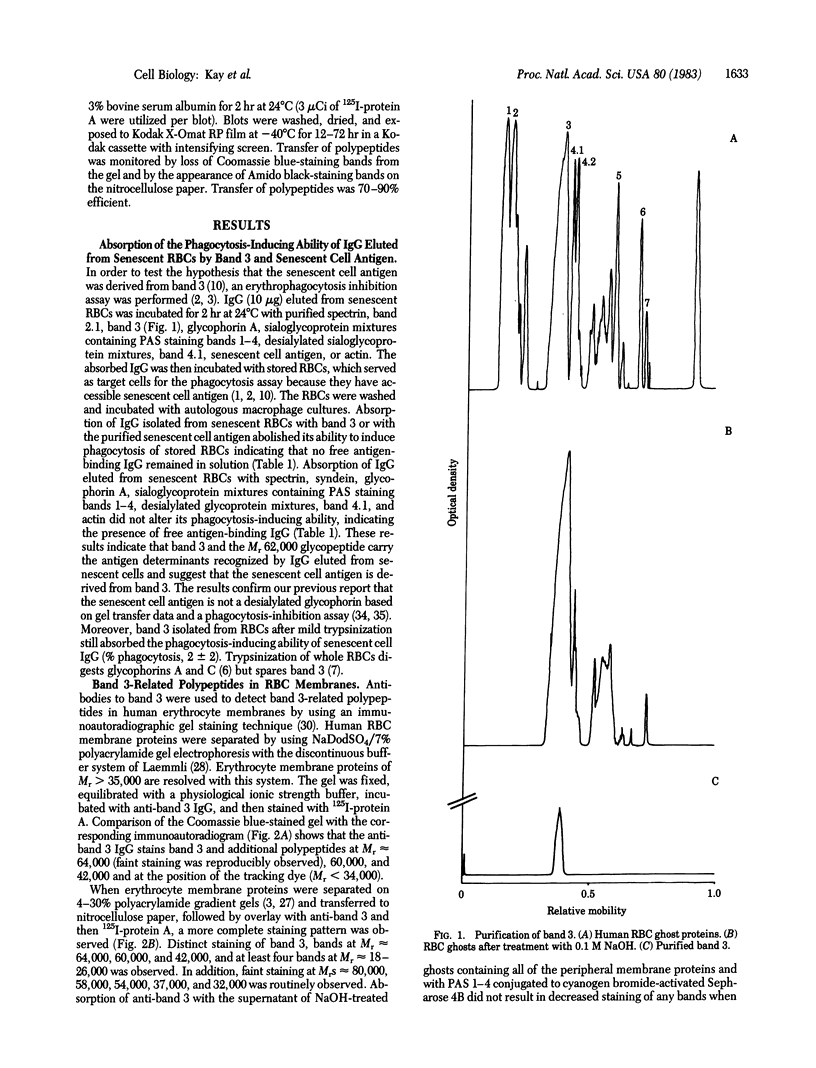
Abstract
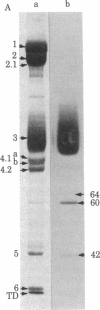
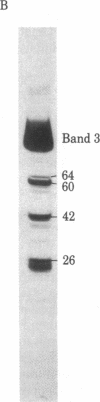
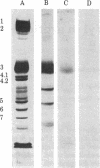
IgG autoantibodies in human serum selectively bind to a glycopeptide antigen that appears on senescent and damaged cells in situ. We identified the membrane protein from which the senescent cell antigen is derived by using a phagocytosis-inhibition assay and immunoautoradiographic gel staining and electroblotting techniques. Results of the phagocytosis-inhibition assay revealed that only the purified transmembrane glycoprotein designated "band 3" and senescent cell antigen inhibited the phagocytosis of erythrocytes induced by IgG eluted from senescent erythrocytes. Purified spectrin, syndein, band 4.1, actin, glycophorin A, and intact or desialylated sialoglycoprotein periodic acid/Schiff (PAS) staining bands 1-4 containing glycophorins A, B, and C did not inhibit phagocytosis. Specific antibodies against the senescent cell antigen and erythrocyte band 3 were used to identify the membrane protein from which the senescent cell antigen is derived. Band 3-related polypeptides (MrS approximately equal to 60,000, 42,000, and 18-26,000) were identified in erythrocyte ghosts prepared in the presence of diisopropyl fluorophosphate, phenylmethylsulfonyl fluoride, and EDTA by immunoautoradiography with antiband 3. Antibodies to senescent cell antigen reacted with band 3 and the same lower Mr band 3-related polypeptides. Thus, the senescent cell antigen is immunologically related to band 3.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S. Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry. 1969 Jan;6(1):43–52. doi: 10.1016/0019-2791(69)90177-3. [DOI] [PubMed] [Google Scholar]
- Bennett G. D., Kay M. M. Homeostatic removal of senescent murine erythrocytes by splenic macrophages. Exp Hematol. 1981 Mar;9(3):297–307. [PubMed] [Google Scholar]
- Bennett V., Branton D. Selective association of spectrin with the cytoplasmic surface of human erythrocyte plasma membranes. Quantitative determination with purified (32P)spectrin. J Biol Chem. 1977 Apr 25;252(8):2753–2763. [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. The membrane attachment protein for spectrin is associated with band 3 in human erythrocyte membranes. Nature. 1979 Aug 9;280(5722):468–473. doi: 10.1038/280468a0. [DOI] [PubMed] [Google Scholar]
- Beutler E., West C., Blume K. G. The removal of leukocytes and platelets from whole blood. J Lab Clin Med. 1976 Aug;88(2):328–333. [PubMed] [Google Scholar]
- Cabantchik Z. I., Rothstein A. Membrane proteins related to anion permeability of human red blood cells. I. Localization of disulfonic stilbene binding sites in proteins involved in permeation. J Membr Biol. 1974;15(3):207–226. doi: 10.1007/BF01870088. [DOI] [PubMed] [Google Scholar]
- England B. J., Gunn R. B., Steck T. L. An immunological study of band 3, the anion transport protein of the human red blood cell membrane. Biochim Biophys Acta. 1980 May 29;623(1):171–182. doi: 10.1016/0005-2795(80)90019-7. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Furthmayr H. Glycophorins A, B, and C: a family of sialoglycoproteins. Isolation and preliminary characterization of trypsin derived peptides. J Supramol Struct. 1978;9(1):79–95. doi: 10.1002/jss.400090109. [DOI] [PubMed] [Google Scholar]
- Goodman S. R., Shiffer K. A., Casoria L. A., Eyster M. E. Identification of the molecular defect in the erythrocyte membrane skeleton of some kindreds with hereditary spherocytosis. Blood. 1982 Sep;60(3):772–784. [PubMed] [Google Scholar]
- Goodman S. R., Weidner S. A. Binding of spectrin alpha 2-beta 2 tetramers to human erythrocyte membranes. J Biol Chem. 1980 Sep 10;255(17):8082–8086. [PubMed] [Google Scholar]
- Goodman S. R., Yu J., Whitfield C. F., Culp E. N., Posnak E. J. Erythrocyte membrane skeletal protein bands 4.1 a and b are sequence-related phosphoproteins. J Biol Chem. 1982 Apr 25;257(8):4564–4569. [PubMed] [Google Scholar]
- Granger B. L., Lazarides E. Synemin: a new high molecular weight protein associated with desmin and vimentin filaments in muscle. Cell. 1980 Dec;22(3):727–738. doi: 10.1016/0092-8674(80)90549-8. [DOI] [PubMed] [Google Scholar]
- Jain S. K., Shohet S. B. Calcium potentiates the peroxidation of erythrocyte membrane lipids. Biochim Biophys Acta. 1981 Mar 20;642(1):46–54. doi: 10.1016/0005-2736(81)90136-x. [DOI] [PubMed] [Google Scholar]
- Karadsheh N. S., Uyeda K. Changes in allosteric properties of phosphofructokinase bound to erythrocyte membranes. J Biol Chem. 1977 Nov 10;252(21):7418–7420. [PubMed] [Google Scholar]
- Kay M. M., Bennett G. D. Isolation and characterization of a senescent cell antigen. Blood. 1982 May;59(5):1111–1112. [PubMed] [Google Scholar]
- Kay M. M. Isolation of the phagocytosis-inducing IgG-binding antigen on senescent somatic cells. Nature. 1981 Feb 5;289(5797):491–494. doi: 10.1038/289491a0. [DOI] [PubMed] [Google Scholar]
- Kay M. M. Mechanism of removal of senescent cells by human macrophages in situ. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3521–3525. doi: 10.1073/pnas.72.9.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay M. M. Multiple labelling technique used for kinetic studies of activated human B lymphocytes. Nature. 1975 Apr 3;254(5499):424–426. doi: 10.1038/254424a0. [DOI] [PubMed] [Google Scholar]
- Kay M. M. Role of physiologic autoantibody in the removal of senescent human red cells. J Supramol Struct. 1978;9(4):555–567. doi: 10.1002/jss.400090409. [DOI] [PubMed] [Google Scholar]
- Kliman H. J., Steck T. L. Association of glyceraldehyde-3-phosphate dehydrogenase with the human red cell membrane. A kinetic analysis. J Biol Chem. 1980 Jul 10;255(13):6314–6321. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lepke S., Fasold H., Pring M., Passow H. A study of the relationship between inhibition of anion exchange and binding to the red blood cell membrane of 4,4'-diisothiocyano stilbene-2,2'-disulfonic acid (DIDS) and its dihydro derivative (H2DIDS). J Membr Biol. 1976 Oct 20;29(1-2):147–177. doi: 10.1007/BF01868957. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Andrews E. P. Glycoproteins: isolation from cellmembranes with lithium diiodosalicylate. Science. 1971 Dec 17;174(4015):1247–1248. doi: 10.1126/science.174.4015.1247. [DOI] [PubMed] [Google Scholar]
- Nakashima K., Beutler E. Comparison of structure and function of human erythrocyte and human muscle actin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):935–938. doi: 10.1073/pnas.76.2.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prohaska R., Koerner T. A., Jr, Armitage I. M., Furthmayr H. Chemical and carbon-13 nuclear magnetic resonance studies of the blood group M and N active sialoglycopeptides from human glycophorin A. J Biol Chem. 1981 Jun 10;256(11):5781–5791. [PubMed] [Google Scholar]
- Salhany J. M., Shaklai N. Functional properties of human hemoglobin bound to the erythrocyte membrane. Biochemistry. 1979 Mar 6;18(5):893–899. doi: 10.1021/bi00572a025. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Ramos B., Strapazon E. Proteolytic dissection of band 3, the predominant transmembrane polypeptide of the human erythrocyte membrane. Biochemistry. 1976 Mar 9;15(5):1153–1161. doi: 10.1021/bi00650a030. [DOI] [PubMed] [Google Scholar]
- Steck T. L. The organization of proteins in the human red blood cell membrane. A review. J Cell Biol. 1974 Jul;62(1):1–19. doi: 10.1083/jcb.62.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strapazon E., Steck T. L. Interaction of the aldolase and the membrane of human erythrocytes. Biochemistry. 1977 Jun 28;16(13):2966–2971. doi: 10.1021/bi00632a025. [DOI] [PubMed] [Google Scholar]
- Tanner M. J., Boxer D. H. Separation and some properties of the major proteins of the human erythrocyte membrane. Biochem J. 1972 Sep;129(2):333–347. doi: 10.1042/bj1290333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler J. M., Hargreaves W. R., Branton D. Purification of two spectrin-binding proteins: biochemical and electron microscopic evidence for site-specific reassociation between spectrin and bands 2.1 and 4.1. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5192–5196. doi: 10.1073/pnas.76.10.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Goodman S. R. Syndeins: the spectrin-binding protein(s) of the human erythrocyte membrane. Proc Natl Acad Sci U S A. 1979 May;76(5):2340–2344. doi: 10.1073/pnas.76.5.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Steck T. L. Isolation and characterization of band 3, the predominant polypeptide of the human erythrocyte membrane. J Biol Chem. 1975 Dec 10;250(23):9170–9175. [PubMed] [Google Scholar]