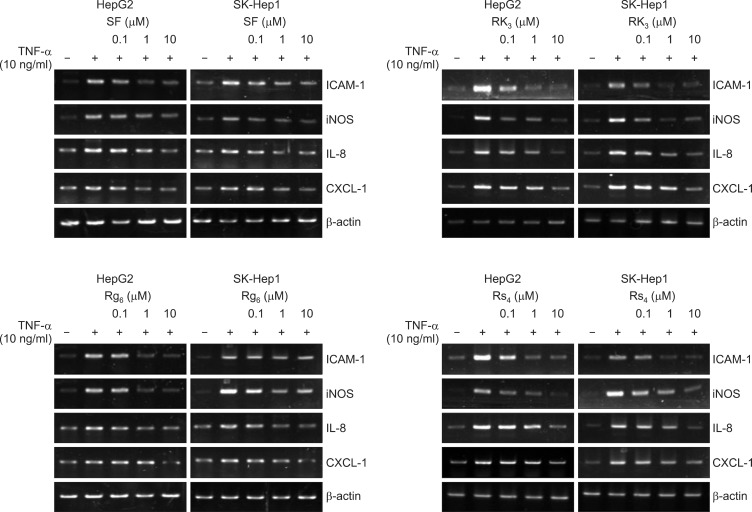
NF-κB regulates several genes involved in immunity, inflammation, and cell proliferation, as well as those that result in the negative feedback of NF-κB signaling (Gasparini and Feldmann, 2012). Therefore, we assessed expression of NF-κB target genes that play an important role in the inflammatory response, including IL-8 (cytokine), CXCL-1 (chemokine), ICAM-1 (migration), and iNOS (inflammatory inducible enzyme), in HepG2 and SK-Hep1 cells treated with ginsenosides (Fig. 4). Consistent with the inhibition of NF-κB, SF, Rk3, Rg6, and Rs4 all inhibited the induction of IL8, CXCL1, iNOS, and ICAM1 mRNA significantly in a dose-dependent manner, suggesting that these compounds reduced the transcription of these genes. Importantly, the expression of the housekeeping protein β-actin was unchanged by ginsenosides.
Fig. 4.
Effect of ginsenosides SF, Rk3, Rg6, and Rs4 on IL-8, CXCL-1, iNOS, and ICAM-1 gene expression in HepG2 and SK-Hep1 hepatocyte-derived cells. HepG2 and SK-Hep1 cells were pretreated with the ginsenosides SF, Rk3, Rg6, and Rs4, or the vehicle (DMSO), for 1 hour, then treated with TNF-α (10 ng/ml) for 6 h. Total mRNA was extracted from the cell pellets using TRIzol reagent. Relative mRNA levels were assessed by RT-PCR. Expression levels are displayed as the ratio of IL-8, CXCL-1, iNOS, and ICAM-1 signal strength to a reference gene (β-actin), compensating for variations in the RNA concentrations.
The ginsenosides SF, Rk3, Rg6, and Rs4 also decreased TNF-α-induced iNOS promoter activity, with IC50 values ranging from 6 to 20 μM (Table 1, Fig. 5). These data suggest that the dammarane-type ginsenosides isolated from steamed FBPG suppress TNF-α-induced NF-κB transcriptional activity via the inhibition of iNOS gene transcription.
Fig. 5.
Effect of ginsenosides SF, Rk3, Rg6, and Rs4 on TNF-α-stimulated iNOS promoter activity. HepG2 and SK-Hep1 cells transiently transfected with iNOS-Luc were pretreated for 1 h with either vehicle (DMSO) or compounds, then treated with TNF-α (10 ng/ml). Unstimulated HepG2 and SK-Hep1 cells acted as a negative control. Cells were harvested and assayed for luciferase activity. Results are expressed as the mean luciferase activity ± S.E.M. (n=3). Statistical significance is indicated as *p<0.05, **p<0.01.
DISCUSSION
The aim of the present study was to identify novel inhibitors of NF-κB, a transcription factor that is a major target in drug discovery due to its causative role in inflammation, cancer, and many other diseases. Eighteen ginsenosides were isolated from the flower buds of Panax ginseng (FBPG), and their ability to inhibit TNF-α-induced NF-κB activation was assessed. Four ginsenosides (22%) inhibited NF-κB activity at a concentration of 10 μM. This high percentage of active compounds was not a surprise, since extracts from ginseng and saponins are recognized as promising NF-κB inhibitors. For example, the NF-κB-inhibiting saponin ginsenosides Rd, Re, and Rp1 are currently being assessed in preclinical and clinical trials (Park et al., 2008; Lee et al., 2012; Wang et al., 2012).
In this study, the ginsenosides SF, Rk3, Rg6, and Rs4 inhibited TNF-α-induced NF-κB promoter activity in hepatocyte-derived HepG2 and SK-Hep1 cells in a dose-dependent manner by modulating gene transcription. They also inhibited the transcription of the NF-κB target genes IL8, CXCL1, iNOS, and ICAM1, as demonstrated by reduced mRNA expression in TNF-α-treated HepG2 and SK-Hep1 cells. To our knowledge, this is the first report of these effects. Of the remaining compounds, ginsenosides F1, Rg1, Rb1, Rb2, (20E)-ginsenoside F4, and pseudoginsenoside RC1 exerted only small inhibitory effects, with IC50 values of 42.51, 45.47, 61.22, 37.46, 89.62, and 98.24 μM, respectively. Furthermore, ginsenosides Rh4, Rg2, Rc, Re, Mb, F4, 6’-acetyl-ginsenoside Rg1, and vinaginsenoside R4 had no effect.
We demonstrated previously that ginsenosides F1, Rg1, Rb1, and Rb2, isolated from the air dried leaves of Panax ginseng, moderately inhibited NF-κB in HepG2 cells (Song et al., 2012). Furthermore, ginsenoside Rc and Re had no effects, consistent with this study.
We also reported that the ginsenoside Rg6 potently inhibited LPS-induced IL-12 production by 82% in bone marrow-derived dendritic cells (Tung et al., 2011). Consistent with this, ginsenoside Rg6 also exerted anti-complement effects, with an IC50 of 174 μM, in antibody-sensitized sheep erythrocytes (EA) as indicator cells (Lee et al., 2011). Ginsenoside Rk3 exerts cardioprotective effects against hypoxia-reoxygenation injury via the phosphatidylinositol 3-kinase (PI3K)/AKT and mitogen-activated protein kinase (MAPK) signaling pathways in H9c2 cardiomyocytes (Sun et al., 2013). In addition, ginsenoside Rk3 and Rs4 exert anti-proliferative effects, with IC50 values of 187 and 20 μM, respectively, in human hepatocarcinoma cells (Kim et al., 1999; Toh et al., 2011). Inhibiting NF-κB by blocking TNF-α resulted in apoptosis in transformed hepatocytes, and inhibited hepatocarcinogenesis. Therefore, the NF-κB signaling pathway may have pro-carcinogenic effects, and so may be a potential target for novel anti-tumor agents (Mantovani et al., 2008). Based on our data, we suggest that the ginsenosides SF, Rk3, Rg6, and Rs4 may act as NF-κB pathway inhibitors that could be applied as targeted therapies for conditions such as inflammatory hepatic cancer and inflammatory hepatitis.
In conclusion, we demonstrated that the ginsenosides SF, Rk3, Rg6, and Rs4, isolated from steamed flower buds of Panax ginseng, suppressed TNF-α-induced NF-κB activation, which subsequently inhibited the expression of IL8, CXCL1, iNOS, and ICAM1 in hepatocyte-derived HepG2 and SK-Hep1 cells. Our data demonstrated that these compounds have therapeutic potential as anti-inflammatory agents. However, the detailed mechanism by which they inhibit TNF-α-induced NF-kB activation remains to be elucidated.
Acknowledgments
This study was supported by a grant from the Priority Research Center Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0093815), Republic of Korea.
REFERENCES
- Baek NI, Kim DS, Lee YH, Park JD, Lee CB, Kim SI. Ginsenoside Rh4, a genuine dammarane glycoside from Korean red ginseng. Planta Med. 1996;62:86–87. doi: 10.1055/s-2006-957816. [DOI] [PubMed] [Google Scholar]
- Berasain C, Castillo J, Perugorria MJ, Latasa MU, Prieto J, Avila MA. Inflammation and liver cancer: new molecular links. Ann N Y Acad Sci. 2009;1155:206–221. doi: 10.1111/j.1749-6632.2009.03704.x. [DOI] [PubMed] [Google Scholar]
- Christensen LP. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- Elsharkawy AM, Mann DA. Nuclear factor-kappaB and the hepatic inflammation-fibrosis-cancer axis. Hepatology. 2007;46:590–597. doi: 10.1002/hep.21802. [DOI] [PubMed] [Google Scholar]
- Ernst E. Panax ginseng: An Overview of the Clinical Evidence. J Ginseng Res. 2010;34:259–263. [Google Scholar]
- Farinati F, Piciocchi M, Lavezzo E, Bortolami M, Cardin R. Oxidative stress and inducible nitric oxide synthase induction in carcinogenesis. Dig Dis. 2010;28:579–584. doi: 10.1159/000320052. [DOI] [PubMed] [Google Scholar]
- Funakoshi-Tago M, Nakamura K, Tago K, Mashino T, Kasahara T. Anti-inflammatory activity of structurally related flavonoids, apigenin, luteolin and fisetin. Int Immunopharmacol. 2011;11:1150–1159. doi: 10.1016/j.intimp.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Gasparini C, Feldmann M. NF-κB as a target for modulating inflammatory responses. Curr Pharm Des. 2012;18:5735–5745. doi: 10.2174/138161212803530763. [DOI] [PubMed] [Google Scholar]
- Hofseth LJ, Wargovich MJ. Inflammation, cancer, and targets of ginseng. J Nutr. 2007;137:183S–185S. doi: 10.1093/jn/137.1.183S. [DOI] [PubMed] [Google Scholar]
- Holt AP, Salmon M, Buckley CD, Adams DH. Immune interactions in hepatic fibrosis. Clin Liver Dis. 2008;12:861–882. doi: 10.1016/j.cld.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanishi S, Hiraku Y, Pinlaor S, Ma N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol Chem. 2006;387:365–372. doi: 10.1515/BC.2006.049. [DOI] [PubMed] [Google Scholar]
- Kim SE, Lee YH, Park JH, Lee SK. Ginsenoside-Rs4, a new type of ginseng saponin concurrently induces apoptosis and selectively elevates protein levels of p53 and p21WAF1 in human hepatoma SK-HEP-1 cells. Eur. J. Cancer. 1999;35:507–11. doi: 10.1016/s0959-8049(98)00415-8. [DOI] [PubMed] [Google Scholar]
- Lee IA, Hyam SR, Jang SE, Han MJ, Kim DH. Ginsenoside Re ameliorates inflammation by inhibiting the binding of lipopolysaccharide to TLR4 on macrophages. J Agric Food Chem. 2012;60:9595–9602. doi: 10.1021/jf301372g. [DOI] [PubMed] [Google Scholar]
- Lee JG, Baek SH, Lee YY, Park SY, Park JH. Anti-complementary ginsenosides isolated from processed ginseng. Biol Pharm Bull. 2011;34:898–900. doi: 10.1248/bpb.34.898. [DOI] [PubMed] [Google Scholar]
- Lentsch AB, Ward PA. The NFkappaBb/IkappaB system in acute inflammation. Arch. Immunol. Ther. Exp. (Warsz) 2000;48:59–63. [PubMed] [Google Scholar]
- Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- Liu GY, Li XW, Wang NB, Zhou HY, Wei W, Gui MY, Yang B, Jin YR. Three new dammarane-type triterpene saponins from the leaves of Panax ginseng C.A. Meyer J Asian Nat Prod Res. 2010;12:865–873. doi: 10.1080/10286020.2010.508035. [DOI] [PubMed] [Google Scholar]
- Luqman S, Pezzuto JM. NFkappaB: a promising target for natural products in cancer chemoprevention. Phytother Res. 2010;24:949–963. doi: 10.1002/ptr.3171. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Nam NH. Naturally occurring NF-kappaB inhibitors. Mini Rev Med Chem. 2006;6:945–951. doi: 10.2174/138955706777934937. [DOI] [PubMed] [Google Scholar]
- Park TY, Park MH, Shin WC, Rhee MH, Seo DW, Cho JY, Kim HM. Anti-metastatic potential of ginsenoside Rp1, a novel ginsenoside derivative. Biol Pharm Bull. 2008;31:1802–1805. doi: 10.1248/bpb.31.1802. [DOI] [PubMed] [Google Scholar]
- Song SB, Tung NH, Quang TH, Ngan NT, Kim KE, Kim YH. Inhibition of TNF-α-mediated NF-κB transcriptional activity in HepG2 cells by dammarane-type saponins from Panax ginseng leaves. J Ginseng Res. 2012;36:146–152. doi: 10.5142/jgr.2012.36.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Sun G, Meng X, Wang H, Wang M, Qin M, Ma B, Luo Y, Yu Y, Chen R, Ai Q, Sun X. Ginsenoside RK3_ prevents hypoxia-reoxygenation induced apoptosis in H9c2 cardiomyocytes via AKT and MAPK pathway. Evid Based Complement Alternat Med. 2013;2013:690190. doi: 10.1155/2013/690190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh DF, Patel DN, Chan EC, Teo A, Neo SY, Koh HL. Anti-proliferative effects of raw and steamed extracts of Panax notoginseng and its ginsenoside constituents on human liver cancer cells. Chin Med. 2011;6:4. doi: 10.1186/1749-8546-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung NH, Song GY, Park YJ, Kim YH. Two new dammarane-type saponins from the leaves of Panax ginseng. Chem. Pharm. Bull. (Tokyo) 2009;57:1412–1414. doi: 10.1248/cpb.57.1412. [DOI] [PubMed] [Google Scholar]
- Tung NH, Song GY, Minh CV, Kiem PV, Jin LG, Boo HJ, Kang HK, Kim YH. Steamed ginseng-leaf components enhance cytotoxic effects on human leukemia HL-60 cells. Chem. Pharm. Bull. (Tokyo) 2010a;58:1111–1115. doi: 10.1248/cpb.58.1111. [DOI] [PubMed] [Google Scholar]
- Tung NH, Cho K, Kim JA, Song GY, Kim YH. Dammarane-type glycosides from the steamed flower-buds of Panax ginseng. Bull Korean Chem Soc. 2010b;31:1381–1384. [Google Scholar]
- Tung NH, Song GY, Kim JA, Hyun JH, Kang HK, Kim YH. Dammarane-type saponins from the flower buds of Panax ginseng and their effects on human leukemia Cells. Bioorg Med Chem Lett. 2010c;20:309–314. doi: 10.1016/j.bmcl.2009.10.110. [DOI] [PubMed] [Google Scholar]
- Tung NH, Song GY, Nhiem NX, Ding Y, Tai BH, Jin LG, Lim CM, Hyun JW, Park CJ, Kang HK, Kim YH. Dammarane-type saponins from the flower buds of Panax ginseng and their intracellular radical scavenging capacity. J Agric Food Chem. 2010d;58:868–874. doi: 10.1021/jf903334g. [DOI] [PubMed] [Google Scholar]
- Tung NH, Quang TH, Son JH, Koo JE, Hong HJ, Koh YS, Song GY, Kim YH. Inhibitory effect of ginsenosides from steamed ginseng-leaves and flowers on the LPS-stimulated IL-12 production in bone marrow-derived dendritic cells. Arch Pharm Res. 2011;34:681–685. doi: 10.1007/s12272-011-0419-2. [DOI] [PubMed] [Google Scholar]
- Vuksan V, Sievenpipper J, Jovanovski E, Jenkins AL. Current clinical evidence for Korean red ginseng in management of diabetes and vascular disease: a Toronto’s ginseng clinical testing program. J Ginseng Res. 2010;34:264–273. [Google Scholar]
- Wang H, Peng D, Xie J. Ginseng leaf-stem: bioactive constituents and pharmacological functions. Chin Med. 2009;4:20. doi: 10.1186/1749-8546-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang Y, Wang Z, Li S, Min G, Wang L, Chen J, Cheng J, Wu Y. Inhibitory effect of ginsenoside-Rd on carrageenan-induced inflammation in rats. Can J Physiol Pharmacol. 2012;90:229–236. doi: 10.1139/y11-127. [DOI] [PubMed] [Google Scholar]