Abstract
Background and Aims
Successive vascular cambia are involved in the secondary growth of at least 200 woody species from >30 plant families. In the mangrove Avicennia these successive cambia are organized in patches, creating stems with non-concentric xylem tissue surrounded by internal phloem tissue. Little is known about radial growth and tree stem dynamics in trees with this type of anatomy. This study aims to (1) clarify the process of secondary growth of Avicennia trees by studying its patchiness; and (2) study the radial increment of Avicennia stems, both temporary and permanent, in relation to local climatic and environmental conditions. A test is made of the hypothesis that patchy radial growth and stem dynamics enable Avicennia trees to better survive conditions of extreme physiological drought.
Methods
Stem variations were monitored by automatic point dendrometers at four different positions around and along the stem of two Avicennia marina trees in the mangrove forest of Gazi Bay (Kenya) during 1 year.
Key Results
Patchiness was found in the radial growth and shrinkage and swelling patterns of Avicennia stems. It was, however, potentially rather than systematically present, i.e. stems reacted either concentrically or patchily to environment triggers, and it was fresh water availability and not tidal inundation that affected radial increment.
Conclusions
It is concluded that the ability to develop successive cambia in a patchy way enables Avicennia trees to adapt to changes in the prevailing environmental conditions, enhancing its survival in the highly dynamic mangrove environment. Limited water could be used in a more directive way, investing all the attainable resources in only some locations of the tree stem so that at least at these locations there is enough water to, for example, overcome vessel embolisms or create new cells. As these locations change with time, the overall functioning of the tree can be maintained.
Keywords: Automatic point dendrometers, Avicennia marina, local climate and environmental conditions, mangrove, patchy growth, pinning analysis, radial tree stem dynamics, successive cambia
INTRODUCTION
Patchy growth, i.e. the change of positions of active secondary growth around and along the tree stem with time, is a feature of the mangrove Avicennia (Schmitz et al., 2007, 2008). This patchiness has been suggested to be independent of leaf and lateral branch formation in different Avicennia species (Zamski, 1981). Point dendrometers, registering radial changes in tree stems at high temporal and spatial resolution, can help in clarifying the degree of patchiness in the secondary growth mechanism that results from the formation of successive cambia. They allow for estimation of secondary growth in relation to changes in the environmental conditions and for the study of daily and seasonal changes in tree stem dimensions.
The time difference between water loss by transpiration and water uptake by roots leads to a reduction in the internal water reserves of trees during the day, which are subsequently replaced during the night (e.g. Améglio and Cruiziat, 1992; Herzog et al., 1995; Zweifel et al., 2000; Zweifel et al., 2001; Steppe et al., 2006, 2012). This causes recurrent shrinking of the stem by day and and swelling by night. These changes are mainly attributable to the elastic living phloem tissue, although the xylem also undergoes small fluctuations (Brough et al., 1986; Irvine and Grace, 1997; Zweifel et al., 2000; De Schepper and Steppe, 2010).
It has been suggested that radial transport of water from the bark to the xylem can take place both apoplastically and symplastically, while water flow from the xylem to the bark primarily depends on the apoplastic route (Steppe et al., 2012). Environmental conditions leading to higher transpiration or sap flow rates are thought to boost aquaporin abundance and/or activity (Steppe et al., 2012), easing the symplastic flow of water from the phloem to the xylem and hence into the transpiration stream, which in turn leads to a reduction of the water held in this tissue, thus resulting in stem diameter shrinkage. Changes in the concentration of soluble carbohydrates in the phloem control the osmotic flow of water from or towards the phloem (De Schepper et al., 2010; Sevanto et al., 2011). Xylem and phloem in trees can therefore be considered tightly coupled hydraulically, with no real barrier between them (Sevanto et al., 2011), but rather with layers of tight control of water flow.
Superimposed on these daily fluctuations in the stem diameter of trees is irreversible radial stem growth resulting from cambial activity. Radial growth is dependent on water status as well as on the carbon status of the plant, as cell wall expansion depends on turgor pressure and on incorporation of carbohydrates (Zweifel et al., 2006; De Schepper et al., 2010). In order to obtain a measure of real growth of the stem using dendrometer measurements, the daily fluctuations of the tree stem have to be identified and extracted from the data.
Changes in the diameter of tree stems can be divided into three phases: (1) contraction; (2) expansion; and (3) stem radius increase (Downes et al., 1999; Deslauriers et al., 2007, 2011). The duration of each phase can be determined, as well as the maximum daily shrinkage (MDS) and stem radius increment (ΔR; see fig. 1 in Deslauriers et al., 2011). While ΔR can be used as an estimation of tree growth (Deslauriers et al., 2011), MDS is a robust indicator for water stress (Ortuño et al., 2006; Giovannelli et al., 2007; Conejero et al., 2007). Increasing MDS has been found to be the first detectable morpho-physiological signal of changes in the tree's water status, occurring before detectable changes in ΔR (Goldhamer et al., 1999; Naor and Cohen, 2003; Remorini and Massai, 2003; Conejero et al., 2007; Giovannelli et al., 2007).
In this study, we aim to clarify (1) the process of secondary growth of Avicennia trees and especially its degree of patchiness, and (2) the relationship between the radial increment of Avicennia stems, both temporary and permanent, and the local climatic and environmental conditions. To do this, we monitored tree stem variations at four different positions around and along the stem of two Avicennia marina trees for 1 year in a monospecific natural mangrove forest in Kenya. We test the hypothesis that patchy radial growth and patchy stem dynamics enable Avicennia trees to better survive conditions of extreme physiological drought.
MATERIALS AND METHODS
Pinning analysis
As a preliminary to the dendrometer study, the cambium of six Avicennia marina (Forssk.) Vierh. trees at two locations in the mangrove forest of Gazi Bay, Kenya (site 1, 4°25′43″S, 39°30′36″E; and site 2, 4°25′15″S, 39°30′27″E) were wounded by pinning with a hypodermic needle, 0·8 mm in diameter. This procedure involved inserting the needle into the stem to damage a small region of the cambium. The wound provides a microscopic reference point from which subsequent cambial activity close to the pinning site can be measured. The pinning was done on a monthly basis between February 2006 and January 2007, each time at three different sides of the tree stem at the same height. Each subsequent monthly pinning was carried out several centimetres below the previous one (see Supplementary Data Fig. S1) to avoid interactions with wound wood formation. The compass bearings of the pinnings were noted. Pinned stem parts were harvested in February 2007 and stored in FAA (formalin–acetic acid–alcohol) for subsequent examination. Before felling the trees, the stem circumference at 130 cm height and the stem diameter at the base were measured, and the height of the trees was calculated trigonometrically.
X-ray tomographical images were made for all harvested stem parts, using a multislice spiral CT scanner (CT-scan Brilliance 64 slice, Philips, The Netherlands) with the same settings as described in Robert et al. (2011b). The number of growth layers from the cambial mark outwards was counted and the increment during the period of the cambial marking experiment was measured on both sides of the wound in iQ-VIEW 2·5.0 (IMAGE Information Systems, London, UK). For one tree (site 1), transverse sections of the pinned wood parts were made. Wood samples were embedded in polyethylene glycol (PEG) 1500, after which sections were made with a sliding microtome and double-stained with safranin and alcian blue. The increment during the period since the cambium was marked by pinning was measured using image analysis software AnalySIS Pro 3·2 (Soft Imaging System GmbH, Münster, Germany) via a camera connected to a microscope (Olympus BX60). The increment was measured at about 2 mm distance from the abnormal growth that had occurred in response to wounding, this being the nearest point to the pinning that did not show any effect of the damage caused. This measurement was done on both sides of the wound and the increment of both sides was averaged.
Dendrometer study
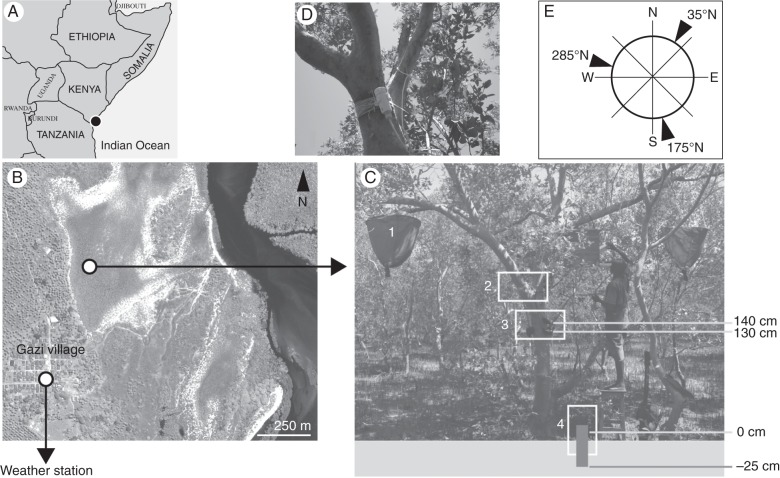
Two adult A. marina trees of 18 cm in diameter at breast height, and approx. 8 m high were selected for the study (4°25′16·2″S, 39°30′28·50″E; Fig. 1). The visual similarity and health status of both trees were assessed before and after the observations. On each tree, four electronic point dendrometers (Ecomatik, Germany) were placed at the beginning of March 2010. Three were placed at 130 cm height (at points 35°N, 175°N and 285°N) and one at 140 cm (285°N) in order to detect the proposed patchy growth of this species (Schmitz et al., 2008; Fig. 1). Radial stem changes with a precision of 0·002 mm were registered automatically at 30 min intervals from 3 March 2010 to 8 March 2011.
Fig. 1.
(A) Map of east Africa, locating Gazi Bay on the Kenyan coast (black dot), and (B) QuickBird satellite image of Gazi Bay acquired in 2002 (Neukermans et al., 2008) showing the locations of the weather station in Gazi village and the dendrometer installation in the mangrove forest (white dots). (C) Picture of the dendrometer installation on one of the studied trees showing the locations of the (1) litter traps, (2) temperature and relative humidity logger (detail shown in D), (3) automatic point dendrometers and (4) conductivity logger. (E) Diagram of the locations of the dendrometers on the stem circumference as a function of the wind directions.
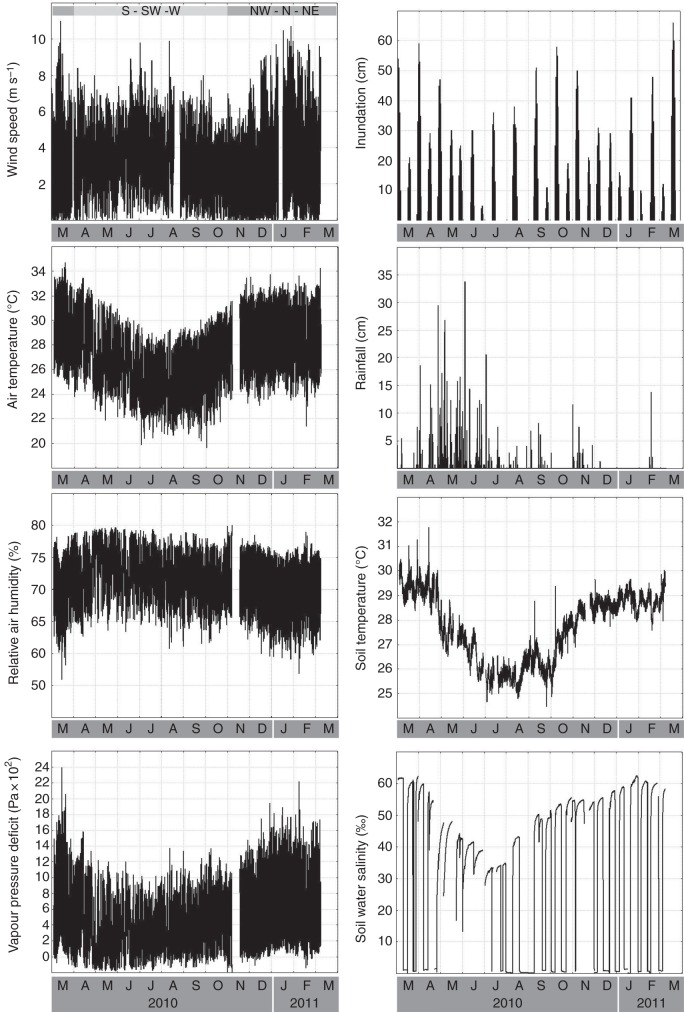
During the same period, the following local environmental variables were logged at the study site (Fig. 2). (1) Air temperature and relative air humidity, at 10 min intervals using a Hobo U23 pro Temperature/Relative Humidity Data Logger (Onset, Bourne, MA, USA), with measurements taken at the study site. (2) Soil water conductivity and soil temperature at approx. 25 cm depth, at 10 min intervals using a HI 9828 Multiparameter Water Quality Portable Meter (Hanna Instruments, Woonsocket, RI, USA) with measurements taken at the study site. (3) Wind speed, wind direction, air temperature (in shadow and in sun), relative air humidity and rainfall, at 1 h intervals using a TFA Nexus Weather Station (TFA Dostmann, Hamburg, Germany), with measurements taken in a nearby village at 500 m from the study site. Calibration of the conductivity meter was carried out on a monthly basis with two calibration solutions (80 000 and 111 800 µS cm−1). Gaps in the automatic rainfall data were corrected through data from a manual self-manufactured pluviometer, based on weighed water volumes. A reference point for height of tides at the site was determined by coating sheets of water-resistant paper with water-soluble ink and attaching them to the stems of trees. After a selected high tide, the height of the tide could be determined by measuring the level at which the ink had been washed away, and this was cross-referenced with local published tide tables for Kilindini Harbour, Mombasa. Maximum daily tidal inundation was calculated based on the tide tables and verified in the field to take the local topography into account, as well as the possible differences in timing and level of inundation between Mombasa and Gazi. Vapour pressure deficit (VPD) was calculated from temperature and relative humidity data according to Schönwiese (2003).
Fig. 2.
Environmental variables measured from 3 March 2010 to 8 March 2011 at the study site in the forest of Gazi Bay (Kenya) (air temperature, relative humidity, soil temperature and soil water salinity) and in Gazi village (wind speed, wind direction and rainfall). Wind direction is shown as the main wind directions for the indicated period. Maximum daily inundation is calculated from local tide tables after determination of the study site's height above sea level datum, while vapour pressure deficit is calculated from air temperature and relative humidity according to Schönwiese (2003). Air temperature, relative humidity and vapour pressure deficit data are mean values from two loggers attached to trees A and B, standing approx. 5 m apart from each other. Steep declines and increases in the salinity curves are an indication of soil drought rather than an abrupt change in soil water salinity.
For both trees, tree phenology was followed on a monthly basis. Leaves, fruits and flowers were counted in three litter traps per tree. The average height of the pen roots of each tree was calculated based on measurements of 100 pen roots per tree.
At the end of the measuring period, wood samples from all dendrometer positions were taken and stored in alcohol (ethanol 70 %). Transverse sections were made with a sliding microtome and double-stained with safranin and alcian blue. Images were made with a camera connected to a microscope (Leica DM LB), after which measurements of the growth segment width and the tissue fractions in the outermost part of the stem at each dendrometer side were made using Image J 1·41k (Wayne Rasband, National Institute of Health, Bethesda, MD, USA) and Axiovision (Carl Zeiss, Germany). Both growth segment width (the sum of the internal phloem and xylem tissue formed by one cambium) and tissue fractions (bark, xylem and internal phloem tissue) were measured along one radius at each dendrometer position. Herein, we use the term internal phloem to indicate the phloem that is situated in between patches of the xylem tissue and thus separated from the outer bark, where the secondary phloem is usually positioned.
Hourly measurements of tree stem variations were extracted and analysed according to Deslauriers et al. (2011). A smoothing level of 2 on a scale from 0 to 10 was chosen in order to preserve significant variation within a day. During the manual correction phase (Deslauriers et al., 2011), cycles of shrinking and swelling based on diameter differences smaller than the detection precision of the dendrometers were corrected. For the visualization of MDS, values have been summed by day if more than one shrinkage phase per day took place. Therefore, in our study, MDS is defined as the total shrinking of a tree per day, not the net shrinkage, i.e. ignoring possible intermediate swelling having occurred.
In order to assess the effects of both inundation (with a tidal pattern and little seasonal variation) and climate (rainy vs. dry season), we chose days with and without rainfall in combination with tidal phases from the extensive data sets. Days were selected such that the dry or wet signature was as pronounced as possible. Selected days were those with the largest amount of rainfall during the study period (6 and 9 May 2010 without inundation, and 27 April and 27 May 2010 with inundation) and the last days without rain after the longest dry period (18 January and 12 February 2011 without inundation, and 23 October and 5 January 2011 with inundation).
The multiparameter used to monitor soil water conductivity could only measure if the soil was sufficiently wet. Steep declines and increases in the salinity curves are therefore an indication of soil drought and water supply, respectively, rather than an abrupt change in soil water salinity. Temperature and relative humidity data were measured within the canopy of both trees, which were standing about 5 m apart. Figures that concern both trees show the mean value of the two, as the data were highly similar (Spearman's rank correlation, r = 0·99, P < 0·05).
RESULTS
Pinning analysis
The pinned positions around and along the stem circumference of the examined trees (breast height diameters ranging from 5·4 to 9·2 cm) were part of different growth segments in the horizontal as well as in the vertical direction in almost all cases. At a given height, the three analysed positions around the stem circumference showed similar amounts of radial increment in some periods but not in others. The same was true for the number of growth segments that were formed. The tree from study site 1, selected for detailed observation using thin sections, is representative for the observations made in all six studied trees, i.e. during some of the observed periods of growth, one sector of the tree was not growing while the other sectors were growing (Table 1). This can be observed, for example, after the pinning of December 2006 (Table 1; Supplementary Data Fig. S2) and the one of August 2006 (Table 1).
Table 1.
Average increment from the moment of pinning up to February 2007, when the tree was harvested
| Pinning date | No. of months | Increment (μm) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| South |
North–west |
East |
||||||||
| Left | Right | Mean | Left | Right | Mean | Left | Right | Mean | ||
| Jan-07 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dec-06 | 2 | 0 | 0 | 0 | 539 | 588 | 564 | 556 | 582 | 569 |
| Nov-06 | 3 | 804 | 732 | 768 | 807 | 958 | 882 | 1632 | 1925 | 1779 |
| Oct-06 | 4 | 758 | 861 | 809 | – | 793 | 793 | 1425 | 1724 | 1575 |
| Sep-06 | 5 | 717 | 704 | 711 | 1356 | 2813 | 2084 | 2115 | 2153 | 2134 |
| Aug-06 | 6 | 997 | 983 | 990 | 0 | 0 | 0 | 2222 | 2268 | 2245 |
| Jul-06 | 7 | – | – | – | 2304 | 1769 | 2036 | 2491 | 2600 | 2546 |
| Jun-06 | 8 | 1506 | 1697 | 1602 | 3293 | 2875 | 3084 | 2393 | 2451 | 2422 |
| May-06 | 9 | 1725 | 1925 | 1825 | 3279 | 3401 | 3340 | 2408 | 2336 | 2372 |
| Apr-06 | 10 | 2100 | 2120 | 2110 | 2743 | 3261 | 3502 | 3981 | 2794 | 3388 |
| Mar-06 | 11 | 2828 | 2909 | 2868 | 3464 | 2987 | 3226 | – | 3180 | 3180 |
| Feb-06 | 12 | 2084 | 2483 | 2283 | 2602 | 2543 | 2573 | 3050 | 3235 | 3142 |
The increment was measured at both sides of the pinning wound (left and right) and averaged. The tree was pinned monthly at three sides of the stem circumference (south, north-west and east) in order to account for patchy growth (Supplementary Data Fig. S1).
–, no data could be obtained.
Dendrometer study
The course of the environmental variables during the year of study is shown in Fig. 2. There was a pronounced rainy season from April to July 2010. Months without rain were December 2010 and January 2011. In February 2011, some days with rainfall interrupted the dry season. In the dry season, wind was stronger and came mainly from the northern sector (north-west to north-east), while during the rest of the year it was generally weaker and mostly south-westerly (south to west), corresponding to the north-east monsoon and the south-west monsoon, respectively. Maximum wind speed per hour followed the same pattern as the wind speed but with more pronounced maxima during the rainy season (data not shown). Air temperature, soil temperature and soil water salinity were lowest in July and August. Relative air humidity was highest from May to June while VPD was lowest. Temperature ranges measured in the forest were in between those measured by the weather station in a full sun and a shaded position (data not shown). Rainfall differed significantly between Gazi village (data used in this study) and the nearest weather station in Mombasa, especially in its daily amount and pattern (data not shown).
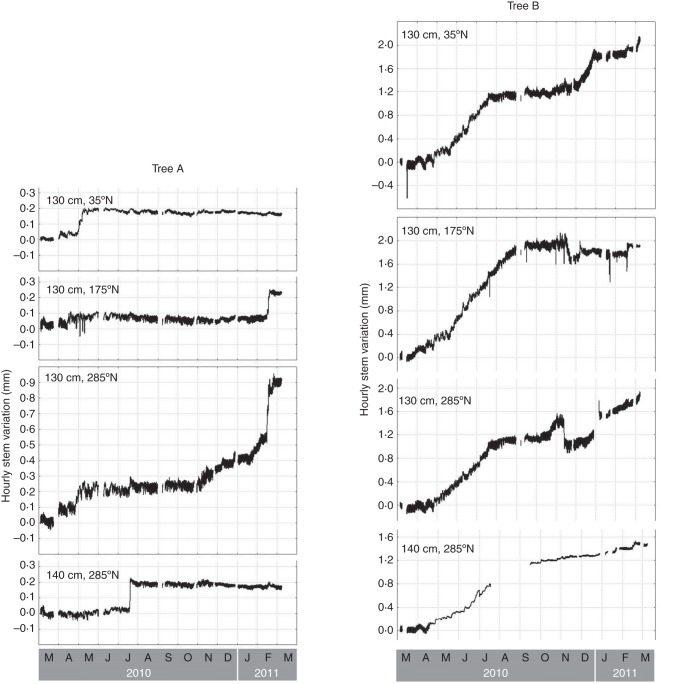
The stem diameter of the two studied trees (tree A and B) showed remarkable differences in total amount of increment after 1 year (Table 2) as well as in the pattern of this increment (Fig. 3). In tree A, the total increment after 1 year was much lower than in tree B for all positions around the stem circumference. Furthermore, the increment after 1 year was the result of a sudden and brief increase in stem diameter at three of the four positions studied. At the fourth position (130 cm, 285°N), the sudden increase was combined with a more gradual increase in April and May 2010 and from the end of October 2010 to February 2011. In tree B, a gradual stem increment mainly occurred from April to July 2010, with some variation among the different positions around the stem circumference. A second phase of slower but still steady increment took place from the end of October 2010 at all except one position.
Table 2.
Difference in stem diameter (mm) between the beginning (March 2010) and the end (March 2011) of the study period, for all four positions on the stems of tree A and B
| Tree A | Tree B | |
|---|---|---|
| Position 1, 130 cm, 35°N | 0·23 | 1·92 |
| Position 2, 130 cm, 175°N | 0·16 | 2·06 |
| Position 3, 130 cm, 285°N | 0·91 | 1·86 |
| Position 4, 140 cm, 285°N | 0·18 | 1·48 |
Fig. 3.
Hourly radial stem variation from 3 March 2010 to 8 March 2011 in tree A (left) and tree B (right) growing in the mangrove forest of Gazi Bay (Kenya). Measurements have been taken at four different positions around the stem of both trees.
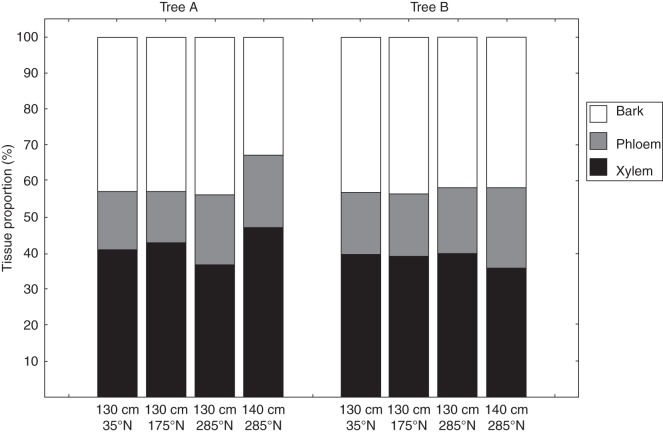
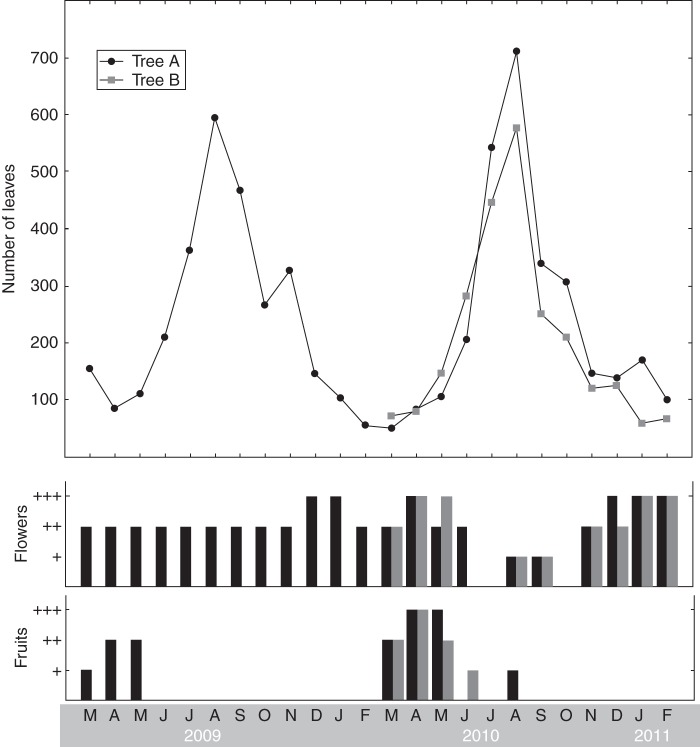
The width of the growth segments in the outermost zone of the tree stems at the locations of the dendrometer measurements ranged from 235 to 1691 µm (Table 3; Supplementary Data Figs S3, S4). No noticeable difference in growth segment width or tissue proportions in the outermost stem part was found between the two trees. The different positions around the stem circumference, however, differed in average, minimum and maximum growth segment width (Table 3) as well as in tissue proportions (Fig. 4). The phenology of both trees had similar patterns in litter fall, fruit production and flower production, with the highest litter fall in July and August and the main fruit production in March to June (Fig. 5). Flowers were present for almost the whole year, with peaks in April to May, and December to February. Average pen root height was 8·0 and 8·4 cm for tree A and B, respectively, with ranges from 3·0 to 25·0 cm (tree A) and 2·7 to 17·0 cm (tree B).
Table 3.
Width of the growth segments visible on the wedges of the stem that were sampled for both tree A and B
| n | Length | Growth segment width |
||||
|---|---|---|---|---|---|---|
| Average | Min | Max | Range | |||
| Tree A | ||||||
| 130 cm, 35°N | 5 | 5006 | 742 | 469 | 1411 | 942 |
| 130 cm, 175°N | 4 | 4779 | 875 | 428 | 1275 | 847 |
| 130 cm, 285°N | 5 | 5176 | 769 | 247 | 944 | 697 |
| 140 cm, 285°N | 5 | 5537 | 910 | 586 | 1691 | 905 |
| Tree B | ||||||
| 130 cm, 35°N | 4 | 4863 | 894 | 633 | 1239 | 606 |
| 130 cm, 175°N | 5 | 5730 | 879 | 741 | 1066 | 325 |
| 130 cm, 285°N | 4 | 3453 | 547 | 270 | 740 | 470 |
| 140 cm, 285°N | 6 | 5293 | 671 | 235 | 974 | 739 |
The number of growth segments (n) and the part of the radius (Length) taken into account for the measurements and calculations are indicated.
Values, except the number of growth segments, are in micrometres.
Fig. 4.
Proportion of xylem, phloem and bark tissue in the outermost 3 mm of each stem part at the four dendrometer positions in trees A and B. Tissue fractions were measured at the radius corresponding to the location of the dendrometer.
Fig. 5.
Tree phenology data from tree A (March 2009 to February 2011) and tree B (March 2010 to February 2011) showing the litter fall (total number of leaves) and the amount of flowers and fruits (in four categories of increasing amount) in three litter traps on each tree.
Detailed graphs of the stem diameter changes and the course of the environmental variables during the days in which tree A showed a sudden increase in its stem diameter at least at one side of the tree can be found in Supplementary Data Fig. S5. The sudden increases in stem diameter systematically took place after rain events (Fig. 6; Supplementary Data Fig. S5). These rain events corresponded to a drop in air temperature, soil temperature and VPD, and an increase in relative humidity. Inundation, soil water salinity and wind speed did not show a clear relationship to the sudden increases of the stem diameter. At the same moment in time, one or two sides of the stem of tree A showed a pronounced and sudden increase in diameter. At those moments, a smaller increment could sometimes, but not systematically, be observed at the other positions around the tree stem of tree A, while tree B showed a similar smaller or larger increase in its stem diameter at some but not all positions of the stem circumference (Fig. 3; Supplementary Data Fig. S5).
Fig. 6.
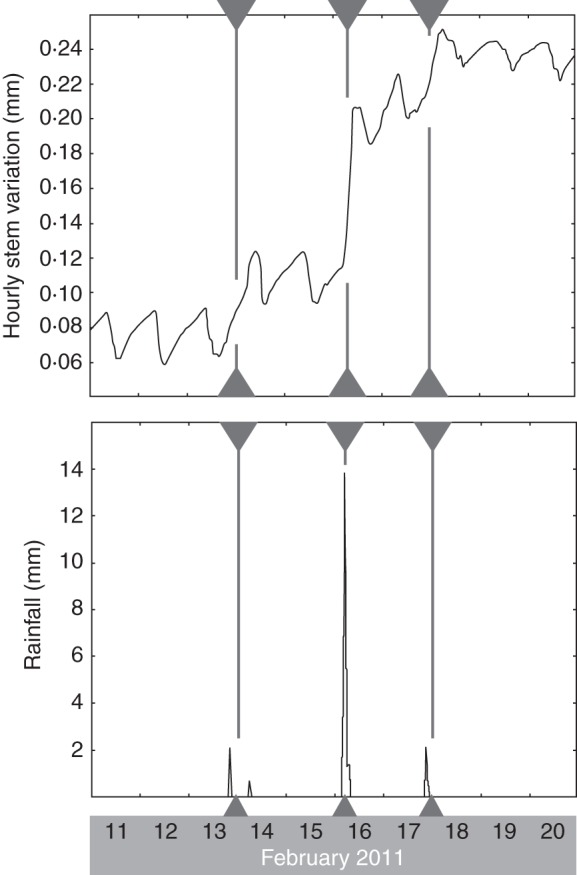
Hourly stem variation at one of the four positions around and along the stem of tree A (130 cm, 175°N) and rainfall data for a period of abrupt increase in the stem diameter of tree A (11–20 February 2010). Grey arrowheads and lines mark the specific hours of strong increase, that are at the base of the abrupt increase in the stem diameter of tree A, observed on the 1-year curves (Fig. 3).
During days without rainfall, the typical pattern in tree stem variation (late morning shrinkage and late afternoon expansion) was found at all except one position around the stem circumference of both trees (Supplementary Data Fig. S6). Inundation did not affect this pattern. Tree stems swelled slowly in the morning up to around 0800 h (approx. 1·5 h after sunrise), then started shrinking to reach a minimum between 1200 h and 1800 h (sunset is at around 1830 h), after which they started swelling more quickly. The period of shrinking corresponded to an increase in air temperature and VPD, and a decrease in relative humidity. Soil temperature increase lagged behind the increase in air temperature but decreased suddenly after inundation. The daily patterns in stem diameter variation did not show a clear relationship to the variations in soil water salinity, wind speed and maximum wind speed. In days with rainfall, the typical pattern in tree stem variation was often disturbed, and large variations between the different positions around the stem circumferences of both trees could be observed.
Maximum daily shrinkage was lower but with larger variation in the rainy season than in the dry season for tree A at three of the four positions around the stem circumference. In tree B, the same decrease in stem diameter during the rainy season could be observed at three of the four positions around the stem circumference; however, there was no large variation. For both trees, the daily shrinkage period was longer in the rainy season than in the dry season. The number of distinct shrinkage phases per day was also more variable in the rainy season. The measurements made at 140 cm were an exception, showing a higher number of distinct shrinkage phases in July and August 2010. At two positions around the stem circumference of tree B (130 cm, 175°N and 285°N) a higher variation in number of distinct shrinkage phases took also place in January to March 2011. The absence of a shrinkage phase was rare and only occured during the rainy season. No general pattern could be observed in the speed of tree stem shrinkage.
DISCUSSION
Patchiness at different levels
The hypothesized patchy growth in Avicennia (Schmitz et al., 2008) is supported by the results of the pinning analysis. Radial increment was not always observed at all positions around and along the tree stems, and small to large variations existed between the different positions (Table 1; Supplementary Data Fig. S2). Absence of a radial increment would indicate a meristematic zone that remained inactive throughout the period of observation. A clear example is the approx. 0·9 and 2 mm increments between August 2006 and February 2007 in an A. marina tree on the southern and eastern sides of the stem, respectively, in contrast to the absence of increment at the north-western side at the same stem height (Table 1). However, the north-western side of this tree did not systematically show a lower increment or absence of increment, confirming that patchiness and irregular growth are occurring. The differences in total increment after 1 year between the different positions around the stem circumference of two Avicennia trees (Table 2) also support a patchy growth mechanism, or at least do not contradict it.
Differences in the amount of increment around the stem circumference are well known in, for example, trees that form reaction wood (Schweingruber et al., 2006), or tropical trees with buttresses (Woodcock et al., 2000). However, little is known about the variation in increment around the stem circumference in trees with concentric stems or those that normally grow concentrically. Radial stem growth is mainly studied in a forestry or forest ecology context, with the general practice being manual or automatic diameter measurements that integrate the diameter increment over the whole stem (Clark et al., 2000). Usually, studies using point dendrometers examine only one side of the tree's stem (e.g. Zweifel and Häsler, 2001; Wimmer et al., 2002; Bouriaud et al., 2005; Turcotte et al., 2009; Biondi and Hartsough, 2010; Hölttä et al., 2010; Volland-Voigt et al., 2011). However, significant differences in radial increment between different compass directions have already been found in Picea abies, Pinus sylvestris, Olea europaea and Podocarpus falcatus (Mäkinen et al., 2003, 2008; Krepkowski et al., 2012; Cherubini et al., 2013). Furthermore, differences in duration patterns and amplitude of daily stem variations with tree height have been recorded in P. abies (Zweifel and Häsler, 2001; Bouriaud et al., 2005; Anfodillo et al., 2012). To better interpret our results, it would be desirable to have more data on the small-scale variation in radial increment around the circumference of temperate and tropical tree species in order to provide a reference point for our high spatial and temporal resolution data.
Patchiness does not occur only in the internal structure (Robert et al., 2011b) and in the radial growth of Avicennia, as it can also be observed in the swelling and shrinking patterns of the trees. Different positions around the stem circumference often showed different swelling and shrinking behaviour (Supplementary Data Figs S5, S6). The differences between different stem parts are especially prominent after rainfall, and this strengthens our hypothesis that patchiness can optimize the interaction of Avicennia trees with their environment. A patchy reaction of the vascular tissues to the environment could allow Avicennia trees to partition the available water over the tree stem. In this way, water, once accessible, could be used in a more directive way, investing all the attainable resources in only some locations of the tree stem so that at least at these locations there is enough water to, for example, overcome vessel embolisms or create new cells. As these locations change with time, the overall functioning of the tree can be maintained.
Patchy growth and a patchy reaction of vascular tissues to the environmental conditions of the mangrove forest is a potential rather than a constant feature in Avicennia trees. Different locations around the stem circumference can react in the same way, but they can also react differently depending on the prevailing environmental conditions. Seemingly, the location of patch growth emerges in a random way. The similarity or dissimilarity occurs at different levels: between trees, between different locations around the stem circumference of one tree, in the overall growth pattern after 1 year and in daily or weekly stem diameter patterns. The variation in stem diameter on days without rain is much smaller than that on rainy days. However, even on dry days without inundation, not all positions around the stem circumference have the same daily pattern of swelling and shrinking. Further studies need to be done on the activity of patches relative to the environment in order to determine whether or not this process is truly random.
Increment during rainy conditions
A gradual increase in the stem diameter, if present in the growth patch, mainly occurred between April and July, in the long rainy season (Fig. 3). This period can be divided into two distinguishable phases: the first, from April to June, with extensive rainfall, decreasing air temperature, soil water salinity and soil temperature, and increasing relative humidity; and a second phase, in July, with less rainfall but with minimum air temperature, soil water salinity and soil temperature. Hence, favourable conditions for radial growth are periods of precipitation, as is the case for many tropical tree species where onset and interruption of cambial activity relate to changes in water availability (Bräuning et al., 2008; Biondi and Hartsough, 2010; Krepkowski et al., 2011; Volland-Voigt et al., 2011). However, the combination of a smaller amount of precipitation, lower air temperature, soil water salinity and soil temperature apparently still permits radial increment in Avicennia trees. This radial increment period could be explained by the combined effect of water availability (small amount of rainfall) and less demanding conditions for the transport of water, i.e. decreased tension in the water column at the atmosphere side (lower air temperatures) as well as at the soil side (lower soil water salinity), making water transport less vulnerable to cavitation and thus enabling a better water supply for cambial activity. As such, periods in which conditions are less demanding for water transport appear to favour radial growth. The wood anatomical features and physiological mechanisms of Avicennia trees have thus apparently evolved as adaptations to minimize the negative effects of cavitation and subsequent vessel embolism in saline conditions (Salleo et al., 1996; Ewers et al., 2007; Robert et al., 2009, 2011b; Carlquist, 2012).
The short rainy season in the year of our study started in the last days of October and continued until the end of November. The start of this short rainy season induced stem increment in four of the eight studied positions around the stem circumference of the two trees (Fig. 3). The period of stem increment lasted to the end of February at most of these positions, with an extensive increment phase at one position of tree B (130 cm, 35°N) in December. Trees appeared to have a pause in radial increment in August to October, between the long and short rainy season, rather than in the dry season, despite a considerable amount of precipitation during this period. A possible explanation for this could be the combined increases of air temperature, soil temperature, soil water salinity and VPD that are caused by less frequent and less intense rainfall as compared with the long rainy season. All these variables are at their highest respective levels in the dry season but without an overall increase in this period. Possibly, the constantly high level of stress that the trees experience in the dry season is less limiting for tree functioning than the steeply increasing stress levels in between the long and the short rainy season. The pause in radial increment also coincided with the peak in litter fall. Peak leaf fall in Avicennia trees occurs after peak leaf emergence (Duke, 1988; Ochieng and Erftemeijer, 2002; Wang'ondu et al., 2010). However, August and September have been reported as months with high leaf production (Wang'ondu et al., 2010), so that in this period Avicennia trees invest available energy and water in leaf production rather than in water storage or wood formation. The hormonal stimuli produced by the leaves formed in this period could then be involved in stimulating radial growth in the period after their formation (Savidge, 1996), i.e. during the short rainy season.
A large difference both in total increment after 1 year and in increment patterns could be observed between the studied trees (Fig. 3; Table 1). This difference could not be explained by any of the measured tree characteristics (general appearance, tree phenology data, pen root height or wood anatomy), and it is unclear whether the observed difference is due to differences at the tree level or at the time level (annual differences in growth in a seemingly homogeneous stand). Longer observation of the stem diameter changes of more trees could show whether one of the two growth patterns is the more general type or whether there is a lot of variability. It could also contribute to establishing whether the two patterns occur in all trees with yearly variation. However, differences of up to several millimetres in yearly increment are not exceptional between trees of the same stand (Deslauriers, 2003; Mäkinen et al., 2003; Volland-Voigt et al., 2011).
In tree A, the increment in 1 year occurred as sudden increases in the stem diameter, depending on the position around the stem circumference. These sudden growth events happened at three different times at one or two sides of the stem circumference and corresponded to environmental changes linked to precipitation events. Since these sudden increases in the stem were not reversed within the period studied and were not larger in width or shorter in time than observed in other species (Deslauriers, 2003; Čufar et al., 2008a, b, 2011), they may be explained by short periods of cell formation. However, the methods used in this study cannot distinguish between radial increment due to water uptake by the vascular tissue or formation of new cells adding to the circumference. Since the internal tissue organization of Avicennia trees is highly variable at a small scale, both in the vertical and in the horizontal direction (Robert et al., 2011a), neither pinning nor coring at short distances from the dendrometer locations could have given an insight into this aspect. The change of location with time of these sudden increases in stem size could allow for an overall concentric growth of the stem diameter, as proposed by Schmitz et al. (2008).
Daily stem diameter fluctuations
Inundation by seawater had no direct effect on daily stem variation patterns in Avicennia trees on days without rain (Supplementary Data Fig. S6). This is in contrast to the effect of fresh water from rain events, as a result of which instant changes could be observed in stem diameter in at least one position around the stem circumference of both studied trees. The large and instant effects of fresh water could indicate that Avicennia trees cannot instantly extract fresh water when inundated with seawater. However, more daily patterns need to be observed in order to evaluate the effects of inundation at different times of the day and the possible delayed effect of tidal inundation. Moreover, rain events are associated with changes in air temperature and relative humidity, while inundation only has an effect on soil temperature. Consequently, the large and instant effects of rain events are more than a reaction by the tree to fresh water availability alone. Processes such as stomatal conductance, known to be instantly influenced by temperature and relative humidity and of high importance for the water fluxes in trees, most probably play an equally crucial role in stem diameter variations during rain events.
The instant effects of rain events were also reflected in the patterns of maximum daily shrinkage of both trees, with generally lower but more varying maximum daily shrinkage in the rainy season. In addition, the occurrence of more than one daily shrinkage phase was more frequent during the rainy season, indicating the division of the diurnal cycle into several shrinking and swelling phases and thus the short-term reaction of the tree stems to changing environmental conditions. Hence, we can conclude that fresh water input has a strong and direct effect on the water flow between the vascular tissues in Avicennia, accounting for both replenishment of the stem with water and stem increment.
We can conclude that patchiness is a prominent characteristic of the hydraulic system of Avicennia trees. Patchiness occurs in the structure of their hydraulic tissues (Robert et al., 2011a), in their radial growth and in the shrinking and swelling patterns of their stems. Patchiness is, however, a feature that is potentially rather than systematically present. By this potential patchiness, Avicennia trees have an extra tool with which to react optimally to the prevailing environmental conditions, leading to ecological success in the highly dynamic mangrove environment.
Radial growth increment was strongly linked to rainfall, and rain events had an instant effect on the daily shrinking and swelling patterns of Avicennia tree stems. This shows the extreme importance of fresh water input for the functioning and water economy of mangrove trees.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We are grateful to Hamisi Ali Kirauni, Hilde Robert and Tom Van der Stocken for their help during the field work, to Achim Bräuning, Julia Krepkowski and Franziska Volland-Voigt for their advice regarding the dendrometer installation and the dendrometer data analysis, to Maarten De Munter for executing the CT scans. and to Sergio Rossi for his help with application of the SAS (Statistical Analysis System) code used. We thank Professor John Barnett for his input on this manuscript. The study was financially supported by the Agency for Innovation by Science and Technology (IWT, Flanders, Belgium), the Research Foundation – Flanders (FWO, Flanders, Belgium), the King Leopold III Fund for Nature Exploration and Conservation (Belgium), the ‘Stichting ter bevordering van wetenschappelijk onderzoek in Afrika’ (SBWOA, Belgium) and a BET/UTB budget of the Royal Museum for Central Africa (RMCA, Tervuren, Belgium). We thank the Department of Radiology of the Universitair Ziekenhuis Brussel for offering free CT scanning. This paper was written under the framework of the project ‘CREC’ (EU IRSES #247514) and is linked to activities conducted within the COST FP1106 network.
LITERATURE CITED
- Améglio T, Cruiziat P. Daily variations of stem and branch diameter: short overview from a developed example. In: Karalis T, editor. Mechanics of swelling. Berlin: Springer-Verlag; 1992. pp. 193–204. [Google Scholar]
- Anfodillo T, Deslauriers A, Menardi R, Tedoldi L, Petit G, Rossi S. Widening of xylem conduits in a conifer tree depends on the longer time of cell expension downwards along the stem. Journal of Experimental Botany. 2012;63:837–845. doi: 10.1093/jxb/err309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biondi F, Hartsough P. Using automated point dendrometers to analyze tropical treeline stem growth at Nevado de Colima, Mexico. Sensors. 2010;10:5827–5844. doi: 10.3390/s100605827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouriaud O, Leban JM, Bert D, Deleuze C. Intra-annual variations in climate influence growth and wood density of Norway spruce. Tree Physiology. 2005;25:651–660. doi: 10.1093/treephys/25.6.651. [DOI] [PubMed] [Google Scholar]
- Bräuning A, Homeier J, Cueva E, Beck E, Günter S. Growth dynamics of trees in tropical mountain ecosystems. Ecological Studies. 2008;198:291–302. [Google Scholar]
- Brough DW, Jones HG, Grace J. Diurnal changes in water content of the stems of apple trees, as influenced by irrigation. Plant, Cell and Environment. 1986;9:1–7. [Google Scholar]
- Carlquist S. How wood evolves: a new synthesis. Botany-Botanique. 2012;90:901–940. [Google Scholar]
- Cherubini P, Humbel T, Beeckman H, et al. Olive tree-ring problematic dating: a comparative analysis on Santorini (Greece) PLoS One. 2013;8 doi: 10.1371/journal.pone.0054730. e54730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark NA, Wynne RH, Schmoldt DL. A review of past research on dendrometers. Forest Science. 2000;46:570–576. [Google Scholar]
- Conejero W, Alarcon JJ, Garcia-Orellana Y, Abrisqueta JM, Torrecillas A. Daily sap flow and maximum daily trunk shrinkage measurements for diagnosing water stress in early maturing peach trees during the post-harvest period. Tree Physiology. 2007;27:81–88. doi: 10.1093/treephys/27.1.81. [DOI] [PubMed] [Google Scholar]
- Čufar K, Prislan P, de Luis M, Gričar J. Tree-ring variation, wood formation and phenology of beech (Fagus sylvatica) from a representative site in Slovenia, SE Central Europe. Trees-Structure and Function. 2008a;22:749–758. [Google Scholar]
- Čufar K, Prislan P, Gričar J. Cambial activity and wood formation in beech (Fagus sylvatica) during the 2006 growth season. Wood Research. 2008b;53:1–12. [Google Scholar]
- Čufar K, Cherubini M, Gričar J, Prislan P, Spina S, Romagnoli M. Xylem and phloem formation in chestnut (Castanea sativa Mill.) during the 2008 growing season. Dendrochronologia. 2011;29:127–134. [Google Scholar]
- De Schepper V, Steppe K. Development and verification of a water and sugar transport model using measured stem diameter variations. Journal of Experimental Botany. 2010;61:2083–2099. doi: 10.1093/jxb/erq018. [DOI] [PubMed] [Google Scholar]
- De Schepper V, Steppe K, Van Labeke M-C, Lemeur R. Detailed analysis of double girdling effects on stem diameter variations and sap flow in young oak trees. Environmental and Experimental Botany. 2010;68:149–156. [Google Scholar]
- Deslauriers A. Dynamique de la croissance radiale et influence météorologique quotidienne chez le sapin baumier (Abies balsamea (L.) Mill.) en fôret boréale. PhD thesis, Université de Quebec, Chicoutimi. 2003 [Google Scholar]
- Deslauriers A, Anfodillo T, Rossi S, Carraro V. Using simple causal modeling to understand how water and temperature affect daily stem radial variation in trees. Tree Physiology. 2007;27:1125–1136. doi: 10.1093/treephys/27.8.1125. [DOI] [PubMed] [Google Scholar]
- Deslauriers A, Rossi S, Turcotte A, Morin H, Krause C. A three-step procedure in SAS to analyze the time series from automatic dendrometers. Dendrochronologia. 2011;29:151–161. [Google Scholar]
- Downes G, Beadle C, Worledge D. Daily stem growth patterns in irrigated Eucalyptus globulus and E. nitens in relation to climate. Trees-Structure and Function. 1999;14:102–111. [Google Scholar]
- Duke NC. Phenologies and litter fall of 2 mangrove trees, Sonneratia alba Sm and Sonneratia caseolaris (L) Engl, and their putative hybrid, S×Gulngai NC Duke. Australian Journal of Botany. 1988;36:473–482. [Google Scholar]
- Ewers FW, Ewers JM, Jacobsen AL, Lopez-Portillo J. Vessel redundancy: modeling safety in numbers. IAWA Journal. 2007;28:373–388. [Google Scholar]
- Giovannelli A, Deslauriers A, Fragnelli G, et al. Evaluation of drought response of two poplar clones (Populus×canadensis Monch ‘I-214’ and P. deltoides Marsh. ‘Dvina’) through high resolution analysis of stem growth. Journal of Experimental Botany. 2007;58:2673–2683. doi: 10.1093/jxb/erm117. [DOI] [PubMed] [Google Scholar]
- Goldhamer DA, Fereres E, Mata M, Girona J, Cohen M. Sensitivity of continuous and discrete plant and soil water status monitoring in peach trees subjected to deficit irrigation. Journal of the American Society for Horticultural Science. 1999;124:437–444. [Google Scholar]
- Herzog KM, Hasler R, Thum R. Diurnal changes in the radius of a sub-alpine Norway spruce stem – their relation to the sap flow and their use to estimate transpiration. Trees-Structure and Function. 1995;10:94–101. [Google Scholar]
- Hölttä T, Mäkinen H, Nöjd P, Mäkelä A, Nikinmaa E. A physiological model of softwood cambial growth. Tree Physiology. 2010;30:1235–1252. doi: 10.1093/treephys/tpq068. [DOI] [PubMed] [Google Scholar]
- Irvine J, Grace J. Continuous measurements of water tensions in the xylem of trees based on the elastic properties of wood. Planta. 1997;202:455–461. [Google Scholar]
- Krepkowski J, Bräuning A, Gebrekirstos A, Strobl S. Cambial growth dynamics and climatic control of different tree life forms in tropical mountain forest in Ethiopia. Trees-Structure and Function. 2011;25:59–70. [Google Scholar]
- Krepkowski J, Braeuning A, Gebrekirstos A. Growth dynamics and potential for cross-dating and multi-century climate reconstruction of Podocarpus falcatus in Ethiopia. Dendrochronologia. 2012;30:257–265. [Google Scholar]
- Mäkinen H, Nöjd P, Saranpää P. Seasonal changes in stem radius and production of new tracheids in Norway spruce. Tree Physiology. 2003;23:959–968. doi: 10.1093/treephys/23.14.959. [DOI] [PubMed] [Google Scholar]
- Mäkinen H, Seo J-W, Nöjd P, Schmitt U, Jalkanen R. Seasonal dynamics of wood formation: a comparison between pinning, microcoring and dendrometer measurements. European Journal of Forest Research. 2008;127:235–245. [Google Scholar]
- Naor A, Cohen S. Sensitivity and variability of maximum trunk shrinkage, midday stem water potential, and transpiration rate in response to withholding irrigation from field-grown apple trees. Hortscience. 2003;38:547–551. [Google Scholar]
- Neukermans G, Dahdouh-Guebas F, Kairo JG, Koedam N. Mangrove species and stand mapping in Gazi bay (Kenya) using Quickbird satellite imagery. Journal of Spatial Science. 2008;53:75–86. [Google Scholar]
- Ochieng CA, Erftemeijer PLA. Phenology, litterfall and nutrient resorption in Avicennia marina (Forssk.) Vierh in Gazi Bay, Kenya. Trees-Structure and Function. 2002;16:167–171. [Google Scholar]
- Ortuño MF, Garcia-Orellana Y, Conejero W, Ruiz-Sanchez MC, Alarcon JJ, Torrecillas A. Stem and leaf water potentials, gas exchange, sap flow, and trunk diameter fluctuations for detecting water stress in lemon trees. Trees-Structure and Function. 2006;20:1–8. [Google Scholar]
- Remorini D, Massai R. Comparison of water status indicators for young peach trees. Irrigation Science. 2003;22:39–46. [Google Scholar]
- Robert EMR, Koedam N, Beeckman H, Schmitz N. A safe hydraulic architecture as wood anatomical explanation for the difference in distribution of the mangroves Avicennia and Rhizophora. Functional Ecology. 2009;23:649–657. [Google Scholar]
- Robert EMR, Schmitz N, Boeren I, et al. Successive cambia: a developmental oddity or an adaptive structure? PLoS One. 2011a;6 doi: 10.1371/journal.pone.0016558. e16558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert EMR, Schmitz N, Okello JA, Boeren I, Beeckman H, Koedam N. Mangrove growth rings: fact or fiction? Trees-Structure and Function. 2011b;25:49–58. [Google Scholar]
- Salleo S, LoGullo MA, DePaoli D, Zippo M. Xylem recovery from cavitation-induced embolism in young plants of Laurus nobilis: a possible mechanism. New Phytologist. 1996;132:47–56. doi: 10.1111/j.1469-8137.1996.tb04507.x. [DOI] [PubMed] [Google Scholar]
- Savidge RA. Xylogenesis, genetic and environmental regulation – a review. IAWA Journal. 1996;17:269–310. [Google Scholar]
- Schmitz N, Verheyden A, Kairo JG, Beeckman H, Koedam N. Successive cambia development in Avicennia marina (Forssk.) Vierh. is not climatically driven in the seasonal climate at Gazi Bay, Kenya. Dendrochronologia. 2007;25:87–96. [Google Scholar]
- Schmitz N, Robert EMR, Verheyden A, Kairo JG, Beeckman H, Koedam N. A patchy growth via successive and simultaneous cambia: key to success of the most widespread mangrove species Avicennia marina? Annals of Botany. 2008;101:49–58. doi: 10.1093/aob/mcm280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönwiese C-D. Klimatologie. Stuttgart: Ulmer (UTB); 2003. [Google Scholar]
- Schweingruber FH, Börner A, Schulze E-D. Berlin: Springer-Verlag; 2006. Atlas of woody plant stems. [Google Scholar]
- Sevanto S, Hölttä T, Holbrook NM. Effects of the hydraulic coupling between xylem and phloem on diurnal phloem diameter variation. Plant, Cell and Environment. 2011;34:690–703. doi: 10.1111/j.1365-3040.2011.02275.x. [DOI] [PubMed] [Google Scholar]
- Steppe K, De Pauw DJW, Lemeur R, Vanrolleghem PA. A mathematical model linking tree sap flow dynamics to daily stem diameter fluctuations and radial stem growth. Tree Physiology. 2006;26:257–273. doi: 10.1093/treephys/26.3.257. [DOI] [PubMed] [Google Scholar]
- Steppe K, Cochard H, Lacointe A, Ameglio T. Could rapid diameter changes be facilitated by a variable hydraulic conductance? Plant, Cell and Environment. 2012;35:150–157. doi: 10.1111/j.1365-3040.2011.02424.x. [DOI] [PubMed] [Google Scholar]
- Turcotte A, Morin H, Krause C, Deslauriers A, Thibeault-Martel M. The timing of spring rehydration and its relation with the onset of wood formation in black spruce. Agricultural and Forest Meteorology. 2009;149:1403–1409. [Google Scholar]
- Volland-Voigt F, Bräuning A, Ganzhi O, Peters T, Maza H. Radial stem variations of Tabebuia chrysantha (Bignoniaceae) in different tropical forest ecosystems of southern Ecuador. Trees-Structure and Function. 2011;25:39–48. [Google Scholar]
- Wang'ondu VW, Kairo JG, Kinyamario JI, et al. Phenology of Avicennia marina (Forsk.) Vierh in a disjunctly-zoned mangrove stand in Kenya. Western Indian Ocean Journal of Marine Science. 2010;9:135–144. [Google Scholar]
- Wimmer R, Downes GM, Evans R. High-resolution analysis of radial growth and wood density in Eucalyptus nitens, grown under different irrigation regimes. Annals of Forest Science. 2002;59:519–524. [Google Scholar]
- Woodcock DW, Dos Santos G, Taylor D. The buttressed blue marble tree: wood and growth characteristics of Elaeocarpus angustifolius (Elaeocarpaceae) Annals of Botany. 2000;85:1–6. [Google Scholar]
- Zamski E. Does successive cambia differentiation in Avicennia depend on leaf and branch initiation. Israel Journal of Botany. 1981;30:57–64. [Google Scholar]
- Zweifel R, Häsler R. Dynamics of water storage in mature subalpine Picea abies: temporal and spatial patterns of change in stem radius. Tree Physiology. 2001;21:561–569. doi: 10.1093/treephys/21.9.561. [DOI] [PubMed] [Google Scholar]
- Zweifel R, Item H, Häsler R. Stem radius changes and their relation to stored water in stems of young Norway spruce trees. Trees-Structure and Function. 2000;15:50–57. [Google Scholar]
- Zweifel R, Item H, Häsler R. Link between diurnal stem radius changes and tree water relations. Tree Physiology. 2001;21:869–877. doi: 10.1093/treephys/21.12-13.869. [DOI] [PubMed] [Google Scholar]
- Zweifel R, Zimmermann L, Zeugin F, Newbery DM. Intra-annual radial growth and water relations of trees: implications towards a growth mechanism. Journal of Experimental Botany. 2006;57:1445–1459. doi: 10.1093/jxb/erj125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.