Abstract
Angiotensin II (Ang II), an endogenous peptide hormone, plays critical roles in the pathophysiological modulation of cardiovascular functions. Ang II is the principle effector of the renin-angiotensin system for maintaining homeostasis in the cardiovascular system, as well as a potent stimulator of NAD(P)H oxidase, which is the major source and primary trigger for reactive oxygen species (ROS) generation in various tissues. Recent accumulating evidence has demonstrated the importance of oxidative stress in Ang II-induced heart diseases. Here, we review the recent progress in the study on oxidative stress-mediated effects of Ang II in the cardiovascular system. In particular, the involvement of Ang II-induced ROS generation in arrhythmias, cell death/heart failure, ischemia/reperfusion injury, cardiac hypertrophy and hypertension are discussed. Ca2+/calmodulin-dependent protein kinase II is an important molecule linking Ang II, ROS and cardiovascular pathological conditions.
Keywords: Angiotensin II, Oxidative stress, Mitochondria, Arrhythmias, Ischemia-reperfusion, Hypertrophy, Hypertension
INTRODUCTION
Synthesis and metabolism of angiotensin II
Angiotensin (Ang) II, since it was first found in bovine brain in 1985[1], has been well recognized as an endogenous vasoactive octapeptide with a wide biological profile in the development of various cardiovascular disorders, such as hypertension, hypotrophy, arrhythmias, coronary ischemia and congestive heart failure[2–4]. Ang II is a critical component of the renin-angiotensin system (RAS) for the regulation of blood pressure and cardiovascular homeostasis. It derives from an enzymatic cascade[5] and is traditionally considered as a systemic hormone. Renin is released primarily from the juxtaglomerular cells of the kidney into the blood, where it proteolytically cleaves angiotensinogen to form the decapeptide Ang I. Ang I can be subsequently cleaved by angiotensin converting enzyme (ACE) and generates the octapeptide Ang II within the pulmonary circulation[6,7]. In addition to the circulating RAS, many other tissues are also shown to synthesize Ang II in the presence of angiotensinogen, renin and ACE, including heart, vasculature, kidney and brain, which implies the paracrine and intracrine effects of Ang II[6–9].
Ang I is thought to be physiologically inactive. As the major effector for RAS, Ang II can be further degraded to Ang III[10] and Ang IV[11] under the action of aminopeptidase, and both of them exhibit much less biological activity than Ang II. On the other hand, Ang I converting enzyme 2 was later identified from a heart failure ventricle cDNA library and was shown to be a converter for Ang II to Ang (1–7)[12], which can be further cleaved to Ang (1–5) and Ang (1–4)[13]. Ang (1–7) has functional roles to counteract many effects of Ang II. For example, it can lower the blood pressure by directly acting on endothelium cells to promote the synthesis and releasing of nitric oxide (NO) and PGI2[14].
Ang II receptors and cellular signal transduction
Angiotensin receptors[15] with seven transmembrane domains belong to the G-protein coupled receptor superfamily. Four angiotensin receptors (AT1–4R) have been identified so far. They are sensitive to angiotensinogen metabolites and responsible for their signal transductions. Before the cloning of the cDNAs, they were initially defined on their pharmacological and biochemical basis.
AT1R is the best-elucidated angiotensin receptor and is involved in most of the well-known effects of Ang II, such as cell growth, oxidative stress generation and vasoconstriction, etc[16]. AT1R can be found ubiquitously in the cardiovascular system, e.g., the heart, blood vessels, liver, kidney and adrenal gland[17]. It is selectively blocked by losartan or candesartan and couples to various signal transduction pathways that involve G-proteins, intracellular second messengers and protein kinases[18–20]. While it is well accepted that AT1R stimulation promotes NAD(P)H oxidase activation, the detailed mechanism(s) has not been completely understood yet. Some studies have shown that NAD(P)H oxidase activation requires upstream PKC in various tissues, such as brain, vascular smooth muscle (VSM) and mesangial cells[21,22]. In our recent study, however, we did not observe any inhibitory effect of chelerythrine or GF 109203X (selective PKC blockers) on Ang II-induced early after deplorizations (EADs), suggesting PKC may not be involved in NAD(P)H oxidase activation in cardiac myocytes[23]. Consistent with this notion, a novel signal transduction pathway of Ang II-induced reactive oxygen species (ROS) production in cardiomyocytes was reported by Nishida et al[24], who demonstrated that AT1R stimulation by Ang II activates Gα12/13 proteins, which in turn cause Rho/ROCK-mediated Rac1 activation. Rac, one of the small GTP-binding proteins, promotes the production of ROS by activating NAD(P)H oxidase. In addition, another recent study has revealed that the epidermal growth factor receptor (EGFR) kinase and PI-3 kinase may serve as upstream activators for NAD(P)H oxidase in cardiac myocytes[25].
It is well known that Ang II receptors couple to the Gαq protein-phospholipase C (PLC) pathway, in which multiple second messengers such as phosphatidylinositol 4,5-bisphosphate (PIP2), inositol-1,4,5-trisphosphate (IP3), diacylglycerol and Ca2+ are included. This pathway activates PKC and thereby causes phosphorylation of membrane ion channels, for example the ICa,L, as shown by Aiello et al[26] in cat. Whereas, another study carried out by Endoh’s group showed that Ang II may increase ICa,L via AT1R in adult rabbit ventricular myocytes in a PKC-independent manner[27]. Zhao et al[23] have also examined this hypothesis and shown that blocking PKC by chelerythrine or GF 109203X had no effect on Ang II-induced EADs, suggesting direct phosphorylation of ICa,L or INa plays less important roles under our experimental conditions. Although we do not have a ready explanation for these discrepancies, animal species or heart regions might be the potential reasons. Indeed, the wide range species-related differences of Ang II in inotropic effects have been revealed in mammalian cardiac muscle[28].
The intracellular C-terminal of AT1R has been highlighted for receptor signaling and internalization[29]. Previous studies suggested that the C-terminal domain of AT1R provides a binding site for receptor dimerization[30] and also directly interacts with signaling molecules JAK2 and PLCγ 1[31,32]. Specific binding proteins, such as AT1R-associated protein[33] and AT1R C-terminal tail-associated proteins (GLP and ARAP1), were also identified[34,35].
In contrast, the roles of AT2R have not been well established. It was distinguished from AT1R for its high affinity to PD123319, PD123177 and poor affinity to losartan and candesartan[20]. AT2R is highly clustered in the developing fetus, while its expression level is very limited in the adult[17]. However, the expression of AT2R can be up-regulated during pathological states when the tissue remodeling and inflammation takes place[36]. Although the roles of AT2R are still under debate, accumulating evidence implied AT2R may play beneficial roles to counterbalance the deleterious actions of AT1R. For example, as a vasodilator[37], AT2R was hypothesized to couple with bradykinin B2 receptor (B2R) and modulate NO production[38–40]. Abadir et al[41] has recently demonstrated that both NO and cGMP levels were enhanced while the functional heterodimer formed between AT2R and B2R.
AT2R stimulates specific serine/threonine phosphatases such as protein phosphatase 2A, MAPK phosphatase and tyrosine phosphatase SHP-1 in different cell types[37]. As the activation of growth factor requires the phosphorylation of tyrosine kinases, AT2R can attenuate the AT1R-derived effects by phosphorylation at those serine/threonine sites via dephosphorylation at tyrosine. AT1R-induced effects can be antagonized via direct binding of agonist when co-expressed with AT2R subunit[42] and the AT1/2R heterodimers have been detected in fetal fibroblasts and myometrial biopsies.
Other angiotensin receptors showed less pathophysiological significance. AT3R was first obtained by adrenal cortex library screening in 1992[43] but its physiological significance remains unclear. AT4R has been identified in a wide range of tissues, including heart, kidney and VSM cells. Ang IV has a high binding affinity to AT4R[44], while Ang II shows 1000-times lower affinity[45].
Electrophysiological effects on cardiac cells
Ang II plays roles in atrial fibrillation (AF) and other types of cardiac arrhythmias. Zankov et al[46] have studied the effect of Ang II on action potentials (APs) and the slow component of delayed rectifier potassium current (Iks) in guinea pig atrial myocytes. Ang II increases the Iks and shortens AP duration in a concentration-dependent manner and Ang II-potentiated Iks is attenuated by the AT1 receptor blocker valsartan and PKC inhibitors, indicating that the enhanced Iks is mediated via a PKC signaling cascade[46]. Furthermore, selective AT1 receptor blocker losartan attenuates Ether-A-Go-Go Related Gene (HERG) currents (or IKr), as well as prolongs the duration of APs and affects QT dispersion[18].
Ang II also activates other potassium currents in ventricular myocytes. AT1R blocker irbesartan induces time-dependent and concentration-dependent blockage of hKv1.5 and Kv4.3 channels, which are thought to form transient outward potassium currents (Ito)[47]. Yu et al[48] made an interesting observation on the transmural gradient of Ito in dog ventricle. A notch due to the presence of Ito was seen in a typical AP measured on epicardium but not endocardium. However, this notch can be induced in endocardium by a pretreatment with losartan without altering the AT1R expression level, suggesting the up-regulation of Ito at the endocardial layer.
In addition to the potassium currents, outward rectifying Cl− current is also activated by Ang II in the rabbit sino-atrial node[49] and ventricular myocytes[50]. Ang II-activated Cl− current is blocked by losartan and shown to be mediated by the PKC pathway[49]. It has been demonstrated that Ang II may inhibit cardiac protein kinase A-dependent Cl− conductance through inhibition of adenylate cyclase via pertussis toxin-sensitive G proteins[51].
As mentioned above, ICa,L is activated by Ang II in either a PKC-dependent[26] or -independent manner[27]. We have determined that Ang II-induced activation of ICa,L is most likely mediated by the activation of Ca2+/Calmodulin- Dependent Protein Kinase II (CaMKII) oxidized by ROS[23].
Ang II and oxidative stress
Oxidative stress describes an imbalance state while the production of ROS, including superoxide (O2−), hydrogen peroxide (H2O2) and hydoxyl radicals (OH), exceeds antioxidant defenses. There are several enzyme systems contributing to the formation of ROS, including NAD(P)H oxidase, xanthine oxidase and mitochondrial electron leakage from electron transport chain. ROS are normally generated as a natural byproduct of oxygen metabolism and play important roles in cell signaling. However, ROS levels can be increased dramatically under oxidative stress conditions, such as heart failure, ischemia-reperfusion and aging.
NAD(P)H oxidase, which has seven NOX isoforms, is a membrane-bound, heteromeric enzyme complex distributed throughout the endothelial cells, VSM cells[52] and cardiac myocytes[53]. NAD(P)H oxidase-generated ROS was initially recognized as a major source for vasculature ROS[54] and later for cardiac ROS as well[53]. Previous studies have demonstrated that the Ang II can double the vascular oxidants (ROS) production in vivo in a NAD(P)H-dependent manner[55]. It is well accepted that AT1R activation stimulates NAD(P)H oxidase. Using intracellular NAD(P)H and NADH as electron donors, the activated NAD(P)H oxidase will catalyze the conversion of extracellular molecular oxygen to O2−[52,56]. O2 − is unstable; it will be rapidly modified by superoxide dismutase (SOD) and generate a more stable and membrane permeable form as H2O2[52].
It is interesting that the NAD(P)H oxidase-generated ROS was proposed to serve as an initial triggering for further ROS generation by other sources, such as mitochondria. This process is referred to as mitochondrial “ROS-induced ROS release” (RIRR)[57]. Mitochondria are the major source of ROS in cells. Both complex I and complex III of the mitochondrial electron transport chain (METC) are involved in the generation of O2 to O2−. Two principles in regard to the increasing of mitochondrial ROS formation have been suggested as (1) increased O2− generation at the METC; and (2) decreased elimination of O2− and H2O2 in mitochondrial matrix[58]. At least three mechanisms have been proposed to be involved in RIRR: (1) increased triggering of ROS causes mitochondrial depolarization via activation of the mitochondrial permeability transition pore (mPTP) and subsequently gives rise to a short-lived burst of ROS originating from METC; (2) increased ROS reaches a threshold level that triggers opening of the requisite mitochondrial membrane anion channels, which causes a brief increase in METC-derived ROS; and (3) mitochondrial ROS can be produced under the stimulation of cytosolic ROS by opening redox sensitive mitochondrial ATP-sensitive potassium (mitoKATP) channels[59–61].
Furthermore, recent studies have hypothesized that Ang II may promote the ROS generation in mitochondria via the RIRR mechanism[59,61]. The NAD(P)H oxidase-derived ROS stimulated by Ang II may serve as a trigger to induce mitoKATP channel opening and mPTP formation, depolarize mitochondrial membrane potential (Δψm) and lead to the mitochondrial ROS burst[62].
Although the detailed molecular mechanisms for the Ang II signaling on NAD(P)H oxidase are still under investigation, a range of possible upstream mediators for NAD(P)H activation have been hypothesized. For instance, PLA2, PKD, PKC, EGFR, PI3K, Rac and c-Src are in the candidate lists. Ang II can activate the PLA2, PLD and PKC phosphorylation pathway via binding to AT1R and subsequently activate a cytosolic oxidase subunit p47phox, which may migrate to the plasma membrane to participate in the oxidation process[63]. Mollnau et al[64] discovered the importance of the PKC regulation pathway. Their results showed the Ang II-derived ROS production was reduced by the treatment with the PKC inhibitor chelerythrine, which also dramatically inhibited the up-regulation of NAD(P)H oxidase subunits.
Previous evidence has shown that Ang II stimulates the activation of MAPK in vivo[65]. Kimura et al[66] further suggested that cardiac mitochondrial RIRR may be mediated by JNK and MAPK. In addition, a recent study[67] revealed that SS-31, a mitochondria targeted antioxidant peptide, reduces mitochondrial ROS in neonatal cardio-myocytes and subsequently inhibits the downstream signaling for fibrosis and apoptosis with reduced activation of MAPK. It has also been reported that Rac GTPase directly modulates NAD(P)H oxidase activity by interacting with its components (e.g., gp91phox, p22phox, p47phox and p67phox subunits) and forming an active enzyme complex[68].
Recent studies by Montezano et al[69] have identified the roles of Nox5, a homolog of gp91phox, in endothelial cells for Ang II and endothelium-1 signaling, which is calcium/calmodulin-dependent but Rac-1-independent. These data link the calcium/calmodulin regulation pathway to the ROS generation and imply its potential importance in various cardiovascular conditions. Palomeque et al[70] postulated that the Ang II-induced ROS production is dependent on the activity of CaMKII; the presence of Ang II or ROS can reset the activation of CaMKII for apoptotic cascade signaling at subdiastolic calcium concentrations. We have also shown that either exogenously applied ROS (H2O2) or endogenously generated ROS by Ang II stimulation can induce ventricular arrhythmias via the CaMKII signaling pathway[23,71]. By the treatment with the CaMKII inhibitor KN-93, the occurrence of Ang II-induced EADs is attenuated.
In addition to its direct action on the activity of enzymes, Ang II facilitates the nitric oxide synthase (NOS) uncoupling by enhancing the superoxide production and the uncoupled NOS further up-regulates the superoxide level in vasculature and accelerates the endothelial dysfunction[64].
Therefore, Ang II has been recognized as a potent activator for NAD(P)H oxidase. NAD(P)H oxidase-derived ROS further promotes the ROS generation from mitochondria (i.e., RIRR). In the following sections, we will discuss the involvement of Ang II in detail, especially via oxidative stress activation in several severe cardiovascular disorders.
ARRHYTHMIAS
Compelling evidence has suggested that the ROS are arrhythmogenic factors[72]. AF is the most common cardiac arrhythmia with high morbidity and mortality risk and chronic AF causes myocardial ischemia with abnormal calcium handling and ventricular perfusion[73]. In patients with chronic AF, the densities of Ica,L, Ito and Ikur were markedly reduced[74]. A recent study further pointed out that AT1R is the key factor for arrhythmia generation, as the major arrhythmic effects usually occur followed by the activation of AT1R[75]. Zankov et al[46] exhibited the activation of AT1 receptor led to marked potentiation on IKs current in atrial myocytes. Such potentiation can be induced by Ang II at nM range and indicates a potential mechanism for Ang II-induced AF. In another study, Goette et al[76] noted the occurrence of AF involves the down-regulation of AT1R and up-regulation of AT2R, suggesting AT2R may also be involved. Goette et al[77] revealed the importance of RAS as a potential molecular basis to link oxidase stress and microvascular flow abnormalities in ventricles. In their study, atrial tachyarrhythmia was induced by rapid atrial pacing in pigs and abnormal coronary flow reserve was measured. The changes in microcirculatory blood flow was accompanied with the elevation in ventricular expression levels for Nox2, lectinlike oxidized low-density lipoprotein receptor-1 (LOX-1) and F2-isoprostane levels; these increases cannot be found in animals treated with AT1R inhibitor irbesartan, suggesting the roles of oxidative stress in abnormal flow regulation. Evidence showed beneficial effects of selective AT1R blocker candesartan and ACE inhibitor captopril on atrial electrical remodeling caused by rapid atrial pacing[78].
We have also shown the potential mechanisms for abnormal afterdepolarizations and triggered activities induced by oxidative stress in rabbit ventricular myocytes[23,71]. The correlation between ROS generation by NAD(P)H oxidase and EADs induction by Ang II has been clearly demonstrated[23]. The EADs were induced after the application of Ang II (1–2 µmol/L) and were eliminated after exposure to NAD(P)H oxidase inhibitor (aposanin) and antioxidants trolox or MnTMPyP, supporting the hypothesis that the NAD(P)H oxidase-derived ROS are involved in EAD generation. The involvement of Ca2+/Calmodulin-sensitive CaMKII was examined using a CaMKII inhibitor and the data showed that Ang II generated EADs were suppressed by KN-93 and inhibitory peptide AIP but not KN-92, an inactive analogue of KN-93. ICa,L and late sodium current were suggested to be the essential factors for the generation of ROS-induced EADs. Ang II potentiates the amplitudes of both ICa.L and late INa significantly and these enhancements can be attenuated by application of antioxidants, e.g., MnTMPyP and trolox. These pieces of evidence clearly link the activation of AT1R to endogenous generation of ROS, oxidation of CaMKII, activation of ICa,L and late INa and the EAD genesis.
Other studies have revealed that Ang II activates ICa,L through stimulating PKC regulation pathway and may be involved in the genesis of reperfusion-induced ventricular arrhythmias by elevating cellular Ca2+ loading[78–80]. Application of ACE antagonist TCV116 also reduced the reperfusion induced ventricular arrhythmia[81]. Touyz et al[82] further examined the cellular Ca2+ concentration levels in atrial and ventricular myocytes and disclosed that the calcium overload caused impairment on atrial myocytes is more severe. This damage may also be attributed to the higher expression level of AT1R in atria under heart failure conditions[83].
A recent study has demonstrated that Ang II plays roles in increasing transmural dispersion of repolarization in the ventricles, which promotes the re-entrant ventricular arrhythmias[84]. In the transgenic rats harboring the human renin and angiotensinogen genes, the mRNA expressions for Kv4.3 subunit and gap junction protein connexin 43 were predominantly reduced compared to the controls. Cardiac magnetic field mapping further revealed the lengthening in depolarization and repolarization and enhancement in APD inhomogeneity and application of the AT1R blocker losartan ameliorated the disturbances.
Although AT1Rs are suggested to be predominantly involved in most of the well-known effects of Ang II, a recent study by Gopinathannair et al[85] discovered a potential role of AT2R in ischemic focal ventricular tachycardia. In a dog model with coronary artery occlusion, Ang II infusion caused sustained focal ventricular tachycardia from Purkinje origin. Ang II enhanced the in vitro triggered activities in Purkinje cells, which were blocked by selective AT2R PD-123319 but not AT1R blocker losartan. These results imply the significant role of AT2R in arrhythmogenesis during ischemia and the protective effect of AT2R blocker in myocardial ischemia.
The “cross-talk” between Ang II and catecholamine was also hypothesized. Evidence indicates the interactions between the adrenergic system and RAAS, as norepinephrine (NE) can be released from sympathetic nerves via activation of prejunctional AT1R[86]. This indicates that Ang II might increase ectopic automaticity of atria and ventricles through stimulating the production of catecholamine.
CELL DEATH/APOPTOSIS AND HEART FAILURE
Ang II-induced ROS generation has been suggested to promote heart failure[87]. Erickson et al[88,89] showed that Ang II stimulates NAD(P)H oxidase and activates CaMKII by oxidation on Met281/282. CaMKII is initially activated by binding to calcified calmodulin (Ca2+/CaM) but Met oxidation permits persistent CaMKII activity even in the absence of Ca2+/CaM. Ang II-induced oxidation of CaMKII promotes myocardial dysfunction and heart failure in part by increasing apoptosis in ventricular myocytes[88,89]. Supporting the finding of Erickson et al[88,89], Palomeque et al[70] revealed that the Ang II-induced apoptosis is mediated by p38MAPK activation induced by ROS-dependent CaMKII activation. They also suggested the presence of Ang II or ROS can reset the activation of CaMKII for apoptotic cascade signaling at subdiastolic calcium concentrations.
A very recent study by Swaminathan et al[90] implicated the roles of Ang II, ROS and oxidation of CaMKII to the development of sino-atrial node dysfunction (SND). SND is mostly characterized by sinus bradycardia (abnormal slow heart rate). It commonly occurs in the setting of heart failure and hypertension. Swaminathan et al[90] found that the patients with heart failure and SND and dogs with pacing-induced SND had higher levels of oxidized CaMKII in right atrial tissue compared to non-SND controls. They also showed elegantly that Ang II infusion in mice causes sino-atrial nodal cell apoptosis/death and SND by activating NAD(P)H oxidase and oxidation of CaMKII. Moreover, the mice lacking functional NAD(P)H oxidase (p47−/−mice) and mice with CaMK II inhibition (AC3-I mice) have higher resistance to sino-atrial nodal cell apoptosis and SND induced by Ang II. These results unambiguously suggested that: (1) the activation of NAD(P)H oxidase, ROS and oxidation of CaMKII are involved in Ang II-induced SND; and (2) the inhibition of CaMKII in the sino-atrial node prevents/ delays the occurrence of SND induced by Ang II.
Evidence consistent with a central role of Ang II in the pathophysiology of heart failure comes from the fact that the Ang II receptor blockers or ACE inhibitors are clinical medications used to treat high blood pressure and heart failure. The recent study by Swaminathan et al[90] also suggested that early inhibition of ACE, Ang II receptor or CaMKII in high-risk patients (heart failure) may be beneficial for preventing SND.
ISCHEMIA/REPERFUSION INJURY AND CARDIAC PROTECTION
Early studies have revealed the involvement of the RAS in ischemia-reperfusion injury of the heart. The RAS is up-regulated during ischemia, infarction and reperfusion[91,92]. Evidence suggests that the binding of Ang II to either AT1R or AT2R can enhance ischemia-reperfusion injury. Yang et al[93,94] showed that, compared to the control, total myocardial Ang II receptor expression is greater in hearts subjected to the global ischemia-reperfusion. AT1R accounts for most of this increase, while AT2R is unaffected. In the hearts suffering ischemia-reperfusion, the AT1R blocker losartan can attenuate the damage. Consistent with this observation, Flynn et al[95] demonstrated that treatment of isolated rat hearts with losartan before global ischemia-reperfusion resulted in significant cardioprotective effect, leading to decrease in end-diastolic pressure and reduction in infarct size. On the other hand, in isolated working rat hearts, Xu et al[96] showed that ischemia-reperfusion decreased AT2R mRNA and protein level. The AT2R blocker PD-123319 increases AT2R mRNA and protein levels and improves functional recovery, suggesting a potential link between increased AT2R protein expression and cardioprotection. On the contrary, the AT1R blocker losartan increased AT1R mRNA and impaired functional recovery[96]. Thus, it seems that the AT1R blockade is not universally beneficial in all ischemia-reperfusion models in all species. Nevertheless, both ACE inhibitors and AT1R or AT2R inhibitors have been suggested as cardiac protection strategies.
Rac1 GTPase is important for ischemia-reperfusion injury induced NAD(P)H oxidase activation, superoxide production and oxidative stress[97,98]. The NAD(P)H oxidative activity and superoxide level rapidly increased and reached a peak at 3 h post-reperfusion, whereas this effect can be significantly attenuated by treatment with Rac GTPase inhibitor NSC23766 before or after ischemia/reperfusion[97]. This observation is supported by an in vivo study using the ischemia-reperfusion mouse model[98]. The size of infarct in Rac-1 knockout mice is much smaller than in control mice. This study further showed that the disruption of Rac1 signaling inhibits the cellular and mitochondria ROS production and exerts protective effects to myocardium during ischemia-reperfusion conditions. These findings provide some possible mechanisms for the roles of Ang II in the development of ischemia-reperfusion injury.
CARDIAC HYPERTROPHY
Cardiac hypertrophy may develop as a physiological adaptive response to heart volume overload and pressure following intense physical training. However, sustained hypertrophy due to intrinsic cardiomyopathic stimuli or extrinsic stimuli (e.g., hypertension and valvular diseases) will lead to severe pathophysiological conditions, with increased risk of morbidity and sudden cardiac death, especially for left ventricular hypertrophy[99]. Cardiac hypertrophy represents a heart enlargement, which involves two components: (1) cardiac myocyte enlargement; and (2) cardiac fibroblast proliferation and collagen formation. Cardiac hypertrophy further leads to an extensive remodeling of ion channels, gap junctions, calcium handling and cytoskeleton. These remodeling changes subsequently reduce the excitability of cardiac myocytes, induce arrhythmias and eventually cause heart pump failure[99].
The renin-angiotensin-aldosterone system (RAAS) has been recognized as one of the most important non-hemodynamic factors for the development of left ventricular hypertrophy, apart from the sympathetic nervous system. The circulating concentrations of Ang II and aldosterone in plasma are suggested to be involved in the development of left ventricular hypertrophy. Previous evidence[100–102] showed that Ang II can increase the cell size, RNA and protein synthesis levels and gene expressions for cardiac myocytes.
Direct effects of Ang II were shown in cultured embryonic chick myocytes. Aceto et al[103] observed that the cellular protein synthesis rate was increased over 25% after 7 d exposure to [Sar1] Ang II (an analogue of Ang II that is an agonist AT1Rs), which was inhibited in the presence of Ang II receptor antagonist, suggesting the stimulation is receptor-linked. They also performed in vivo experiments using rat model with developed pressure-overload cardiac hypertrophy and found the increase in left ventricular mass can be totally prevented in animals fed with ACE inhibitor enalapril maleate[104]. In addition, a fibroblast-derived factor was identified as responsible for the biochemical process by which Ang II stimulates the cardiomyocyte hypertrophy[105]. Ang II stimulates protein synthesis in neonatal myocytes by over 40%, while about 50% of the increase can be blocked using fibroblast proliferation inhibitor bromodeoxyuridine. Further experiments showed that the Ang II stimulated protein synthesis can be abolished by the AT1R blocker losartan[105], indicating the direct role of Ang II in the production of some fibroblasts factor.
The effect of intracellular Ang II to the development of hypertrophy was examined by over-expressing Ang II peptide in mouse heart using a plasmid-mediated gene delivery system. As such, Ang II was retained intracellularly. Significant cardiac hypertrophy and cardiac gene expression were observed in this model, suggesting Ang II acts as an “intracrine” in stimulating the cellular hypertrophy[106]. A previous study by Mazzolai et al[107] also demonstrated the development of ventricular hypertrophy in transgenic mice over-expressing the angiotensinogen gene is independent from fibrosis and hypertension, indicating the significance of local Ang II in mediating hypertrophic response in vivo. New evidence has demonstrated the critical role of mitochondrial ROS in the development of Ang II-derived cardiac hypertrophy. The mice over-expressed catalase targeted to mitochondria has shown to be resistant to cardiac hypertrophy, fibrosis and mitochondrial damage induced by Ang II. Therefore, mitochondrial-targeted antioxidants have been suggested as promising agents for prevention and treatment of hypertensive cardiomyopathy[67,108].
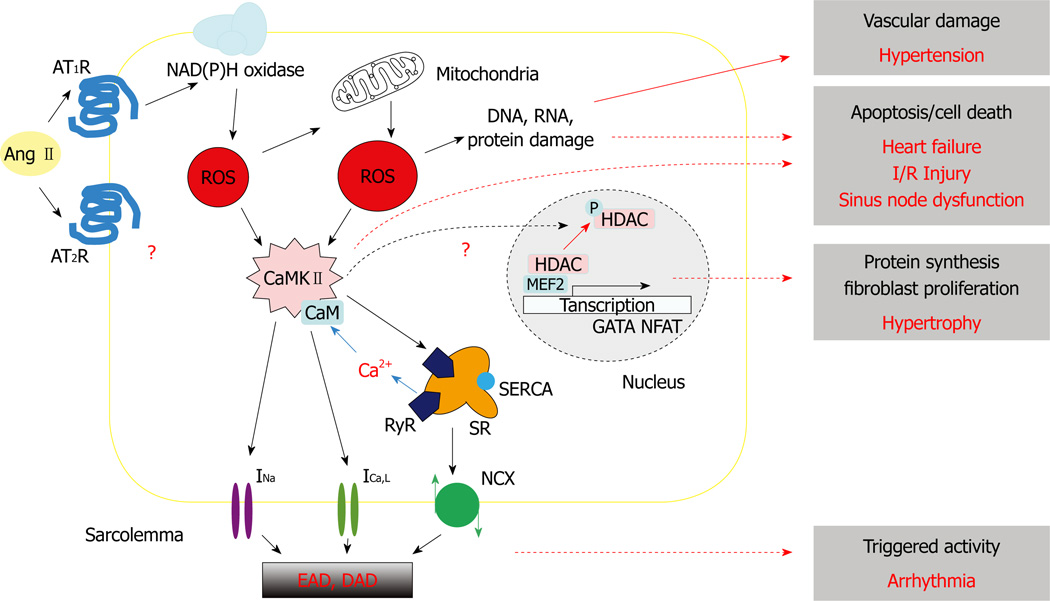
The activation of CaMKII via the Gq/PLC/InsP3 signaling pathway has been believed to contribute to the hypertrophic growth and gene expression in response to Ang II and other G-protein couple receptor stimulations, such as by NE and endothelin (ET-1)[109]. The G-protein couple receptor stimulations alter the expression of a range of hypertrophy-associated genes, including atrial natriuretic peptide, brain natriuretic peptide, myosin light chain-2 and α- and β-myosin heavy chains, which can be prevented by the inhibition of CaMKII[110–112]. The expression and phosphorylation levels of CaMKII are increased in the mouse with transverse aortic constriction induced cardiac hypertrophy[113]. It has also been demonstrated that nuclear CaMKII activated by envelope IP3 Receptor-mediated Ca release can cause histone deacetylase (HDAC) phosphorylation and nuclear export. This relieves HDAC-dependent suppression of myocyte enhancer factor 2-driven transcriptions and contributes to hypertrophy (Figure 1). However, it remains to be studied whether ROS-activated cytosolic CaMKII can also mediate Ang II-induced hypertrophy.
Figure 1. A schematic diagram illustrating the involvement of ROS signaling pathways in numeral Ang II-induced cardiovascular diseases.
See main text for details. Ang II: Angiotensin II; AT1R: Angiotensin II type 1 receptor; AT2R: Angiotensin II type 2 receptor; ROS: Reactive Oxygen Species; CaMKII: calcium/calmodulin-dependent protein kinase II; INa: Sodium current; ICa,L: L-type calcium current; NCX: Sodium-Calcium current; SR: Sarcoplasmic reticulum; RyR: Ryanodine receptor; SERCA: SR Ca2+ ATPase; EAD: Early afterdepolarization; DAD: Delayed afterdepolarization.
HYPERTENSION
Accumulating data from molecular, cellular and in vivo animal studies implicate a central role of ROS in the pathogenesis of hypertension (refer to a review article by Briones, 2010)[114]. Ang II-induced hypertension is also associated with an increase of ROS production in vasculature[55]. Infused Ang II leads to the increase of systolic blood pressure and marked up-regulation of vascular ROS production, which can be blocked by losartan. The underlined regulation pathway is further characterized as NAD(P)H dependent. Laursen et al[115] showed that the Ang II infusion induced hypertension in rats is likely due to the degradation of endothelium-derived NO, as well as increased superoxide levels, since the treatment with liposome-encapsulated SOD decreases blood pressure in Ang II-infused rats and had no effect in positive control. A similar phenomenon was revealed by Virdis et al[116], who showed that Ang II caused increase in systolic blood pressure and structural alterations in small resistant arteries, which was prevented by the NAD(P)H oxidase inhibitor apocynin. Taking these together, NAD(P)H oxidase activity is essential for vascular structural and functional changes in Ang II-dependent hypertension.
Oxidative stress has critical roles in vascular damages. ROS may affect the vascular tone by altering the bioactivities or signaling of antioxidant and vascular NAD(P)H oxidase. In VSM cells, the NAD(P)H oxidase p22phox has been shown to be important in the arachidonic acid metabolite-mediated hypertrophy[117], as transfection of antisense p22phox can attenuate NAD(P)H oxidase expression and inhibit Ang II-stimulated protein synthesis.
In patients with inherent structural membrane abnormalities in essential hypertension, in which blood cell membranes contain less unsaturated fatty acyl composition, the levels of free radical and SOD were examined. As a result, the untreated patients with severe hypertension have significantly higher levels of oxygen derived free radicals but lower levels of SOD[118]. Berry et al[119] further measured the superoxide production in vasculatures and revealed the basal level of superoxide in arteries is greater than those in veins. As there are more VSM cells in arteries, this indicated the role of arteries in ROS generation. They also suggested that Ang II could stimulate superoxide anion production and this Ang II mediated effect can be blocked by either losartan or NAD(P)H inhibitor DPI.
In genetic hypertension models, such as the spontaneous hypertensive rats (SHR) and stroke-prone SHRs (SHRSP), enhanced O2− production associated with NAD(P)H were observed in resistance arteries, aorta and kidneys. In SHR 30 wk old, significantly higher levels of p22phox mRNA, NAD(P)H induced superoxide anion production and aorta damage were measured compared to the control WKY rats[120]. Vitamin C and E have shown antioxidant properties. They were used to treat adult SHRSP models and have shown preventive effects on the progress of hypertension[121]. In this study, it was found that both vitamin-treated groups improve the total antioxidant status, with decreased activation of vascular NAD(P)H oxidase and significantly increased activation of SOD.
CaMKII inhibition, which has been proposed as a novel therapy for arrhythmias and heart failure, was also linked to Ang II-mediated VSM hypertrophy and hypertension in a recent study[122].
CONCLUSION
Oxidative stress plays key roles in the development and progression of myocardial dysfunction and cardiovascular diseases (Table 1 and Figure 1). In spite of the clinical relevance of Ang II-induced ROS being unclear, recent evidence strongly implicates the benefit of inhibition on Ang II in reducing the risk for cardiovascular remodeling and inflammation. Several novel ROS modulation pathways on which Ang II exerts direct effects have been identified. Apart from its crucial roles in vasoconstriction modulation, recent studies further emphasized the importance of Ang II in heart function. Knowledge linking oxidative stress to clinical cardiac disorders is potentially of exceptional importance. Besides, there are great interests in developing therapeutic strategies by targeting the AT1R and Ang II-induced ROS transduction cascades.
Table 1.
Reactive oxygen species signaling pathways and Ang II-induced cardiovascular diseases
| Disease | Receptor(s) | NAD(P)H oxidase | ROS | CaMKII | Other signaling molecules | Ref. |
|---|---|---|---|---|---|---|
| Arrhythmias | AT1R/AT2R | + (Nox2) | + | + | Ca2+ handing, RyR, ICa,L, INa, INCX | [23,71,74–85] |
| Cell death/heart failure | AT1R ? | + (p47phox) | + | + | p38MARK | [70,88–90] |
| I/R injury | AT1R/AT2R | + | + | ? | Rac1 GTPase | [91–98] |
| Hypertrophy | AT1R | + | + | +, ? | Transcription factors | [67,103–113] |
| Hypertension | AT1R | + (p22phox) | + | + | Nitric oxide | [114–122] |
+: Involved; ?: Not clear; AT1R: Ang II type 1 receptor; AT2R: Ang II type 2 receptor; RyR: Ryanodine receptor; ICa,L: L-type calcium current; INa: Sodium current; INCX: Sodium-calcium exchange current; p38MARK: p38 mitogen-activated protein kinase.
Acknowledgments
Supported by A NIH R01 Grant, No. HL097979 (to Xie LH)
Footnotes
Author contributions: Wen H and Xie LH drafted the manuscript; Wen H, Gwathmey JK and Xie LH edited and revised the manuscript and approved the final version of manuscript.
Peer reviewer: Carlos Escobar, Dr., Hospital Infanta Sofia, Paseo de Europa 34, 28702 San Sebastian de los Reyes, Spain
Contributor Information
Hairuo Wen, Department of Cell Biology and Molecular Medicine, UMDNJ-New Jersey Medical School, Newark, NJ 07103, United States.
Judith K Gwathmey, Gwathmey Inc., Cambridge, MA 02138, United States; School of Optometry, Massachusetts College of Pharmacy and Health Sciences, Worcester, MA 01608, United States.
Lai-Hua Xie, Department of Cell Biology and Molecular Medicine, UMDNJ-New Jersey Medical School, Newark, NJ 07103, United States.
REFERENCES
- 1.Kaneko S, Tamura S, Takagi H. Purification and identification of endogenous anti-opioid substances from bovine brain. Biochem Biophys Res Commun. 1985;126:587–593. doi: 10.1016/0006-291x(85)90646-1. [DOI] [PubMed] [Google Scholar]
- 2.Dostal DE, Baker KM. The cardiac renin-angiotensin system: conceptual, or a regulator of cardiac function? Circ Res. 1999;85:643–650. doi: 10.1161/01.res.85.7.643. [DOI] [PubMed] [Google Scholar]
- 3.Dzau VJ. Cell biology and genetics of angiotensin in cardiovascular disease. J Hypertens Suppl. 1994;12:S3–S10. [PubMed] [Google Scholar]
- 4.Gavras I, Gavras H. Angiotensin II as a cardiovascular risk factor. J Hum Hypertens. 2002;16(Suppl 2):S2–S6. doi: 10.1038/sj.jhh.1001392. [DOI] [PubMed] [Google Scholar]
- 5.Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system--an endocrine and paracrine system. Endocrinology. 2003;144:2179–2183. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- 6.Campbell DJ. Circulating and tissue angiotensin systems. J Clin Invest. 1987;79:1–6. doi: 10.1172/JCI112768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnston CI. Franz Volhard Lecture. Renin-angiotensin system: a dual tissue and hormonal system for cardiovascular control. J Hypertens Suppl. 1992;10:S13–S26. [PubMed] [Google Scholar]
- 8.Phillips MI, Speakman EA, Kimura B. Levels of angiotensin and molecular biology of the tissue renin angiotensin systems. Regul Pept. 1993;43:1–20. doi: 10.1016/0167-0115(93)90403-u. [DOI] [PubMed] [Google Scholar]
- 9.Vinson GP, Ho MM, Puddefoot JR. The distribution of angiotensin II type 1 receptors, and the tissue renin-angiotensin systems. Mol Med Today. 1995;1:35–39. doi: 10.1016/1357-4310(95)80018-2. [DOI] [PubMed] [Google Scholar]
- 10.Pendleton RG, Gessner G, Horner E. Studies defining minimal receptor domains for angiotensin II. J Pharmacol Exp Ther. 1989;250:31–36. [PubMed] [Google Scholar]
- 11.Mustafa T, Lee JH, Chai SY, Albiston AL, McDowall SG, Mendelsohn FA. Bioactive angiotensin peptides: focus on angiotensin IV. J Renin Angiotensin Aldosterone Syst. 2001;2:205–210. doi: 10.3317/jraas.2001.032. [DOI] [PubMed] [Google Scholar]
- 12.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 13.Allred AJ, Diz DI, Ferrario CM, Chappell MC. Pathways for angiotensin-(1–7) metabolism in pulmonary and renal tissues. Am J Physiol Renal Physiol. 2000;279:F841–F850. doi: 10.1152/ajprenal.2000.279.5.F841. [DOI] [PubMed] [Google Scholar]
- 14.Ferrario CM, Chappell MC, Tallant EA, Brosnihan KB, Diz DI. Counterregulatory actions of angiotensin-(1–7) Hypertension. 1997;30:535–541. doi: 10.1161/01.hyp.30.3.535. [DOI] [PubMed] [Google Scholar]
- 15.Dinh DT, Frauman AG, Johnston CI, Fabiani ME. Clin Sci. Vol. 100. Lond: 2001. Angiotensin receptors: distribution, signalling and function; pp. 481–492. [PubMed] [Google Scholar]
- 16.Sandberg K. Structural analysis and regulation of angiotensin II receptors. Trends Endocrinol Metab. 1994;5:28–35. doi: 10.1016/1043-2760(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 17.Takeda H, Kondo S. Immunohistochemical study of angiotensin receptors in normal human sweat glands and eccrine poroma. Br J Dermatol. 2001;144:1189–1192. doi: 10.1046/j.1365-2133.2001.04229.x. [DOI] [PubMed] [Google Scholar]
- 18.Goette A, Lendeckel U. Electrophysiological effects of angiotensin II. Part I: signal transduction and basic electrophysiological mechanisms. Europace. 2008;10:238–241. doi: 10.1093/europace/eum283. [DOI] [PubMed] [Google Scholar]
- 19.Saito Y, Berk BC. Angiotensin II-mediated signal transduction pathways. Curr Hypertens Rep. 2002;4:167–171. doi: 10.1007/s11906-002-0042-1. [DOI] [PubMed] [Google Scholar]
- 20.Timmermans PB, Wong PC, Chiu AT, Herblin WF, Benfield P, Carini DJ, Lee RJ, Wexler RR, Saye JA, Smith RD. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993;45:205–251. [PubMed] [Google Scholar]
- 21.Garrido AM, Griendling KK. NADPH oxidases and angiotensin II receptor signaling. Mol Cell Endocrinol. 2009;302:148–158. doi: 10.1016/j.mce.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- 23.Zhao Z, Fefelova N, Shanmugam M, Bishara P, Babu GJ, Xie LH. Angiotensin II induces afterdepolarizations via reactive oxygen species and calmodulin kinase II signaling. J Mol Cell Cardiol. 2011;50:128–136. doi: 10.1016/j.yjmcc.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishida M, Tanabe S, Maruyama Y, Mangmool S, Urayama K, Nagamatsu Y, Takagahara S, Turner JH, Kozasa T, Kobayashi H, Sato Y, Kawanishi T, Inoue R, Nagao T, Kurose H. G alpha 12/13- and reactive oxygen species-dependent activation of c-Jun NH2-terminal kinase and p38 mitogen-activated protein kinase by angiotensin receptor stimulation in rat neonatal cardiomyocytes. J Biol Chem. 2005;280:18434–18441. doi: 10.1074/jbc.M409710200. [DOI] [PubMed] [Google Scholar]
- 25.Ren Z, Raucci FJ, Browe DM, Baumgarten CM. Regulation of swelling-activated Cl(−) current by angiotensin II signalling and NADPH oxidase in rabbit ventricle. Cardiovasc Res. 2008;77:73–80. doi: 10.1093/cvr/cvm031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aiello EA, Cingolani HE. Angiotensin II stimulates cardiac L-type Ca(2+) current by a Ca(2+)- and protein kinase C-dependent mechanism. Am J Physiol Heart Circ Physiol. 2001;280:H1528–H1536. doi: 10.1152/ajpheart.2001.280.4.H1528. [DOI] [PubMed] [Google Scholar]
- 27.Ichiyanagi O, Ishii K, Endoh M. Angiotensin II increases L-type Ca2+ current in gramicidin D-perforated adult rabbit ventricular myocytes: comparison with conventional patch-clamp method. Pflugers Arch. 2002;444:107–116. doi: 10.1007/s00424-002-0808-y. [DOI] [PubMed] [Google Scholar]
- 28.Ishihata A, Endoh M. Species-related differences in inotropic effects of angiotensin II in mammalian ventricular muscle: receptors, subtypes and phosphoinositide hydrolysis. Br J Pharmacol. 1995;114:447–453. doi: 10.1111/j.1476-5381.1995.tb13247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo DF, Sun YL, Hamet P, Inagami T. The angiotensin II type 1 receptor and receptor-associated proteins. Cell Res. 2001;11:165–180. doi: 10.1038/sj.cr.7290083. [DOI] [PubMed] [Google Scholar]
- 30.AbdAlla S, Lother H, Quitterer U. AT1-receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature. 2000;407:94–98. doi: 10.1038/35024095. [DOI] [PubMed] [Google Scholar]
- 31.Ali MS, Sayeski PP, Dirksen LB, Hayzer DJ, Marrero MB, Bernstein KE. Dependence on the motif YIPP for the physical association of Jak2 kinase with the intracellular carboxyl tail of the angiotensin II AT1 receptor. J Biol Chem. 1997;272:23382–23388. doi: 10.1074/jbc.272.37.23382. [DOI] [PubMed] [Google Scholar]
- 32.Venema RC, Ju H, Venema VJ, Schieffer B, Harp JB, Ling BN, Eaton DC, Marrero MB. Angiotensin II-induced association of phospholipase Cgamma1 with the G-protein-coupled AT1 receptor. J Biol Chem. 1998;273:7703–7708. doi: 10.1074/jbc.273.13.7703. [DOI] [PubMed] [Google Scholar]
- 33.Daviet L, Lehtonen JY, Tamura K, Griese DP, Horiuchi M, Dzau VJ. Cloning and characterization of ATRAP, a novel protein that interacts with the angiotensin II type 1 receptor. J Biol Chem. 1999;274:17058–17062. doi: 10.1074/jbc.274.24.17058. [DOI] [PubMed] [Google Scholar]
- 34.Guo DF, Chenier I, Tardif V, Orlov SN, Inagami T. Type 1 angiotensin II receptor-associated protein ARAP1 binds and recycles the receptor to the plasma membrane. Biochem Bio-phys Res Commun. 2003;310:1254–1265. doi: 10.1016/j.bbrc.2003.09.154. [DOI] [PubMed] [Google Scholar]
- 35.Guo DF, Tardif V, Ghelima K, Chan JS, Ingelfinger JR, Chen X, Chenier I. A novel angiotensin II type 1 receptor-associated protein induces cellular hypertrophy in rat vascular smooth muscle and renal proximal tubular cells. J Biol Chem. 2004;279:21109–21120. doi: 10.1074/jbc.M401544200. [DOI] [PubMed] [Google Scholar]
- 36.Sales VL, Sukhova GK, Lopez-Ilasaca MA, Libby P, Dzau VJ, Pratt RE. Angiotensin type 2 receptor is expressed in murine atherosclerotic lesions and modulates lesion evolution. Circulation. 2005;112:3328–3336. doi: 10.1161/CIRCULATIONAHA.105.541714. [DOI] [PubMed] [Google Scholar]
- 37.Lemarié CA, Schiffrin EL. The angiotensin II type 2 receptor in cardiovascular disease. J Renin Angiotensin Aldosterone Syst. 2010;11:19–31. doi: 10.1177/1470320309347785. [DOI] [PubMed] [Google Scholar]
- 38.Katada J, Majima M. AT(2) receptor-dependent vasodilation is mediated by activation of vascular kinin generation under flow conditions. Br J Pharmacol. 2002;136:484–491. doi: 10.1038/sj.bjp.0704731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu YH, Yang XP, Sharov VG, Nass O, Sabbah HN, Peterson E, Carretero OA. Effects of angiotensin-converting enzyme inhibitors and angiotensin II type 1 receptor antagonists in rats with heart failure. Role of kinins and angiotensin II type 2 receptors. J Clin Invest. 1997;99:1926–1935. doi: 10.1172/JCI119360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seyedi N, Xu X, Nasjletti A, Hintze TH. Coronary kinin generation mediates nitric oxide release after angiotensin receptor stimulation. Hypertension. 1995;26:164–170. doi: 10.1161/01.hyp.26.1.164. [DOI] [PubMed] [Google Scholar]
- 41.Abadir PM, Periasamy A, Carey RM, Siragy HM. Angiotensin II type 2 receptor-bradykinin B2 receptor functional heterodimerization. Hypertension. 2006;48:316–322. doi: 10.1161/01.HYP.0000228997.88162.a8. [DOI] [PubMed] [Google Scholar]
- 42.AbdAlla S, Lother H, Abdel-tawab AM, Quitterer U. The angiotensin II AT2 receptor is an AT1 receptor antagonist. J Biol Chem. 2001;276:39721–39726. doi: 10.1074/jbc.M105253200. [DOI] [PubMed] [Google Scholar]
- 43.Sandberg K, Ji H, Clark AJ, Shapira H, Catt KJ. Cloning and expression of a novel angiotensin II receptor subtype. J Biol Chem. 1992;267:9455–9458. [PubMed] [Google Scholar]
- 44.Swanson GN, Hanesworth JM, Sardinia MF, Coleman JK, Wright JW, Hall KL, Miller-Wing AV, Stobb JW, Cook VI, Harding EC. Discovery of a distinct binding site for angiotensin II (3–8), a putative angiotensin IV receptor. Regul Pept. 1992;40:409–419. doi: 10.1016/0167-0115(92)90527-2. [DOI] [PubMed] [Google Scholar]
- 45.Miller-Wing AV, Hanesworth JM, Sardinia MF, Hall KL, Wright JW, Speth RC, Grove KL, Harding JW. Central angiotensin IV binding sites: distribution and specificity in guinea pig brain. J Pharmacol Exp Ther. 1993;266:1718–1726. [PubMed] [Google Scholar]
- 46.Zankov DP, Omatsu-Kanbe M, Isono T, Toyoda F, Ding WG, Matsuura H, Horie M. Angiotensin II potentiates the slow component of delayed rectifier K+ current via the AT1 receptor in guinea pig atrial myocytes. Circulation. 2006;113:1278–1286. doi: 10.1161/CIRCULATIONAHA.104.530592. [DOI] [PubMed] [Google Scholar]
- 47.Moreno I, Caballero R, González T, Arias C, Valenzuela C, Iriepa I, Gálvez E, Tamargo J, Delpón E. Effects of irbesartan on cloned potassium channels involved in human cardiac repolarization. J Pharmacol Exp Ther. 2003;304:862–873. doi: 10.1124/jpet.102.042325. [DOI] [PubMed] [Google Scholar]
- 48.Yu H, Gao J, Wang H, Wymore R, Steinberg S, McKinnon D, Rosen MR, Cohen IS. Effects of the renin-angiotensin system on the current I(to) in epicardial and endocardial ventricular myocytes from the canine heart. Circ Res. 2000;86:1062–1068. doi: 10.1161/01.res.86.10.1062. [DOI] [PubMed] [Google Scholar]
- 49.Bescond J, Bois P, Petit-Jacques J, Lenfant J. Characterization of an angiotensin-II-activated chloride current in rabbit sinoatrial cells. J Membr Biol. 1994;140:153–161. doi: 10.1007/BF00232903. [DOI] [PubMed] [Google Scholar]
- 50.Morita H, Kimura J, Endoh M. Angiotensin II activation of a chloride current in rabbit cardiac myocytes. J Physiol. 1995;483(Pt 1):119–130. doi: 10.1113/jphysiol.1995.sp020572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Obayashi K, Horie M, Xie LH, Tsuchiya K, Kubota A, Ishida H, Sasayama S. Angiotensin II inhibits protein kinase A-dependent chloride conductance in heart via pertussis toxin-sensitive G proteins. Circulation. 1997;95:197–204. doi: 10.1161/01.cir.95.1.197. [DOI] [PubMed] [Google Scholar]
- 52.Griendling KK, Sorescu D, Lassègue B, Ushio-Fukai M. Modulation of protein kinase activity and gene expression by reactive oxygen species and their role in vascular physiology and pathophysiology. Arterioscler Thromb Vasc Biol. 2000;20:2175–2183. doi: 10.1161/01.atv.20.10.2175. [DOI] [PubMed] [Google Scholar]
- 53.Xiao L, Pimentel DR, Wang J, Singh K, Colucci WS, Sawyer DB. Role of reactive oxygen species and NAD(P)H oxidase in alpha(1)-adrenoceptor signaling in adult rat cardiac myocytes. Am J Physiol Cell Physiol. 2002;282:C926–C934. doi: 10.1152/ajpcell.00254.2001. [DOI] [PubMed] [Google Scholar]
- 54.Brandes RP, Kreuzer J. Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res. 2005;65:16–27. doi: 10.1016/j.cardiores.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 55.Rajagopalan S, Kurz S, Münzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci. 2002;59:1428–1459. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maack C, Böhm M. Targeting mitochondrial oxidative stress in heart failure throttling the afterburner. J Am Coll Cardiol. 2011;58:83–86. doi: 10.1016/j.jacc.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 59.Brady NR, Hamacher-Brady A, Westerhoff HV, Gottlieb RA. A wave of reactive oxygen species (ROS)-induced ROS release in a sea of excitable mitochondria. Antioxid Redox Signal. 2006;8:1651–1665. doi: 10.1089/ars.2006.8.1651. [DOI] [PubMed] [Google Scholar]
- 60.Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta. 2010;1797:897–906. doi: 10.1016/j.bbabio.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 61.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochim Biophys Acta. 2006;1757:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 62.Zhang GX, Lu XM, Kimura S, Nishiyama A. Role of mitochondria in angiotensin II-induced reactive oxygen species and mitogen-activated protein kinase activation. Cardiovasc Res. 2007;76:204–212. doi: 10.1016/j.cardiores.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 63.Hitomi H, Kiyomoto H, Nishiyama A. Angiotensin II and oxidative stress. Curr Opin Cardiol. 2007;22:311–315. doi: 10.1097/HCO.0b013e3281532b53. [DOI] [PubMed] [Google Scholar]
- 64.Mollnau H, Wendt M, Szöcs K, Lassègue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Förstermann U, Meinertz T, Griendling K, Münzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res. 2002;90:E58–E65. doi: 10.1161/01.res.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Z, Lai GH, Sirica AE. Celecoxib-induced apoptosis in rat cholangiocarcinoma cells mediated by Akt inactivation and Bax translocation. Hepatology. 2004;39:1028–1037. doi: 10.1002/hep.20143. [DOI] [PubMed] [Google Scholar]
- 66.Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, Rahman M, Abe Y. Mitochondria-derived reactive oxygen species and vascular MAP kinases: comparison of angiotensin II and diazoxide. Hypertension. 2005;45:438–444. doi: 10.1161/01.HYP.0000157169.27818.ae. [DOI] [PubMed] [Google Scholar]
- 67.Dai DF, Chen T, Szeto H, Nieves-Cintrón M, Kutyavin V, Santana LF, Rabinovitch PS. Mitochondrial targeted antioxidant Peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol. 2011;58:73–82. doi: 10.1016/j.jacc.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bokoch GM, Diebold BA. Current molecular models for NADPH oxidase regulation by Rac GTPase. Blood. 2002;100:2692–2696. doi: 10.1182/blood-2002-04-1149. [DOI] [PubMed] [Google Scholar]
- 69.Montezano AC, Burger D, Paravicini TM, Chignalia AZ, Yusuf H, Almasri M, He Y, Callera GE, He G, Krause KH, Lambeth D, Quinn MT, Touyz RM. Nicotinamide adenine dinucleotide phosphate reduced oxidase 5 (Nox5) regulation by angiotensin II and endothelin-1 is mediated via calcium/calmodulin-dependent, rac-1-independent pathways in human endothelial cells. Circ Res. 2010;106:1363–1373. doi: 10.1161/CIRCRESAHA.109.216036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Palomeque J, Rueda OV, Sapia L, Valverde CA, Salas M, Petroff MV, Mattiazzi A. Angiotensin II-induced oxidative stress resets the Ca2+ dependence of Ca2+-calmodulin protein kinase II and promotes a death pathway conserved across different species. Circ Res. 2009;105:1204–1212. doi: 10.1161/CIRCRESAHA.109.204172. [DOI] [PubMed] [Google Scholar]
- 71.Xie LH, Chen F, Karagueuzian HS, Weiss JN. Oxidative-stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res. 2009;104:79–86. doi: 10.1161/CIRCRESAHA.108.183475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jeong EM, Liu M, Sturdy M, Gao G, Varghese ST, Sovari AA, Dudley SC. Metabolic stress, reactive oxygen species, and arrhythmia. J Mol Cell Cardiol. 2012;52:454–463. doi: 10.1016/j.yjmcc.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iwasaki YK, Nishida K, Kato T, Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124:2264–2274. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- 74.Naccarelli GV, Peacock F. Angiotensin II receptor blockers in the prevention of complications from atrial fibrillation. Vasc Health Risk Manag. 2009;5:783–791. [PMC free article] [PubMed] [Google Scholar]
- 75.von Lewinski D, Kockskämper J, Rübertus SU, Zhu D, Schmitto JD, Schöndube FA, Hasenfuss G, Pieske B. Direct pro-arrhythmogenic effects of angiotensin II can be suppressed by AT1 receptor blockade in human atrial myocardium. Eur J Heart Fail. 2008;10:1172–1176. doi: 10.1016/j.ejheart.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 76.Goette A, Arndt M, Röcken C, Spiess A, Staack T, Geller JC, Huth C, Ansorge S, Klein HU, Lendeckel U. Regulation of angiotensin II receptor subtypes during atrial fibrillation in humans. Circulation. 2000;101:2678–2681. doi: 10.1161/01.cir.101.23.2678. [DOI] [PubMed] [Google Scholar]
- 77.Goette A, Bukowska A, Dobrev D, Pfeiffenberger J, Morawietz H, Strugala D, Wiswedel I, Röhl FW, Wolke C, Bergmann S, Bramlage P, Ravens U, Lendeckel U. Acute atrial tachyarrhythmia induces angiotensin II type 1 receptor-mediated oxidative stress and microvascular flow abnormalities in the ventricles. Eur Heart J. 2009;30:1411–1420. doi: 10.1093/eurheartj/ehp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nakashima H, Kumagai K, Urata H, Gondo N, Ideishi M, Arakawa K. Angiotensin II antagonist prevents electrical remodeling in atrial fibrillation. Circulation. 2000;101:2612–2617. doi: 10.1161/01.cir.101.22.2612. [DOI] [PubMed] [Google Scholar]
- 79.Brock TA, Alexander RW, Ekstein LS, Atkinson WJ, Gimbrone MA. Angiotensin increases cytosolic free calcium in cultured vascular smooth muscle cells. Hypertension. 1985;7:I105–I109. doi: 10.1161/01.hyp.7.3_pt_2.i105. [DOI] [PubMed] [Google Scholar]
- 80.Griendling KK, Rittenhouse SE, Brock TA, Ekstein LS, Gimbrone MA, Alexander RW. Sustained diacylglycerol formation from inositol phospholipids in angiotensin II-stimulated vascular smooth muscle cells. J Biol Chem. 1986;261:5901–5906. [PubMed] [Google Scholar]
- 81.Yoshiyama M, Kim S, Yamagishi H, Omura T, Tani T, Yanagi S, Toda I, Teragaki M, Akioka K, Takeuchi K. Cardioprotective effect of the angiotensin II type 1 receptor antagonist TCV-116 on ischemia-reperfusion injury. Am Heart J. 1994;128:1–6. doi: 10.1016/0002-8703(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 82.Touyz RM, Sventek P, Larivière R, Thibault G, Fareh J, Reudelhuber T, Schiffrin EL. Cytosolic calcium changes induced by angiotensin II in neonatal rat atrial and ventricular cardiomyocytes are mediated via angiotensin II subtype 1 receptors. Hypertension. 1996;27:1090–1096. doi: 10.1161/01.hyp.27.5.1090. [DOI] [PubMed] [Google Scholar]
- 83.Urata H, Healy B, Stewart RW, Bumpus FM, Husain A. Angiotensin II receptors in normal and failing human hearts. J Clin Endocrinol Metab. 1989;69:54–66. doi: 10.1210/jcem-69-1-54. [DOI] [PubMed] [Google Scholar]
- 84.Fischer R, Dechend R, Gapelyuk A, Shagdarsuren E, Gruner K, Gruner A, Gratze P, Qadri F, Wellner M, Fiebeler A, Dietz R, Luft FC, Muller DN, Schirdewan A. Angiotensin II-induced sudden arrhythmic death and electrical remodeling. Am J Physiol Heart Circ Physiol. 2007;293:H1242–H1253. doi: 10.1152/ajpheart.01400.2006. [DOI] [PubMed] [Google Scholar]
- 85.Gopinathannair R, Chaudhary AK, Xing D, Ely D, Zheng W, Martins JB. Angiotensin II effects on ischemic focal ventricular tachycardia are predominantly mediated through myocardial AT(2) receptor. Am J Physiol Heart Circ Physiol. 2009;297:H1889–H1898. doi: 10.1152/ajpheart.00080.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goette A, Lendeckel U, Klein HU. Signal transduction systems and atrial fibrillation. Cardiovasc Res. 2002;54:247–258. doi: 10.1016/s0008-6363(01)00521-1. [DOI] [PubMed] [Google Scholar]
- 87.Tojo A, Onozato ML, Kobayashi N, Goto A, Matsuoka H, Fujita T. Angiotensin II and oxidative stress in Dahl Salt-sensitive rat with heart failure. Hypertension. 2002;40:834–839. doi: 10.1161/01.hyp.0000039506.43589.d5. [DOI] [PubMed] [Google Scholar]
- 88.Erickson JR, He BJ, Grumbach IM, Anderson ME. CaMKII in the cardiovascular system: sensing redox states. Physiol Rev. 2011;91:889–915. doi: 10.1152/physrev.00018.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O’Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Swaminathan PD, Purohit A, Soni S, Voigt N, Singh MV, Glukhov AV, Gao Z, He BJ, Luczak ED, Joiner ML, Kutschke W, Yang J, Donahue JK, Weiss RM, Grumbach IM, Ogawa M, Chen PS, Efimov I, Dobrev D, Mohler PJ, Hund TJ, Anderson ME. Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J Clin Invest. 2011;121:3277–3288. doi: 10.1172/JCI57833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dudley DT, Panek RL, Major TC, Lu GH, Bruns RF, Klinkefus BA, Hodges JC, Weishaar RE. Subclasses of angiotensin II binding sites and their functional significance. Mol Pharmacol. 1990;38:370–377. [PubMed] [Google Scholar]
- 92.Horiuchi M, Akishita M, Dzau VJ. Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension. 1999;33:613–621. doi: 10.1161/01.hyp.33.2.613. [DOI] [PubMed] [Google Scholar]
- 93.Yang B, Li D, Phillips MI, Mehta P, Mehta JL. Myocardial angiotensin II receptor expression and ischemia-reperfusion injury. Vasc Med. 1998;3:121–130. doi: 10.1177/1358836X9800300206. [DOI] [PubMed] [Google Scholar]
- 94.Yang BC, Phillips MI, Ambuehl PE, Shen LP, Mehta P, Mehta JL. Increase in angiotensin II type 1 receptor expression immediately after ischemia-reperfusion in isolated rat hearts. Circulation. 1997;96:922–926. doi: 10.1161/01.cir.96.3.922. [DOI] [PubMed] [Google Scholar]
- 95.Flynn JD, Akers WS. Effects of the angiotensin II subtype 1 receptor antagonist losartan on functional recovery of isolated rat hearts undergoing global myocardial ischemia-reperfusion. Pharmacotherapy. 2003;23:1401–1410. doi: 10.1592/phco.23.14.1401.31947. [DOI] [PubMed] [Google Scholar]
- 96.Xu Y, Menon V, Jugdutt BI. Cardioprotection after angiotensin II type 1 blockade involves angiotensin II type 2 receptor expression and activation of protein kinase C-epsilon in acutely reperfused myocardial infarction in the dog. Effect of UP269-6 and losartan on AT1 and AT2-receptor expression and IP3 receptor and PKCepsilon proteins. J Renin Angiotensin Aldosterone Syst. 2000;1:184–195. doi: 10.3317/jraas.2000.024. [DOI] [PubMed] [Google Scholar]
- 97.Raz L, Zhang QG, Zhou CF, Han D, Gulati P, Yang LC, Yang F, Wang RM, Brann DW. Role of Rac1 GTPase in NADPH oxidase activation and cognitive impairment following cerebral ischemia in the rat. PLoS One. 2010;5:e12606. doi: 10.1371/journal.pone.0012606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shan L, Li J, Wei M, Ma J, Wan L, Zhu W, Li Y, Zhu H, Arnold JM, Peng T. Disruption of Rac1 signaling reduces ischemia-reperfusion injury in the diabetic heart by inhibiting calpain. Free Radic Biol Med. 2010;49:1804–1814. doi: 10.1016/j.freeradbiomed.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 99.Kahan T, Bergfeldt L. Left ventricular hypertrophy in hypertension: its arrhythmogenic potential. Heart. 2005;91:250–256. doi: 10.1136/hrt.2004.042473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Miyata S, Haneda T. Hypertrophic growth of cultured neonatal rat heart cells mediated by type 1 angiotensin II receptor. Am J Physiol. 1994;266:H2443–H2451. doi: 10.1152/ajpheart.1994.266.6.H2443. [DOI] [PubMed] [Google Scholar]
- 101.Sadoshima J, Izumo S. Molecular characterization of angiotensin II--induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts. Critical role of the AT1 receptor subtype. Circ Res. 1993;73:413–423. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- 102.Sadoshima J, Izumo S. Signal transduction pathways of angiotensin II--induced c-fos gene expression in cardiac myocytes in vitro. Roles of phospholipid-derived second messengers. Circ Res. 1993;73:424–438. doi: 10.1161/01.res.73.3.424. [DOI] [PubMed] [Google Scholar]
- 103.Aceto JF, Baker KM. [Sar1]angiotensin II receptor-mediated stimulation of protein synthesis in chick heart cells. Am J Physiol. 1990;258:H806–H813. doi: 10.1152/ajpheart.1990.258.3.H806. [DOI] [PubMed] [Google Scholar]
- 104.Baker KM, Chernin MI, Wixson SK, Aceto JF. Renin-angiotensin system involvement in pressure-overload cardiac hypertrophy in rats. Am J Physiol. 1990;259:H324–H332. doi: 10.1152/ajpheart.1990.259.2.H324. [DOI] [PubMed] [Google Scholar]
- 105.Sil P, Sen S. Angiotensin II and myocyte growth: role of fibroblasts. Hypertension. 1997;30:209–216. doi: 10.1161/01.hyp.30.2.209. [DOI] [PubMed] [Google Scholar]
- 106.Kumar R, Singh VP, Baker KM. The intracellular renin-angiotensin system: implications in cardiovascular remodeling. Curr Opin Nephrol Hypertens. 2008;17:168–173. doi: 10.1097/MNH.0b013e3282f521a8. [DOI] [PubMed] [Google Scholar]
- 107.Mazzolai L, Nussberger J, Aubert JF, Brunner DB, Gabbiani G, Brunner HR, Pedrazzini T. Blood pressure-independent cardiac hypertrophy induced by locally activated renin-angiotensin system. Hypertension. 1998;31:1324–1330. doi: 10.1161/01.hyp.31.6.1324. [DOI] [PubMed] [Google Scholar]
- 108.Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintrón M, Chen T, Marcinek DJ, Dorn GW, Kang YJ, Prolla TA, Santana LF, Rabinovitch PS. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ Res. 2011;108:837–846. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:468–473. doi: 10.1016/j.yjmcc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ramirez MT, Zhao XL, Schulman H, Brown JH. The nuclear deltaB isoform of Ca2+/calmodulin-dependent protein kinase II regulates atrial natriuretic factor gene expression in ventricular myocytes. J Biol Chem. 1997;272:31203–31208. doi: 10.1074/jbc.272.49.31203. [DOI] [PubMed] [Google Scholar]
- 111.Sei CA, Irons CE, Sprenkle AB, McDonough PM, Brown JH, Glembotski CC. The alpha-adrenergic stimulation of atrial natriuretic factor expression in cardiac myocytes requires calcium influx, protein kinase C, and calmodulin-regulated pathways. J Biol Chem. 1991;266:15910–15916. [PubMed] [Google Scholar]
- 112.Zhu W, Zou Y, Shiojima I, Kudoh S, Aikawa R, Hayashi D, Mizukami M, Toko H, Shibasaki F, Yazaki Y, Nagai R, Komuro I. Ca2+/calmodulin-dependent kinase II and calcineurin play critical roles in endothelin-1-induced cardiomyocyte hypertrophy. J Biol Chem. 2000;275:15239–15245. doi: 10.1074/jbc.275.20.15239. [DOI] [PubMed] [Google Scholar]
- 113.Colomer JM, Mao L, Rockman HA, Means AR. Pressure overload selectively up-regulates Ca2+/calmodulindependent protein kinase II in vivo. Mol Endocrinol. 2003;17:183–192. doi: 10.1210/me.2002-0350. [DOI] [PubMed] [Google Scholar]
- 114.Briones AM, Touyz RM. Oxidative stress and hypertension: current concepts. Curr Hypertens Rep. 2010;12:135–142. doi: 10.1007/s11906-010-0100-z. [DOI] [PubMed] [Google Scholar]
- 115.Laursen JB, Rajagopalan S, Galis Z, Tarpey M, Freeman BA, Harrison DG. Role of superoxide in angiotensin II-induced but not catecholamine-induced hypertension. Circulation. 1997;95:588–593. doi: 10.1161/01.cir.95.3.588. [DOI] [PubMed] [Google Scholar]
- 116.Virdis A, Neves MF, Amiri F, Touyz RM, Schiffrin EL. Role of NAD(P)H oxidase on vascular alterations in angiotensin II-infused mice. J Hypertens. 2004;22:535–542. doi: 10.1097/00004872-200403000-00016. [DOI] [PubMed] [Google Scholar]
- 117.Zafari AM, Ushio-Fukai M, Minieri CA, Akers M, Lassègue B, Griendling KK. Arachidonic acid metabolites mediate angiotensin II-induced NADH/NADPH oxidase activity and hypertrophy in vascular smooth muscle cells. Antioxid Redox Signal. 1999;1:167–179. doi: 10.1089/ars.1999.1.2-167. [DOI] [PubMed] [Google Scholar]
- 118.Sagar S, Kallo IJ, Kaul N, Ganguly NK, Sharma BK. Oxygen free radicals in essential hypertension. Mol Cell Biochem. 1992;111:103–108. doi: 10.1007/BF00229580. [DOI] [PubMed] [Google Scholar]
- 119.Berry C, Hamilton CA, Brosnan MJ, Magill FG, Berg GA, McMurray JJ, Dominiczak AF. Investigation into the sources of superoxide in human blood vessels: angiotensin II increases superoxide production in human internal mammary arteries. Circulation. 2000;101:2206–2212. doi: 10.1161/01.cir.101.18.2206. [DOI] [PubMed] [Google Scholar]
- 120.Zalba G, Beaumont FJ, San José G, Fortuño A, Fortuño MA, Etayo JC, Díez J. Vascular NADH/NADPH oxidase is involved in enhanced superoxide production in spontaneously hypertensive rats. Hypertension. 2000;35:1055–1061. doi: 10.1161/01.hyp.35.5.1055. [DOI] [PubMed] [Google Scholar]
- 121.Chen X, Touyz RM, Park JB, Schiffrin EL. Antioxidant effects of vitamins C and E are associated with altered activation of vascular NADPH oxidase and superoxide dismutase in stroke-prone SHR. Hypertension. 2001;38:606–611. doi: 10.1161/hy09t1.094005. [DOI] [PubMed] [Google Scholar]
- 122.Li H, Li W, Gupta AK, Mohler PJ, Anderson ME, Grumbach IM. Calmodulin kinase II is required for angiotensin II-mediated vascular smooth muscle hypertrophy. Am J Physiol Heart Circ Physiol. 2010;298:H688–H698. doi: 10.1152/ajpheart.01014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]