Abstract
Significance: There are accruing concerns on potential genotoxic agents present in the environment including low-dose ionizing radiation (LDIR) that naturally exists on earth's surface and atmosphere and is frequently used in medical diagnosis and nuclear industry. Although its long-term health risk is being evaluated and remains controversial, LDIR is shown to induce temporary but significant adaptive responses in mammalian cells and animals. The mechanisms guiding the mitochondrial function in LDIR-induced adaptive response represent a unique communication between DNA damage and cellular metabolism. Elucidation of the LDIR-regulated mitochondrial activity may reveal new mechanisms adjusting cellular function to cope with hazardous environmental stress. Recent Advances: Key cell cycle regulators, including Cyclin D1/CDK4 and Cyclin B1/cyclin-dependent kinase 1 (CDK1) complexes, are actively involved in the regulation of mitochondrial functions via phosphorylation of their mitochondrial targets. Accumulating new evidence supports a concept that the Cyclin B1/CDK1 complex acts as a mediator in the cross talk between radiation-induced DNA damage and mitochondrial functions to coordinate cellular responses to low-level genotoxic stresses. Critical Issues: The LDIR-mediated mitochondrial activity via Cyclin B1/CDK1 regulation is an irreplaceable network that is able to harmonize vital cellular functions with adjusted mitochondrial metabolism to enhance cellular homeostasis. Future Directions: Further investigation of the coordinative mechanism that regulates mitochondrial activities in sublethal stress conditions, including LDIR, will reveal new insights of how cells cope with genotoxic injury and will be vital for future targeted therapeutic interventions that reduce environmental injury and cancer risk. Antioxid. Redox Signal. 20, 1463–1480.
Introduction
Humans are consistently exposed to a certain dose range of low levels of ionizing radiation (IR), which includes natural radiation on earth surface, medical radiation, and industrial radioactive materials (31, 92, 141, 198). In contrast to extensive studies collected from the exposure to high doses of IR that cause acute injury resulting in cell death and carcinogenesis (15, 77, 88, 109, 161), the health risks associated with low level of genotoxic agents, including low-dose ionizing radiation (LDIR) (less or equal to 10 cGy), need to be further investigated (169, 213). In addition to the controversial cancer risks evaluated on long-term consequences (27, 34, 56, 169, 170), mammalian cells exposed to a single dose or accumulated doses of LDIR are shown to be able to induce a temporary but significant resistance to subsequent more severe genotoxic agents, such as high doses of IR (3, 4, 22, 67, 68, 98, 122, 186, 192, 240). Further investigation of LDIR-associated adaptive mechanism may reveal new information on unknown cellular capacities that may allow cells to sense and tolerate hazardous environmental conditions. Such studies may also provide effective approaches or targets to reduce radiation-associated injury and cancer risk. Recent evidence suggests that mitochondria play a key role in the orchestrated response to maintain the homeostasis of the cell and organism (129). IR triggers not only the DNA repair (70) but also the detoxification of reactive oxygen species (ROS) that lasts for many hours or weeks depending on the cell or tissue type, and redox imbalance plays a critical role in mitochondria-mediated adaptive response (46, 101, 160, 211, 221, 233). Under IR stress, cells initiate several critical steps to induce an adaptive protection, including the enhancement of free glutathione and superoxide dismutase with a subsequent decrease in lipid peroxidation (65, 80, 201, 242). Additional prosurvival pathways are activated via a cross talk between mitochondria and NADPH oxidase (NOX) (53), which is contrasted with the proapoptotic response induced by mitochondrial dysfunction and subsequent Ca2+ release to the cytoplasm activating protein kinase C (PKC), mitogen-activated kinases (MAPKs), and c-jun N-terminal kinases (JNKs) (129).
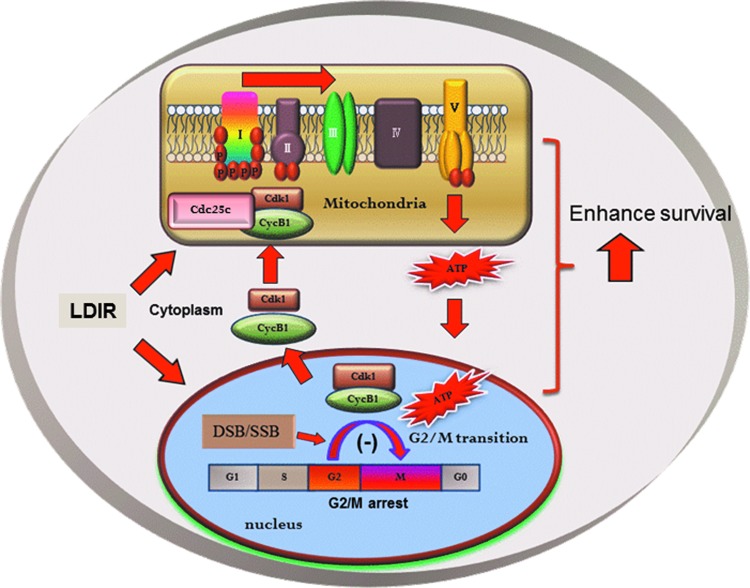
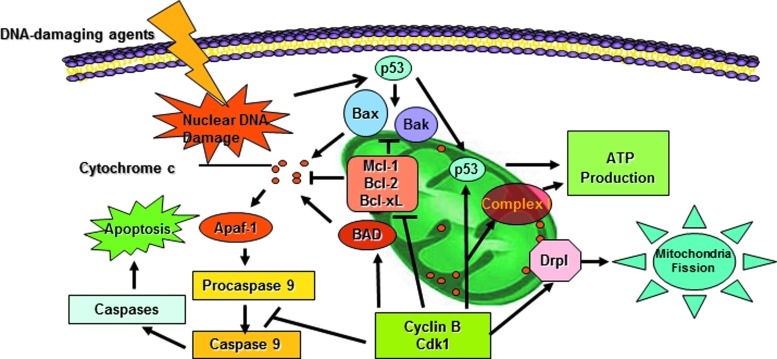
Additionally, mitochondria affect cell fate by interconnecting glycolysis and the pentose phosphate signaling pathways to cell cycle progression and apoptosis (33, 60, 113, 126, 184, 189, 218). Essential nuclear events are shown to be affected by cellular nutrient metabolism via the regulation of D type cyclins, cyclin-dependent kinases (CDKs), p53, and B-cell lymphoma 2 (Bcl-2) proteins (19, 26, 43, 95, 176, 190, 205, 234). These results illustrate a unique signaling network that appears to enable the mitochondria to sense and respond to major nuclear events, such as IR-induced DNA damages and repair. In this review, we demonstrate a pattern of radiation-induced cell adaptive response via the cell cycle regulator-mediated mitochondrial activity. We will focus the role of Cyclin D1/CDK4 and Cyclin B1/CDK1 in LDIR-induced adaptive response. A conceptual new mechanism is proposed to link the nuclear events, such as IR-induced nuclear DNA damages and G2/M division, with mitochondrial regulation (Fig. 1). The elucidation of the cell cycle regulator-guided mitochondrial metabolism in IR-induced adaptive resistance may shed light on how DNA damage can initiate the reprogramming of mitochondrial metabolism. Since the mitochondria-to-nuclear communication has been reviewed well in the literature, we will focus on the mitochondrial functions triggered by IR-induced mitochondrial protein influx, while keeping in mind the hypothesis that this could represent a paradigm for understanding nuclear-to-mitochondria and mitochondria-to-nuclear cross talk. Further elucidation of these communications in genotoxic conditions may define more abnormalities in cellular metabolism and human diseases.
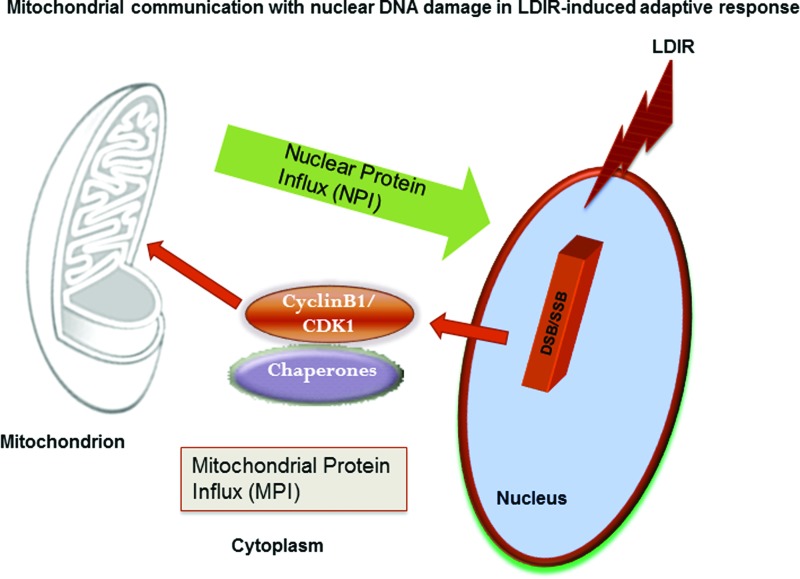
FIG. 1.
Hypothesized communication between nuclear DNA damages and mitochondrial activity. Cyclin B1/CDK1, one of the components of the MPI, is delivered with mitochondrial chaperones to mitochondria and potentially cooperated with NPI (not discussed in this review) in the LDIR adaptive response. CDK1, cyclin-dependent kinase 1; LDIR, low-dose ionizing radiation; MPI, mitochondrial protein influx; NPI, nuclear protein influx. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Features of LDIR-Induced Adaptive Response
Mammalian cells are able to induce an adaptive protective response when exposed to IR with low dose and/or low dose rate. The term of adaptive radioprotection is defined as “the ability of low dose radiation to induce cellular changes that alter the level of subsequent radiation induced or spontaneous damage” (Notice 03-07 of Office of Science, DOE, 2003) (29, 31, 122, 141, 186, 202, 230, 240, 247). Radioadaptive responses have been observed in nearly all the species, including Escherichia coli, protozoa, algae, higher plants, and insects (12, 35, 143, 185, 192, 246, 247). The most significant phenomena of LDIR-induced radioprotection include the reduction of the lethal and mutagenic effects caused by subsequent exposure to higher doses (105, 154, 173, 193, 204, 227), resistance to subsequent radiation-induced genomic instability (96, 105, 121, 142), and activation of stress-sensitive transcription factors and gene regulators (68, 69, 202, 228). Bhattacharjee and Ito (22) reported that whole-body pre-irradiation of Swiss mice with five repeated exposures to small doses of 1 cGy per day reduces the incidence of thymic lymphoma from 46% to 16% following 2 Gy IR (21). Additionally, IR-induced nontargeted effects from astronaut space exploration were mimicked by cranially irradiated Sprague Dawley rats; the results showed that levels of key proteins involved in mitochondrial fatty acid metabolism were reduced, and proteins involved in various cellular defense mechanisms, including antioxidants, were elevated in both irradiated and nonirradiated tissues (2, 81). An adaptive response of human lymphocytes to IR has long been observed (154, 238) (187), and several reports create a strong case for the existence of cellular radioprotective mechanisms activated in response to IR (3, 105, 154, 173, 193, 204, 227). IR induces the expression of a specific cluster of stress responsive genes to repair damaged biomolecules, including DNA, and enhance overall cell survival (7, 28, 63, 68, 69, 85, 120, 140, 199, 202, 228). Recent studies demonstrate that colon carcinoma cells along with transformed mouse embryonic fibroblasts showed an adaptive response when grown either to confluence in vitro or as tumors in the flank of C57BL/6 mice. LDIR-induced survivin expression was linked to the adaptive radioresistance after pre-exposure of 100 mGy or a lower dose. This survivin-mediated adaptive response may affect the outcomes if regularly induced throughout a course of image-guided radiation therapy (82). In another study, the same dose of 100 mGy offered radioprotection to C57BL/6 mice exposed 24 h later to 100 mg/kg of N-ethyl-N-nitrosourea via inducing manganese superoxide dismutase (MnSOD) (83) whose enzymatic activity can be further enhanced by Cyclin B1/CDK1-mediated phosphorylation (36). These results clearly indicate that mitochondria are actively involved in LDIR-induced adaptive response and specific factors that are able to coordinate DNA damages with the mitochondrial activity remain to be identified.
Mitochondrial Activities Guided by Nuclear Genome-Encoded Proteins
Accumulating evidence suggests that mitochondria play an important role in the IR-induced adaptive response via the regulation of mitochondrial metabolism (198). The major function of mitochondria in mammalian cells is to generate adenosine triphosphate (ATP) through oxidative phosphorylation (OXPHOS) to accommodate cellular energy demands (14, 18, 51, 112). The nucleus represents a less oxidizing and hospitable environment for high fidelity storage of large amounts of genetic material necessary to code for the gene products required for organism function (226). Many mitochondrial functions are shown to be regulated by nuclear-encoded proteins and via protein phosphorylation (159). For example, despite the presence of mitochondrial DNA (mtDNA), more than 98% of mitochondrial protein components are encoded by the nuclear genome (174) with only 13 of 1465 mitochondrial proteins identified to date transcribed from the mitochondrial genome (48). Such a “foreigner-taking-over” process in the mitochondrion indicates how adopted organelles with its own genome are well integrated evolutionarily into one living system. As a result, the major function of mitochondria is under the control of the central genome in the host cells. Thus, the nuclear genome is able to efficiently and timely guide the activity of mitochondria. This organization of the regulatory hierarchy likely evolved because coordinated control in cellular fuel generation is required for optimal function of cell proliferation and stress response.
Mitochondrial Activities Guided by Radiation-Induced Cyclins and CDKs
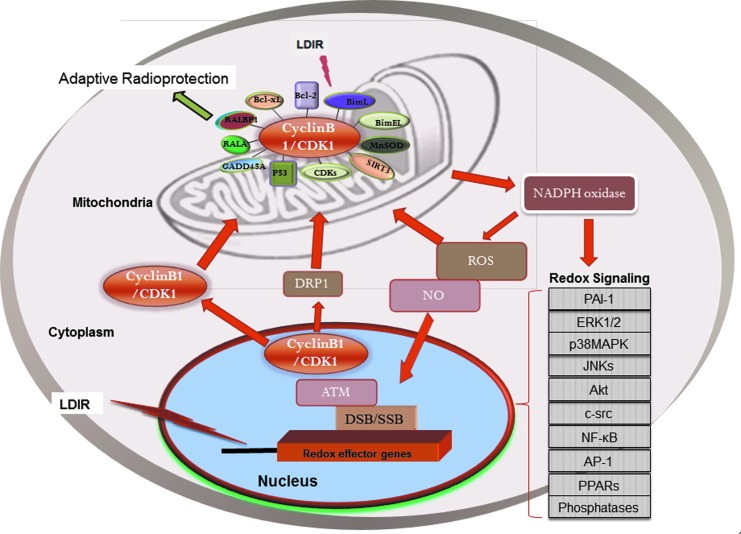
Cell cycle progression depends on highly ordered events controlled by a subset of Cyclins and CDKs (139, 146, 191). Cyclin B1/CDK1 complex specifically regulates the entry into mitosis at the G2/M border (61). Through its cytoplasmic, nuclear, and centrosomal localization, Cyclin B1/CDK1 synchronizes the crucial events of early mitosis, such as nuclear envelope breakdown and centrosome separation (76). Accumulating evidence links mitochondrial dynamics (40) and metabolism (54, 107) with cell proliferation and cell cycle regulation (10, 73, 128, 175, 183). Examples include the G1–S arrest caused by mitochondrial dysfunction (156); involvement of Cyclin D1 in coordinating mitochondrial bioenergetics with G1 progression; (118, 176, 178, 223) and Cyclin E in controlling the formation of high energy-charged mitochondria in the G1/S transition (138). Recent identification of the mitochondrial localization of Cyclin B1/CDK1 (149) as well as its role in the integration of mitochondrial fission with the onset of G2/M transition (207) suggests that Cyclin B1/CDK1 activity is involved in mitochondrial morphological dynamics, mitochondrial bioenergetics, and mitochondria-mediated resistance to IR as shown in Figure 2. Numerous studies have examined the effects of IR on the expression of genes involved in cell cycle control (8, 20, 93, 120, 158, 199). Irradiated MCF7 cells showed a rapid reduction in Cyclin D1 levels before p53 stabilization, indicating that the stability of Cyclin D1 was controlled in a p53-independent manner (48). In addition, Cyclin D1 phosphorylation and proteolysis are linked with cell genomic instability and the regulation of Cyclin D1 degradation is involved in cancer development (132, 166). However, specific cyclins induced by varied IR doses may function differently in mitochondria-mediated responses. In Xenopus embryos, a high dosage of IR induced apoptotic cell death due to the increased levels of Cyclin A1 and Cyclin A1/CDK2 activity (9). In the following review, we will illustrate the protective functions of two cell cycle complexes, Cyclin D1/CDK4 and Cyclin B1/CDK1, in LDIR-induced adaptive responses (Fig. 2).
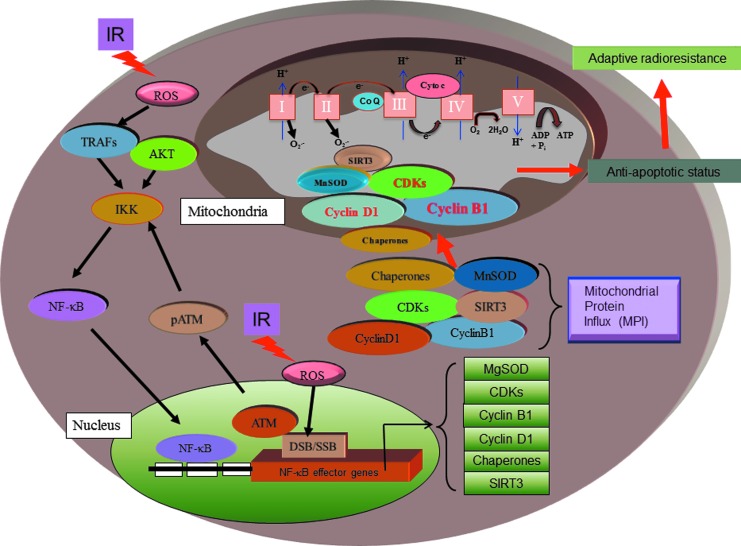
FIG. 2.
Schematic presentation of mitochondrial signaling network in cellular adaptive response to IR. IR generates reactive oxygen species (ROS) to induce nuclear DNA damages and mitochondrial ROS level by accumulating cells in the G2/M phase (235). NF-κB can be activated via the ROS in cytoplasm and damaged DNA in nucleus via the regulation of IκB kinase (IKK). NF-κB in turn regulates an array of IR-responsive effector genes, including mitochondrial antioxidant MnSOD, Cyclins, and CDKs (marked as red color). These effector genes are shown to localize to mitochondria and to regulate the mitochondrial activity to induce the adaptive response after exposure to IR. IR, ionizing radiation; MnSOD, manganese superoxide dismutase.
Cyclin D1/CDK4-Mediated Adaptive Radioprotection
Cell cycle progression is controlled by highly ordered events via Cyclins and CDKs (97, 137, 139, 146, 162). The activity of CDKs is exquisitely controlled by multiple pathways, including the regulation of Cyclins (49), phosphorylation of the catalytic subunits (125), and subcellular localization (209). Cyclins, such as Cyclin D1, is involved in cell cycle arrest in DNA-damage response. A study tested the hypothesis that Cyclin D1 regulates mitochondrial apoptosis. Cyclin D1 was found to complex with chaperon 14-3-3ζ (3). A direct interaction of Cyclin D1 with proapoptotic Bax occurred in LDIR-treated cells and improved mitochondrial membrane potential (Δψm). These results demonstrate the evidence that cytosolic Cyclin D1 is able to regulate apoptosis by interaction with Bax in LDIR-induced adaptive resistance (105).
The LDIR-induced adaptive response is manifested through changes of total mitochondrial protein translocation rate (160). NF-κB upregulates and translocates mitochondrial antioxidant MnSOD from the nucleus to the cytoplasm in (55) cells exposed to IR (67, 85). According to radiation-induced gene expression profiles (6, 200), cell cycle regulators, such as Cyclin D1, are also required for the IR-induced adaptive response. Cyclin D1 is involved in the IR-induced adaptive response when localized in the cytoplasm, and apoptosis is enforced by nuclear relocation of Cyclin D1 (203). Additionally, 14-3-3 chaperones (14-3-3s) are crucial for cell cycle checkpoint control and cell survival after radiation-induced DNA damage (231). Studies show that inhibition of 14-3-3s renders cancer cells sensitive to IR (171). The radioprotective effect of 14-3-3s in normal cells is from both interaction and inhibition of proapoptotic Bcl-2-associated X protein (Bax) (151), relocation of apoptosis-promoting fork-head transcription factor (FKHRL1) to the cytoplasm (32), and sequestration of cytoplasmic c-Abl (237). The mitochondrial proapoptotic Bax and antiapoptotic Bcl-2 are major factors involved in mitochondrial apoptosis and Δψm (103). Bcl-2 antagonizes the proapoptotic activity of Bax through the formation of Bcl-2/Bax heterodimers, and the Bcl-2 to Bax ratio is associated with alterations of Δψm and cell death (243). Bax activity is decreased in cells with enhanced Δψm preventing cell death. An antiapoptotic pathway was induced by Ataxia telangiectasia mutated (ATM)/NF-kB-mediated Cyclin D1 expression after treating human skin keratinocytes with LDIR (5- or 10-cGy X-ray) and mice with whole-body IR. Exposure of normal cells to 5 Gy IR caused nuclear translocation of Cyclin D1, whereas LDIR decreased 14-3-3/Cyclin D1 complex formation resulting in free cytoplasmic Cyclin D1. Higher levels of free cytoplasmic Cyclin D1 further sequestered Bax from mitochondria maintaining Δψm. siRNA-mediated Cyclin D1 inhibition ablated LDIR-induced Cyclin D1/Bax complex formation and decreased Δψm. Thus, the formation of Cyclin D1/Bax in LDIR is able to inhibit mitochondria-mediated cell death (Fig. 3).
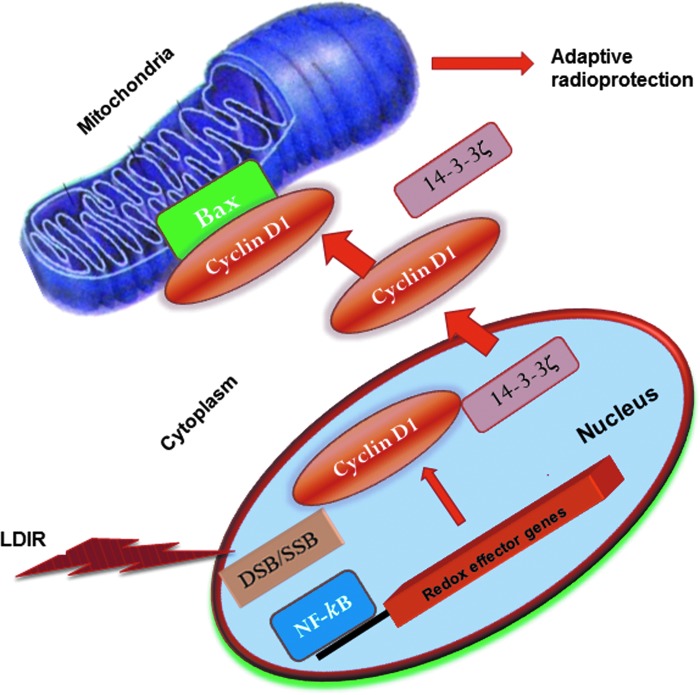
FIG. 3.
Cyclin D1 interacts with Bax in the LDIR-induced adaptive response. LDIR (10 cGy X-ray) induced Cyclin D1 via NF-κB regulation is conjugated with the chaperon 14-3-3 protein in the cytoplasm. Cyclin D1 then interacts and forms a complex with Bax in mitochondria of human karotinocytes and skin tissue from whole-body irradiated mice (3). The NF-κB-Cyclin D1-Bax signaling pathway is critical to the regulation of the level of 14-3-3/Cyclin D1 complex in the LDIR adaptive response. Bax, Bcl-2-associated X protein; 14-3-3ζ, chaperones. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Mitochondrial Relocation of Cyclin B1/CDK1 in Adaptive Radioprotection
Cyclin B1 and its catalytic partner protein CDK1 belong to the fundamental kinase machinery regulating cell cycle progression from G2 to mitosis (61, 164). The cytoplasm-, nucleus- and centrosome-localized Cyclin B1/CDK1 synchronizes critical subcellular events, such as nuclear envelope breakdown and centrosome separation to ensure even segregation of chromosomes into two daughter cells (76). Cyclin B1/CDK1 was not activated in G2 phase until nuclear envelope breakdown, thereby initiating the events of prophase and different levels of Cyclin B1/CDK1 activity required to trigger different mitotic events (76). Mass spectrometry analysis identified a cluster of MnSOD protein–protein interactions in an array of cellular and mitochondrial proteins (63). Cyclin B1/CDK1 regulates the MnSOD activity through reversible serine 106 phosphorylation both in vivo and in vitro, enhancing the MnSOD enzymatic activity and protein stability to improve mitochondrial function in LDIR-induced adaptive protection (36). Also known as the mitotic promoting factor, Cyclin B1/CDK1 phosphorylation governs key steps for mitotic entrance featured by the nuclear envelope breakdown, spindle formation, and chromatin condensation (75). However, the mitochondrial transition of Cyclin B1/CDK1 is hypothesized to be dependent on the total levels of cellular Cyclin B1 and CDK1 (149). Abundance of Cyclin B1 was found to regulate γ-ray radiation-induced apoptosis (167). Under normal growth conditions, mitochondrial localization of Cyclin B1/CDK1 is attenuated during G1 phase due to a lack of cellular Cyclin B1 before gradually accumulating in the mitochondria. Upon entering S/G2 phase, Cyclin B1 expression reaches a maximum level at the G2/M phase, and this will be discussed in the following sections. Upon DNA-damage conditions, such as chemotherapy and IR, induced Cyclin B1/CDK1 expression can delay the cell cycle causing the G2/M border arrest to enable DNA damage repair or initiate apoptosis (147). Following DNA-damaging agents and radiation, at least a fraction of the induced Cyclin B1/CDK1 is translocated to mitochondria and Cyclin B1/CDK1 may phosphorylate mitochondrial targets under both normal and DNA-damaging stress conditions (149). As shown in Figure 4A, mitochondrial localization of Cyclin B1/CDK1 appears to be proportional with the overall expression levels of cellular Cyclin B1/CDK1 throughout the cell cycle phases. Since Cyclin B1/CDK1 is activated in the prophase stage of mitosis (99), the mitochondrial CDK1 may be activated causing the phosphorylation of mitochondrial substrates in prophase. In Figure 4B, mitochondrial ATP generation and ROS production are linked with G2/M transition phase, indicating that mitochondrial Cyclin B1/CDK1 may participate in mitochondrial bioenergetics in DNA damage-associated adaptive response. This is supported by the fact that cell division cycle 25c (Cdc25c), the phosphatase activator of CDK1, is also present in mitochondria and therefore may further enhance the mitochondrial CDK1 kinase activity. We assume that mitochondrial Cdc25c may allow Cyclin B1/CDK1 to function in this particular compartment of cells, whereas the cytoplasmic and nuclear Cyclin B1/CDK1 may remain inactive until the cells progress into prophase. Thus, the mitochondrial Cdc25c may also play a critical role in radiation-induced adaptive protection, which remains to be elucidated.
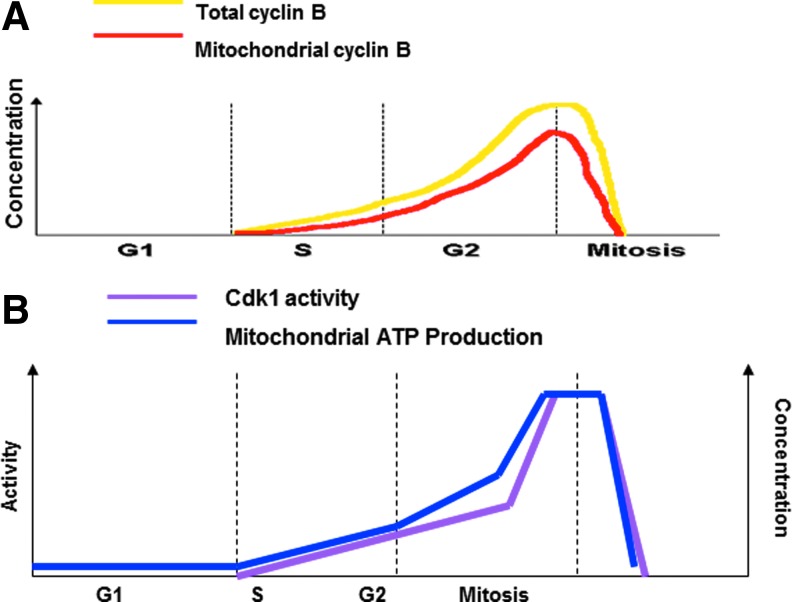
FIG. 4.
Correlation between mitochondrial Cyclin B1/CDK1 activity and mitochondrial bioenergetics in cell cycle progression. (A) Cyclin B1 is expressed at the beginning of S phase, accumulated during the G2 phase, peaked at the late G2/early M-phase before advancing into mitosis, and is rapidly degraded during anaphase (86, 92). Cyclin B1 is not expressed throughout G1 phase. (B) The activity of mitochondrial CDK1 correlated with the level of mitochondrial Cyclin B1 and IR induces Cyclin B1/CDK1-mediated phosphorylation of mitochondrial p53 to mediate the adaptive response. An enhanced mitochondrial ATP generation (149) and mitochondrial ROS are accompanied by upregulation of mitochondrial electron transport chain function, and mitochondrial content is under control of cell cycle checkpoints (235). ATP, adenosine triphosphate. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Redox and Mitochondrial Cyclin B1/CDK1 in the Adaptive Response
Cells under genotoxic stress, such as IR, induce an imbalance in redox reactions caused by altered mitochondrial function, metabolism, and adaptive responses leading to short- or long-term effects in the cells (9, 49, 56, 57, 71–73, 100, 175, 214). A topic on redox reactions in cellular responses to IR has been well reviewed (199). Recent studies revealed that normal stem cells alter their redox homeostasis to adapt to adverse conditions, and radiation-induced oxidative stress in the pluripotent and multipotent human stem cells plays a crucial role in regulating the function and fate of stem cells within tissues compromised by radiation injury (72, 78, 110). IR-mediated ROS generation can directly alter the activity of kinases and transcription factors indirectly modulating cysteine-rich redox-sensitive proteins exemplified by thioredoxin and glutathione S-transferase. ROS-related redox changes in key signaling pathways have been well addressed (1, 11, 79, 86, 199).
Mitochondrial Ca2+ release to the cytoplasm causes the activation of multiple signaling pathways, including PKCs, MAPKs, and JNK (129). Herein, we illustrate two other major lines of evidence supporting mitochondria-centered redox signaling pathways in IR-induced adaptive response. The first is NOX. Under IR stress, NOX-derived ROS can activate MAPKs (extracellular signal-regulated kinase, p38) and JNK, which both participate in the adaptive response signaling network that cross talk with mitochondria (53). Acetylation of MnSOD directs the enzymatic activity responding to cellular nutrient status or oxidative stress (157). JNK-mediated repression of MnSOD and catalase occur via mitochondrial complex I and NOX I (104). Among the main factors involved in the redox balancing in adaptive response to IR (119), ATM, NF-κB, and pre-inflammatory factors (134, 135, 224) may also be regulated via NOX (220). In addition, plasminogen activator inhibitor-1 is a redox-regulated factor involved in the induction of profibrogenic mediators in acute or chronic oxidative stress after exposure to IR or H2O2 (244). A second redox-regulated factor is Dynamin-related protein 1 (DRP1), a guanosine triphosphate hydrolase enzyme (GTPase) regulating mitochondrial fission during cell cycle progression (236). A recent study reported that excessive nitric oxide could also lead to S-nitrosylation of Drp1 at cystine 644 (130). S-nitrosylation of Drp1 (resulting in SNO-Drp1) induces Drp1 dimerization, which functions as fundamental elements for higher order structures of Drp1 to activate Drp1 GTPase (148). Hypoxia-inducible factor-1α (HIF-1α) activation leads to mitochondrial fission by Cyclin B1/CDK1-dependent phosphorylation of DRP1 at serine 616 (130). These results together with the identification of mitochondria-localized Cyclin B1/CDK1 (36, 149) indicate that Cyclin B1/CDK1 cooperates with the redox-mediated signaling network to regulate the mitochondrial activity (Fig. 5).
FIG. 5.
NADPH oxidase redox reaction imbalance in IR-induced adaptive protection. Cyclin B1/CDK1 phosphorylation of the ubiquinone-binding site of core complex I confers transition of G2 to M phase. ATP mitochondrial generation mediates cell division by G2 to M phase transition.
Chaperones for Cyclin B1/CDK1 Mitochondrial Influx
Although mitochondria possess their own transcriptional machinery, merely 1% of mitochondrial proteins are synthesized inside the organelle; thus, the transportation of the nuclear-encoded proteins into mitochondria is an indispensable process alluding to multiple cross talk signaling pathways resulting in the nuclear to mitochondrial translocation of proteins. For proteins containing mitochondrial targeting sequences (MTS), the chaperone Translocase of Inner Mitochondrial Membrane 23 complex located on the mitochondrial membrane mediates their mitochondrial translocation to the matrix of the mitochondria (217). However, few mitochondrial proteins, including Cyclin B1 and CDK1, are identified to contain an MTS. Thus, specific chaperone proteins are required to assist their mitochondrial translocation. Although chaperone proteins, such as heat shock protein (HSP)60, HSP10, and HSP70, are suggested to be involved in mitochondrial translocation of many proteins (38, 150), the exact mechanism and the chaperones responsible for trafficking the cell cycle regulators to mitochondria remains largely unknown. Both 14-3-3ζ, a well-defined chaperon for mitochondrial protein flux, and Cyclin B1 are activated by radiation (67, 85), indicating the possibility that 14-3-3ζ may be the vehicle responsible for delivering Cyclin B1/CDK1 to the mitochondria under genotoxic stress. Interestingly, 14-3-3ζ is known to bind to Cdc25c (74) and thus may also be responsible for transporting Cdc25c to mitochondria to activate the mitochondrial CDK1 activity. Taken together, although the exact mechanism underlying mitochondrial relocation of Cyclin B1/CDK1 is unknown, current data implicate that chaperone proteins, including 14-3-3ζ, are involved in facilitating Cyclin B1/CDK1 mitochondrial relocation. In addition to the 14-3-3s, Cyclin B1/CDK1 is shown to interact with other chaperone proteins, such as HSP70-2, HSP90, and cell division cycle 37 (144, 214, 248). These chaperones should be able to assist Cyclin B1/CDK1 mitochondrial translocation. The specific pathways involved in a timely fashion of Cyclin B1/CDK1 mitochondrial transportation, especially under cell cycle progression and nuclear DNA damaged conditions, remain to be elucidated.
Cyclin B1/CDK1 in Mitochondrial Fission
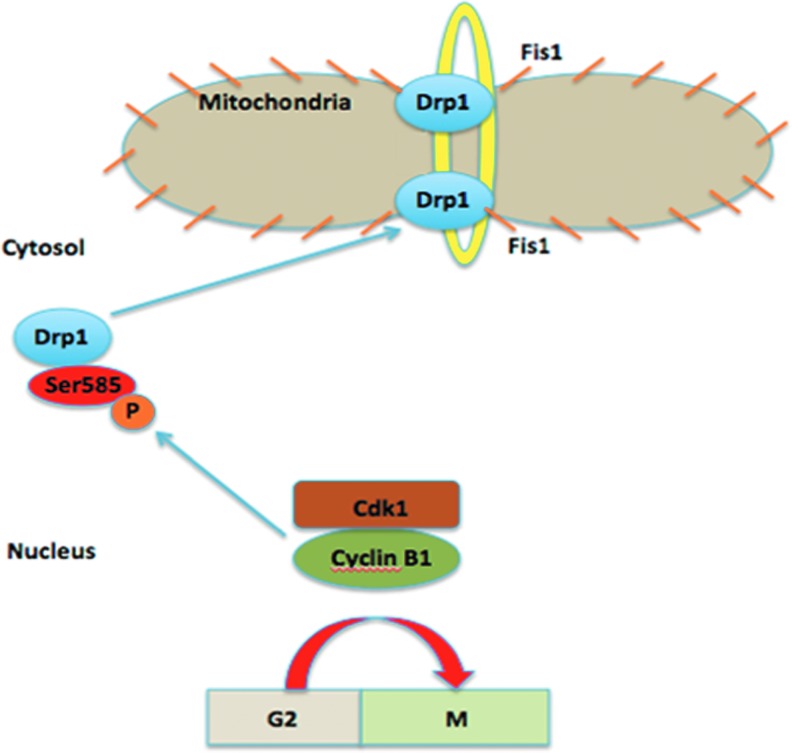
Cyclin B1/CDK1 may regulate mitochondrial fission and fusion in radiation-induced adaptive responses. Mitochondria proliferate only from existing mitochondria (168) via complementary fission and fusion events. A balance between these opposing processes contributes to mitochondrial membrane dynamics (152, 225). In mammalian cells, the fusion events are carried out by a mitochondrial transmembrane GTPase known as mitofusin (66), whereas Drp1 is responsible for mitochondrial fission events (195). The post-translational modification on Drp1 is shown to play a critical role in determining the GTPase activity during mitochondrial fission (179). The contribution of Cyclin B1/CDK1 in the regulation of mitochondrial functions is not limited to directing the kinase targeting to mitochondria but also is involved in the morphological regulation. It has long been recognized that the mitochondrial number coordinates with the cell cycle phase with a 50% increase during S phase due to fission (58). However, the mechanism connecting cell cycle and regulation of mitochondrial fission and fusion remains obscure. Taguchi et al. demonstrated that in addition to chromatid segregation, Cyclin B1/CDK1 is the kinase regulating the mitotic mitochondrial fragmentation, also known as mitochondrial fission during mitosis (207). Interestingly, phosphorylation of Drp1 by Cyclin B1/CDK1 at serine 585 residue during mitosis was found to be required to translocate Drp1 from cytosol to the mitochondrial outer membrane, which is necessary for mitochondrial fission (Fig. 6) (194, 207). Cyclin B1/CDK1 coordinates mitochondrial fission with the onset of G2/M transition via phosphorylation of Drp1 by Cyclin B1/CDK1 (207). Activated Drp1 then punctuates holes on the mitochondrial membrane to proceed with membrane constriction and fission directed by mitochondrial fission 1 protein (Fis1) (41, 111). Drp1 reportedly assembles into rings and spirals that encircle and constrict the mitochondria during fission (57). The separation of mitochondria during cytokinesis is essential to the survival of the two daughter cells, and the mitochondrial fragmentation modulated by Drp1 allows equal distribution of the mother mitochondria into two daughter cells as the fission events occur during the cell cycle. Exogenous expression of unphosphorylated mutant Drp1S585A leads to reduced mitotic mitochondrial fragmentation (207). In addition to morphological alterations, the Drp1 activity has shown to be essential for mitochondrial bioenergetics supported by the fact that ATP production is severely impaired in Drp1−/− deficient cells (17). On the contrary, phosphorylation at serine 637 residue by cyclic adenosine monophosphate-dependent protein kinase on Drp1 has been shown to suppress its GTPase activity by decreasing the intramolecular interaction that drives GTP hydrolysis (42, 108), which can be removed by the phosphatase calcineurin (52). Mutation in Drp1 also leads to the highly elongated mitochondrial filaments and reduction in mitotic mitochondrial fragmentation (24, 207). This hypothetic pathway is illustrated in Figure 6. Further definition of the relationship between Cyclin B1/CDK1 and Drp1 during mitochondrial fission may reveal a new role of mitochondrial Cyclin B1/CDK1 in regulating Drp1 to prepare mitochondria for a successful cell division, which may be interrupted or enhanced under different doses of genotoxic agents.
FIG. 6.
Phosphorylation of Drp1 by Cyclin B1/CDK1 at the serine 585 residue during mitosis is required to translocate Drp1 from cytosol to the mitochondrial outer membrane during mitochondrial fission. Activated Drp1 results in membrane constriction and fission directed by Fis1. Drp1, dynamin-related protein 1. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Mitochondrial Cyclin B1/Cdk1 in Cell Cycle Progression
Dynamic alterations in mitochondrial mass and Δψm together with cellular ATP levels were detected during cell cycle progression (206). Depletion of nutrients induced mtDNA replication but not nuclear DNA replication during the development of Dictyostelium discoideum cells (188). Mitochondrial regulation of cell cycle progression during development was revealed by the permanent mutation in Drosophila, which caused a reduction of intracellular ATP that was still sufficient to maintain cell survival, growth, and differentiation, but not adequate for progression through the cell cycle (128). This suggests that a group of mitochondrial proteins were regulated during cell cycle progression. We have identified mitochondrial protein targets of Cyclin B1/CDK1 in an array of cancer cells that were treated with high-dose IR (5 Gy) (149). As shown in Figure 7, a model of Cyclin B1/CDK1-mediated mitochondrial function in cell cycle progression is proposed for the IR-induced adaptive response. In this model, adaptive response-inducing DNA damage, such as those triggered by LDIR, stimulates the translocation of Cyclin B1 and CDK1 to mitochondria where the kinase activity of Cyclin B1/CDK1 is activated by cdc25 to phosphorylate the cluster of subunits in the OXPHO machinery. This leads to increased mitochondrial respiration, ATP production, and Δψm, which facilitates DNA repair and cell cycle progression. This may be significant for rapidly growing cells, and radiation may block cellular mitosis by causing G2/M arrest.
FIG. 7.
Cyclin B1/CDK1 phosphorylation of the ubiquinone-binding site of core complex I confers transition of G2 to M phase. DNA-damage IR stimulates the gene expression and translocation of Cyclin B1 and CDK1 from the nucleus to the mitochondria. Mitochondrial Cyclin B1 and CDK1 form a complex to phosphorylate the ubiquinone-binding site of core complex I, which leads to increased ATP production and inhibition of mitochondria-mediated apoptosis. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Specific Cyclin B1/CDK1 Targets in OXPHO
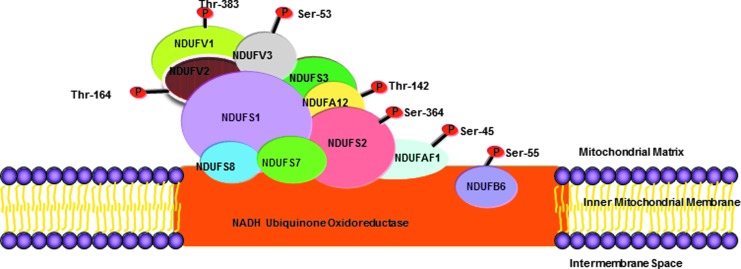
Mitochondria, the powerhouse in mammalian cells, derive energy from both the tricarboxylic acid cycle and OXPHOS. Although the metabolic activity is believed to be a crucial determinant for cell proliferative growth, the exact pathways linking mitochondrial energy output and cell cycle regulation are unknown (7). During division, cells require additional amounts of ATP to enter mitosis at the G2/M phase (206). OXPHOS supplies more than 90% of cellular ATP required for eukaryotic cells (91), and the mitochondrial OXPHOS is irrefutably critical for the progression of the cell cycle as the level of ATP determines the fate of cell division (133). A mitochondrial protein database for the CDK1 consensus phosphorylation motif (S/T P×R/K) (196, 215) reveals 12 OXPHOS subunits that can be potentially phosphorylated by CDK1 on the mitochondrial respiration chain (Complex I–V) (Fig. 8). This phosphorylation has the potential to modulate the activity of the protein complexes thereby regulating energy production. The majority of those subunits are the components of the mitochondrial Complex I (nicotinamide adenine dinucleotide-ubiquinone oxidoreductase), the essential complex in the OXPHOS system (180, 216). The potential targets of phosphorylation by Cyclin B1/CDK1 are the ubiquinone-binding sites located on the core of the complex I facing the matrix side of the mitochondria (Fig. 8). Since the ubiquinone of complex III and an unknown component of complex I functions as the major sites of ROS generation (25, 62, 127), the mitochondrial Cyclin B1/CDK1 may regulate the surge in mitochondrial ATP production required for the critical boost of cellular energy reserves to repair DNA damage. In addition, radiation-induced Cyclin B1/CDK1 is also required for many cellular functions, such as DNA repair in the nucleus. Therefore, a cooperation of events occurring in the nucleus and mitochondria by the same regulator highlights a tight connection of cell cycle progression with mitochondrial activity.
FIG. 8.
The location of potential Cyclin B1/CDK1-targeted mitochondrial complex I subunits corresponding to the structure of complex I (NADH-ubiquinone oxidoreductase) in the mitochondrial inner membrane. Three of the phosphorylation targeted subunits are the central subunits for NADH reduction (30). The phosphorylation on these subunits leads to the enhanced function of the complex I, particularly during the G2/M phase when Cyclin B1 expression is at the peak level (Fig. 3). The model structure is derived from Sazanov and Hinchliffe (181). NADH, nicotinamide adenine dinucleotide. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Cyclin B1/CDK1 in Mitochondria-Mediated Apoptosis
It has been generally accepted that the initiation of apoptosis is a unique function of mitochondria in mammalian cells (64, 84, 115–117, 165). Cyclin B1/CDK1 is known to be responsible for initiating mitochondria-mediated apoptosis under cell damage conditions by phosphorylation of several pro- and antiapoptotic proteins (37, 219, 229). CDK activity is involved in the mitochondrial translocation of Bax, which plays an important role in the mitochondrial membrane permeability transition during apoptotic progression (47). Under different levels of genotoxic stresses, including IR, the phosphorylation of several Bcl-2 family proteins, such as Bcl-2, Bcl-2-associated death protein, and B-cell lymphoma-extra large (Bcl-xL) by Cyclin B1/CDK1 can alter mitochondrial membrane permeability resulting in loss of cytochrome c to the cytosol (100, 149, 212, 219, 232). Cyclin B1/CDK1 thus may be the determining factor in deciding cell apoptosis. Abnormal activities and aberrant expression of Cyclin B1 have been observed in a number of human cancers, including esophageal squamous cell carcinoma (145), laryngeal squamous cell carcinoma, nonsmall cell lung cancer (197), and colorectal carcinoma (59, 114, 208, 222). Most importantly, Cyclin B1 also potentially causes chemo- and radioresistance in cancer cells (89, 90). Deficiency of Cyclin B1 leads to profound inhibition of cell proliferation and activation of apoptosis (241). Activated Cyclin B1 is linked with the prosurvival pathway regulated by NF-κB (158). Therefore, Cyclin B1/CDK1 serves as an important component in NF-κB-induced cellular resistance to genotoxic insults. However, Cyclins and CDKs appear to have dual functions in both promoting and suppressing apoptosis in mammalian cells. Examples include that phosphorylation on pro-caspase-9 by Cyclin B1/CDK1 or survivin (Baculoviral inhibitor of apoptosis repeat-containing 5) by p34cdc2 leads to inhibition of apoptosis (5, 153). These results suggest that although Cyclin B1/CDK1 is a key factor determining the fate of an irradiated cell, cell survival appears to be dependent on not only the degree of genomic injury and instability but also Cyclin B1/CDK1-associated mitochondrial targets of phosphorylation with the end result of increased or decreased energy production.
Cyclin B1/CDK1-Mediated Antiapoptotic Pathway
Tumor suppressor p53 is well characterized to regulate mitochondria-mediated apoptosis at protein and mRNA levels (45, 136). p53 initiates the apoptotic cascade by inducing expression or interacting directly with cytoplasmic proteins in the Bcl-2 family (44). Localization of p53 to mitochondria conventionally resembles a major starting signal for mitochondria-mediated apoptosis (245). However, mitochondrial p53 may not necessarily induce apoptosis (71). Vital functions of mitochondrial p53 have been reported, including mtDNA transcription, DNA repair, mitochondrial biogenesis (13, 177, 239), as well as in ATP production since p53 also regulates mitochondrial respiratory genes, synthesis of cytochrome c oxidase (SCO2), and phosphate-activated mitochondrial glutaminase (GLS-2) (131, 205). Therefore, the role of mitochondria-localized p53 should be considered broadly, depending on its cooperative and differential phosphorylation in addition to apoptotic signals (102). Although several kinases reportedly phosphorylate 17 residues on p53, the regulation of phosphorylation on mitochondrial p53 is not yet known. In an attempt to identify kinases for mitochondrial p53, our group recently reported direct evidence showing Cyclin B1/CDK1 phosphorylation on mitochondrial p53 (149). Upon stress stimulus, the levels p53, Cyclin B1, and CDK1 in mitochondria were all elevated leading to the phosphorylation of mitochondrial p53 at serine 315 residue, the only putative site for CDK1 phosphorylation (23). This phosphorylation, together with the phosphorylation of other Cyclin B1/CDK1 targets in the mitochondria and elevated OXPHO, could compromise the proapoptotic function of mitochondrial p53 by sequestering it from binding to Bcl-2 and Bcl-xL (Fig. 9). As a result, its proapoptotic potential is reversed into the prosurviving function by maintaining mitochondrial integrity and increasing ATP production as a possible supplement for the DNA repair processes. The finding of Cyclin B1/CDK1-phosphorylated mitochondrial p53 reinstates the prosurvival function of Cyclin B1/CDK1, an important insight in the nuclear-guided mitochondrial functions.
FIG. 9.
Schematic representation of signaling transduction for Cyclin B1/CDK1 in mitochondria. Cyclin B1/CDK1 serves as a kinase on several mitochondria-translocated proteins involved in bioenergetics and apoptotic functions. The Cyclin B1/CDK1 complex also serves as a direct link between cell cycle and mitochondrial responses in response to stress signals. During the G2 phase, the Cyclin B1/CDK1 serves as an apoptotic suppressor when the cells undergo a G2 checkpoint and repair DNA damage. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Conclusion
Citing a model of cellular adaptive response to LDIR, this review discussed a new feature of nuclear-to-mitochondrial communication mediated by the cell cycle G2/M regulator Cyclin B1/CDK1. A myriad of Cyclin B1/CDK1 protein targets remain to be characterized (94, 124, 215). Promising novel mitochondrial targets of Cyclin B1/CDK1 may play an important role in coordinating cellular respiration related to cell cycle progression and the adaptive response to genotoxic stress. Thus, Cyclin B1/CDK1 may be considered one of the key harmonizers for the regulation of mitochondrial functions in cellular adaptive response under genotoxic stress, including LDIR.
The variation in cellular energy at different stages of the cell cycle requires the precise control and communication with mitochondria that produces the major resource of ATP for proliferation. The production of ATP from a glucose molecule is ∼13-fold higher with aerobic respiration compared to anaerobic metabolism (172). The targets of mitochondria regulated by Cyclin B1/CDK1 may serve as one of the many mechanisms for cells to communicate with mitochondria under different growth or stress conditions. Since the function of this kinase complex varies at different stages of the cell cycle and can be induced by IR, Cyclin B1/CDK1 seems to synchronize mitochondrial energy production in concomitant with the nuclear DNA repair. In addition, the Warburg effect, described as cancer cells utilizing energy produced from glycolysis rather than OXPHOS (106), may be perturbed or reversed under stress conditions leading to mitochondrial metabolism reprograming. Unknown mechanisms remain to be elucidated to understand why cancer cells avoid utilizing mitochondrial machinery for its major energy source and how it is adjusted under anticancer radiotherapy. A potential critical mechanism involving sirtuin3 (SIRT3), a mitochondrial sirtuin protein (50), was proposed to guide mitochondrial energetics in normal cells versus glycolysis in tumor cells described as the Warburg effect (16, 39, 123, 155, 163, 182). A recent study showed that tumors from SIRT3-deficient mice have high levels of ROS that induce genomic instability and elevate HIF-1α protein levels (87). Importantly, the acetylation/deacetylation status of MnSOD was found to regulate the MnSOD enzymatic activity responding to cellular nutrient status or oxidative stress (157). Thus, an integration of signals between Cyclin B1/CDK1-mediated MnSOD phosphorylation (36) and SIRT3-mediated MnSOD acetylation status (210, 249) may coordinate the radiation-induced adaptive response at the subcellular level.
Abbreviations Used
- Δψm
mitochondrial membrane potential
- 14-3-3ζ
chaperones
- Apaf-1
apoptotic peptidase activating factor 1
- ATM
ataxia telangiectasia mutated
- ATP
adenosine triphosphate
- BAD
Bcl-2-associated death protein
- Bak
Bcl-2 homologous antagonist/killer
- Bax
Bcl-2-associated X protein
- Bcl-2
B-cell lymphoma 2
- Bcl-xL
B-cell lymphoma-extra large
- BIRC5
Baculoviral inhibitor of apoptosis repeat-containing 5
- cAMP
cyclic adenosine monophosphate
- Cdc25c
cell division cycle 25c
- Cdc37
cell division cycle 37
- CDK
cyclin-dependent kinases
- CDK1
cyclin-dependent kinase 1
- DRP1, Drp1
dynamin-related protein 1
- ERK
extracellular signal-regulated kinase
- FKHRL1
apoptosis-promoting fork-head transcription factor
- GLS-2
phosphate-activated mitochondrial glutaminase
- GTPase
guanosine triphosphate hydrolase enzyme
- HIF-1α
hypoxia-inducible factor 1
- HSP
heat shock protein
- IR
ionizing radiation
- JNKs
c-jun N-terminal kinases
- LDIR
low-dose ionizing radiation
- MAPKs
mitogen-activated kinases
- Mfn
mitofusin
- MnSOD
manganese superoxide dismutase
- MPF
maturation promoting factor
- MPI
mitochondrial protein influx
- mtDNA
mitochondrial DNA
- MTS
mitochondria targeting sequence
- NADH
nicotinamide adenine dinucleotide
- NDUFA12
NADH dehydrogenase ubiquinone 1 alpha subcomplex subunit 12
- NDUFB6
NADH dehydrogenase ubiquinone 1 beta subcomplex subunit 6
- NDUFS2
NADH dehydrogenase ubiquinone iron-sulfur protein 2
- NDUFV1
NADH dehydrogenase ubiquinone flavoprotein 1
- NDUFV3
NADH dehydrogenase ubiquinone flavoprotein 3
- NOX
NADPH oxidase
- NPI
nuclear protein influx
- OXPHOS
oxidative phosphorylation
- PAI-1
plasminogen activator inhibitor-1
- PKA
cAMP-dependent protein kinase
- PKC
protein kinase c
- SCO2
synthesis of cytochrome c oxidase
- sirtuin3
SIRT3
- Thr
threonine (T)
- Tyr
tyrosine (Y)
Acknowledgments
We appreciate Danupon Nantajit and Doug Spitz for initiating the discussion on this topic; Cheikh Menaa, Chris Liu, and Guochun Jiang for help in article preparation. The authors acknowledge Gayle Woloschak, David Grdina, and Daret St. Clair for their communication and suggestions to the research work related to this review. The authors also acknowledge grant support from the National Institutes of Health RO1 CA133402, CA152313, and the U.S. Department of Energy, Office of Science DE-SC0001271.
References
- 1.Adler V, Yin Z, Tew KD, and Ronai Z. Role of redox potential and reactive oxygen species in stress signaling. Oncogene 18: 6104–6111, 1999 [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal P, Lessie MD, Lin DI, Pontano L, Gladden AB, Nuskey B, Goradia A, Wasik MA, Klein-Szanto AJ, Rustgi AK, Bassing CH, and Diehl JA. Nuclear accumulation of cyclin D1 during S phase inhibits Cul4-dependent Cdt1 proteolysis and triggers p53-dependent DNA rereplication. Genes Dev 21: 2908–2922, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed KM, Fan M, Nantajit D, Cao N, and Li JJ. Cyclin D1 in low-dose radiation-induced adaptive resistance. Oncogene 27: 6738–6748, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed KM. and Li JJ. NF-kappa B-mediated adaptive resistance to ionizing radiation. Free Radic Biol Med 44: 1–13, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allan LA. and Clarke PR. Phosphorylation of caspase-9 by CDK1/cyclin B1 protects mitotic cells against apoptosis. Mol Cell 26: 301–310, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Amundson SA, Bittner M, Chen Y, Trent J, Meltzer P, and Fornace AJ., Jr.Fluorescent cDNA microarray hybridization reveals complexity and heterogeneity of cellular genotoxic stress responses. Oncogene 18: 3666–3672, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Amundson SA, Bittner M, Meltzer P, Trent J, and Fornace AJ., Jr.Induction of gene expression as a monitor of exposure to ionizing radiation. Radiat Res 156: 657–661, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Amundson SA, Grace MB, McLeland CB, Epperly MW, Yeager A, Zhan Q, Greenberger JS, and Fornace AJ., Jr.Human in vivo radiation-induced biomarkers: gene expression changes in radiotherapy patients. Cancer Res 64: 6368–6371, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Anderson JA, Lewellyn AL, and Maller JL. Ionizing radiation induces apoptosis and elevates cyclin A1-Cdk2 activity before but not after the midblastula transition in Xenopus. Mol Biol Cell 8: 1195–1206, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arakaki N, Nishihama T, Owaki H, Kuramoto Y, Suenaga M, Miyoshi E, Emoto Y, Shibata H, Shono M, and Higuti T. Dynamics of mitochondria during the cell cycle. Biol Pharm Bull 29: 1962–1965, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Azzam EI, De Toledo SM, Spitz DR, and Little JB. Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from alpha-particle-irradiated normal human fibroblast cultures. Cancer Res 62: 5436–5442, 2002 [PubMed] [Google Scholar]
- 12.Azzam EI, Raaphorst GP, and Mitchel RE. Radiation-induced adaptive response for protection against micronucleus formation and neoplastic transformation in C3H 10T1/2 mouse embryo cells. Radiat Res 138: S28–S31, 1994 [PubMed] [Google Scholar]
- 13.Bakhanashvili M, Grinberg S, Bonda E, Simon AJ, Moshitch-Moshkovitz S, and Rahav G. p53 in mitochondria enhances the accuracy of DNA synthesis. Cell Death Differ 15: 1865–1874, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Balaban RS. Regulation of oxidative phosphorylation in the mammalian cell. Am J Physiol 258: C377–C389, 1990 [DOI] [PubMed] [Google Scholar]
- 15.Barlow JC. and Sellers EA. Effect of exposure to cold on response of the rat to whole body radiation. Am J Physiol 172: 147–151, 1953 [DOI] [PubMed] [Google Scholar]
- 16.Bell EL. and Guarente L. The SirT3 divining rod points to oxidative stress. Mol Cell 42: 561–568, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benard G, Bellance N, James D, Parrone P, Fernandez H, Letellier T, and Rossignol R. Mitochondrial bioenergetics and structural network organization. J Cell Sci 120: 838–848, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Benard G, Faustin B, Passerieux E, Galinier A, Rocher C, Bellance N, Delage JP, Casteilla L, Letellier T, and Rossignol R. Physiological diversity of mitochondrial oxidative phosphorylation. Am J Physiol Cell Physiol 291: C1172–C1182, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, Gottlieb E, and Vousden KH. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 126: 107–120, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Bernhard EJ, Maity A, Muschel RJ, and McKenna WG. Effects of ionizing radiation on cell cycle progression. A review. Radiat Environ Biophys 34: 79–83, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Bhattacharjee D. Role of radioadaptation on radiation-induced thymic lymphoma in mice. Mutat Res 358: 231–235, 1996 [DOI] [PubMed] [Google Scholar]
- 22.Bhattacharjee D. and Ito A. Deceleration of carcinogenic potential by adaptation with low dose gamma irradiation. In Vivo 15: 87–92, 2001 [PubMed] [Google Scholar]
- 23.Bischoff JR, Friedman PN, Marshak DR, Prives C, and Beach D. Human p53 is phosphorylated by p60-cdc2 and cyclin B-cdc2. Proc Natl Acad Sci U S A 87: 4766–4770, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bleazard W, McCaffery JM, King EJ, Bale S, Mozdy A, Tieu Q, Nunnari J, and Shaw JM. The dynamin-related GTPase Dnm1 regulates mitochondrial fission in yeast. Nat Cell Biol 1: 298–304, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bleier L. and Drose S. Superoxide generation by complex III: from mechanistic rationales to functional consequences. Biochim Biophys Acta 1827: 1320–1331, 2013 [DOI] [PubMed] [Google Scholar]
- 26.Bohnsack BL. and Hirschi KK. Nutrient regulation of cell cycle progression. Annu Rev Nutr 24: 433–453, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Bouffler S, Silver A, and Cox R. Mechanistic and genetic studies of radiation tumorigenesis in the mouse—implications for low dose risk estimation. J Radiol Prot 22: A11–A16, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Bourke E, Brown JA, Takeda S, Hochegger H, and Morrison CG. DNA damage induces Chk1-dependent threonine-160 phosphorylation and activation of Cdk2. Oncogene 29: 616–624, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Braby LA, Brooks AL, and Metting NF. Cellular effects of individual high-linear energy transfer particles and implications for tissue response at low doses. Radiat Res 148: S108–S114, 1997 [PubMed] [Google Scholar]
- 30.Brandt U. Energy converting NADH:quinone oxidoreductase (complex I). Annu Rev Biochem 75: 69–92, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Brooks AL. Developing a scientific basis for radiation risk estimates: goal of the DOE low dose research program. Health Phys 85: 85–93, 2003 [DOI] [PubMed] [Google Scholar]
- 32.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, and Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857–868, 1999 [DOI] [PubMed] [Google Scholar]
- 33.Buchakjian MR. and Kornbluth S. The engine driving the ship: metabolic steering of cell proliferation and death. Nat Rev Mol Cell Biol 11: 715–727 [DOI] [PubMed] [Google Scholar]
- 34.Busby CC. Very low dose fetal exposure to Chernobyl contamination resulted in increases in infant leukemia in Europe and raises questions about current radiation risk models. Int J Environ Res Public Health 6: 3105–3114, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cai L. and Liu SZ. Study on the mechanism of cytogenetic adaptive response induced by low dose radiation. Chin Med J (Engl) 105: 277–283, 1992 [PubMed] [Google Scholar]
- 36.Candas D, Fan M, Nantajit D, Vaughan AT, Murley JS, Woloschak GE, Grdina DJ, and Li JJ. CyclinB1/Cdk1 phosphorylates mitochondrial antioxidant MnSOD in cell adaptive response to radiation stress. J Mol Cell Biol 5: 166–175, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carter AD, Wroble BN, and Sible JC. Cyclin A1/Cdk2 is sufficient but not required for the induction of apoptosis in early Xenopus laevis embryos. Cell Cycle 5: 2230–2236, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Chacinska A, Koehler CM, Milenkovic D, Lithgow T, and Pfanner N. Importing mitochondrial proteins: machineries and mechanisms. Cell 138: 628–644, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chalkiadaki A. and Guarente L. Sirtuins mediate mammalian metabolic responses to nutrient availability. Nat Rev Endocrinol 8: 287–296, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell 125: 1241–1252, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol 22: 79–99, 2006 [DOI] [PubMed] [Google Scholar]
- 42.Chang CR. and Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem 282: 21583–21587, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Chesney J, Mitchell R, Benigni F, Bacher M, Spiegel L, Al-Abed Y, Han JH, Metz C, and Bucala R. An inducible gene product for 6-phosphofructo-2-kinase with an AU-rich instability element: role in tumor cell glycolysis and the Warburg effect. Proc Natl Acad Sci U S A 96: 3047–3052, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chipuk JE. and Green DR. Dissecting p53-dependent apoptosis. Cell Death Differ 13: 994–1002, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, and Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 303: 1010–1014, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Chiueh CC, Andoh T, and Chock PB. Induction of thioredoxin and mitochondrial survival proteins mediates preconditioning-induced cardioprotection and neuroprotection. Ann N Y Acad Sci 1042: 403–418, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Choi JS, Shin S, Jin YH, Yim H, Koo KT, Chun KH, Oh YT, Lee WH, and Lee SK. Cyclin-dependent protein kinase 2 activity is required for mitochondrial translocation of Bax and disruption of mitochondrial transmembrane potential during etoposide-induced apoptosis. Apoptosis 12: 1229–1241, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Choo DW, Baek HJ, Motoyama N, Cho KH, Kim HS, and Kim SS. ATM is required for rapid degradation of cyclin D1 in response to gamma-irradiation. Biochem Biophys Res Commun 378: 847–850, 2009 [DOI] [PubMed] [Google Scholar]
- 49.Clute P. and Pines J. Temporal and spatial control of cyclin B1 destruction in metaphase. Nat Cell Biol 1: 82–87, 1999 [DOI] [PubMed] [Google Scholar]
- 50.Cooper HM. and Spelbrink JN. The human SIRT3 protein deacetylase is exclusively mitochondrial. Biochem J 411: 279–285, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Cousineau B, Leclerc F, and Cedergren R. On the origin of protein synthesis factors: a gene duplication/fusion model. J Mol Evol 45: 661–670, 1997 [DOI] [PubMed] [Google Scholar]
- 52.Cribbs JT. and Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep 8: 939–944, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daiber A. Redox signaling (cross-talk) from and to mitochondria involves mitochondrial pores and reactive oxygen species. Biochim Biophys Acta 1797: 897–906, 2010 [DOI] [PubMed] [Google Scholar]
- 54.Dang CV. Links between metabolism and cancer. Genes Dev 26: 877–890, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Daosukho C, Kiningham K, Kasarskis EJ, Ittarat W, and St. Clair DK. Tamoxifen enhancement of TNF-alpha induced MnSOD expression: modulation of NF-kappaB dimerization. Oncogene 21: 3603–3610, 2002 [DOI] [PubMed] [Google Scholar]
- 56.Dendy P. Low dose radiation risk: UKRC 2004 debate. Br J Radiol 78: 1–2, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Detmer SA. and Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol 8: 870–879, 2007 [DOI] [PubMed] [Google Scholar]
- 58.Dewey WC. and Fuhr MA. Quantification of mitochondria during the cell cycle of Chinese hamster cells. Exp Cell Res 99: 23–30, 1976 [DOI] [PubMed] [Google Scholar]
- 59.Dong Y, Sui L, Watanabe Y, Sugimoto K, and Tokuda M. Clinical relevance of cyclin B1 overexpression in laryngeal squamous cell carcinoma. Cancer Lett 177: 13–19, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Donohoe DR, Wali A, Brylawski BP, and Bultman SJ. Microbial regulation of glucose metabolism and cell-cycle progression in Mammalian colonocytes. PLoS One 7: e46589, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Doxsey S, Zimmerman W, and Mikule K. Centrosome control of the cell cycle. Trends Cell Biol 15: 303–311, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Drose S. and Brandt U. Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv Exp Med Biol 748: 145–169, 2012 [DOI] [PubMed] [Google Scholar]
- 63.Eldridge A, Fan M, Woloschak G, Grdina DJ, Chromy BA, and Li JJ. Manganese superoxide dismutase interacts with a large scale of cellular and mitochondrial proteins in low-dose radiation-induced adaptive radioprotection. Free Radic Biol Med 53: 1838–1847, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Endlich B, Radford IR, Forrester HB, and Dewey WC. Computerized video time-lapse microscopy studies of ionizing radiation-induced rapid-interphase and mitosis-related apoptosis in lymphoid cells. Radiat Res 153: 36–48, 2000 [DOI] [PubMed] [Google Scholar]
- 65.Epperly MW, Kagan VE, Sikora CA, Gretton JE, Defilippi SJ, Bar-Sagi D, and Greenberger JS. Manganese superoxide dismutase-plasmid/liposome (MnSOD-PL) administration protects mice from esophagitis associated with fractionated radiation. Int J Cancer 96: 221–231, 2001 [DOI] [PubMed] [Google Scholar]
- 66.Eura Y, Ishihara N, Yokota S, and Mihara K. Two mitofusin proteins, mammalian homologues of FZO, with distinct functions are both required for mitochondrial fusion. J Biochem 134: 333–344, 2003 [DOI] [PubMed] [Google Scholar]
- 67.Fan M, Ahmed KM, Coleman MC, Spitz DR, and Li JJ. Nuclear factor-kappaB and manganese superoxide dismutase mediate adaptive radioresistance in low-dose irradiated mouse skin epithelial cells. Cancer Res 67: 3220–3228, 2007 [DOI] [PubMed] [Google Scholar]
- 68.Feinendegen LE. The role of adaptive responses following exposure to ionizing radiation. Hum Exp Toxicol 18: 426–432, 1999 [DOI] [PubMed] [Google Scholar]
- 69.Feinendegen LE. Reactive oxygen species in cell responses to toxic agents. Hum Exp Toxicol 21: 85–90, 2002 [DOI] [PubMed] [Google Scholar]
- 70.Feinendegen LE, Bond VP, Sondhaus CA, and Altman KI. Cellular signal adaptation with damage control at low doses versus the predominance of DNA damage at high doses. C R Acad Sci III 322: 245–251, 1999 [DOI] [PubMed] [Google Scholar]
- 71.Ferecatu I, Bergeaud M, Rodriguez-Enfedaque A, Le Floch N, Oliver L, Rincheval V, Renaud F, Vallette FM, Mignotte B, and Vayssiere JL. Mitochondrial localization of the low level p53 protein in proliferative cells. Biochem Biophys Res Commun 387: 772–777, 2009 [DOI] [PubMed] [Google Scholar]
- 72.Fike JR, Rosi S, and Limoli CL. Neural precursor cells and central nervous system radiation sensitivity. Semin Radiat Oncol 19: 122–132, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Finkel T. and Hwang PM. The Krebs cycle meets the cell cycle: mitochondria and the G1-S transition. Proc Natl Acad Sci U S A 106: 11825–11826, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fu H, Subramanian RR, and Masters SC. 14-3-3 proteins: structure, function, and regulation. Annu Rev Pharmacol Toxicol 40: 617–647, 2000 [DOI] [PubMed] [Google Scholar]
- 75.Gautier J, Minshull J, Lohka M, Glotzer M, Hunt T, and Maller JL. Cyclin is a component of maturation-promoting factor from Xenopus. Cell 60: 487–494, 1990 [DOI] [PubMed] [Google Scholar]
- 76.Gavet O. and Pines J. Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev Cell 18: 533–543, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Geraci JP, Jackson KL, and Mariano MS. An estimate of the radiation-induced cancer risk from the whole-body stray radiation exposure in neutron radiotherapy. Eur J Cancer Clin Oncol 18: 1187–1195, 1982 [DOI] [PubMed] [Google Scholar]
- 78.Giedzinski E, Rola R, Fike JR, and Limoli CL. Efficient production of reactive oxygen species in neural precursor cells after exposure to 250 MeV protons. Radiat Res 164: 540–544, 2005 [DOI] [PubMed] [Google Scholar]
- 79.Goswami PC, Higashikubo R, and Spitz DR. Redox control of cell cycle-coupled topoisomerase II alpha gene expression. Methods Enzymol 353: 448–459, 2002 [DOI] [PubMed] [Google Scholar]
- 80.Grdina DJ, Kataoka Y, and Murley JS. Amifostine: mechanisms of action underlying cytoprotection and chemoprevention. Drug Metabol Drug Interact 16: 237–279, 2000 [DOI] [PubMed] [Google Scholar]
- 81.Grdina DJ, Murley JS, and Kataoka Y. Radioprotectants: current status and new directions. Oncology 63Suppl 2: 2–10, 2002 [DOI] [PubMed] [Google Scholar]
- 82.Grdina DJ, Murley JS, Miller RC, Mauceri HJ, Sutton HG, Li JJ, Woloschak GE, and Weichselbaum RR. A survivin-associated adaptive response in radiation therapy. Cancer Res 14: 4418–4428, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grdina DJ, Murley JS, Miller RC, Mauceri HJ, Sutton HG, Thirman MJ, Li JJ, Woloschak GE, and Weichselbaum RR. A manganese superoxide dismutase (SOD2)-mediated adaptive response. Radiat Res 170: 115–124, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Green DR. and Reed JC. Mitochondria and apoptosis. Science 281: 1309–1312, 1998 [DOI] [PubMed] [Google Scholar]
- 85.Guo G, Yan-Sanders Y, Lyn-Cook BD, Wang T, Tamae D, Ogi J, Khaletskiy A, Li Z, Weydert C, Longmate JA, Huang TT, Spitz DR, Oberley LW, and Li JJ. Manganese superoxide dismutase-mediated gene expression in radiation-induced adaptive responses. Mol Cell Biol 23: 2362–2378, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haas AF, Wong JW, Iwahashi CK, Halliwell B, Cross CE, and Davis PA. Redox regulation of wound healing? NF-kappaB activation in cultured human keratinocytes upon wounding and the effect of low energy HeNe irradiation. Free Radic Biol Med 25: 998–1005, 1998 [DOI] [PubMed] [Google Scholar]
- 87.Haigis MC, Deng CX, Finley LW, Kim HS, and Gius D. SIRT3 is a mitochondrial tumor suppressor: a scientific tale that connects aberrant cellular ROS, the Warburg effect, and carcinogenesis. Cancer Res 72: 2468–2472, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Haley TJ. and Harris DH. Response of the guinea pig to 200 roentgens acute whole body X irradiation. Science 111: 88–90, 1950 [DOI] [PubMed] [Google Scholar]
- 89.Hassan KA, Ang KK, El-Naggar AK, Story MD, Lee JI, Liu D, Hong WK, and Mao L. Cyclin B1 overexpression and resistance to radiotherapy in head and neck squamous cell carcinoma. Cancer Res 62: 6414–6417, 2002 [PubMed] [Google Scholar]
- 90.Hassan KA, El-Naggar AK, Soria JC, Liu D, Hong WK, and Mao L. Clinical significance of cyclin B1 protein expression in squamous cell carcinoma of the tongue. Clin Cancer Res 7: 2458–2462, 2001 [PubMed] [Google Scholar]
- 91.Hatefi Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu Rev Biochem 54: 1015–1069, 1985 [DOI] [PubMed] [Google Scholar]
- 92.Hei TK, Zhou H, Chai Y, Ponnaiya B, and Ivanov VN. Radiation induced non-targeted response: mechanism and potential clinical implications. Curr Mol Pharmacol 4: 96–105, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hirai Y, Hayashi T, Kubo Y, Hoki Y, Arita I, Tatsumi K, and Seyama T. X-irradiation induces up-regulation of ATM gene expression in wild-type lymphoblastoid cell lines, but not in their heterozygous or homozygous ataxia-telangiectasia counterparts. Jpn J Cancer Res 92: 710–717, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Holt IJ. Mitochondrial DNA replication and repair: all a flap. Trends Biochem Sci 34: 358–365, 2009 [DOI] [PubMed] [Google Scholar]
- 95.Hu W, Zhang C, Wu R, Sun Y, Levine A, and Feng Z. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc Natl Acad Sci U S A 107: 7455–7460, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Huang L, Kim PM, Nickoloff JA, and Morgan WF. Targeted and nontargeted effects of low-dose ionizing radiation on delayed genomic instability in human cells. Cancer Res 67: 1099–1104, 2007 [DOI] [PubMed] [Google Scholar]
- 97.Hunt T. Cell biology. Cell cycle gets more cyclins. Nature 350: 462–463, 1991 [DOI] [PubMed] [Google Scholar]
- 98.Ikushima T. Radio-adaptive response: characterization of a cytogenetic repair induced by low-level ionizing radiation in cultured Chinese hamster cells. Mutat Res 227: 241–246, 1989 [DOI] [PubMed] [Google Scholar]
- 99.Jackman M, Lindon C, Nigg EA, and Pines J. Active cyclin B1-Cdk1 first appears on centrosomes in prophase. Nat Cell Biol 5: 143–148, 2003 [DOI] [PubMed] [Google Scholar]
- 100.Jacotot E, Ferri KF, and Kroemer G. Apoptosis and cell cycle: distinct checkpoints with overlapping upstream control. Pathol Biol (Paris) 48: 271–279, 2000 [PubMed] [Google Scholar]
- 101.Ji LL, Gomez-Cabrera MC, and Vina J. Exercise and hormesis: activation of cellular antioxidant signaling pathway. Ann N Y Acad Sci 1067: 425–435, 2006 [DOI] [PubMed] [Google Scholar]
- 102.Kapoor M, Hamm R, Yan W, Taya Y, and Lozano G. Cooperative phosphorylation at multiple sites is required to activate p53 in response to UV radiation. Oncogene 19: 358–364, 2000 [DOI] [PubMed] [Google Scholar]
- 103.Karbowski M, Norris KL, Cleland MM, Jeong SY, and Youle RJ. Role of Bax and Bak in mitochondrial morphogenesis. Nature 443: 658–662, 2006 [DOI] [PubMed] [Google Scholar]
- 104.Katiyar S, Casimiro MC, Dettin L, Ju X, Wagner EF, Tanaka H, and Pestell RG. C-jun inhibits mammary apoptosis in vivo. Mol Biol Cell 21: 4264–4274, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kelsey KT, Memisoglu A, Frenkel D, and Liber HL. Human lymphocytes exposed to low doses of X-rays are less susceptible to radiation-induced mutagenesis. Mutat Res 263: 197–201, 1991 [DOI] [PubMed] [Google Scholar]
- 106.Kim JW. and Dang CV. Cancer's molecular sweet tooth and the Warburg effect. Cancer Res 66: 8927–8930, 2006 [DOI] [PubMed] [Google Scholar]
- 107.Kim JW, Tchernyshyov I, Semenza GL, and Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 3: 177–185, 2006 [DOI] [PubMed] [Google Scholar]
- 108.Knott AB, Perkins G, Schwarzenbacher R, and Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci 9: 505–518, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lamerton LF, Elson LA, and Christensen WR. A study of the phases of radiation response in the rat. I. The effects of uniform whole body irradiation. Br J Radiol 26: 510–518, 1953 [DOI] [PubMed] [Google Scholar]
- 110.Lan ML, Acharya MM, Tran KK, Bahari-Kashani J, Patel NH, Strnadel J, Giedzinski E, and Limoli CL. Characterizing the radioresponse of pluripotent and multipotent human stem cells. PLoS One 7: e50048, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee YJ, Jeong SY, Karbowski M, Smith CL, and Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell 15: 5001–5011, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lemasters JJ. and Sowers AE. Phosphate dependence and atractyloside inhibition of mitochondrial oxidative phosphorylation. The ADP-ATP carrier is rate-limiting. J Biol Chem 254: 1248–1251, 1979 [PubMed] [Google Scholar]
- 113.Levine AJ. and Puzio-Kuter AM. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science 330: 1340–1344, 2010 [DOI] [PubMed] [Google Scholar]
- 114.Li JQ, Kubo A, Wu F, Usuki H, Fujita J, Bandoh S, Masaki T, Saoo K, Takeuchi H, Kobayashi S, Imaida K, Maeta H, Ishida T, and Kuriyama S. Cyclin B1, unlike cyclin G1, increases significantly during colorectal carcinogenesis and during later metastasis to lymph nodes. Int J Oncol 22: 1101–1110, 2003 [PubMed] [Google Scholar]
- 115.Li K, Li Y, Shelton JM, Richardson JA, Spencer E, Chen ZJ, Wang X, and Williams RS. Cytochrome c deficiency causes embryonic lethality and attenuates stress-induced apoptosis. Cell 101: 389–399, 2000 [DOI] [PubMed] [Google Scholar]
- 116.Li LY, Luo X, and Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 412: 95–99, 2001 [DOI] [PubMed] [Google Scholar]
- 117.Li P, Nijhawan D, and Wang X. Mitochondrial activation of apoptosis. Cell 116: S57–S59, 2 p following S59, 2004 [DOI] [PubMed] [Google Scholar]
- 118.Li Z, Jiao X, Wang C, Ju X, Lu Y, Yuan L, Lisanti MP, Katiyar S, and Pestell RG. Cyclin D1 induction of cellular migration requires p27(KIP1). Cancer Res 66: 9986–9994, 2006 [DOI] [PubMed] [Google Scholar]
- 119.Li Z, Khaletskiy A, Wang J, Wong JY, Oberley LW, and Li JJ. Genes regulated in human breast cancer cells overexpressing manganese-containing superoxide dismutase. Free Radic Biol Med 30: 260–267, 2001 [DOI] [PubMed] [Google Scholar]
- 120.Li Z, Xia L, Lee ML, Khaletskiy A, Wang J, Wong JYC, and Li JJ. Effector genes altered in MCF-7 human breast cancer cells after exposure to fractionated ionizing radiation. Radiat Res 155: 543–553, 2001 [DOI] [PubMed] [Google Scholar]
- 121.Limoli CL, Ponnaiya B, Corcoran JJ, Giedzinski E, Kaplan MI, Hartmann A, and Morgan WF. Genomic instability induced by high and low LET ionizing radiation. Adv Space Res 25: 2107–2117, 2000 [DOI] [PubMed] [Google Scholar]
- 122.Liu SZ, Cai L, and Sun SQ. Induction of a cytogenetic adaptive response by exposure of rabbits to very low dose-rate gamma-radiation. Int J Radiat Biol 62: 187–190, 1992 [DOI] [PubMed] [Google Scholar]
- 123.Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV, Jr., Weissman S, Verdin E, and Schwer B. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol 27: 8807–8814, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Loog M. and Morgan DO. Cyclin specificity in the phosphorylation of cyclin-dependent kinase substrates. Nature 434: 104–108, 2005 [DOI] [PubMed] [Google Scholar]
- 125.Lundgren K, Walworth N, Booher R, Dembski M, Kirschner M, and Beach D. mik1 and wee1 cooperate in the inhibitory tyrosine phosphorylation of cdc2. Cell 64: 1111–1122, 1991 [DOI] [PubMed] [Google Scholar]
- 126.Macfarlane M, Robinson GL, and Cain K. Glucose-a sweet way to die: metabolic switching modulates tumor cell death. Cell Cycle 11: 3819–3925, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Malinska D, Kulawiak B, Kudin AP, Kovacs R, Huchzermeyer C, Kann O, Szewczyk A, and Kunz WS. Complex III-dependent superoxide production of brain mitochondria contributes to seizure-related ROS formation. Biochim Biophys Acta 1797: 1163–1170, 2010 [DOI] [PubMed] [Google Scholar]
- 128.Mandal S, Guptan P, Owusu-Ansah E, and Banerjee U. Mitochondrial regulation of cell cycle progression during development as revealed by the tenured mutation in Drosophila. Dev Cell 9: 843–854, 2005 [DOI] [PubMed] [Google Scholar]
- 129.Manoli I, Alesci S, Blackman MR, Su YA, Rennert OM, and Chrousos GP. Mitochondria as key components of the stress response. Trends Endocrinol Metab 18: 190–198, 2007 [DOI] [PubMed] [Google Scholar]
- 130.Marsboom G, Toth PT, Ryan JJ, Hong Z, Wu X, Fang YH, Thenappan T, Piao L, Zhang HJ, Pogoriler J, Chen Y, Morrow E, Weir EK, Rehman J, and Archer SL. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ Res 110: 1484–1497, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, Hurley PJ, Bunz F, and Hwang PM. p53 regulates mitochondrial respiration. Science 312: 1650–1653, 2006 [DOI] [PubMed] [Google Scholar]
- 132.Matsunaga T, Maeda Y, Yoshino T, Takeyama H, Takahashi M, Ginya H, Aasahina J, and Tajima H. Fully automated immunoassay for detection of prostate-specific antigen using nano-magnetic beads and micro-polystyrene bead composites, ‘Beads on Beads’. Anal Chim Acta 597: 331–339, 2007 [DOI] [PubMed] [Google Scholar]
- 133.McBride HM, Neuspiel M, and Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol 16: R551–R560, 2006 [DOI] [PubMed] [Google Scholar]
- 134.McBride WH, Pajonk F, Chiang CS, and Sun JR. NF-kappa B, cytokines, proteasomes, and low-dose radiation exposure. Mil Med 167: 66–67, 2002 [PubMed] [Google Scholar]
- 135.McGowan AJ, Bowie AG, O'Neill LA, and Cotter TG. The production of a reactive oxygen intermediate during the induction of apoptosis by cytotoxic insult. Exp Cell Res 238: 248–256, 1998 [DOI] [PubMed] [Google Scholar]
- 136.Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, and Moll UM. p53 has a direct apoptogenic role at the mitochondria. Mol Cell 11: 577–590, 2003 [DOI] [PubMed] [Google Scholar]
- 137.Minshull J, Golsteyn R, Hill CS, and Hunt T. The A- and B-type cyclin associated cdc2 kinases in Xenopus turn on and off at different times in the cell cycle. EMBO J 9: 2865–2875, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mitra K, Wunder C, Roysam B, Lin G, and Lippincott-Schwartz J. A hyperfused mitochondrial state achieved at G1-S regulates cyclin E buildup and entry into S phase. Proc Natl Acad Sci U S A 106: 11960–11965, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Morgan DO. Principles of CDK regulation. Nature 374: 131–134, 1995 [DOI] [PubMed] [Google Scholar]
- 140.Morgan WF. Is there a common mechanism underlying genomic instability, bystander effects and other nontargeted effects of exposure to ionizing radiation? Oncogene 22: 7094–7099, 2003 [DOI] [PubMed] [Google Scholar]
- 141.Morgan WF. Will radiation-induced bystander effects or adaptive responses impact on the shape of the dose response relationships at low doses of ionizing radiation? Dose Response 4: 257–262, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Morgan WF. and Schwartz JL. Environmental mutagen society symposium on ‘Risks of low dose, low dose rate exposures of ionizing radiation to humans’. Int J Radiat Biol 83: 491–499, 2007 [DOI] [PubMed] [Google Scholar]
- 143.Mothersill C. and Seymour C. Radiation-induced bystander effects: evidence for an adaptive response to low dose exposures? Dose Response 4: 283–290, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Munoz MJ. and Jimenez J. Genetic interactions between Hsp90 and the Cdc2 mitotic machinery in the fission yeast Schizosaccharomyces pombe. Mol Gen Genet 261: 242–250, 1999 [DOI] [PubMed] [Google Scholar]
- 145.Murakami H, Furihata M, Ohtsuki Y, and Ogoshi S. Determination of the prognostic significance of cyclin B1 overexpression in patients with esophageal squamous cell carcinoma. Virchows Arch 434: 153–158, 1999 [DOI] [PubMed] [Google Scholar]
- 146.Murray AW. Recycling the cell cycle: cyclins revisited. Cell 116: 221–234, 2004 [DOI] [PubMed] [Google Scholar]
- 147.Muschel RJ, Zhang HB, Iliakis G, and McKenna WG. Cyclin B expression in HeLa cells during the G2 block induced by ionizing radiation. Cancer Res 51: 5113–5137, 1991 [PubMed] [Google Scholar]
- 148.Nakamura T. and Lipton SA. Redox regulation of mitochondrial fission, protein misfolding, synaptic damage, and neuronal cell death: potential implications for Alzheimer's and Parkinson's diseases. Apoptosis 15: 1354–1363, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nantajit D, Fan M, Duru N, Wen Y, Reed JC, and Li JJ. Cyclin B1/Cdk1 phosphorylation of mitochondrial p53 induces anti-apoptotic response. PLoS One 5: e12341, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Neupert W. and Herrmann JM. Translocation of proteins into mitochondria. Annu Rev Biochem 76: 723–749, 2007 [DOI] [PubMed] [Google Scholar]
- 151.Nomura M, Shimizu S, Sugiyama T, Narita M, Ito T, Matsuda H, and Tsujimoto Y. 14-3-3 interacts directly with and negatively regulates pro-apoptotic Bax. J Biol Chem 278: 2058–2065, 2003 [DOI] [PubMed] [Google Scholar]
- 152.Nunnari J, Marshall WF, Straight A, Murray A, Sedat JW, and Walter P. Mitochondrial transmission during mating in Saccharomyces cerevisiae is determined by mitochondrial fusion and fission and the intramitochondrial segregation of mitochondrial DNA. Mol Biol Cell 8: 1233–1242, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.O'Connor DS, Grossman D, Plescia J, Li F, Zhang H, Villa A, Tognin S, Marchisio PC, and Altieri DC. Regulation of apoptosis at cell division by p34cdc2 phosphorylation of survivin. Proc Natl Acad Sci U S A 97: 13103–13107, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Olivieri G, Bodycote J, and Wolff S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science 223: 594–597, 1984 [DOI] [PubMed] [Google Scholar]
- 155.Onyango P, Celic I, McCaffery JM, Boeke JD, and Feinberg AP. SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc Natl Acad Sci U S A 99: 13653–13658, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Owusu-Ansah AK. and David RJ. Mortality risk of small infants varies with their mother's birthweight and race. Paediatr Perinat Epidemiol 22: 145–154, 2008 [DOI] [PubMed] [Google Scholar]
- 157.Ozden O, Park SH, Kim HS, Jiang H, Coleman MC, Spitz DR, and Gius D. Acetylation of MnSOD directs enzymatic activity responding to cellular nutrient status or oxidative stress. Aging (Albany NY) 3: 102–107, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ozeki M, Tamae D, Hou DX, Wang T, Lebon T, Spitz DR, and Li JJ. Response of cyclin B1 to ionizing radiation: regulation by NF-kappaB and mitochondrial antioxidant enzyme MnSOD. Anticancer Res 24: 2657–2663, 2004 [PMC free article] [PubMed] [Google Scholar]
- 159.Pagliarini DJ. and Dixon JE. Mitochondrial modulation: reversible phosphorylation takes center stage? Trends Biochem Sci 31: 26–34, 2006 [DOI] [PubMed] [Google Scholar]
- 160.Pandey BN, Gordon DM, De Toledo SM, Pain D, and Azzam EI. Normal human fibroblasts exposed to high- or low-dose ionizing radiation: differential effects on mitochondrial protein import and membrane potential. Antioxid Redox Signal 8: 1253–1261, 2006 [DOI] [PubMed] [Google Scholar]
- 161.Pearson AE. and Phelps TA. Radiation effects on mouse incisor teeth following whole-body doses of up to 16 gray. Int J Radiat Biol Relat Stud Phys Chem Med 39: 409–417, 1981 [DOI] [PubMed] [Google Scholar]
- 162.Pines J. and Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell 58: 833–846, 1989 [DOI] [PubMed] [Google Scholar]
- 163.Pirinen E, Lo Sasso G, and Auwerx J. Mitochondrial sirtuins and metabolic homeostasis. Best Pract Res Clin Endocrinol Metab 26: 759–770, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pohlit W. and Heyder IR. Growth of cells on solid culture medium. II. Cell physiological data of stationary yeast cells and the initiation of cell cycle in nutrient free buffer solution. Radiat Environ Biophys 14: 213–230, 1977 [DOI] [PubMed] [Google Scholar]
- 165.Polyak K, Xia Y, Zweier JL, Kinzler KW, and Vogelstein B. A model for p53-induced apoptosis. Nature 389: 300–305, 1997 [DOI] [PubMed] [Google Scholar]
- 166.Pontano LL, Aggarwal P, Barbash O, Brown EJ, Bassing CH, and Diehl JA. Genotoxic stress-induced cyclin D1 phosphorylation and proteolysis are required for genomic stability. Mol Cell Biol 28: 7245–7258, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Porter LA, Singh G, and Lee JM. Abundance of cyclin B1 regulates gamma-radiation-induced apoptosis. Blood 95: 2645–2650, 2000 [PubMed] [Google Scholar]
- 168.Posakony JW, England JM, and Attardi G. Mitochondrial growth and division during the cell cycle in HeLa cells. J Cell Biol 74: 468–491, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Prasad KN, Cole WC, and Hasse GM. Health risks of low dose ionizing radiation in humans: a review. Exp Biol Med (Maywood) 229: 378–382, 2004 [DOI] [PubMed] [Google Scholar]
- 170.Puskin JS. and Nelson CB. Estimates of radiogenic cancer risks. Health Phys 69: 93–101, 1995 [DOI] [PubMed] [Google Scholar]
- 171.Qi W. and Martinez JD. Reduction of 14-3-3 proteins correlates with increased sensitivity to killing of human lung cancer cells by ionizing radiation. Radiat Res 160: 217–223, 2003 [DOI] [PubMed] [Google Scholar]
- 172.Rich PR. The molecular machinery of Keilin's respiratory chain. Biochem Soc Trans 31: 1095–1105, 2003 [DOI] [PubMed] [Google Scholar]
- 173.Robson T, Price ME, Moore ML, Joiner MC, McKelvey-Martin VJ, McKeown SR, and Hirst DG. Increased repair and cell survival in cells treated with DIR1 antisense oligonucleotides: implications for induced radioresistance. Int J Radiat Biol 76: 617–623, 2000 [DOI] [PubMed] [Google Scholar]
- 174.Ryan MT. and Hoogenraad NJ. Mitochondrial-nuclear communications. Annu Rev Biochem 76: 701–722, 2007 [DOI] [PubMed] [Google Scholar]
- 175.Saavedra RA, Cordoba C, and Anderson GR. LDHk in the retina of diverse vertebrate species: a possible link to the Warburg effect. Exp Eye Res 41: 365–370, 1985 [DOI] [PubMed] [Google Scholar]
- 176.Sakamaki T, Casimiro MC, Ju X, Quong AA, Katiyar S, Liu M, Jiao X, Li A, Zhang X, Lu Y, Wang C, Byers S, Nicholson R, Link T, Shemluck M, Yang J, Fricke ST, Novikoff PM, Papanikolaou A, Arnold A, Albanese C, and Pestell R. Cyclin D1 determines mitochondrial function in vivo. Mol Cell Biol 26: 5449–5469, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Saleem A, Adhihetty PJ, and Hood DA. Role of p53 in mitochondrial biogenesis and apoptosis in skeletal muscle. Physiol Genomics 37: 58–66, 2009 [DOI] [PubMed] [Google Scholar]
- 178.Sankaran VG, Orkin SH, and Walkley CR. Rb intrinsically promotes erythropoiesis by coupling cell cycle exit with mitochondrial biogenesis. Genes Dev 22: 463–475, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Santel A. and Frank S. Shaping mitochondria: the complex posttranslational regulation of the mitochondrial fission protein DRP1. IUBMB Life 60: 448–455, 2008 [DOI] [PubMed] [Google Scholar]
- 180.Saraste M. Oxidative phosphorylation at the fin de siecle. Science 283: 1488–1493, 1999 [DOI] [PubMed] [Google Scholar]
- 181.Sazanov LA. and Hinchliffe P. Structure of the hydrophilic domain of respiratory complex I from Thermus thermophilus. Science 311: 1430–1436, 2006 [DOI] [PubMed] [Google Scholar]
- 182.Scher MB, Vaquero A, and Reinberg D. SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress. Genes Dev 21: 920–928, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Schieke SM, McCoy JP, Jr., and Finkel T. Coordination of mitochondrial bioenergetics with G1 phase cell cycle progression. Cell Cycle 7: 1782–1787, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Schuster S, Fell DA, and Dandekar T. A general definition of metabolic pathways useful for systematic organization and analysis of complex metabolic networks. Nat Biotechnol 18: 326–332, 2000 [DOI] [PubMed] [Google Scholar]
- 185.Seong J. and Kim GE. Adaptive response to ionizing radiation induced by low dose of gamma ray in human hepatoma cell lines. Yonsei Med J 35: 77–83, 1994 [DOI] [PubMed] [Google Scholar]
- 186.Shadley JD, Afzal V, and Wolff S. Characterization of the adaptive response to ionizing radiation induced by low doses of X rays to human lymphocytes. Radiat Res 111: 511–517, 1987 [PubMed] [Google Scholar]
- 187.Shadley JD. and Wolff S. Very low doses of X-rays can cause human lymphocytes to become less susceptible to ionizing radiation. Mutagenesis 2: 95–96, 1987 [DOI] [PubMed] [Google Scholar]
- 188.Shaulsky G. and Loomis WF. Mitochondrial DNA replication but no nuclear DNA replication during development of Dictyostelium. Proc Natl Acad Sci U S A 92: 5660–5663, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shen L, Sun X, Fu Z, Yang G, Li J, and Yao L. The fundamental role of the p53 pathway in tumor metabolism and its implication in tumor therapy. Clin Cancer Res 18: 1561–1567, 2012 [DOI] [PubMed] [Google Scholar]
- 190.Sherr CJ. D-type cyclins. Trends Biochem Sci 20: 187–190, 1995 [DOI] [PubMed] [Google Scholar]
- 191.Shiekhattar R, Mermelstein F, Fisher RP, Drapkin R, Dynlacht B, Wessling HC, Morgan DO, and Reinberg D. Cdk-activating kinase complex is a component of human transcription factor TFIIH. Nature 374: 283–287, 1995 [DOI] [PubMed] [Google Scholar]
- 192.Skov KA. Molecular, cellular, and genetic basis of radiosensitivity at low doses: a case of inducible repair? Radiat Res 138: S1–S4, 1994 [PubMed] [Google Scholar]
- 193.Skov KA. Perspectives on the adaptive response from studies on the response to low radiation doses (or to cisplatin) in mammalian cells. Hum Exp Toxicol 18: 447–451, 1999 [DOI] [PubMed] [Google Scholar]
- 194.Smirnova E, Griparic L, Shurland DL, and van der Bliek AM. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 12: 2245–2256, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Smirnova E, Shurland DL, Ryazantsev SN, and van der Bliek AM. A human dynamin-related protein controls the distribution of mitochondria. J Cell Biol 143: 351–358, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Songyang Z, Blechner S, Hoagland N, Hoekstra MF, Piwnica-Worms H, and Cantley LC. Use of an oriented peptide library to determine the optimal substrates of protein kinases. Curr Biol 4: 973–982, 1994 [DOI] [PubMed] [Google Scholar]
- 197.Soria JC, Jang SJ, Khuri FR, Hassan K, Liu D, Hong WK, and Mao L. Overexpression of cyclin B1 in early-stage non-small cell lung cancer and its clinical implication. Cancer Res 60: 4000–4004, 2000 [PubMed] [Google Scholar]
- 198.Spitz DR. Metabolic oxidative stress and low dose radiation responses: are mitochondria involved. Health Phys 100: 295, 2011 [DOI] [PubMed] [Google Scholar]
- 199.Spitz DR, Azzam EI, Li JJ, and Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology. Cancer Metastasis Rev 23: 311–322, 2004 [DOI] [PubMed] [Google Scholar]
- 200.Sreekumar A, Nyati MK, Varambally S, Barrette TR, Ghosh D, Lawrence TS, and Chinnaiyan AM. Profiling of cancer cells using protein microarrays: discovery of novel radiation-regulated proteins. Cancer Res 61: 7585–7593, 2001 [PubMed] [Google Scholar]
- 201.Srinivasan M, Sudheer AR, Pillai KR, Kumar PR, Sudhakaran PR, and Menon VP. Lycopene as a natural protector against gamma-radiation induced DNA damage, lipid peroxidation and antioxidant status in primary culture of isolated rat hepatocytes in vitro. Biochim Biophys Acta 1770: 659–665, 2007 [DOI] [PubMed] [Google Scholar]
- 202.Stecca C. and Gerber GB. Adaptive response to DNA-damaging agents: a review of potential mechanisms. Biochem Pharmacol 55: 941–951, 1998 [DOI] [PubMed] [Google Scholar]
- 203.Sumrejkanchanakij P, Tamamori-Adachi M, Matsunaga Y, Eto K, and Ikeda MA. Role of cyclin D1 cytoplasmic sequestration in the survival of postmitotic neurons. Oncogene 22: 8723–8730, 2003 [DOI] [PubMed] [Google Scholar]
- 204.Suzuki K, Kodama S, and Watanabe M. Extremely low-dose ionizing radiation causes activation of mitogen-activated protein kinase pathway and enhances proliferation of normal human diploid cells. Cancer Res 61: 5396–5401, 2001 [PubMed] [Google Scholar]
- 205.Suzuki S, Tanaka T, Poyurovsky MV, Nagano H, Mayama T, Ohkubo S, Lokshin M, Hosokawa H, Nakayama T, Suzuki Y, Sugano S, Sato E, Nagao T, Yokote K, Tatsuno I, and Prives C. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proc Natl Acad Sci U S A 107: 7461–7466, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sweet S. and Singh G. Changes in mitochondrial mass, membrane potential, and cellular adenosine triphosphate content during the cell cycle of human leukemic (HL-60) cells. J Cell Physiol 180: 91–96, 1999 [DOI] [PubMed] [Google Scholar]
- 207.Taguchi N, Ishihara N, Jofuku A, Oka T, and Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem 282: 11521–11529, 2007 [DOI] [PubMed] [Google Scholar]
- 208.Takeno S, Noguchi T, Kikuchi R, Uchida Y, Yokoyama S, and Muller W. Prognostic value of cyclin B1 in patients with esophageal squamous cell carcinoma. Cancer 94: 2874–2881, 2002 [DOI] [PubMed] [Google Scholar]
- 209.Takizawa CG. and Morgan DO. Control of mitosis by changes in the subcellular location of cyclin-B1-Cdk1 and Cdc25C. Curr Opin Cell Biol 12: 658–665, 2000 [DOI] [PubMed] [Google Scholar]
- 210.Tao R, Coleman MC, Pennington JD, Ozden O, Park SH, Jiang H, Kim HS, Flynn CR, Hill S, Hayes McDonald W, Olivier AK, Spitz DR, and Gius D. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell 40: 893–904, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Taylor CT. and Moncada S. Nitric oxide, cytochrome C oxidase, and the cellular response to hypoxia. Arterioscler Thromb Vasc Biol 30: 643–647, 2010 [DOI] [PubMed] [Google Scholar]
- 212.Terrano DT, Upreti M, and Chambers TC. Cyclin-dependent kinase 1-mediated Bcl-xL/Bcl-2 phosphorylation acts as a functional link coupling mitotic arrest and apoptosis. Mol Cell Biol 30: 640–656, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Tucker JD. Low-dose ionizing radiation and chromosome translocations: a review of the major considerations for human biological dosimetry. Mutat Res 659: 211–220, 2008 [DOI] [PubMed] [Google Scholar]
- 214.Turnbull EL, Martin IV, and Fantes PA. Activity of Cdc2 and its interaction with the cyclin Cdc13 depend on the molecular chaperone Cdc37 in Schizosaccharomyces pombe. J Cell Sci 119: 292–302, 2006 [DOI] [PubMed] [Google Scholar]
- 215.Ubersax JA, Woodbury EL, Quang PN, Paraz M, Blethrow JD, Shah K, Shokat KM, and Morgan DO. Targets of the cyclin-dependent kinase Cdk1. Nature 425: 859–864, 2003 [DOI] [PubMed] [Google Scholar]
- 216.Ugalde C, Vogel R, Huijbens R, Van Den Heuvel B, Smeitink J, and Nijtmans L. Human mitochondrial complex I assembles through the combination of evolutionary conserved modules: a framework to interpret complex I deficiencies. Hum Mol Genet 13: 2461–2472, 2004 [DOI] [PubMed] [Google Scholar]
- 217.van der Laan M, Hutu DP, and Rehling P. On the mechanism of preprotein import by the mitochondrial presequence translocase. Biochim Biophys Acta 1803: 732–739, 2010 [DOI] [PubMed] [Google Scholar]
- 218.Vanamala J, Radhakrishnan S, Reddivari L, Bhat VB, and Ptitsyn A. Resveratrol suppresses human colon cancer cell proliferation and induces apoptosis via targeting the pentose phosphate and the talin-FAK signaling pathways-A proteomic approach. Proteome Sci 9: 49, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Vantieghem A, Xu Y, Assefa Z, Piette J, Vandenheede JR, Merlevede W, De Witte PA, and Agostinis P. Phosphorylation of Bcl-2 in G2/M phase-arrested cells following photodynamic therapy with hypericin involves a CDK1-mediated signal and delays the onset of apoptosis. J Biol Chem 277: 37718–37731, 2002 [DOI] [PubMed] [Google Scholar]
- 220.Venkatachalam P, de Toledo SM, Pandey BN, Tephly LA, Carter AB, Little JB, Spitz DR, and Azzam EI. Regulation of normal cell cycle progression by flavin-containing oxidases. Oncogene 27: 20–31, 2008 [DOI] [PubMed] [Google Scholar]
- 221.Vina J, Borras C, Gomez-Cabrera MC, and Orr WC. Part of the series: from dietary antioxidants to regulators in cellular signalling and gene expression. Role of reactive oxygen species and (phyto)oestrogens in the modulation of adaptive response to stress. Free Radic Res 40: 111–119, 2006 [DOI] [PubMed] [Google Scholar]
- 222.Wang A, Yoshimi N, Ino N, Tanaka T, and Mori H. Overexpression of cyclin B1 in human colorectal cancers. J Cancer Res Clin Oncol 123: 124–127, 1997 [DOI] [PubMed] [Google Scholar]
- 223.Wang C, Li Z, Lu Y, Du R, Katiyar S, Yang J, Fu M, Leader JE, Quong A, Novikoff PM, and Pestell RG. Cyclin D1 repression of nuclear respiratory factor 1 integrates nuclear DNA synthesis and mitochondrial function. Proc Natl Acad Sci U S A 103: 11567–11572, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wang T, Zhang X, and Li JJ. The role of NF-kappaB in the regulation of cell stress responses. Int Immunopharmacol 2: 1509–1520, 2002 [DOI] [PubMed] [Google Scholar]
- 225.Westermann B. Merging mitochondria matters: cellular role and molecular machinery of mitochondrial fusion. EMBO Rep 3: 527–531, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Willkomm DK. and Hartmann RK. Intricacies and surprises of nuclear-mitochondrial co-evolution. Biochem J 399: e7–e9, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wolff S. Are radiation-induced effects hormetic? Science 245: 575, 621, 1989 [DOI] [PubMed] [Google Scholar]
- 228.Wolff S. The adaptive response in radiobiology: evolving insights and implications. Environ Health Perspect 106Suppl 1: 277–283, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wu J, Feng Y, Xie D, Li X, Xiao W, Tao D, Qin J, Hu J, Gardner K, Judge SI, Li QQ, and Gong J. Unscheduled CDK1 activity in G1 phase of the cell cycle triggers apoptosis in X-irradiated lymphocytic leukemia cells. Cell Mol Life Sci 63: 2538–2545, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Wyrobek AJ, Manohar CF, Krishnan VV, Nelson DO, Furtado MR, Bhattacharya MS, Marchetti F, and Coleman MA. Low dose radiation response curves, networks and pathways in human lymphoblastoid cells exposed from 1 to 10 cGy of acute gamma radiation. Mutat Res 722: 119–130, 2011 [DOI] [PubMed] [Google Scholar]
- 231.Xiao B, Smerdon SJ, Jones DH, Dodson GG, Soneji Y, Aitken A, and Gamblin SJ. Structure of a 14-3-3 protein and implications for coordination of multiple signalling pathways. Nature 376: 188–191, 1995 [DOI] [PubMed] [Google Scholar]
- 232.Xu N, Libertini S, Black EJ, Lao Y, Hegarat N, Walker M, and Gillespie DA. Cdk-mediated phosphorylation of Chk1 is required for efficient activation and full checkpoint proficiency in response to DNA damage. Oncogene 31: 1086–1094, 2012 [DOI] [PubMed] [Google Scholar]
- 233.Xu Y, Fang F, St Clair DK, Josson S, Sompol P, Spasojevic I, and St. Clair WH. Suppression of RelB-mediated manganese superoxide dismutase expression reveals a primary mechanism for radiosensitization effect of 1alpha,25-dihydroxyvitamin D(3) in prostate cancer cells. Mol Cancer Ther 6: 2048–2056, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yalcin A, Clem BF, Simmons A, Lane A, Nelson K, Clem AL, Brock E, Siow D, Wattenberg B, Telang S, and Chesney J. Nuclear targeting of 6-phosphofructo-2-kinase (PFKFB3) increases proliferation via cyclin-dependent kinases. J Biol Chem 284: 24223–24232, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Yamamori T, Yasui H, Yamazumi M, Wada Y, Nakamura Y, Nakamura H, and Inanami O. Ionizing radiation induces mitochondrial reactive oxygen species production accompanied by upregulation of mitochondrial electron transport chain function and mitochondrial content under control of the cell cycle checkpoint. Free Radic Biol Med 53: 260–270, 2012 [DOI] [PubMed] [Google Scholar]
- 236.Yamano K. and Youle RJ. Coupling mitochondrial and cell division. Nat Cell Biol 13: 1026–1027, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Yoshida K, Yamaguchi T, Natsume T, Kufe D, and Miki Y. JNK phosphorylation of 14-3-3 proteins regulates nuclear targeting of c-Abl in the apoptotic response to DNA damage. Nat Cell Biol 7: 278–285, 2005 [DOI] [PubMed] [Google Scholar]
- 238.Yoshida N, Imada H, Kunugita N, and Norimura T. Low dose radiation-induced adaptive survival response in mouse spleen T-lymphocytes in vivo. J Radiat Res (Tokyo) 34: 269–276, 1993 [DOI] [PubMed] [Google Scholar]
- 239.Yoshida Y, Izumi H, Torigoe T, Ishiguchi H, Itoh H, Kang D, and Kohno K. P53 physically interacts with mitochondrial transcription factor A and differentially regulates binding to damaged DNA. Cancer Res 63: 3729–3734, 2003 [PubMed] [Google Scholar]
- 240.Youngblom JH, Wiencke JK, and Wolff S. Inhibition of the adaptive response of human lymphocytes to very low doses of ionizing radiation by the protein synthesis inhibitor cycloheximide. Mutat Res 227: 257–261, 1989 [DOI] [PubMed] [Google Scholar]
- 241.Yuan J, Yan R, Kramer A, Eckerdt F, Roller M, Kaufmann M, and Strebhardt K. Cyclin B1 depletion inhibits proliferation and induces apoptosis in human tumor cells. Oncogene 23: 5843–5852, 2004 [DOI] [PubMed] [Google Scholar]
- 242.Zhang H, Zheng RL, Wei ZQ, Li WJ, Gao QX, Chen WQ, Wang ZH, He J, Liang JP, Han GW, Huang T, Li Q, Xie HM, Zhang SM, and Cai XC. Effects of pre-exposure of mouse testis with low-dose (16)O8+ ions or 60Co gamma-rays on sperm shape abnormalities, lipid peroxidation and superoxide dismutase (SOD) activity induced by subsequent high-dose irradiation. Int J Radiat Biol 73: 163–167, 1998 [DOI] [PubMed] [Google Scholar]
- 243.Zhang L, Yu J, Park BH, Kinzler KW, and Vogelstein B. Role of BAX in the apoptotic response to anticancer agents. Science 290: 989–992, 2000 [DOI] [PubMed] [Google Scholar]
- 244.Zhao W, Spitz DR, Oberley LW, and Robbins ME. Redox modulation of the pro-fibrogenic mediator plasminogen activator inhibitor-1 following ionizing radiation. Cancer Res 61: 5537–5543, 2001 [PubMed] [Google Scholar]
- 245.Zhao Y, Chaiswing L, Velez JM, Batinic-Haberle I, Colburn NH, Oberley TD, and St. Clair DK. p53 translocation to mitochondria precedes its nuclear translocation and targets mitochondrial oxidative defense protein-manganese superoxide dismutase. Cancer Res 65: 3745–3750, 2005 [DOI] [PubMed] [Google Scholar]
- 246.Zhestianikov VD, Janovska E, Savel'eva GE, and Brabec V. [Effect of gamma radiation, cis-diamminedichloroplatinum and its derivates on the Escherichia coli cell survival and potentiality for adaptive response]. Tsitologiia 43: 1067–1074, 2001. [Article in Russian] [PubMed] [Google Scholar]
- 247.Zhou PK, Liu XY, Sun WZ, Zhang YP, and Wei K. Cultured mouse SR-1 cells exposed to low dose of gamma-rays become less susceptible to the induction of mutagenesis by radiation as well as bleomycin. Mutagenesis 8: 109–111, 1993 [DOI] [PubMed] [Google Scholar]
- 248.Zhu D, Dix DJ, and Eddy EM. HSP70–2 is required for CDC2 kinase activity in meiosis I of mouse spermatocytes. Development 124: 3007–3014, 1997 [DOI] [PubMed] [Google Scholar]
- 249.Zhu Y, Park SH, Ozden O, Kim HS, Jiang H, Vassilopoulos A, Spitz DR, and Gius D. Exploring the electrostatic repulsion model in the role of Sirt3 in directing MnSOD acetylation status and enzymatic activity. Free Radic Biol Med 53: 828–833, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]