Abstract
A new method using ultra-fast liquid chromatography and tandem mass spectrometry (UFLC–MS/MS) was developed for the simultaneous determination of buprenorphine and the metabolites norbuprenorphine, buprenorphine-3β-glucuronide, and norbuprenorphine-3β-glucuronide in plasma and urine. Sample handling, sample preparation and solid-phase extraction procedures were optimized for maximum analyte recovery. All four analytes of interest were quantified by positive ion electrospray ionization tandem mass spectrometry after solid-phase microextraction. The lower limits of quantification in plasma were 1 pg/mL for buprenorphine and buprenorphine glucuronide, and 10 pg/mL for norbuprenorphine and norbuprenorphine glucuronide. The lower limits of quantitation in urine were 10 pg/mL for buprenorphine, norbuprenorphine and their glucuronides. Overall extraction recoveries ranged from 68–100% in both matrices. Interassay precision and accuracy was within 10% for all four analytes in plasma and within 15% in urine. The method was applicable to pharmacokinetic studies of low-dose buprenorphine.
Keywords: Buprenorphine, Norbuprenorphinem, Glucuronide, Mass spectrometry
1. Introduction
Buprenorphine is an opioid used for decades in treating acute and chronic pain [1–3]. A transdermal formulation was recently approved for treating moderate-severe chronic pain. Buprenorphine sublingual tablets or films are also used for opioid addiction therapy [4], and for reducing addiction-related infectious diseases [5]. Clinical advantages of buprenorphine in addiction treatment include approval for use by private (non-specialized) practitioners, and a narrow dose range enabling rapid dose titration [5]. Reported advantages in pain therapy include a ceiling effect for respiratory depression but not analgesia at clinically relevant doses, fewer adverse events than other opioids, and the rarity of withdrawal symptoms [6–10].
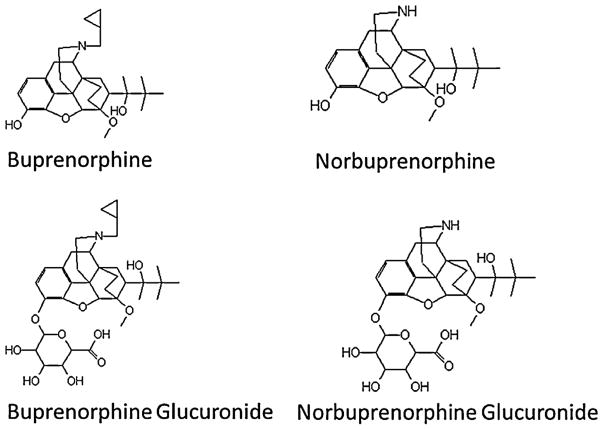
Buprenorphine is extensively metabolized, primarily to norbuprenorphine, and both also undergo glucuronidation, to buprenorphine-3β-glucuronide and the secondary metabolite, norbuprenorphine-3β-glucuronide (Fig. 1) [11,12]. Plasma metabolite concentrations can approximate or exceed those of the parent drug, and relative norbuprenorphine, buprenorphine-3β-glucuronide, and norbuprenorphine-3β-glucuronide exposures, based on molar area under the plasma concentration vs time curves, are 200%, 100%, and 600% those of buprenorphine [13]. These buprenorphine metabolites are pharmacologically active in animals [14–17]. Metabolism therefore may potentially constitute a bioactivation pathway, although the role of these metabolites in mediating buprenorphine effects in humans is unknown. Additional pathways of buprenorphine metabolism have also been recognized [18]. Clinical disposition and metabolism of buprenorphine are subject to well-described drug interactions [19–21].
Fig. 1.
Structures of buprenorphine and the metabolites norbuprenorphine, buprenorphine-3β-glucuronide and norbuprenorphine-3β-glucuronide.
Methods for quantification of buprenorphine and one or more major metabolites in plasma and/or urine include immunoassay [22,23], gas chromatography with various detectors [24–28], HPLC with single quadrupole or tandem quadrupole mass spectrometry [13,29–35], and most recently UPLC methods with mass spectrometry [36–40]. Various methods of sample preparation have also been described, including protein precipitation [40], liquid/liquid extraction [39], and solid-phase extraction [37,41,42]. The most sensitive of the above assays generally have a lower limit of quantification of approximately 0.1 ng/mL for buprenorphine and 0.25 ng/mL for norbuprenorphine, and the glucuronide metabolites are often not analyzed.
Increasing interest in the clinical use of buprenorphine, recognition of metabolites formation and their potential clinical significance, use of lower buprenorphine doses for pain, and the desire to study buprenorphine drug interactions in healthy volunteers (at much lower doses than used in patients for prevention of opioid withdrawal) have accentuated interest in analytical methods with greater sensitivity for buprenorphine and metabolites in both plasma and urine. Buprenorphine is commonly administered sublingually at 4–32 mg/d for addiction maintenance therapy, with steady-state plasma buprenorphine concentrations averaging 5–10 ng/mL, and 2–16 mg intravenous buprenorphine results in peak buprenorphine and norbuprenorphine concentrations of 20–130 and 0.5–3.5 ng/mL respectively [43]. In contrast, lower doses (0.15–0.2 mg intravenously, 2 mg sublingually) must be used in non-dependent healthy volunteers [44]. Assuming linear pharmacokinetics, 0.2 mg/kg intravenously would have an expected peak plasma concentration (Cmax) of approximately 2 ng/mL, declining to approximately 0.005 ng/mL after 72 h, and a 2 mg sublingual dose gives an expected Cmax of 1.6 ng/mL [45]. With transdermal buprenorphine patches delivering 5, 10 and 20 μg/h maximum plasma buprenorphine concentrations were 176, 191 and 476 pg/mL, respectively [10]. These studies exemplify the need for assay sensitivity.
Therefore, the purpose of this investigation was to develop and validate an analytical method for the analysis of buprenorphine and the metabolites norbuprenorphine, buprenorphine-3β-glucuronide, and norbuprenorphine-3β-glucuronide, in plasma and urine, with greater sensitivity, particularly over the expected plasma concentration range of low pg/mL to low ng/mL. A target limit of quantification of 1 pg/mL was established.
2. Experimental
2.1. Reagents
Buprenorphine, buprenorphine-d4, norbuprenorphine, norbuprenorphine-d3, buprenorphine-3β-glucuronide (B3G) and norbuprenorphine-3β-glucuronide (N3G) were all purchased from Cerilliant Corporation (Round Rock, TX). Phosphoric acid, formic acid, glacial acetic acid, methanol and acetonitrile were all obtained from Sigma-Aldrich (St. Louis, MO). Ammonium hydroxide was from JT Baker (Center Valley, PA). Water was filtered by a Milli-Q water filtration system, Millipore (Billerica, MA). Outdated human plasma and human urine were obtained from the local university hospital. Oasis MCS 96 well microextraction plates for method development purposes were kindly donated by Waters (Milford, MA).
2.2. Instrumentation
LC–MS/MS analysis was performed on an ultra-fast liquid chromatography system from Shimadzu Scientific Instruments (Columbia, MD) consisting of a CMB-20A system controller, two LC-20ADXR pumps, a DGU-20A3 degasser, a SIL-20AC autosampler, and a CTO-20A column oven. An external Valco switching valve was installed between the chromatography system and the mass spectrometer. The chromatography system was coupled to an API 4000 QTrap LC–MS/MS linear ion trap triple quadrupole tandem mass spectrometer from Applied Biosystems/MDS Sciex (Foster City, CA).
2.3. Solutions
Methanol stock solutions of buprenorphine, norbuprenorphine, B3G and N3G (100 μg/mL) from Cerilliant Corporation were stored at −20 °C. Composite 1 μg/mL standard solutions containing buprenorphine, norbuprenorphine, B3G, and N3G were prepared in duplicate in methanol from the 100 μg/mL stock solutions (CS1 and CS2). CS1 was utilized to make the working standard solutions for the standard curve and CS2 was used to make the working standard solutions for the quality control samples. Composite standards (10 μg/mL) were prepared and used to make urine calibrators and quality control samples (UCS1 and UCS2) which were prepared in a similar manner to the plasma standards. Internal standard solutions were made by spiking 100 ug/mL methanol stocks of buprenorphine-d4 and norbuprenorphine-d3 into 5% phosphoric acid to a final concentration of 0.5 ng/mL of each compound. This solution was found to be stable for several months stored at 4 °C. The solid-phase extraction (SPE) elution solution was acetonitrile, methanol and 30% ammonium hydroxide (12:8:1).
2.4. Standard and quality control preparation
Working plasma standards were prepared in methanol at the following concentrations of buprenorphine, norbuprenorphine, B3G and N3G by spiking the appropriate amount of CS1 or dilutions of CS1 into methanol: 0, 0.02, 0.06, 0.2, 0.6, 2, 6, 20, 60 and 200 ng/mL. Working urine standards were prepared in methanol at the following concentrations of the four analytes of interest by spiking methanol with UCS1 or dilutions of UCS1: 0.2, 0.6, 1, 6, 20, 60, 200, 600, 2000 and 6000 ng/mL. Working plasma quality control solutions were prepared in methanol at the following concentrations of all four analytes by spiking the appropriate amount of CS2 or dilutions of CS2 into methanol: 0.12, 1.2 and 12 ng/mL. These working standard solutions were verified to be stable for four weeks when stored at −20 °C. Working urine quality control solutions were also prepared in methanol for all for analytes by spiking the appropriate amount of UCS2 or dilutions of UCS2 into methanol: 0.12, 1.2, 12, 120 and 1200 ng/mL.
Standards and quality control samples were prepared in pooled control plasma or urine from 5 anonymous donors. To a glass culture tube containing 475 μl of control plasma or urine, 25 μl of each working standard or working quality control solution was added. The final concentrations of standards were 0.001, 0.003, 0.01, 0.03, 0.1, 0.3, 1, 3 and 10 ng/mL for plasma and 0.01, 0.03, 0.1, 0.3, 1, 3, 10, 30, 100 and 300 ng/mL for urine. The final low, mid and high quality control (QC) concentrations (LQC, MQC and HQC respectively) were 0.001, 0.1, and 10 ng/mL for buprenorphine and buprenorphine glucuronide and 0.01, 0.3 and 10 ng/mL for norbuprenorphine and norbuprenorphine glucuronide in plasma, and 0.06, 0.6, and 60 ng/mL for all analytes in urine.
2.5. Sample preparation procedure
Plasma or urine (420 μl) from calibration standards, QC samples, or subject samples was pipetted to a clean glass culture tube. An equal volume of internal standard mix, 0.5 ng/mL of each buprenorphine d4 and norbuprenorphine d3 in 5% phosphoric acid, was added to each tube. Samples were capped and mixed on a vortex mixer for approximately 30 s.
Solid-phase microextraction was then performed on an Oasis MCX 96 well μextraction plate. The plate was prepared by first conditioning the plate with 200 μl of methanol followed by 200 μl of MilliQ water. Next, a 750 μl aliquot of the plasma sample was then passed through the plate under vacuum as recommended by the manufacturer (10–15 mm Hg). The plate was then washed with 200 μl of 0.2% acetic acid, followed by 200 μl of methanol, again under vacuum. The plates were thoroughly dried under vacuum for 2 min. The analytes were eluted into a clean 96 well collection plate by vacuum with two separate additions of 25 μl of SPE elution solution (acetonitrile, methanol, ammonium hydroxide). Eluents were then transferred to autosampler vials with glass inserts and placed in the autosampler tray.
2.6. Analytical method
Chromatographic separation of buprenorphine, norbuprenorphine, B3G, N3G and the internal standards buprenorphine-d4, and norbuprenorphine-d3 was achieved using a Kinetix core shell analytical column (100 × 2.1 mm, 2.6 μm) coupled with a Security Guard ULTRA cartridge UHPLC C18 (2.1 mm) guard cartridge (Phenomenex, Torrance, CA). The flow rate was 0.7 mL/min with a mobile phase of 0.1% formic acid in water (A) and acetonitrile (B). The column was equilibrated with 10% B for 0.5 min, a linear gradient to 85% B was applied over 6.5 min. The column was then ramped linearly to 100% B over 0.1 min, held at 100% B for 0.5 min, then reverted back to 10% B over the next 0.3 min and allowed to re-equilibrate at 10% B for 2.2 min. The total run time was 10 min per sample. The column oven was kept at 55 °C and the autosampler cooler was maintained at 15 °C. The injection volume was 15 μl. The same chromatographic conditions were utilized for plasma and urine samples.
The mass spectrometer was equipped with an electro-spray ion source which was operated in positive ion multiple reaction monitoring (MRM) mode for the detection of all of the analytes and internal standards. The [M+H]+ transitions were optimized for each analyte and were selected as follows: m/z 468 → 363 and m/z 468 → 101 for buprenorphine, m/z 414 → 115 and m/z 414 → 101 for norbuprenorphine, m/z 644 → 396 and m/z 644 → 468 for buprenorphine-3-glucuronide, m/z 590 → 414 and m/z 590 → 101 for norbuprenorphine-3-glucuronide, m/z 472 → 101 for buprenorphine-d4 and m/z 417 → 101 for norbuprenorphine-d3. The first mass pair for buprenorphine, norbuprenorphine, B3G and N3G was used for quantitation and the second mass pair was used for qualitative identification. The mass spectrometer settings for the declustering potential (DP), the collision energy (CE) and the collision cell exit potential (CXP) were optimized for each of these transitions. The entrance potential (EP) was optimized at 10 V for all of the transitions. Buprenorphine was used to optimize the source temperature and gas settings through flow injection. The optimal source temperature was 600 °C with the selected mobile phase and flow rate. Nitrogen was used for all gasses and the curtain gas was set at 35 psig, CAD gas set to high, Gas 1 40 psig and Gas 2 60 psig.
Raw data were acquired and processed using Analyst software version 1.5.2 (Applied Biosystems/MDS Sciex, Foster City, CA). Calibration curves of peak area ratio vs analyte concentration were fit using linear least-squares analysis and 1/x2 weighting.
2.7. Method validation
The validation was performed as per the United States Food and Drug Administration (US-FDA) Bioanalytical Method Validation Guidance [46]. The following assessments were made to demonstrate the rigor of this assay: recovery, accuracy and precision, selectivity, stability and matrix effect. The method was then deemed rigorous enough to analyze sub-clinical doses of buprenorphine that had been dosed in healthy volunteers.
2.7.1. Recovery
Recovery of buprenorphine, norbuprenorphine, B3G and N3G from plasma or urine was determined by comparing a sample of pure compound with internal standard in solvent at the nominal high, medium or low concentrations, to the same concentration of sample in plasma or urine extracted as described above.
2.7.2. Accuracy and precision
The precision and accuracy for each analyte was determined at a low, mid and high concentration levels in human control plasma or urine over three separate batch runs. Precision was determined as the coefficient of variation (%CV), calculated by dividing the standard deviation by the mean. Accuracy was determined as the percent relative error, calculated by subtracting the theoretical concentration from the observed mean, and dividing by the theoretical concentration.
2.7.3. Selectivity
Chromatography of buprenorphine and metabolites in pooled blank plasma and urine was optimized to achieve separation from potential interferences. Signal to noise ratios were greater than 5:1 at the limit of quantification for each analyte, as recommended by the FDA guidance [46]. No carryover was observed when a blank plasma sample or blank urine sample was injected directly following the highest calibration standard.
2.7.4. Stability
Stability of the spiked plasma and urine samples was determined at the low and high quality control concentrations after the samples were held at room temperature for 24 hr, after one freeze/thaw cycle (−20 °C), and after three −20 °C freeze/thaw cycles.
2.7.5. Matrix effect
Matrix effect was studied by comparing pure compound samples prepared in mobile phase, to extracted quality control samples in plasma. The chromatographic separation was optimized to ensure that any potentially interfering peaks would be chromatographically separated from the peak of interest.
3. Results and discussion
3.1. Method development
Various mobile phases and HPLC columns were evaluated for their usefulness. Initially investigated was a Zorbax Extend C18 Rapid Resolution C18 HT 2.1 × 100 mm, 1.8 μm column with a 600 bar limit (Agilent, Santa Clara, CA) and a mobile phase of acetonitrile and 10 mM ammonium bicarbonate in water (pH 9.0). The small particle size was chosen to give sharper and taller peaks and therefore improved sensitivity. However, interferences with norbuprenorphine prevented a limit of quantitation below 50 ng/mL for this analyte. Also, upon method validation, extended use of the ammonium bicarbonate mobile phase with the extracted plasma samples required frequent column washing to keep the operating pressure within the recommended operating range. The mobile phase was switched to an ammonia/methanol mobile phase to assess if that would provide a lower LOQ for norbuprenorphine and a more robust column life and operation. The pressure again was very high after each individual run requiring extensive column washing to regain adequate operating pressures. The Kinetix core shell column ultimately used afforded the requisite selectivity, and had a slightly larger particle size than the Zorbax extend column (2.6 μm vs 1.8 μm), necessitating less extensive column washing than was necessary with the smaller particle size Zorbax column.
The mass spectrometer settings were optimized for each of the analytes dissolved in methanol. Flow injection allowed optimization of the system gas settings and temperature with a mobile phase system of 0.1% formic acid in water and either methanol or acetonitrile, both with and without added formic acid at various flow rates. Buprenorphine and norbuprenorphine were used to optimize the gas and temperature settings.
Extraction procedures which were initially assessed all utilized traditional solid-phase extraction techniques with either a Strata XC plate (Phenomenex, Torrance, CA) or a Varian Bond Elute Plexa plate (Agilent, Santa Clara, CA). These systems did not give adequate sample concentration to achieve the target limit of detection necessary due to many processing steps, drying down, and transfer procedures which increased the loss of compound at each consecutive transfer step. This was most notable for buprenorphine, which is known to stick to plasticware.
The final microextraction procedure was optimized for high recovery of the analytes from plasma and its applicability to urine samples was then confirmed. The microextraction procedure step with the greatest effect on percent recovery was the wash step with 0.2% acetic acid. Various acids and strengths of acid were tested, with a more dilute acid giving a greater recovery than more concentrated acids. Optimization of recovery was of great importance as the concentrations in subject samples was expected to be very low. Also, minimization of steps and elimination of the dry down steps necessary with traditional solid-phase extraction plates increased the recovery of all analytes.
Sample concentration achieved by using microextraction, combined with a 2.6 μm HPLC column that could be run at pressures up to 600 bar (thereby decreasing peak width and increasing peak height), along with careful optimization of the mass spectrometer, resulted in a limit of quantitation in plasma for buprenorphine and buprenorphine glucuronide of 1 pg/mL and 10 pg/mL for norbuprenorphine and norbuprenorphine glucuronide.
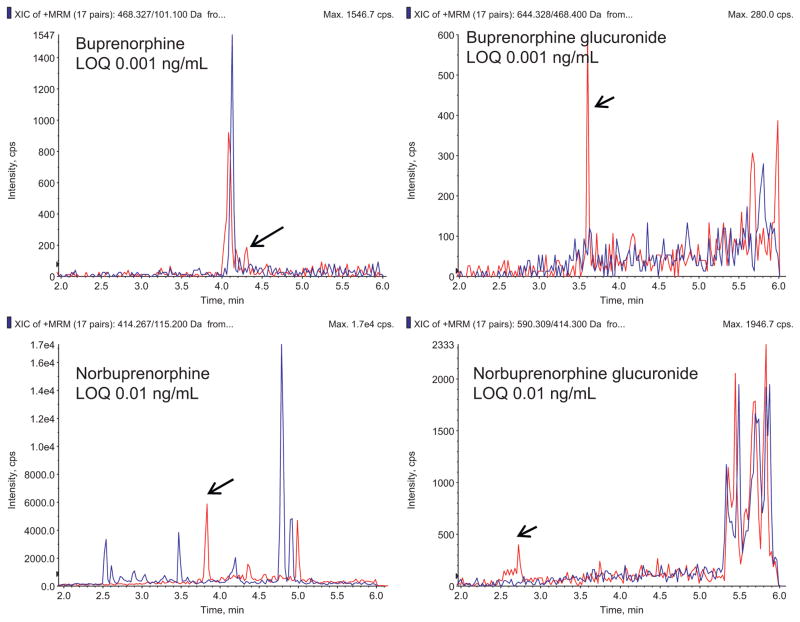
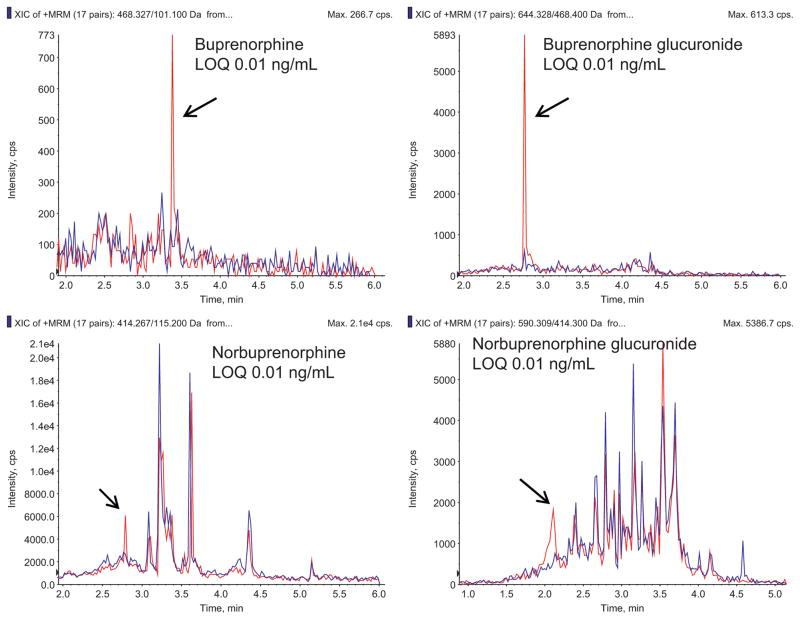
Fig. 2 shows representative chromatograms for buprenorphine and metabolites in plasma at the limit of quantification, as well as chromatograms of blank plasma. Fig. 3 shows representative chromatograms for buprenorphine and metabolites in urine at the limit of quantification, and chromatograms of blank plasma. Calibration curves were linear over the concentration range 0.001–10, 0.01–10, 0.001–10, and 0.01–10 ng/mL in plasma for buprenorphine, norbuprenorphine, buprenorphine glucuronide and norbuprenorphine glucuronide, with typical correlation coefficients (r2) of 0.999, 0.998, 0.999, and 0.997, respectively. Calibration curves in urine were linear over the range 0.01–100 ng/mL for all four analytes, with typical correlation coefficients of 0.997, 0.998, 0.998 and 0.997 for buprenorphine, norbuprenorphine, buprenorphine glucuronide and norbuprenorphine glucuronide, respectively.
Fig. 2.
Representative MRM chromatogram of a human plasma standard containing each analyte of interest at the assay limit of quantification (0.001 ng/ml buprenorphine, 0.01 ng/ml norbuprenorphine, 0.001 ng/ml buprenorphine-3β-glucuronide, and 0.010 ng/ml norbuprenorphine-3β-glucuronide). In each panel, the blue trace is blank plasma, and the red trace is the analyte at the limit of quantification.
Fig. 3.
Representative MRM chromatogram of a human urine standard containing each analyte of interest at the assay limit of quantification (0.01 ng/ml for all). In each panel, the blue trace is blank plasma, and the red trace is the analyte at the limit of quantification.
3.2. Method validation
Recovery data for the four analytes in plasma and urine are provided in Tables 1 and 2, respectively. Plasma recovery ranged from 92 to 104% for buprenorphine and buprenorphine glucuronide, and from 89% to 107% from urine. Recovery of norbuprenorphine and norbuprenorphine glucuronide from plasma was lower, generally approximately 80%. Analyte recovery from urine was generally higher for all analytes, with all in the range of 90–100%. Inter-day and intraday accuracy and precision for analyte determination in plasma and urine are shown in Tables 3 and 4, respectively. Precision and accuracy of buprenorphine was high in both urine and plasma with a coefficient of variation of less than 10% in both matrices. The other three analytes all had coefficients of variation of less than 15% in both matrices. Optimized chromatographic separation of analytes from matrix interferences in both plasma and urine minimized matrix interferences, thereby enabling the low coefficients of variation. Precision and accuracy of 10 fold urine dilution samples were also tested and passed acceptance criteria of being within 10% of the original measurement (Table 4). Stability results in plasma and urine are shown in Tables 5 and 6. All four compounds were stable in both plasma and urine for 24 h on the benchtop at room temperature, and after undergoing up to three freeze thaw cycles. It was also verified that prepared samples were stable up to one week when stored at 4 °C (data not shown).
Table 1.
Analyte recovery from plasma.
| Analyte | Theoretical concentration (ng/mL) | Recovered concentration (ng/mL)* | Recovery (%) | CV (%) |
|---|---|---|---|---|
| Buprenorphine | 0.001 | 0.00096 ± 0.00011 | 96.4 | 11.8 |
| 0.1 | 0.0966 ± 0.0058 | 96.6 | 6.0 | |
| 10 | 10.4 ± 0.77 | 104 | 7.3 | |
| Norbuprenorphine | 0.01 | 0.00815 ± 0.00064 | 81.5 | 7.9 |
| 0.3 | 0.247 ± 0.032 | 82.4 | 12.8 | |
| 10 | 10.9 ± 1.7 | 109 | 16.0 | |
| Buprenorphine Glucuronide | 0.001 | 0.00092 ± 0.00012 | 92.5 | 12.4 |
| 0.1 | 0.098 ± 0.010 | 98.2 | 10.5 | |
| 10 | 9.45 ± 0.66 | 94.5 | 6.9 | |
| Norbuprenorphine Glucuronide | 0.01 | 0.0068 ± 0.0006 | 68.4 | 8.7 |
| 0.3 | 0.252 ± 0.063 | 84.2 | 24.9 | |
| 10 | 8.04 ± 2.00 | 80.4 | 25.0 |
Mean ± SD (N = 3).
Table 2.
Analyte recovery from urine.
| Analyte | Theoretical concentration (ng/mL) | Recovered concentration (ng/mL)* | Recovery (%) | CV (%) |
|---|---|---|---|---|
| Buprenorphine | 0.06 | 0.0647 ± 0.0052 | 108 | 8.1 |
| 6 | 6.2 ± 0.41 | 103 | 6.5 | |
| 60 | 53.5 ± 2.0 | 89.1 | 3.8 | |
| Norbuprenorphine | 0.06 | 0.0636 ± 0.0029 | 106 | 4.5 |
| 6 | 6.54 ± 0.42 | 109 | 6.4 | |
| 60 | 57.7 ± 3.5 | 96.2 | 6.1 | |
| Buprenorphine glucuronide | 0.06 | 0.0594 ± 0.0082 | 99.0 | 13.8 |
| 6 | 5.63 ± 0.74 | 93.9 | 13.1 | |
| 60 | 60.3 ± 5.9 | 100 | 9.8 | |
| Norbuprenorphine glucuronide | 0.06 | 0.0627 ± 0.0050 | 105 | 8.0 |
| 6 | 6.08 ± 0.56 | 101 | 9.2 | |
| 60 | 61.6 ± 5.3 | 103 | 8.6 |
Mean ± SD (N = 3).
Table 3.
Accuracy and precision in plasma.
| Buprenorphine | Norbuprenorphine | Buprenorphine glucuronide | Norbuprenorphine glucuronide | |
|---|---|---|---|---|
| Intra-assay precision and accuracy | ||||
| Low | 99.9 ± 3.3 | 99.9 ± 7.4 | 99.0 ± 6.6 | 100.4 ± 13.0 |
| Medium | 104.7 ± 7.7 | 102.8 ± 8.0 | 96.0 ± 12.1 | 98.6 ± 7.6 |
| High | 88.9 ± 2.6 | 102.4 ± 5.5 | 101.3 ± 6.5 | 103.4 ± 5.2 |
| Inter-assay precision and accuracy | ||||
| Low | 99.3 ± 3.3 | 100.6 ± 8.9 | 100.7 ± 5.6 | 103.1 ± 12.8 |
| Medium | 107.3 ± 7.0 | 97.2 ± 9.3 | 96.7 ± 13.2 | 98.9 ± 9.5 |
| High | 89.3 ± 2.5 | 101.1 ± 5.3 | 103.6 ± 5.7 | 103.6 ± 5.9 |
Results are % of target value (mean ± standard deviation; N = 5 for intra-assay and N = 9 for inter-assay). Low, medium and high quality control concentrations were 0.001, 0.1, and 10 ng/mL for buprenorphine and buprenorphine glucuronide and 0.01, 0.3 and 10 ng/mL for norbuprenorphine and norbuprenorphine glucuronide.
Table 4.
Accuracy and precision in urine.
| Buprenorphine | Norbuprenorphine | Buprenorphine glucuronide | Norbuprenorphine glucuronide | |
|---|---|---|---|---|
| Intra-assay precision and accuracy | ||||
| Low | 106.0 ± 6.2 | 101.4 ± 10.6 | 107.0 ± 9.9 | 111.0 ± 2.6 |
| Medium | 99.1 ± 7.2 | 113.3 ± 2.1 | 85.8 ± 0.5 | 97.8 ± 7.2 |
| High | 99.9 ± 9.6 | 100.0 ± 4.5 | 101.4 ± 5.2 | 99.8 ± 8.1 |
| Inter-assay precision and accuracy | ||||
| Low | 102.9 ± 9.7 | 96.2 ± 10.4 | 97.8 ± 12.2 | 105.7 ± 7.5 |
| Medium | 99.3 ± 8.1 | 102.3 ± 9.0 | 94.9 ± 12.2 | 99.9 ± 5.9 |
| High | 88.3 ± 5.5 | 93.8 ± 4.8 | 93.5 ± 10.3 | 89.3 ± 6.4 |
| 10 fold dilution | 98.3 ± 9.8 | 100.5 ± 4.2 | 101.4 ± 5.2 | 99.3 ± 7.5 |
Results are the % of target value (mean ± standard deviation; N = 3 for intra-assay and N = 9 for inter-assay).
Low, medium and high quality control concentrations were 0.06, 0.6, and 60 ng/mL for all analytes.
Table 5.
Stability in plasma.
| % of original value
|
||||||
|---|---|---|---|---|---|---|
| Room temp.
|
Freeze/thaw x1
|
Freeze/thaw x3
|
||||
| Low | High | Low | High | Low | High | |
| Buprenorphine | 101.3 ± 14.1 | 102.1 ± 9.1 | 113.0 ± 10.3 | 104.4 ± 10.6 | 106.0 ± 6.6 | 101.5 ± 12.6 |
| Norbuprenorphine | 99.1 ± 9.9 | 100.6 ± 1.4 | 109.3 ± 14.4 | 102.7 ± 5.2 | 101.4 ± 15.0 | 101.4 ± 6.3 |
| Buprenorphine glucuronide | 108.4 ± 8.2 | 108.9 ± 7.4 | 116.9 ± 10.9 | 111.5 ± 6.6 | 114.8 ± 10.3 | 112.8 ± 4.9 |
| Norbuprenorphine glucuronide | 114.2 ± 6.7 | 102.3 ± 14.2 | 105.1 ± 13.5 | 102.3 ± 8.5 | 116.7 ± 9.4 | 103.0 ± 5.6 |
Results are the mean ± standard deviation (N = 3). Low concentration was 0.01 ng/mL and high concentration was 10 ng/mL.
Table 6.
Stability in urine.
| % of original value
|
||||||
|---|---|---|---|---|---|---|
| Room temp
|
Freeze/thaw x1
|
Freeze/thaw x3
|
||||
| Low | High | Low | High | Low | High | |
| Buprenorphine | 87.7 ± 1.9 | 101.1 ± 5.9 | 92.9 ± 4.3 | 95.5 ± 3.4 | 94.1 ± 13.7 | 97.7 ± 9.8 |
| Norbuprenorphine | 109.8 ± 1.1 | 104.1 ± 7.0 | 82.5 ± 7.3 | 89.4 ± 8.4 | 101.8 ± 5.6 | 92.1 ± 10.8 |
| Buprenorphine glucuronide | 108.7 ± 3.5 | 82.4 ± 8.5 | 101.3 ± 11.6 | 100.4 ± 11.6 | 97.3 ± 7.1 | 96.6 ± 8.8 |
| Norbuprenorphine glucuronide | 101.9 ± 10.1 | 86.2 ± 11.5 | 105.6 ± 4.8 | 93.5 ± 8.6 | 101.5 ± 8.3 | 104.1 ± 10.6 |
Results are the mean ± standard deviation (N = 3). Low concentration was 0.06 ng/mL and high concentration was 60 ng/mL.
3.3. Application to a pharmacokinetic study
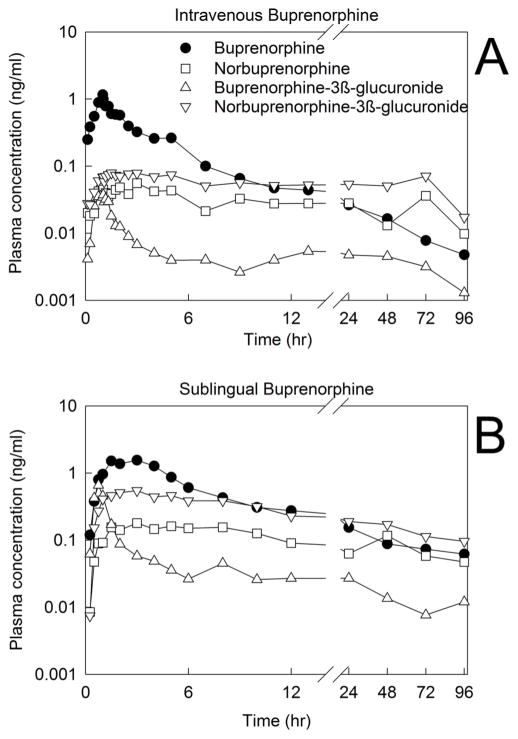
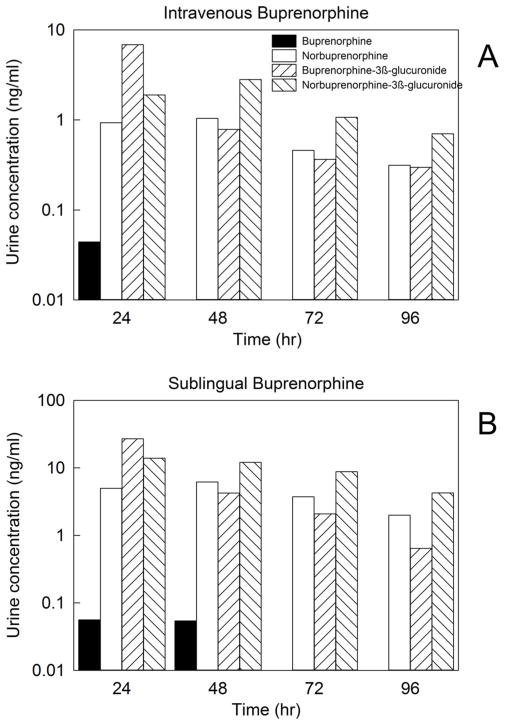
The method described here was successfully utilized to quantify plasma and urine concentrations of buprenorphine and metabolites in a healthy volunteer subject enrolled in a clinical study to evaluate the disposition of low dose intravenous (0.2 mg) and sublingual (2 mg) buprenorphine. Plasma and urine concentrations are shown in Figs. 4 and 5, respectively. Analytes were quantifiable for 96 h after dosing.
Fig. 4.
Plasma concentrations of buprenorphine and metabolites in a research subject administered (A) intravenous buprenorphine (0.2 mg) or (B) sublingual buprenorphine (2 mg).
Fig. 5.
Urine concentrations of buprenorphine and metabolites in a research subject administered (A) intravenous buprenorphine (0.2 mg) or (B) sublingual buprenorphine (2 mg). Urine was collected in 24 hr intervals (days 1–4) after drug dosing.
3.4. Assay comparison
The investigation aimed to develop and validate an analytical method with improved sensitivity for analysis of buprenorphine, β-glucuronide, and norbuprenorphine-3β-glucuronide, in plasma and urine. The intended application was analysis of pharmacokinetic studies of low dose intravenous and sublingual buprenorphine, with measurement of plasma and urine concentrations for up to 96 h after dosing (the entire measurement period), to capture three buprenorphine half-lives. The anticipated plasma concentration range needed was low pg/mL to low ng/mL with a target limit of quantification of 1 pg/mL. The degree of sensitivity afforded by this assay was sufficient to determine buprenorphine concentrations in the clinical study, and achieved the target of a lower limit of quantitation of 1 pg/mL for buprenorphine and buprenorphine glucuronide in plasma. Limits of quantification were higher for norbuprenorphine and norbuprenorphine glucuronide. Nonetheless, because plasma concentrations of these metabolites were higher than those of buprenorphine glucuronide, plasma concentrations were quantifiable for up to 96 h (the experimental measurement period). Buprenorphine and all metabolites were readily quantifiable in urine.
No analytical methods with sufficient sensitivity, for all four analytes, had been reported at the time this assay was developed. Two recently reported higher sensitivity assays, one using liquid–liquid extraction for buprenorphine and norbuprenorphine, and another using unspecified solid-phase extraction for buprenorphine and norbuprenorphine glucuronide [47]. Assay range for the four analytes was 20–10,000 pg/mL, and the lower limit of quantification was 20 pg/mL for buprenorphine and norbuprenorphine, and 25 pg/mL for buprenorphine and norbuprenorphine glucuronide. A high-sensitivity assay for buprenorphine and norbuprenorphine, but not their glucuronides, in plasma, using unspecified mixed-mode cation extraction plates, was also recently reported [48]. Recovery of buprenorphine and norbuprenorphine was 76% and 82%, respectively, and the lower limit of quantification was 25 pg/mL for both. The present assay represents a significant improvement in sensitivity, and applicability to all four analytes (buprenorphine, norbuprenorphine, buprenorphine glucuronide, and norbuprenorphine glucuronide), and in both matrices of interest (plasma and urine), compared with even these recent methods.
4. Conclusion
A highly selective and sensitive UFLC–MS/MS assay for buprenorphine and the metabolites norbuprenorphine, buprenorphine-3β-glucuronide and norbuprenorphine-3β-glucuronide in human plasma and urine was developed and validated. The method follows the guidance for biolanalytical assays outlined by the US FDA. The method uses two deuterated internal standards for quantification, minimizes the number of steps to increase ruggedness of the assay. The use of solid-phase microextraction plates combined with UFLC instrumentation increased both the speed of sample processing and the sensitivity of the assay. The results presented support the assay validation, including recovery, precision, accuracy, specificity, selectivity and stability. This is the first assay to present a robust method that can be used for routine sample analysis of buprenorphine, norbuprenorphine, buprenorphine glucuronide and norbuprenorphine glucuronide in plasma in the pg/mL concentration range utilizing solid phase microextraction and core shell column technology combined with a UFLC instrument. This method is fast, efficient, robust and has successfully been utilized to analyze clinical samples.
Footnotes
National Institutes of Health grants R01-DA025931 and K24-DA00417 (to EDK) and UL1-TR000448 (to the Washington University in St. Louis Institute of Clinical and Translational Sciences).
References
- 1.Heel RC, Brogden RN, Speight TM, Avery GS. Drugs. 1979;17:81. doi: 10.2165/00003495-197917020-00001. [DOI] [PubMed] [Google Scholar]
- 2.Johnson RE, Fudala PJ, Payne R. J Pain Symptom Manage. 2005;29:297. doi: 10.1016/j.jpainsymman.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 3.Kress HG. Eur J Pain. 2009;13:219. doi: 10.1016/j.ejpain.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Lobmaier P, Gossop M, Waal H, Bramness J. Eur J Clin Pharmacol. 2010;66:537. doi: 10.1007/s00228-010-0793-6. [DOI] [PubMed] [Google Scholar]
- 5.Metzger DS, Zhang Y. Curr HIV/AIDS Rep. 2010;7:220. doi: 10.1007/s11904-010-0059-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clin Pharmacol Ther. 1994;55:569. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- 7.Dahan A, Yassen A, Romberg R, Sarton E, Teppema L, Olofsen E, Danhof M. Br J Anaesth. 2006;96:627. doi: 10.1093/bja/ael051. [DOI] [PubMed] [Google Scholar]
- 8.Wolff RF, Aune D, Truyers C, Hernandez AV, Misso K, Riemsma R, Kleijnen J. Curr Med Res Opin. 2012;28:833. doi: 10.1185/03007995.2012.678938. [DOI] [PubMed] [Google Scholar]
- 9.Hans G, Robert D. J Pain Res. 2009;2:117. doi: 10.2147/jpr.s6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plosker GL. Drugs. 2011;71:2491. doi: 10.2165/11208250-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Zacny JP, Conley K, Galinkin J. J Pharmacol Exp Ther. 1997;282:1187. [PubMed] [Google Scholar]
- 12.Moody DE, Chang Y, Huang W, McCance-Katz EF. Basic Clin Pharmacol Toxicol. 2009;105:211. doi: 10.1111/j.1742-7843.2009.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moody DE, Slawson MH, Strain EC, Laycock JD, Spanbauer AC, Foltz RL. Anal Biochem. 2002;306:31. doi: 10.1006/abio.2002.5673. [DOI] [PubMed] [Google Scholar]
- 14.Ohtani M, Kotaki H, Sawada Y, Iga T. J Pharm Exp Ther. 1995;272:505. [PubMed] [Google Scholar]
- 15.Ohtani M, Kotaki H, Nishitateno K, Yasufumi S, Iga T. J Pharmacol Exp Ther. 1997;281:428. [PubMed] [Google Scholar]
- 16.Yassen A, Kan J, Olofsen E, Suidgeest E, Dahan A, Danhof M. J Pharmacol Exp Ther. 2007;321:598. doi: 10.1124/jpet.106.115972. [DOI] [PubMed] [Google Scholar]
- 17.Brown SM, Holtzman M, Kim T, Kharasch ED. Anesthesiology. 2011;115:1251. doi: 10.1097/ALN.0b013e318238fea0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang Y, Moody DE, McCance-Katz EF. Drug Metab Dispos. 2006;34:440. doi: 10.1124/dmd.105.006148. [DOI] [PubMed] [Google Scholar]
- 19.Bruce RD, McCance-Katz E, Kharasch ED, Moody DE, Morse GD. Clin Infect Dis. 2006;43(Suppl 4):S216. doi: 10.1086/508186. [DOI] [PubMed] [Google Scholar]
- 20.Gruber VA, McCance-Katz EF. Curr HIV/AIDS Rep. 2010;7:152. doi: 10.1007/s11904-010-0048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saber-Tehrani AS, Bruce RD, Altice FL. Am J Drug Alcohol Abuse. 2011;37:1. doi: 10.3109/00952990.2010.540279. [DOI] [PubMed] [Google Scholar]
- 22.Hand CW, Baldwin D, Moore RA, Allen MC, McQuay HJ. Ann Clin Biochem. 1986;23:47. doi: 10.1177/000456328602300105. [DOI] [PubMed] [Google Scholar]
- 23.De Giovanni N, Fucci N, Scarlata S, Donzelli G. Clin Chem Lab Med. 2005;43:1377. doi: 10.1515/CCLM.2005.235. [DOI] [PubMed] [Google Scholar]
- 24.Ohtani M, Kotaki H, Uchino K, Sawada Y, Iga T. Drug Metab Dispos. 1994;22:2. [PubMed] [Google Scholar]
- 25.Kuhlman JJ, Jr, Lalani S, Magluilo J, Jr, Levine B, Darwin WD. J Anal Toxicol. 1996;20:369. doi: 10.1093/jat/20.6.369. [DOI] [PubMed] [Google Scholar]
- 26.Lisi AM, Kazlauskas R, Trout GJ. J Chromatog B. 1997;692:67. doi: 10.1016/s0378-4347(96)00496-3. [DOI] [PubMed] [Google Scholar]
- 27.Nath RP, Upton RA, Everhart ET, Cheung P, Shwonek P, Jones RT, Mendelson JE. J Clin Pharmacol. 1999;39:619. doi: 10.1177/00912709922008236. [DOI] [PubMed] [Google Scholar]
- 28.Wang YS, Lin DL, Yang SC, Wu MY, Liu RH, Su LW, Cheng PS, Liu C, Fuh MR. J Chromatogr A. 2010;1217:1688. doi: 10.1016/j.chroma.2010.01.038. [DOI] [PubMed] [Google Scholar]
- 29.Tracqui A, Kintz P, Mangin P. J Forensic Sci. 1997;42:111. [PubMed] [Google Scholar]
- 30.Hoja H, Marquet P, Verneuil B, Lotfi H, Dupuy JL, Lachâtre G. J Anal Toxicol. 1997;21:160. doi: 10.1093/jat/21.2.160. [DOI] [PubMed] [Google Scholar]
- 31.Kronstrand R, Selden TG, Josefsson M. J Anal Toxicol. 2003;27:464. doi: 10.1093/jat/27.7.464. [DOI] [PubMed] [Google Scholar]
- 32.Yue H, Borenstein MR, Jansen SA, Raffa RB. J Pharmacol Toxicol Methods. 2005;52:314. doi: 10.1016/j.vascn.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Hegstad S, Khiabani HZ, Øiestad EL, Berg T, Christophersen AS. J Anal Toxicol. 2007;31:214. doi: 10.1093/jat/31.4.214. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Rosas ME, Lofwall MR, Strain EC, Siluk D, Wainer IW. J Chromatogr B. 2007;850:538. doi: 10.1016/j.jchromb.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 35.Concheiro M, Shakleya DM, Huestis MA. Forensic Sci Int. 2009;188:144. doi: 10.1016/j.forsciint.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oiestad EL, Johansen U, Oiestad AM, Christophersen AS. J Anal Toxicol. 2011;35:280. doi: 10.1093/anatox/35.5.280. [DOI] [PubMed] [Google Scholar]
- 37.Verplaetse R, Tytgat J. Forensic Sci Int. 2012;215:136. doi: 10.1016/j.forsciint.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 38.McMillin GA, Davis R, Carlisle H, Clark C, Marin SJ, Moody DE. J Anal Toxicol. 2012;36:81. doi: 10.1093/jat/bkr020. [DOI] [PubMed] [Google Scholar]
- 39.Berg T, Jorgenrud B, Strand DH. J Anal Toxicol. 2013;37:159. doi: 10.1093/jat/bkt005. [DOI] [PubMed] [Google Scholar]
- 40.Luthi G, Blangy V, Eap CB, Ansermot N. J Pharm Biomed Anal. 2013;77 C:1. doi: 10.1016/j.jpba.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 41.Al-Asmari AI, Anderson RA. J Anal Toxicol. 2007;31:394. doi: 10.1093/jat/31.7.394. [DOI] [PubMed] [Google Scholar]
- 42.Liu AC, Lin TY, Su LW, Fuh MR. Talanta. 2008;75:198. doi: 10.1016/j.talanta.2007.10.050. [DOI] [PubMed] [Google Scholar]
- 43.Huestis MA, Cone EJ, Pirnay SO, Umbricht A, Preston KL. Drug Alcohol Depend. 2013;131:258. doi: 10.1016/j.drugalcdep.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Escher M, Daali Y, Chabert J, Hopfgartner G, Dayer P, Desmeules J. Clin Ther. 2007;29:1620. doi: 10.1016/j.clinthera.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 45.McAleer SD, Mills RJ, Polack T, Hussain T, Rolan PE, Gibbs AD, Mullins FG, Hussein Z. Drug Alcohol Depend. 2003;72:75. doi: 10.1016/s0376-8716(03)00188-1. [DOI] [PubMed] [Google Scholar]
- 46.US Food and Drug Administration, Center for Drug Evaluation and Research. [accessed 25.08.2013];2001 http://www.fda.gov/downloads/Drugs/Guidances/ucm070107.pdf.
- 47.Kapil RP, Cipriano A, Michels GH, Perrino P, O’Keefe SA, Shet MS, Colucci SV, Noveck RJ, Harris SC. Clin Drug Investig. 2012;32:583. doi: 10.1007/BF03261913. [DOI] [PubMed] [Google Scholar]
- 48.Al-Tawil N, Odar-Cederlof I, Berggren AC, Johnson HE, Persson J. Eur J Clin Pharmacol. 2013;69:143. doi: 10.1007/s00228-012-1320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]