Abstract
Background and Aims Pterostylis
is an Australasian terrestrial orchid genus of more than 400 species, most of which use a motile, touch-sensitive labellum to trap dipteran pollinators. Despite studies dating back to 1872, the mechanism of pollinator attraction has remained elusive. This study tested whether the fungus gnat-pollinated Pterostylis sanguinea secures pollination by sexual deception.
Methods
The literature was used to establish criteria for confirming sexual deception as a pollination strategy. Observations and video recordings allowed quantification of each step of the pollination process. Each floral visitor was sexed and DNA barcoding was used to evaluate the degree of pollinator specificity. Following observations that attraction to the flowers is by chemical cues, experimental dissection of flowers was used to determine the source of the sexual attractant and the effect of labellum orientation on sexual attraction. Fruit set was quantified for 19 populations to test for a relationship with plant density and population size.
Key Results
A single species of male gnat (Mycetophilidae) visited and pollinated the rewardless flowers. The gnats often showed probing copulatory behaviour on the labellum, leading to its triggering and the temporary entrapment of the gnat in the flower. Pollen deposition and removal occurred as the gnat escaped from the flower via the reproductive structures. The labellum was the sole source of the chemical attractant. Gnats always alighted on the labellum facing upwards, but when it was rotated 180 ° they attempted copulation less frequently. Pollination rate showed no relationship with orchid population size or plant density.
Conclusions
This study confirms for the first time that highly specific pollination by fungus gnats is achieved by sexual deception in Pterostylis. It is predicted that sexual deception will be widespread in the genus, although the diversity of floral forms suggests that other mechanisms may also operate.
Keywords: Orchid, pollination, sexual deception, fungus gnat, Pterostylis, semiochemicals, specialization, Mycetophilidae
INTRODUCTION
Sexual deception is a pollination strategy in which rewardless flowers secure pollination by the sexual attraction of male insects by chemical and/or physical mimicry of a female, with pollination often achieved during attempted courtship or copulatory behaviour (Peakall, 1990; Schiestl, 2005; Gaskett, 2011). While primarily known from the Orchidaceae (Schiestl, 2005; Gaskett, 2011), sexual deception has recently been discovered cases in the Asteraceae (Ellis and Johnson, 2010) and the Iridaceae (Vereecken et al., 2012), suggesting that the phenomenon may be much more taxonomically widespread than currently appreciated. Within the Orchidaceae, work in comparatively poorly studied tropical floras has revealed several new cases of sexual deception, greatly increasing the range of orchid tribes and pollinator groups involved (Singer, 2002; Singer et al., 2004; Blanco and Barboza, 2005; Ciotek et al., 2006). Taken together, these discoveries suggest that the evolution of sexual deception may be much more frequent than presently documented.
As a consequence of mimicking the specific sexual signals of female insects, pollination by sexual deception is a highly specialized strategy, with most species reliant on the chemical attraction of just a single pollinator species (Paulus and Gack, 1990; Phillips et al., 2009; Peakall et al., 2010; Phillips, 2010). However, as sexually deceptive systems are studied in increasing depth, a diversity of floral adaptations is being revealed. For example, the chemical attractants involved in the long-range attraction of pollinators encompass a range of chemical classes, such as chiloglottones (2,5-dialkycyclohexane-1,3-diones), pyrazines, unsaturated hydrocarbons and oxygenated acids (Schiestl et al., 1999, 2003; Ayasse et al., 2003; Franke et al., 2009; Bohman et al., 2012a, b). The importance and role of floral colour likely vary between systems, but at short range it may enhance attraction by direct mimicry (Gaskett and Herberstein, 2010), maximizing colour contrast to aid detectability (Streinzer et al., 2009) or exploiting sensory bias (Gaskett, 2011). Using a case of pollinator sharing between the unrelated sexually deceptive orchids Drakaea livida and Caladenia pectinata, Phillips et al. (2013) showed that floral traits that affect pollinator behaviour have direct consequences for plant fecundity. In D. livida, which releases its sexual attractant from the labellum, there is higher pollination success than in C. pectinata, which produces its attractant from sepals distal from the reproductive parts of the flower (Phillips et al., 2013). Consequently, understanding the range of floral adaptations to pollinator behaviour will prove important for determining the traits underpinning the evolution and function of sexually deceptive systems.
While most sexual deceptive pollination systems involve Hymenoptera (Schiestl, 2005), there are many orchid genera pollinated by Diptera that may harbour such systems. For example, in the Pleurothallidinae, which contains over 4100 species, almost all records of pollination concern Diptera, but the mechanism of pollinator attraction has been resolved in only a handful of species (Pridgeon, 2005; but see Borba and Semir, 2001; Barbosa et al., 2009; Melo et al., 2011). Until now, the only well documented cases of pollination by sexual deception of Diptera in the Orchidaceae are in the large neotropical orchid genus Lepanthes (>700 species; Blanco and Barboza, 2005). Observations of the pollinator behaviour in Lepanthes revealed that the courtship and copulatory behaviour of sciarid fungus gnats explained floral adaptations not seen in other sexually deceptive systems. For example, the presentation of the flowers against the surface of the leaf matches the calling position of the female gnat (Blanco and Barboza, 2005). Consequently, the discovery of sexual deception of Diptera by other orchids is expected to yield important insights beyond those achieved for species pollinated by Hymenoptera.
The diverse Australasian orchid genus Pterostylis (>400 species; Jones, 2006; Brown et al., 2008) has long been known to be pollinated by small Diptera (Sargent, 1909), with most recorded floral visitors being members of the Mycetophilidae (Hyett, 1960; Bernhardt, 1995; Brown et al., 1997; Gaskett, 2011; but see Coleman, 1934). However, despite a history of study dating back to the 19th century (Cheeseman, 1872), the mechanism of pollinator attraction has not been resolved for a single species (Adams and Lawson, 1993; Gaskett, 2011). All species investigated are recorded as being nectarless and use a touch-sensitive, motile labellum to trap pollinating insects within a galeate fusion of the lateral and dorsal sepals (Sargent, 1909; Erickson, 1951; Jones, 2006). While all species are dull green, brown or red-brown, there is considerable variation in floral morphology, leading to sexual deception and shelter site both being suggested as potential pollination strategies (Adams and Lawson, 1993; Bernhardt, 1995). In particular, in some species complexes, the insectiform labella and anecdotal accounts of pollinator behaviour (Jones, 2003; Gaskett, 2011) are suggestive of the strategy of sexual deception.
Our discovery of male fungus gnats as the pollinators of Pterostylis sanguinea provided an opportunity to objectively assess whether or not pollination by sexual deception might be operating in Pterostylis. Because claims of sexual deception in Pterostylis are either speculative or based only on anecdotal observations, we first established a set of criteria that characterizes other sexually deceptive systems. We conducted observations and experiments to assess the case for sexual deception in P. sanguinea against these objective criteria. The sexual behaviour of the pollinator with the flower was quantified, and we used experiments to investigate the role of floral odour and morphology in sexual attraction. Using DNA barcoding of insects attracted to the orchid, we tested for the high pollinator specificity evident in other sexually deceptive systems. We also evaluated the relationships of fruit set with population size and density to understand the ecological consequences of pollination by sexual deception of fungus gnats.
MATERIALS AND METHODS
Pterostylis sanguinea is a common and widespread species ranging across south-western Australia, southern South Australia, Victoria and Tasmania (Jones, 2006; Hoffman and Brown, 2011). Flowering occurs in winter, with peak flowering in June and July (Hoffman and Brown, 2011). Two to five flowers are typically produced per stem, but large individuals can produce 12 or more flowers with up to four flowers open at any one time (Fig. 1; Hoffman and Brown, 2011). Flower colours range between red-brown and green, with various colour forms often co-occurring within a population. Pterostylis sanguinea is self-compatible but reliant on a pollen vector for pollination (Retter, 2009). Thus far, the pollinator of P. sanguinea has not been recorded, although an unidentified species of fungus gnat (Mycetophilidae) has been found dead in some flowers (Retter, 2009).
Fig. 1.

Pterostylis sanguinea, illustrating flowers and growth habit. In the study region, larger individuals regularly grow to 40 cm. Photograph by R. D. Phillips.
Observations of the trap mechanism and movement of the pollinator through the flower have been published for the closely related Pterostylis vittata (Sargent, 1909). However, based on the region where Sargent made his observations, it is likely that these plants were actually P. sanguinea. For pollination to occur, the pollinator must make contact with the labellum, which then swings upwards, trapping the gnat within the galea (Sargent, 1909) (Fig. 2). The labellum remains in this position, keeping the pollinator trapped within the flower, where it flies upwards, keeping it in regular contact with the column (Sargent, 1909). After several minutes the pollinator crawls through the passage formed by the column wings (lined with angled bristles to ensure one-way movement of the insect), bringing it into contact with the lightly hinged anther, which then deposits the pollinia as it leaves the flower (Sargent, 1909) (Fig. 2). This one-way passage of the pollinator effectively prevents self-pollination. Sargent (1909) estimated that the labellum takes approximately 2 h to reset, but noted that the time was delayed by cool conditions.
Fig. 2.

Demonstration of the hinge mechanism and internal structure of the galea of Pterostylis sanguinea. (A) Flower with the hinge ready to trap a pollinator. (B) The labellum has triggered and moved into the ‘up’ position and the column wing has been removed to show the passage through which the gnat must move to exit the flower. The flower is approximately 10 mm across the galea. Photographs by K. W. Dixon.
Confirmation and description of pollination by sexual deception
Criteria for confirming sexual deception
We define pollination by sexual deception as a strategy in which plants lure pollinators with signals that are sexually attractive to the pollinator. While there is considerable diversity among sexually deceptive systems, meeting one or more of the criteria listed below provides confirmation of sexual deception (Table 1). It should be noted that the level of sexual response can vary markedly between individual visitors, meaning that in many cases only a portion of the insects arriving at a flower will show the full potential repertoire of sexual behaviour at the flower (e.g. approach, alight and attempted copulation; Peakall, 1990; Phillips et al., 2013). Further, the level of the sexual response can also vary with the time of day and stage in the season (Paulus and Gack, 1990).
Pre-mating behaviour. Any behaviour associated with courtship (e.g. wing fanning of gnats towards Lepanthes; Blanco and Barboza, 2005) or the initiation of mating behaviour towards the flower (e.g. male thynnine wasps attempting to grasp a Drakaea labellum while in flight; Peakall, 1990). The identification of pre-mating behaviour requires an understanding of the sexual behaviour of mating pairs of the pollinator.
Attempted copulation. An attempt to initiate copulation with the flower (e.g. Coleman, 1928; Kullenberg, 1961; Peakall, 1989; Paulus and Gack, 1990; Schiestl et al., 1999; Blanco and Barboza, 2005; Phillips et al., 2013).
Ejaculation. Sperm release during attempted copulation with the flower. Ejaculation is known for pollinators of two sexually deceptive orchid genera (Blanco and Barboza, 2005; Gaskett et al., 2008), but appears unlikely to occur in most others.
Chemical mimicry of sex pheromones. Flowers produce semiochemicals that mimic the chemical composition of the sex pheromone of the pollinator (e.g. Schiestl et al., 1999, 2003; Stokl et al., 2007; Ayasse et al., 2011). Bioassays with synthetic semiochemicals are required to confirm that the specific compounds (or specific blends) elicit sexual attraction and sexual behaviour (e.g. Peakall et al., 2010). Sexual deception may still be involved when a pollinator exhibits a flight pattern indicative of tracking an odour plume, but fails to show any subsequent sexual behaviour at the flower. This distinction may be particularly important in cases where entrapment may prevent the full repertoire of sexual behaviour. Here, sexual attraction could be tested by presenting the scent-producing parts in isolation from the remainder of the flower or by adding chemical extracts from the flower to a dummy female.
Table 1.
Evidence required for confirmation that pollination occurs via sexual deception
| Characteristics of sexual deception |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chemical attractant |
Confirmation of sexual deception |
||||||||||||
| Species | Pollinator | MALE | POL (N) | REWARD | INFORM | RA | OP | EX | PRE-MATE | COPULA | EJAC | Pheromone | Reference |
| Orchidaceae | |||||||||||||
| Caladenia pectinata | Thynnine wasp | Yes | 1 | No | No | Yes | Yes | Yes | Yes | Yes | Not likely | Unknown | Phillips et al. (2013) |
| Calochilus holtzei | Scoliid wasp | Yes | 1? | No | Yes | Yes | X | X | Yes | Yes | Not likely | Unknown | Jones and Gray (1974) |
| Chiloglottis trapeziformis | Thynnine wasps | Yes | 2 | No | Yes | Yes | Yes | Yes | Yes | Yes | Not likely | Chiloglottones | Schiestl et al. (2003) |
| Cryptostylis erecta | Ichneumonid wasp | Yes | 1 | No | Yes | Yes | Yes | Yes | ? | Yes | Yes | Unknown | Gaskett et al. (2008) |
| Disa atricapilla | Sphecid wasp | Yes | 1 | No | No | X | Yes | X | ? | No | No | Unknown | Steiner et al. (1994) |
| Drakaea glyptodon | Thynnine wasp | Yes | 1 | No | Yes | Yes | Yes | Yes | Yes | Yes | Not likely | Pyrazines | Peakall (1990), Bohman et al. (2012a) |
| Geoblasta penicillata | Scoliid wasp | Yes | 1 | No | Yes | X | Yes | X | ? | Yes | Not likely | Unknown | Ciotek et al. (2006) |
| Lepanthes glicensteinii | Sciarid gnat | Yes | 1 | No | Yes | X | Yes | X | Yes | Yes | Yes? | Unknown | Blanco and Barboza (2005) |
| Leporella fimbriata | Formicid ant | Yes | 1 | No | Yes | No | Yes | Yes | ? | Yes | Not likely | Unknown | Peakall (1989) |
| Mormolyca ringens | Apid bees | Yes | 2 | No | Yes | X | X | X | ? | Yes | Not likely | Alkenes and alkanes? | Singer et al. (2004); Flach et al. (2006) |
| Ophrys sphegodes | Andrenid bee | Yes | 5? | No | Yes | Yes | Yes | Yes | ? | Yes | Not likely | Alkenes and alkanes | Schiestl et al. (1999), Gaskett (2011) |
| Orchis galilaea | Halictid bee | Yes | 1 | No | No | X | Yes | X | No | No | No | Unknown | Bino et al. (1982) |
| Paracaleana nigrita | Thynnine wasp | Yes | X | No | Yes | Yes | Yes | X | X | X | X | Unknown | Hopper and Brown (2006) |
| Pterostylis sanguinea | Mycetophilid gnat | Yes | 1 | No | Yes | Yes | Yes | Yes | Yes | Yes | Not likely | Unknown | Present study |
| Serapias lingua | Apid bee | Yes | 1? | No | Yes | X | X | X | ? | Yes | Not likely | Unknown | Vereecken et al. (2012) |
| Trigonidium obtusum | Meliponine bee | Yes | 1 | No | No | X | No? | X | ? | Yes | Not likely | Unknown | Singer (2002) |
| Spiculaea ciliata | Thynnine wasp | Yes | 1 | No | Yes | Yes | Yes | Yes | Yes | Yes | Not likely | Unknown | Alcock (2000) |
| Asteraceae | |||||||||||||
| Gorteria diffusa | Bombyliid fly | No | 1 | Yes | Yes | No | No | X | Yes | Yes | No | No pheromone? | De Jager and Ellis (2012) |
| Iridaceae | |||||||||||||
| Iris paradoxa | Apid bee | Yes | 1? | X | Yes | X | X | X | Yes | Yes | Not likely | Unknown | Vereecken et al. (2012) |
Examples from the literature are used to show the characteristics of sexual deception and identify gaps in the literature: X, this element has not been tested or quantified; MALE, only males are pollinators; POL (N), number of pollinator species. REWARD, a reward is provided to the pollinator; INFORM, insectiform flower.
A chemical attractant can be confirmed with three lines of evidence: RA, rapid attraction of pollinators; OP, the pollinator behaves as if it is tracking an odour plume; EX, experiments such as covering of flowers. PRE-MATE, pre-mating behaviour such as courtship signals or grasping prior to copulation; COPULA, attempts copulation with the flower; EJAC, ejaculates on the flower.
Note that (1) not all pollinators responding to a given species show the full repertoire of behaviours, and (2) data in this table only relate to pollinators attracted by sexual deception.
In the absence of observations that meet the above criteria, other lines of evidence may be indicative of pollination by sexual deception. However, these traits alone are insufficient to confirm sexual deception (Table 1).
Males only. All known cases of sexual deception involve the attraction of male pollinators (Gaskett, 2011), but female-only attraction remains a possibility. It is worth noting that for some insects, such as thynnine wasps, in which the females are wingless, males are the only sex available as potential pollinators (Peakall, 1990). Therefore, when observing pollination by males only, the biology of the insect species needs to be considered before interpreting this as evidence for sexual deception.
Food reward absent. There are no confirmed cases of sexually deceptive orchids rewarding pollinators with nectar. However, in Gorteria diffusa (Asteraceae), male and female pollinators take nectar and pollen from flowers, while males are also sexually attracted to the dark spots present in some morphotypes (De Jager and Ellis, 2012).
Highly specific. In most species, only one or two pollinator species are attracted via sexual deception (see review in Gaskett, 2011). However, in some sexually deceptive orchids, food-foraging insects can potentially act as pollinators (Steiner et al., 1994).
Chemical attractant. All known sexually deceptive orchids attract their pollinators via floral odour. By contrast, in G. diffusa attraction of sexually deceived pollinators is by visual cues (De Jager and Ellis, 2012). Chemical attraction can be confirmed by attracting pollinators to covered flowers (Kullenberg, 1961), an approach flight associated with insects following an odour plume (Stoutamire, 1983) or the rapid attraction of males to floral odours released from artificially presented flowers (Peakall, 1990).
Insectiform floral structure. Most species of sexually deceptive orchids have an insectiform floral structure (e.g. Coleman, 1928; Kullenberg, 1961; Stoutamire, 1974; Paulus and Gack, 1990), though there are exceptions (e.g. Phillips et al., 2009). We consider insectiform orchid flowers to have some or all of the following traits: dull coloured, inconspicuous flowers; reduced petals and sepals; a large labellum relative to the remaining petals and sepals; the presence of hairs and/or a pronounced texture. In the case of G. diffusa, dark floral spots on the otherwise brightly coloured ray florets are associated with sexual behaviour (De Jager and Ellis, 2012).
Description of behaviour of the pollinator on the flower
Observations of pollinator behaviour were made in Kings Park, Western Australia, with additional observations at nearby Star Swamp Reserve (Table 2). At both sites, P. sanguinea was present. Flower-visiting gnats were most active between 1000 and 1600 h, though flight times were delayed on cold mornings (e.g. <5 °C). All observations were made between 1000 and 1600 h across 7 days between 21 June and 11 July 2013 (487 min of observation in total). During the observation periods temperature ranged between 17·5 and 22 °C, as measured with a Tinytag data logger (Gemini Data Loggers) suspended 30 cm above the ground (approximating flower height).
Table 2.
Study populations used to survey for pollinators of Pterostylis sanguinea
| Site | Latitude/longitude | Flowers dissected (late June) | Flowers dissected (mid-July) | Minutes of baiting | Gnats with pollen |
|---|---|---|---|---|---|
| Alfreton Reserve | 31·82691 °S, 115·78108 °E | 44 | Not surveyed | 60 (1) | 0 |
| Breckler Park | 31·89863 °S, 115·86318 °E | 55 | 35 | Not surveyed | 0 |
| Kings Park, population 1 | 31·95623 °S, 115·83578 °E | 30 | Not surveyed | 60 | 0 |
| Kings Park, population 2 | 31·95469 °S, 115·83208 °E | 25 (1) | 21 | 60 | 0 |
| Kings Park, population 3 | 31·96215 °S, 115·82569 °E | Not surveyed | 10 | Not surveyed | 0 |
| Kings Park, population 4 | 31·95727 °S, 115·84156 °E | Not surveyed | Not surveyed | 307 (134) | 6 |
| Paloma Park | 31·86520 °S, 115·75229 °E | 20 | 32 | Not surveyed | 0 |
| Pinnaroo Valley | 31·81411 °S, 115·77867 °E | 20 (1) | 20 | 60 | 1 |
| Shepherds Bush Reserve | 31·81004 °S, 115·79347 °E | 33 | 20 | 60 | 0 |
| Shenton Park | 31·8004 °S, 115·79347 °E | 32 (2) | 25 | 60 | 1 |
| Star Swamp Reserve, population 1 | 31·85824 °S, 115·75948 °E | 33 (2) | 30 (1) | 120 (25) | 2 |
| Star Swamp Reserve, population 2 | 31·85464 °S, 115·76125 °E | 11 | 8 | Not surveyed | 0 |
| Trigg Reserve | 31·87030 °S, 115·76025 °E | 31 | 35 | 60 | 0 |
| Total | 334 (6) | 236 (1) | 487 (160) | 10 |
Numbers in parentheses are the number of gnats detected.
Bold indicates the surveys at which gnats were detected.
We conducted observations of pollinator behaviour based on the baiting method of Stoutamire (1974) and Peakall (1990), in which picked flowers are used to attract pollinators. As observed in sexually deceptive orchids pollinated by thynnine wasps, relocating bait flowers to a new location initiates a renewed response. Picked flowers were maintained in vials of water at all times and stored in a refrigerator at 4 °C between experiments. Three flowering stems were used simultaneously as an attractant, with one to three flowers open per stem. We baited at random positions within the population of fungus gnats, with consecutive positions greater than 3 m apart. Baiting was undertaken for 5-min periods in each position. If no gnats were attracted during this period, orchids were moved to another position. If gnats were attracted, we continued to make observations until visitation ceased. A total of 32 observation periods were conducted.
Pollinator behaviour at the flower was recorded for subsequent detailed evaluation with a Sony Digital HD Video Camera Recorder. Since no courting or mating pairs were observed during the study, the curling of the abdomen below the thorax was taken as evidence of courtship behaviour [as illustrated in Blanco and Barboza (2005) for sciarid gnats], while vigorous probing with the tip of the abdomen was interpreted as attempted copulatory behaviour. The position of the abdomen during copulatory probing was quantified for 29 separate visits. Probes were classed as being made at the labellum apex, along the lower section of the surface of the labellum, towards the upper section of the labellum or immediately below the callus (Fig. 3).
Fig. 3.

Labellum of Pterostylis sanguinea. Letters refer to landmarks used when describing the copulatory behaviour of the pollinator on the flower. A, apex; LS, lower section; US, upper section; C, callus.
During field observations, pollinator behaviour was quantified across a hierarchy of responses following Peakall (1990) and Phillips et al. (2013). For each floral visitor (Fig. 4) we recorded the presence of pollen on arrival, whether or not the pollinator touched the labellum, the duration of any attempted copulation and the incidence of labellum triggering. If the labellum was triggered, we recorded the duration of pollinator entrapment and whether or not pollination was achieved. For each visitor, the time taken to arrive at an artificially presented bait flower and the total time spent on the flower were also recorded.
Fig. 4.

Male fungus gnat (genus Mycomya) showing copulatory behaviour with the labellum of Pterostylis sanguinea. Photograph by R. D. Phillips.
Due to the difficulty of observing such small insects in the wild (body length of approximately 3·5 mm), it was only possible to observe their approach to the flower when the sun was low in the sky and so illuminating their wings in flight. Therefore, we could only make general observations of how they approached the flower.
How long does the labellum take to reset?
On 12 July we conducted an experiment in Kings Park bushland to quantify how long it takes the labellum to reset after being trigged. The experiment was conducted between 1430 and 1630 h, coinciding with the period of peak gnat activity. Temperature ranged between 15·7 and 19·1 °C in sunny weather, following rain the previous day. We randomly selected one flower from each of 20 different plants in a population over an area of 15 × 15 m. At 1430 h a wooden skewer was used to trigger all 20 flowers. Each flower was then checked every 2 min to record whether the labellum had returned to the down position as required for pollination.
Specificity of the pollination system
How many species of fungus gnats are involved?
Thirteen populations of P. sanguinea were visited to collect pollinators (Table 2), using two approaches: (1) baiting for 12 periods of 5 min at each site, during which any visitors to the flower were collected; (2) following observations that gnats are sometimes terminally trapped in Pterostylis flowers (Sargent, 1909; Bernhardt, 1995; Retter, 2009), flowers were dissected to check for the presence of gnats and whether or not they were carrying pollen. The floral dissections were done twice, once early and once late in the flowering season (n = 19–90 flowers per population, 570 flowers in total; Table 2). All gnats collected were placed in 80 % ethanol for subsequent identification and DNA extraction. Any gnats collected carrying pollen were observed under a dissecting microscope (Leica DCF450) to determine the positioning of the pollen on the animal.
To assess the degree of pollinator specificity, we sequenced the mitochondrial CO1 region, a region commonly used for DNA barcoding in Diptera (Meier et al., 2008). A total of 24 gnats were sequenced from the three populations where live gnats were collected (Kings Park, Shenton Park and Star Swamp). DNA was extracted from whole gnats using a modified salt extraction method (Bruford et al., 1998). Briefly, the gnats were added to 250 μl TNES buffer with 30 μl proteinase K (10 mg ml−1), mixed gently and incubated at 55 °C for a minimum of 18 h. The buffer was then transferred to a tube containing 95 μl of 4 m ammonium acetate, mixed, incubated on ice for 20 min, then centrifuged for 20 min at 16 000 × g. The intact pollinator was returned to storage in 80 % ethanol. Following centrifugation, the supernatant (400 μl) was transferred to a clean tube and 1 mL of cold 100 % ethanol was added and the tube contents were thoroughly mixed before incubation for at least 18 h to precipitate the DNA. A pellet of DNA was formed by spinning at 16 000 × g for 20 min. The supernatant was discarded and the DNA pellet was washed with 500 μl of cold 70 % ethanol and allowed to dry before resuspending in 80 μl TE.
Amplification and sequencing followed the methods of Griffith et al. (2011). We used the primers of Folmer et al. (1994) as recommended by the Consortium for the Barcoding of Life (http://www.barcoding.si.edu/protocols.html). Sequences were edited and aligned using Geneious v6·1·6 created by Biomatters Ltd. A phylogenetic analysis was undertaken using the Phyml plugin in Geneious, using a substitution model of GTR + G. To quantify the genetic divergence within morphologically defined gnat species, we calculated the average Kimura 2 parameter (K2P) distances (Kimura, 1980) and the average percentage of varying base pairs using MEGA 5·05 (Tamura et al., 2011).
Are all populations of Pterostylis sanguinea attractive to the same pollinator species?
To test whether all study populations of P. sanguinea attracted the same pollinator, two flowering stems from each population were picked for baiting in the Kings Park gnat population. When possible we collected individuals representing both green and brown colour forms. Picked orchids were artificially presented within the gnat population and left exposed until a gnat arrived at the flower and was observed to attempt copulation. These gnats were then captured and included in the DNA sequencing dataset.
What is the role of the site of scent release and morphology in sexual attraction?
Is pollinator attraction by floral odour or visual cues?
The ability of gnats to locate hidden flowers was investigated by obscuring flowers in a black plastic screen made from a washed 1·5 L PET bottle. The screen was 15 cm high but open at the top and raised 5 cm off the ground, allowing access to the concealed flowers. Each trial consisted of a 2-min presentation without flowers within the screen, followed by a 2-min presentation with the flowers. The number of gnats approaching the screen and locating the hidden flowers was recorded for 30 trials in bushland in Kings Park.
Site of production of the sexual attractant
Following the observation that copulatory behaviour was only ever observed on the labellum, we tested the hypothesis that the labellum is the sole source of the chemical attractant by dissecting flowers. Each trial consisted of the sequential presentation of the flower without the labellum for the first 5 min, followed by simultaneous presentation of both the flower (minus labellum) and the labellum only (each presented pinned on a separate wooden skewer). The number of gnats responding was recorded along with evidence of sexual behaviour. This trial was repeated on five occasions per flower, for a total of nine flowers.
Does the morphology of the labellum determine the orientation of the pollinator?
Since gnats typically alighted on the labellum facing upwards, we tested whether the orientation of the labellum influenced their behaviour. We removed the labella and affixed them to wooden skewers using UHU All Purpose Adhesive. Labella were either positioned in the natural vertical position or rotated 180 °. Trials were undertaken by presenting a labellum to the fungus gnats until a response was achieved. For every response we alternated between the treatments used. We recorded whether each gnat faced upwards, downwards or sideways and whether they exhibited sexual behaviour (bending and/or probing of the abdomen). Each subsequent trial was conducted at least 1 m from the previous location. If no gnats were attracted within 5 min the trial was terminated. This experiment was conducted using labella from six different plants, for a total of 32 observations per treatment. We employed G-tests calculated in GenAlEx 6·5 (Peakall and Smouse, 2006, 2012) to compare both the proportion of gnats copulating with the flower and the proportion showing abdomen bending between treatments.
Ecological consequences of deception of fungus gnats
The influence of population size and density on fruit set
Fruit set (percentage of flowers in a population setting fruit) was recorded for 19 populations in 2008 and 2012. Population size ranged from five to 127 plants. The position of all individuals, as measured with a GPS, was uploaded to ArcGIS 9·3·1 and the minimum convex polygon was calculated to determine the area and density of the population. Regression analysis was conducted in PASW statistics 18 (SPSS Inc., 2009) to determine whether there is a relationship between fruit set and plant population size or density. The 2008 and 2012 data sets were analysed separately. As a test for resource-limited fruit set, in 2008 all flowers on six plants were cross-pollinated by hand in 13 populations. Pollen was sourced from plants more than 5 m from the pollen recipient.
RESULTS
Confirmation and description of pollination by sexual deception
Confirmation of pollination by sexual deception
All insects collected at the flowers (n = 33) were male fungus gnats belonging to the family Mycetophilidae (genus Mycomya). Ten male gnats visiting flowers were observed carrying pollen of P. sanguinea (Table 2). Of the nine found dead in flowers, two were carrying pollen of P. sanguinea (Fig. 5). Five gnats were observed to remove pollinia following passage through the flower. One pollen-laden gnat was followed through the entire pollination sequence from alighting on the flower, through attempted copulation, labellum triggering, entrapment, pollen transfer and pollen removal, and departure from the flower. These results confirm that males mycetophilids are legitimate pollinators of P. sanguinea (Supplementary Data Video).
Fig. 5.
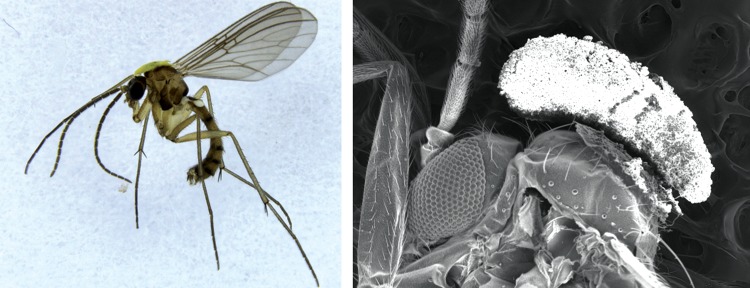
Male fungus gnat (genus Mycomya) showing the position of pollen deposition. Photographs by D. Scaccabarozzi (left) and K. W. Dixon (right).
Of the 135 gnats alighting on the flower, 57·8 % exhibited sexual behaviour with the labellum. When attempting copulation with the flower (Fig. 4), gnats regularly probed at the labellum apex (55·2 %), the lower section of the labellum surface (51·7 %), the upper section of the labellum surface (51·7 %) and the labellum callus (37·9 %). Attempted copulations lasted on average 3·5 ± 0·2 s (minimum = 1; maximum = 26 s; mode = 2). Males that attempted copulation averaged 2·9 ± 0·3 copulation attempts per flower (minimum = 1; maximum = 10; mode = 1).
Description of behaviour of the pollinator on the flower
During behavioural observations, a total of 135 gnats were observed alighting on the study flowers. Due to the small size of this gnat species, it was not possible to accurately judge how many gnats approached the flowers in flight but then flew away without alighting. Similarly, it was difficult to observe gnat behaviour as they arrived at the flowers in flight. However, on the few occasions when the sunlight was at a suitable angle, it was observed that the gnats arrived at the flower after flying 10–30 cm above the ground, in a rapid zig-zag flight. The highest number of gnats arriving at the flower was within the first 30 s after the trial commenced, with visitations gradually decreasing (Fig. 6).
Fig. 6.
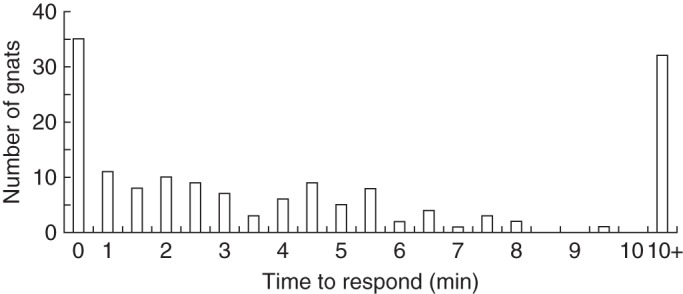
Time taken by gnats to respond to experimentally presented bait flowers of Pterostylis sanguinea. The rapid response is characteristic of some families of insects that are attracted to flowers by the release of floral odours that mimic sex pheromones (e.g. thynnine wasps and ichneumonid wasps).
All but two of the 135 gnats observed alighting on the flower landed directly on the labellum. The labellum triggered on 13 occasions, with six triggerings resulting in entrapment. Consequently, only 4·4 % of visitations were likely to lead to contact with the stigma. On the three occasions when triggering of the labellum was filmed, the gnat was either copulating with the labellum apex (twice) or the callus (once) while grasping the labellum and appeared to pull the labellum towards its body. Once inside, gnats were observed flying inside the bulbous portion of the galea, likely bringing them into frequent contact with the stigma. Pollen was evenly distributed over the stigmatic surface (n = 10), indicating that the pollen is gradually smeared over the surface rather than deposited as intact pollinia. Gnats were trapped within the flower for between 1 and 7 min (mean = 226 ± 63 s).
On 18 occasions a gnat alighted on a flower when the labellum had already been triggered. In each of these cases the gnat attempted to grasp the labellum. Twice the gnat was observed to reset the labellum by pulling it down. While the gnats began to attempt copulation, the labellum did not trigger. On six occasions the gnats showed at least some copulatory behaviour with the triggered labellum.
How long does the labellum take to reset?
The labellum showed considerable variation in the length of time to reset, ranging from 6 to 112 min (mean = 52·5 ± 7·7, n = 20).
Specificity of the pollination system
How many species of fungus gnats are sexually attracted?
The taxonomy of fungus gnats in Australia is poorly resolved, with the only substantial taxonomic paper post-1900 being a generic-level revision (Tonnoir, 1929). Colless (1970) estimated that only one-third of the Australian fauna had been described. Using the generic key in Tonnoir (1929), we identified the gnats as a single species of the genus Mycomya. However, due to a paucity of relevant literature, we have been unable to identify the gnat at the species level. It should be noted that the revision of Tonnoir (1929) included no specimens from the western half of the continent, suggesting that this is likely to represent an undescribed taxon. Morphological examination suggest that all of the specimens we collected belong to a single species based on consistency of the morphology of the genitalia.
Sequencing of the mt DNA CO1 region confirmed that all gnats attracted to P. sanguinea belong to a single species. All floral visitors formed a clade with 100 % bootstrap support, without any support for internal structure (Supplementary Data Fig. S1). Intraspecific divergence was low, with an average of only 0·8 % sequence divergence using both the KP2 and proportion of base pairs methods. This value is lower than the mean of 1·3 % for 334 species of Diptera reported in Meier et al. (2008). There are no CO1 sequences from other species of Mycomya currently available on GenBank, preventing a rigorous comparison of the level of genetic divergence within and among species.
Baiting with picked flowers from eight populations from the Kings Park gnat population showed that specimens from all populations, including both green and brown colour forms, were attractive to the same gnat species.
What is the role of the site of scent release and morphology in sexual attraction?
Is pollinator attraction by floral odour or visual cues?
A total of 27 gnats were attracted to orchids concealed within the screen, demonstrating that long-range attraction (>1 m) is by floral odour. Of the gnats attracted, 18 alighted on the labellum of the concealed orchids. The remaining nine gnats circled the outside of the screen, staying within close proximity for several seconds. No gnats were observed to fly towards the screen when the orchids were absent.
Site responsible for the production of the sexual attractant
In the floral dissection experiment, a total of 50 gnats were attracted to the labellum while none were attracted to the remainder of the flower. Of those trials where gnats responded, on average 2·5 ± 0·3 gnats alighted on the labellum per trial. Further, 29 of the 50 gnats showed sexual behaviour with the labellum. All 29 showed bending of the abdomen, with 26 of these also probing at the labellum with the abdomen.
Does the morphology of the labellum determine the orientation of the pollinator?
All 32 gnats alighting on the dissected labellum glued in its natural vertical position faced vertically upwards. Similarly, 28 out of the 29 gnats landing on the upside-down labella also faced vertically upwards. When the labellum was in its natural position 75 % of gnats showed bending of the abdomen, while 71·8 % of gnats both probed at the labellum and bent their abdomen. However, when the labellum faced downwards the proportion of gnats showing sexual behaviour was significantly lower, with only 27·6 % showing bending of the abdomen (G = 14·26; P < 0·001) and 20·7 % probing at the labellum (G = 16·8l; P < 0·001). Thus the sexual response is strongly influenced by labellum orientation in relation to the gnats' preference for upright landing.
Ecological consequences of deception of fungus gnats
Influence of plant population size and density on fruit set
Across all 19 populations, fruit set averaged 23·7 ± 4·4 % (s.e.) in 2008 and 27·7 ± 3·6 % in 2012. Following hand pollination in 2008, fruit set averaged 82·0 ± 2·6 %, demonstrating that fruit set is primarily limited by pollination. There was no significant relationship between fruit set and either population size or density in 2008 (population size, P = 0·410; density, P = 0·436) or 2012 (population size, P = 0·095; density, P = 0·355).
DISCUSSION
Evaluation of sexual deception in Pterostylis sanguinea
Following a history of over 100 years of study (e.g. Cheeseman, 1872; Sargent, 1909; Coleman, 1934; Hyett, 1960; Bernhardt, 1995), here we confirm for the first time that pollination by sexual deception occurs in the genus Pterostylis, with P. sanguinea pollinated solely by sexual deception of male fungus gnats. Our discovery represents the first case of sexual deception involving a member of the Mycetophilidae.
As is typical for most species of sexually deceptive orchid, only one pollinator species was recorded (see Gaskett, 2011, for review). The males appear to show courtship behaviour with the flower in the form of curling of the abdomen, though unlike sciarids (the sister family to Mycetophilidae), the males did not exhibit wing fanning during courtship (Liu et al., 2002; Blanco and Barboza, 2005). However, they regularly engaged in vigorous copulatory probing with the abdomen over the upper surface and apex of the labellum. Thus, P. sanguinea meets two of the criteria needed to confirm pollination by sexual deception: pre-mating behaviour and attempted copulation (Table 1).
As observed in other sexually deceptive genera of the Orchidaceae (e.g. Kullenberg, 1973; Stoutamire, 1983; Shiestl et al., 2003; Ayasse et al., 2003), long-range attraction of pollinators by P. sanguinea is achieved by floral odour. However, this contrasts with the fly-pollinated daisy G. diffusa, to which male bombyliid flies are attracted to the visual cue of black spots that mimic the appearance of females (Ellis and Johnson, 2010; De Jager and Ellis, 2012). The response of the male gnats to the floral odour of P. sanguinea is rapid, with peak numbers arriving in the first minute. This represents an interesting parallel to the sexually deceptive systems involving thynnine wasps, which show a similar response (Peakall, 1990).
While we have confirmed the presence of sexual deception in P. sanguinea, we have been unable to ascertain whether ejaculation occurs on the flower. Though rare in sexually deceptive systems, ejaculation on the flower has been confirmed in Australian species of Cryptostylis, all of which are pollinated by sexual deception of an ichneumonid wasp (Coleman, 1928; Erickson, 1951; Gaskett et al., 2008). Blanco and Barboza (2005) also provided evidence that ejaculation occurs in the Lepanthes pollinated by sciarids. In Lepanthes, the pollinator grasps the labellum appendix with genitalic claspers before assuming the tail-to-tail copulatory position (Blanco and Barboza, 2005). By contrast, the pollinators of P. sanguinea probe with their abdomen at multiple places over the labellum, without the abdomen ever fixing on a single point. When combined with the rapid triggering of the labellum and subsequent entrapment of the pollinator, this behaviour suggests that ejaculation is unlikely in this pollination system.
The importance of labellum morphology for sexual attraction
While floral odour is critical for long-range attraction, morphological features on the surface of the labellum also appear to be important for sexual attraction to the flower. When the labellum was rotated 180 °, the frequency of sexual behaviour greatly declined, suggesting that the texture and shape of the surface of the labellum may be critical for both the positioning of the pollinator (e.g. Kullenberg, 1961) and for stimulating further sexual behaviour. However, the gnats observed attempted to copulate with multiple parts of the labellum surface, suggesting that they are not reliant on a precise morphological cue. An interesting possibility is that the floral scent may be released from a specific part of the labellum which, when combined with appropriate morphology, elicits stronger sexual behaviour from the gnat.
At present, we cannot rule out colour as a possible additional signal leading to sexual attraction. However, to human eyes the colour of the labellum is highly variable between individual orchids, and we did not detect any evidence for differential pollinator behaviour among the floral colour morphs. Unlike the labellum of some sexually deceptive orchids, that of P. sanguinea does not appear to bear a close resemblance to the colour of fungus gnats, though this interpretation needs to be supported by spectral reflectance data in concert with a dipteran vision model (e.g. Gaskett and Herberstein, 2010).
Comparison of sexual deception in Pterostylis sanguinea with that in other orchid genera
Our detailed quantification of the pollination process for P. sanguinea permits comparison of the efficiency of the pollination process with that of other sexually deceptive genera. Compared with the thynnine wasp-pollinated D. livida, P. sanguinea has a very low rate of converting pollinator attraction into potential column contact (Phillips et al., 2013) (Table 3). While in P. sanguinea the proportion of pollinators alighting on the labellum and attempting copulation is very high, the inefficiency occurs with the low incidence of labellum triggering. It may be that only particularly large or particularly vigorous gnats succeed in triggering the labellum. Alternatively, the low frequency of the labellum triggering could be an artefact of using bait flowers. When wild plants are touched by the observer, the labellum of most flowers triggers immediately. By contrast, we observed that bait flowers tended to be less sensitive after they reset, following their initial triggering when picked.
Table 3.
Quantification of the behaviour of fungus gnat pollinators on Pterostylis sanguinea with data on thynnine wasp-pollinated species (Phillips et al., 2013)
|
Pterostylis sanguinea/Mycomya sp. |
Caladenia pectinata/Zaspilothynnus nigripes |
Drakaea livida/Zaspilothynnus nigripes |
||||
|---|---|---|---|---|---|---|
| % | n | % | n | % | n | |
| Alighting | X | X | 60·1 | 233 | 80·5 | 313 |
| Touched labellum | 99·3 | 135 | 66·4 | 233 | 98·8 | 313 |
| Courtship | 54·1 | 133 | X | X | X | X |
| Copulation with labellum | 49·6 | 133 | 0 | 233 | 34·5 | 313 |
| Labellum triggered | 9·6 | 135 | X | X | 81·3 | 313 |
| Potential column contact | 4·4 | 135 | 3·6 | 233 | 42·5 | 313 |
In the case of Drakaea livida the hinge in the labellum was flipped through the momentum of the pollinator rather than actively moving.
X, not applicable or not recorded for that species.
Evolution of sexual deception in Pterostylis
Of the over 400 species of Pterostylis, a large number are likely to be pollinated by sexual deception. Several large species groups, which form a clade (Janes et al., 2010; Clements et al., 2011), share very similar floral morphology with P. sanguinea, all being characterized by an insectiform labellum presented in front of deflexed lateral sepals (Janes et al., 2010). Evidence from other lineages of sexually deceptive orchids suggests that this pollination strategy is characterized by rapid speciation through pollinator-mediated isolation (Cozzolino and Widmer, 2005; Peakall et al., 2010). If this is the case, the evolution of pollination by sexual deception could have driven the large radiation of species evident in some groups of Pterostylis.
Many Australian orchids possess labella that are connected by a short hinge that allows some movement during pollination. However, our confirmation of pollination by sexual deception in P. sanguinea strengthens an interesting trend among Australian sexually deceptive orchids, where many species have evolved touch-sensitive, motile or moveable labella. For example, Paracaleana and Caleana use a hinged trigger mechanism to trap pollinators (Hopper and Brown, 2006). Similarly, Arthrochilus, Drakaea, Spiculaea and some species of sexually deceptive Caladenia exhibit highly developed hinged labella (Peakall, 1990; Alcock, 2000; Jones, 2006; Phillips et al., 2009), which appear to aid in the precise positioning of the insect for pollination (Phillips et al., 2013). We hypothesize that the evolution of such precise motile or moveable labella is more likely in sexually deceptive systems because (1) sexually deceived animals are more vigorous with the flower, increasing the effectiveness of a trigger or hinge mechanism, (2) the extreme pollinator specificity means that mechanisms reliant on a specific body size or weight of pollinator are more likely to evolve, and (3) sexually deceived visitors are strongly attracted to the site of scent release, creating the opportunity for this specific positioning to be exploited.
While it is likely that many of the Pterostylis with similar morphology to P. sanguinea are using sexual deception, the pollination system of the full diversity of floral forms in the genus remains a mystery. In the species groups that have upswept lateral sepals forming urn-shaped flowers, the labellum is often entirely concealed within the flower. The obscured labellum suggests that if sexual deception is involved the pollinator must be lured into the flower by scent and then trapped rather than relying on an insectiform labellum to position the pollinator. Alternatively, these groups may be attracting pollinators as a shelter site or some other strategy (Adams and Lawson, 1993). Pterostylis appears to be a notable contrast with most other sexually deceptive species, which have evolved from lineages primarily pollinated by food-foraging hymenoptera (Steiner et al., 1994; Kores et al., 2001; Cozzolino and Widmer, 2005; Phillips et al., 2009). The intriguing morphology and evolutionary history of Pterostylis suggests that if more pollination systems can be resolved, Pterostylis will yield novel insights into the evolution of pollination by sexual deception.
Ecological consequences of deception of fungus gnats
Fruit set in P. sanguinea is higher than reported for most species of sexually deceptive orchid (references in Gaskett, 2011). Further, following investigation of 19 populations, P. sanguinea maintained a similar level of fruit set across all population sizes and densities. In the sexually deceptive Drakaea glyptodon, which is pollinated by a single species of thynnine wasp (Peakall, 1990), pollination rates were highest in small populations (fewer than ten plants; Phillips, 2010). Thus, maintaining frequent fruit set in small populations may represent an advantage of sexual deception over pollination by food-foraging insects, which in many cases show a decrease in pollination rate at small population sizes (e.g. Ghazoul, 2005; Jacquemyn et al., 2007; Brys et al., 2008; Johnson et al., 2009; Duffy et al., 2013; though see Johnson et al., 2012). In the case of sexually deceptive systems, it may be that the chemical attractant causes pollinators to seek out orchids even when they occur in low numbers or at low density, thereby avoiding the negative consequences of small population size (Peakall and Beattie, 1996).
Should sexual deception prove to be widespread in Pterostylis, this will have important consequences for the conservation of many species. In south-western Australia, sexually deceptive orchids are characterized by a high incidence of rarity, most likely as a by-product of a specialized pollination strategy (Phillips et al., 2011). Studies of extremely specialized pollination systems have confirmed that these plants are highly vulnerable to a decline in the pollinator species, leading to reduced fecundity (Anderson et al., 2011; Pauw and Bond, 2011; Pauw and Hawkins, 2011). In undisturbed landscapes, natural scarcity of the pollinator can contribute to rarity of the orchid through reducing the availability of suitable habitat (Phillips, 2010). While these studies suggest a need to understand the ecology of the pollinators of Pterostylis, lack of knowledge on the ecology and taxonomy of fungus gnats may represent an impediment to effective conservation efforts.
Conclusions
Here we confirm for the first time that pollination by sexual deception operates in Pterostylis. Our discovery also represents the first known case of the sexual deception of the Mycetophilidae. While remarkable inroads are being made into the pollination biology of some groups of sexually deceptive orchids, this discovery in Pterostylis highlights the prospect that many future discoveries of this pollination strategy are still possible, both within and beyond the Orchidaceae. In particular, an expansion of natural history studies with a focus on the large radiation of tropical orchids pollinated by Diptera is likely to yield other candidate systems. However, a clearer understanding of the evolution of this intriguing pollination strategy and its ecological consequences will be best achieved by integrated studies that combine the fields of ecology, genetics, phylogeny and chemical ecology (Peakall et al., 2010; Xu et al., 2011; Peakall and Whitehead, 2014). In the light of the likely large differences in chemical communication, mating systems and sexual behaviour between Diptera and Hymenoptera, future multidisciplinary studies of Pterostylis and other fly-pollinated genera promise to yield many novel insights into the ecology and evolution of sexually deceptive systems.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Rauno Väisänen for advice on the identification of pollinators, Marinus De Jager and Michael Whitehead for comments on the manuscript and the numerous local shires that provided permission to undertake research on their land. This work was supported by an Australian Research Council Linkage grant (LP110100408) to R.P. and K.W.D.
LITERATURE CITED
- Adams PB, Lawson SD. Pollination in Australian orchids: a critical assessment of the literature 1882–1992. Australian Journal of Botany. 1993;41:553–575. [Google Scholar]
- Alcock J. Interactions between the sexually-deceptive orchid Spiculaea ciliata and its wasp pollinator Thynnoturneria sp. (Hymenoptera: Thynninae) Journal of Natural History. 2000;34:629–636. [Google Scholar]
- Anderson SH, Kelly D, Ladley JJ, Molloy S, Terry J. Cascading effects of bird functional extinction reduce pollination and plant density. Science. 2011;331:1068–1070. doi: 10.1126/science.1199092. [DOI] [PubMed] [Google Scholar]
- Ayasse M, Schiestl FP, Paulus HF, Ibarra F, Francke W. Pollinator attraction in a sexually deceptive orchid by means of unconventional chemicals. Proceedings of the Royal Society of London B: Biological Sciences. 2003;270:517–522. doi: 10.1098/rspb.2002.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayasse M, Stokl J, Francke W. Chemical ecology and pollinator-driven speciation in sexually deceptive orchids. Phytochemistry. 2011;72:1667–1677. doi: 10.1016/j.phytochem.2011.03.023. [DOI] [PubMed] [Google Scholar]
- Barbosa AR, Melo MC, Borba EL. Self-incompatibility and myophily in Octomeria (Orchidaceae; Pleurothallidinae) species. Plant Systematics and Evolution. 2009;283:1–8. [Google Scholar]
- Bernhardt P. Notes on the anthecology of Pterostylis curta (Orchidaceae) Cunninghamia. 1995;4:1–8. [Google Scholar]
- Bino RJ, Dafni A, Meeuse ADJ. The pollination ecology of Orchis galilea (Bornm. Et Schulze) Schltr. (Orchidaceae) New Phytologist. 1982;90:315–319. [Google Scholar]
- Blanco MA, Barboza G. Pseudocopulatory pollination in Lepanthes (Orchidaceae: Pleurothallidinae) by fungus gnats. Annals of Botany. 2005;95:763–772. doi: 10.1093/aob/mci090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohman B, Jeffares L, Flematti G, et al. The discovery of 2-hydroxymethyl-3-(3-methylbutyl)-5-methylpyrazine: A semiochemical in orchid pollination. Organic Letters. 2012;14:2576–2578. doi: 10.1021/ol300864u. [DOI] [PubMed] [Google Scholar]
- Bohman B, Jeffares L, Flematti G, et al. Discovery of tetra-substituted pyrazines as semiochemicals in a sexually deceptive orchid. Journal of Natural Products. 2012;75:1589–1594. doi: 10.1021/np300388y. [DOI] [PubMed] [Google Scholar]
- Borba EL, Semir J. Pollinator specificity and convergence in fly-pollinated Pleurothallis (Orchidaceae) species: a multiple population approach. Annals of Botany. 2001;88:75–88. [Google Scholar]
- Brown AP, Dundas P, Dixon KW, Hopper SD. Orchids of Western Australia. Perth: University of Western Australia Press; 2008. [Google Scholar]
- Brown EM, Burbidge AH, Dell J, Edinger D, Hopper SD, Wills RT. Pollination in Western Australia: a database of animals visiting flowers. Handbook No. 15. Perth: Western Australia Naturalists' Club; 1997. [Google Scholar]
- Bruford MW, Hanotte O, Brookfield JFY, Burke T. Molecular genetic analysis of populations: A practical approach. Oxford: IRL Press; 1998. Multilocus and single-locus DNA fingerprinting. In: Hoelzel AR. ed 2nd edition. [Google Scholar]
- Brys R, Jacquemyn H, Hermy M. Pollination efficiency and reproductive patterns in relation to local plant density, population size, and floral display in the rewarding Listera ovata (Orchidaceae) Botanical Journal of the Linnean Society. 2008;157:713–721. [Google Scholar]
- Cheeseman TF. On the fertilisation of the New Zealand species of Pterostylis. Transactions and Proceedings of the New Zealand Institute. 1872;5:352–357. [Google Scholar]
- Ciotek L, Giorgis P, Benitez-Vieyra S, Cocucci AA. First confirmed case of pseudocopulation in terrestrial orchids of South America: pollination of Geoblasta pennicillata (Orchidaceae) by Campsomeris bistrimacula (Hymenoptera, Scoliidae) Flora. 2006;201:365–369. [Google Scholar]
- Clements MA, Otero JT, Miller JT. Phylogenetic relationships in Pterostylidinae (Cranichideae: Orchidaceae): combined evidence from nuclear ribosomal and plastid DNA sequences. Australian Journal of Botany. 2011;59:99–117. [Google Scholar]
- Coleman E. Pollination of an Australian orchid by the male ichneumonid Lissopimpla semipunctata, Kirby. Transactions of the Entomological Society of London. 1928;2:533–539. [Google Scholar]
- Coleman E. Pollination of Pterostylis acuminata R.Br. and Pterostylis falcata Rogers. Victorian Naturalist. 1934;50:248–252. [Google Scholar]
- Colless DH. The Mycetophilidae (Diptera) of Australia. Part 1. Introduction, key to subfamilies, and review of Ditomyiinae. Journal of the Australian Entomological Society. 1970;9:83–99. [Google Scholar]
- Cozzolino S, Widmer A. Orchid diversity: an evolutionary consequence of deception? Trends in Ecology and Evolution. 2005;20:487–494. doi: 10.1016/j.tree.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Duffy KJ, Patrick KL, Johnson SD. Does the likelihood of an Allee affect on plant fecundity depend on the type of pollinator? Journal of Ecology. 2013;101:953–962. [Google Scholar]
- Ellis AG, Johnson SD. Floral mimicry enhances pollen export: the evolution of pollination by sexual deceit outside of the Orchidaceae. American Naturalist. 2010;176:E143–E151. doi: 10.1086/656487. [DOI] [PubMed] [Google Scholar]
- Erickson R. Orchids of the West. Perth: Paterson Brokensha; 1951. [Google Scholar]
- Flach A, Marsaioli AJ, Singer RB, et al. Pollination by sexual mimicry in Mormolyca ringens: a floral chemistry that remarkably matches the pheromones of virgin queens of Scaptotrigona sp. Journal of Chemical Ecology. 2006;32:59–70. doi: 10.1007/s10886-006-9351-1. [DOI] [PubMed] [Google Scholar]
- Franke S, Ibarra F, Schulz CM, et al. The discovery of 2,5-dialkylcyclohexan-1,3-diones as a new class of natural products. Proceedings of the National Academy of Sciences of the USA. 2009;106:8877–8882. doi: 10.1073/pnas.0900646106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmer O, Black M, Hoen W, Lutz W, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology. 1994;3:294–299. [PubMed] [Google Scholar]
- Gaskett AC. Orchid pollination by sexual deception: pollinator perspectives. Biological Reviews. 2011;86:33–75. doi: 10.1111/j.1469-185X.2010.00134.x. [DOI] [PubMed] [Google Scholar]
- Gaskett AC, Herberstein ME. Colour mimicry and sexual deception by tongue orchids (Cryptostylis) Naturwissenschaften. 2010;97:97–102. doi: 10.1007/s00114-009-0611-0. [DOI] [PubMed] [Google Scholar]
- Gaskett AC, Winnick CG, Herberstein ME. Orchid sexual deceit provokes ejaculation. American Naturalist. 2008;171:E206–E212. doi: 10.1086/587532. [DOI] [PubMed] [Google Scholar]
- Ghazoul J. Pollen and seed dispersal among dispersed plants. Biological Reviews. 2005;80:413–443. doi: 10.1017/s1464793105006731. [DOI] [PubMed] [Google Scholar]
- Griffith KE, Trueman JWH, Brown GR, Peakall R. Molecular genetic analysis and ecological evidence reveals multiple cryptic species among thynnine wasp pollinators of sexually deceptive orchids. Molecular Phylogenetics and Evolution. 2011;59:195–205. doi: 10.1016/j.ympev.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Hoffman N, Brown AP. Orchids of south-west Australia. 3rd edn. Perth: Scott Print; 2011. [Google Scholar]
- Hopper SD, Brown AP. Australia's wasp-pollinated flying duck orchids revised (Paracaleana: Orchidaceae) Australian Systematic Botany. 2006;19:211–244. [Google Scholar]
- Hyett J. Pollination of the nodding greenhood. Victorian Naturalist. 1960;76:240–241. [Google Scholar]
- Jacquemyn H, Vandepitte K, Brys R, Honnay O, Roldán-Ruiz I. Fitness variation and genetic diversity in small remnant populations of the food deceptive orchid Orchis purpurea. Biological Conservation. 2007;139:203–210. [Google Scholar]
- De Jager ML, Ellis AG. Gender-specific pollinator preference for floral traits. Functional Ecology. 2012;26:1197–1204. [Google Scholar]
- Janes JK, Steane DA, Vaillancourt RE, Duretto MF. A molecular phylogeny of the subtribe Pterostylidinae (Orchidaceae): resolving the taxonomic confusion. Australian Systematic Botany. 2010;23:248–259. [Google Scholar]
- Johnson SD, Torninger E, Årgen J. Relationships between population size and pollen fates in a moth-pollinated orchid. Biology Letters. 2009;5:282–285. doi: 10.1098/rsbl.2008.0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SD, Hollens H, Kuhlmann M. Competition versus facilitation: conspecific effects on pollinator visitation and seed set in the iris Lapeirousia oreogena. Oikos. 2012;121:545–550. [Google Scholar]
- Jones DL. Pterostylis pollination. In: Pridgeon A, Cribb P, Chase M, Rasmussen F, editors. Genera Orchidacearum. Vol. 2. New York: Oxford University Press; 2003. pp. 161–162. [Google Scholar]
- Jones DL. A Complete Guide to Native Orchids of Australia including the Island Territories. Frenchs Forest, NSW: Reed New Holland; 2006. [Google Scholar]
- Jones DL, Gray B. The pollination of Calochilus holtzei. American Orchid Society Bulletin. 1974;43:604–606. [Google Scholar]
- Kimura K. A simple method for estimating evolutionary rates of based substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Kores PJ, Molvray M, Weston PH, et al. A phylogenetic analysis of Diurideae (Orchidaceae) based on plastid DNA sequence data. American Journal of Botany. 2001;88:1903–1914. [PubMed] [Google Scholar]
- Kullenberg B. Studies in Ophrys pollination. Zoologiska Bidrag från Uppsala. 1961;34:1–340. [Google Scholar]
- Kullenberg B. New observations on the pollination of Ophrys L. (Orchidaceae) Zoon Supplement. 1973;1:9–14. [Google Scholar]
- Liu YN, Honda H, Kohno Y. Mating behaviour and its regulatory factors in the black fungus gnat, Bradysia paupera (Diptera: Sciaridae) Japanese Journal of Applied Entomology and Zoology. 2002;46:23–30. [Google Scholar]
- Meier R, Zhang G, Ali F. The use of mean instead of smallest interspecific distances exaggerates the size of the ‘barcoding gap’ and leads to misidentification. Systematic Biology. 2008;57:809–813. doi: 10.1080/10635150802406343. [DOI] [PubMed] [Google Scholar]
- Melo MC, Tauce PPG, Borba EL. Reproductive biology and isolation mechanisms in rupicolous species of the Acianthera prolifera complex (Orchidaceae) occurring in southeastern Brazil. Plant Systematics and Evolution. 2011;293:161–176. [Google Scholar]
- Paulus HF, Gack C. Pollinators as prepollinating isolation factors: evolution and speciation in Ophrys (Orchidaceae) Israel Journal of Botany. 1990;39:43–79. [Google Scholar]
- Pauw A, Bond WJ. Mutualisms matter: pollination rate limits the distribution of oil-secreting orchids. Oikos. 2011;120:1531–1538. [Google Scholar]
- Pauw A, Hawkins JA. Reconstruction of historical pollination rates reveals linked declines of pollinators and plants. Oikos. 2011;120:344–349. [Google Scholar]
- Peakall R. The unique pollination of Leporella fimbriata (Orchidaceae): pollination by pseudocopulating male ants (Myrmecia urens, Formicidae) Plant Systematics and Evolution. 1989;167:137–148. [Google Scholar]
- Peakall R. Responses of male Zaspilothynnus trilobatus Turner wasps to females and the sexually deceptive orchid it pollinates. Functional Ecology. 1990;4:159–167. [Google Scholar]
- Peakall R, Beattie AJ. Ecological and genetic consequences of pollination by sexual deception in the orchid Caladenia tentaculata. Evolution. 1996;50:2207–2220. doi: 10.1111/j.1558-5646.1996.tb03611.x. [DOI] [PubMed] [Google Scholar]
- Peakall R, Smouse PE. GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Notes. 2006;6:288–295. doi: 10.1093/bioinformatics/bts460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peakall R, Smouse PE. GenAlEx 6·5: genetic analysis in Excel. Population genetic software for teaching and research – an update. Bioinformatics. 2012;28:2537–2539. doi: 10.1093/bioinformatics/bts460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peakall R, Whitehead MR. Floral odour chemistry defines species boundaries and underpins strong reproductive isolation in sexually deceptive orchids. Annals of Botany. 2014;113:341–355. doi: 10.1093/aob/mct199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peakall R, Ebert D, Poldy J, et al. Pollinator specificity, floral odour chemistry and the phylogeny of Australian sexually deceptive Chiloglottis orchid: implications for pollinator-driven speciation. New Phytologist. 2010;188:437–450. doi: 10.1111/j.1469-8137.2010.03308.x. [DOI] [PubMed] [Google Scholar]
- Phillips RD. Landscape, pollinator and mycorrhizal specificity and their contribution to rarity in Drakaea (hammer orchids) 2010 Unpublished PhD thesis, University of Western Australia. [Google Scholar]
- Phillips RD, Faast R, Bower CC, Brown GR, Peakall R. Implications of pollination by food and sexual deception for pollinator specificity, fruit set, population genetics and conservation of Caladenia (Orchidaceae) Australian Journal of Botany. 2009;57:287–306. [Google Scholar]
- Phillips RD, Brown AP, Dixon KW, Hopper SD. Orchid biogeography and the factors associated with rarity in a biodiversity hotspot: the Southwest Australian Floristic Region. Journal of Biogeography. 2011;38:487–501. [Google Scholar]
- Phillips RD, Xu T, Hutchinson MF, Dixon KW, Peakall R. Convergent specialisation – the sharing of pollinators by sympatric genera of sexually deceptive orchids. Journal of Ecology. 2013;101:826–835. [Google Scholar]
- Pridgeon AM. Subtribe Pleurothallidinae. In: Pridgeon A, Cribb P, Chase M, Rasmussen F, editors. Genera Orchidacearum. Vol. 2. New York: Oxford University Press; 2005. pp. 319–327. [Google Scholar]
- Retter BA. Factors affecting pollination success and offspring viability of orchids in a fragmented landscape. 2009 Unpublished Honours thesis, University of Western Australia. [Google Scholar]
- Sargent OH. Notes on the life-history of Pterostylis. Annals of Botany. 1909;23:265–274. [Google Scholar]
- Schiestl FP. On the success of a swindle: pollination by deception in orchids. Naturwissenschaften. 2005;92:255–264. doi: 10.1007/s00114-005-0636-y. [DOI] [PubMed] [Google Scholar]
- Schiestl FP, Ayasse M, Paulus HF, et al. Orchid pollination by sexual swindle. Nature. 1999;399:421–422. [Google Scholar]
- Schiestl FP, Peakall R, Mant JG, et al. The chemistry of sexual deception in an orchid-wasp pollination system. Science. 2003;302:437–438. doi: 10.1126/science.1087835. [DOI] [PubMed] [Google Scholar]
- Singer RB. The pollination mechanism in Trigonidium obtusum Lindl. (Orchidaceae: Maxillariinae): sexual mimicry and trap-flowers. Annals of Botany. 2002;89:157–163. doi: 10.1093/aob/mcf021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer RB, Flach A, Koehler S, Marsaioli AJ, Amaral MDCE. Sexual mimicry in Mormolyca ringens (Lindl.) Schltr. (Orchidaceae: Maxillariinae) Annals of Botany. 2004;93:755–762. doi: 10.1093/aob/mch091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner KE, Whitehead VB, Johnson SD. Floral and pollinator divergence in two sexually deceptive South African orchids. American Journal of Botany. 1994;81:185–194. [Google Scholar]
- Stoutamire WP. Australian terrestrial orchids, thynnid wasps, and pseudocopulation. American Orchid Society Bulletin. 1974;43:13–18. [Google Scholar]
- Stoutamire WP. Wasp-pollinated species of Caladenia (Orchidaceae) in South-western Australia. Australian Journal of Botany. 1983;31:383–394. [Google Scholar]
- Stokl J, Twele R, Erdmann DH, Francke W, Ayasse M. Comparison of the flower scent of the sexually deceptive orchid Ophrys iricolor and the female sex pheromone of its pollinator Andrena morio. Chemoecology. 2007;17:231–233. [Google Scholar]
- Streinzer M, Paulus HF, Spaethe J. Floral colour signal increases short-range detectability of a sexually deceptive orchid to its bee pollinator. Journal of Experimental Biology. 2009;212:1365–1370. doi: 10.1242/jeb.027482. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnoir AL. Australian Mycetophilidae. Synopsis of the genera. Proceedings of the Linnean Society of New South Wales. 1929;54:584–614. [Google Scholar]
- Vereecken NJ, Wilson CA, Hötling S, Schulz S, Banketov SA, Mardulyn P. Pre-adaptations and the evolution of pollination by sexual deception: Cope's rule of specialization revisited. Proceedings of the Royal Society of London B: Biological Sciences. 2012;279:4786–4794. doi: 10.1098/rspb.2012.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu SQ, Schluter PM, Scopece G, et al. Floral isolation is the main reproductive barrier among closely related sexually deceptive orchids. Evolution. 2011;65:2606–2620. doi: 10.1111/j.1558-5646.2011.01323.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


