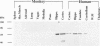Abstract
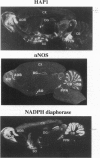
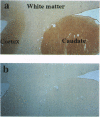
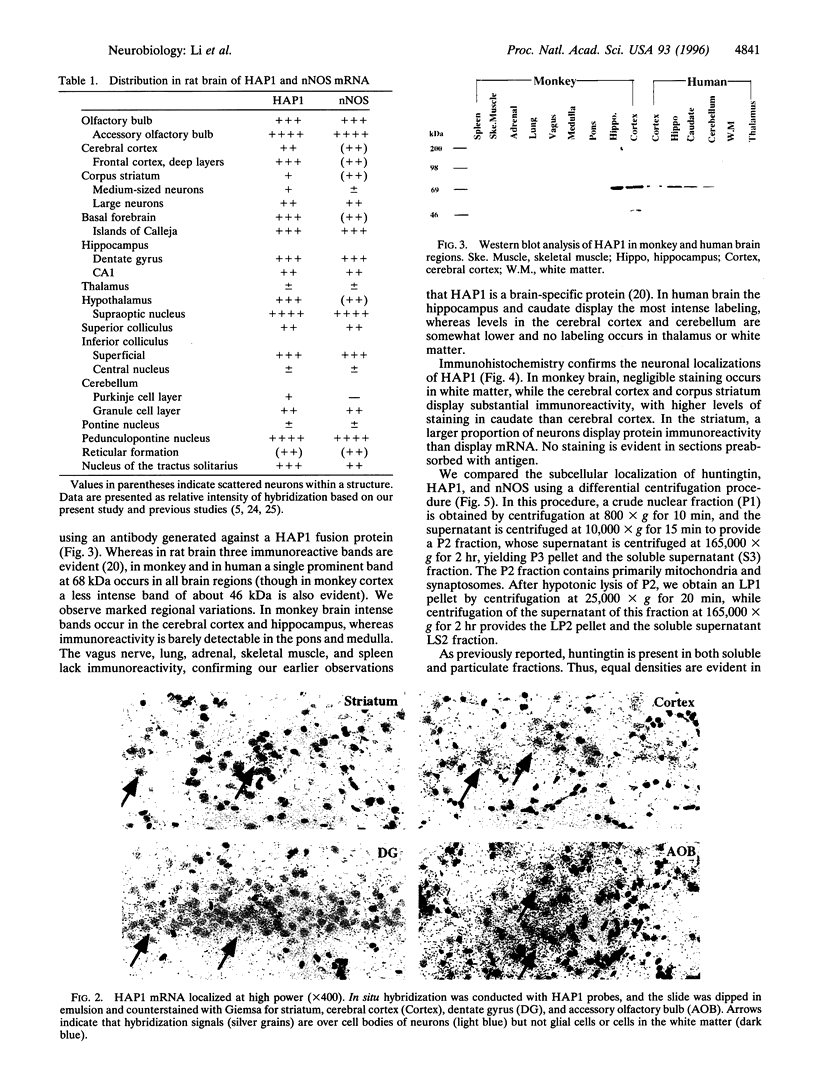
Huntington disease stems from a mutation of the protein huntingtin and is characterized by selective loss of discrete neuronal populations in the brain. Despite a massive loss of neurons in the corpus striatum, NO-generating neurons are intact. We recently identified a brain-specific protein that associates with huntingtin and is designated huntingtin-associated protein (HAP1). We now describe selective neuronal localizations of HAP1. In situ hybridization studies reveal a resemblance of HAP1 and neuronal nitric oxide synthase (nNOS) mRNA localizations with dramatic enrichment of both in the pedunculopontine nuclei, the accessory olfactory bulb, and the supraoptic nucleus of the hypothalamus. Both nNOS and HAP1 are enriched in subcellular fractions containing synaptic vesicles. Immunocytochemical studies indicate colocalizations of HAP1 and nNOS in some neurons. The possible relationship of HAP1 and nNOS in the brain is reminiscent of the relationship of dystrophin and nNOS in skeletal muscle and suggests a role of NO in Huntington disease, analogous to its postulated role in Duchenne muscular dystrophy.
Full text
PDF

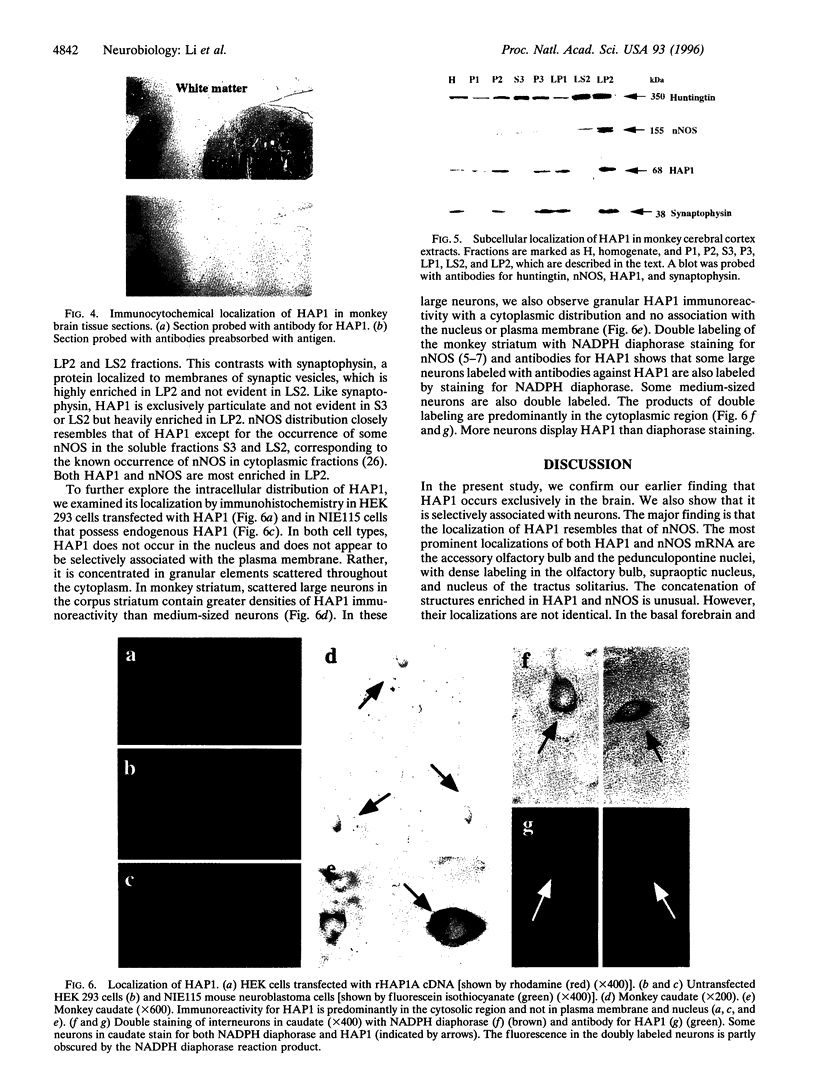


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albin R. L., Greenamyre J. T. Alternative excitotoxic hypotheses. Neurology. 1992 Apr;42(4):733–738. doi: 10.1212/wnl.42.4.733. [DOI] [PubMed] [Google Scholar]
- Albin R. L., Tagle D. A. Genetics and molecular biology of Huntington's disease. Trends Neurosci. 1995 Jan;18(1):11–14. doi: 10.1016/0166-2236(95)93943-r. [DOI] [PubMed] [Google Scholar]
- Andrew S. E., Goldberg Y. P., Kremer B., Telenius H., Theilmann J., Adam S., Starr E., Squitieri F., Lin B., Kalchman M. A. The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington's disease. Nat Genet. 1993 Aug;4(4):398–403. doi: 10.1038/ng0893-398. [DOI] [PubMed] [Google Scholar]
- Beal M. F. Aging, energy, and oxidative stress in neurodegenerative diseases. Ann Neurol. 1995 Sep;38(3):357–366. doi: 10.1002/ana.410380304. [DOI] [PubMed] [Google Scholar]
- Beal M. F., Brouillet E., Jenkins B. G., Ferrante R. J., Kowall N. W., Miller J. M., Storey E., Srivastava R., Rosen B. R., Hyman B. T. Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. J Neurosci. 1993 Oct;13(10):4181–4192. doi: 10.1523/JNEUROSCI.13-10-04181.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal M. F., Brouillet E., Jenkins B., Henshaw R., Rosen B., Hyman B. T. Age-dependent striatal excitotoxic lesions produced by the endogenous mitochondrial inhibitor malonate. J Neurochem. 1993 Sep;61(3):1147–1150. doi: 10.1111/j.1471-4159.1993.tb03633.x. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Ferris C. D., Snyder S. H. Nitric oxide synthase regulatory sites. Phosphorylation by cyclic AMP-dependent protein kinase, protein kinase C, and calcium/calmodulin protein kinase; identification of flavin and calmodulin binding sites. J Biol Chem. 1992 Jun 5;267(16):10976–10981. [PubMed] [Google Scholar]
- Bredt D. S., Glatt C. E., Hwang P. M., Fotuhi M., Dawson T. M., Snyder S. H. Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron. 1991 Oct;7(4):615–624. doi: 10.1016/0896-6273(91)90374-9. [DOI] [PubMed] [Google Scholar]
- Brenman J. E., Chao D. S., Xia H., Aldape K., Bredt D. S. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995 Sep 8;82(5):743–752. doi: 10.1016/0092-8674(95)90471-9. [DOI] [PubMed] [Google Scholar]
- Brouillet E., Hantraye P., Ferrante R. J., Dolan R., Leroy-Willig A., Kowall N. W., Beal M. F. Chronic mitochondrial energy impairment produces selective striatal degeneration and abnormal choreiform movements in primates. Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):7105–7109. doi: 10.1073/pnas.92.15.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle J. T., Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993 Oct 29;262(5134):689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Dawbarn D., De Quidt M. E., Emson P. C. Survival of basal ganglia neuropeptide Y-somatostatin neurones in Huntington's disease. Brain Res. 1985 Aug 12;340(2):251–260. doi: 10.1016/0006-8993(85)90921-7. [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Bredt D. S., Fotuhi M., Hwang P. M., Snyder S. H. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7797–7801. doi: 10.1073/pnas.88.17.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson V. L., Dawson T. M., Bartley D. A., Uhl G. R., Snyder S. H. Mechanisms of nitric oxide-mediated neurotoxicity in primary brain cultures. J Neurosci. 1993 Jun;13(6):2651–2661. doi: 10.1523/JNEUROSCI.13-06-02651.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson V. L., Kizushi V. M., Huang P. L., Snyder S. H., Dawson T. M. Resistance to neurotoxicity in cortical cultures from neuronal nitric oxide synthase-deficient mice. J Neurosci. 1996 Apr 15;16(8):2479–2487. doi: 10.1523/JNEUROSCI.16-08-02479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFiglia M. Excitotoxic injury of the neostriatum: a model for Huntington's disease. Trends Neurosci. 1990 Jul;13(7):286–289. doi: 10.1016/0166-2236(90)90111-m. [DOI] [PubMed] [Google Scholar]
- DiFiglia M., Sapp E., Chase K., Schwarz C., Meloni A., Young C., Martin E., Vonsattel J. P., Carraway R., Reeves S. A. Huntingtin is a cytoplasmic protein associated with vesicles in human and rat brain neurons. Neuron. 1995 May;14(5):1075–1081. doi: 10.1016/0896-6273(95)90346-1. [DOI] [PubMed] [Google Scholar]
- Duyao M., Ambrose C., Myers R., Novelletto A., Persichetti F., Frontali M., Folstein S., Ross C., Franz M., Abbott M. Trinucleotide repeat length instability and age of onset in Huntington's disease. Nat Genet. 1993 Aug;4(4):387–392. doi: 10.1038/ng0893-387. [DOI] [PubMed] [Google Scholar]
- Ferrante R. J., Kowall N. W., Beal M. F., Richardson E. P., Jr, Bird E. D., Martin J. B. Selective sparing of a class of striatal neurons in Huntington's disease. Science. 1985 Nov 1;230(4725):561–563. doi: 10.1126/science.2931802. [DOI] [PubMed] [Google Scholar]
- Giuili G., Luzi A., Poyard M., Guellaën G. Expression of mouse brain soluble guanylyl cyclase and NO synthase during ontogeny. Brain Res Dev Brain Res. 1994 Sep 16;81(2):269–283. doi: 10.1016/0165-3806(94)90313-1. [DOI] [PubMed] [Google Scholar]
- Greene J. G., Porter R. H., Eller R. V., Greenamyre J. T. Inhibition of succinate dehydrogenase by malonic acid produces an "excitotoxic" lesion in rat striatum. J Neurochem. 1993 Sep;61(3):1151–1154. doi: 10.1111/j.1471-4159.1993.tb03634.x. [DOI] [PubMed] [Google Scholar]
- Gutekunst C. A., Levey A. I., Heilman C. J., Whaley W. L., Yi H., Nash N. R., Rees H. D., Madden J. J., Hersch S. M. Identification and localization of huntingtin in brain and human lymphoblastoid cell lines with anti-fusion protein antibodies. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8710–8714. doi: 10.1073/pnas.92.19.8710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecker M., Mülsch A., Busse R. Subcellular localization and characterization of neuronal nitric oxide synthase. J Neurochem. 1994 Apr;62(4):1524–1529. doi: 10.1046/j.1471-4159.1994.62041524.x. [DOI] [PubMed] [Google Scholar]
- Henshaw R., Jenkins B. G., Schulz J. B., Ferrante R. J., Kowall N. W., Rosen B. R., Beal M. F. Malonate produces striatal lesions by indirect NMDA receptor activation. Brain Res. 1994 May 30;647(1):161–166. doi: 10.1016/0006-8993(94)91412-5. [DOI] [PubMed] [Google Scholar]
- Hope B. T., Michael G. J., Knigge K. M., Vincent S. R. Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2811–2814. doi: 10.1073/pnas.88.7.2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobzik L., Reid M. B., Bredt D. S., Stamler J. S. Nitric oxide in skeletal muscle. Nature. 1994 Dec 8;372(6506):546–548. doi: 10.1038/372546a0. [DOI] [PubMed] [Google Scholar]
- Li X. J., Li S. H., Sharp A. H., Nucifora F. C., Jr, Schilling G., Lanahan A., Worley P., Snyder S. H., Ross C. A. A huntingtin-associated protein enriched in brain with implications for pathology. Nature. 1995 Nov 23;378(6555):398–402. doi: 10.1038/378398a0. [DOI] [PubMed] [Google Scholar]
- Monaco A. P., Neve R. L., Colletti-Feener C., Bertelson C. J., Kurnit D. M., Kunkel L. M. Isolation of candidate cDNAs for portions of the Duchenne muscular dystrophy gene. Nature. 1986 Oct 16;323(6089):646–650. doi: 10.1038/323646a0. [DOI] [PubMed] [Google Scholar]
- O'Hearn E., Zhang P., Molliver M. E. Excitotoxic insult due to ibogaine leads to delayed induction of neuronal NOS in Purkinje cells. Neuroreport. 1995 Aug 21;6(12):1611–1616. doi: 10.1097/00001756-199508000-00006. [DOI] [PubMed] [Google Scholar]
- Pasqualotto B. A., Hope B. T., Vincent S. R. Citrulline in the rat brain: immunohistochemistry and coexistence with NADPH-diaphorase. Neurosci Lett. 1991 Jul 22;128(2):155–160. doi: 10.1016/0304-3940(91)90250-w. [DOI] [PubMed] [Google Scholar]
- Ross C. A. When more is less: pathogenesis of glutamine repeat neurodegenerative diseases. Neuron. 1995 Sep;15(3):493–496. doi: 10.1016/0896-6273(95)90138-8. [DOI] [PubMed] [Google Scholar]
- Schulz J. B., Matthews R. T., Jenkins B. G., Ferrante R. J., Siwek D., Henshaw D. R., Cipolloni P. B., Mecocci P., Kowall N. W., Rosen B. R. Blockade of neuronal nitric oxide synthase protects against excitotoxicity in vivo. J Neurosci. 1995 Dec;15(12):8419–8429. doi: 10.1523/JNEUROSCI.15-12-08419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz J. B., Matthews R. T., Jenkins B. G., Ferrante R. J., Siwek D., Henshaw D. R., Cipolloni P. B., Mecocci P., Kowall N. W., Rosen B. R. Blockade of neuronal nitric oxide synthase protects against excitotoxicity in vivo. J Neurosci. 1995 Dec;15(12):8419–8429. doi: 10.1523/JNEUROSCI.15-12-08419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp A. H., Loev S. J., Schilling G., Li S. H., Li X. J., Bao J., Wagster M. V., Kotzuk J. A., Steiner J. P., Lo A. Widespread expression of Huntington's disease gene (IT15) protein product. Neuron. 1995 May;14(5):1065–1074. doi: 10.1016/0896-6273(95)90345-3. [DOI] [PubMed] [Google Scholar]
- Stine O. C., Pleasant N., Franz M. L., Abbott M. H., Folstein S. E., Ross C. A. Correlation between the onset age of Huntington's disease and length of the trinucleotide repeat in IT-15. Hum Mol Genet. 1993 Oct;2(10):1547–1549. doi: 10.1093/hmg/2.10.1547. [DOI] [PubMed] [Google Scholar]
- Sugaya K., McKinney M. Nitric oxide synthase gene expression in cholinergic neurons in the rat brain examined by combined immunocytochemistry and in situ hybridization histochemistry. Brain Res Mol Brain Res. 1994 Apr;23(1-2):111–125. doi: 10.1016/0169-328x(94)90217-8. [DOI] [PubMed] [Google Scholar]
- Trottier Y., Devys D., Imbert G., Saudou F., An I., Lutz Y., Weber C., Agid Y., Hirsch E. C., Mandel J. L. Cellular localization of the Huntington's disease protein and discrimination of the normal and mutated form. Nat Genet. 1995 May;10(1):104–110. doi: 10.1038/ng0595-104. [DOI] [PubMed] [Google Scholar]
- Webster C., Silberstein L., Hays A. P., Blau H. M. Fast muscle fibers are preferentially affected in Duchenne muscular dystrophy. Cell. 1988 Feb 26;52(4):503–513. doi: 10.1016/0092-8674(88)90463-1. [DOI] [PubMed] [Google Scholar]