Abstract
Background and Aims
Throughout the history of fern classification, familial and generic concepts have been highly labile. Many classifications and evolutionary schemes have been proposed during the last two centuries, reflecting different interpretations of the available evidence. Knowledge of fern structure and life histories has increased through time, providing more evidence on which to base ideas of possible relationships, and classification has changed accordingly. This paper reviews previous classifications of ferns and presents ideas on how to achieve a more stable consensus.
Scope
An historical overview is provided from the first to the most recent fern classifications, from which conclusions are drawn on past changes and future trends. The problematic concept of family in ferns is discussed, with a particular focus on how this has changed over time. The history of molecular studies and the most recent findings are also presented.
Key Results
Fern classification generally shows a trend from highly artificial, based on an interpretation of a few extrinsic characters, via natural classifications derived from a multitude of intrinsic characters, towards more evolutionary circumscriptions of groups that do not in general align well with the distribution of these previously used characters. It also shows a progression from a few broad family concepts to systems that recognized many more narrowly and highly controversially circumscribed families; currently, the number of families recognized is stabilizing somewhere between these extremes. Placement of many genera was uncertain until the arrival of molecular phylogenetics, which has rapidly been improving our understanding of fern relationships. As a collective category, the so-called ‘fern allies’ (e.g. Lycopodiales, Psilotaceae, Equisetaceae) were unsurprisingly found to be polyphyletic, and the term should be abandoned. Lycopodiaceae, Selaginellaceae and Isoëtaceae form a clade (the lycopods) that is sister to all other vascular plants, whereas the whisk ferns (Psilotaceae), often included in the lycopods or believed to be associated with the first vascular plants, are sister to Ophioglossaceae and thus belong to the fern clade. The horsetails (Equisetaceae) are also members of the fern clade (sometimes inappropriately called ‘monilophytes’), but, within that clade, their placement is still uncertain. Leptosporangiate ferns are better understood, although deep relationships within this group are still unresolved. Earlier, almost all leptosporangiate ferns were placed in a single family (Polypodiaceae or Dennstaedtiaceae), but these families have been redefined to narrower more natural entities.
Conclusions
Concluding this paper, a classification is presented based on our current understanding of relationships of fern and lycopod clades. Major changes in our understanding of these families are highlighted, illustrating issues of classification in relation to convergent evolution and false homologies. Problems with the current classification and groups that still need study are pointed out. A summary phylogenetic tree is also presented. A new classification in which Aspleniaceae, Cyatheaceae, Polypodiaceae and Schizaeaceae are expanded in comparison with the most recent classifications is presented, which is a modification of those proposed by Smith et al. (2006, 2008) and Christenhusz et al. (2011). These classifications are now finding a wider acceptance and use, and even though a few amendments are made based on recently published results from molecular analyses, we have aimed for a stable family and generic classification of ferns.
Keywords: Bibliography, classification, convergence, cryptogams, Cyatheaceae, fern family concepts, fern allies, ferns, homology, lycopods, monilophytes, Polypodiaceae, pteridophytes, history of botany
INTRODUCTION
One can only understand current classifications of a particular group of organisms after a thorough review of past systems. Classification of ferns has been particularly unstable in the past, and to understand why this has been so problematic we review the excellent article by Tryon (1952), in which the history of classification was discussed up until that time. Since that date, many other classifications have been proposed, some of which were discussed by Pichi-Sermolli (1973). We build upon these and provide an overview of our understanding of fern relationships and fern classification from the early 1970s to the present, addressing the influence of molecular phylogenetic studies in redefining genera and families. We will highlight some of the major changes in classification and identify genera that need further study to come to a better understanding of fern phylogeny. Many of these problematic genera were already suggested by Holttum (1973) to need monographic study. We summarize current knowledge of fern relationships in an annotated classification and point out where foci for future studies should be directed.
In commemoration of the 250th birthday of Carolus Linnaeus, Pichi-Sermolli (1958) provided an overview of higher classification of pteridophyta (understood to mean at that time ferns and ‘fern allies’). He stated that classifications until then had reflected four trends; (1) accepting four major lineages usually treated as classes: Psilopsida, Lycopsida, Sphenopsida and Pteropsida, the last including ferns and seed plants (e.g. Jeffrey, 1903; Arnold, 1948); (2) treating ferns and ‘fern allies’ as a group but excluding Psilopsida, treating the latter as related to Bryophyta (Lam, 1948) or as an independent lineage together with the fossil Psilophytopsida (Rothmaler, 1951); (3) maintaining Pteridophyta in their classical sense, including all vascular cryptogams (e.g. Campbell, 1940; Reimers, 1954); and (4) recognizing the independent divisions Psilophyta, Lycophyta, Sphenophyta, Pteridophyta and Spermatophyta (Benson, 1957). Pichi-Sermolli effectively dismissed trends 1, 2 and 4, concluding that all should be treated in a single division in the classical sense, placing them next to Spermatophyta. His classification scheme is discussed further below, but we highlight these four approaches here so they can be compared with the more detailed discussion of trends below.
The concept of family has always been fluid in ferns and, up to now, no clear family concept has been established. Families are generally defined as one or more genera that share several morphological characteristics, and many angiosperm families have had a long history of recognition based on shared floral and fruit features. However, in ferns, it has never been clear from what part of the plant these features should be derived, and this has led to a shifting number of families being recognized and differing circumscriptions for many of the more frequently recognized families.
Characters and terminology of ferns
Most readers will have a general idea of what a fern is, but a review of their typical characters and life histories will help focus attention on their key characters (those typically used in fern classification); this section of course can be skipped by pteridologists. There are several profound differences from flowering plants and mosses that define ferns and allow different characters to be used in the classification of this group. We therefore thought it appropriate to include a short introduction to the terminology of ferns and highlight the features important in their classification.
Ferns are vascular plants that produce spores and undergo an alternation of generations (with separate gametophyte and sporophyte generations that exist as free-living plants). Lycopods are similar to ferns in this regard, but ferns are the sister group of the seed plants (gymnosperms plus angiosperms), whereas the lycopods are sister to all other vascular plants (ferns plus the seed plants). Mosses, hornworts and liverworts (bryophytes) are also spore-producing plants with alternation of generations, but their sporophytes are reduced in size and dependent on the dominant gametophyte stage.
Alternation of generations
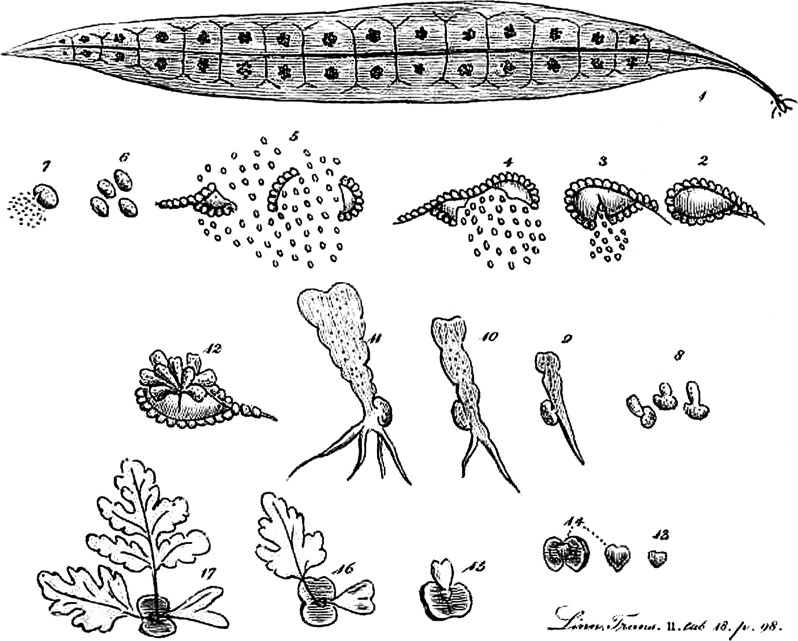
The leafy fern plant we observe in the fields, marshes and forests is called a sporophyte (Fig. 1B), a vascular plant that through meiosis produces spores with half the number of chromosomes found in the mother plant. When a spore lands in a suitable place, it grows into a free-living gametophyte (Fig. 1A), a haploid plant. Typically gametophytes are photosynthetic, but there are mycoheterotrophic gametophytes in some fern genera and families (Merckx et al., 2012). Gametophytes, often called prothalli, produce gametes through mitosis: male gametes, formed in antheridia, swim to the archegonia where female gametes are produced. The mobile, free-swimming, flagellate male cells are the reason for the water dependence of ferns; water is required for their movement from one gametophyte to another. Gametophytes can be bisexual or unisexual, and some can also reproduce vegetatively (a few may have lost the sporophyte stage altogether). From the fertilized female gamete, a new sporophyte develops. This life-history pattern differs from that in mosses, in which sporophytes are parasitic on the photosynthetic gametophyte, and from seed plants. In the latter, male and female gametophytes develop inside the pollen and ovule, respectively, with ovules being retained on the parental sporophyte until after fertilization, when at some point it is released as a seed with the new sporophyte partly developed inside. Spore and gametophyte morphology and the number of sperm flagellae have been used for classification of ferns. Alternation of generations in ferns was first described by Lindsay in 1794 (Fig. 2).
Fig. 1.
Characters of ferns. (A) Gametophyte [Ptisana attenuata (Labill.) Murdock, Marattiaceae]. (B) Sporophyte [Ptisana sp. nov. (Kamau & Christenhusz 638, EA, K), Marattiaceae]. (C) Circinnate vernation (Sphaeropteris excelsa (Endl.) R.M.Tryon, Cyatheaceae). (D) Sporocarps [Salvinia natans (L.) All., Salviniaceae]. (E) Discrete sori with indusia [Polystichum falcatum (L.f.) Diels non Fée, Polypodiaceae]. (F) Acrostichoid sori [Acrostichum durvillei (Fée) C.Presl, Pteridaceae].
Fig. 2.
The first illustration of the germination of spores by Lindsay (1794). 1. Leaf with sori. 2–5. Sporangium opening, showing annulus and spores. 6–7. Spores. 8–11. Developing gametophyte. 15–17. Developing sporophyte.
Sporophyte structure
A fern sporophyte consists of a stem, which is often called a rhizome, even if it is above ground or forms a 20 m tall trunk, as in some tree ferns. The rhizome can have various forms of stelar structures and orientation and scales, all of which are commonly used characters for identification and classification. From the rhizomes, leaves (also called fronds) grow, which in ferns are megaphylls (in contrast to lycopods, which have a similar life cycle but bear microphylls: simple leaves with single unbranched veins). Leaves emerge as fiddleheads (Fig. 1C; circinnately coiled), although some groups have different types of vernation; circinnate vernation is not exclusive to ferns and also occurs, for example, in the carnivorous sundews, Droseraceae, and in the gymnosperm genus Stangeria T.Moore (Zamiaceae). The petiole, or stipe, is used as a diagnostic character and in particular the number of vascular strands is important. Leaf blades can be simple or highly divided, and this has in the past been used for classification; however, in most cases, leaf division does not apply above the rank of species. Fertile leaves bear spore-producing sporangia, which are typically organized in groups, called sori. Usually sori are on the lower side of the leaf, and some groups have specialized structures associated with their sporangia and sori.
Sporangia
Spores are formed in sporangia. In eusporangiate ferns, sporangia are formed from a group of cells, which is the plesiomorphic state. Eusporangia are found in all other vascular plants, except in the leptosporangiate ferns, where the sporangium develops from a single cell into a structure with a stalk, wall and spores. Leptosporangiate ferns form a clade that includes the bulk of fern species, but eusporangiate ferns are composed of several independent groups. In some families, most notably Marattiales, eusporangia are fused together, forming a complex synangium. The numbers of cells in the stalk of a leptosporangium and the shape and orientation of the annulus, the structure that splits to release spores, have been used as diagnostic characters.
Sori
Sporangia can be organized in various ways. Sometimes they cover the entire lower lamina of the blade, especially towards its apex, which is called an acrostichoid sorus (Fig. 1F). In most taxa, sporangia are organized in discrete sori (Fig. 1E). The typical leptosporangiate sorus consists of a stalk to which sporangia are fixed; a sorus can be covered with a scale-like structure called the indusium (Fig. 1E). The shape and organization of the indusium has been an important character for higher classification of leptosporangiate ferns and is still employed today, although usually in combination with other characters. It was clear, even before the advent of molecular systematics, that there is a great deal of convergence in sorus characters, so on their own they have not reliably revealed a great deal about relationships.
Indument
Fern leaves can be glabrous or densely covered in hairs or scales. The shape and anatomy of these hairs are often used to diagnose species, but this is rarely useful at higher levels of classification. Scales of the rhizomes have been demonstrated to be conservative and phylogenetically informative, especially if clathrate scales are present (these are translucent like a stained-glass window).
Spores
Spore characters are useful in classification and phylogenetically informative, and they have also allowed calibration of DNA-based phylogenetic trees with the ages of fossil spores. Most remarkable are perhaps the heterosporous ferns. Heterospory is a condition in which a single sporophyte produces a smaller number of large spores, megaspores, that develop into female gametophytes and, more typically, numerous microspores that develop into male gametophytes. In ferns, this condition is only found in two groups of aquatics: Marsileaceae and Salviniaceae; it is clearly an adaptation to their aquatic environments, where the female gametophyte develops inside the megaspore and is thus protected from the surrounding water. These heterosporous ferns were often placed among ‘fern allies’, together with the unrelated heterosporous lycopods (Selaginellaceae, Isoëtaceae), but heterospory has evolved independently in these lineages (and again in seed plants, which are also heterosporous) and is not necessarily associated with aquatic habitats in those lineages. Spores in heterosporous ferns are formed in special structures called sporocarps (Figs 1D, 4I). All other ferns and Lycopodiaceae are homosporous, the plesiomorphic condition.
With this explanation of terms, we hope to have clarified the various characters used in fern classification, which should make a historical overview easier to follow.
HISTORICAL OVERVIEW
Difficulties with artificial classification and ‘natural’ systems
As with all botanical studies, the nomenclatural history of fern taxonomy starts with Linnaeus (1753, 1754), who first attempted to organize ferns based on the shape and position of sori on leaves; this use of a single character produced an artificial but easily used classification. Fern diversity and anatomy had been addressed in earlier studies (e.g. Cesalpino, 1583; Grew, 1682; Malpighi, 1675; Morison, 1699; Van Rheede tot Drakestein, 1678–1703; Plumier, 1705; Petiver, 1712), but it is important to note that at the time little was known about the life cycle of ferns. Linnaeus's sexual system, based on the number of stamens and pistils (the so-called ‘sex organs’ in flowering plants), functioned well for artificially classifying angiosperms, but it was impossible to place ferns in a system based on numbers of male and female parts. Ferns were therefore placed in Cryptogamae, among mosses, algae, fungi and even some animals (corals, sponges, etc). In his Cryptogamia filices Linnaeus (1753, Sp. Pl. 2: 1085–1100) recognized 16 genera and 174 species: Equisetum L. (6), Onoclea L. (1), Ophioglossum L. (6), Osmunda L. (17), Acrostichum L. (25), Pteris L. (19), Blechnum L. (2), Hemionitis L. (2), Lonchitis L. (3), Asplenium L. (20), Polypodium L. (58), Adiantum L. (15), Trichomanes L. (11), Marsilea L. (2), Pilularia L. (1) and Isoëtes L. (1). All but the last are still classifed as ferns; Isoëtes is a lycopod and does not have a close relationship to the other genera. Linnaeus's classification was artificial, with species of clearly distant relationships placed together. This was the case for Linnaeus' classifications in general, but it served the purpose of organizing the numerous newly discovered species until a more ‘natural’ system would be available. Species had to be assigned to a genus as a requirement in Linnaeus's binomial system, which is the reason why so many fern species were often placed seemingly randomly in a genus. This seems illogical now, but it made sense at a time when ferns were biologically so poorly understood. In many cases, the author may not have known in which genus to place a new species and thus a guess was made, which was subsequently corrected when genera became better known or the generic concepts changed, a process that still continues.
Indusium characters (as ‘fructifications’) were traditionally employed, and we still use these today (but always in combination with other characters). James E. Smith (1793) used characters of the indusium and recognized 20 genera, paying attention in particular to ontogeny and the method of indusial rupture. It was the first fern classification presented as a ‘natural system’, albeit that much diversity in opinion existed even then on what constituted a natural genus in ferns.
It was Lindsay (1794), a British surgeon stationed in Jamaica, who gathered ‘dust’ from several weedy ferns, sowed them in a flower pot on his window sill and observed in detail germination of spores and formation of gametophytes, followed by development of sporophytes (Fig. 2). This discovery sparked anatomical study of ferns, aiding the progress of understanding their structural biology. Gradually, this improved understanding of the anatomy and biology of ferns led to many changes in interpretation of fern morphology and to what was hoped would be a more natural classification for them.
Swartz (1801) published an early handbook on ferns, in which he treated some 670 species in 30 genera. In his slightly later Synopsis filicum, Swartz (1806; 38 genera, 720 species) followed the characters of sori and indusia as established by J. E. Smith (1793) and presented an elaborated version of Smith's classification. Despite knowing at the time that Swartz's genera were artificially delimited, this classification was followed by subsequent authors for 30 years, probably as a result of no further insights into other characters that could be used in addition to or in place of sori and indusia. They were also, for instance, the basis of a classification by Desvaux (1827), who, due to a more detailed study of soral characters, increased the number of genera to 79 in five families (Marsilées, Lycopodiées, Osmondées, Marattiées, Filicées), improving somewhat on the intrinsic naturalness of Swartz's classification. This informal use of families by Desvaux contrasted with all other fern taxonomists, who did not apply the family concept to ferns and preferred to use other categories. An impressive 1666 species were presented by Desvaux (1827), of which many species were new, but they were mechanically placed in genera solely following the classification of Swartz (1801). This system was soon challenged because species too different in other characters, and obviously not closely related, were often placed in the same genus.
An essentially new principle of classification was employed by Presl (1836, 1845, 1851), who also added to his system vegetative characters (especially venation, habit, and rhizome and petiole anatomy) in addition to the traditional fertile characters. Spore characters were also discussed and illustrated, but these were not used to group taxa. In total, Presl recognized 176 genera. Independent of Presl, John Smith (1841, 1843) compiled a classification, which, although differing in generic delimitation, essentially used similar characters to distinguish the 138 genera recognized. Both should be credited for establishing modern pteridology in the sense that they eschewed simpler methods of relying only upon soral and indusial characters to define a genus and instead emphasized that key generic characters could be drawn from any part of a fern. This was certainly an improvement in terms of putting together species that were morphologically and anatomically similar (in many cases), but it was much more difficult to use; to many of their contemporaries, Presl's and J. Smith's shifting emphases on soral characters to define one genus, petiole anatomy for another and venation for a third made their systems look arbitrary and flew in the face of the standard practice of the time.
However these classifications were criticized by Hooker, who initially accepted many of Presl's genera (Hooker and Bauer, 1842), but later changed his opinion and only recognized 63 genera based on classical characters of the sorus; nonetheless, he clearly saw the value of these other characters and treated many of Presl's and J. Smith's genera at subgeneric levels. It appears that he attempted to provide a consensus between the large artificially delimited but well-established genera of Swartz (1801, 1806) and the newer treatments based on intrinsic characters that were considered ‘more natural’, the definition of which meant different things to different authors and changed over time, particularly once the concept of evolution was proposed. Hooker emphasized that characters of ‘fruiting parts’ were more important than characters of ‘vegetative parts’ for classifying all plants at the generic level. This worked better with angiosperms, for which the concept of family was long established; many families had a long history of recognition, with a consensus of ideas about which genera were to be included. This sort of consistency of circumscription of the major angiosperm families is without parallel in ferns, and the emphasis on ‘fruiting parts’ for ferns did not result in either a consistent or a widely accepted system. Hooker's authority, as the director of the Royal Botanic Gardens, Kew, led to the re-application of the Swartzian system, which lasted for another 50 years as the most often used fern classification.
Although sporangium and sorus characters remained predominant as the basic framework for fern classification thanks to Hooker's prominence, some interest in applying other characters to classification continued; Fée, who published the elaborate series Mémoires sur la famille des fougères (Fée, 1844–1873), which included Genera filicum (6th and 7th Mémoire), emphasized an even wider range of characters for generic delimitation than Presl and recognized approx. 188 genera. The first Mémoire dealt entirely with characters of ferns useful for classification and focused on venation. It also reviewed other characters, seeking new ones in fertile structures. He applied, for instance, characters of the spores and number of cells in the annulus. The last character was not taken up by subsequent pteridologists, but it was re-applied nearly a century later in the classification of Copeland (1947).
John Smith, who had become curator of the living fern collection at Kew, knew his plants intimately, and his Historia filicum (Smith, 1875) presented his views on classification and incorporated the genera of Presl and Fée, accounting for 212 genera. Even though the classification of Fée found followers in the French Empire, his and the classifications of Smith and Presl remained in the shadow of the more influential one of Hooker until the end of the 19th century.
Evolution enters the scene: a diminished importance of sori and sporangia as pre-eminent characters for classification
The classification of Christ (1897) emphasized the importance of vegetative characters, and this was adopted and expanded by Diels (1898–1900) in his treatment for Die natürlichen Pflanzenfamilien (German for ‘the natural plant families’). Diels presented a phyletic classification, a discipline in its early stages, and he applied the modern usage of family as a formal category, previously often referred to at higher taxonomic ranks (e.g. suborders and orders). The classification of Diels was heavily criticized by most subsequent workers, even though, or maybe because, it was the first classification to take Darwinian evolution into consideration.
Christ's (1897) evaluation of previous systematic works gives us an insight into the perception of that work at the time. He wrote, for instance, about Die Farnkräuter of Schkuhr (1809) that the ‘nomenclature is outdated but that it contains many good images’, and of Fée's Mémoires (1844–1873) that ‘it contains many species that were later not recognised, but that it contains valuable material, in particular the images’. In contrast, he cited Mettenius (1856) as the ‘founding work for a natural systematics’ of ferns, but that classification was only natural in a pre-Darwinian sense and the work of Mettenius was largely ignored outside the German language sphere. Christ clearly attributed the shift from a classification based on the sorus to a classification based on the entire anatomy of the ferns to Mettenius, which is only partly true (Presl and Fée had already applied this concept earlier), but Christ and Mettenius shared a nationality, which may explain the preference. Christ accepted 99 genera of ferns in 13 families and divided them into Leptosporangiatae and Eusporangiatae, possibly the first use of the character of sorus development as a primary character.
At the turn of the century, phyletic classifications of ferns were also being compiled elsewhere, the first being a treatment by the British botanist Bower (1889), who based his ideas on his elaborate studies on anatomy, development and morphology. In his three-volume work, The ferns, Bower (1923–1928) recognized 12 families, and in Polypodiaceae, the first major use of this now ubiquitously applied name, he recognized six evolutionary lineages. This phyletic classification was, however, problematic, mainly due to an emphasis on the location of sori on the leaf blade.
Copeland (1929), who attempted to arrange the East Asian genera into a phyletic sequence, was the first to address this problem and suggested that a larger Polypodiaceae would be more natural. The tree-fern lineages were included, not making Polypodiaceae natural in the Bowerian sense, but this wider concept of Polypodiaceae resulted in the name being variously applied ever since.
Christensen's influential Index filicum (1906) listed 147 genera and followed almost exactly the treatment of Diels, except for some name changes to conform with the rules and an increase in number to account for new genera described during the six years between these publications. In subsequent supplements, the number of genera grew to 213. Christensen (1938) advanced from a bibliographer to a researcher in his own right, taking up the advances in Bowerian ‘phylogeny’, and – like Bower – he recognized 12 families, but divided his Polypodiaceae into 15 subfamilies, about which he stated that these should maybe be treated at the family level. His approx. 247 genera were based on a variety of vegetative (mostly hairs and scales) and soral characters, but several genera were pointed out to be of uncertain delimitation or status. Even though his classification was highly regarded and widely followed, Christensen himself regarded it as a tentative scheme.
Ching (1940) divided ‘Polypodiaceae’ into 33 families, grouped into seven ‘lines of evolution’ corresponding more or less to Copeland's families (1929), in which the essential unnaturalness of ‘Polypodiaceae’ was accepted. Holttum (1947), at that time the dominant personality in fern taxonomy, did not agree with certain relationships expressed by Bower, and he presented a revised classification of Polypodiaceae in which five families were recognized, but he admitted that some are difficult to define morphologically.
Microscopic characters added to the mix
Copeland (1947) was one of the first systematists to propose that ‘valid’ taxa should reflect ‘naturalness’ and ‘convenience’, which by this time meant that taxa must correspond to a single evolutionary lineage and be well circumscribed, supported by characteristics and easy to define. He based his Genera filicum (1947) on extensive fieldwork in tropical Asia, and altogether he recognized 305 genera, many of which included just a single species. He divided Trichomanes but treated Asplenium in the broad sense, which was prescient. On the other hand, Holttum (1913) stated that ‘Copeland's limitation seems to be his lack of understanding of Christensen's discovery of the great importance of the details of hairs and scales as evidence of relationships’, which is understandable from a field botanist's point of view. Microscopic characters are often difficult to observe in the field when all you have is a hand lens, especially when other characters such as habit are more obvious to distinguish groups. Copeland also provided a good overview of the concepts of fern classification preceding his magnum opus. He presented a similar number of taxa to Ching (1940), which Christensen stated had much to do with the two having the same generic concept. However, Ching still recognized 28 genera more than Copeland, so Christensen's criticism was not entirely justified.
Holttum (1947) revised the classification of Christensen (1938), which resulted from a detailed study of Malayan ferns. It had been customary to place all leptosporangiate ferns in a broad Polypodiaceae, apart from a few easily recognizable families such as Osmundaceae (considered intermediate between leptosporangiate and eusporangiate ferns), Cyatheaceae (the tree ferns with a helicogyrate annulus), Gleicheniaceae (with a strange leaf morphology) and Hymenophyllaceae (the filmy ferns with leaves one cell layer thick and cup-shaped sori). Christensen (1938) also followed this, but stated that the subfamilies he treated in Polypodiaceae ‘were perhaps better dealt with as families’, which Holttum (1947) did. He redefined Polypodiaceae, including Dipteris Reinw., but excluding Grammitidaceae. He also segregated Thelypteris Schmid. from Christensen's Dryopteridoideae into Thelypteridaceae, and the remaining subfamilies except Gymnogrammoideae, Vittarioideae and Onocleoideae were placed by Holttum in a large and diverse Dennstaedtiaceae with 11 subfamilies. The other group of tree ferns, Dicksoniaceae, were also segregated, even though they were previously thought to be closely related to Dennstaedtia Bernh. Gymnogrammoideae was renamed Adiantaceae, and Holttum included Vittarioideae because they are undoubtedly related. Onocleaceae remained unplaced. Many matters remained unsolved in Holttum's scheme, and he did not claim that all major groupings had been solved. Although it was easier to recognize a broad circumscription of Polypodiaceae and only segregate a few clearly divergent families, such as Osmundaceae, Cyatheaceae and Hymenophyllaceae, all authors of the time believed that this was unsatisfactory and most split Polypodiaceae into several families. Which characters should be emphasized and which families should be recognized was far from uniform, and substantial disagreements among authors were the standard.
Starting from the beginning (again), fossils included
Tryon (1952) concluded his historical overview predicting that ‘the conflict between utility and naturalness will remain an issue’ in future classifications. He suggested that taxa based on evolutionarily circumscribed groups would not necessarily have characters that are useful for identification, and indeed for some modern fern families this is a major issue today. Current examples of continuing contentious issues are the numerous families in the tree-fern lineage, segregation of Lonchitis, Saccoloma Kaulf. and Cystodium J.Sm. from Lindsaeaceae (Lehtonen et al., 2010), merging of Hymenophyllopsidaceae with Cyathea Sm. (Christenhusz, 2009b), family placement of Nephrolepis Schott (Smith et al., 2006; Hennequin et al., 2010) and difficulties with morphological circumscription of Pteridaceae, to name a few.
Reimers (1954) produced a thorough overview of Pteridophyta and included fossil taxa in his classification, setting it apart from earlier classifications that dealt only with extant or fossil taxa. He first defined his concept of Pteridophyta as ‘chlorophyllous, autotrophic land plants with antithetic (heterophasic) change of generations, and both generations organically independent’. In this way he included the lycopods with ferns in a single lineage. Reimers provided a developmental scheme in which fern lineages were set out on a time scale, all essentially originating from Psilophytales (in which he included the fossils Rhynia R.Kidston & W.H.Lang and Psilophyton J.W.Dawson) in the early Devonian (320 million years ago). Modern Psilotales are directly derived from them in his scheme, which was the general idea at the time and one that prevailed until this century. We will not go into the classification of his fossil taxa here, although we admit that to come to a good classification of ferns, fossil lineages will have to be included. It is, however, not within the remit of this paper to compare fossil classifications with those for extant ferns. Reimers concluded that there were four extant lineages (as classes) in Pteridophyta: Lycopsida (Lycopodiales, Selaginellales and Isoëtales), Psilotopsida (Psilotales), Articulatae (Equisetales) and Filices (Ophioglossales, Marattiales, Filicales, Marsileales and Salviniales). In Psilotopsida, two extant families were accepted, Psilotaceae and Tmesipteridaceae, because the two extant genera ‘appear to be relict forms’. What is interesting is that Reimers mentioned that the lack of roots in these genera cannot be seen as proof for any relationship with Psilophytopsida (which he placed in a different class, in spite of the linearity shown in the scheme). Articulatae included a great number of fossil taxa, but within this lineage Equisetum was placed in its own order because he stated that ‘relationships between this and fossil Calamitales are not yet clear’. The largest group, Filices, were divided into four subclasses, one extinct (Primofilicales) and three with extant members: Eusporangiatae and Leptosporangiatae with Osmundidae as a ‘transition group’. Eusporangiatae included the obvious non-leptosporangiate orders Ophioglossales and Marattiales, the latter divided into five families, of which one is the fossil Asterothecaceae (which includes the giant trees, Psaronius B.Cotta), and which should still be accepted as separate from extant Marattiaceae (Christenhusz, 2007). Leptosporangiate ferns were divided into three orders, Filicales, Marsileales and Salviniales, on the basis of heterospory of the last two orders; Filicales included a dozen families, of which the largest, Polypodiaceae, was divided into 14 subfamilies. Reimers, however, stated that Polypodiaceae would undoubtedly be polyphyletic in this concept, noting that Dennstaedtioideae closely resembled Dicksoniaceae, that the relationship of Dipteridaceae to Polypodiaceae was unclear and that the origin of the obviously primitive Woodsioideae and Onocleoideae required further study. We now know that if the heterosporous ferns and Parkeriaceae are included, this would make a monophyletic group (and could in theory be treated as a single family), and most subfamilies treated here correspond to many of the currently accepted families or subfamilies. Differences lie mostly in the placement of single problematic genera, e.g. Nephrolepis in Davallioideae, Pteridium Gled. and Stenochlaena J.Sm. in Pteridoideae, Diacalpe Blume and Peranema D.Don in Woodsioideae (now part of Dryopteris Adans., see Zhang and Zhang, 2012), Cyclosorus Link, Lomariopsis Fée and Thelypteris in Dryopteridoideae and Cheiropleuria C.Presl in Polypodiaceae. In spite of obvious misplacement of some genera, this work actually sets a good example for how the fossil record can be used to aid in placement of extant taxa, and it has therefore been unfortunate that this classification did not find wider acceptance.
Alston (1956), while preparing an account for the Flora of West Tropical Africa, came to disagree with the classification of Holttum (1947). He segregated Lindsaeaceae from Dennstaedtiaceae and maintained Vittariaceae as separate from Adiantaceae [although this had already been done by Reimers (1954) at the subfamilial level]. He also segregated Aspleniaceae, Aspidiaceae, Athyriaceae and Blechnaceae from Holttum's Dennstaedtiaceae, and he described Athyriaceae and Lomariopsidaceae. The movement of genera by various authors between Athyriaceae, Aspidiaceae and Dennstaedtiaceae shows, however, that relationships among these genera were far from understood.
Pichi-Sermolli (1958) subdivided Pteridophyta into six classes, which were mixtures of living and fossil taxa with the two exceptions noted: Lycopsida, Sphenopsida, Noeggerathiopsida (fossil taxa only), Psilotopsida, Psilophytopsida (fossil taxa only) and Filicopsida. He used Filicopsida because the name Pteropsida was then often used in a broader sense to include all vascular plants. Within Lycopsida, he placed Isoëtaceae amid Lepidodendridae. The two genera in Psilotales were placed in their own families, and he stated ‘that these show no close affinity with the other groups of Pteridophyta’ and should be placed ‘near the Psilotophytopsida purely as a matter of convenience, since their derivation from them is very questionable’, a return to artificial classification in the absense of evidence of relatedness. Moving on to his Filicopsida, he divided this class into seven subclasses, of which Primofilicidae are extinct, and all others have living members. For his classes Ophioglossidae and Maratiidae, by most earlier authors joined in a single group ‘Eusporangiatae’, Pichi-Sermolli agreed that these two lineages differ greatly in morphology, and he dropped the terms Eusporangiatae and Leptosporangiatae ‘because the origin of the sporangia does not offer us a sharply taxonomical distinction’. In his opinion, these terms ‘should be used as descriptive morphological terms, not as names of taxa’. Osmundidae were maintained in a separate subclass because of their ‘antiquity and their paleontological history’. The living heterosporous ferns are placed in two subclasses: Marsileidae and Salviniidae. Formerly placed together, the two were not considered to be related and were treated by Nakai (1949) as classes, but ‘it is clear that they are related to homosporous Filicidae’. Within Filicidae, Pichi-Sermolli agreed that delimitation and classification had been difficult ‘and pteridologists are very far from agreeing about their classification’. The number of families had at that time increased due to splitting of the traditional Polypodiaceae (e.g. Ching, 1940; Holttum, 1947; Alston, 1956; Pichi-Sermolli, 1957), and this led to the suggestion they should be organized in orders, which Pichi-Sermolli (1958) did in a tentative scheme.
Cytology enters the picture
Prior to 1950, cytological aspects of ferns were practically unknown, and what was known was mainly due to the pioneering work of Manton (1950, 1954, 1958; Manton and Sledge, 1954) and Brownlie (1957, 1958); as cytological information became more widely available, it began to be applied in classifications. As more chromosome counts accumulated over the next decades, the position of certain species and wider affinities of genera, families and higher ranks could be postulated. By the 1970s, chromosomes of around 15 % of fern species had been counted, and Walker (1973) provided a good review of how cytological information could help in unravelling previous problems. Manton (1958) demonstrating, for instance, that Pteridaceae of Copeland (1947) did not form a uniform lineage, with Pteris, Cheilanthes Sw. and Adiantum having chromosome numbers based on 29 or 30, and the other group (corresponding to the ‘dennstaedtioid group’) showing a wide range of basic numbers, even within a single genus. Chromosome numbers continued to play a role in classification for several decades, only to be replaced by molecular phylogenetics in the 1990s, providing important insights into relationships of species, genera and sometimes families. Mehra (1961) finalized the application of chromosome numbers and described phyletic lineages in terms of their cytological evolution, but this information was not included in later fern classifications. Cytological data on ferns are well documented and may in the future provide good additional insights into fern relationships and evolution.
The beginning of a new approach to fern classification, before DNA
In 1961, Wagner wrote an essay discussing the problems related to fern classification. He correctly concluded that due to the problems of assumed homology of morphological traits and associated convergent evolution abundant in ferns, classification had been unstable and the concept of a fern family had become meaningless. A few years later, Wagner (1969) addressed the question of how a system of relationships is constructed. He discussed non-evolutionary classifications and the then-modern phenetic systems of numerical taxonomy. Numerical taxonomy in which all information is included, he believed, could lead to circumscription of evolutionary groupings, but he stated that he was concerned about parallelisms and convergences and that in the case of ferns he did not have enough data needed to make an objective classification. He also made a significant and relevant statement: ‘In botany it may be that purely phenetic systems, no matter how detailed, will not produce results that coincide with evolutionary relationships. This possibility very much needs to be tested’. Wagner illustrated the subjectivity of phenetics in assuming homology of similar forms, when in fact they have evolved independently. As an example, he used evolution of heterospory, which, although appearing highly similar, certainly evolved several times. Of course this argument over phenetic and evolutionary classifications – the latter in which certain characters are weighted and exhibit a trend from primitive to derived – is still ongoing, and therefore the classification given in this review below is as much biased and subjective as previous ones, were it not that it is based on far more data than were available in the 1960s.
Similarly, Nayar (1970) criticized the classifications proposed in the 1940s and 1950s because they diverged substantially, which he blamed on the differing importance various authors gave to morphological characters. He proposed a new classification based on the ‘significant additions to our knowledge relating to several morphological criteria of the pteridophytes’, which he enumerated, but neither justified nor cited sources for this ‘knowledge’. In spite of his argument that subjective classifications are bad practice, his classification was also based on subjective interpretation of morphological characters. Even though the genera were better known than before, this again resulted in movement of genera into different families and changes between family and subfamily names. He did attempt to place his families and subfamilies into an evolutionary scheme, although also here there are some odd placements. Plagiogyriaceae, for instance, are in his scheme derived from Osmundaceae, which is a strange placement for a group that has sporangia bearing remarkable similarities to those of Cyathea (Hooker and Bauer, 1842). Blechnaceae, to which Plagiogyria (Kunze) Mett. generally was thought to be related due to superficial similarity, are the only unplaced lineage in Nayar's scheme in which there had been no attempt to justify postulated relationships.
Because Pichi-Sermolli (1970b) published a detailed catalogue of fern family names, Holttum (1971) felt obliged to comment on it and pointed out that ‘in the case of family names we are very far from being able to make a good taxonomic judgement’. Holttum stated that it is difficult to decide which family names would be the correct ones to use because ‘most family names of ferns have had such different meanings’ and ‘that such names are only intelligible if we associate them with the names of particular authors’. Holttum therefore regarded ‘all family names of ferns as informal and tentative’, a comment that may still be relevant today. Against this background, genetic studies of ferns were proposed, helping us understand processes of speciation and inheritance in ferns (e.g. Klekowski, 1971); this sort of publication did not involve such genetic studies, but rather hinted at the advantages of using a fern system rather than an angiosperm system for genetic analyses (due to their alternation of generations producing haploid gametophytes).
The view in 1972: fern classification is still a jungle for the user, unless …
In April 1972, a symposium was held in London bringing pteridologists from around the world together to discuss phylogeny and classification of ferns. In the resulting conference volume, Holttum (1973) stated that it would be impossible to come to a new classification without additional good monographic studies. He was of the opinion that ‘phylogenetic understanding will develop as classification improves’, but that classification ‘is still in a very imperfect state’. Nevertheless, Holttum proposed a tentative scheme, showing possible relationships, setting his understanding of fern inter-relationships apart from the schemes of Nayar and Kaur (1971) and Bierhorst (1971). The groups that Holttum suggested should be revised taxonomically [e.g. Adiantum, Asplenium, Cheilanthes, Ctenitis (C.Ch.) C.Ch., Diplazium, Dryopteris and Tectaria Cav.] are often groups that even today are still in need of taxonomic study, and some still need an improved generic circumscription.
In the same volume, Pichi-Sermolli (1973) provided an historical review of fern classification, in which he showed how classifications had changed over time and how unstable they had been. He concluded that no real progress in fern classification based on morphology could be made without the collaboration of palaeobotanists. He placed his hopes in numerical approaches due to their ‘being more objective’, which ‘will placate the fight between splitters and lumpers’. We now know that this was a forlorn hope and has not turned out to be the case, and arguments about splitting and lumping are still a major problem in taxonomy of all groups despite large amounts of genetic (DNA) data having been collected. Knowing the phylogenetic tree does not, of course, tell us how to delineate families or genera.
Mickel (1974) criticized the development of phyletic classifications over the preceding years, stating that circumscription was often ‘ill-founded and the relationships were often based on speculation rather than solid evidence’. Mickel stressed the need for proper evaluation of homologous characters, and he discussed some of the major issues of homology, for instance the different types of scales and development of stelar structures. He also pointed out the usefulness of stomata morphology to infer phyletic relationships. He then presented a freely drawn phylogram, which is backed by a discussion of characters, but, even though the evidence was sound, the choice of characters used to develop this phyletic classification was still subjective.
Crabbe et al. (1975) discussed the rearrangement of pteridophytes at the British Museum of Natural History and New York Botanical Garden with a large number of pteridologists and provided a new generic sequence for fern herbaria. They provided a complete list of genera, which was helpful in making sense of the numerous names proposed and used in herbaria. Even though many groupings do not seem natural, it reflected knowledge of fern classification at the time. They did suggest an alphabetical system as an alternative, which is easy for filing, but they pointed out the limitation in implementation of generic changes without major reorganization of the entire herbarium in that case. Placement of the heterosporous ferns was still uncertain, and they were therefore placed at the end of the sequence. Some genera were placed where we would not expect them based on their morphology (e.g. Plagiogyria among eusporangiate ferns). Despite its ambitions, this sequence never found much of a following in herbaria.
Development of fern classification and further study of the structures of ferns were summarized in Tryon and Tryon (1982) and Kramer and Green (1990). Kramer and Green (1990) was the most influential classification until the arrival of molecular phylogenetics, although it should be stated that at that time Polypodiaceae was generally taken in a broad sense, including most leptosporangiate lineages, and within that it was preferred to place genera alphabetically, a practice that is still followed in some books and herbaria. It may make it easy to find a genus quickly (if you happen to know in which genus a species is placed), but it can be difficult and laborious to update the herbarium when generic changes take place or to find a species when it has previously been placed in more than one genus.
Molecular systematics (DNA) and fern classification
The advent of molecular phylogenetics has rapidly changed our understanding of fern relationships through phylogenetic analyses of DNA sequence data. Studies of plastid DNA data slowly started emerging in the mid 1990s. Plastid data became mostly used initially in seed plants, and maternal, biparental (Sears, 1980; Corriveau and Coleman, 1988; Harris and Ingram, 1991) and paternal (Neale and Sederoff, 1988) inheritance of plastids is known in seed plants, but ferns still had not been thoroughly studied. Gastony and Yatskievych (1992) studied a natural hybrid of Pellaea Link with known parentage, which demonstrated that plastid DNA is maternally inherited in this cheilanthoid fern.
In an early phylogenetic study, Hasebe et al. (1993) sequenced four ferns to infer the relationships of ferns to other land plants. Sequencing was still a laborious process, but, due to the development and improvement of in vitro DNA amplification [polymerase chain reaction (PCR)], a much larger number of taxa could be studied, and rbcL data for ferns quickly accumulated. Hasebe et al. (1994) developed effective primers to sequence rbcL in ferns; their paper focused on leptosporangiate lineages, and it showed monophyly of heterosporous ferns within the leptosporangiate clade. They identified all main lineages in ferns and showed that Dennstaedtiaceae were not monophyletic when lindsaeoids were included; they also found Vittariaceae resolved close to Adiantum. Possible changes in classification were suggested, but not formally proposed. Wolf et al. (1995a) sequenced 45 species of dennstaedtioid ferns, which produced similar results to the work of Hasebe et al. (1994), but here too no formal recircumscriptions of groupings were proposed because a much wider sampling of taxa was needed to assess monophyly of such a diverse group of plants.
A large pteridophyte symposium was held at the Royal Botanic Gardens, Kew, in memory of Holttum in 1995. This resulted in a volume called Pteridology in perspective (Camus et al., 1995) in which a wide range of topics in fern research were addressed, including several early molecular phylogenetic studies, family, genus and species concepts, speciation and systematics. In this volume, Pahnke et al. (1995) discussed the utility of the internal transcribed spacer (ITS) in phylogenetics of ferns. Wolf (1995b), who earlier discussed the usage of plastid sequences in fern phylogenetics (1995a), evaluated the use of a combination of genes in molecular phylogenetic studies. Comparisons of morphology with molecular phylogenetic results for tree ferns (Conant et al., 1995) and filmy ferns (Dubuisson, 1995) were also provided, increasing our understanding of these lineages. A discussion of the use of phylogenetic hypotheses for formal scientific classifications was presented by Hennipman (1995), and he stated that ‘modern higher classifications of ferns are a jungle for the user’, showing the great need to simplify fern classification. Hennipman discussed criteria used in the past for family and generic classifications but did not provide a clear-cut way on how to define a family in ferns; he emphasized lineages that he considered monophyletic and which ones were in his opinion clearly paraphyletic. He divided ferns into four orders, Marattiales, Ophioglossales, Psilotales and Filicales, and his order Filicales, which corresponds to the leptosporangiate ferns, is divided into 14 families (Aspleniaceae, Cyatheaceae, Davalliaceae, Dennstaedtiaceae, Dryopteridaceae, Gleiche-niaceae, Hymenophyllaceae, Lindsaeaceae, Marsileaceae, Osmundaceae, Polypodiaceae, Pteridaceae, Schizaeaceae and Thelypteridaceae). The classification of Hennipman formed the backbone on which more recent classifications have been based because this simplified organization of the ferns was much more accessible than previous schemes. Of course with the molecular analysis of previously unsequenced genera, this simplified classification was destabilized and new families were again added.
The number of taxa studied increased when Hasebe et al. (1995) analysed rbcL sequences of 107 species. Their results showed that Grammitidaceae are embedded in Polypodiaceae, and Davalliaceae are the closest relative of the Polypodiaceae clade. Tectaria was found to be closer to Oleandraceae rather than to Dryopteridaceae, with which it was usually associated. Again, Dennstaedtiaceae were found to be polyphyletic, but this time with three lineages emerging; Lonchitis and Orthiopteris Copel. fell in a clade separate from Lindsaea Dryand. Vittariaceae were found deeply embedded in Pteridaceae and strongly supported as sister to Adiantum. Tree ferns (including Metaxyaceae, Loxsomataceae and Plagiogyriaceae) were monophyletic and diverged early from the rest of the leptosporangiate taxa, just after the divergence of heterosporous ferns. No clear placement was found for Psilotaceae, but in one of their analyses (neighbor joining), Psilotum Sw. and Tmesipteris Bernh. were sister to Ophioglossaceae, a portent of what would be later found.
Although the techniques were still laborious, data for the major groups began to accumulate rapidly, and certain problematic fern groups were logically targeted using rbcL sequences, e.g. cheilanthoid ferns (Gastony and Yatskievych, 1992; Gastony and Rollo, 1995), dennstaedtioid ferns (Wolf, 1995a) and vittarioid ferns (Crane et al., 1995), helping to better understand relationships of genera in these groups.
Despite the molecular revolution in fern phylogenetics, morphology was not forgotten, and Smith (1995) summarized non-molecular ideas of fern relationships. He hypothesized that the eusporangiate families Ophioglossaceae and Marattiaceae constituted isolated lineages and that Osmundaceae, Schizaeaceae, tree ferns and a few other small families represent early branches off the main lineage leading to other leptosporangiates. This was based on fossil and living plant morphology and became the most broadly accepted classification. Morphologically most leptosporangiate families were recognizable, but inter-relationships among these were still unknown and widely disputed. Fern systematics had been diverted from producing phyletic schemes based on morphological characters, and thus these ideas were never robustly tested. Smith (1995) posed 16 challenging questions and invited readers to answer as many as possible. Many of these questions related to placement of genera with difficult to interpret morphology – Ceratopteris Brongn., Hymenophyllopsis K.I.Goebel, Monachosorum Kunze, Plagiogyria, Pleurosoriopsis Fomin, Psilotum, Saccoloma Kaulf. – or to relationships of entire larger groups – Dennstaedtiaceae, Grammitidaceae, Hymeno-phyllaceae, Lindsaeaceae, Schizaeaceae, Thelypteridaceae, Vittariaceae and heterosporous ferns. Delimitation of Dryopteridaceae and other higher leptosporangiate fern families also needed sorting out because at the time their relationships were hotly debated. Hasebe et al. (1994) had already resolved some of these issues in their phylogenetic analysis of rbcL, showing Adiantum to be sister to Vittariaceae, Monachosorum sister to Dennstaedtiaceae, Lindsaeaceae sister to all higher leptosporangiates separate from other dennstaedtioids, Plagiogyria and Metaxya C.Presl in the tree-fern lineage, and the heterosporous ferns forming a lineage in leptosporangiate ferns (see also Fig. 3). The base of their tree did not have good support, but it was after all a first attempt at using DNA sequences to examine broader relationships within ferns; many relationships had to be left for resolution in subsequent studies.
Fig. 3.
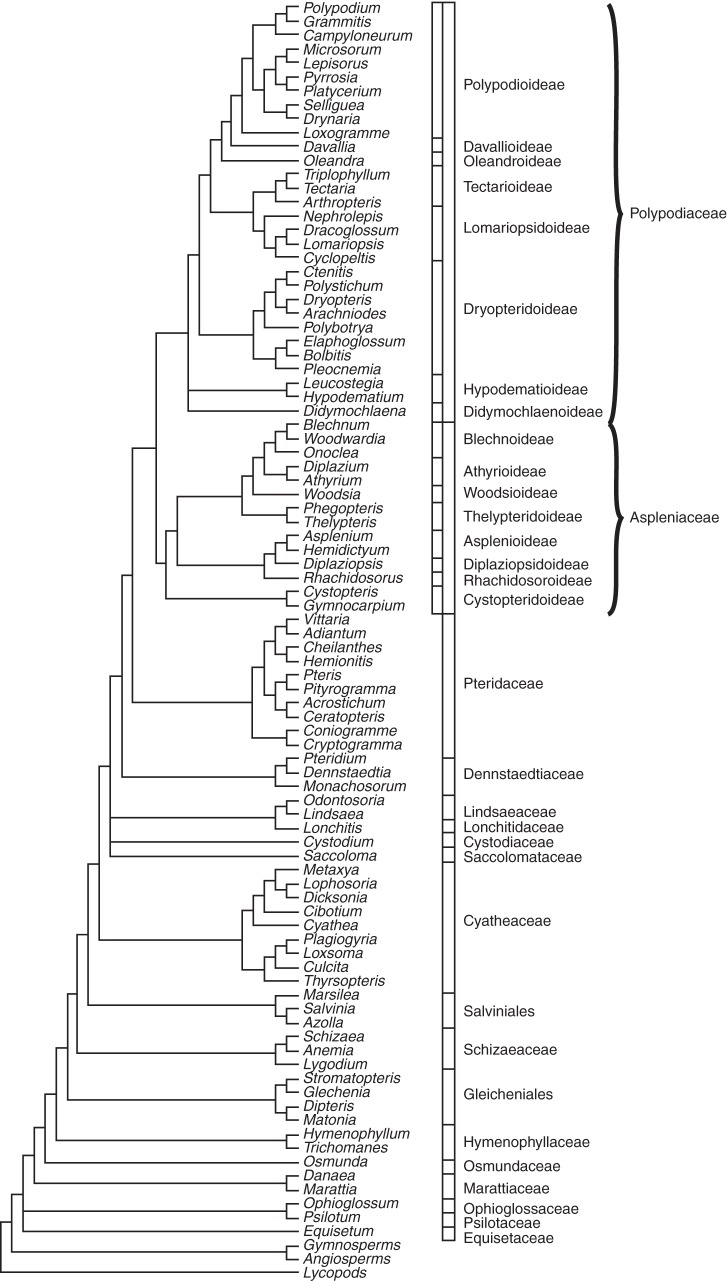
Summary phylogenetic tree showing relationships of a representative selection of fern genera based on molecular (DNA) data, modified from Schuettpelz and Pryer (2007), Lehtonen (2011), Rothfels et al. (2012) and Schneider et al. (2013).
Smith's review (1995) soon led to analysis of combined molecular and morphological data sets. Pryer et al. (1995) presented the first cladistic analysis based on morphology combined with rbcL data for the same 50 taxa. They also found Ceratopteris and Vittaria Sm. among pteridoid ferns and Plagiogyria in the tree-fern lineage. Dennstaedtiaceae formed two unresolved lineages, but with Lindsaea not grouping with either of these. Heterosporous ferns and Schizaeaceae formed two independent lineages also at unresolved positions within leptosporangiate ferns.
Plastid 16S ribosomal DNA sequences were then applied to address the placement of ‘fern allies’. Manhart (1995) found Psilotum as sister to Tmesipteris, together forming a well-supported sister group to Ophioglossaceae. This relationship seemed odd at first, but it is now supported by morphological characters (Schneider, 2013). When the problematic lycopod Selaginella P.Beauv. was removed from their matrix (which perhaps had been causing some sort of spurious attraction of Equisetum to it), Equisetum also fell within the fern lineage but without strong support. Hasebe et al. (1995) further addressed the problems posed by Smith (1995) in an expanded rbcL phylogenetic analysis, which supported the sister relationship of Psilotaceae and Ophioglossaceae and showed Dennstaedtiaceae, Polypodiaceae, Pteridaceae and Dryopteridaceae in their traditional senses to be polyphyletic.
Placement of Hymenophyllopsis, a peculiar genus from the tepuis in the Guayana Shield, has always been problematic, and it was therefore often placed in its own family, Hymenophyllopsidaceae. The sorus structure is unique in ferns, although superficially similar to filmy ferns. Wolf et al. (1999) had shown that these are related to the tree fern genus Cyathea, with which they share scale and spore morphology. More recent studies have shown that Hymenophyllopsis is embedded in Cyathea (Korall et al., 2006, 2007), and thus it should not even be treated as a genus, let alone as a family or order. Christenhusz (2009b) and others now treat it as part of Cyathea.
Lophosoria quadripinnata (J.F.Gmel.) C.Ch., a common montane species in the Neotropics, was placed in its own family by Pichi-Sermolli (1970b), who removed it from the mosly extinct Protocyatheaceae, where it had been placed by Bower (1923–1928) and Reimers (1954). The relationship of this trunkless species to the tree ferns was never disputed, but Wolf et al. (1999) showed it to be most closely related to Dicksoniaceae.
Placement of Equisetum was evaluated again by Pryer et al. (2001a), who showed with strong support that it belongs to the fern lineage. In the process, they refuted the prevailing theory that horsetails and ferns form a transitional grade between lycopods and seed plants. As a replacement name for the ferns including Equisetaceae, Kenrick and Crane (1997) had proposed the infradivision Moniliformopses, an invalid name under the current nomenclatural code (McNeill et al., 2012). The term ‘monilophytes’, loosely based on this infradivision, became established as a result of the paper of Pryer et al. (2004), in which phylogenetic relationships of ferns were inferred. Why this new term not based on a generic name is useful in the context of a cladistic study is unclear to us. Nevertheless, it was quickly picked up by the community and rendered into the vernacular as ‘monilophyte’ (e.g. Pryer et al., 2001a, 2004; Schneider et al., 2004; Schneider, 2013). ‘Moniliform’ means ‘bead-shaped’, but it was never stated to what part of a fern, or to which fern, this refers, although it was suggested that this might refer to the stele structure of some extinct Devonian taxa. It seems that a new colloquial name has been coined for no particular reason other than providing a scientific-sounding synonym for the word ‘fern’; in fact, it is often used in parentheses after the word fern, so ‘monilophyte’ is superfluous. It is an alternative to the word ‘pteridophyte’, which traditionally included all ferns plus lycopods; thus, an alternative, similar sounding word was coined, without taking notice of its etymology: ‘bead-plant’ being as uninformative as ‘wolf-plant’ or ‘lycophyte’, which should correctly be called clubmoss (in the English vernacular) or lycopod (based on Lycopodium and referring to the vernacular ‘wolf-claw’ of many languages).
Psilotaceae were not included in the study of Kenrick and Crane (1997), but Pryer et al. (2004) included these to show they also belong to the fern clade. Critical taxa were included in this data set and additional plastid (rbcL, atpB and rps4) and nuclear (18S rDNA) regions were added to those previously used. As expected, Osmundaceae were sister to the leptosporangiate ferns. Hymenophyllaceae – which had recently been shown to consist of two monophyletic groups (Pryer et al., 2001b) – was found to be sister to the gleichenioid ferns, and tree ferns and heterosporous ferns were shown to belong to ‘core leptosporangiates’.
In a high-profile paper, Schneider et al. (2004) studied the age of fern diversification and concluded that the higher leptosporangiates, to which the majority of extant ferns belong, diversified in the Cretaceous, most probably in response to diversification of the angiosperms. The hypothesis is that, because of the increase in diversity of angiosperms, numerous new niches appeared in which ferns could diversify. Schneider et al. (2004) also introduced the term ‘eupolypods’, defined as the polypods excluding ‘basal’ polypods (e.g. Dennstaedtiaceae and Lindsaeaceae) and pteridoids (Pteridaceae). The eupolypods were found to be represented by two clades (numbered I and II), on which we will elaborate below. This showed that many fern lineages are of similar age to or younger than many angiosperm families, refuting the usage of clade age as a useful character for defining a fern family.
Classification appeared to be more stable after publication of the fern classification by Smith et al. (2006), which summarized molecular findings to that date and provided synapomorphies for the accepted families. Schuettpelz et al. (2006) added plastid atpA to their earlier fern data set to gain better support at deeper nodes. They continued their work on fern phylogeny and performed a global analysis to serve as the basis for addressing large-scale evolutionary questions (Schuettpelz and Pryer, 2007). They assembled and analysed the most inclusive molecular data set to date with three plastid regions (rbcL, atpB and atpA) including 400 leptosporangiate fern species. Their findings were of great value for the placement of genera that were previously unavailable for molecular study and were dependent on the generous contributions of many fern collectors.
Meanwhile, a number of families were studied in greater phylogenetic detail, resolving many taxonomic problems (e.g. Korall et al., 2006, 2007; Nagalingum et al., 2006, 2007; Christenhusz et al., 2008; Hennequin et al., 2008; Rothfels et al., 2008). New findings were incorporated in the updated classification of Smith et al. (2008), although this did not substantially differ from his classification published two years earlier.
Schneider (2007) tested the congruence of molecular and morphological data with the idea of incorporating fossil taxa in phylogenetic studies, which is currently being further developed (Corvez et al., 2011). Morphological data have since been combined with molecular data in analyses with some important insights (e.g. Schneider, 2007; Lehnert et al., 2009; Lehtonen et al., 2010; Sundue, 2010; Sundue et al., 2010).
In a new edition of A. Engler's Syllabus of Plant Families, Fischer (2009) adopted findings of recent molecular analyses and treated Lycophytina as separate from Euphyllophytina. Within the latter, he accepted super-class ‘Moniliformopses’ of Kenrick and Crane (1997), comprising all ferns, Equisetaceae and Psilotaceae. A synopsis was provided on classification of this group, and it included all fossil taxa, which in many cases were placed in families consisting of exclusively fossil taxa. We did not check all fossil families, but we noticed that nomenclatural priority of family names was not always taken into account. For the extant taxa, it simply followed the classification of Smith et al. (2006), although here and there with some reservations and occasionally following some subclassifications that had already been shown to be incorrect. The acceptance of subfamily Stenochlaenoideae as separate from Blechnoideae, for instance, is inappropriate if Woodwardia Sm. is included in either of them.
The classifications of Smith (2006, 2008) initiated a discussion about morphology being incompatible with the molecular results, and indeed some groups such as Hypodematiaceae, Lomariopsidaceae, Pteridaceae and Woodsiaceae (sensu Smith) were difficult to define morphologically. Therefore, an analysis with morphological data alone was carried out (Schneider et al., 2009), and this recovered the same deep phylogenetic relationships (recognizing the four major clades in ferns: Equisetidae, Ophioglossidae, Marattiidae and Polypodiidae) found in previous studies of DNA sequence data. It would appear that molecular data and morphology are compatible, provided they are treated in similar ways.
To provide a guide for herbaria to organize their specimens in a phylogenetic way, a linear classification for all vascular plants was composed of which here the lycopods and ferns are of consequence (Christenhusz et al., 2011). This linear sequence was not intended as a new classification and followed in many ways Smith et al. (2006, 2008), but major changes were made in the treatment of some ‘eupolypods II’, with the disintegration of Smith's polyphyletic Woodsiaceae and with descriptions of Diplaziopsidaceae, Rhachidosoraceae and Hemidictyaceae (Christenhusz and Schneider, 2011; Christenhusz et al., 2011). Also, placement of Equisetaceae as sister to all ferns seems to be better than as sister to Marattiidae (as in Smith et al., 2006). The position of Equisetum is poorly supported but always among the deeper nodes of the fern clade, and therefore it could end up in various places, depending on the method of analysis or data set used (Lehtonen, 2011). Placement as sister to all other ferns is also in agreement with palaeontological studies that show its early appearance in the fossil record (Taylor et al., 2009).
The classifications of Smith et al. (2006, 2008) and Christenhusz et al. (2011) mostly reduced the number of genera, resulting in an expansion of several (e.g. Asplenium, Blechnum, Cyathea and Hymenophyllum Sm.), but also resulted in the acceptance of narrower generic concepts in other groups (e.g. Hymenophyllaceae, Polypodiaceae and Pteridaceae). These classifications are, however, remarkable in that they combine morphological and molecular data to come to a consensus classification, aiming to define monophyletic families and genera with synapomorphies.
Some of the notable discrepancies are due to the traditionally broader generic concepts used by workers in the Neotropics compared with those in tropical Asia. Now we can combine the data on these taxa and come to a consensus classification resulting in evolutionarily defined natural genera and families. With the wealth of data currently available (e.g. Eiserhardt et al., 2011; Lehtonen, 2011; Lehtonen et al., 2012; Rothfels et al., 2012; Zhang and Zhang, 2012; Liu et al., 2013; Schneider, 2013; Schneider et al., 2013) it is now possible to compare generic concepts across continents, applying a global concept of family and genus.
DISCUSSION AND SOME HIGHLIGHED GROUPS
Concepts of higher classification of ferns and lycopods have changed considerably throughout history, and we therefore discuss a few groups with regard to their previous and current delimitation.
Fern allies have previously been loosely defined as vascular spore-bearing plants that are not ferns. They have variously included the lycopods (Huperzia Bernh., Lycopodiella Holub, Lycopodium, Isoëtes and Selaginella), horsetails (Equisetum, Fig. 4A), whisk ferns (Psilotum and Tmesipteris, Fig. 4C), Ophioglossaceae (Fig. 4B), water ferns (Marsileaceae, Fig. 4I, Salviniaceae and Ceratopteris) and sometimes even members of Schizaeaceae and Gleicheniaceae. Of course this was never considered a natural group but just included genera that according to subsequent authors could not be placed among the ferns proper for various reasons. Because of its vague circumscription and now evident non-monophyly, the term ‘fern ally’ should be avoided. In a similar way, the term ‘eusporangiate fern’ is to be avoided because this includes ferns that are not leptosporangiate; it just describes the plesiomorphic state (eusporangia are also present in seed plants and lycopods) and the taxa included do not form a clade.
Fig. 4.
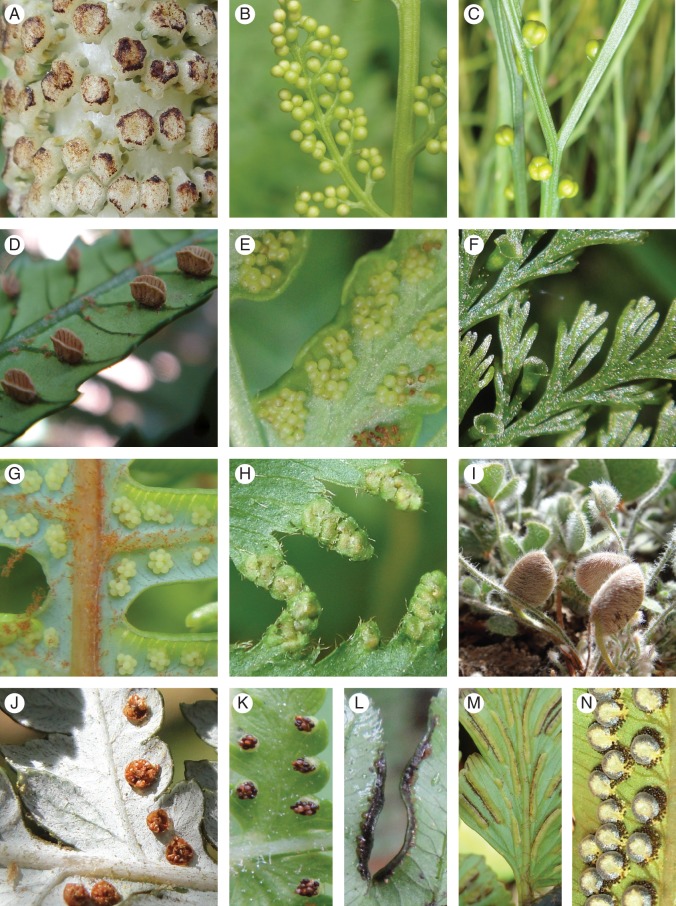
Sori of major fern lineages. (A) Equisetales, Equisetum telmateia Ehrh. (B) Ophioglossales, Botrychium virginianum (L.) Sw. (C) Psilotales, Psilotum nudum (L.) P.Beauv. (D) Marattiales, Marattia cicutifolia Kaulf. (E) Osmundales, Todea barbara T.Moore. (F) Hymenophyllales, Trichomanes cupressoides Desv. (G) Gleicheniales, Dicranopteris linearis (Burm.f.) Underw. (H) Schizaeales, Lygodium volubile Sw. (I) Salviniales, Marsilea drummondii A.Braun. (J) Cyatheales, Alsophila dealbata (G.Forst.) C.Presl. (K) Polypodiales, Dennstaedtia punctilobula (Michx.) T.Moore. (L) Pteris usambarensis Hieron. (M) Eupolypods, Asplenium caudatum G.Forst. (N) Dryopteris sieboldii (T.Moore) Kunze.
The water ferns (Salviniales, Fig. 4I) were previously placed among ‘fern allies’ because they are heterosporous like Selaginella and Isoëtes. They have, however, been found to be sister to the polypods, so they are deeply embedded in the homosporous fern clade. Heterospory in general evolved several times independently in lycopods, ferns and seed plants, but this evolution is not necessarily the result of adaptation to an aquatic life form, although it appears as a plausible association for heterosporous ferns.
Even though it is not a new concept (Linnaeus, 1753, already included Equisetum in his ‘Cryptogamia filices’), the term ‘monilophyte’ was coined (Pryer et al., 2001a) for the fern clade including Equisetaceae and Psilotaceae, in contrast to the ‘lycophytes’, both of which are linguistically erroneous and superfluous terms. This clade has been traditionally called pteridophytes, but it has been suggested that ‘pteridophyte’ cannot be used because in some classifications it has included the lycopods. However, the origin of the term ‘monilophyte’ is unclear and has never been published as a formal taxon and, moreover, its etymology is obscure. ‘Sphenophytes’ (a term commonly used for extant horsetails and their fossil relatives) share a common ancestor with ferns, even though their exact placement within the fern lineage is still uncertain. It is therefore preferable to use the term ‘fern’, which as noted above has in the past often included Equisetaceae and Psilotaceae, although alternatively the early branching lineages of the fern clade could be treated as independent lineages (‘sphenophytes’, ‘psilotophytes’, ‘marattiophytes’), although this does not reflect their membership in a clade with the rest of the ferns.
To illustrate the changing ideas of how ferns should be classified, we focus below on a number of groups and genera and describe the variation in opinions that have existed about how best to handle their taxonomy.
Leptosporangiate ferns are a natural group, forming the crown group of ferns including the vast majority of species. In the past, most of these have at one time been included in a single family, Polypodiaceae (or variants such as Filices or Dennstaedtiaceae, variously including Osmundaceae, tree ferns and gleichenioid ferns), resulting in great variability in application and circumscription of this family. The concept changed from including the majority of the leptosporangiate ferns to the most recent concept of those having scaly creeping rhizomes with abaxial (rarely marginal), rounded to elliptic, elongate or acrostichoid, exindusiate sori. The grammitid ferns were traditionally not included, but molecular studies have shown that this group is deeply embedded and therefore also part of Polypodiaceae, expanding the description above to include taxa occasionally having green spores and a petiole not always cleanly abscising (as is the case with most other polypods; Smith et al., 2008). Generic classification of grammitid ferns has been unstable during the last 20 years, and they are currently divided in a great number of genera, even though they form a subclade within Polypodiaceae. It would be better for general users if these are treated as a single genus (with approx. 700 species, making it a large genus, the size of Asplenium, but smaller than Elaphoglossum or Thelypteris) and treat most currently recognized genera at the subgeneric level. Polypodiaceae as a family should probably be expanded again to include all families placed in ‘eupolypods I’, avoiding the need to recognize a great number of monogeneric families; this approach is taken in the classification presented below.
Placement and number of genera in the filmy fern family Hymenophyllaceae (Fig. 4F) have long been debated. Traditionally two genera have been recognized (Hymenophyllum and Trichomanes) on the basis of differences in soral morphology, but Iwatsuki (1977) pointed out that the morphological distinction is not clear-cut. The morphological diversity in the family has resulted in recognition of a number of additional genera; however, several molecular studies (e.g. Pryer et al., 2001b) have shown that only two lineages are present, more or less corresponding to the two traditionally recognized genera. Ebihara et al. (2006) proposed on the basis of his analysis to maintain Hymenophyllum in a broad sense, but meanwhile accepted eight genera in the Trichomanes clade, which in our opinion may not be warranted. As is the case for grammitid ferns, it seems a better solution for general users to treat these as subgenera of Trichomanes, accepting only two genera in Hymenophyllaceae.
Tree ferns (Fig. 4J) are sister to ‘polypods’ sensu Smith et al. (2006) or Polypodiales sensu Christenhusz et al. (2011; see Fig. 3). The tree-fern clade is usually divided into eight small families, with Cyatheaceae being the largest (Korall et al., 2007; Lehtonen, 2011). Tree ferns are all minimally genetically divergent, which may be a result of the much longer generation time of these long-lived plants (palms are a similar example from the angiosperms; e.g. Asmussen and Chase, 2001). It is therefore possible to merge all families of Cyatheales in a single family, which has generally not been done because there are few universally diagnostic characters. The best character is the helicogyroid annulus of the sporangium, which links all these taxa together and which has been used as the synapomorphy for the order Cyatheales. This leads to a discussion of what defines an order relative to a family. One character used is the age of divergence between the individual lineages, which in the case of Cyatheales is Late Jurassic (Schneider et al., 2004), similar to the age of many angiosperm families. Cyatheales are too highly divided at the family level, and the lineages should still be revised taxonomically on the basis of synapomorphies and monophyly. In the classification below, the established families are treated at the subfamilial level, allowing movement of genera between them without altering their family placement. Not all genera in Cyatheaceae sensu lato are tree like; they include trunkless Plagiogyria, Metaxya and Loxsoma R.Br. They also include Hymenophyllopsis (Lellinger, 1984), a group of small ferns from the Guayana Highlands in South America. This genus was difficult to place on a morphological basis and was thus placed in its own family or even its own order. It is now known that these are embedded in Cyathea (Korall et al., 2007), and combinations in Cyathea have been made for all eight species (Christenhusz, 2009b).
Lonchitis has also been variously treated in the past, the genus previously including many species, but now reduced to two (most of the remaining species were moved to Blotiella Tryon, Dennstaedtiaceae). Placement of Lonchitis has been contentious in recent times. Earlier it was placed in Polypodiaceae subfamily Dennstaedtioideae or Dennstaedtiaceae, but Smith (2006) placed it in Lindsaeaceae. This makes the last difficult to define morphologically; hence, it was placed in its own family Lonchitidaceae (Schomburgk, 1848; Christenhusz, 2009a; Lehtonen et al., 2010; Christenhusz et al., 2011), a family placed near Saccolomataceae and Cystodiaceae among the early branching polypods, even though the deep nodes of the polypods are not well supported in most studies (Lehtonen et al., 2012).
Lomaria Willd. has, since its publication, been widely accepted, so much so that Smith (1875) stated that it is a natural genus containing a considerable number of species of great uniformity of habit. Hooker (in Hooker and Bauer, 1842) agreed with this to a certain extent, but he pointed out that a number of species, including L. spicant (L.) Desv. ‘vacillate between Lomaria and Blechnum …, but such or similar gradations are common to other genera of ferns, and if made an excuse for abolishing a long-established one, the number of genera would undergo a great reduction’. Hooker further discussed inclusion of Plagiogyria in Lomaria, and he pointed out that there is an ‘affinity of those species with Cyatheaceae’ (to which we now know Plagiogyria is related), especially in characters of the ‘capsules’ (the annulus of the sporangium is oblique, e.g. helicogyrate), ‘yet the habit and sori are so entirely in accordance with Lomaria that’ the genus Plagiogyria is difficult to identify. He further discussed differences in the number of genera accepted in this group according to other authors. Mettenius (1857), who excluded Plagiogyria, stated that several species depart from the pteridoid character and completely merge into Blechnum, such that Blechnum and Lomaria are no longer separate, whereas Presl (1851) kept them separate and divided them into a multitude of genera based on minor characters. Some of these genera are still in use today, but recent phylogenetic studies (Lehtonen, 2011) have shown Salpichlaena J.Sm. and Stenochlaena (which had been separated from other genera on the basis of their climbing habit) to be embedded in Blechnum (specifically related to B. serrulatum Rich.), which should also include Brainea J.Sm., Doodia R.Br., Pteridoblechnum Hennipm. and Sadleria Kaulf. Lomaria was based on the Australian L. nuda (Labill.) Willd., and Blechnum is based on the Neotropical B. occidentale L.; the European species that has characters of the two was placed in Spicanta C.Presl, an intermediate genus created to solve the problem of intergrading morphology. The current consensus is to apply a broad concept of Blechnum, but further studies on the evolution of Blechnaceae are needed to assess which, if any, of the segregate genera should be maintained.
Acrostichum was a genus applied initially to all ferns with sori that were acrostichoid (Fig. 1F), i.e. distributed as a solid mass across the back of the frond rather than organized in separate, discrete structures. We know today that an acrostichoid fertile lamina can be formed in a variety of ways, and thus it is not useful for classification, although it is definitely a distinctive structure. Therefore, ontogeny of this sorus type is more important for the placement of these ferns than its product. Initially genera such as Blechnum, Ceratopteris, Danaea Sm., several species of Asplenium, Bolbitis Schott, Tectaria, etc. were included, but it was soon realized that these confluent sori were covered by indusia at least at the younger stages, or, as in the case of Danaea, they were fused sporangia with pores on the apex (Christenhusz, 2010). It even included genera as different from each other as Actiniopteris Link, Anetium Splitg., Anogramma Link, Cheiropleuria, Hemionitis, Platycerium Desv., Polybotrya Willd., Schizaea Sm. and Todea Bernh. It was Schott (1834) who separated Elaphoglossum Schott from Acrostichum, which was followed by Moore (1857) who provided numerous new combinations. This process was completed by Christensen (1906), after which Elaphoglossum was widely accepted and Acrostichum (with A. aureum L. as the type species) achieved its modern sense and was restricted to a few species of mangrove ferns.
The name Thelypteris was coined by Schmidel (1763) based on Acrostichum thelypteris L., but the name Thelypteris had already been published in the same year by Adanson (his name is a synonym of Pteris L.), making Schmidel's name illegitimate. This caused confusion, and the name was not accepted by subsequent authors. Pichi-Sermolli (1953) discussed this nomenclature, and Thelypteris of Schmidel was subsequently conserved (Holttum, 1968), resulting in the transferral of numerous species, mainly from Aspidium Sw., Dryopteris and Nephrodium Rich., to Thelypteris. It was soon recognized as a natural group and broadly accepted. Thelypteridaceae as a family were published [including related genera such as Cyclosorus, Macrothelypteris (H.Ito) Ching, Meniscium Schreb., Phegopteris (C.Presl) Fée, Oreopteris Holub, etc.] by Pichi-Sermolli (1970a), and molecular studies soon showed five subgroups in this family, resulting in five genera being recognized as comprising it, although it may be better to accept only three at the generic level (see classification below).
Eupolypods form two subclades often called eupolypods I and II. The first includes the families Davalliaceae, Dryopteridaceae, Hypodematiaceae, Lomariopsidaceae, Nephrolepidaceae, Oleandraceae, Polypodiaceae and Tectariaceae (sensu Christenhusz et al., 2011). These families are morphologically divergent, and it is difficult to see them as a single taxonomic entity, especially if Dryopteridaceae and Polypodiaceae sensu stricto are compared; nevertheless, they share characters of soral structure, which are microscopic, and in the vascular structure of petioles (generally an arch of vascular strands in a U-shape, except in Hypodematiaceae). The second lineage (eupolypods II) includes Aspleniaceae sensu stricto, Athyriaceae, Blechnaceae, Cysto-pteridaceae, Diplaziopsidaceae, Hemidictyaceae, Onocleaceae, Rhachidosoraceae, Thelypteridaceae and Woodsiaceae (sensu Christenhusz et al., 2011), which share the character of a laterally attached indusium (except in some Thelypteridaceae) and two vascular bundles in their petioles. Even though synapomorphies are few and not visible to the naked eye, it is possible to treat these as two broad families instead of the current 18 (or 20) based on their shared characters. Within these two lineages, a plethora of families has recently been proposed (including the recent segregation of Arthropteridaceae from Tectariaceae; Liu et al., 2013), and many genera have changed family (e.g. Christenhusz et al., 2013) when new phylogenetic analyses have become available. Such a broad familial circumscription, if accepted, would make the taxonomy of these groups more stable, allowing further changes at subfamilial levels without affecting the rank of family, which is the one above the genus most frequently used by non-specialists.
A summary of published studies showing relationships of major groups of ferns as well as some problematic genera discussed above is given in Fig. 3. This tree is further discussed below.
CONCLUSIONS
As often observed in taxonomy of many organisms, there has been a trend from an initial artificial classification towards more natural ones over time, although the definition of the latter has changed several times. Artificial classifications are useful for identification purposes, which were often the only intention of early authors (such as Linneaus with his sexual system of plant classification), and these were not intended to reflect phylogenetic relationships between taxa; of course, phylogenetic classification was not a conceptual framework available to taxonomists at that time. These artificial and early ‘natural’ groupings often resulted in species that are superficially similar being placed together, which does not take convergent evolution into account; some such cases in ferns required the application of DNA phylogenetics to demonstrate that they were not reflective of relationships. On the other hand, natural classifications may separate species that are highly similar, particularly in terms of ‘key characters’ that were judged (often subjectively) to be important, in completely unrelated families, which makes these families difficult to tell apart despite their distant relationship. Fern classification is particularly full of such problems, and now that we can use DNA studies to obtain information about where problematic species and genera should be placed, we often find that we lose the capacity to diagnose many families with clear sets of morphological characteristics. As has been observed previously, the more natural a classification becomes, the more difficult it typically is to use, especially for researchers not intimately familiar with the details of fern taxonomy. This is of course not unique to ferns, and it can also be observed in recent classifications of some angiosperm families such as Asparagaceae, Liliaceae and Xanthorrhoeaceae in monocots and Acanthaceae, Gesneriaceae, Lamiaceae and Plantaginaceae among eudicots in APG III (2009). There are few characters that can be easily used to distinguish these families, and their history has been as complicated and controversial as those for most fern families.
A second common trend has been the use of minute characters to circumscribe narrowly circumscribed genera, and some authors have used phylogenetic trees as the basis for placing every pair of sister species into separate genera. Following such episodes, another trend typically emerges, which expands generic limits and subsumes such small genera into larger ones, often redefining the original generic concept to fit this greater number of species with more divergent morphology. Even though this waxing and waning of generic limits may eventually produce a consensus taxonomy, it destabilizes nomenclature and confuses the users. Ultimately, such instability hinders research on ferns by non-taxonomic researchers, thereby retarding progress on understanding their biology, conservation, ecology and population dynamics. Of course, in an ideal world, complete revisions at a generic level should be a prerequisite before familial revisions, but monographic study is time consuming, material for study is not always available, species sampling is often incomplete and funding for such research is scant, resulting in many groups not having been studied in their entirety. Nevertheless, when type species of genera are sequenced and analysed, phylogenetic positions of genera can be inferred and, even though a genus may be found to be polyphyletic, the type species and its associated original generic concept will remain, merely resulting in the transfer of species to other genera, but not genera to different families. Ferns have been notorious in the past for having an unstable higher level classification, but molecular phylogenetic techniques have helped to better understand relationships between genera, and this has resulted in the three most recent classifications (Smith et al., 2006, 2008; Christenhusz et al., 2011) being highly similar, especially when compared with most earlier classifications. There are still likely to be changes in the treatment of families recognized in future classifications. Numerous small families in the eupolypods could be merged with a larger family to which they are sister, such as Davalliaceae with Polypodiaceae, Nephrolepidaceae with Lomariopsidaceae, Hypodematiaceae with Dryopteridaceae, and Hemidictyaceae with Aspleniaceae. We would actually argue that to stabilize fern familial classification, the two clades informally called eupolypods I and II should be treated at the family level, Polypodiaceae and Aspleniaceae, respectively, in which the corresponding families of Christenhusz et al. (2011) would be treated at the subfamilial level. This may now seem like it would be further destabilizing the fern classification, but it will in the longer term stabilize matters, especially when taking into account the poor knowledge of several currently recognized large families, such as Woodsiaceae and Tectariaceae. Within a broader family concept, the genera may be moved between subfamilies but will remain in the same larger family, which is the only higher taxonomic level frequently used by non-taxonomists.
Current consensus classification of extant Lycopodiophyta (lycopods) and Polypodiophyta (ferns)
Numbers in parentheses after families correspond to estimated numbers of genera and species (genera/species). Currently accepted genera are listed; for a full synonymy of genera see Christenhusz et al. (2011). Estimated species numbers for some of the larger genera are also provided. Sori of all major lineages are illustrated in Fig. 4.
[lycopods; 3 families, 5 genera, approx. 1300 species]
Lycopodiidae Bek.
Lycopodiales DC.
Lycopodiaceae P.Beauv.1 (3/approx. 400)
(Huperzia Bernh., Lycopodiella Holub, Lycopodium L.)
Selaginellales Prantl
Selaginellaceae Willk. (1/approx. 750)
(Selaginella P.Beauv.)
Isoëtales Prantl
Isoëtaceae Reichenb. (1/approx. 140)
(Isoëtes L.)
[ferns; 21 families, approx. 212 genera, approx. 10 535 species]
Equisetidae Warm.
Equisetales DC.
Equisetaceae Michx. (1/20)
(Equisetum L.)
Ophioglossidae Klinge
Ophioglossales Link
Ophioglossaceae Martinov2 (4/approx. 80)
(Botrychium Sw., Helminthostachys Kaulf.,
Mankyua B.Y.Sun, M.H.Kim & C.H.Kim, Ophioglossum L.)
Psilotales Prantl
Psilotaceae J.W.Griff. & Henfr. (2/12)
(Psilotum Sw., Tmesipteris Bernh.)
Marattiidae Klinge
Marattiales Link
Marattiaceae Kaulf.3 (6/approx. 130)
subfamily Danaeoideae J.Williams
(Danaea Sm.).
subfamily Marattioideae C.Presl
(Angiopteris Hoffm., Christensenia Maxon, Eupodium J.Sm., Marattia Sw.,
Ptisana Murdock)
Polypodiidae Cronquist, Takht. & Zimmerm.
Osmundales Link
Osmundaceae Martinov4 (4/approx. 25)
(Leptopteris C.Presl, Osmundastrum C.Presl, Osmunda L., Todea Willd.)
Hymenophyllales A.B.Frank
Hymenophyllaceae Mart.5 (2/approx. 650)
(Hymenophyllum Sm., Trichomanes L.)
Gleicheniales Schimp.
Gleicheniaceae C.Presl (6/approx. 165)
[Dicranopteris Bernh., Diplopterygium (Diels) Nakai, Gleichenella Ching,
Gleichenia Sm., Sticherus C.Presl, Stromatopteris Mett.]
Dipteridaceae Seward & E.Dale (2/9)
(Cheiropleuria C.Presl, Dipteris Reinw.)
Matoniaceae C.Presl (2/4)
(Matonia R.Br., Phanerosorus Copel.)
Schizaeales Schimp.
Schizaeaceae Kaulf. (4/approx. 190)
subfamily Lygodioideae Christenh.6
(Lygodium Sw.)
subfamily Schizaeoideae Lindl.
(Actinostachys Wallich, Schizaea Sm.)
subfamily Anemioideae C.Presl
(Anemia Sw.)
Salviniales Bartl.
Marsileaceae Mirb. (3/approx. 65)
(Marsilea L., Pilularia L., Regnellidium Lindman)
Salviniaceae Martinov (2/approx. 20)
(Azolla Lam., Salvinia Ség.)
Cyatheales A.B.Frank
Cyatheaceae Kaulf. (approx. 14/approx. 700)
subfamily Thyrsopteridoideae B.K.Nayar
(Thyrsopteris Kunze)
subfamily Loxsomatoideae Christenh.7
(Loxsoma R.Br., Loxsomopsis Christ)
subfamily Culcitoideae Christenh.8
(Culcita C.Presl)
subfamily Plagiogyrioideae Christenh.9
[Plagiogyria (Kunze) Mett.]
subfamily Cibotioideae Nayer
(Cibotium Kaulf.)
subfamily Cyatheoideae Endl.10
(Alsophila R.Br., Cyathea Sm., Gymnosphaera Blume, Sphaeropteris Bernh.)
subfamily Dicksonioideae Link
[Calochlaena (Maxon) R.A.White & M.D.Turner, Dicksonia L'Hér., Lophosoria C.Presl]
subfamily Metaxyoideae B.K.Nayar
(Metaxya C.Presl)
Polypodiales Link
Cystodiaceae J.R.Croft (1/1)
(Cystodium J.Sm.)
Lonchitidaceae C.Presl (1/2)
(Lonchitis L.)
Lindsaeaceae C.Presl (6/approx. 220)
[Lindsaea Dryander, Nesolindsaea Lehtonen & Christenh., Odontosoria Fée,
Osmolindsaea (K.U.Kramer) Lehtonen & Christenh., Sphenomeris Maxon,
Tapeinidium (C.Presl) C.Chr.]
Saccolomataceae Doweld11 (2/approx. 12)
(Orthiopteris Copel., Saccoloma Kaulf.)
Dennstaedtiaceae Lotsy (10/approx. 240)
[Blotiella Tryon, Dennstaedtia Bernh., Histiopteris (J.Agardh) J.Sm.,
Hypolepis Bernh., Leptolepia Prantl, Microlepia C.Presl, Monachosorum Kunze,
Oenotrichia Copel., Paesia St.-Hil., Pteridium Gled.]
Pteridaceae E.D.M.Kirschn. (approx. 44/approx. 1150)
subfamily Cryptogrammoideae S.Linds.
(Coniogramme Fée, Cryptogramma R.Br., Llavea Lag.)
subfamily Ceratopteridoideae R.M.Tryon
(Acrostichum L., Ceratopteris Brongn.)
subfamily Pteridoideae C.Chr.12
[Actiniopteris Link, Anogramma Link, Aspleniopsis Mett.,
Austrogramme E.Fourn., Cerosora (Baker) Domin, Cosentinia Todaro,
Jamesonia Hook. & Grev., Nephopteris Lell., Onychium Kaulf.,
Pityrogramma Link, Pteris L., Pterozonium Fée, Syngramma J.Sm., Taenitis Willd.]
subfamily Cheilanthoideae W.C.Shieh13
[Allosorus Bernh., Aspidotis (Nutt.) Copel., Bommeria E.Fourn.,
Calciphilopteris Yesilyurt & H.Schneid., Cheilanthes Sw., Doryopteris J.Sm., Hemionitis L., Mildella Trevis., Myriopteris Fée, Notholaena R.Br.,
Pellaea Link, Pentagramma Yatsk., Windham & E.Wollenw., Pteridella Mett. ex Kuhn]
subfamily Vittarioideae Link
[Adiantum L. (approx. 200), Ananthacorus Underw. & Maxon, Anetium Splitg.,
Anthrophyum Kaulf., Haplopteris C.Presl, Hecistopteris J.Sm.,
Monogramma Comm., Polytaenium Desv., Radiovittaria (Benedict) E.H.Crane,
Scoliosorus T.Moore, Vittaria Sm.]
Aspleniaceae Newman = eupolypods II14 (approx. 22/approx. 2780)
subfamily Cystopteridoideae Ching & Z.R.Wang
(Acystopteris Nakai, Cystoathyrium Ching, Cystopteris Bernh., Gymnocarpium Newman)
subfamily Rhachidosoroideae M.L.Wang & Y.T.Hsieh
(Rachidosorus Ching)
subfamily Diplaziopsidoideae Christenh.15
(Diplaziopsis C.Chr., Homalosorus Small)
subfamily Asplenioideae Link
[Asplenium L. (approx. 700), Hemidictyum C.Presl16, Hymenasplenium Hayata]
subfamily Thelypteridoideae C.F.Reed17
[Macrothelypteris (H.Ito) Ching, Phegopteris (C.Presl) Fée,
Thelypteris Schmid. (approx. 1100)]
subfamily Woodsioideae Schmakov
(Woodsia R.Br.)
subfamily Athyrioideae B.K.Nayar
[Athyrium Roth (approx. 200), Cornopteris Nakai, Deparia Hook. & Grev.,
Diplazium Sw. (approx. 350)]
subfamily Blechnoideae Hook.
[Blechnum L. (approx. 250), Onoclea L.18, Stenochlaena J.Sm., Woodwardia Sm.]19
Polypodiaceae J.Presl & C.Presl = eupolypods I20 (approx. 76/approx. 4070)
subfamily Didymochlaenoideae Christenh.21
(Didymochlaena Desv.)
subfamily Hypodematioideae Christenh.22
(Hypodematium Kunze, Leucostegia C.Presl)
subfamily Dryopteridoideae Link23
[Adenoderris J.Sm., Arachniodes Blume (approx. 110), Arthrobotrya J.Sm., Bolbitis Schott, Coveniella Tindale, Ctenitis (C.Chr.) C.Chr, Cyclodium C.Presl, Cyrtomidictyum Ching, Dryopolystichum Copel., Dryopsis Holttum & P.J. Edwards, Dryopteris Adanson (approx. 375), Elaphoglossum Schott (approx. 750), Lastreopsis Ching, Lomagramma J.Sm., Maxonia C.Chr., Megalastrum Holttum, Mickelia R.C.Moran, Labiak & Sundue, Olfersia Raddi, Phanerophlebia C.Presl, Pleocnemia C.Presl, Polybotrya Humb. & Bonpl., Polystichopsis (J.Sm.) Holttum, Polystichum Roth (approx. 370), Pseudotectaria Tardieu, Rumohra Raddi, Stenolepia Alderw., Stigmatopteris C.Chr., Teratophyllum Mett.]
subfamily Lomariopsidoideae Crabbe, Jermy & Mickel24
(Cyclopeltis J.Sm., Dracoglossum Christenh., Lomariopsis Fée, Nephrolepis Schott)
subfamily Tectarioideae B.K.Nayar25
[Aenigmopteris Holttum, Arthropteris J.Sm., Hypoderris R.Br.,
Pteridrys C.Chr. & Ching, Tectaria Cav. (approx. 250),
Triplophyllum Holttum]
subfamily Oleandroideae Crabbe, Jermy & Mickel
(Oleandra Cav.)
subfamily Davallioideae Hook.26
[Davallia Sm., Davallodes (Copel.) Copel.]
subfamily Polypodioideae B.K.Nayar
tribe Loxogrammeae R.M.Tryon & A.F.Tryon
[Dictymia J.Sm., Loxogramme (Blume) C.Presl]
tribe Drynarieae Chandra27
[Drynaria (Bory) J.Sm., Selliguea Bory (approx. 145)]
tribe Platycerieae Christenh., trib. nov.28
(Platycerium Desv., Pyrrosia Mirb.)
tribe Microsoreae V.N.Tu
[Dendroconche Copel., Goniophlebium (Blume) C.Presl, Lecanopteris Reinw.,
Lemmaphyllum C.Presl, Lepidomicrosorium Ching & K.H.Shing, Lepisorus (J.Sm.) Ching, Leptochilus Kaulf., Microsorum Link, Neocheiropteris Christ, Neolepisorus Ching, Paragramma (Blume) T.Moore, Phymatosorus Pic.Serm., Podosorus Holttum, Thylacopteris Kunze, Tricholepidium Ching]29
tribe Polypodieae Hook. & Lindl. ex Duby30
[Campyloneurum C.Presl, Grammitis Sw. (approx. 700), Microgramma C.Presl,
Pecluma M.G.Price, Phlebodium (R.Br.) J.Sm., Pleopeltis Humb. & Bonpl.,
Pleurosoriopsis Fomin, Polypodium L. (approx. 70), Serpocaulon A.R.Sm.,
Syngramma C.Presl]
ACKNOWLEDGEMENTS
We are grateful to Mike Fay for inspiration and for suggesting that we write this review. Samuli Lehtonen and Harald Schneider have kindly discussed their ideas and knowledge about relationships of ferns. We greatly appreciated the constructive comments by the two anonymous reviewers, whose thorough reviews improved our manuscript substantially.
Footnotes
The number of genera in Lycopodiaceae is not yet certain, but molecular phylogenetic studies (Wikström and Kenrick, 1997) have clearly identified three clades, corresponding to the genera Huperzia, Lycopodiella and Lycopodium. A treatment of three genera will result in the loss of some well-established names such as Diphasiastrum Holub as a synonym of Lycopodium and Phylloglossum Kunze in Huperzia. Use of these names has resulted in the recognition of a great number of genera in Lycopodiaceae, and as a result some authors have treated the three broader clades as subfamilies (e.g. Øllgaard, 2012). We see no benefit in destabilizing the taxonomy of these genera to accommodate these additional groups at the generic level, and these should therefore be treated at the subgeneric level if some form of formal classification of these groups is desired.
Delimitation of genera in Ophioglossaceae is generally accepted, although many segregate genera have been proposed, subdividing Botrychium and Ophioglossum. The latter includes epiphytic Cheiroglossa C.Presl and Ophioderma (Blume) Endl., which can be maintained as separate, but, since they form a clade with core Ophioglossum, we have included them there.
Marattiaceae can be divided into two subfamilies, although one will be monogeneric, which calls into question the need for subfamilies, especially when there are only six genera recognized in total. Danaea is nevertheless sufficiently genetically and morphologically distinct from other extant genera in the family that it could be recognized at the subfamilial level. Archangiopteris Christ & Giesenh. is embedded in Angiopteris, and, to prevent Angiopteris from being synonymized with Marattia, the last has been divided into three genera (Murdock, 2008).
Phylogenetic analysis of Osmundaceae clearly placed the cinnamon fern as sister to all other Osmundaceae, which should thus be treated as Osmundastrum cinnamomeum (L.) C.Presl (Yatabe et al., 1999). Todea and Leptopteris form a clade and could be merged as a single genus, although to maintain stability we have tentatively accepted Leptopteris here.
The filmy ferns are composed of two subclades, corresponding to the traditional genera Hymenophyllum and Trichomanes, which we accept here. The subdivision of Trichomanes into eight genera as done by Ebihara et al. (2006) is possible, and the genera are stable and backed up by morphological characters. However, we think that non-specialist users may find these genera difficult to apply, and these are thus probably better treated at the subgeneric level within Trichomanes.
Subfamily Lygodioideae Christenh., subfam. nov. is based on full and direct reference to the Latin diagnosis of Lygodium by Swartz (1801: 106). The genus Lygodium is the type of this subfamily.
Subfamily Loxsomatoideae (C.Presl) Christenh., stat. nov. is based on full and direct reference to the Latin description and type of Loxsomataceae in Presl (1847: 31). The two genera in Loxsomatoideae are similar, and their separation is mainly based on geographical isolation. They may be better treated as a single genus.
Subfamily Culcitoideae (Pic.Serm.) Christenh., stat. nov. is based on full and direct reference to the Latin description and type of Culcitaceae in Pichi-Sermolli (1970a: 207).
Subfamily Plagiogyrioideae (Doweld) Christenh., stat. nov. is based on full and direct reference to the Latin description and type of Plagiogyriidae in Doweld (2001: XII).
Even though Cyatheoideae are in need of further study, there appears to be a current consensus of four genera (Korall, 2006, 2007; Christenhusz et al., 2011), although Gymnosphaera is not yet well defined and, because many combinations are not yet made, it can be argued that the subfamily is best treated as including a single large genus. Hymenophyllopsis, previously placed in its own family, and Cnemidaria C.Presl are embedded in Cyathea and should be treated as part of that genus in any classification of the subfamily.
We still cannot make an informed decision about whether or not to maintain Orthiopteris as separate from Saccoloma. Phylogenetic study and monographic revision of Saccolomataceae are needed, although the two genera are undoubtedly closely related.
Several issues remain in the variable subfamily Pteridoideae. Anogramma is polyphyletic with regard to Pityrogramma, Cerosora and Cosentinia (Schneider et al., 2013). Perhaps these are better treated under a single genus or maybe as two genera, merging Cerosora and Anogramma chaerophylla (Desv.) Link with Pityrogramma and Cosentinia with core Anogramma. The status of Aspleniopsis, Austrogramma and Syngramma has not yet been studied, and some of these may not remain after phylogenetic revision of this group. Pteris may be polyphyletic, and it is possible that up to three genera should be accepted or some genera may have to be merged with it to achieve monophyly. Pteris in the broad sense includes Afropteris Alston, Anopteris Prantl, Campteria C.Presl, Litobrochia C.Presl, Neurocallis Fée and Platyzoma R.Br. (Schuettpelz and Pryer, 2007; Christenhusz et al., 2011).
The genera in this subfamily are in great need of recircumscription. Most previously recognized genera, such as Cheilanthes, Doryopteris and Pellaea, are not monophyletic, although about 14 clades can be recognized (Eiserhardt et al., 2011). Bommeria, Calciphilopteris and Pentagramma appear to be monophyletic. Pellaea, Astrolepis D.M.Benham & Windham, Paraceterach Copel., Paragymnopteris K.H.Shing and Platyloma J.Sm. form a single clade and should be united, but which species actually belong here is still debated. It is also unclear if Argyrochosma (J.Sm.) Windham should be excluded. Pellaea doniana (J.Sm.) Hook., type of the genus Pteridella, does not group with core Pellaea, but falls in a clade that includes Hemionitis and some Doryopteris. Aspidotis needs to be redefined and should most probably include some Cheilanthes spp. and the recently described genus Gaga Pryer, F.W.Li & Windham. Cassebeera Kaulf., Ormopteris J.Sm. and core Doryopteris form a clade (Eiserhardt et al., 2011) and could be merged. Aleuritopteris Fée and Sinopteris C.Chr. & Ching form a clade with Allosorus, the last having priority (Christenhusz, 2012), and all species should be moved to it. Cheilanthes, Doryopteris, Pellaea and Notholaena will have to be redefined in morphological terms. Even though work is still needed on this group, on the basis of Eiserhardt et al. (2011) we can conclude that this subfamily probably consists of the following 14 clades, representing these genera: Allosorus (including Aleuritopteris, Leptolepidium K.H.Shing & S.K.Wu and Sinopteris), Aspidotis (including Gaga), Bommeria, Calciphilopteris, Cheilanthes sensu stricto, Doryopteris (including Adiantopsis, Choristosoria, Ormopteris and Trachypteris), Hemionitis, Mildella, Myriopteris (the complex surrounding Cheilanthes tomentosa Link and C. allosuroides Mett., including Cheilosoria), Notholaena (including Cheiloplecton Fée), Pellaea (including Argyrochosma, Astrolepis, Paraceterach, Paragymnopteris and Platyloma), Pentagramma, Pteridella and an undescribed genus based on Cheilanthes skinneri (Hook.) T.Moore. Whether it is better to accommodate additional species in already redefined genera (as suggested above) or place the entire Cheilanthoideae in the single genus Hemionitis is a subject of debate, but in either case many new combinations will be needed.
Eupolypods II are here treated as one large family, Aspleniaceae. If this is done, however, few synapomorphies remain, especially with inclusion of Thelypteridaceae, but all members share the two vascular bundles in the petiole and (except Thelypteridoideae) the laterally attached indusium. This treatment would reduce the number of monogeneric families, and one large family that is difficult to define morphologically is preferable to many smaller families that are equally problematic to define morphologically and differ only in relatively trivial characters that are typically difficult to observe.
Subfamily Diplaziopsidoideae (X.C.Zhang & Christenh.) Christenh., stat. nov. is based on full reference to the description and type of Diplaziopsidaceae X.C.Zhang & Christenh. in Christenhusz et al. (2011), but excluding Hemidictyum.
Hemidictyaceae are here included in Asplenioideae, which are here treated in the broader sense. The two share many characters and form a clade.
The consensus on this subfamily has been that it includes five genera, but problems in this group remain (Smith and Cranfill, 2002). Phegopteris and Pseudophegopteris form a clade and are here combined under Phegopteris. Cyclosorus is sometimes divided into a number of genera, some of which have nomenclatural priority over it (notably Meniscium and Stegnogramma Blume). The genus in the broad sense is monophyletic and includes the type species of Thelypteris. It is preferable to treat this as a single large genus under the last, and, when the taxonomy of this group is better understood, division into subgenera is a better option than dividing the genus and moving species around, needing new combinations and continuing to destabilize the taxonomy of this group.
The tiny family Onocleaceae used to be divided into four genera, but, with only five species, this is too complicated. They form a well-supported sister (Gastony and Ungerer, 1997) to Blechnoideae and should be treated as a single genus Onoclea (including Matteuccia Tod., Onocleopsis F.Ballard and Pentarhizidium Hayata) in that subfamily with which it shares several characters.
Blechnoideae comprise three major clades, one corresponding to Onoclea sensu lato, a second corresponding to Woodwardia, sister to all other species that can be treated as the single genus Blechnum. However, the subclade sister to the rest of Blechnum sensu lato contains the vining taxa Stenochlaena, Salpichlaena J.Sm. and a few non-vining Blechnum species with long-creeping rhizomes, which may have to be accepted at the generic level pending further studies. Brainea, Doodia, Pteridoblechnum and Sadleria belong to Blechnum sensu lato (Cranfill, 2001; Lehtonen, 2011).
As with eupolypods II, eupolypods I are here also subsumed into the single family Polypodiaceae sensu lato, reducing issues with placement of certain genera, especially in Dryopteridaceae and Tectariaceae and the polymorphic families Lomariopsidaceae and Hypodematiaceae. Despite their morphological diversity, they share some soral characters and usually have three vascular strands in their petioles (except Hypodematioideae). This does somewhat destabilize the current consensus classification, however, and many family names familiar to users are reduced to the subfamilial level; however, it is a better option than adding many more monogeneric families, such as Arthropteridaceae or Didymochlaenaceae, in order to maintain better known names. Such proliferation of families makes the sysem difficult to use and remember, and is frustrating for students, who continually come across families they were never taught.
Didymochlaena does not form a clade with Hypodematium and Leucostegia, with which it was previously associated. It also shares no morphological characters with any other, which is why it is here placed as the sole genus in a separate subfamily, Didymochlaenoideae Christenh., subfam. nov. Characters: terrestrial ferns with erect, thick scaly rhizomes. The scales long and narrow, almost hair-like. Leaves bipinnate, long-petiolate. Petioles with several vascular bundles arranged in a half-circle. Pinnae articulated, all more or less similar in size and shape, somewhat rectangular in outline. Veins free, forked, with thickened endings before reaching the margin. Sori terminating a vein, indusiate, often somewhat sunken in the blade, forming bumps on the upper side. Indusia elongate, centrally attached along a line, opening on either side. Sporangia long-stalked. Spores monolete, ellipsoidal to globose, tuberculate and echinate. Type: Didymochlaena Desv.
Subfamily Hypodematioideae (Ching) Christenh., comb. & stat. nov. is based on full reference to the Latin description and type of Hypodematiaceae in Ching (1975: 96). It includes the genera Hypodematium and Leucostegia.
Cyrtogonellum Ching and Cyrtomium C.Presl are embedded in Polystichum. Dryopsis is perhaps better not united with Dryopteris (as done by Zhang, 2012) because Dryopteris may then not be monophyletic. Maxonia, Olfersia and Polybotrya could also be merged, although these genera are well established and monophyletic. The Ctenitis assembly including Rumohra, Lastreopsis, Megalastrum and Pseudotectaria needs more study. The two subfamilies proposed by Christenhusz et al. (2011) have not been supported in more recent analyses and are therefore merged here.
Dracoglossum is placed here following recent findings of Christenhusz et al. (2013). Nephrolepis probably also belongs here (as stated by Smith et al., 2006). The unsampled Thysanosoria Gepp from New Guinea is here tentatively merged with Lomariopsis on morphological grounds: Lomariopsis dimorphophylla (Gepp) Christenh., comb. nov. Basionym: Thysanosoria dimorphophylla Gepp, Contr. Phytogeog. Fl. Arfak Mts. 193. 1917. Lomariopsis pteridiformis (Ces.) Christenh., comb. nov. Basionym: Gymnogramma pteridiformis Ces., Rendiconti della Reale Accademia delle Scienze Fisiche e Matematiche di Napoli 16: 30. 1877.
A number of genera previously placed in the Tectaria group by Holttum (1991) appear to belong to either the Ctenitis assembly (Dryopteridoideae) or Lomariopsidoideae. Further studies should show the relationships within this subfamily. Psammiosorus C.Chr. belongs to Arthropteris (e.g. Lehtonen, 2011; Liu et al., 2013).
Some controversy remains about the generic classification of Davallioideae, due to the work of Kato and Tsutsumi (2009), Tsutsumi and Kato (2006) and Tsutsumi et al. (2008). Even though clades can be recognized as genera in the Davallia alliance, the type of the genus, D. canariensis (L.) Sm., has only recently been included in a study (Liu & Schneider, 2013), supporting the concept of two genera. Davallia is better treated in its broad sense including Araiostegiella M.Kato & Tsutsumi and Humata Cav. because characters proposed to define these genera do not hold up to closer scrutiny, which does not warrant splitting them from Davallia. Araiostegia Copel. is tentatively included in Davallodes, but it may warrant recognition.
Currently being studied, tribe Drynarieae probably only consist of two genera (Drynaria and Selliguea), but how these are to be defined is still being investigated (H. Schneider, pers. comm.).
Tribe Platycerieae (B.K.Nayar) Christenh., stat. nov., based on the Latin description and type of subfamily Platycerioideae in Nayar (1970: 233). They include the genera Platycerium and Pyrrosia.
Tribe Microsoreae need major study to determine generic delimitation, especially Microsorum. Colysis C.Presl and Kontumia S.K.Wu & P.K. Lôc belong to Leptochilus (Kim et al., 2013). Leptochilus heterophylla (S.K.Wu & P.K.Lôc) Christenh., comb. nov. Basionym: Kontumia heterophylla S.K.Wu & P.K.Lôc, Novon 15: 245. 2005. Many small genera are still commonly applied, and larger genera are poorly defined, but with phylogenetic analyses it should be possible to apply a broader generic concept and redefine these as larger more natural entities.
As stated earlier in this paper, the grammitid ferns are all treated as a single genus in this classification. Few of the numerous relatively new genera have been properly evaluated for monophyly, and some of those that have been studied were found to be polyphyletic (Ranker et al., 2004; Sundue et al., 2010; Hirai et al., 2011), resulting in the description of yet more genera. The broad approach taken for Asplenium and Hymenophyllum seems to be a better fit here. Of course, when this is done, the remaining genera in tribe Polypodieae also need to be addressed. Niphidium J.Sm. and Campyloneurum are difficult to separate and should therefore be merged, better to be treated at the subgeneric level, with the latter name having nomenclatural priority. Polypodium and its close relatives should also be reassessed, and generic delimitation may have to be addressed in the future.
LITERATURE CITED
- Alston A. The subdivision of the Polypodiaceae. Taxon. 1956;5:23–25. [Google Scholar]
- APG III. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society. 2009;161:105–121. [Google Scholar]
- Arnold CA. Classification of gymnosperms from the viewpoint of paleobotany. Botanical Gazette. 1948;110:2–12. [Google Scholar]
- Asmussen CB, Chase MW. Coding and noncoding plastid DNA in palm systematics. American Journal of Botany. 2001;88:1103–1117. [PubMed] [Google Scholar]
- Benson L. Plant classification. Boston: Heath; 1957. [Google Scholar]
- Bierhorst D. Morphology of vascular plants. London: Macmillan; 1971. [Google Scholar]
- Bower FO. The comparative examination of the meristems of ferns, as a phylogenetic study. Annals of Botany. 1889;3:305–392. [Google Scholar]
- Bower FO. Cambridge: Cambridge University Press; 1923–1928. The ferns, vols 1–3. [Google Scholar]
- Brownlie G. Cyto-taxonomic studies on New Zealand Pteridaceae. New Phytologist. 1957;56:207–209. [Google Scholar]
- Brownlie G. Chromosome numbers in New Zealand ferns. Transactions of the Royal Society of New Zealand. 1958;85:213–216. [Google Scholar]
- Campbell DH. The evolution of the land plants. London: Stanford University Press; 1940. [Google Scholar]
- Camus GM, Gibby M, Johns RJ, editors. Pteridology in perspective – Pteridophyte symposium ‘95. Proceedings of the Holttum Memorial Pteridophyte Symposium. Richmond: Kew Publishing; 1995. [Google Scholar]
- Cesalpino A. De plantis libri, XVI. Florence: Marescottum; 1583. [Google Scholar]
- Ching R-C. On natural classification of the family ‘Polypodiaceae. Sunyatsenia. 1940;5:201–268. [Google Scholar]
- Ching R-C. Two new fern families. Acta Phytotaxonomica Sinica. 1975;13:96–98. [Google Scholar]
- Christ H. Die Farnkräuter der Erde – Beschreibende Darstellung der Geschlechter und wichtigeren Arten der Farnpflanzen. Jena: Gustav Fischer; 1897. [Google Scholar]
- Christenhusz MJM. Evolutionary history and taxonomy of Neotropical marattioid ferns: studies of an ancient lineage of plants. Annales Universitatis Turkuensis ser. AII. 2007;216:1–77. [Google Scholar]
- Christenhusz MJM. Index pteridophytorum Guadalupensium, or a revised checklist to the ferns and club mosses of Guadeloupe (French West Indies) Botanical Journal of the Linnean Society. 2009a;161:213–277. [Google Scholar]
- Christenhusz MJM. New combinations and an overview of Cyathea subg. Hymenophyllopsis (Cyatheaceae) Phytotaxa. 2009b;1:37–42. [Google Scholar]
- Christenhusz MJM. Danaea (Marattiaceae) revisited: biodiversity, a classification and ten new species of a Neotropical fern genus. Botanical Journal of the Linnean Society. 2010;163:360–385. [Google Scholar]
- Christenhusz MJM. Pteridaceae. In: Greuter W, von Raab-Straube E, editors. Euro+Med Notulae, 6. Willdenowia. Vol. 42. 2012. pp. 284–284. [Google Scholar]
- Christenhusz MJM, Schneider H. Corrections to Phytotaxa 19: Linear sequence of lycophytes and ferns. Phytotaxa. 2011;28:50–52. [Google Scholar]
- Christenhusz MJM, Tuomisto H, Metzgar JS, Pryer KM. Evolutionary relationships within the Neotropical, eusporangiate fern genus Danaea (Marattiaceae) Molecular Phylogenetics and Evolution. 2008;46:34–48. doi: 10.1016/j.ympev.2007.09.015. [DOI] [PubMed] [Google Scholar]
- Christenhusz MJM, Zhang X-C, Schneider H. A linear sequence of extant lycophytes and ferns. Phytotaxa. 2011;19:7–54. [Google Scholar]
- Christenhusz MJM, Jones M, Lehtonen S. Phylogenetic placement of the enigmatic fern genus Dracoglossum. American Fern Journal. 2013;103:131–138. [Google Scholar]
- Christensen C. Index filicum, sive Enumeratio omnium generum specierumque filicum et Hydropteridum ab anno 1753 ad finem anni 1905 descriptorium: adjectis synonymis principalibus, area geographica, etc. Copenhagen: Hagerup; 1906. [Google Scholar]
- Christensen C. Filicinae. In: Verdoorn F, editor. Manual of pteridology. The Hague: Nijhof; 1938. pp. 522–550. [Google Scholar]
- Conant DS, Raubeson LA, Attwood DK, Perera S, Zimmer EA, Sweere JA, Stein DB. Phylogenetic and evolutionary implications of combined analysis of DNA and morphology in Cyatheaceae. In: Camus GM, Gibby M, Johns RJ, editors. Pteridology in perspective. Richmond: Kew Publishing; 1995. pp. 231–248. [Google Scholar]
- Copeland EB. The oriental genera of Polypodiaceae. University of California Publications in Botany. 1929;16:45–128. [Google Scholar]
- Copeland EB. Genera filicum. Waltham, MA: Chronica Botanica; 1947. [Google Scholar]
- Corriveau JL, Coleman AW. Rapid screening method to detect potential biparental inheritance of plastid DNA and results for over 200 angiosperm species. American Journal of Botany. 1988;75:1443–1458. [Google Scholar]
- Corvez A, Galtier J, Barriel V, Dubuisson J.-Y. New insights on the phylogeny of Paleozoic ferns and implication on foliar organ evolution. IBC2011 Abstract Book, International Botanical Congress, Melbourne. 2011 http://www.ibc2011.com/ (accessed 13 March 2013) [Google Scholar]
- Crabbe JA, Jermy AC, Mickel JT. A new generic sequence for the pteridophyte herbarium. Fern Gazette. 1975;11:141–162. [Google Scholar]
- Crane EH, Farrar DR, Wendel JF. Phylogeny of the Vittariaceae: convergent simplification leads to a polyphyletic Vittaria. American Fern Journal. 1995;85:283–305. [Google Scholar]
- Cranfill RB. Phylogenetic Studies in the Polypodiales (Pteridophyta) with an emphasis on the family Blechnaceae. 2001. PhD thesis, University of California, Berkeley. [Google Scholar]
- Desvaux M. Prodrome de la famille des Fougeres. Mémoires de la Société Linnéenne de Paris, 2me partie. 1827;6:171–337. [Google Scholar]
- Diels L. Pteridophyta. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien 1. Teil, Abteilung 4. Leipzig: Engelmann; 1898–1900. [Google Scholar]
- Doweld A. Prosyllabus Tracheophytorum, tentamen systematis plantarum vascularium (Tracheophyta). Moscow: Geos; 2001. [Google Scholar]
- Dubuisson J-Y. Evolutionary relationships within the genus Trichomanes sensu lato (Hymenophyllaceae) based on anatomical and morphological characters and a comparison with rbcL nucleotide sequences: preliminary results. In: Camus GM, Gibby M, Johns RJ, editors. Pteridology in perspective. Richmond: Kew Publishing; 1995. pp. 203–215. [Google Scholar]
- Ebihara A, Dubuisson J-Y, Iwatsuki K, Hennequin S, Ito M. A taxonomic revision of Hymenophyllaceae. Blumea. 2006;51:221–280. [Google Scholar]
- Eiserhardt WL, Rohwer JG, Russell SJ, Yesilyurt JC, Schneider H. Evidence for radiations of cheilanthoid ferns in the greater Cape floristic region. Taxon. 2011;60:1269–1283. [Google Scholar]
- Fée ALA. Mémoires sur la famille des fougères. olvols 1–13. Strasbourg: Berger-Levrault; 1844–1873. [Google Scholar]
- Fischer E. Moniliformopses (‘Pteridophyta’) In: Frey W, editor. Syllabus of plant families, Adolf Engler's Syllabus der Pflanzenfamilien. 13th edn. Berlin: Gebr. Borntraeger; 2009. pp. 311–390. [Google Scholar]
- Gastony GJ, Rollo DR. Phylogeny and generic circumscriptions of cheilanthoid ferns (Pteridaceae: Cheilanthoideae) inferred from rbcL nucleotide sequences. American Fern Journal. 1995;85:341–360. [Google Scholar]
- Gastony GJ, Ungerer MC. Molecular systematics and a revised taxonomy of the onocleoid ferns (Dryopteridaceae: Onocleeae) American Journal of Botany. 1997;84:840–849. [PubMed] [Google Scholar]
- Gastony GJ, Yatskievych G. Maternal inheritance of the chloroplast and mitochondrial genomes in cheilanthoid ferns. American Journal of Botany. 1992;78:716–722. [Google Scholar]
- Grew N. The anatomy of plants. London: Rawlins; 1682. [Google Scholar]
- Harris SA, Ingram R. Chloroplast DNA and biosystematics: the effects of intraspecific diversity and plastid transmission. Taxon. 1991;40:393–412. [Google Scholar]
- Hasebe M, Ito M, Kofuji R, Ueda K, Iwatsuki K. Phylogenetic relationships of ferns deduced from rbcL gene sequence. Journal of Molecular Evolution. 1993;37:476–482. doi: 10.1007/BF00160428. [DOI] [PubMed] [Google Scholar]
- Hasebe M, Omori T, Nakazawa M, Sano T, Kato M, Iwatsuki K. rbcL gene sequences provide evidence for the evolutionary lineages of leptosporangiate ferns. Proceedings of the National Academy of Sciences, USA. 1994;91:5730–5734. doi: 10.1073/pnas.91.12.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasebe M, Wolf PG, Pryer KM, et al. Fern phylogeny based on rbcL nucleotide sequences. American Fern Journal. 1995;85:134–181. [Google Scholar]
- Hennequin S, Schuettpelz E, Pryer KM, Ebihara A, Dubuisson J-Y. Divergence times and the evolution of epiphytism in filmy ferns (Hymenophyllaceae) revisited. International Journal of Plant Sciences. 2008;169:1278–1287. [Google Scholar]
- Hennequin S, Hovenkamp P, Christenhusz MJM, Schneider H. Phylogenetics and biogeography of Nephrolepis – a tale of old settlers and young tramps. Botanical Journal of the Linnean Society. 2010;164:113–127. [Google Scholar]
- Hennipman E. Scientific consensus classification of pteridophyta. In: Camus GM, Gibby M, Johns RJ, editors. Pteridology in perspective. Richmond: Kew Publishing; 1995. pp. 191–202. [Google Scholar]
- Hirai RY, Rouhan G, Labiak PH, Ranker TA, Prado J. Moranopteris: a new Neotropical genus of grammitid ferns (Polypodiaceae) segregated from Asian Micropolypodium. Taxon. 2011;60:1123–1137. [Google Scholar]
- Holttum RE. Copeland's contribution to fern taxonomy. Philippine Agriculturist. 1913;57:17–20. [Google Scholar]
- Holttum RE. A revised classification of leptosporangiate ferns. Botanical Journal of the Linnean Society. 1947;53:123–158. [Google Scholar]
- Holttum RE. Proposal to conserve the name Thelypteris Schmidel against Thelypteris Adanson. Taxon. 1968;17:330–331. [Google Scholar]
- Holttum RE. The family names of ferns. Taxon. 1971;20:527–532. [Google Scholar]
- Holttum RE. Posing the problems The phylogeny and classification of the ferns. In: Jermy AC, Crabbe JA, Thomas BA, editors. Botanical Journal of the Linnean Society. Suppl. 1. Vol. 67. 1973. pp. 1–10. [Google Scholar]
- Holttum RE. Flora Malesiana. Series II, Pteridophyta: ferns and fern allies. pt 1. Vol. 2. Leiden: Foundation Flora Malesiana; 1991. Tectaria group. [Google Scholar]
- Hooker WJ, Bauer F. Genera filicum, or illustrations of the ferns, and other allied genera. London: Bohn; 1842. [Google Scholar]
- Iwatsuki K. Studies in the systematics of filmy ferns III. An observation on the involucres. Botanical Magazine (Tokyo) 1977;90:259–267. [Google Scholar]
- Jeffrey EC. The structure and development of the stem in the pteridophyta and gymnosperms. Philosophical Transactions of the Royal Society B: Biological Sciences. 1903;195:119–146. [Google Scholar]
- Kato M, Tsutsumi C. Generic classification of Davalliaceae. Acta Phytotaxonomica Geobotanica. 2009;59:1–14. [Google Scholar]
- Kenrick P, Crane PR. The origin and early diversification of land plants. A cladistic study. Washington: Smithsonian Institution Press; 1997. [Google Scholar]
- Kim C, Zha H-G, Deng T, Sun H, Wu S-G. Phylogenetic position of Kontumia (Polypodiaceae) inferred from four chloroplast DNA regions. Journal of Systematics and Evolution. 2013;51:154–163. [Google Scholar]
- Klekowski EJ., Jr Ferns and genetics. Bioscience. 1971;21:317–322. [Google Scholar]
- Korall P, Pryer KM, Metzgar JS, Schneider H, Conant DS. Tree ferns: monophyletic groups and their relationships as revealed by four protein-coding plastid loci. Molecular Phylogenetics and Evolution. 2006;39:830–845. doi: 10.1016/j.ympev.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Korall P, Conant DS, Metzgar JS, Schneider H, Pryer KM. A molecular phylogeny of scaly tree ferns (Cyatheaceae) American Journal of Botany. 2007;94:873–886. doi: 10.3732/ajb.94.5.873. [DOI] [PubMed] [Google Scholar]
- Kramer KU, Green PS. Pteridophytes and gymnosperms. In: Kubitzki K, editor. The families and genera of vascular plants. Vol. 1. Berlin: Springer; 1990. [Google Scholar]
- Lam HJ. Classification and the new morphology. Acta Biotheoretica. 1948;8:107–154. [Google Scholar]
- Lehnert M, Kessler M, Schmidt-Lebuhn AN, Klimas SA, Fehlberg SD, Ranker TA. Phylogeny of the fern genus Melpomene (Polypodiaceae) inferred from morphology and chloroplast DNA analysis. Systematic Botany. 2009;34:17–27. [Google Scholar]
- Lehtonen S. Towards resolving the complete fern tree of life. PLoS One. 2011;6 doi: 10.1371/journal.pone.0024851. e24851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtonen S, Tuomisto H, Rouhan G, Christenhusz MJM. Phylogenetics and classification of the pantropical fern family Lindsaeaceae. Botanical Journal of the Linnean Society. 2010;163:305–359. [Google Scholar]
- Lehtonen S, Wahlberg N, Christenhusz MJM. Diversification of lindsaeoid ferns and phylogenetic uncertainty of early polypod relationships. Botanical Journal of the Linnean Society. 2012;170:489–503. [Google Scholar]
- Lellinger BD. Hymenophyllopsidaceae. In: The botany of the Guayana Highland – pt XII. Memoires of the New York Botanical Garden. 1984;38:2–9. [Google Scholar]
- Lindsay J. Account of the germination and raising of ferns from the seed. Transactions of the Linnean Society of London. 1794;2:93–100. [Google Scholar]
- Linnaeus C. Species plantarum, etc. Stockholm: Salvius; 1753. [Google Scholar]
- Linnaeus C. Genera plantarum, etc. Stockholm: Salvius; 1754. [Google Scholar]
- Liu H, Schneider H. Evidence supporting Davallia canariensis as a Late Miocene relict endemic to Macaronesia and Atlantic Europe. Australian Journal of Botany. 2013;26:378–385. [Google Scholar]
- Liu H-M, Jiang R-H, Guo J, Hovenkamp P, Perrie L, Shepherd L, Hennequin S, Schneider H. Towards a phylogenetic classification of the climbing fern genus Arthropteris. Taxon. 2013;62:688–700. [Google Scholar]
- Malpighi M. Anatome plantarum. London: Martyn; 1675. [Google Scholar]
- Manhart JR. Chloroplast 16S rDNA sequences and phylogenetic relationships of fern allies and ferns. American Fern Journal. 1995;85:182–192. [Google Scholar]
- Manton I. Problems of cytology and evolution in the Pteridophyta. Cambridge: Cambridge University Press; 1950. [Google Scholar]
- Manton I. Cytological notes on one hundred species of Malayan ferns. In: Holttum RE, editor. Flora of Malaya. II. Ferns. Singapore: Government Printing House; 1954. pp. 623–627. [Google Scholar]
- Manton I. Chromosomes and fern phylogeny with special reference to ‘Pteridaceae. Journal of the Linnean Society, Botany. 1958;56:73–92. [Google Scholar]
- Manton I, Sledge WA. Observations on the cytology and taxonomy of the pteridophyte flora of Ceylon. Philosophical Transactions of the Royal Society B: Biological Sciences. 1954;238:127–185. [Google Scholar]
- McNeill J, Barrie FR, Buck WR, et al. Regnum vegetabile. Koenigstein: Koeltz; 2012. International code of nomenclature for algae, fungi, and plants (Melbourne Code) [Google Scholar]
- Mehra P. Cytological evolution of ferns with particular reference to Himalayan forms. Proceedings of the 48th Indian Science Congress. 1961;2:1–24. [Google Scholar]
- Merckx V, Freudenstein J, Kissling J, Christenhusz MJM, Stotler RE, Crandall-Stotler B, Wickett N, Rudall PJ, Maas-van de Kamer H, Maas PJM. Taxonomy and classification. In: Merckx V, editor. Mycoheterotrophy, the biology of plants living on fungi. Heidelberg: Springer; 2012. pp. 19–104. [Google Scholar]
- Mettenius GH. Filices horti botanici Lipsiensis, die Farne des botanischen Gartens zu Leipzig. Leipzig: Voss; 1856. [Google Scholar]
- Mettenius GH. Über einige Farngattungen. Frankfurt: Brönner; 1857. [Google Scholar]
- Mickel JT. Phyletic lines in the modern ferns. Annals of the Missouri Botanical Garden. 1974;61:474–482. [Google Scholar]
- Moore T. Index filicum, etc. London: Pamplin; 1857. [Google Scholar]
- Morison R. Plantarum historiae universalis Oxoniensis pars tertia, post auctoris mortem expleta et absoluta a Jacobo Bobartio. Oxford: Sheldonian; 1699. [Google Scholar]
- Murdock AG. A taxonomic revision of the eusporangiate fern family Marattiaceae, with description of a new genus Ptisana. Taxon. 2008;57:737–755. [Google Scholar]
- Nagalingum NS, Schneider H, Pryer KM. Comparative morphology of reproductive structures in heterosporous water ferns and a reevaluation of the sporocarp. International Journal of Plant Sciences. 2006;167:805–815. [Google Scholar]
- Nagalingum NS, Schneider H, Pryer KM. Molecular phylogenetic relationships and morphological evolution in the heterosporous fern genus Marsilea. Systematic Botany. 2007;32:16–25. [Google Scholar]
- Nakai T. Classes, ordines, familiae, subfamiliae, tribus, genera nova quae attinent ad plantas Koreanas. Journal of Japanese Botany. 1949;24:8–14. [Google Scholar]
- Nayar BK. A phylogenetic classification of the homosporous ferns. Taxon. 1970;19:229–236. [Google Scholar]
- Nayar BK, Kaur S. Gametophytes of homosporous ferns. Botanical Review. 1971;37:295–396. [Google Scholar]
- Neale DB, Sederoff RR. Inheritance and evolution of conifer organelle genomes. In: Hannover JW, Keathley DE, editors. Genetic manipulation of woody plants. New York: Plenum; 1988. pp. 251–264. [Google Scholar]
- Øllgaard B. New combinations in Neotropical Lycopodiaceae. Phytotaxa. 2012;57:10–22. [Google Scholar]
- Pahnke J, Gorenykin V, Bobrova V, Troitsky A, Antonov A, Martin W. Utility of rDNA internal transcribed spacer sequences from the inverted repeat of chloroplast DNA in pteridophyte molecular phylogenies. In: Camus GM, Gibby M, Johns RJ, editors. Pteridology in perspective. Richmond: Kew Publishing; 1995b. pp. 217–230. [Google Scholar]
- Petiver J. Pteri-graphia Americana, icones continens plusquam 400 filicum variarum specierum, etc. London: Royal Society; 1712. [Google Scholar]
- Pichi-Sermolli REG. The nomenclature of some fern-genera. Webbia. 1953;9:387–454. [Google Scholar]
- Pichi-Sermolli REG. Adumbratio florae Aethiopicae – 5. Parkeriaceae, Adiantaceae, Vittariaceae. Webbia. 1957;12:645–704. [Google Scholar]
- Pichi-Sermolli REG. The higher taxa of the Pteridophyta and their classification. In: Hedberg O, editor. Systematics of today. Uppsala Universitets Årsskrift. Vol. 6. Uppsala: Lundequist; 1958. pp. 70–90. [Google Scholar]
- Pichi-Sermolli REG. Fragmenta pteridologica II. Webbia. 1970a;24:699–722. [Google Scholar]
- Pichi-Sermolli REG. A provisional catalogue of the family names of living pteridophytes. Webbia. 1970b;25:219–297. [Google Scholar]
- Pichi-Sermolli REG. An historical review of the higher classification of the Filicopsida The phylogeny and classification of the ferns. In: Jermy AC, Crabbe JA, Thomas BA, editors. Botanical Journal of the Linnean Society. Vol. 67. 1973. pp. 11–40. [Google Scholar]
- Plumier C. Traité des fougères de l'Amérique. Paris: Imprimérie Royale; 1705. [Google Scholar]
- Presl CB. Tentamen pteridographiae, etc. Prague: Haase; 1836. [Google Scholar]
- Presl CB. Supplementum tentaminis Pteriodographiae. Abhandlungen der Böhmischen Gesellschaft der Wissenschaften n. ser. 1845;4:261–380. [Google Scholar]
- Presl CB. Die Gefässbündel im Stipes der Farrn. 1847 Prague: K. Böhmische Gesellschaft der Wissenschaften. [Google Scholar]
- Presl CB. Epimeliae botanicae. Abhandlungen der Böhmischen Gesellschaft der Wissenschaften n. ser. 1851;6:361–624. [Google Scholar]
- Pryer KM, Smith AR, Skog JE. Phylogenetic relationships of extant pteridophytes based on evidence from morphology and rbcL sequences. American Fern Journal. 1995;85:205–282. [Google Scholar]
- Pryer KM, Schneider H, Smith AR, Cranfill R, Wolf PG, Hunt JS, Sipes SD. Horsetails and ferns are a monophyletic group and the closest living relatives to seed plants. Nature. 2001a;409:618–622. doi: 10.1038/35054555. [DOI] [PubMed] [Google Scholar]
- Pryer KM, Smith AR, Hunt JS, Dubuisson J-Y. rbcL data reveal two monophyletic groups of filmy ferns (Filicopsida: Hymenophyllaceae) American Journal of Botany. 2001b;88:1118–1130. [PubMed] [Google Scholar]
- Pryer KM, Schuettpelz E, Wolf PG, Schneider H, Smith AR, Cranfill R. Phylogeny and evolution of ferns (monilophytes) with a focus on the early leptosporangiate divergences. American Journal of Botany. 2004;91:1582–1598. doi: 10.3732/ajb.91.10.1582. [DOI] [PubMed] [Google Scholar]
- Ranker TA, Smith AR, Parris BS, Geiger JMO, Haufler CH, Straub SCK, Schneider H. Phylogeny and evolution of grammitid ferns (Grammitidaceae): a case of rampant morphological homoplasy. Taxon. 2004;53:415–428. [Google Scholar]
- Reimers H. XV. Abteilung: Pteridophyta. Farnpflanzen. In: Melchior H, Werdermann E, editors. A. Engler's Syllabus der Pflanzenfamilien. Berlin-Nikolassee: Borntraeger; 1954. pp. 269–311. [Google Scholar]
- Rothfels CJ, Windham MD, Grusz AL, Gastony GJ, Pryer KM. Towards a monophyletic Notholaena (Pteridaceae): resolving patterns of evolutionary convergence in xeric-adapted ferns. Taxon. 2008;57:712–724. [Google Scholar]
- Rothfels CJ, Larsson A, Kuo L-Y, Korall P, Chiou W-L, Pryer KM. Overcoming deep roots, fast rates, and short internodes to resolve the ancient rapid radiation of eupolypod II ferns. Systematic Biology. 2012;16:490–509. doi: 10.1093/sysbio/sys001. [DOI] [PubMed] [Google Scholar]
- Rothmaler W. Die Abteilungen und Klassen der Pflanzen. Feddes Repertorium. 1951;54:256–266. [Google Scholar]
- Schkuhr CS. Vier und zwanzigste Klasse des Linnéischen Pflanzensystems oder Kryptogamische Gewäsche (Farnkräuter). 1809 Wittenburg: bei dem Verfasser. [Google Scholar]
- Schmidel CC. Icones plantarum. 1763;3 Nuremberg. [Google Scholar]
- Schneider H. Plant morphology as the cornerstone to the integration of fossils and extant taxa in phylogenetic systematics. Species, Phylogeny and Evolution. 2007;1:65–74. [Google Scholar]
- Schneider H. Evolutionary morphology of ferns (monilophytes) Annual Plant Reviews. 2013;45:115–140. [Google Scholar]
- Schneider H, Schuettpelz E, Pryer KM, Cranfill R, Megallon S, Lupia R. Ferns diversified in the shadow of angiosperms. Nature. 2004;428:553–557. doi: 10.1038/nature02361. [DOI] [PubMed] [Google Scholar]
- Schneider H, Smith AR, Pryer KM. Is morphology really at odds with molecules in estimating fern phylogeny? Systematic Botany. 2009;34:455–475. [Google Scholar]
- Schneider H, He L, Hennequin S, Zhang X-C. Towards a natural classification of Pteridaceae: inferring the relationships of enigmatic pteridoid fern species occurring in the Sino-Himalaya and Afro-Madagascar. Phytotaxa. 2013;77:49–60. [Google Scholar]
- Schomburgk MR. Versuch einer Fauna und Flora von Britisch-Guiana. Leipzig: Weber; 1848. [Google Scholar]
- Schott HW. Genera filicum. Vienna: Wallishausser; 1834. [Google Scholar]
- Schuettpelz E, Pryer KM. Fern phylogeny inferred from 400 leptosporangiate species and three plastid genes. Taxon. 2007;56:1037–1050. [Google Scholar]
- Schuettpelz E, Korall P, Pryer KM. Plastid atpA data provide improved support for deep relationships among ferns. Taxon. 2006;55:987–906. [Google Scholar]
- Sears BB. Elimination of plastids during spermatogenesis and fertilization in the plant kingdom. Plasmid. 1980;4:233–255. doi: 10.1016/0147-619x(80)90063-3. [DOI] [PubMed] [Google Scholar]
- Smith AR. Non-molecular phylogenetic hypotheses for ferns. American Fern Journal. 1995;85:104–122. [Google Scholar]
- Smith AR, Cranfill RB. Intrafamilial relationships of the thelypteroid ferns (Thelypteridaceae) American Fern Journal. 2002;92:131–149. [Google Scholar]
- Smith AR, Pryer KM, Schuettpelz E, Korall P, Schneider H, Wolf PG. A classification for extant ferns. Taxon. 2006;55:705–731. [Google Scholar]
- Smith AR, Pryer KM, Schuettpelz E, Korall P, Schneider H, Wolf PG. Fern classification. In: Ranker TA, Haufler CH, editors. Biology and evolution of ferns and lycophytes. Cambridge: Cambridge University Press; 2008. pp. 417–467. [Google Scholar]
- Smith J. An arrangement and definition of the genera of ferns. Journal of Botany, British and Foreign. 1841;4(38–70):147–198. [Google Scholar]
- Smith J. An arrangement and definition of the genera of ferns. London Journal of Botany. 1843;1:419–438. 659–668; 2: 378–394. [Google Scholar]
- Smith J. Historia filicum, etc. London: MacMillan; 1875. [Google Scholar]
- Smith J.E. Tentamen botanicum de filicum generibus dorsiferarum. Mémoires de l'Académie Royale des Sciences, Turin. 1793;5:401–422. [Google Scholar]
- Sundue MA. A morphological cladistic analysis of Terpsichore (Polypodiaceae) Systematic Botany. 2010;35:716–729. [Google Scholar]
- Sundue MA, Islam MB, Ranker TA. Systematics of grammitid ferns (Polypodiaceae): using morphology and plastid sequence data to resolve the circumscription of Melpomene and the polyphyletic genera Lellingeria and Terpsichore. Systematic Botany. 2010;35:701–715. [Google Scholar]
- Swartz O. Genera et species filicum, etc. In: Schrader H.A., editor. Journal für die Botanik. Vol. 1800. 1801. pp. 1–120. [Google Scholar]
- Swartz O. Synopsis filicum, etc. 1806. Kiel: Imp. Bibliopolii Novi Academici. [Google Scholar]
- Taylor TN, Taylor EL, Krings M. Palaeobotany, the biology and evolution of fossil plants. 2nd edn. Burlington: Elsevier; 2009. [Google Scholar]
- Tryon RM. A sketch of the history of fern classification. Annals of the Missouri Botanical Garden. 1952;39:255–262. [Google Scholar]
- Tryon RM, Tryon AF. Ferns and allied plants with special reference to tropical America. New York: Springer; 1982. [Google Scholar]
- Tsutsumi C, Kato M. Evolution of epiphytes in Davalliaceae and related ferns. Botanical Journal of the Linnean Society. 2006;151:495–510. [Google Scholar]
- Tsutsumi C, Zhang X-C, Kato M. Molecular phylogeny of Davalliaceae and implications for generic classification. Systematic Botany. 2008;33:44–48. [Google Scholar]
- Van Rheede tot Draakestein HA. Amsterdam: Someren & van Dyck; 1678–1703. Hortus Indicus Malabaricus, 12 vols. [Google Scholar]
- Wagner WH., Jr Problems in the classification of ferns. Recent Advances in Botany. 1961;1:841–844. [Google Scholar]
- Wagner WH., Jr . The construction of a classification. In: Sibley CG chairman, editor. Systematic Biology: proceedings of an international conference. Washington: US National Academy of Science Press; 1969. pp. 67–90. [National Academy of Sciences] Publication 1692. [Google Scholar]
- Walker TG. Evidence from cytology in the classification of ferns The phylogeny and classification of the ferns. In: Jermy AC, Crabbe JA, Thomas BA, editors. Botanical Journal of the Linnean Society. Suppl. 1. Vol. 67. 1973. pp. 91–110. [Google Scholar]
- Wikström N, Kenrick P. Phylogeny of Lycopodiaceae (Lycopsida) and the relationships of Phylloglossum drummondii Kunze based on rbcL sequences. International Journal of Plant Sciences. 1997;158:862–871. [Google Scholar]
- Wolf PG. Phylogenetic analysis of rbcL and nuclear ribosomal RNA gene sequences in Dennstaedtiaceae. American Fern Journal. 1995a;85:306–327. [Google Scholar]
- Wolf PG. Pteridophyte phylogenies based on analysis of DNA sequences: a multiple gene approach. In: Camus GM, Gibby M, Johns RJ, editors. Pteridology in perspective. Richmond: Kew Publishing; 1995b. pp. 203–215. [Google Scholar]
- Wolf PG, Sipes SD, White MR, Martines ML, Pryer KM, Smith AR, Ueda K. Phylogenetic relationships of the enigmatic fern families Hymenophyllopsidaceae and Lophosoriaceae: evidence from rbcL nucleotide sequences. Plant Systematics and Evolution. 1999;219:263–270. [Google Scholar]
- Yatabe Y, Nishida H, Murakami N. Phylogeny of Osmundaceae inferred from rbcL nucleotide sequences and comparison to the fossil evidences. Journal of Plant Research. 1999;112:397–404. [Google Scholar]
- Zhang L-B, Zhang L. The inclusion of Acrophorus, Diacalpe, Nothoperanema, and Peranema in Dryopteris: the molecular phylogeny, systematics and nomenclature of Dryopteris subg. Nothoperanema (Dryopteridaceae) Taxon. 2012;61:1199–1216. [Google Scholar]
- Zhang L-B. Reducing the fern genus Dryopsis to Dryopteris and the systematics and nomenclature of Dryopteris subgenus Erythrovariae section Dryopsis (Dryopteridaceae) Phytotaxa. 2012;71:17–27. [Google Scholar]