Abstract
OBJECTIVE
To review the original surgical records from the Johns Hopkins Hospital, and analyze the records of patients Cushing treated for pituitary disorders from 1896 to 1912.
METHODS
Following IRB approval, and through the courtesy of the Alan Mason Chesney Archives, we reviewed the original surgical files from the Johns Hopkins Hospital. Patients presenting with pituitary-related symptoms, who underwent surgical treatment directed at the pituitary gland, were selected for further review.
RESULTS
Thirty-seven patients who underwent surgical intervention for pituitary disorders were found. Of these patients, 12 were mentioned only briefly in Cushing’s 1912 monograph, whereas 6 were not described at all. The remaining 19 were documented by Cushing in his 1912 monograph. Cushing used three main surgical approaches to the pituitary: transsphenoidal, transcranial, and the subfrontal “omega incision.” There were 6 inpatient deaths. The mean time to last follow-up was 41.0 months. At follow-up, headache was the most common unresolved symptom.
CONCLUSION
This review highlights Cushing’s accomplishments in the surgical treatment of suspected pituitary pathology during his early career as a young attending at Johns Hopkins Hospital. It reveals new information about patients whom Cushing did not include in his publications detailing his surgical experience at the Johns Hopkins Hospital.
Keywords: Harvey Cushing, Pituitary
INTRODUCTION
Although the varied and important roles played by the pituitary are firmly established in contemporary medicine, in the 19th century, the field of endocrinology was in its infancy. Indeed, while the roles of the pancreas (3, 44) and thyroid (33) were described by the late 19th century, it was not until the early 20th century that the physiologic (1, 8, 9, 15, 24, 29, 41) and pathophysiologic roles (4, 13, 11, 24, 30-32) of the pituitary gland began to be understood.
In this setting of new knowledge, surgical intervention for suspected pituitary disorders became a rapidly expanding field Operative interventions involved various approaches and techniques, but they were generally classified as either transcranial or transsphenoidal operations. Transcranial approaches appeared first, with F. T. Paul and Caton publishing the first report of such a procedure in 1893 (6). The approach was refined and expanded by Sir Victor Horsley, who published in 1906 the results of a series of 13 patients (23). At Johns Hopkins Hospital, Cushing began surgical treatment of the pituitary in 1905, with a transcranial approach. As surgeons became more experienced with pituitary procedures, the pendulum swung toward transsphenoidal approaches, with the first such operation reported in 1907 by Schloffer (40). This approach became popular among the European neurosurgeons (17, 22, 26, 38, 43), and made its way across the Atlantic to Baltimore, where Cushing performed a number of similar procedures.
Although many surgeons attempted their first operations on acromegalic patients (24), Cushing’s first surgical intervention for a pituitary disorder was a series of three palliative decompressive operations, performed on a 16-year-old woman between February and March of 1902 (12). Although this case was described in Cushing’s 1912 monograph as Case III (12), the patient record was not available in the archived surgical records. The first surgical record recovered, documenting intervention for a pituitary disorder, was atranscranial approach with bilateral bone flaps, performed in 1905 on a 26-year-old with a chief complaint of headache, without signs of acromegaly. This case was described as Case XIV, and described as “chromophobe struma” (12). Cushing later operated on two acromegalic patients, through a subfrontal approach, using what he described as the “omega incision” (34).
Cushing was instrumental in popularizing the transsphenoidal approach (14, 24); however, Cushing began advocating for a transcranial approach in the late 1920s (10, 24, 39), leading other neurosurgeons of the day to abandon the transsphenoidal approach. Although Oscar Hirsch and Norman Dott, among other neurosurgeons, continued to employ the transsphenoidal approach, it was not until the 1960s that the transsphenoidal approach to the pituitary began to regain popularity (2, 5, 18-20, 24, 28). The transsphenoidal approach allowed surgeons to perform resections of hormonally active adenomas, in patients who were otherwise too ill to undergo more-invasive or open techniques without significant morbidity and mortality (24).
In his 1912 monograph The Pituitary Body and Its Disorders, Cushing described 52 patients with symptoms referable to the pituitary. This selection included patients who did not undergo surgical treatment, patients who underwent surgical treatment unrelated to their pituitary disorder, and patients with symptoms Cushing attributed to nonpituitary causes (hydrocephalus, pineal tumors). Cushing’s monograph does not include operative details for many patients treated at the end of 1911 through 1912.
The original microfilm revealed operative details for 12 primary surgical interventions occurring between September 1, 1911, and February 1, 1912, as well as six primary surgical interventions occurring after February 1, 1912. The series of 37 cases described here offered insight into Cushing’s surgical innovations (34, 36), as well as his use of postmortem exams in defining the pathophysiology of pituitary disorders (35). This review of Cushing’s surgical treatment of pituitary disorders while at Johns Hopkins Hospital outlines the operative approaches he favored, documents the immediate postoperative and long-term outcomes for patients, and offers new information that complements the extensive publications prepared by Cushing himself.
MATERIALS AND METHODS
Following IRB approval, and through the courtesy of the Alan Mason Chesney Archives, we reviewed the Johns Hopkins Hospital surgical files from 1896 to 1912; approximately 26,000 surgical interventions were reviewed. All neurosurgical cases performed by Cushing, and a selection of nonneurosurgical cases performed by Cushing, were further analyzed. This review uncovered 37 patients whom Cushing treated with surgical intervention directed at the pituitary gland.
The series does not include all patients Cushing documented who met the inclusion criteria described above. The absence of these files is unfortunate, and is secondary to some patient files not being included in the original microfilm, as well as the microfilm archived records being badly damaged in some parts. In determining out-come of the patients, the condition at the time of discharge as documented in the surgical record was used verbatim. Other out-comes measures were length of hospital stay, time to last follow-up, symptoms during follow-up, and time to death. Patient death was determined using written correspondence and other documentation in the surgical chart. Symptoms during follow-up were culled from written correspondence between the patients and Cushing, contained in the charts; therefore, not all possible symptoms were documented for each patient during follow-up.
RESULTS
Patient Characteristics
Of the 37 patients identified in this study, 19 (51%) were female. The mean age was 35.5 years, ranging from 14 to 56 (Table 1). The most common presenting symptoms were headache (81%); vision changes (87%); and changes in mental status, including somnolence, confusion, and memory loss (46%) (Table 2).
Table 1.
Baseline Characteristics at Presentation, Primary Intervention Approach, and Follow-Up Data for Patients Treated for Suspected Pituitary Pathology by Dr. Harvey Cushing at the Johns Hopkins Hospital, 1896 to 1912
| Number (%) of Patients | Mean ± SD | |
|---|---|---|
| Total patients | 37 | |
| Gender | ||
| Male | 18(49) | |
| Female | 19(51) | |
| Age (years) | 35.5 ± 10.0 | |
| Range: 14-56 | ||
| Length of stay (days) | 41.9 ± 34.0 | |
| Range: 6-122 | ||
| Primary intervention approach | ||
| Transphenoidal | 25(68) | |
| Transcranial | 10(27) | |
| Omega incision | 2(5.4) | |
| Outcome at discharge | ||
| Well/improved | 18(49) | |
| Unimproved | 13(35) | |
| Dead | 6(16) | |
| Follow-up time (months) | ||
| Time to last follow-up | 41.0 ± 74.9 | |
| Range: 0-325 | ||
| Time to death | 18.4 ± 27.2 | |
| Range: 0-93 | ||
| Follow-up outcomes | ||
| Well/improved | 9(31) | |
| Unimproved | 7(24) | |
| Dead | 13(45) |
Table 2.
Symptoms at Initial Presentation and During Follow-Up for Patients Undergoing Surgical Intervention for Suspected Pituitary Pathology by Dr. Harvey Cushing at the Johns Hopkins Hospital, 1896 to 1912
| Number (%) of Patients | Number (%) of Patients Who Reported About Symptom at Follow-Up | |||
|---|---|---|---|---|
| Symptoms | At Presentation | Resolved at Follow-Up | Remained at Follow-Up | New Symptom at Follow-Up |
| Headache | 30(81) | 3(20) | 12(80) | 0(0) |
| Vision changes | 32 (87) | 8(44) | 9(50) | 1 (5.6) |
| Polyuria | 10(27) | 1 (33) | 2(67) | 0(0) |
| Polydipsia | 1 (2.7) | 0(0) | 0(0) | 0(0) |
| Weight gain | 16(43) | 1 (17) | 4(67) | 1 (17) |
| Constipation | 14(38) | 0(0) | 0(0) | 1 (17) |
| Decreased libido | 8 (44)* | 1 (25) | 2(50) | 1 (25) |
| Amenorrhea | 8 (42)† | 2(67) | 1 (33) | 0(0) |
| Dry skin/brittle nails | 10(27) | 1 (20) | 1 (20) | 3(60) |
| Changed mental status | 17(46) | 1 (11) | 7(78) | 1 (11) |
| Cold intolerance | 5(14) | 1 (50) | 1 (50) | 0(0) |
| Heat intolerance | 2 (5.4) | 0(0) | 0(0) | 0(0) |
| Acromegaly | 10(27) | 0(0) | 6(100) | 0(0) |
Percentages are given as a total of the appropriate population for each symptom.
Data collected only in male patients.
Data collected only in female patients.
Surgical Approaches
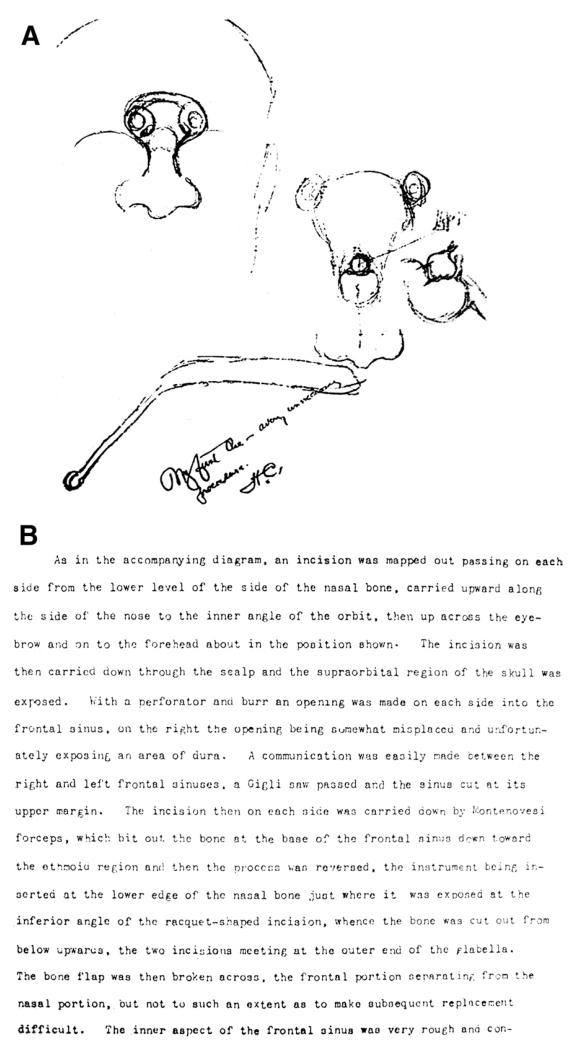
Records reveal that Dr. Cushing used three main operative approaches for patients with suspected pituitary lesions during his time at Hopkins: transsphenoidal, transcranial, and the omega incision (Table 1). The omega incision (Figure 1) was used by Cushing solely in the treatment of acromegalic patients and has been described in detail elsewhere (7, 34).
Figure 1.
(A) Cushing’s operative illustration of the “omega incision” (previously published in Pituitary) (B) Cushing’s operative note describing the omega incision.
Outcomes
The mean hospital stay was 41.9 days, ranging from 6 to 122 days; the longest mean hospitalization was found in patients undergoing transcranial approaches (Table 3). However, patients undergoing multiple operations at the time of the first operative intervention had an increased length of stay (87.9 days) compared to patients undergoing a single operation at the time of the first operative intervention (31.7 days). At the time of discharge, 49% of patients were documented in the surgical records as doing well or having improvement of their condition. There were 6 inpatient deaths (16%) among 37 treated patients, compared to Cushing’s monograph, which documents 5 deaths in 43 patients undergoing operative treatment. The mean time to death, calculated from the date of operation, and including inpatient and outpatient deaths, was 18.4 months, ranging from 0 to 33 months. Moreover, one patient in whom Cushing documented no complications was documented in her surgical chart as having a serious postoperative complication: blindness.
Table 3.
Postoperative and Follow-Up Outcomes for Patients Undergoing Primary Surgical Intervention for Suspected Pituitary Pathology, by Dr. Harvey Cushing at the Johns Hopkins Hospital, 1896 to 1912
| Number (%) of Patients | Mean ± SD | |
|---|---|---|
| Transsphenoidal | 25 | |
| Hospital stay (days) | 34.8 ± 10.0 | |
| Outcome | ||
| “Well/Improved” | 16(64) | |
| “Not improved” | 5(20) | |
| “Dead” | 4(16) | |
| Time to death (months) | 18.7 ± 24.8 | |
| Time to last follow-up (months) | 37.0 ± 62.7 | |
| Outcome at last follow-up | ||
| “Well/Improved” | 9(47) | |
| “Not Improved” | 1 (5.3) | |
| “Dead” | 9(48) | |
| Transcranial | 10 | |
| Hospital stay (days) | 47.9 ± 45.5 | |
| Outcome | ||
| “Well/Improved” | 0(0) | |
| “Not improved” | 8(80) | |
| “Dead” | 2(20) | |
| Time to death (months) | 19.5 ± 36.1 | |
| Time to last follow-up (months) | 69.4 ± 110 | |
| Outcome at last follow-up | ||
| “Well/Improved” | 0(0) | |
| “Not Improved” | 5(63) | |
| “Dead” | 3(38) | |
| “Omega Incision” | 2 | |
| Hospital stay (days) | 26.0 ± 1.4 | |
| Outcome | ||
| “Well/Improved” | 2(100) | |
| “Not improved” | 0(0) | |
| “Dead” | 0(0) | |
| Time to death (months) | 6.5 | |
| Time to last follow-up (months) | 86.4 ± 114 | |
| Outcome at last follow-up | ||
| “Well/Improved” | 0(0) | |
| “Not Improved” | 1 (50) | |
| “Dead” | 1 (50) |
Patients followed up with Cushing via written correspondence, which was filed in the surgical records. The mean time to last follow-up was 41.0 months, ranging from 0.4 to 325 months (Table 3). At follow-up, headache was the most common unresolved symptom (80%); changes in mental status (78%), polyuria (67%), and weight gain (67%) were other commonly unresolved symptoms (Table 2).
CASE ILLUSTRATIONS
Transsphenoidal
Case 1.
On July 10, 1910, a 24-year-old woman, working as a bookkeeper in Baltimore, presented to Johns Hopkins Hospital complaining of suboccipital headaches, recent weight gain, amenorrhea for 2 years, dry skin, and chronic otitis media. She was diagnosed with hypopituitarism, treated with 3 g of whole gland pituitary powder, and discharged without surgical intervention, on July 18, 1910.
The patient returned on October 17, 1910, complaining of the same symptoms as during her previous admission. Cushing took her to the operating room on October 26, 1910, for a “mastoid operation for chronic otitis media” (Figure 2). Her postoperative course was, again, uneventful, and she was discharged in improved condition on post-operative day 8. She returned on January 19, 1911, and was again treated with whole gland pituitary extract.
Figure 2.

Cushing’s handwritten note documenting the reasons for the mastoid operation in Transsphenoidal Case 1. “It was conjectured that the chronic suppurative process might bear some relation to the ductless gland disturbance. Hence the operation. HC.”
On October 19, 1911, she returned to Johns Hopkins Hospital complaining of failing vision, amenorrhea, and headaches (Figure 3). The history was negative for constipation, polyuria, vomiting, and jaundice; it was positive for a weight gain of 68 pounds in 6 months, choking sensation in her throat, pain in the eyes, partial deafness, syncope, vertigo, irritability, drowsiness, dry skin, alopecia, brittle nails, and epistaxis. The surgical record does not document an x-ray of the sella, although Cushing’s monograph indicates that a number of skiagrams were taken between January 1911 and October 1911, which demonstrated growth of the sella (12). Cushing brought her to the operating room on October 25, 1911, for a sellar decompression. His operative note describes the approach:
Blood removed for sugar test after primary anaesthesia. Patient some-what cyanosed at the time which may affect the sugar percentage.
Figure 3.
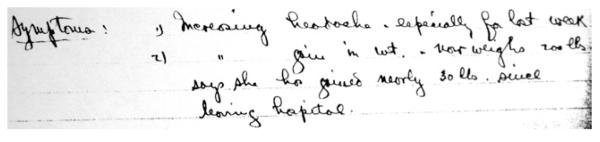
Cushing’s handwritten note documenting presenting symptoms for Transsphenoidal Case 1, at the time of her third admission. Reads: “1) Increasing headache, especially for last week. 2) [increasing] gain in wt. now weighs 200 lbs says she has gained nearly 30 lbs since leaving hospital.”
Usual approach to the hypophysis under the lobe. A simple easy case. Large synovial cells opened and bulging floor of sella seen. Bone of paper thinness, chipped away, exposing a larger gland than usual. Excellent view obtained. Dura was split in two directions and two minute fragments of gland removed for histological study. No bleeding.
No pathology report was available, although Cushing’s monograph documented “chromophobe struma.” The patient had an uneventful postoperative course, and was “well” when discharged on November 2, 1911.
Case 2.
On October 4, 1911, a 43-year-old housewife presented to Johns Hopkins Hospital complaining of headaches, loss of vision (Figure 4), tinnitus, deafness, and amenorrhea for 16 years. Cushing brought her to the operating room on October 12, 1911, and his operative note offers details regarding the transsphenoidal approach he employed:
After the usual preparation a sub labial incision was made: then as heretofore in the later cases, the mucous membrane was separated from the septum on either side of the synovial region. The septum was then taken out in its lower half including the vomer. The operation thus far was carried on without difficulty. The synovial cells were then rongeured away and it became evident that there was a bulging tumor in the synovial region. The floor of the sella was practically taken away. The dura was incised and a soft strumous tumor began to extrude itself. The dura was opened widely. Small pieces of tumor were taken out for histological study. Mucous membrane flaps were then replaced, two catgut sutures were taken in the sublabial incision. Vaseline and cotton plugs were placed in each nostril.
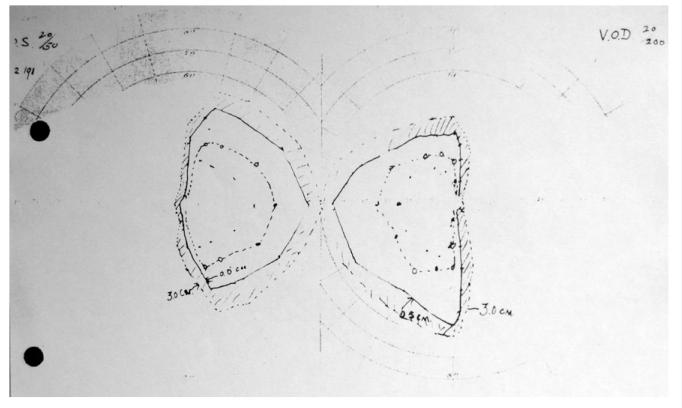
Figure 4.
Visual fields taken postoperatively from Transsphenoidal Case 2, the accompanying note describes “bitemporal hemianopsia still present, some improvement possibly in fields.”
The patient experienced severe nausea and vomiting immediately postoperatively, but was discharged in improved condition on November 1, 1911. A letter from Dr. D.A. Flexner, the patient’s physician, dated December 20, 1911, states that the patient died on that date, following a brief illness:
I regret to tell you that Mrs. [name omitted here for privacy] died this morning. I was called back there a week ago after she had been ill for three days, but the progress downwards could not be stopped …. She was progressing well for a time then got angry with me about my bill and quit her treatment and did some very foolish things.
Transcranial
On March 11, 1905, a 26-year-old sales-woman presented to Johns Hopkins Hospital complaining of severe headache. The history was negative for nausea, vomiting, and syncope; it was positive for headache, visual disturbances, 10-pound weight loss, and dizzy spells. Cushing brought the patient to the operating room on March 14, 1905. His operative note documents the approach:
Bilateral palliative intramuscular craniotomy.
A tourniquet was applied but in this instance did not seem to have the desired result in lessening bleeding from the scalp. An incision was made over the temporal region first on the right side, namely that of subjective symptoms. The fibres of the temporal muscle were separated and an opening about 5 cm in diameter was Rongeured away from the underlying squamous portion of the temporal and lower edge of the parietal bones. The underlying dura was not tense. There was no indication from its appearance of any increase of intra-cranial tension. The dura was opened in one small place and the brain found to be normal in appearance, though somewhat injected and contused apparently from the operative traumatism. In rongeuring away the lower part of the temporal the posterior branch of the meningeal was torn and it apparently stopped bleeding after crowding a piece of gauze down into the neighborhood of the vessel so as to force it away from its bony connection and allow it to contract …. A similar operation was carried out on the left side of the head with a similar injury to the meningeal branch.
The patient was discharged on March 25, 1905, in unimproved condition (Figure 5). As the operation during her first admission was primarily decompressive, her symptoms continued to progress. She returned to Johns Hopkins Hospital in February 1912. An x-ray demonstrated “marked distortion of the sellar region, outlines completely obscured.” At that time she underwent a transsphenoidal operation for sellar decompression. Although Cushing documented no complications in his monograph (12), the surgical chart notes that she experienced new-onset blindness postoperatively, which gradually improved during her admission. She was discharged in un-improved condition following the second operation; no further information was available in the chart.
Figure 5.

Cushing’s post-discharge note for Transcranial Case 1. Reads: “1906. I think this case is akin to that of [omitted] an [illegible] girl with infantile pelvic viscera, headaches, optic atrophy and in whom at autopsy was found a congenital mixed tumor, [illegible], size pigeons egg situated just behind the optic convergence—HC.”
DISCUSSION
Surgical treatment of pituitary pathology has advanced tremendously since the field developed in the late 19th century. In 1882, Hyrtl stated that the sphenoid sinus remained unreachable for the surgeon (16). In 1906, Horsley described a series of 10 cases in which he approached the pituitary through a transcranial approach (23, 25). Throughout the early 20th century, European neurosurgeons began experimenting with the transsphenoidal approach, which Cushing pioneered and popularized within American practice. Cushing rather unexpectedly abandoned the transsphenoidal approach in the late 1920s, adopting instead the transcranial approach. This decision, and his publications advocating the transcranial method (10, 39), led to a shift in the operative landscape of American neurosurgery.
In explaining this transition, Cushing offered the explanation that the transcranial approach led to more reliable improvement in visual symptoms, and offered a better operative corridor, which was of particular importance in cases with uncertain diagnoses. This review demonstrates that Cushing’s experience with the two approaches at Johns Hopkins Hospital had similar inpatient mortality rates, with the transsphenoidal approach having a higher percentage of patients discharged in well/improved condition (64% transsphenoidal vs. 0 transcranial). Patients undergoing transsphenoidal procedures were more likely to have an out-come of well/improved at last follow-up, compared to those undergoing transcranial procedures (47% vs. 0). However, patients undergoing transsphenoidal procedures had a slightly higher rate of mortality at last follow-up than those patients undergoing transcranial procedures (48% vs. 38%) (Table 3). Of patients commenting on visual field symptoms during follow-up correspondence, those who underwent transsphenoidal procedures reported better resolution of symptoms postoperatively (54% transsphenoidal vs. 25% transcranial), and slightly less persistence of symptoms postoperatively (46% transsphenoidal vs. 50% transcranial). However, the follow-up data are limited by patient correspondence, and symptoms that patients spontaneously described in letters; only 13 patients undergoing transsphenoidal procedures and 4 patients undergoing transcranial procedures reported on the presence or absence of visual symptoms.
Contemporary scholars have commented that the operative mortality was not a factor in the transition from transsphenoidal to transcranial approaches (24). This review bears that out, with similar inpatient mortality in the transsphenoidal (16%) and transcranial (20%) groups. Notably, the two patients undergoing the omega incision were both discharged in well condition, with no inpatient mortality (34). In comparison, Horsley’s series of 12 operations for pituitary pathology, as reported by Verga in 1911, demonstrated the death of four patients (33%) (25, 37, 42). However, Cushing later reported an operative mortality of 5.6% for a series of 200 cases (10, 21). This was likely due to a combination of factors: operative experience, better hemostasis, and improvement of sterile technique.
Although patients undergoing transsphenoidal and transcranial approaches had similar mortality rates, the condition at discharge varied between the two groups; of the patients who underwent a transsphenoidal approach for the primary operative intervention 60% were discharged in “improved” condition and 20% were discharged in “unimproved” condition, compared to patients who underwent a transcranial approach for primary operative intervention, where 80% were discharged in “unimproved” condition and none were “improved.” Despite these outcomes, Cushing continued to perform transcranial approaches, in particular subtemporal decompressions, throughout his tenure at the Johns Hopkins Hospital, although his operative practice shifted toward the transsphenoidal approach (Table 4).
Table 4.
Diagnosis as Documented in the Surgical Records and Excerpts from Operative Notes for Patients Undergoing Surgical Intervention for Suspected Pituitary Pathology, by Dr. Harvey Cushing at the Johns Hopkins Hospital 1896 to 1912, Arranged Chronologically by Date of Primary Surgical Intervention
| Case | Diagnosis (Verbatim from Chart) | Surgery Date(s) | Approach (Excerpts from Operative Notes) |
|---|---|---|---|
| 1 | Hypophyseal struma, hypopituitarism | 3/14/1905 | “Bilateral palliative intramuscular craniotomy … an incision was made over the temporal region" |
| 2 | Sinus thrombosis (?) cerebral Tumor? Hypophyseal Tumor!!” |
7/17/1908 | “Right subtemporal decompression … the usual vertical incision through soft parts was made” |
| 3 | Cerebral tumor; hypophyseal tumor?; hypopituitarism |
3/15/1909 | “A cycle-shaped incision like the magnified incision of the form used in the ordinary ganglion operation was made in the left temporal region … the zygoma was divided and loosened without being removed in toto” |
| 4 | Acromegaly | 3/25/1909 | “An incision was mapped out passing on each side from the lower level of the side of the nasal bone, carried upward along the side of the nose to the inner angle of the orbit” |
| 5 | Tumor hypophysis cerebri, hypopituitarism, acromegaly |
10/12/1909 | “Rose position. Horseshoe-shaped incision from base of nasal bones over roof of nose. Mid-frontal incision carried up from the arch of this first incision for a distance of an inch in a deep median furrow.” |
| 6 | Tumor hypophysis (hypopituitarism), hemianopsia, hypophyseal struma |
4/20/1910 | “An incision was made under the upper lip and the nose was entered as usual.” |
| 5/26/1910 | “The old incision under the lip was reopened.” | ||
| 7 | Hypopituitarism | 4/21/1910 | “Elevating the lip by a transverse incision the nasal fossa were opened” |
| 8 | Acromegaly, cerebellar cyst | 5/10/1910 | “The usual incision in the lip was made” |
| 9 | Hypophyseal tumor, primary optic atrophy, hemianopsia |
5/10/1910 | “Incision under upper lip” |
| 10 | Hypophysis tumor | 8/3/1910 | “Usual incision under lip” |
| 11 | Acromegalic gigantism; former hyperpituitarism, present hyperpituitarism |
12/17/1910 | “The sublabial incision made with an opening into the nares without however opening the actual cavity.” |
| 12 | Hypophyseal tumor, epileptoid attacks, paroxysmal headache |
12/27/1910 | “L subtemporal decompression … R Ventricle Puncture at Kocher's point” |
| 13 | Hypophyseal tumor (struma) | 3/18/1911 | “Hypophyseal decompression… practically conducted throughout without opening the nasal cavities” |
| 14 | Hypophyseal tumor, intra and extra sellar, hypopituitarism |
4/20/1911 | “Usual route followed” |
| 15 | Hypophyseal adenoma, hypopituitarism | 5/18/1911 | “Usual sublabial approach … the usual incision was made.” |
| 16 | Hypophyseal tumor, blindness | 6/16/1911 | “Small one inch incision into lip … usual approach to the sphenoidal cells.” |
| 17 | Infundibular tumor, hypopituitarism (secondary obstruction), adiposity |
8/10/1911 | “Exploratory craniotomy… the bone flap was reflected without complication.” |
| 18 | Hypophyseal struma, dyspituitarism, primary optic atrophy, bitemporal hemianopsia |
8/15/1911 | “Sublabial approach.” |
| 19 | Hypopituitarism, hypoadrenalism | 9/6/1911 | “Subtemporal decompression … the flap was reflected.” |
| 20 | Hypophyseal cyst with hypopituitarism, pronounced neighborhood symptoms, blindness |
10/11/1911 | “Sublabial approach … incision under upper lip.” |
| 21 | Hypophysis tumor–hypopituitarism, bitemporal hemianopsia |
10/12/1911 | “Sellar decompression … a sub labial incision was made” |
| 22 | Acromegaly, extrasellar extension of struma | 10/19/1911 | “Sellar decompression” |
| 23 | Hypophyseal hypertrophy, hypopituitarism | 10/23/1911 | “Sellar decompression … usual approach to the hypophysis under the lobe” |
| 24 | Hypophyseal struma, dyspituitarism: incipient acromegaly, bitemporal (increasing) hemianopsia |
10/20/1911 | “The usual sublabial route was followed” |
| 11/4/1911 | “A large bone flap was turned down from the right hemisphere” | ||
| 11/23/1911 | “The original wound in the lip was reopened” | ||
| 25 | Interpeduncular (hypophyseal?) tumor; blindness; hypopituitarism |
11/1/1911 | “Sellar decompression ... the approach was somewhat easier than usual.” |
| 12/4/1911 | “Right subtemporal decompression ... usual incision was made.” | ||
| 26 | Hypophyseal tumor struma(?), uncinate gyrus attacks |
11/9/1911 | “The usual sublabial approach” |
| 27 | Infundibular tumor | 11/9/1911 | “A large bone flap was turned down from the right hemisphere anteriorly” |
| 11/18/1911 | “A simple approach though the mucous membrane of the nares was somewhat torn” |
||
| 12/7/1911 | “The old bone flap on the right side was re-elevated” | ||
| 28 | Infundibular cyst | 11/21/1911 | “Usual preparation and approach” |
| 29 | Hypopituitarism, interpeduncular cyst, bitemporal hemianopsia |
11/30/1911 | Right subtemporal decompression |
| 12/15/1911 | “Sellar decompression ... a rather difficult approach and some tearing of mucous membrane. |
||
| 2/9/1912 | “A small 2 inch opening of the old right decompression incision was made.” |
||
| 30 | Hypophyseal cyst, bitemporal hemianopsia | 1/4/1912 | “Usual approach was made by sublabial incision” |
| 1/10/1912 | “The old incision under the lip was reopened” | ||
| 31 | Hypophyseal struma, dyspituitarism, incipient acromegaly, bitemporal hemianopsia |
1/27/1912 | “Usual sublabial approach” |
| 32 | Hypophyseal/cerebral tumor, hypopituitarism | 2/28/1912 | “The transsphenoidal approach was made without difficulty” |
| 33 | Acromegaly, dyspituitarism | 3/16/1912 | “The approach was made as usual with a sublabial incision” |
| 34 | Hypophyseal tumor, right homonymous hemianopsia |
3/18/1912 | Right subtemporal decompression; “the usual approach was made” |
Cushing published several reports on his experience with the surgical treatment of pituitary disorders, and his 1912 publication offers thorough descriptions of 52 patients presenting to the Johns Hopkins Hospital with complaints related to the pituitary gland. In fact, this publication is the most complete collection of his experience with pituitary disorders at the Johns Hopkins Hospital. However, the publication lacks detail about a number of patients who were treated after the manuscript was complete, but who are included in a table appended to the manuscript. Additionally, the publication excludes patients who presented for treatment following February 1, 1912. The monograph offers an explanation for the omissions:
Numbers 29 to 39 of the above table include cases which have come under observation subsequent to the completion of Part II of this monograph in September, 1911, and before the completion of this last section in February, 1912 …. Between February 1 and April 1, while the manuscript has been in press, there have been nine additional cases, most of them with lesions demanding operative intervention. (12)
This review of the original surgical records uncovered 12 primary interventions that Cushing incompletely described, as well as six primary interventions that were not published. The resulting series of 37 patients, though not complete because of imperfections in the archived records, provides a broader insight into Cushing’s experience of operative treatment of pituitary pathology while at the Johns Hopkins Hospital. It was discovered that Cushing’s surgical innovation in these patients pushed the limits of available surgical and pharmacologic technologies; in 1912, Cushing performed two pituitary gland transplantations from still-born fetuses into the cerebral cortex of a single patient (36).
Additionally, the original surgical records provide detailed information that allow for retrospective diagnosis: The clinical manifestations (weight gain, amenorrhea, and dry skin) and pathologic description (“chromophobe struma”) of Case 1 suggest a diagnosis of nonfunctioning pituitary adenoma with secondary hypothyroidism and possibly hypogonadism. The immediate postoperative course in Case 2 suggests a disturbance in water balance or untreated adrenal insufficiency, and the rapid deterioration in her condition after she “quit her treatment,” in the context of a “brief illness,” suggests adrenal insufficiency as the cause of death. In Case 3, the symptoms of weight loss and dizziness again suggest un-treated adrenal insufficiency prior to operation. It is worthwhile to note that the complications of hypopituitarism following neurosurgical intervention had limited treatment options. It was not until 1913 that posterior pituitary extract began to be used in the treatment of diabetes insipidus. Anterior and posterior pituitary extracts were available from animal sources, but targeted therapies such as antidiuretic hormone and its analogs were not available until the 1950s (27). One has to wonder to what extent the favorable outcome in Case 1 was due to appropriate recognition and treatment of preoperative hypopituitarism.
This analysis highlights Cushing’s accomplishments in the surgical treatment of suspected pituitary pathology during his early career as a young attending at Johns Hopkins Hospital. Although he went on to polish and perfect his operative techniques and clinical knowledge while at the Peter Bent Brigham Hospital, this review demonstrates that the seeds of Cushing’s fascination with the pituitary were sown during his first cases in Baltimore.
ACKNOWLEDGMENTS
Figures provided courtesy of the Alan Mason Chesney Archives.
Footnotes
Conflict of interest statement: C.P. was supported by an HHMI-Ivy Foundation Research Training Grant. H.A. was supported by VSBfonds and The Prins Bernhard Cultuurfonds. N.M. was supported by an NIH T32 Grant. A.Q.H. was funded by the KO8 NIH grant and an HHMI grant.
REFERENCES
- 1.Aschner B. Ueber die Funktion der Hypophyse. Pflugers Arch Gesamte Physiol Menschen Tiere. 1912;146:1–146. [Google Scholar]
- 2.Bateman GH. Trans-sphenoidal hypophysectomy. A review of 70 cases treated in the past two years. Trans Am Acad Ophthalmol Otolaryngol. 1962;66:103–110. [PubMed] [Google Scholar]
- 3.Bayliss WM, Starling EH. The mechanism of pancreatic secretion. J Physiol. 1902;28:325–353. doi: 10.1113/jphysiol.1902.sp000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benda C. Beitrage zur normalen und pathologischen histologie der menschlichen hypophysis cerebri. Klin Wochenschr. 1900;52:1205–1210. [Google Scholar]
- 5.Bouche J, Guiot G, Freche C. Evaluation of hypophysectomy by low transseptal approach based on 120 operations. Ann Otolaryngol Chir Cervicofac. 1966;83:466–471. [PubMed] [Google Scholar]
- 6.Caton R, Paul FT. Note on a case of acromegaly treated by operation. Br Med J. 1893;2:1421–1423. doi: 10.1136/bmj.2.1722.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen-Gadol AA, Liu JK, Laws ER., Jr Cushing’s first case of transsphenoidal surgery: the launch of the pituitary surgery era. J Neurosurg. 2005;103:570–574. doi: 10.3171/jns.2005.103.3.0570. [DOI] [PubMed] [Google Scholar]
- 8.Comte L. Enlargement of the pituitary during pregnancy. Beitr Pathol Anat. 1898;23:90. [Google Scholar]
- 9.Crowe SJ, Cushing HW, Homans J. Experimental hypophysectomy. Bull Johns Hopkins Hosp. 1910;21:127–169. [Google Scholar]
- 10.Cushing H. Intracranial tumours, Notes Upon a Series of Two Thousand Verified Cases with Surgical-Mortality Percentages Pertaining Thereto. Spring-field, IL, C. C. Thomas; 1932. [Google Scholar]
- 11.Cushing H. III. Partial Hypophysectomy for acromegaly: with remarks on the function of the hypophysis. Ann Surg. 1909;50:1002–1017. doi: 10.1097/00000658-190912000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cushing H. The Pituitary Body and Its Disorders, Clinical States Produced by Disorders of the Hypophysis Cerebri. Philadelphia: J.B. Lippincott. 1912 [Google Scholar]
- 13.Cushing H. Sexual infantilism with optic atrophy in cases of tumor affecting the hypophysis cerebri. J Nerv Ment Dis. 33:704–1906. [Google Scholar]
- 14.Cushing H. Surgical experiences with pituitary adenoma. JAMA. 1914;63:1515–1525. [Google Scholar]
- 15.Dostoiewsky A. Uerer Den Bau Der Vorderlappen Des Hirmanhanges. Arch Mikrosk Anat Bonn. 1885;26:592–598. [Google Scholar]
- 16.Frazier CH. I. An Approach to the Hypophysis through the Anterior Cranial Fossa. Ann Surg. 1913;57:145–150. doi: 10.1097/00000658-191302000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goris C. Une Intervention sur l’hypophyse pour tumeur hypophysaire avec symptomes acromegaliques. Nevraxe. 13:1912. [Google Scholar]
- 18.Hamberger CA, Hammer G, Marcusson G. Experiences in transantrosphenoidal hypophysectomy. Trans Pac Coast Otoophthalmol Soc Annu Meet. 1961;42:273–286. [PubMed] [Google Scholar]
- 19.Hamberger CA, Hammer G, Norlen G, Sjogren B. Transantrosphenoidal hypophysectomy. Arch Otolaryngol. 1961;74:2–8. doi: 10.1001/archotol.1961.00740030005002. [DOI] [PubMed] [Google Scholar]
- 20.Hardy J, Ciric IS. Selective anterior hypophysectomy in the treatment of diabetic retinopathy. A transsphenoidal microsurgical technique. JAMA. 1968;203:73–78. [PubMed] [Google Scholar]
- 21.Henderson WR. The pituitar adenomata. A follow-up study of the surgical results in 338 cases (Dr Harvey Cushing’s series) Br J Surg. 1939;26:911–921. [Google Scholar]
- 22.Hochenegg J. Zur Therapie von Hyophysentumoren. Deutsche Zeitschrift fur Chirurgie. 1909;100:317–327. [Google Scholar]
- 23.Horsley V. Address in surgery on the technique of operations on the central nervous system. Lancet. 1906;2:484–490. [Google Scholar]
- 24.Jane JA, Thapar K, Laws ER. A history of pituitary surgery. Operative Tech Neurosurg. 2002;5:200–209. [Google Scholar]
- 25.Jefferson G. Sir Victor Horsley, 1857-1916, centenary lecture. Br Med J. 1957;1:903–910. doi: 10.1136/bmj.1.5024.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kocher T. Ein Fall von Hypophysis—Tumor mit Operativer Heilung. Deutsche Zeitschrift fur Chirurgie. 1909;100:13–37. [Google Scholar]
- 27.Lindholm J. Diabetes insipidus: historical aspects. Pituitary. 2004;7:33–38. doi: 10.1023/b:pitu.0000044633.52516.e1. [DOI] [PubMed] [Google Scholar]
- 28.Liu JK, Cohen-Gadol AA, Laws ER, Jr, Cole CD, Kan P, Couldwell WT. Harvey Cushing and Oskar Hirsch: early forefathers of modern transsphenoidal surgery. Neurosurg. 2005;103:1096–1104. doi: 10.3171/jns.2005.103.6.1096. [DOI] [PubMed] [Google Scholar]
- 29.Lothringer S. Untersuchungen ander Hypophysee Elniger Saugethiere Und des menschen. Arch Mikrosk Anat Bonn. 1886;28:257. [Google Scholar]
- 30.Marie P. Sur deux cas d’acromegalie. Revista Medica del Instituto Mexicano del Seguro Social. 1886;6:297. [Google Scholar]
- 31.Massalongo R. Sull’acromegalia. Riforma Med. 1892;8 [Google Scholar]
- 32.Minkowski O. Ueber einen Fall von Akromegalie. Berl Kliln Wochenschr. 1887;24:371–374. [Google Scholar]
- 33.Moebius PJ. Ueber Insufficienz der Convergenz Bei Morbus Basedowii. Arch Psychol. 1886;27:301–321. [Google Scholar]
- 34.Pendleton C, Adams H, Salvatori R, Wand G, Quinones-Hinojosa A. On the shoulders of giants: Harvey Cushing’s experience with acromegaly and gigantism at the Johns Hopkins Hospital, 1896-1912. Pituitary. doi: 10.1007/s11102-010-0258-z. [DOI] [PubMed] [Google Scholar]
- 35.Pendleton C, Wand G, Quinones-Hinojosa A. The autopsy was conducted “Under most inauspicious circumstances:” John Turner, Harvey Cushing’s case XXXII, and his unwitting contributions to the early understanding of acromegaly. Pituitary. doi: 10.1007/s11102-010-0239-2. [DOI] [PubMed] [Google Scholar]
- 36.Pendleton C, Zaidi HA, Pradilla G, Cohen-Gadol AA, Quinones-Hinojosa A. Harvey Cushing’s attempt at the first human pituitary transplantation. Nat Rev Endocrinol. 2010;6:48–52. doi: 10.1038/nrendo.2009.223. [DOI] [PubMed] [Google Scholar]
- 37.Pollock JR, Akinwunmi J, Scaravilli F, Powell MP. Transcranial surgery for pituitary tumors performed by Sir Victor Horsley. Neurosurgery. 2003;52:914–925. doi: 10.1227/01.neu.0000053148.34310.bb. discussion 925-926. [DOI] [PubMed] [Google Scholar]
- 38.Proust R. La Chirurgie de l’hypophyse. J Chir (Paris) 1908;1:665–680. [Google Scholar]
- 39.Rosegay H. Cushing’s legacy to transsphenoidal surgery. J Neurosurg. 1981;54:448–454. doi: 10.3171/jns.1981.54.4.0448. [DOI] [PubMed] [Google Scholar]
- 40.Schloffer H. Erfolgreiche Operationen eines Hypophysentumors auf nasalem Wege. Wien Klin Wochenschr. 1907;20:621–624. [Google Scholar]
- 41.Vassale G, Sacchi E. Sulla Distruzione della Ghiandola Pituitaria. Riv Sper Freniatr. 1892;18:525–561. [Google Scholar]
- 42.Verga G. Patologia chirurgicale delle l’ipofisii. Pavia. 1911 [Google Scholar]
- 43.von Eiselsberg A, von Frankl-Hochwart L. Uber die Operative Behandlung der Tumoren der Hypophysisgegend. Neurologisches Centrablatt. 1907;26:994–1001. [Google Scholar]
- 44.von Mering JV, Minkowski O. Diabetes mellitus nach pankreasexstirpation. Zentralblatt fur Klinische Medizin. 1889;10:393. [Google Scholar]