Abstract
Background and Aims
Cold is a major constraint for cereal cultivation under temperate climates. Winter-hardy plants interpret seasonal changes and can acquire the ability to resist sub-zero temperatures. This cold acclimation process is associated with physiological, biochemical and molecular alterations in cereals. Brachypodium distachyon is considered a powerful model system to study the response of temperate cereals to adverse environmental conditions. To date, little is known about the cold acclimation and freezing tolerance capacities of Brachypodium. The main objective of this study was to evaluate the cold hardiness of seven diploid Brachypodium accessions.
Methods
An integrated approach, involving monitoring of phenological indicators along with expression profiling of the major vernalization regulator VRN1 orthologue, was followed. In parallel, soluble sugars and proline contents were determined along with expression profiles of two COR genes in plants exposed to low temperatures. Finally, whole-plant freezing tests were performed to evaluate the freezing tolerance capacity of Brachypodium.
Key Results
Cold treatment accelerated the transition from the vegetative to the reproductive phase in all diploid Brachypodium accessions tested. In addition, low temperature exposure triggered the gradual accumulation of BradiVRN1 transcripts in all accessions tested. These accessions exhibited a clear cold acclimation response by progressively accumulating proline, sugars and COR gene transcripts. However, whole-plant freezing tests revealed that these seven diploid accessions only have a limited capacity to develop freezing tolerance when compared with winter varieties of temperate cereals such as wheat and barley. Furthermore, little difference in terms of survival was observed among the accessions tested despite their previous classification as either spring or winter genotypes.
Conclusions
This study is the first to characterize the freezing tolerance capacities of B. distachyon and provides strong evidence that some diploid accessions such as Bd21 have a facultative growth habit.
Keywords: Brachypodium distachyon, cold acclimation, COR413, flowering, freezing tolerance, ice recrystallization inhibition, fructans, phenological development, proline, vernalization, VRN1, winter hardiness
INTRODUCTION
Cold is one of the major constraints restricting cereal cultivation under temperate climates (Kosova et al., 2011). Freezing temperatures encountered late in spring, early in autumn and during winter severely alter plant survival and thus represent a significant impediment for yield improvement. To circumvent some of the limitations imposed by freezing temperatures, hardy cereals efficiently interpret and respond in a co-ordinated manner to environmental cues that signal seasonal changes. As a consequence, winter genotypes can acquire the ability to resist freezing temperatures, whereas spring genotypes will remain limited in their ability to resist freezing (Limin et al., 2007). Freezing tolerance in plants is thus not a constitutive trait and is expressed following exposure to low non-freezing temperatures, a process known as cold acclimation (Guy, 1990; Thomashow, 1999). Numerous studies have characterized in depth the cold acclimation process in cereals and other plants such as Arabidopsis thaliana and have established that this process is associated with numerous physiological, biochemical and molecular alterations (Houde et al., 1992; Danyluk et al., 1994; Crosatti et al., 1996; Limin et al., 1997; Stockinger et al., 1997; Grossi et al., 1998; Jaglo-Ottosen et al., 1998; Thomashow, 1999; NDong et al., 2002; Vogel et al., 2005; Van Buskirk and Thomashow, 2006; Badawi et al., 2007; Galiba et al., 2009). These alterations are regulated by a complex multigenic system that revolves around the induced expression of several cold-regulated (COR) genes (Thomashow, 2001). This transcriptional response is thus essential for the development of freezing tolerance and will ultimately promote self-adaptive features, such as increased levels of carbohydrates, soluble proteins, proline and organic acids, photosynthetic adjustments, appearance of new enzyme isoforms, and modifications in the lipid membrane composition, necessary for the plant to protect critical cell structures and vital physiological processes (Hughes and Dunn, 1996; Hüner et al., 1998; McNeil et al., 1999; Thomashow, 1999).
Despite these self-adaptive features, sub-zero temperatures remain fatal to the sensitive floral meristems. Hardy cereals thus have a vernalization requirement that delays the transition from the vegetative to the reproductive phase. Exposure to a period of low non-freezing temperatures modulates the expression of specialized vernalization genes, which in turn promotes flowering only in the milder conditions of spring. Vernalization is thus initiated within the same time frame as the induction of the cold acclimation pathway. Despite this temporal overlap, no direct molecular link has been clearly established between vernalization and the cold acclimation processes. Nonetheless, several studies have pointed out that the characterization of the vernalization requirements of a cereal provides key information about its freezing tolerance capacity by showing that the transition from the vegetative to the reproductive phase is associated with the downregulation of COR genes and the upregulation of the vernalization gene VRN-1 (Fowler et al., 1996a, b; Mahfoozi et al., 2001a, b; Dhillon et al., 2010). As a result, full expression of cold hardiness genes only occurs in the vegetative phase, and plants in the reproductive phase have a limited ability to accumulate COR gene transcripts and to cold acclimate. In addition, plants that are still in the vegetative phase have the ability to re-acclimate even after periods of exposure to warm temperatures, whereas plants in the reproductive phase only have a poor ability to re-acclimate (Mahfoozi et al., 2001a).
In the context of increasing demand on crops for food and biofuel production, breeding for cold tolerance holds promises for enhanced yield and extended cultivation seasons. It is thus important to gain in-depth knowledge about the cold response mechanisms of cereal plants and the intricate molecular circuitry involved. In order to support research initiatives, it is crucial to have a model plant which, in addition to possessing a small physical size, rapid life cycle and undemanding growth requirements, can make it amenable to perform high-throughput screening routines, functional analyses and transformation procedures (Draper et al., 2001).
With its status of a major staple food and its compact genome of 441 Mbp (Bennett et al., 2000), rice (Oryza sativa) was initially proposed as such a model for cereal plants. However, its use as a model organism for temperate cereals and forages has remained a matter of debate despite extensive international efforts that have led to the development of comprehensive genetic maps and imposing expressed sequence tag (EST) and germplasm collections. Nevertheless, the lack of microsynteny conservation, the presence of multiple small rearrangements and the fact that rice does not necessarily exhibit all the traits relevant to temperate cereal crops such as freezing tolerance and vernalization have considerably hampered the initial interest in this plant (Draper et al., 2001; Faricelli et al., 2010).
Brachypodium distachyon is an annual temperate wild grass that originates from Mediterranean and Middle East countries where sub-zero temperatures are frequently observed (Opanowicz et al., 2008; Vogel et al., 2010). It has many appealing biological attributes including self-fertility, small stature, short generation time and efficient transformation (Garvin, 2007; Alves et al., 2009; Vogel and Bragg, 2009). Moreover, Brachypodium has a small sequenced genome (272 Mbp), and spring and winter diploid accessions have been classified according to the capacity to flower with or without cold exposure (Vogel et al., 2010). There is strong chromosomal synteny between Brachypodium and other temperate cereals, and about 77 % of the genes retrieve significant matches in Triticeae EST databases (Huo et al., 2009). This body of evidence has led researchers to propose Brachypodium as an appropriate model to study the response of temperate cereals to their environment. As a result, the Brachypodium model has proved its value in a number of biotic and abiotic stress tolerance studies (Schwartz et al., 2010; Luo et al., 2011; Peraldi et al., 2011), clearly demonstrating its potential for understanding and ultimately improving abiotic stress tolerance in temperate cereals.
To date, little is known about the capacities of Brachypodium to cold acclimate and develop freezing tolerance. A recent study by Li et al. (2012) has demonstrated that Brachypodium has the molecular circuitry necessary to activate COR gene expression. Despite this leap forward, the extent of Brachypodium's capacity to resist freezing is still unknown. In this study, we investigated the cold hardiness capacity of Brachypodium. To achieve this goal, an integrated approach involving the monitoring of double-ridge (DR) formation and final leaf number (FLN) was used to verify the growth habit classification of seven diploid Brachypodium accessions. In addition, the cellular concentration of soluble sugars and proline were determined, along with the transcript accumulation profiles of orthologues of the major vernalization regulator VRN1 and two COR genes at different stages of cold acclimation. Finally, whole-plant freezing tests (WPFTs) were performed in order to characterize fully the freezing tolerance capacity of Brachypodium. This study is thus critical to evaluate the potential contribution of B. distachyon to cold hardiness research.
MATERIALS AND METHODS
Plant material and growth conditions
Seeds of Brachypodium distachyon spring accessions Bd2-3, Bd3-1, Bd21 and Bd30-1, and winter accessions Bd1-1, Bd18-1 and Bd29-1 were soaked for 2 h in sterile distilled water at room temperature, after which the lemma was removed. The seeds were then sterilized in 70 % ethanol, rinsed with sterile distilled water and sterilized again in 1·3 % sodium hypochlorite solution according to Vain et al. (2008) and Alves et al. (2009). The seeds were placed between two sterile filter papers imbibed with sterile distilled water in a Petri dish at 4 °C in the dark for 1 week. This stratification treatment is essential for the synchronization of germination of all Brachypodium accessions. Seeds were sown in pots containing Agro Mix® (Plant Products Co. Ltd) and grown until the three-leaf stage (approx. 10 d) at 20 °C with a 16 h photoperiod and a photosynthetic photon flux density of 150 µmol m−2 s−1. At the end of this period, control non-acclimated plants were harvested (NA0) or maintained under the same light and temperature conditions for 5 (NA5), 7 (NA7), 21 (NA21) and 45 d (NA45) to provide adequate controls for the different cold acclimation (CA) time points. Cold acclimation was performed by subjecting plants at the three-leaf stage to a temperature of 4 ± 1 °C under either an 8 h photoperiod [short day (SD)] or a 16 h photoperiod [long day (LD)] at a photosynthetic photon flux density of 150 µmol m−2 s−1 for different periods of time as specified for each experiment. Deacclimated plants (DA1) were exposed to cold for 28 d (sugar and proline assays) or 42 d [quantitative real-time PCR (qPCR)] and returned to 20 °C under a 16 h photoperiod for 1 d. All plant samples were collected at the same time of day, 4 h after the beginning of the light period.
Double-ridge formation
A dissection of the crown and an analysis of the shoot apex development identified the DR stage (Kirby and Appleyard, 1987; Kirby, 1990). Three-leaf stage plants were maintained under control conditions or exposed to 4 °C for the specified periods of time. After each treatment, plants were returned to 20 °C under a 16 h photoperiod until DR formation. An average of ten plants per accession were dissected at each of the ten low temperature (LT) treatments, and the mean number of days required to achieve DR formation was recorded to determine the influence of the LT treatment on the rate of phenological development. The experiment was repeated three times with independent biological replicates.
Final leaf number measurements
Leaves on the main shoot were numbered and the plants were grown until the flag leaf emerged and the FLN could be determined (Wang et al., 1995). Vernalization saturation was reached once the LT treatment no longer reduced the FLN. A minimum of ten plants was used at each of the ten LT treatments under both SD and LD conditions. The experiment was repeated three times with independent biological replicates.
Whole-plant freezing test
For the WPFTs, 21 seedlings (three of each of the seven Brachypodium accessions tested) were grown until the three-leaf stage in a circular pattern in 4 inch round pots at 20 °C. Plants were either kept under the same conditions for an additional 5 d (NA5 plants) or cold acclimated at 4 °C for 28 d (CA28). Following acclimation, WPFTs were performed in a programmable Percival low-temperature chamber (LT-36VL) specifically designed to measure cold hardiness in plants. After a 4 h equilibration period at –2 °C during which plants were seeded with ice chips to initiate freezing (Charron et al., 2008), the temperature was lowered by 1 °C h−1, the temperature being decreased in the first 5 min followed by a 55 min plateau. Plants were tested between –7 and –12 °C. At the end of each temperature plateau, three randomly selected pots of NA and CA plants were withdrawn from the chambers for a total of six pots per plateau. To minimize light stress effects after the freezing treatment, plants were thawed at 4 °C for 24 h in the dark before returning to normal growth conditions. After 2 weeks, survival counts were taken and the 50 % lethal temperature (LT50) calculated. A total of nine plants per accession, per treatment, per plateau, per experiment were removed from the incubator. The experiment was repeated four times with independent biological replicates.
Proline and water-soluble sugar assays
For free proline and water-soluble sugar (WSS) content assays, the aerial parts of nine plants for each accession were used at each of the seven LT treatments. The experiment was repeated three times with nine independent biological replicates.
Free proline content was analysed using a colorimetric assay (Ábrahám et al., 2010). Proline was extracted by grinding 100 mg of dried plant material with 500 µL of 3 % (w/v) sulfosalicylic acid. The extract was centrifuged at 13 000 rpm for 5 min at room temperature. A 0·1 mL aliquot of the extract was mixed with 0·5 mL of a solution of acidic ninhydrin [40 % acidic ninhydrin (8·8 µm nihydrin, 10·5 m glacial acetic acid, 2·4 m orthophosphoric acid), 40 % glacial acetic acid and 20 % of 3 % sulfosalicylic acid]. The samples were incubated for 60 min at 96 °C and the reaction was terminated by incubating the samples on ice for 5 min. The samples were then extracted by adding 1 mL of toluene and vortexing for 20 s. The absorbance at 520 nm was measured using toluene as a reference. The standard curve was made using l-proline in a range of 0–57·5 µg mL−1. Free proline content [μmol g−1 fresh weight (f. wt)] was calculated according to Bates et al. (1973):
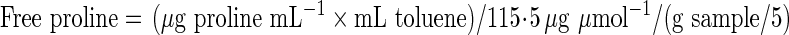 |
(1) |
Total WSS content was assessed according to Luo et al. (2011) with modifications to allow the data to be collected using a microplate reader (Galicia et al., 2009). Total WSS was extracted from 5 mg of plant dry tissues with 1 mL of distilled water by vortexing. The extract was incubated at 70 °C for 45 min, vortexed every 15 min and centrifuged at 13 000 rpm for 20 min at room temperature. The supernatant was diluted 1:10 and 50 µL of this sample was mixed with 100 µL of anthrone solution [100 mg of anthrone in 100 mL of 95 % (w/w) H2SO4] in a 96-well microplate. The microplates were shaken at 150 rpm for 10 min at room temperature and incubated at 100 °C for 4 min. After cooling, the absorbance at 630 nm was measured. The standard curve was made using sucrose in a range of 0–200 µg mL−1. The WSS content in mg of sucrose g−1 of dry plant material was calculated as:
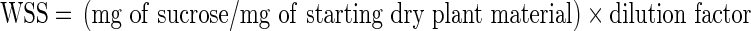 |
(2) |
Quantification of fructans
Dried ground material (100–200 mg) was incubated in 6 mL of deionized H2O at 80 °C for 20 min. The extracts were then incubated overnight at 4 °C and were subsequently centrifuged for 10 min at 1500 g. A 1 mL sub-sample of the supernatant was collected for quantification of fructans. High degree of polymerization (HDP, from DP 15 to DP 200) fructans and LDP (DP 10) fructans were analysed using a Waters HPLC analytical system controlled by the Empower II software. Samples were kept at 4 °C throughout the analysis within the Waters 717plus autosampler. The HDP fructans were separated on a Shodex KS-804 column preceded by a Shodex KS-G pre-column (Shodex, Tokyo, Japan) eluted isocratically at 50 °C with deionized water at a flow rate of 1·0 mL min−1, and were detected on a refractive index detector (Model 2410, Waters). The DP of HDP fructans was estimated by reference to a standard curve established with seven polymaltotriose pullulan standards (Shodex Standard P-82) ranging from a molecular weight of 0·58 × 104 to 85·3 × 104. The retention time on the Shodex column is a function of the log of the molecular weight of pullulan molecules. The concentrations of both HDP and LDP fructans are expressed on an equivalent fructose basis.
RNA extraction, cDNA synthesis and quantitative real-time PCR analysis
Total RNA was isolated from the aerial parts of plants (n = 3 plants per time point) using the RNeasy plant mini kit (Qiagen). All RNAs were treated with DNase I (Qiagen) to remove genomic DNA. RNA integrity was visually assessed with agarose gel electrophoresis. A 2 µg aliquot of total RNA was then reverse transcribed using the AffinityScript QPCR cDNA Synthesis Kit (Agilent Technologies) according to the manufacturer's recommendations. Parallel reactions were run for each RNA sample in the absence of AffinityScript reverse transcriptase (no reverse transcriptase control) to assess any genomic DNA contamination. The cDNA products were diluted in water to 400 ng μL−1 and stored at –20 °C.
Quantitative PCR assays were conducted in triplicate in an Mx3000 real-time thermal cycler (Agilent Technologies) with Brilliant III Ultra-Fast SYBR® Green QPCR master mix (Agilent Technologies). First-strand cDNA was used as template for PCR amplification using gene-specific primers (Table 1). Amplification of the 18S rRNA gene was used as an internal standard. Amplification was performed in a 15 µL reaction containing 1× SYBR Green master mix, 350 nm of each primer, 30 nm reference dye ROX and 80 ng of cDNA template. The PCR thermal cycling parameters were 95 °C for 2 min followed by 40 cycles of 95 °C for 5 s and 60 °C for 20 s. Three technical replicates were used, and the experiment was repeated three times with different biological replicates. Controls without template were included for all primer pairs.
Table 1.
List of primers used in this study
| Primer name | Primer sequence (5′ to 3′) | Amplicon size (bp) |
|---|---|---|
| BradiVRN1-F | 5′-CAGATCCAGAAAGAACCAGCTAA-3′ | 220 |
| BradiVRN1-R | 5′-GCGATTACTGATATTTGTTGTTGG-3′ | |
| BradiCOR413-F | 5′-AGGTTGGTTGCTGGATTGCGTTC-3′ | 76 |
| BradiCOR413-R | 5′-TCCAGCCAATCAGGAAAGTGGCG-3′ | |
| BradiIRI-F | 5′-AACTGGCAACAACAACGCCGTG-3′ | 147 |
| BradiIRI-R | 5′-ACGATGTGGTTGCTCCCGGATAC-3′ | |
| Bradi18S-F | 5′-GAAGTTTGAGGCAATAACAGGTCT-3′ | 131 |
| Bradi18S-R | 5′-ATCACGATGAATTTCCCAAGATTAC-3′ |
Raw fluorescent data (background-subtracted data) provided by the Mx-Pro QPCR software (Agilent Technologies) were analysed using the PCR Miner program (http://miner.ewindup.info/miner; Zhao and Fernald, 2005). For each LT treatment, data were expressed as a ratio of gene of interest expression to 18S expression.
Statistical analysis
Unless stated otherwise, all the experiments were carried out three times in a complete randomized design with a minimum of three replicates. All data were subjected to an analysis of variance (ANOVA) using the general linear model (GLM) procedure in SAS (SAS Institute, Cary, NC, USA) to identify significant treatment effects. Comparisons between means were made using least significant differences (LSDs) at a 0·05 probability level when ANOVA indicated model and treatment significances.
RESULTS
Phenological development
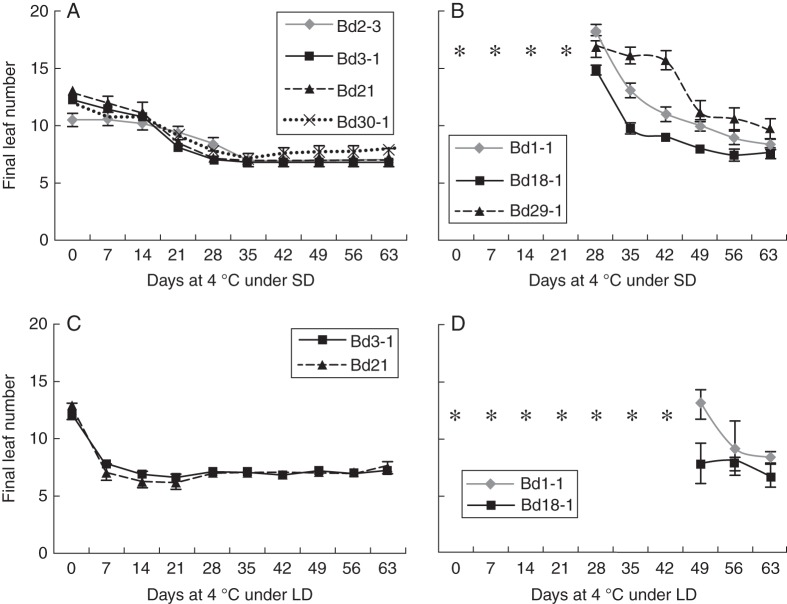
Molecular and physiological studies aimed at understanding the mechanism of freezing tolerance in cereals have revealed that this process is closely associated with the vernalization response (Fowler et al., 1996a; Danyluk et al., 2003; Dhillon et al., 2010). Thus, with the goal of unravelling the cold acclimation pattern of seven Brachypodium accessions, we initially opted for a classical approach by determining the effects of low non-freezing temperatures on their phenological development. As a first step, we tested the effects of cold exposure on the FLN of different accessions under SD and LD conditions. In all Brachypodium accessions tested in the current study, exposure to cold and SD conditions had noticeable effects on their FLN. The FLN of the four accessions able to flower without being exposed to LT clearly decreased following the cold treatment under SD conditions (Fig. 1A). Their FLN declined from 11–13 leaves under normal conditions to 7–8 leaves after 28 d of cold treatment. Exposure to cold for periods >28 d did not further reduce the FLNs of accessions Bd3-1, Bd21 and Bd30-1.
Fig. 1.
Final leaf number of seven diploid Brachypodium distachyon accessions in response to cold exposure. Final leaf number was compiled for Brachypodium accessions previously shown to have spring (left panel) or winter (right panel) growth habits when cold acclimated under (A and B) SD and (C and D) LD conditions. Time points where plants did not flower during the course of the experiment are indicated by an asterisk. Statistically significant differences (P < 0·05) in final leaf number were observed until 28 d for Bd3-1, Bd21 and Bd30-1, until 35 d for Bd2-3, until 49 d for Bd29-1 and until 56 d for accessions Bd1-1 and Bd18-1.
The three winter accessions did not transition from the vegetative to the reproductive phase in the time frame of the experiment unless they were cold treated under SD conditions for a minimum of 28 d. At this time point, these accessions showed elevated FLN (15–18 leaves), and a steady decrease in the FLN was observed during the cold treatment until no noticeable changes (7–10 leaves) were observed for exposure longer than 49–56 d (Fig. 1B).
We initially chose to perform cold treatment under SDs as this experimental set-up resembles the natural conditions observed during the autumn and is known to promote strong cold acclimation responses in temperate cereals (Fowler et al., 2001). However, this experimental design introduces an obvious confounding factor by simultaneously varying the temperature and the photoperiod. Thus, to determine the respective contributions of LT and daylength reduction to the FLN changes observed in Fig. 1A and B, we monitored fluctuations in the FLNs of Brachypodium accessions cold treated under LD conditions. Under these conditions, we observed a significant FLN reduction in two Brachypodium accessions able to flower without being exposed to LT (Bd3-1 and Bd21; Fig. 1C). The FLNs of these accessions declined, respectively, from 11 and 13 leaves under normal conditions to six and seven leaves after only 14 d of cold treatment. Interestingly, LD conditions significantly delayed the flowering transition of winter accessions since these accessions only formed spikes after spending a minimum of 49 d at 4 °C. At this time point, the winter accessions tested (Bd1-1 and Bd18-1) had FLNs of eight and 13, respectively, and prolonged LT exposure up to 63 d further reduced their FLNs to seven and eight (Fig. 1D). Together, the results presented in Fig. 1 demonstrate that a LT treatment reduces the FLN of Brachypodium accessions in both SD and LD conditions and that the rate of this reduction is photoperiod dependent.
In order to expand our phenological characterization, we monitored at different stages of cold exposure under SDs the formation of the DR structure, a well-known indicator of the transition from the vegetative to the reproductive stages in grasses (Kirby, 2002). As expected, dissection of the shoot apices established that four of the accessions, Bd2-3, Bd3-1, Bd21 and Bd30-1, entered the reproductive phase when grown at 20 °C for their complete life cycle. This observation confirms that those accessions do not require a cold treatment in order to flower (point 0 day; Fig. 2A). The days of growth required until DR formation (from the end of the vernalization treatment to the DR formation) varied markedly among those accessions, clearly indicating natural genetic variation. Indeed, accession Bd2-3 needed the most days to reach the DR stage, in contrast to accessions Bd3-1, Bd21 and Bd30-1 that presented very similar profiles when maintained under control conditions (point 0 day; Fig. 2A). Interestingly, a cold treatment under SD conditions reduced the numbers of days required until DR formation following LT treatment in these four accessions (Fig. 2A).
Fig. 2.
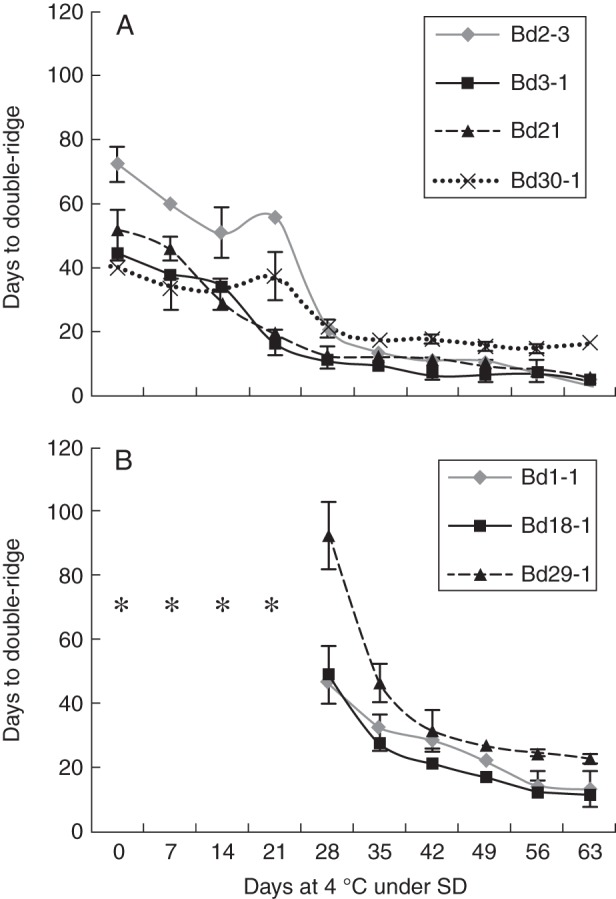
Apical development of seven diploid Brachypodium distachyon accessions exposed to cold (4 °C) for the indicated time. Double-ridge formation was compiled for Brachypodium accessions previously shown to have (A) spring or (B) winter growth habits. Time points where plants never formed the double-ridge structure during the course of the experiment are indicated by an asterisk. Statistically significant differences (P < 0·05) in days to double-ridge values were observed until 28 d for Bd21 and Bd 30-1, until 42 d for Bd2-3 and Bd 3-1 and until 49 d of treatment for Bd1-1, Bd18-1 and Bd29-1.
Contrasting results were obtained with the three other accessions tested (Bd1-1, Bd18-1 and Bd29-1; Fig. 2B). Under SD conditions, these accessions only formed the DR structure after spending a minimum of 28 d at 4 °C, indicating that unlike accessions Bd2-3, Bd3-1, Bd21 and Bd30-1 (Fig. 2A), they have a strong vernalization requirement and are true winter accessions. For these accessions, vernalization saturation was reached after a cold treatment of 49 d (Fig. 2B).
The results presented in Fig. 2 also indicate that a cold treatment under SD conditions can promote the formation of the DR structure earlier in the development of most Brachypodium accessions with no absolute vernalization requirement. Indeed, by adding the number of days of growth before the vernalization treatment (14 d), the duration of the vernalization treatment at a given time point and the corresponding number of days needed to observe the DR structure once the plants are returned to normal conditions, it is possible to estimate whether an LT treatment under SD conditions can stimulate early flowering in Brachypodium plants. In comparison with untreated plants, 21 d of exposure to LT and SD conditions accelerated the DR formation in accessions Bd3-1 and Bd21 by 6 and 11 d, respectively, while 28 and 35 d exposures accelerated the flowering transition of accession Bd3-1 by 23 and 24 d. On the other hand, accession Bd30-1 did not develop the DR structure earlier following the nine LT treatments.
Molecular response to low temperatures
In order to substantiate our phenological development observations and further to highlight the link between the vernalization and the cold acclimation responses in Brachypodium, we opted to juxtapose the expression profiles of orthologues of the vernalization regulator VRN1 and of two COR genes already shown to be differentially expressed in response to cold in monocots (Breton et al., 2003; Tremblay et al., 2005; Li et al., 2012).
In agreement with Higgins et al. (2010), Bradi1g08340·1, hereafter named BradiVRN1, was selected for this study because of its high level of sequence homology to the wheat VRN1 (Supplementary Data Fig. S1). The protein encoded by BradiVRN1 shares high homology with Triticum monococcum VRN1 (Pidal et al., 2009) (89 % identity and 92 % similarity) and Triticum aestivum VRT-1 (Danyluk et al., 2003) (84 % identity and 89 % similarity; Fig. S1). Like most MADS box transcription factors, the protein encoded by BradiVRN1 contains the usual three conserved regions (MADS, I and K domains) and a C-terminal domain. As expected, lower sequence similarity is observed in the C-terminal region since this region was shown to diverge substantially between MADS box orthologues (Theissen et al., 1996). Sequence analysis further revealed that BradiVRN1 possesses a bipartite nuclear targeting domain that is conserved in all the MADS box transcription factors as well as conserved phosphorylation sites in the C-terminal region (Fig. S1; Danyluk et al., 2003).
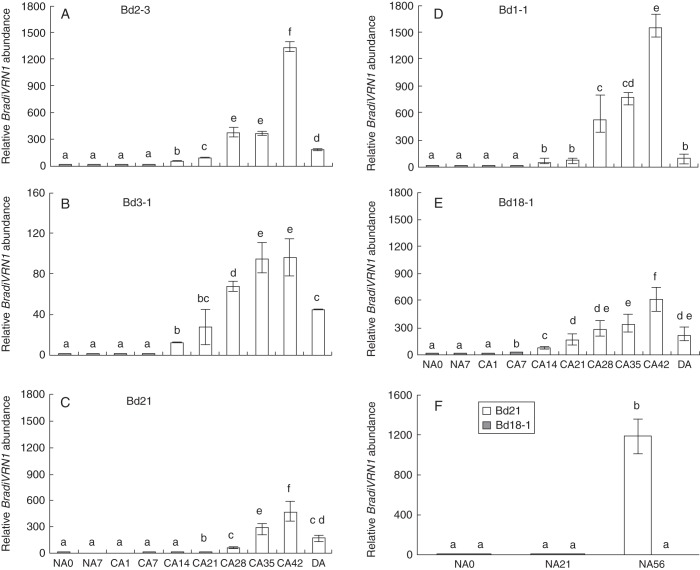
Brachypodium accessions Bd1-1, Bd2-3, Bd3-1, Bd18-1 and Bd21 were selected to highlight variations in transcript accumulation of BradiVRN1 in response to cold exposure because of their contrasting differences in phenological development. Non-acclimated (NA0 and NA7) plants and plants cold acclimated for 1 and 7 d (CA1 and CA7) showed minimal BradiVRN1 transcript accumulation, whereas progressively longer cold exposure led to a gradual accumulation of the transcript in all accessions tested (Fig. 3A–E). Maximum accumulation was reached after 42 d, and transcript levels remained elevated after 1 d of deacclimation in all accessions (DA1; Fig. 3A–E). The fold change values in BradiVRN1 transcripts varied greatly among accessions, with overall differences ranging from 100-fold (Bd3-1) to 1500-fold (Bd1-1) increases after 42 d of cold treatment. No obvious correlation was observed between the magnitude of the variations in BradiVRN1 transcripts and the growth habits of the accessions tested (Fig. 3A–E). Interestingly, increased accumulation of BradiVRN1 transcripts is also observed in ageing Bd21 plants never exposed to 4 °C, whereas the level of this transcript remains low and stable in ageing Bd18-1 plants (Fig. 3F).
Fig. 3.
Expression analysis of BradiVRN1 in response to low temperatures. (A–C) Relative transcript accumulation of BradiVRN1 in Brachypodium accessions Bd2-3, Bd3-1 and Bd21. (D and E) Relative transcript accumulation of BradiVRN1 in winter Brachypodium accessions Bd1-1 and Bd18-1. (F) Relative transcript accumulation of BradiVRN1 in Bd21 and Bd18-1 plants not exposed to 4 °C. Letters above bars represent statistical significance (P < 0·05); different letters indicate statistically different fold expression.
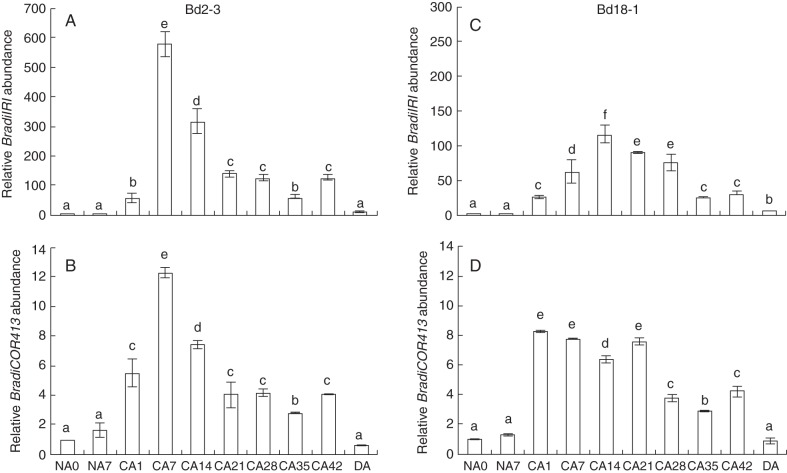
Variations in the expression of COR genes can be correlated to the level of freezing tolerance in cereals (Houde et al., 1992; Danyluk et al., 1994; Fowler et al., 1996a; Limin et al., 1997; Danyluk et al., 1998; Breton et al., 2003). In this study, we thus explored the possibility of using BradiIRI (Bradi5g27350·1) and Bradicor413 (Bradi1g07440·1) as potential markers for freezing tolerance in Brachypodium. As expected, the accumulation of BradiIRI increased following cold exposure in accessions Bd2-3 and Bd18-1 (Fig. 4A, C). In Bd2-3, maximum transcript accumulation was reached after 7 d at 4 °C, after which a steady decrease in accumulation was observed. In Bd18-1, the level of BradiIRI transcripts gradually increased until 14 d of cold exposure, after which a decrease in accumulation was also observed. The level of BradiIRI transcripts returned to control levels in both accessions after 1 d of deacclimation (Fig. 4A, C).
Fig. 4.
Accumulation of COR gene transcripts during cold acclimation in Brachypodium distachyon. Relative transcript accumulation of (A, B) BradiIRI and (C, D) BradiCOR413 in Bd2-3 and Bd18-1 plants exposed to LT for up to 42 d. Relative transcript accumulation in above-ground tissues was measured by quantitative real-time PCR and normalized to 18S rRNA transcript levels. Letters above bars represent statistical significance (P < 0·05); different letters indicate statistically different fold expression.
The cold-regulated 413 gene was first identified in wheat and arabidopsis where the accumulation of its transcripts was correlated with the capacity of the plant to develop freezing tolerance (Breton et al., 2003). Sequence homology searches allowed us to identify Bradi1g07440·1 as the Brachypodium orthologue of COR413 (Supplementary Data Fig. S2). A clear accumulation of BradiCOR413 transcripts was observed after 1 d of cold treatment in both accessions tested (Fig. 4B, D). In Bd2-3, the maximum accumulation was observed after 7 d of cold exposure. Following that point, a gradual decrease in transcript abundance was observed until 21 d of cold treatment, after which the levels remained constant until the end of the cold treatment (Fig. 4B, D). In Bd18-1, BradiCOR413 transcripts levels remained high during the first 3 weeks of cold treatment, after which a slight decrease in transcript levels was noticed (Fig. 4B, D). Upon deacclimation, BradiCOR413 levels returned to control levels in both accessions (Fig. 4).
Freezing tolerance determination
In order to determine the freezing tolerance capacities of the seven diploid Brachypodium accessions used in this study, WPFTs were conducted with plants grown under normal conditions (NA5) and plants that were cold acclimated for 28 d (CA28; Fig. 5A). The 28 d time point was selected based on our phenological development observations and gene accumulation data (Figs 1–4), as well as on reported distinctive variation in freezing tolerance between spring and winter wheat varieties observed after 4 weeks of cold exposure (Fowler and Limin, 2004). Furthermore, preliminary tests performed with control plants and plants exposed to cold for 7 and 14 d did not reveal significant differences in freezing tolerance between NA and CA Brachypodium plants (data not shown). Prior to WPFTs, no noticeable morphological differences that could explain variations in freezing tolerance were apparent between NA5 and CA28 Brachypodium plants. Following WPFTs, plants that did not survive the procedure were heavily damaged and evidently dead. A survival rate of 50 % was obtained for NA5 plants at temperatures ranging from –7·6 °C (Bd21) to –8·8 °C (Bd18-1). A 28 d period of cold exposure allowed an approx. 2 °C decrease in the LT50 values for all accessions, with survival rates of 50 % observed at temperatures ranging from –10·3 °C (Bd3-1) to –10·6 °C (Bd18-1). Overall, no obvious difference in freezing tolerance was observed between the group of accessions that do not require vernalization to flower (Bd2-3, Bd3-1, Bd21 and Bd30-1) and the winter type accessions (Bd1-1, Bd18-1 and Bd29-1; Fig. 5A).
Fig. 5.
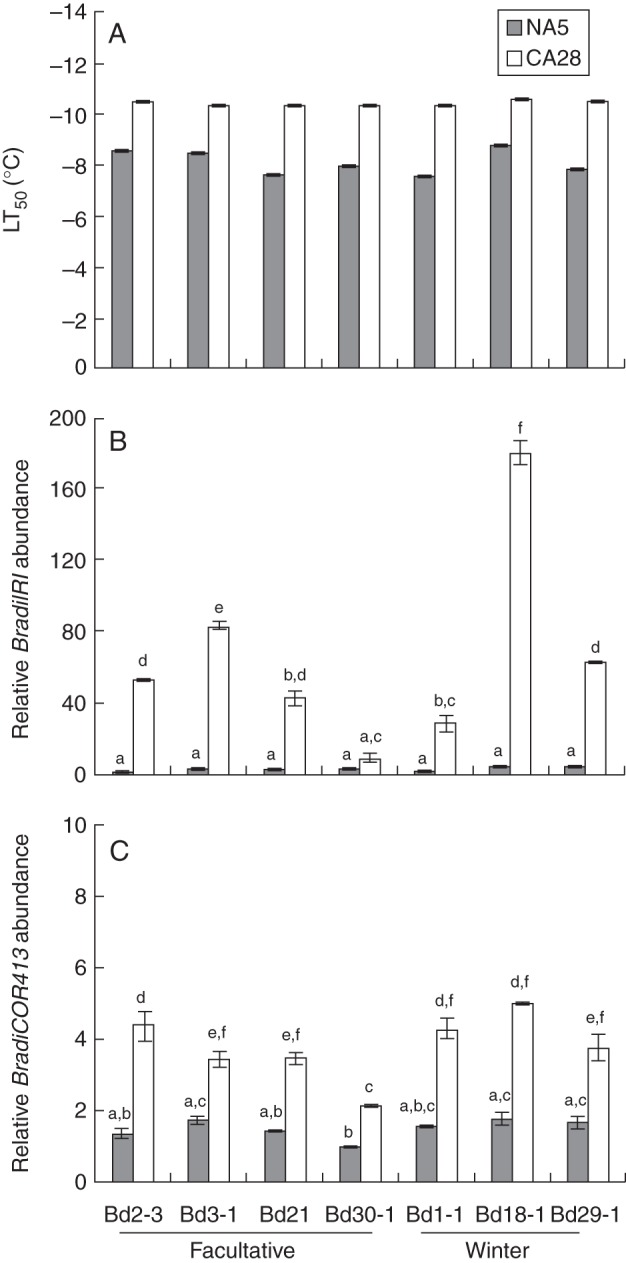
Freezing temperature tolerance (LT50) of seven diploid Brachypodium distachyon accessions. (A) Plants at the three-leaf stage were either kept under normal conditions (non-acclimated) for 5 d (NA5) or cold acclimated for 28 d (CA28) before being subjected to WPFTs. After 2 weeks in normal growth conditions, survival counts were taken and the LT50 was calculated. The experiment was repeated four times, and error bars indicate the standard deviation of the mean. (B and C) Relative transcript abundance of BradiIRI (B) and BradiCOR413 (C) in NA5 and CA28 plants used for WPFTs of seven diploid Brachypodium accessions. Letters above bars represent statistical significance (P < 0·05); different letters indicate statistically different fold expression.
The observed homogeneity in freezing tolerance levels between the seven Brachypodium accessions tested suggests similar COR gene transcripts levels after 28 d of cold acclimation. The accumulation levels of BradiIRI and BradiCOR413 transcripts were thus monitored in NA5 and CA28 samples taken prior to the WPFTs. The accumulation levels of BradiIRI transcripts did not reflect the WPFT observations since pronounced variations in BradiIRI transcript levels were observed among the different accessions (Fig. 5B). After 28 d of cold acclimation, the strongest accumulation of BradiIRI transcripts was detected in Bd18-1, while 20 times fewer transcripts were detected in Bd3-1. On the other hand, BradiCOR413 transcript accumulation was relatively stable among CA28 plants of all accessions, which better reflects their homogenous freezing tolerance capacities (Fig. 5C).
Metabolic adjustments in response to low temperatures
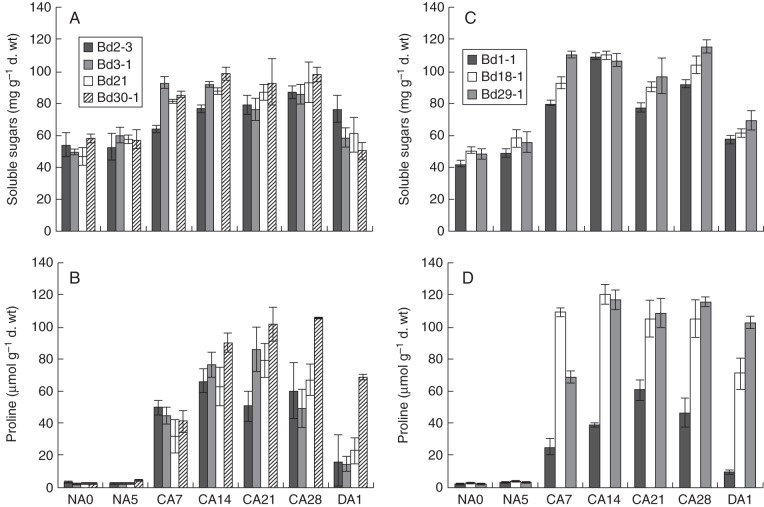
In order to characterize further the freezing tolerance of Brachypodium, we determined the effect of prolonged cold exposure on the content of WSS. Non-acclimated Brachypodium tissues contained minimum values of WSS, ranging from 43 µg mg−1 dry weight (d. wt) (Bd1-1) to 57 µg mg−1 d. wt (Bd30-1; Fig. 6A, C). A significant increase in the WSS concentration was noticed in all accessions when the plants were exposed to cold temperatures (Fig. 6A, C). After a 1 d de-acclimation period under normal growth conditions, a clear reduction of the WSS concentration was observed in all accessions (Fig. 6A, C). Interestingly, the WSS content was high in all Brachypodium accessions at the 28 d time point. However, these values varied greatly among accessions, which could suggest that a global assessment of WSS does not have the specificity needed to estimate precisely the freezing tolerance capacity of Brachypodium plants. To circumvent this limitation, we opted to measure fructans, a well-characterized group of storage carbohydrates known to accumulate during cold acclimation in temperate grasses (Livingston et al., 2009). The chromatographic characterization of fructans revealed that both LDP and HDP fructans are present in above-ground tissues of Brachypodium plants (Table 2). In all accessions tested, LDP and HDP fructans were found to increase in response to LT treatment. In comparison with LDP fructans, higher concentrations of HDP fructans were observed in the seven Brachypodium accessions tested (Table 2). Furthermore, HDP fructan values in cold-acclimated plants did not show any significant variation among the different accessions, which suggest that this measurement could potentially be used as a freezing tolerance indicator for the Brachypodium model.
Fig. 6.
Water-soluble sugar and proline concentration in leaves of seven diploid Brachypodium distachyon accessions exposed to cold. (A and C) WSS and (B and D) proline concentration of non-acclimated plants (NA0 and NA5), cold-acclimated plants (CA7, CA14, CA21 and CA28) and de-acclimated plants (DA1) of accessions previously shown to have a spring growth habit (left) or winter growth habit (right). The error bars indicate standard deviation of the mean.
TABLE 2.
Changes in LDP and HDP fructans in above-ground tissues of seven diploid Brachypodium distachyon accessions in response to cold acclimation
| Treatment | Accession | Growth habit | LDP (mg g−1 d. wt) | HDP (mg g−1 d. wt) |
|---|---|---|---|---|
| NA5 | Bd2-3 | Facultative | 0·16 ± 0·02a | 1·05 ± 0·08a |
| Bd3-1 | Facultative | 0·11 ± 0·03a | 1·10 ± 0·10a | |
| Bd21 | Facultative | 0·06 ± 0·02a | 1·12 ± 0·09a | |
| Bd30-1 | Facultative | 0·08 ± 0·02a | 1·51 ± 0·13a | |
| Bd1-1 | Winter | 0·04 ± 0·02a | 0·80 ± 0·06a | |
| Bd18-1 | Winter | 0·05 ± 0·01a | 1·04 ± 0·04a | |
| Bd29-1 | Winter | 0·08 ± 0·01a | 1·29 ± 0·05a | |
| CA28 | Bd2-3 | Facultative | 1·13 ± 0·09e | 2·66 ± 0·21ab |
| Bd3-1 | Facultative | 4·31 ± 0·22f | 5·45 ± 0·62bc | |
| Bd21 | Facultative | 2·57 ± 0·11d | 5·96 ± 2·90bc | |
| Bd30-1 | Facultative | 2·22 ± 0·11c | 5·95 ± 0·43bc | |
| Bd1-1 | Winter | 1·99 ± 0·13c | 7·55 ± 2·79bc | |
| Bd18-1 | Winter | 1·62 ± 0·05b | 5·97 ± 0·09bc | |
| Bd29-1 | Winter | 1·38 ± 0·09be | 8·74 ± 0·64c |
Different letters indicate significant differences between means (P < 0·05).
We also wanted to determine if proline could be used as an indicator of the freezing tolerance level of Brachypodium accessions. Under control conditions, similar basal proline contents were observed in all accessions (Fig. 6B, D). As observed in the case of WSS, an elevated proline content was observed in all accessions throughout the cold treatment. Winter accessions Bd18-1 and Bd29-1 displayed the most robust proline profile throughout the cold treatment (Fig. 6B, D), whereas the lowest pool of proline was observed in winter accession Bd1-1. A decrease in proline concentration was observed in all accessions when plants were returned to normal conditions for 1 d (DA1). However, proline levels remained elevated in DA1 plants of accessions Bd18-1, Bd29-1 and Bd30-1, whereas levels returned close to control values in accession Bd1-1, Bd2-3, Bd3-1 and Bd21 (Fig. 6B, D).
DISCUSSION
As part of our long-term objective of complete characterization of the freezing tolerance components of Brachypodium distachyon, we devised a classical approach involving the monitoring of phenological, molecular and metabolic indicators. Temperate cereal germplasms can be divided into three broad growth habit classifications – winter, facultative and spring. Winter varieties are freezing tolerant and require vernalization. In contrast, spring varieties barely tolerate freezing temperatures and do not require vernalization. The facultative growth habit lacks a clear definition and is considered by some as a sub-class of the winter growth habit. The facultative habit genotypes are usually freezing tolerant like winter varieties but lack vernalization requirements (von Zitzewitz et al., 2005).
The B. distachyon diploid inbred lines used in this study were previously classified as either spring (Bd2-3, Bd3-1, Bd21 and Bd30-1) or winter genotypes (Bd1-1, Bd18-1 and Bd29-1) according to their capacity to flower or not without prior exposure to cold (Schwartz et al., 2010). Our results, obtained from the monitoring of phenological indicators and the profiling of BradiVRN1 transcript accumulation, corroborate the winter habit classification of accessions Bd1-1, Bd18-1 and Bd29-1. These accessions did not flower without being previously exposed to 4 °C for a minimum of 28 d under SD conditions (Figs. 1, 2). Non-acclimated winter accession plants were eventually grown for >200 d without any signs of flowering (data not shown), clearly highlighting their absolute vernalization requirement. The fact that sharp decreases in FLN and in the number of days to DR formation are observed for theses accessions is in agreement with reports on wheat and rye winter varieties (Limin et al., 1996b). On the other hand, the trend observed for these two phenological indicators in Brachypodium ‘spring’ accessions did not exhibit the stable characteristic profiles generally observed in rye and wheat spring varieties (Fowler et al., 1996a, b). A closer examination of the profiles obtained for the two phenological indicators used in this study revealed a closer match to facultative cereal varieties where a small but significant acceleration of the phenological development is usually observed when plants are exposed to cold. The acceleration in response to LT of the phenological development of accessions Bd2-3, Bd3-1, Bd21 and Bd30-1 observed during this study was unexpected, and implies that these accessions are facultative genotypes since this type of behaviour is uncommon for spring genotypes. At first sight, these results do not agree with the study of Schwartz et al. (2010) that reported no significant effect of vernalization (in terms of number of days) on the vegetative to reproductive phase transition in accessions Bd3-1, Bd21 and Bd30-1. However, this discrepancy can most probably be explained by the age difference of the plant material used in both studies. Indeed, Schwartz et al. (2010) used plants that were closer to the transition point before performing the vernalization treatment (4-week-old plants grown under a 20 h light/4 h dark photoperiod), wheras Brachypodium plants at the three-leaf stage (approx. 14 d old) were exposed to cold in the current study.
The genome of Brachypodium has been reported to contain multiple orthologues of the temperate cereal gene VRN1. On one hand, Schwartz et al. (2010) proposed that two genes (Bradi1g08340·1 and Bradi1g59250·1) could potentially be considered as VRN1 orthologues, while another study clearly identified Bradi1g08340·1 as the only VRN1 candidate in Brachypodium (Higgins et al., 2010). For this study, Bradi1g08340·1 was selected based on its close sequence identity to wheat VRN1. Although further work is needed to confirm BradiVRN1 (Bradi1g08340·1) as VRN1, the expression profiles presented in this study undoubtedly revealed several key characteristics that establish this gene as a vernalization gene (Yan et al., 2003). The transcript accumulation patterns observed among accessions Bd1-1, Bd2-3, Bd3-1, Bd21 and Bd18-1 over a period of 42 d of cold exposure are consistent with profiles commonly observed in winter wheat varieties. These winter varieties generally express VRN1 at low levels during the early stages of cold exposure, and progressively higher levels of this transcript can be detected until the 49–56 d mark, where a distinctive plateau is usually reached (Kane et al., 2005). Furthermore, the abundance of this transcript remains elevated upon deacclimation after 42 d of cold treatment. While this expression pattern was expected for winter habit genotypes Bd1-1 and Bd18-1, it does not support the ‘spring habit’ classification of Bd2-3, Bd3-1 and Bd21 accessions. Spring cereal genotypes such as Manitou (wheat) and Morex (barley) constitutively express VRN1 regardless of their vernalization status (Danyluk et al., 2003; Kane et al., 2005; von Zitzewitz et al., 2005). Surprisingly, the transcript accumulation pattern of BradiVRN1 in accessions Bd2-3, Bd3-1 and Bd21 (Fig. 3A, B, C) resembles the profiles observed in winter cereal varieties and is a very close match to the VRN1 profile observed in the facultative barley variety Dicktoo (Danyluk et al., 2003; Kane et al., 2005; von Zitzewitz et al., 2005). In addition, the present study demonstrates that LT is not necessarily the only determinant responsible for the increased accumulation of VRN1 in facultative accessions, since this transcript accumulates at late developmental stages in accession Bd21 grown under control conditions (Fig. 3F). This observation, together with our DR and FLN data (Figs 1, 2), clearly suggests that accession Bd21 has a facultative growth habit.
The possibility that no spring habit Brachypodium accession can be found in the group of diploid accessions tested is further substantiated by our WPFT observations. Even if a cold acclimation period of 28 d only resulted in a modest 2 °C gain in freezing tolerance in Brachypodium, the fact that this gain is similarly observed among all accessions is another good indication that these accessions can be classified as facultative or winter habits. In addition, the LT50 values observed for non-acclimated plants are relatively low in comparison with values previously obtained for barley (approx. –2 °C; Limin et al., 2007) and winter wheat varieties (approx. –4 °C; Fowler and Limin, 2004) grown under similar conditions. This suggests that B. distachyon has stronger constitutive freezing tolerance mechanisms compared with modern cultivated cereal varieties that rely on inducible mechanisms to develop freezing tolerance.
The BradiIRI and BradiCOR413 transcript abundance analyses reported here provide additional evidence that some of the molecular mechanisms of freezing tolerance observed in hardy grasses are present in Brachypodium. Furthermore, the differential transcript accumulation patterns observed for both COR genes emphasizes the possibility that accessions Bd2-3, Bd3-1, Bd21 and Bd30-1 have facultative growth habits. Facultative genotypes, like winter types, are known to rely on induced COR gene expression to induce their freezing tolerance capacities but lack an absolute vernalization requirement (Karsai et al., 2005). In addition, the decline observed in BradiIRI and BradiCOR413 transcript accumulation in Bd2-3 and Bd18-1 (Fig. 4) is negatively correlated with the increase in BradiVRN1 transcript levels (Fig. 3A, E). This suggests that COR gene transcripts become less abundant when the floral transition is about to be reached in these accessions. However, our results suggest that BradiIRI is not a reliable freezing tolerance indicator/marker for Brachypodium. This observation agrees well with results reported in Li et al. (2012), where 7-week-old cold-acclimated Brachypodium plants of accessions Bd1-1, Bd21-1 and Bd29-1 were shown to accumulate various levels of BradiIRI transcripts. On the other hand, COR413 accumulation has been tightly correlated to the plant's capacity to develop freezing tolerance in wheat (Breton et al., 2003), and our observations suggest that this gene could potentially be used as a freezing tolerance indicator for Brachypodium as well. Nevertheless, accessions or eventually transformants with clear phenotypic variations for freezing tolerance will have to be investigated either to support or to refute this assertion.
Several metabolites have been reported to give instantaneous snapshots of the physiology of a plant cell (Levitt, 1980). This is best exemplified by the existence of positive correlations between the level of freezing tolerance of cereals and their capacities to accumulate compatible solutes such as proline and WSS (Olien and Clark, 1993; Hurry et al., 1995; Yoshida et al., 1998; Vágújfalvi et al., 1999). The distinctive difference in WSS concentration generally observed between spring and winter cereal genotypes was not observed among the Brachypodium diploid accessions tested. Again, this can probably be explained by the facultative behaviour displayed by accessions Bd2-3, Bd3-1, Bd21 and Bd30-1 throughout this study. Nonetheless, the lack of a clear trend among the WSS profiles observed in this study might just be a characteristic that sets apart the Brachypodium model, since Luo et al. (2011) did not find a distinctive difference in WSS among Brachypodium accessions even if clear differences in drought tolerance capacities were observed. However, our study provides evidence that the characterization of HDP fructans could serve as an alternative to the broad characterization of WSS in order to evaluate the freezing tolerance capacities of Brachypodium accessions. Our results revealed that Brachypodium accessions with similar freezing tolerance capacities accumulate HDP fructans at very comparable levels after 28 d of cold treatment. This finding does not seem to be limited to the Brachypodium model since similar correlations have been established in winter wheat, triticale and rye cultivars (Suzuki and Nass, 1988). However, while HDP fructans do accumulate in response to cold in Brachypodium, it is worth noting that the overall concentration values of HDP and LDP fructans remain low in comparison with values observed in cold-hardy grasses such as winter wheat varieties and annual bluegrass genotypes that can accumulate up to 100 mg g−1 d. wt under similar conditions (Suzuki and Nass, 1988; Yoshida et al., 1998; Bertrand et al., 2011).
Our results also indicate that although exposure to cold temperatures triggers the accumulation of proline in Brachypodium, the size of the proline pool of a given accession cannot be used to predict its freezing tolerance behaviour accurately since accessions with markedly different proline levels such as Bd29-1 and Bd1-1 exhibited very similar LT50 values (Figs 5, 6). This observation agrees well with the fact that no correlation could be established between variations in the proline pool during cold exposure and the level of freezing tolerance for arabidopsis and Thellungiella sp. (Lee et al., 2012).
Conclusions
This study demonstrates that cold acclimation and freezing tolerance mechanisms exist in Brachypodium distachyon. In response to LT, Brachypodium plants were able to accumulate COR gene transcripts and increase their pools of osmoprotectants. These changes are most probably responsible for the decrease in the LT50 values observed in all Brachypodium accessions tested and support our findings that cold acclimation modestly increases the freezing tolerance capacity of the Brachypodium model. Together with earlier findings that the CBF gene family is present in the genome of Brachypodium and that BradiCBF1 is regulated by LT (Li et al., 2012), the results presented here demonstrate that Brachypodium is able to acclimate to LT and to develop freezing tolerance.
This study also demonstrates that little to no natural genetic variation in terms of freezing tolerance exists among the Brachypodium diploid accessions tested and is the first to demonstrate that some Brachypodium accessions that do not necessitate a cold treatment in order to flower possess a facultative growth habit. We demonstrated that Brachypodium accessions Bd2-3, Bd3-1, Bd21 and Bd30-1 advanced to DR formation more rapidly and progressively reduced their FLN when exposed to cold for several days. Furthermore, a gradual accumulation of BradiVRN1 transcripts and a synchronized decrease in the level of COR gene transcript were also observed in these accessions during cold acclimation.
Taken together, this study is the first to characterize the freezing tolerance capacities of the model plant Brachypodium and to demonstrate that the widely used Bd21 accession has a facultative growth habit.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
The authors are grateful to Thomas Nicole, Lisa Rosenberger, Purva Karia and Philippe Seguin for their help during the course of the experiments. We also thank David F. Garvin for providing Brachypodium distachyon seeds. K.C.G. was supported by an Alexander-Graham-Bell scholarship from the Natural Sciences and Engineering Research Council (NSERC). The authors also acknowledge the support from the Centre SEVE. This work was supported by the NSERC of Canada [Discovery grant 386537 to J.B.C].
LITERATURE CITED
- Ábrahám E, Hourton-Cabassa C, Erdei L, Szabados L. Methods for determination of proline in plants. Methods in Molecular Biology. 2010;639:317–331. doi: 10.1007/978-1-60761-702-0_20. [DOI] [PubMed] [Google Scholar]
- Alves S, Worland B, Thole V, Snape J, Bevan M, Vain P. A protocol for Agrobacterium-mediated transformation of Brachypodium distachyon community standard line Bd21. Nature Protocols. 2009;4:638–649. doi: 10.1038/nprot.2009.30. [DOI] [PubMed] [Google Scholar]
- Badawi M, Danyluk J, Boucho B, Houde M, Sarhan F. The CBF gene family in hexaploid wheat and its relationship to the phylogenetic complexity of cereal CBFs. Molecular Genetics and Genomics. 2007;277:533–554. doi: 10.1007/s00438-006-0206-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates L, Waldren R, Teare I. Rapid determination of free proline for water-stress studies. Plant and Soil. 1973;39:205–207. [Google Scholar]
- Bennett MD, Bhandol P, Leitch IJ. Nuclear DNA amounts in angiosperms and their modern uses – 807 new estimates. Annals of Botany. 2000;86:859–909. [Google Scholar]
- Bertrand A, Castonguay Y, Azaiez A, Hsiang T, Dionne J. Cold-induced responses in annual bluegrass genotypes with differential resistance to pink snow mold (Microdochium nivale) Plant Science. 2011;180:111–119. doi: 10.1016/j.plantsci.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Breton G, Danyluk J, Charron JB, Sarhan F. Expression profiling and bioinformatic analyses of a novel stress-regulated multispanning transmembrane protein family from cereals and Arabidopsis. Plant Physiology. 2003;132:64–74. doi: 10.1104/pp.102.015255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charron J, Ouellet F, Houde M, Sarhan F. The plant Apolipoprotein D ortholog protects Arabidopsis against oxidative stress. BMC Plant Biology. 2008;8:86. doi: 10.1186/1471-2229-8-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosatti C, Nevo E, Stanca A, Cattivelli L. Genetic analysis of the accumulation of COR14 proteins in wild (Hordeum spontaneum) and cultivated (Hordeum vulgare) barley. Theoretical and Applied Genetics. 1996;93:975–981. doi: 10.1007/BF00224101. [DOI] [PubMed] [Google Scholar]
- Danyluk J, Houde M, Rassart É, Sarhan F. Differential expression of a gene encoding an acidic dehydrin in chilling sensitive and freezing tolerant gramineae species. FEBS Letters. 1994;344:20–24. doi: 10.1016/0014-5793(94)00353-x. [DOI] [PubMed] [Google Scholar]
- Danyluk J, Perron A, Houde M, et al. Accumulation of an acidic dehydrin in the vicinity of the plasma membrane during cold acclimation of wheat. The Plant Cell. 1998;10:623–638. doi: 10.1105/tpc.10.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danyluk J, Kane N, Breton G, Limin A, Fowler D, Sarhan F. TaVRT-1, a putative transcription factor associated with vegetative to reproductive transition in cereals. Plant Physiology. 2003;132:1849–1860. doi: 10.1104/pp.103.023523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon T, Pearce SP, Stockinger EJ, et al. Regulation of freezing tolerance and flowering in temperate cereals: the VRN-1 connection. Plant Physiology. 2010;153:1846–1858. doi: 10.1104/pp.110.159079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper J, Mur LA, Jenkins G, et al. Brachypodium distachyon A new model system for functional genomics in grasses. Plant Physiology. 2001;127:1539–55. [PMC free article] [PubMed] [Google Scholar]
- Faricelli ME, Valarik M, Dubcovsky J. Control of flowering time and spike development in cereals: the earliness per se Eps-1 region in wheat, rice, and Brachypodium. Functional and Integrative Genomics. 2010;10:293–306. doi: 10.1007/s10142-009-0146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler D, Limin A. Interactions among factors regulating phenological development and acclimation rate determine low-temperature tolerance in wheat. Annals of Botany. 2004;94:717–724. doi: 10.1093/aob/mch196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler B, Chauvin L, Limin A, Sarhan F. The regulatory role of vernalization in the expression of low-temperature-induced genes in wheat and rye. Theoretical and Applied Genetics. 1996a;93:554–559. doi: 10.1007/BF00417947. [DOI] [PubMed] [Google Scholar]
- Fowler D, Limin A, Wang S, Ward R. Relationship between low-temperature tolerance and vernalization response in wheat and rye. Canadian Journal of Plant Science. 1996b;76:37–42. [Google Scholar]
- Fowler D, Breton G, Limin A, Mahfoozi S, Sarhan F. Photoperiod and temperature interactions regulate low-temperature-induced gene expression in barley. Plant Physiology. 2001;127:1676–1681. [PMC free article] [PubMed] [Google Scholar]
- Galiba G, Vágújfalvi A, Li C, Soltész A, Dubcovsky J. Regulatory genes involved in the determination of frost tolerance in temperate cereals. Plant Science. 2009;176:12–19. [Google Scholar]
- Galicia L, Nurit E, Rosales A, Palacios-Rojas N. Laboratory protocols 2009: maize nutrition quality and plant tissue analysis laboratory. Mexioc, D.F: CIMMYT; 2009. [Google Scholar]
- Garvin D. Brachypodium distachyon: a new model system for structural and functional analysis of grass genomes. In: Varshney RK, Koebner RMD, editors. Model plants and crop improvement. Boca Raton, CRC: Taylor & Francis; 2007. pp. 109–123. [Google Scholar]
- Grossi M, Giorni E, Rizza F, Stanca AM, Cattivelli L. Wild and cultivated barleys show differences in the expression pattern of a cold-regulated gene family under different light and temperature conditions. Plant Molecular Biology. 1998;38:1061–1069. doi: 10.1023/a:1006079916917. [DOI] [PubMed] [Google Scholar]
- Guy C. Cold acclimation and freezing stress tolerance: role of protein metabolism. Annual Review of Plant Biology. 1990;41:187–223. [Google Scholar]
- Higgins JA, Bailey PC, Laurie DA. Comparative genomics of flowering time pathways using Brachypodium distachyon as a model for the temperate grasses. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010065. e10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde M, Dhindsa R, Sarhan F. A molecular marker to select for freezing tolerance in Gramineae. Molecular and General Genetics. 1992;234:43–48. doi: 10.1007/BF00272343. [DOI] [PubMed] [Google Scholar]
- Hughes M, Dunn M. The molecular biology of plant acclimation to low temperature. Journal of Experimental Botany. 1996;47:291–305. [Google Scholar]
- Hüner NPA, Öquist G, Sarhan F. Energy balance and acclimation to light and cold. Trends in Plant Science. 1998;3:224–230. [Google Scholar]
- Huo N, Vogel JP, Lazo GR, et al. Structural characterization of Brachypodium genome and its syntenic relationship with rice and wheat. Plant Molecular Biology. 2009;70:47–61. doi: 10.1007/s11103-009-9456-3. [DOI] [PubMed] [Google Scholar]
- Hurry VM, Strand A, Tobiaeson M, Gardestrom P, Oquist G. Cold hardening of spring and winter wheat and rape results in differential effects on growth, carbon metabolism, and carbohydrate content. Plant Physiology. 1995;109:697–706. doi: 10.1104/pp.109.2.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaglo-Ottosen K, Gilmour S, Zarka D, Schabenberger O, Thomashow M. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science. 1998;280:104–106. doi: 10.1126/science.280.5360.104. [DOI] [PubMed] [Google Scholar]
- Kane N, Danyluk J, Tardif G, et al. TaVRT-2, a member of the StMADS-11 clade of flowering repressors, is regulated by vernalization and photoperiod in wheat. Plant Physiology. 2005;138:2354–2363. doi: 10.1104/pp.105.061762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsai I, Szűcs P, Meszaros K, et al. The Vrn-H2 locus is a major determinant of flowering time in a facultative×winter growth habit barley (Hordeum vulgare L.) mapping population. Theoretical and Applied Genetics. 2005;110:1458–1466. doi: 10.1007/s00122-005-1979-7. [DOI] [PubMed] [Google Scholar]
- Kirby E. Botany of the wheat plant. In: Curtis BC, Rajaram S, Gómez Macpherson H, editors. FAO Plant Production and Protection Series. Food and Agriculture Organization of the United Nations (FAO); 2002. [Google Scholar]
- Kirby E, Appleyard M. Cereal development guide. Warwickshire, UK: Arable Unit National Agricultural Centre; 1987. [Google Scholar]
- Kirby EJM. Co-ordination of leaf emergence and leaf and spikelet primordium initiation in wheat. Field Crops Research. 1990;25:253–264. [Google Scholar]
- Kosova K, Vitamvas P, Prasil IT. Expression of dehydrins in wheat and barley under different temperatures. Plant Science. 2011;180:46–52. doi: 10.1016/j.plantsci.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Lee YP, Babakov A, de Boer B, Zuther E, Hincha DK. Comparison of freezing tolerance, compatible solutes and polyamines in geographically diverse collections of Thellungiella sp. and Arabidopsis thaliana accessions. BMC Plant Biology. 2012;12:131. doi: 10.1186/1471-2229-12-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt J. Responses of plants to environmental stresses: chilling, freezing and high temperature stresses. New York: Academic Press; 1980. [Google Scholar]
- Li C, Rudi H, Stockinger EJ, et al. Comparative analyses reveal potential uses of Brachypodium distachyon as a model for cold stress responses in temperate grasses. BMC Plant Biology. 2012;12 doi: 10.1186/1471-2229-12-65. 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limin A, Danyluk J, Chauvin L, Fowler D, Sarhan F. Chromosome mapping of low-temperature induced Wcs120 family genes and regulation of cold-tolerance expression in wheat. Molecular and General Genetics. 1997;253:720–727. doi: 10.1007/s004380050376. [DOI] [PubMed] [Google Scholar]
- Limin A, Corey A, Hayes P, Fowler D. Low-temperature acclimation of barley cultivars used as parents in mapping populations: response to photoperiod, vernalization and phenological development. Planta. 2007;226:139–146. doi: 10.1007/s00425-006-0475-x. [DOI] [PubMed] [Google Scholar]
- Livingston DP, 3rd, Hincha D, Heyer A. Fructan and its relationship to abiotic stress tolerance in plants. Cell and Molecular Life Sciences. 2009;66:2007–2023. doi: 10.1007/s00018-009-0002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo N, Liu J, Yu X, Jiang Y. Natural variation of drought response in Brachypodium distachyon. Physiologia Plantarum. 2011;141:19–29. doi: 10.1111/j.1399-3054.2010.01413.x. [DOI] [PubMed] [Google Scholar]
- Mahfoozi S, Limin A, Fowler D. Developmental regulation of low-temperature tolerance in winter wheat. Annals of Botany. 2001a;87:751–757. [Google Scholar]
- Mahfoozi S, Limin A, Fowler D. Influence of vernalization and photoperiod responses on cold hardiness in winter cereals. Crop Science. 2001b;41:1006–1011. [Google Scholar]
- McNeil S, Nuccio M, Hanson A. Betaines and related osmoprotectants. Targets for metabolic engineering of stress resistance. Plant Physiology. 1999;120:945–949. doi: 10.1104/pp.120.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NDong C, Danyluk J, Wilson KE, Pocock T, Huner NPA, Sarhan F. Cold-regulated cereal chloroplast late embryogenesis abundant-like proteins: molecular characterization and functional analyses. Plant Physiology. 2002;129:1368–1381. doi: 10.1104/pp.001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olien C, Clark J. Changes in soluble carbohydrate composition of barley, wheat, and rye during winter. Agronomy Journal. 1993;85:21–21. [Google Scholar]
- Opanowicz M, Vain P, Draper J, Parker D, Doonan J. Brachypodium distachyon: making hay with a wild grass. Trends in Plant Science. 2008;13:172–177. doi: 10.1016/j.tplants.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Peraldi A, Beccari G, Steed A, Nicholson P. Brachypodium distachyon: a new pathosystem to study Fusarium head blight and other Fusarium diseases of wheat. BMC Plant Biology. 2011;11 doi: 10.1186/1471-2229-11-100. 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidal B, Yan L, Fu D, Zhang F, Tranquilli G, Dubcovsky J. The CArG-box located upstream from the transcriptional start of wheat vernalization gene VRN1 is not necessary for the vernalization response. Journal of Heredity. 2009;100 doi: 10.1093/jhered/esp002. 355. [DOI] [PubMed] [Google Scholar]
- Schwartz C, Doyle M, Manzaneda A, Rey P, Mitchell-Olds T, Amasino R. Natural variation of flowering time and vernalization responsiveness in Brachypodium distachyon. BioEnergy Research. 2010;3:38–46. [Google Scholar]
- Stockinger E, Gilmour S, Thomashow M. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Proceedings of the National Academy of Sciences, USA. 1997;94:1035–1040. doi: 10.1073/pnas.94.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Nass HG. Fructan in winter wheat, triticale and fall rye cultivars of varying cold hardiness. Canadian Journal of Botany. 1988;66:1723–1728. [Google Scholar]
- Theissen G, Kim JT, Saedler H. Classification and phylogeny of the MADS-box multigene family suggest defined roles of MADS-box gene subfamilies in the morphological evolution of eukaryotes. Journal of Molecular Evolution. 1996;43:484–516. doi: 10.1007/BF02337521. [DOI] [PubMed] [Google Scholar]
- Thomashow M. Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annual Review of Plant Physiology and Plant Molecular Biology. 1999;50:571–599. doi: 10.1146/annurev.arplant.50.1.571. [DOI] [PubMed] [Google Scholar]
- Thomashow M. So what's new in the field of plant cold acclimation? Lots! Plant Physiology. 2001;125:89. doi: 10.1104/pp.125.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay K, Ouellet F, Fournier J, Danyluk J, Sarhan F. Molecular characterization and origin of novel bipartite cold-regulated ice recrystallization inhibition proteins from cereals. Plant and Cell Physiology. 2005;46:884–891. doi: 10.1093/pcp/pci093. [DOI] [PubMed] [Google Scholar]
- Vágújfalvi A, Kerepesi I, Galiba G, Tischner T, Sutka J. Frost hardiness depending on carbohydrate changes during cold acclimation in wheat. Plant Science. 1999;144:85–92. [Google Scholar]
- Vain P, Worland B, Thole V, et al. Agrobacterium-mediated transformation of the temperate grass Brachypodium distachyon (genotype Bd21) for T-DNA insertional mutagenesis. Plant Biotechnology Journal. 2008;6:236–245. doi: 10.1111/j.1467-7652.2007.00308.x. [DOI] [PubMed] [Google Scholar]
- Van Buskirk HA, Thomashow MF. Arabidopsis transcription factors regulating cold acclimation. Physiologia Plantarum. 2006;126:72–80. [Google Scholar]
- Vogel JT, Zarka DG, Van Buskirk HA, Fowler SG, Thomashow MF. Roles of the CBF2 and ZAT12 transcription factors in configuring the low temperature transcriptome of Arabidopsis. The Plant Journal. 2005;41:195–211. doi: 10.1111/j.1365-313X.2004.02288.x. [DOI] [PubMed] [Google Scholar]
- Vogel J, Bragg J. Brachypodium distachyon, a new model for the Triticeae. In: Feuillet C, Muehlbauer GJ, editors. Genetics and genomics of the Triticeae (Plant genetics and genomics: crops and models). New York: Springer; 2009. pp. 427–449. [Google Scholar]
- Vogel J, Garvin D, Mockler T, et al. Genome sequencing and analysis of the model grass Brachypodium distachyon. Nature. 2010;463:763–768. doi: 10.1038/nature08747. [DOI] [PubMed] [Google Scholar]
- Wang S, Ward R, Ritchie J, Fischer R, Schulthess U. Vernalization in wheat I. A model based on the interchangeability of plant age and vernalization duration. Field Crops Research. 1995;41:91–100. [Google Scholar]
- Yan L, Loukoianov A, Tranquilli G, Helguera M, Fahima T, Dubcovsky J. Positional cloning of the wheat vernalization gene VRN1. Proceedings of the National Academy of Sciences, USA. 2003;100 doi: 10.1073/pnas.0937399100. 6263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Abe J, Moriyama M, Kuwabara T. Carbohydrate levels among winter wheat cultivars varying in freezing tolerance and snow mold resistance during autumn and winter. Physiologia Plantarum. 1998;103:8–16. [Google Scholar]
- Zhao S, Fernald RD. Comprehensive algorithm for quantitative real-time polymerase chain reaction. Journal of Computational Biology. 2005;12:1047–1064. doi: 10.1089/cmb.2005.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zitzewitz J, Szucs P, Dubcovsky J, et al. Molecular and structural characterization of barley vernalization genes. Plant Molecular Biology. 2005;59:449–467. doi: 10.1007/s11103-005-0351-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.