Abstract
The present study was aimed to evaluate the antioxidant defense system of cinnamaldehyde in normal, diabetic rats and its possible protection of pancreatic β-cells against its gradual loss under diabetic conditions. In vitro free radical scavenging effect of cinnamaldehyde was determined using DPPH (1,1-diphenyl-2-dipicrylhydrazyl), superoxide radical, and nitric oxide radical. Streptozotocin (STZ) diabetic rats were orally administered with cinnamaldehyde at concentrations of 5, 10 and 20 mg/kg body weight for 45 days. At the end of the experiment, the levels of plasma lipid peroxides and antioxidants such as vitamin C, vitamin E, ceruloplasmin, catalase, superoxide dismutase, reduced glutathione and glutathione peroxidase were determined. A significant increase in the levels of plasma glucose, vitamin E, ceruloplasmin, and lipid peroxides and significant decrease in the levels of plasma insulin and reduced glutathione were observed in the diabetic rats. Also the activities of pancreatic antioxidant enzymes were altered in the STZ-induced diabetic rats. The altered enzyme activities were reverted to near-normal levels after treatment with cinnamaldehyde and glibenclamide. Histopathological studies also revealed a protective effect of cinnamaldehyde on pancreatic β-cells. Cinnamaldehyde enhances the antioxidant defense against reactive oxygen species produced under hyperglycemic conditions and thus protects pancreatic β-cells against their loss and exhibits antidiabetic properties.
Keywords: Cinnamaldehyde, Diabetes, β-Islets, Streptozotocin, Cinnamonum zeylanicum
INTRODUCTION
Diabetes have been estimated to affect 180 million people worldwide in 2000, and this number is expected to double by 2030 (WHO, 2008). Diabetes is associated with an increased generation of reactive oxygen species (ROS) or impaired antioxidant defense systems which results in oxidative damage leading to ROS-mediated diabetic pathogenesis (Pitozzi et al., 2003). Moreover, it has been reported that an enhancement of lipid peroxidation, an alteration in antioxidant enzymes, and impaired glutathione metabolism may cause disturbances to the antioxidant systems in diabetes (Bagri et al., 2009). Chronic hyperglycemia is the key clinical manifestation of diabetes and leads to a series of biochemical events those results in the production of high levels of ROS and eventual oxidative stress (Rajarajeswari and Pari, 2011). The increased generation of free radicals and the subsequent impairment of antioxidant defense capabilities indicate that these processes play a central role in the onset, progression and complications of diabetes (Rolo and Palmeira, 2006). Hyperglycemia increases oxidative stress via the overproduction of ROS (Nogueira et al., 2005). The concentrations of ROS have been modulated by nonenzymatic and enzymic antioxidants (Saxena et al., 1993). Alterations in the activities of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) which are the three primary antioxidant enzymes have been demonstrated in different tissues of diabetic animals (Kakkar et al., 1995).
Free radicals are continuously produced in the body as a result of normal metabolic processes and interactions with environmental stimuli. Oxidative stress results from an imbalance between radical-generating and radical-scavenging systems due to increased free radical production, reduced antioxidant defense activity or both. The implication of oxidative stress in the pathogenesis of diabetes is suggested not only by the generation of oxygen free radicals but also by non-enzymatic protein glycation, the auto-oxidation of glucose (Mullarkey et al., 1990), impaired glutathione metabolism, alterations in antioxidant enzymes, the formation of lipid peroxides and a decrease in ascorbic acid levels (Ravi et al., 2004). In addition to reduced glutathione (GSH), there are other defense mechanisms that may play a major role in protecting against free radicals by eliminating superoxide anion radicals (O2•−), hydrogen peroxide (H2O2), hydroxyl radicals (•OH) and singlet oxygen (1O2) (Soto et al., 2003).
Recent investigations demonstrated that the antioxidant properties of plants can be correlated with the defense mechanism against oxidative stress and different human diseases including cancer, and arteriosclerosis and aging processes (Rolo and Palmeira, 2006). Antioxidants can interfere with the oxidative process by reacting with free radicals, chelating free catalytic metals and acting as oxygen scavengers. Phenolic antioxidants function as free radical terminators and sometimes as metal chelators (Sanchez-Moreno et al., 1999). Thus, antioxidant defense systems have been co-evolved with aerobic metabolism to counteract oxidative damage from ROS.
We have isolated the potential antidiabetic compound cinnamaldehyde from the bark of Cinnamonum zeylanicum (Subash-Babu et al., 2007). It has been well established that cinnamaldehyde possesses a wide variety of bioactive properties (Lee, 2002; Lee et al., 2003; Qin et al., 2003). However, no information is available regarding the determination of the antioxidant defense system using cinnamaldehyde treatment in streptozotocin (STZ) diabetic rats. We investigated the antioxidant properties of cinnamaldehyde to evaluate its medicinal value and to characterize its natural antioxidant effect which could be used as a food supplement to the diabetic people. In this study, we evaluated the antioxidant defense system of cinnamaldehyde (20 mg/kg body weight [bw]) in normal and diabetic rats and determined its possible protection of pancreatic β-cell against their gradual loss under diabetic conditions.
MATERIALS AND METHODS
Chemicals
STZ was purchased from Sigma Chemicals Co., St. Louis, MO, USA. All other chemicals used were of analytical grade.
Plant material
Cinnamonum zeylanicum Blume. (Lauraceae) bark was collected from the Kanyakumari district, Tamil Nadu, India, and dried in the shade. The species was identified and authenticated by Dr. D. Narasimhan, Taxonomist, Department of Botany, Madras Christian College, Chennai, and a voucher specimen (MPC-301) was deposited at the Ethnopharmacology Research Unit, Loyola College, Chennai, Tamil nadu, India.
Isolation and identification of the active compounds
Based on a bioassay-guided fractionation, the active compound was isolated and identified; this compound was shown to decrease plasma glucose levels. The active isolate was purified by repeat of column chromatography, and the structure of cinnamaldehyde was determined on the basis of a spectral analysis (Subash-Babu et al., 2007).
In vitro free radical scavenging assay
In vitro DPPH assay: The hydrogen-donating ability of cinnamaldehyde was examined in the presence of a DPPH stable radical using the method of Blois with a slight modification (Blois, 1958). The reaction mixture contained 1.0 ml of 0.1 mM DPPH-ethanol solution, 1.0 ml of ethanol, 0.95 ml of 0.05 M Tris-HCl buffer (pH 7.4), and 50 μl of cinnamaldehyde in different concentrations (2.5, 5, 10, 20 and 40 μg). Reduction of the DPPH free radical was measured by reading the absorbance at 517 nm at exactly 30 sec after adding different concentrations of cinnamaldehyde. Vitamin C was used as a positive control. The inhibition percentage (%) of the radical scavenging activity was calculated using the following equation:
Inhibition (%) = (Ac-As/Ac)×100, where Ac is the absorbance of the control, and As is the absorbance of the sample at 515 nm.
From the inhibition (%), the amount of the sample (μg) reducing the absorbance by 50% was determined (IC50).
Superoxide anion scavenging activity
The superoxide anion scavenging activity of cinnamalde-hyde was determined according to the method of Nishimiki et al. (1972) with slight modifications. Briefly, generation of su-peroxide anion was measured in a reaction mixture containing 160 mM NADH, 40 mM Nitro blue tetrazolium (NBT) and 8 mM phenazine methosulphate (PMS) in phosphate buffered saline (PBS) at pH 7.4. The reduction of NBT was followed by measuring the change in absorbance at 560 nm for 2 min, a period in which the absorbance increased linearly. Different doses of cinnamaldehyde were prepared in dimethylsulfoxide (DMSO) and added to the reaction mixture to give a final concentration of 0.7% DMSO. The activity of the test compound was determined in comparison with the control sample.
Scavenging of NO radical
Nitric oxide was generated from sodium nitropruside and measured by Griess’ reaction as described by Green et al. (1982). Sodium nitropruside (5 mM) in standard phosphate buffer saline solution (0.025 M, pH: 7.4) was incubated with different concentrations (2–200 μg/ml) of cinnamaldehyde dissolved in phosphate buffer saline (0.025 M, pH: 7.4) and the tubes were incubated at 27°C for 5 hr. A control without the test compound but with an equivalent amount of methanol was taken. After incubation 5 ml of incubation mixture was removed and diluted with 0.5 ml of Griess’ reagent (1% sulphanilamide, 2% O-phosparic acid and 0.1% naphthylethylenediamine dihydrochloride). The absence of chromophore formed during diazotization of nitrite with sulphanilamide and is subsequent coupling with napthyl ethylene diamine was read at 546 nm against blank. The experiment was repeated in triplicate.
In vivo biochemical assays
Estimation of thiobarbituric acid reactive substances (TBARS) and hydroperoxides (HP): Plasma thiobarbituric acid reactive substances (TBARS) and hydroperoxides (HP) were determined by the methods of Yagi (1976) and Jiang et al. (1992) respectively. Briefly, 0.1 ml of Plasma/tissue homogenate was treated with 2 ml of (1:1:1 ratio) TBA-TCA-HCL (TBA 0.37%, 0.25 N HCL and 15% TCA) reagent and placed in water bath for 15 mints, cooled and centrifuged and then clear supernatant was measured at 535 nm against reference blank.
Hydroperoxides was expressed as mM/dl. 0.1 ml of Plasma/tissue homogenate was treated with 0.9 ml of Fox reagent (88 mg BHT, 7.6 mg xylenol orange and 0.8 mg ammonium iron sulphate were added to 90 ml of methanol and 10 ml of 250 mM sulphuric acid) and incubated at 37°C for 30 mint. The colour developed was read at 560 nm.
Determination of non-enzymatic antioxidants
Reduced glutathione (GSH) was determined by the method of Ellman (1959). 1 ml of plasma/tissue homogenate was taken and 0.5 ml of Ellmans’s reagent (0.0198% DTNB in 1% sodium citrate) and 3 ml of phosphate buffer (pH-8.0) were added. The colour developed was read at 412 nm.
Ascorbic acid (vitamin C) concentration was measured by Omaye et al. (1979) method. To 0.5 ml of plasma/tissue homogenate 1.5 ml of 6% TCA was added and centrifuged (3,500 g, 20 min). To 0.5 ml of supernatant, 0.5 ml of DNPH reagent (2% DNPH and 4% thiourea in 9N sulphuric acid) was added and incubated for 3 hours at room temperature. After incubation 2.5 ml of 85% sulphuric acid was added and colour developed was read at 530 nm after 30 min.
Vitamin E was estimated by the method of Desai (1984). Vitamin E was extracted from plasma/tissue homogenate by addition of 1.6 ml ethanol and 2.0 ml petroleum ether to 0.5 ml plasma and centrifuged. The supernatant was separated and evaporated. To the residue, 0.2 ml of 0.2% 2,2’-dipyridyl, 0.2 ml of 0.5% Ferric chloride was added and kept in dark for 5 min. An intense red colored layer obtained on addition of 4 ml butanol was read at 520 nm. And plasma ceruloplasmin was determined according to the methods of Ravin (1961).
Determination of enzymic antioxidants
Superoxide dismutase (SOD) activity was determined by the modified method of NADH-Phenazinemethosulphate-nitroblue tetrazolium formazon inhibition reaction spectrophotometrically at 560 nm (Misra and Fridovich, 1972). A single unit of enzyme was expressed as 50% inhibition of NBT (Nitroblue tetrazolium) reduction/min/mg protein. Catalase (CAT) was assayed colorimetrically as described by Takahara et al. (1960) using dichromate-acetic acid reagent (5% potassium dichromate and glacial acetic acid were mixed in 1:3 ratio). The intensity was measured at 620 nm and the amount of hydrogen peroxide hydrolyzed was calculated for the catalase activity.
Glutathione peroxidase (GPx) activity was measured by the method described by Rotruck et al. (1973). The activity was expressed based on inhibition of GSH. Protein was determined by the method of Lowry et al. (1951) using Bovine Serum Albumin (BSA) as standard, at 660 nm.
Histopathological study
A portion of pancreatic tissue was fixed in 10% buffered neutral formal saline for histological studies. After fixation, tissues were embedded in paraffin; solid sections were cut at 5 μm and stained with aldehyde. The sections were examined under light microscope and photomicrographs were taken.
Statistical Analysis
Statistical analysis was performed using the SPSS software package, version 6.0. The values were analyzed by one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test (DMRT) (Duncan, 1957). All the results were expressed as the mean ± SD for six rats in each group. p values less than 0.05 were considered significant.
RESULTS
In vitro free radical scavenging effect of cinnamaldehyde
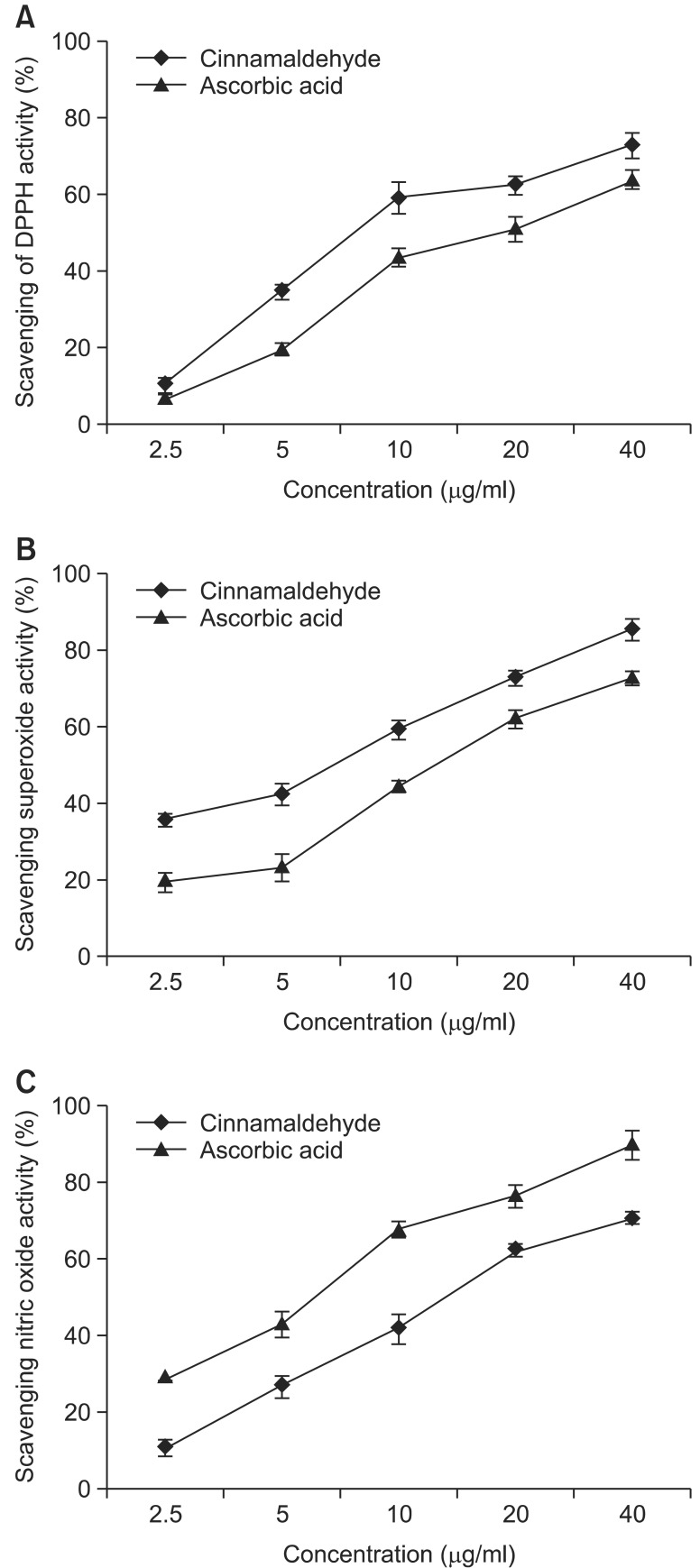
The free radical scavenging effects of cinnamaldehyde on DPPH, superoxide radical, and NO radical were determined. The results are shown in Fig. 1. On a comparative basis, cinnamaldehyde exhibited better free radical scavenging activity in quenching DPPH, with an IC50 value of 8.2 μg/ml, and the superoxide radical, with an IC50 value of 13.3 μg/ml. Cinnamaldehyde also exhibited a better response in quenching NO radicals, with an IC50 value of 12.1 μg/ml. The results were compared with ascorbic acid as a positive control, indicating the potential antioxidant properties of cinnamaldehyde.
Fig. 1.
Comparison of abilities to scavenge DPPH (A), superoxide (B) and nitric oxide (C) radicals in vitro between cinnamaldehyde and ascorbic acid.
Effect of cinnamaldehyde on plasma glucose levels
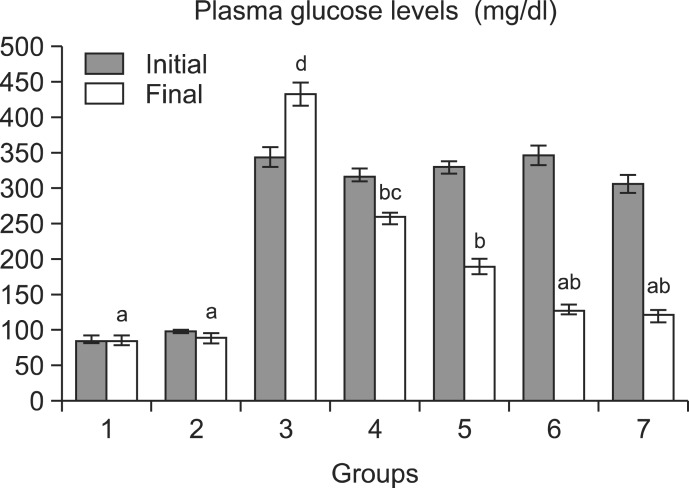
Fig. 2 shows the plasma glucose levels in the normal and experimental rats. The STZ-diabetic rats showed a significant increase in the levels of plasma glucose compared with normal rats. Oral administration of cinnamaldehyde at a dose of 20 mg/kg bw resulted in a highly significant (p<0.05) effect compared with 5 and 10 mg/kg bw doses. Therefore, cinnamaldehyde was selected for further biochemical studies.
Fig. 2.
Effect of cinnamaldehyde on plasma glucose levels (mg/dl) in streptozotocin-induced diabetic male Wistar rats. Group 1: Normal; Group 2: Normal+Cinnamaldehyde (20 mg/Kg bw); Group 3: Diabetic control; Group 4: Diabetic+Cinnamaldehyde (5 mg/kg bw); Group 5: Diabetic+Cinnamaldehyde (10 mg/kg bw); Group 6: Diabetic+Cinnamaldehyde (20 mg/kg bw); Group 7: Diabetic+Glibenclamide (0.6 mg/kg bw). Each value is mean ± SD for 6 rats in each group. Values not sharing a common superscript differ significantly at p<0.05 (DMRT).
Effect of cinnamaldehyde on plasma insulin levels
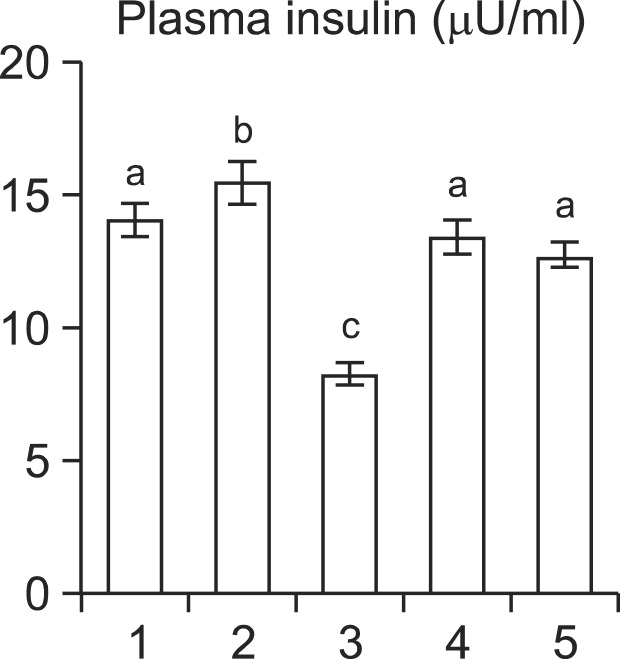
Fig. 3 shows the plasma insulin levels of normal and experimental rats. Plasma insulin level was significantly (p<0.05) decreased in diabetic rats when compared to the normal levels. Oral administration of cinnamaldehyde significantly (p<0.05) increased the plasma insulin levels compared with the levels observed in the untreated diabetic rats. The results were compared with glibenclamide, a standard reference drug.
Fig. 3.
Effect of cinnamaldehyde on plasma insulin levels (μU/ml) in streptozotocin-induced diabetic male Wistar rats. Group1: Normal; Group 2: Normal+Cinnamaldehyde (20 mg/Kg bw); Group 3: Diabetic control; Group 4: Diabetic+Cinnamaldehyde (20 mg/kg bw); Group 5: Diabetic+Glibenclamide (0.6 mg/kg bw). Each value is mean ± SD for 6 rats in each group. Values not sharing a common superscript differ significantly at p<0.05 (DMRT).
In vivo antioxidant effect of cinnamaldehyde
Table 1 shows the levels of TBARS and HP and nonenzymic antioxidants such as GSH, vitamin C, vitamin E and ceruloplasmin in the plasma of normal and STZ-diabetic rats. The rats treated with cinnamaldehyde alone showed increased levels of TBARS, HP and ceruloplasmin levels when compared with normal rats, but the differences were not significant. The diabetic rats showed a significant (p<0.05) increase in plasma TBARS, hydroperoxides, vitamin E and ceruloplasmin levels, whereas the diabetic rats showed a significantly (p<0.05) decreased levels of vitamin C compared with normal rats. The altered levels of lipid peroxides and non-enzymic antioxidants in diabetic rats have been significantly (p<0.05) restored to near control levels by the administration of cinnamaldehyde and glibenclamide.
Table 1.
Effect of cinnamaldehyde on plasma levels of TBARS, hydroperoxides, and non-ezymatic antioxidants in male Wistar rats with and without diabetes
| Groups | Normal | Normal + cinnamaldehyde (20 mg/kg bw) | Diabetic control | Diabetic + cinnamaldehyde (20 mg/kg bw) | Diabetic + glibenclamide (0.6 mg/kg bw) |
|---|---|---|---|---|---|
| TBARS (nMoles/ml) | 2.02 ± 0.12a | 2.18 ± 0.05a,b | 4.98 ± 0.15d | 2.24 ± 0.08b | 2.65 ± 0.09c |
| Hydroperoxides (valuex10−5 mMoles/dl) | 7.23 ± 0.28a | 7.84 ± 0.61a,b | 14.97 ± 0.74d | 8.10 ± 0.25b | 9.18 ± 0.29c |
| GSH (mg/dl) | 28.81 ± 1.19a | 30.10 ± 1.27a,b | 17.92 ± 0.77c | 26.37 ± 0.82a | 24.16 ± 0.57b |
| Vitamin C (mg/dl) | 1.71 ± 0.14a | 1.83 ± 0.10a | 0.85 ± 0.04c | 1.67 ± 0.03a | 1.59 ± 0.08a,b |
| Vitamin E (mg/dl) | 1.82 ± 0.25a | 1.78 ± 0.42a | 3.42 ± 0.37b | 1.68 ± 0.40a | 1.70 ± 0.39a |
| Ceruloplasmin (mg/dl) | 18.23 ± 1.75a | 22.94 ± 1.19a,b | 32.18± 2.01c | 20.63 ± 1.41a,b | 22.11 ± 1.26b |
Each value is mean ± SD for 6 rats in each group.
Values not sharing a common superscript differ significantly at p<0.05 (DMRT).
Table 2 shows the concentration of TBARS, HP and GSH and the activities of enzymic antioxidants such as GPx, SOD and CAT in the pancreas of normal and diabetic rats. In diabetic rats, we observed significantly (p<0.05) increased levels of TBARS and HP and diminished levels of GSH, SOD, CAT and GPx compared with the normal rats. We also observed decreased activities of GPx and CAT in the rats treated with cinnamaldehyde alone compared with the normal rats. The oral administration of cinnamaldehyde and glibenclamide significantly (p<0.05) decreased the levels of lipid peroxides and increased the enzymic antioxidant activities and the GSH levels in the pancreas of diabetic rats compared with the diabetic control rats.
Table 2.
Effect of cinnamaldehyde on pancreatic levels of TBARS, hydroperoxides, GSH, and enzymatic antioxidants in male Wistar rats with and without diabetes
| Groups | Normal | Normal + cinnamaldehyde (20 mg/kg bw) | Diabetic control | Diabetic + cinnamaldehyde (20 mg/kg bw) | Diabetic + glibenclamide (0.6 mg/kg bw) |
|---|---|---|---|---|---|
| TBARS | 32.40 ± 2.20a | 31.75 ± 2.30a | 67.28 ± 3.90c | 38.91 ± 3.10b | 36.27 ± 2.90a,b |
| Hydroperoxides | 12.65 ± 0.56a | 12.66 ± 1.18a | 22.16 ± 0.87b | 12.24 ± 0.51a | 13.03 ± 0.35a |
| GSH | 19.34 ± 0.62b | 20.46 ± 1.14b,c | 11.67 ± 1.18a | 21.43 ± 0.80c | 20.94 ± 0.61c |
| GPx | 28.31 ± 1.08c | 25.081.65b | 17.13 ± 0.56a | 26.44 ± 1.54b,c | 25.95 ± 1.44b,c |
| SOD | 4.05 ± 0.17b | 4.930.22c | 2.53 ± 0.34a | 3.96 ± 0.23b | 3.67 ± 0.22b |
| CAT | 17.90 ± 0.54e | 16.090.47d | 7.81 ± 0.29a | 15.17 ± 0.58c | 14.40 ± 0.42b |
Units: TBARS- nmols/100 g tissue; HP- nmols/100 g tissue; GSH- m moles/ g wet tissue; GPX - μg GSH consumed/ min/ mg protein; SOD-Enzyme concentration required to inhibit the O.D at 560nm of chromogen production by 50% in one min / mg protein; CAT- μmoles of H2O2 consumed/ min/ protein.
Each value is mean ± SD for 6 rats in each group.
Values not sharing a common superscript differ significantly at p<0.05 (DMRT).
Histopathological observation of the pancreas
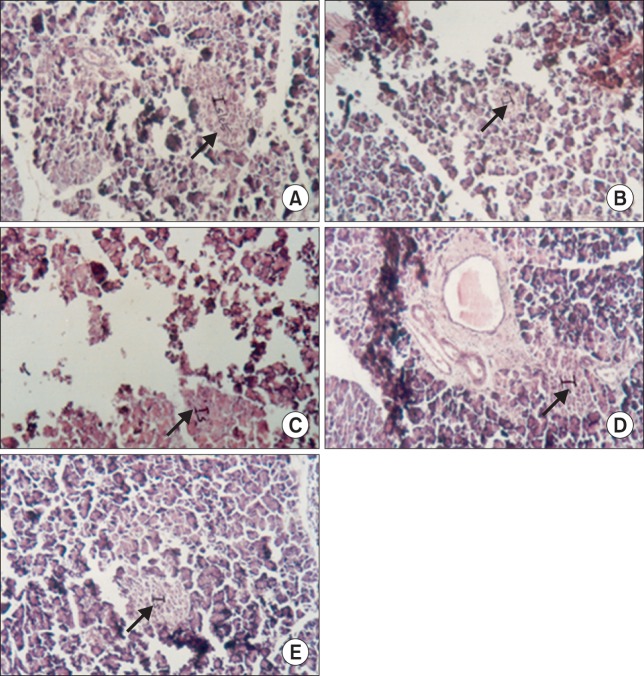
The histopathological examination revealed a preventive effect of cinnamaldehyde administration on extensive alteration in the pancreas of STZ-induced diabetic rats (Fig. 4). Fig. 4A depicts the pancreas of a normal rat, showing a normal architecture of the islets. Fig. 4B depicts the pancreas of a normal rat treated with cinnamaldehyde. The pancreas appeared to have normal islets. Fig. 4C depicts the pancreas of a diabetic rat, showing pancreatic acini and shrunken islet cells. Fig. 4D depicts the pancreas of a diabetic rat treated with cinnamaldehyde, showing regenerated pancreatic islets. Fig. 4E depicts the pancreas of a diabetic rat treated with glibenclamide, showing the regeneration of the pancreatic islets.
Fig. 4.
Histopathological studies of normal and experimental diabetic rat pancreas (A–E). (A) Normal rat pancreas. Pancreas showing β-islets. (B) Normal+Cinnamaldehyde treated rat pancreas. Normal acini with islet cells. (C) Diabetic rat pancreas. Pancreatic acini with destroyed islet cells. (D) Diabetic+Cinnamaldehyde treated rats pancreas. Regenerating islet cells. (E) Diabetic+glibenclamide treated rat pancreas. Normal islets cells with mild fatty change.
DISCUSSION
Diabetes is one of the pathological processes known to be related to an unbalanced production of ROS, such as hydroxyl radicals (•OH), superoxide anions (O2), and H2O2. Therefore, cells must be protected from this oxidative injury by antioxidant enzymes (Mullarkey et al., 1990). Antioxidants may offer resistance against oxidative stress by scavenging free radicals, inhibiting lipid peroxidation by many other mechanisms and preventing disease (Qujeq and Rezvani, 2007). DPPH is a stable free radical that is often used to evaluate the antioxidant activity of several natural compounds (Miller and Rice-Evans, 1997). Antioxidants interact with DPPH and thus neutralize its free-radical character.
SOD catalyzes the dismutation of the highly reactive su-peroxide anion to form oxygen and hydrogen peroxide (Rajarajeswari and Pari, 2011). The superoxide anion is the first reduction product of oxygen, which is measured in terms of the inhibition of generation of O2•. NO is a free radical produced in mammalian cells and is involved in the regulation of various physiological processes. However, an excessive production of NO is associated with several diseases (Yokozawa et al., 1998). NO is a very unstable species under aerobic conditions. NO reacts with O2 to produce the products nitrate and nitrite via the intermediates NO2, N2O4 and N3O4. The level of NO is estimated using Griess’ reagent. NO produced by a solution of sodium nitroprusside was scavenged by the presence of cinnamaldehyde, and the production of nitrous acid has been scavenged by the presence of cinnamaldehyde. This result may be due to the antioxidant principle whereby cinnamaldehyde competes with oxygen to react with nitric oxide (Ray and Husain, 2002).
STZ-diabetes resulted an increase in lipid peroxidation, which occurs indirectly by the intensified free radical production (Ialenti et al., 1993). Most of the tissue damage is considered to be mediated by free radicals, which attack the membrane via the peroxidation of unsaturated fatty acids (Maritim et al., 2003). Increased levels of peroxides are observed in the plasma due to the consequence of the increased free radical production, and the lipid peroxides are liberated into circulation due to pathological changes. A marked increase in the levels of TBARS may be due to an increased susceptibility of pancreas to lipid peroxidation (Mullarkey et al., 1990). The oral administration of cinnamaldehyde significantly protects against the formation of lipid peroxides. This result suggests that the protective role of cinnamaldehyde could be due to the antioxidative effect of cinnamaldehyde. In addition, oral administration of glibenclamide also significantly decreased TBARS and HP levels in the pancreas of diabetic rats. The alterations in lipid peroxide and antioxidant levels in the rats treated with cinnamaldehyde alone were not significant, which may be due to the counter-defense mechanism between the native and developed antioxidant defense systems.
The plasma protein ceruloplasmin is a powerful free radical scavenger that inhibits lipid peroxidation by binding to copper (Cao et al., 1997). Ceruloplasmin levels increased under conditions leading to the generation of oxygen products such as the superoxide radical and hydrogen peroxide. The observed rise in the plasma ceruloplasmin levels in the diabetic rats may be due to the increased levels of lipid peroxides (Halliwell and Gutteridge, 1990).
GSH, the most important biomolecule protecting against chemically induced toxicity, can participate in the elimination of reactive intermediates via reduction of hydroperoxides in the presence of GPx (Rajarajeswari and Pari, 2011). The observed decrease in the GSH level in diabetic rats represents an increased utilization of GSH due to oxidative stress (Dormandy, 1980). It appears that generation of oxygen radicals by increased levels of glucose causes utilization of GSH and thus lowers GSH levels in plasma and pancreas. Oral administration of cinnamaldehyde and glibenclamide increased the glutathione content in the diabetic animals.
Vitamin C, or L-ascorbic acid, is considered to be the most important antioxidant in extracellular fluids, and it has many cellular activities of an antioxidant nature (Stocker and Frei, 1991). Vitamin C functions as a free radical scavenger of active and stable oxy radicals. The marked decrease in the plasma levels of ascorbic acid levels might be due to its increased utilization in antioxidant defense against increased levels of ROS or to a decrease in the GSH levels because GSH is required for the recycling of vitamin C (Moser and Bendich, 1991). Ascorbic acid can also protect membranes against peroxidation by enhancing the activity of a tocopherol, the chief lipid-soluble, chain breaking antioxidant (Wefers and Sies, 1988). α-Tocopherol interrupts the chain reaction of lipid peroxidation by reacting with lipid peroxy radicals and protects cell membranes against damage by lipid hydroperoxides (Bhatia and Jain, 2003). The elevated levels of α-tocopherol observed in diabetic rats play a protective role against increased lipid peroxidation in diabetes mellitus (Kinalski et al., 2000). In our study, oral administration of cinnamaldehyde and glibenclamide gradually decreased the α-tocopherol level in a manner that was directly proportional to the production of free radicals by STZ.
Pancreatic islet cells possess very low levels of free radical scavenging enzymes, including SOD, CAT and GPx and are vulnerable to free radical toxicity (Subash Babu and Prince, 2004). Pancreatic β-cells are considered to be exceptionally vulnerable to the cytotoxic actions of oxygen free radicals because of their low levels of antioxidant enzymes (Simmons, 2006). STZ-induced cytotoxicity of islets is reduced by anti-oxidant enzymes. SOD in particular is considered to be the first cellular defense against superoxide radical toxicity (Bhattacharya et al., 1997). The antioxidant activity of cinnamalde-hyde may be due to the inhibition of the glycation of the anti-oxidant enzymes SOD, CAT and GPx. Glucose, which forms a Schiff base with proteins, has been reported to have a high affinity for proteins, especially those containing transition metal ions. Increased glycation of Cu-Zn-SOD has been reported in diabetes. Many reports have shown that curcumin inhibits the formation of advanced glycation end products in STZ-induced diabetic rats (Jia et al., 2009). In our previous study, we observed the decreased glycosylation of hemoglobin (HbA1c) in cinnamaldehyde-treated diabetic rats (Subash-Babu et al., 2007). In the present study, we concluded that the increased activities of CAT and GPx in the cinnamaldehyde-treated STZ-diabetic rats might be due to the prevention of glycation.
An increase in the activities of the enzymes SOD and CAT may protect β-cells against damage from ROS. In this context, treatment with the antioxidant N-acetyl cysteine in diabetic mice was shown to suppress apoptosis in β-cells and decrease blood glucose levels (Subash-Babu et al., 2009). A histopathological observation also revealed that an alteration occurred in the architecture of the pancreatic islets in STZ-diabetic rats.
Reduction of β-cell mass is critical in the pathogenesis of diabetes mellitus. The discovery of agents that induce regeneration of pancreatic β-cells would be useful to develop a new therapeutic approach to treat diabetes. After 45 days of oral administration of cinnamaldehyde, blood glucose returned to normal levels, and the islets of Langerhans showed an improvement in the β-cell granulation. The plasma glucose lowering activity was compared with glibenclamide a standard hypoglycemic drug. Glibenclamide has been used for many years to treat diabetes by stimulating insulin secretion from pancreatic β-cells. Sharma et al. (2003) have also reported an improvement of the islet after one month of oral feeding of E. jambolana seed powder and some alkaloids. Our results are consistent with previous reports suggesting that the regeneration of islet cells by dietary components and the stimulation of insulin secretion by different plant extracts have been achieved (Sharma et al., 2003).
In conclusion, the present study demonstrates that cinnamaldehyde decreased the levels of lipid peroxidation products and increased the activities of antioxidant enzymes. The results of the present study indicated the glucose lowering effect of cinnamaldehyde due to the insulin release from pancreatic β-cells of the islets of Langerhans. Cinnamaldehyde augmented the release of insulin many folds probably by protecting β-cells from free radicals through its antioxidant activity and stimulation of β-cells resembling direct insulin secretagogue effect. Histological assessment also showed that the damage caused by STZ in pancreatic β-cells was markedly reduced, and that the damaged pancreatic β-cells were regenerated by the administration of cinnamaldehyde. Thus, it is suggested that cinnamaldehyde plays an antioxidant role in addition to its antidiabetic activity.
Acknowledgments
The authors would like to extend their sincere thanks to the Deanship of Scientific Research, King Saud University for its funding of this research through the Research Group Project no: RGP- VPP-276.
REFERENCES
- Bagri P, Ali M, Aeri V, Bhowmik M, Sultana S. Antidiabetic effect of Punica granatum flowers: effect on hyperlipidemia, pancreatic cells lipid peroxidation and antioxidant enzymes in experimental diabetes. Food Chem Toxicol. 2009;47:50–54. doi: 10.1016/j.fct.2008.09.058. [DOI] [PubMed] [Google Scholar]
- Bhatia AL, Jain M. Amaranthus paniculatus(Linn.) improves learning after radiation stress. J Ethnopharmacol. 2003;85:73–79. doi: 10.1016/s0378-8741(02)00337-9. [DOI] [PubMed] [Google Scholar]
- Bhattacharya SK, Satyan KS, Chakrabarti A. Effect of trasina, an ayurvedic herbal formulation on pancreatic islet su-peroxide dismutase activity in hyperglycaemic rats. Indian J Exp Biol. 1997;35:297–299. [PubMed] [Google Scholar]
- Blois MS. Antioxidant determinations by the use of stable free radical. Nature. 1958;181:1199–1200. [Google Scholar]
- Cao G, Sofic E, Prior RL. Antioxidant and prooxidant behaviour of flavonoides: structure-activity relationships. Free Radic Bio Med. 1997;22:749–760. doi: 10.1016/s0891-5849(96)00351-6. [DOI] [PubMed] [Google Scholar]
- Desai ID. Vitamin E analysis methods for animal tissues. Methods Enzymol. 1984;105:138–147. doi: 10.1016/s0076-6879(84)05019-9. [DOI] [PubMed] [Google Scholar]
- Dormandy TL. Free radical reactions in biological systems. Ann R Coll Surg Engl. 1980;62:188–194. [PMC free article] [PubMed] [Google Scholar]
- Duncan BD. Multiple range tests for correlated and heteroscedastic means. Biometrics. 1957;13:164–176. [Google Scholar]
- Ellman GC. Tissue Sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Green LC, Wanger DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM. The antioxidants of human extracellular fluids. Arch Biochem Biophys. 1990;280:1–8. doi: 10.1016/0003-9861(90)90510-6. [DOI] [PubMed] [Google Scholar]
- Ialenti S, Moncada M, Rosa D. Modulation of adjuvant arthritis by endogenous nitric oxide. Brit J Pharmacol. 1993;110:701–706. doi: 10.1111/j.1476-5381.1993.tb13868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia J, Zhang X, Hu YS, Wu Y, Wang QZ, Li NN, Guo QC, Dong XC. Evaluation of in vivo antioxidant activities of Ganoderma lucidum polysaccharides in STZ-diabetic rats. Food Chem. 2009;115:32–36. [Google Scholar]
- Jiang ZY, Hunt JV, Wolff SP. Ferrous ion oxidation in the presence of Xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem. 1992;202:384–389. doi: 10.1016/0003-2697(92)90122-n. [DOI] [PubMed] [Google Scholar]
- Kakkar R, Kalra J, Mantha SV, Prasad K. Lipid peroxidation and activity of antioxidant enzymes in diabetic rats. Mol Cell Biochem. 1995;151:113–119. doi: 10.1007/BF01322333. [DOI] [PubMed] [Google Scholar]
- Kinalski M, Sledziewski A, Telejko B, Zarzycki W, Kinalska I. Lipid peroxidation and scavenging enzyme activity in streptozotocin-induced diabtetes. Acta Diabetol. 2000;37:179–183. doi: 10.1007/s005920070002. [DOI] [PubMed] [Google Scholar]
- Lee HS. Inhibitary activity of Cinnamomum cassia bark derived component against rat lens aldolase reductase. J Pharm Pharm Sci. 2002;5:226–230. [PubMed] [Google Scholar]
- Lee JS, Jeon SM, Park EM, Huk TL, Kwon OS, Lee MK, Cois MS. Cinnamate supplementation enhances hepatic lipid metabolism and antioxidant defense systems in high cholesterol-fed rats. J. Med. Food. 2003;6:183–191. doi: 10.1089/10966200360716599. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosenbrough NJ, Farr AL, Randall R. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Maritim AC, Sanders RA, Watkins JB. Effects of alpha-lipoic acid on biomarkers of oxidative stress in streptozotocin-induced diabetic rats. J Nutr Biochem. 2003;14:288–94. doi: 10.1016/s0955-2863(03)00036-6. [DOI] [PubMed] [Google Scholar]
- Miller NJ, Rice-Evans C. Factors influencing the antioxidant activity determined by the ABTS.s radical cation assay. Free Radic Res. 1997;26:195–199. doi: 10.3109/10715769709097799. [DOI] [PubMed] [Google Scholar]
- Misra HP, Fridovich I. The role of superoxide anion in the auto oxidation of epinephrine and a simple assay of superoxide dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- Moser U, Bendich A. Vitamin C. In: Machlin LJ, editor. Handbook of vitamins. 2nd Ed. Marcel Dekker; New York: 1991. pp. 195–232. [Google Scholar]
- Mullarkey CJ, Edelstein D, Brownlee L. Free radical generation by early glycation products: a mechanism for accelerated atherogenesis in diabetes. Biochem Biophys Res Commun. 1990;173:932–939. doi: 10.1016/s0006-291x(05)80875-7. [DOI] [PubMed] [Google Scholar]
- Nishimiki M, Rao NA, Yagi K. The occurance of super-oxide anion in the reaction of reduced phenazine methosulphate and molecular oxygen. Biochem Biophys Res Commun. 1972;46:849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- Nogueira FN, Carvalho AM, Yamaguti PM, Nicolau J. Antioxidants parameters and lipid peroxidation in salivary glands of streptozotocin-induced diabetic rats. Clin. Chim. Acta. 2005;353:133–139. doi: 10.1016/j.cccn.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Omaye ST, Turnabull JC, Sanberlick HE. Selected methods for the determination of ascorbic acid in animal cells, tissues and fluids. Methods Enzymol. 1979;62:3–11. doi: 10.1016/0076-6879(79)62181-x. [DOI] [PubMed] [Google Scholar]
- Pitozzi V, Giovannelli L, Bardini G, Rotella CM, Dolara P. Oxidative DNA damage in peripheral blood cells in type 2 diabetes mellitus: higher vulnerability of polymorphonuclear leukocytes. Mutat Res. 2003;529:129–133. doi: 10.1016/s0027-5107(03)00114-3. [DOI] [PubMed] [Google Scholar]
- Qin B, Nagasaki M, Ren M, Bajotto G, Oshida Y, Sato Y. Cinnamon extract (traditional herb) potentiates in vivo insulin regulated glucose utilization via enhancing insulin signaling in rats. Diabetes Res Clin Pract. 2003;62:139–148. doi: 10.1016/s0168-8227(03)00173-6. [DOI] [PubMed] [Google Scholar]
- Qujeq D, Rezvani T. Catalase (antioxidant enzyme) activity in streptozotocin- induced diabetic rats. Int J Diabetes Metab. 2007;15:22–24. [Google Scholar]
- Rajarajeswari N, Pari L. Antioxidant role of coumarin on streptozotocin-nicotinamide-induced type 2 diabetic rats. J Biochem Mol Toxicol. 2011;25:355–361. doi: 10.1002/jbt.20395. [DOI] [PubMed] [Google Scholar]
- Ravi K, Ramachandran B, Subramaniyan S. Effect of Eugenia jambolana seed kernel on antioxidant defense system in streptozotocin-induced diabetes in rats. Life Sci. 2004;75:2717–2731. doi: 10.1016/j.lfs.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Ravin HA. An improved colorimetric enzymatic assay of ceruloplasmin. J Lab Clin Med. 1961;58:161–168. [PubMed] [Google Scholar]
- Ray G, Husain SA. Oxidants, antioxidants and carcino-genesis. Indian J Exp Biol. 2002;40:1213–1232. [PubMed] [Google Scholar]
- Rolo AP, Palmeira CM. Diabetes and mitochondrial function: role of hyperglycemia and oxidative stress. Toxicol Appl Pharmacol. 2006;212:167–178. doi: 10.1016/j.taap.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium; Biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Sanchez-Moreno C, Larrauri JA, Saura-Calixto F. Free radical scavenging capacity and inhibition of lipid peroxidation of wines, grape juices and related polyphenolic constituents. Food Res Int. 1999;32:407–412. [Google Scholar]
- Saxena AK, Srivastava P, Kale RK, Baquer NZ. Impaired antioxidant status in diabetic rat liver: effect of vanadate. Biochem Pharmacol. 1993;45:539–542. doi: 10.1016/0006-2952(93)90124-f. [DOI] [PubMed] [Google Scholar]
- Sharma SB, Nasir A, Prabhu KM, Murthy PS, Dev G. Hypoglycemic and hypolipidemic effect of ethanolic extract of seeds of Eugenia jambolana in alloxan induced diabetic rabbits. J Ethnopharmacol. 2003;85:201–206. doi: 10.1016/s0378-8741(02)00366-5. [DOI] [PubMed] [Google Scholar]
- Simmons RA. Developmental origins of diabetes: the role of oxidative stress. Free Radic Biol Med. 2006;40:917–922. doi: 10.1016/j.freeradbiomed.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Soto C, Recoba R, Barron H, Alvarez C, Favari L. Silymarin increases antioxidant enzymes in alloxan induced diabetes in rat pancreas. Comp Biochem Physiol C Toxicol Pharmacol. 2003;136:205–212. doi: 10.1016/s1532-0456(03)00214-x. [DOI] [PubMed] [Google Scholar]
- Stocker R, Frei B. In: Endogenous antioxidant defense in human blood plasma. Sies H, editor. Oxidative Stress: Oxidants and antioxidants; Academic Press; London: 1991. pp. 213–243. [Google Scholar]
- Subash Babu P, Prince PSM. Antihyperglycemic and antioxidant effect of Hyponidd, an ayurvedic herbomineral formulation in streptozotocin induced diabetic rats. J Pharm Pharmacol. 2004;56:1435–1442. doi: 10.1211/0022357044607. [DOI] [PubMed] [Google Scholar]
- Subash-Babu P, Prabuseenivasan S, Ignacimuthu S. Cinnamaldehyde- A potential antidiabetic agent. Phytomedicine. 2007;14:15–22. doi: 10.1016/j.phymed.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Subash-Babu P, Ignacimuthu S, Agastian P, Varghese B. Partial regeneration of beta-cells in the islets of Langerhans by Nymphayol a sterol isolated from Nymphaea stellata (Willd.) flowers. Bioorg Med Chem. 2009;17:2864–2870. doi: 10.1016/j.bmc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- Takahara S, Hamilton BH, Nell JV, Kobara TY, Ogura Y, Nishimura ET. Hypocatalasemia, a new genetic carrier state. J Clin Inv. 1960;39:610–619. doi: 10.1172/JCI104075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wefers H, Sies H. The protection by ascorbate and glutathione against microsomal lipid peroxidation is dependent on vita-min E. Eur J Biochem. 1988;174:353–357. doi: 10.1111/j.1432-1033.1988.tb14105.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization Prevalence data of diabetes worldwide. 2008 Available at http://www.who.int/mediacentre/factsheets/fs312/en/index.html, Accessed 24.04.2008. [Google Scholar]
- Yagi K. A simple fluorometric assay for lipid peroxide in blood plasma. Biochem Med. 1976;15:212–216. doi: 10.1016/0006-2944(76)90049-1. [DOI] [PubMed] [Google Scholar]
- Yokozawa T, Chen CP, Dong E, Tanaka T, Nonaka GI, Nishioka I. Study on the inhibitory effect of tannins and flavonoids against the 1,1-diphenyl-2-picrylhydrazyl radical. Biochem Pharmacol. 1998;56:213–222. doi: 10.1016/s0006-2952(98)00128-2. [DOI] [PubMed] [Google Scholar]