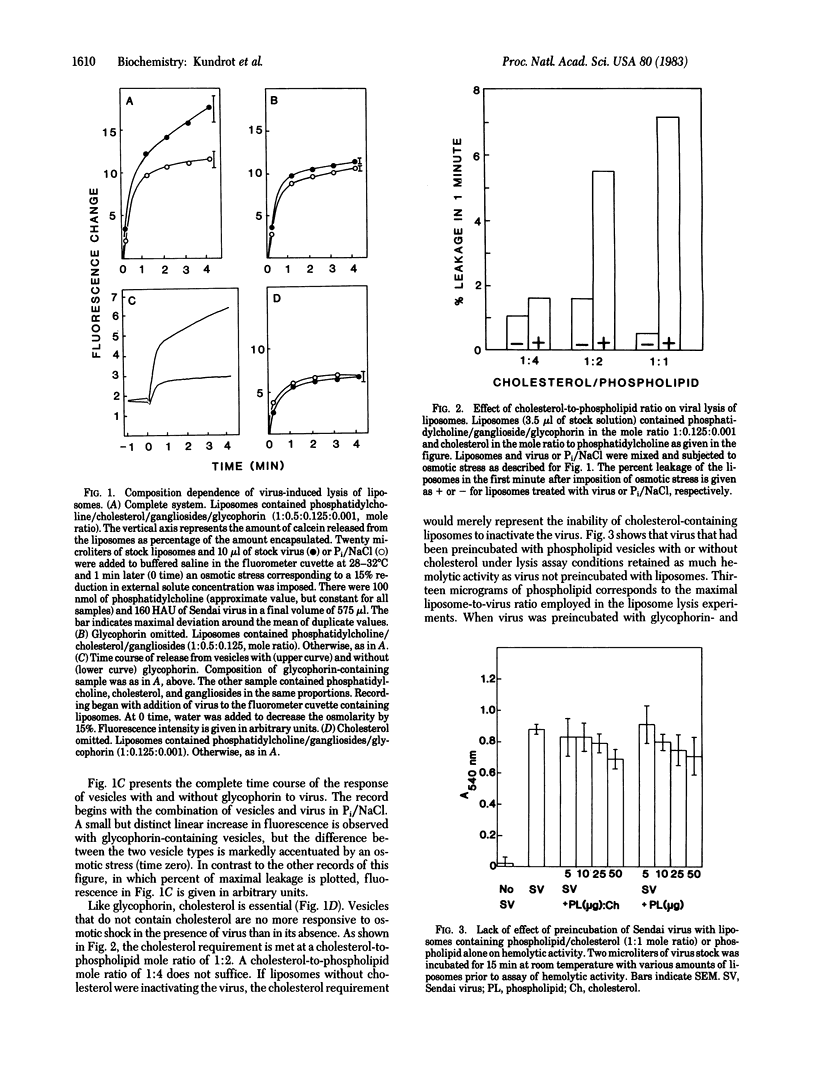
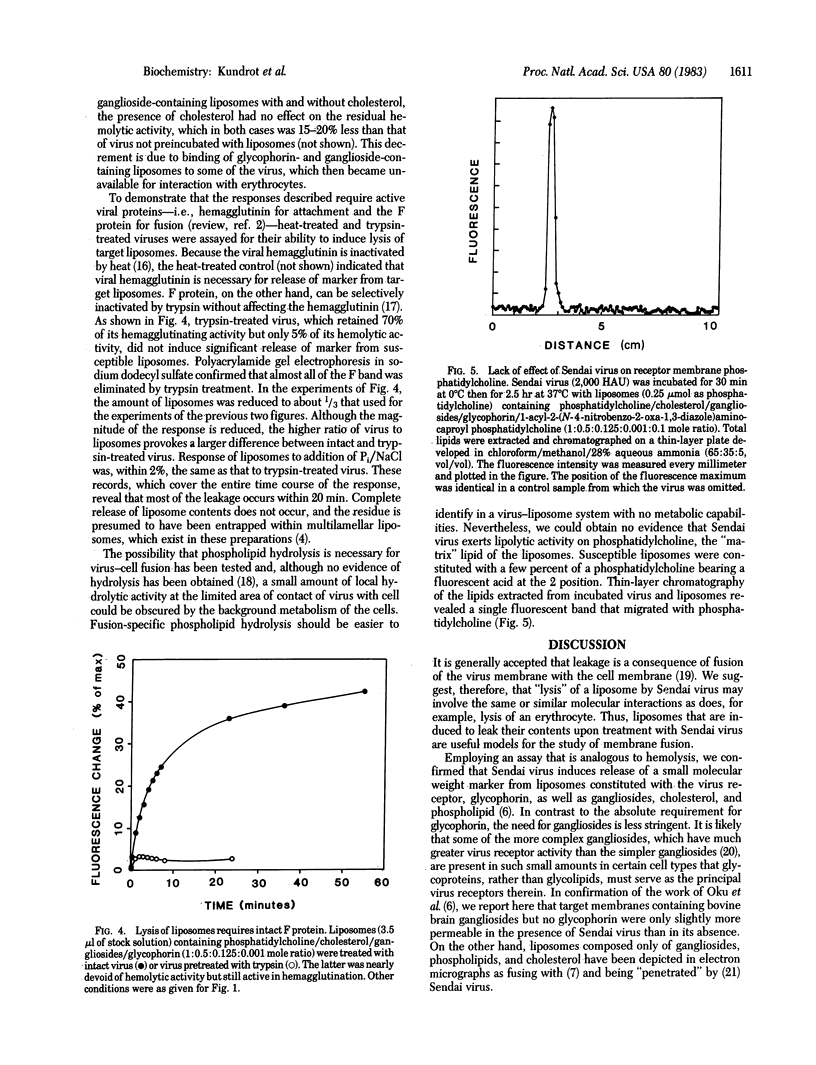
Abstract
Vesicles were constituted with glycophorin, the Sendai virus receptor of human erythrocytes, and loaded with calcein, a polar derivative of fluorescein, at self-quenching concentrations. On exposure to Sendai virus and mild hypo-osmotic stress, vesicles of the appropriate composition released a significant portion of their internal contents, as indicated by an increase in calcein fluorescence. Susceptible liposomes were not induced to leak by heat-inactivated virus or by trypsin-treated virus. The response of the vesicles to virus attachment is thus analogous to virus-induced hemolysis and presumably involves fusion of the vesicle and virus membranes. In addition to glycophorin and phosphatidylcholine, cholesterol was absolutely required for the lytic response to the virus. The need for cholesterol was not attributable to inactivation of the virus by liposomes without cholesterol. The presence of gangliosides increased the encapsulated volume of the liposomes, but gangliosides did not effectively substitute for glycophorin. Thin-layer chromatography of lipid extracted from incubated virus and liposomes containing a small amount of a fluorescent phosphatidylcholine indicated that phosphatidylcholine in the vesicle is not chemically altered by functional interaction with the virus.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen T. M., Cleland L. G. Serum-induced leakage of liposome contents. Biochim Biophys Acta. 1980 Apr 10;597(2):418–426. doi: 10.1016/0005-2736(80)90118-2. [DOI] [PubMed] [Google Scholar]
- Aloj S. M., Lee G., Consiglio E., Formisano S., Minton A. P., Kohn L. D. Dansylated thyrotropin as a probe of hormone-receptor interactions. J Biol Chem. 1979 Sep 25;254(18):9030–9039. [PubMed] [Google Scholar]
- Brûlet P., McConnell H. M. Structural and dynamical aspects of membrane immunochemistry using model membranes. Biochemistry. 1977 Mar 22;16(6):1209–1217. doi: 10.1021/bi00625a028. [DOI] [PubMed] [Google Scholar]
- Cohen F. S., Akabas M. H., Finkelstein A. Osmotic swelling of phospholipid vesicles causes them to fuse with a planar phospholipid bilayer membrane. Science. 1982 Jul 30;217(4558):458–460. doi: 10.1126/science.6283637. [DOI] [PubMed] [Google Scholar]
- De Wolf M. J., Fridkin M., Kohn L. D. Tryptophan residues of cholera toxin and its A and B protomers. Intrinsic fluorescence and solute quenching upon interacting with the ganglioside GM1, oligo-GM1, or dansylated oligo-GM1. J Biol Chem. 1981 Jun 10;256(11):5489–5496. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- GAMMACK D. B. PHYSICOCHEMICAL PROPERTIES OF OX-BRAIN GANGLIOSIDES. Biochem J. 1963 Aug;88:373–383. doi: 10.1042/bj0880373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haywood A. M., Boyer B. P. Initiation of fusion and disassembly of Sendai virus membranes into liposomes. Biochim Biophys Acta. 1981 Aug 6;646(1):31–35. doi: 10.1016/0005-2736(81)90268-6. [DOI] [PubMed] [Google Scholar]
- Haywood A. M. Characteristics of Sendai virus receptors in a model membrane. J Mol Biol. 1974 Mar 15;83(4):427–436. doi: 10.1016/0022-2836(74)90504-x. [DOI] [PubMed] [Google Scholar]
- Haywood A. M. Letter to the editor: Fusion of Sendai viruses with model membranes. J Mol Biol. 1974 Aug 15;87(3):625–628. doi: 10.1016/0022-2836(74)90107-7. [DOI] [PubMed] [Google Scholar]
- Homma M., Shimizu K., Shimizu Y. K., Ishida N. On the study of Sendai virus hemolysis. I. Complete Sendai virus lacking in hemolytic activity. Virology. 1976 May;71(1):41–47. doi: 10.1016/0042-6822(76)90092-1. [DOI] [PubMed] [Google Scholar]
- Hope M. J., Bruckdorfer K. R., Hart C. A., Lucy J. A. Membrane cholesterol and cell fusion of hen and guinea-pig erythrocytes. Biochem J. 1977 Aug 15;166(2):255–263. doi: 10.1042/bj1660255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenard J., Miller D. K. Uncoating of enveloped viruses. Cell. 1982 Jan;28(1):5–6. doi: 10.1016/0092-8674(82)90368-3. [DOI] [PubMed] [Google Scholar]
- Lenard J., Rothman J. E. Transbilayer distribution and movement of cholesterol and phospholipid in the membrane of influenza virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):391–395. doi: 10.1073/pnas.73.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi V. T., Andrews E. P. Glycoproteins: isolation from cellmembranes with lithium diiodosalicylate. Science. 1971 Dec 17;174(4015):1247–1248. doi: 10.1126/science.174.4015.1247. [DOI] [PubMed] [Google Scholar]
- Miller C., Arvan P., Telford J. N., Racker E. Ca++-induced fusion of proteoliposomes: dependence on transmembrane osmotic gradient. J Membr Biol. 1976;30(3):271–282. doi: 10.1007/BF01869672. [DOI] [PubMed] [Google Scholar]
- Mooney J. J., Dalrymple J. M., Alving C. R., Russell P. K. Interaction of Sindbis virus with liposomal model membranes. J Virol. 1975 Feb;15(2):225–231. doi: 10.1128/jvi.15.2.225-231.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore N. F., Patzer E. J., Shaw J. M., Thompson T. E., Wagner R. R. Interaction of vesicular stomatitis virus with lipid vesicles: depletion of cholesterol and effect on virion membrane fluidity and infectivity. J Virol. 1978 Aug;27(2):320–329. doi: 10.1128/jvi.27.2.320-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada Y., Murayama F., Yamada K. Requirement of energy for the cell fusion reaction of Ehrlich ascites tumor cells by HVJ. Virology. 1966 Jan;28(1):115–130. doi: 10.1016/0042-6822(66)90312-6. [DOI] [PubMed] [Google Scholar]
- Oku N., Inoue K., Nojima S., Sekiya T., Nozawa Y. Electron microscopic study on the interaction of Sendai virus with liposomes containing glycophorin. Biochim Biophys Acta. 1982 Sep 24;691(1):91–96. doi: 10.1016/0005-2736(82)90217-6. [DOI] [PubMed] [Google Scholar]
- Oku N., Nojima S., Inoue K. Studies on the interaction of HVJ (Sendai Virus) with liposomal membranes. HVJ-induced permeability increase of liposomes containing glycophorin. Virology. 1982 Jan 30;116(2):419–427. doi: 10.1016/0042-6822(82)90136-2. [DOI] [PubMed] [Google Scholar]
- Oku N., Nojima S., Inoue K. Studies on the interaction of Sendai virus with liposomal membranes. Sendai virus-induced agglutination of liposomes containing glycophorin. Biochim Biophys Acta. 1981 Aug 6;646(1):36–42. doi: 10.1016/0005-2736(81)90269-8. [DOI] [PubMed] [Google Scholar]
- Pasternak C. A., Micklem K. J. The biochemistry of virus-induced cell fusion. Changes in membrane integrity. Biochem J. 1974 Jun;140(3):405–411. doi: 10.1042/bj1400405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patzer E. J., Wagner R. R., Dubovi E. J. Viral membranes: model systems for studying biological membranes. CRC Crit Rev Biochem. 1979;6(2):165–217. doi: 10.3109/10409237909102563. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Isida N. The smallest protein of Sendi virus: its candidate function of binding nucleocaspsid to envelope. Virology. 1975 Oct;67(2):427–437. [PubMed] [Google Scholar]
- Tozawa H., Watanabe M., Ishida N. Structural components of Sendai virus. Serological and physicochemical characterization of hemagglutinin subunit associated with neuraminidase activity. Virology. 1973 Sep;55(1):242–253. doi: 10.1016/s0042-6822(73)81027-x. [DOI] [PubMed] [Google Scholar]
- White J., Helenius A. pH-dependent fusion between the Semliki Forest virus membrane and liposomes. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3273–3277. doi: 10.1073/pnas.77.6.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]


