Abstract
Systemic artemin promotes regeneration of dorsal roots to the spinal cord following crush injury. However, it is unclear whether systemic artemin can promote peripheral nerve regeneration and functional recovery distal to the dorsal root ganglion (DRG). In the present investigation, male SD rats received axotomy, ligation, or crush of the L5 spinal nerve or sham surgery. Starting the day of injury, animals received intermittent s.c. artemin or vehicle across 2 weeks. Sensory thresholds to tactile or thermal stimuli were monitored for 6 weeks following injury. Immunohistochemical analyses of the DRG and nerve regeneration were performed at the 6 week timepoint. Artemin transiently reversed tactile and thermal hypersensitivity following axotomy, ligation or crush injury. Thermal and tactile hypersensitivity re-emerged within 1 week of treatment termination. However, artemin treated rats with nerve crush, but not axotomy or ligation, subsequently showed gradual return of sensory thresholds to pre-injury baseline levels by 6 weeks post-injury. Artemin normalized labeling for NF200, IB4 and CGRP in nerve fibers distal to the crush injury, suggesting persistent normalization of nerve-crush induced neurochemical changes. Sciatic and intradermal administration of dextran or CTB distal to the crush injury site resulted in labeling of neuronal profiles in the L5 DRG, suggesting functional restoration of non-myelinated and myelinated fibers across the injury site into cutaneous tissue. Artemin also diminished ATF3 and caspase-3 expression in the L5 DRG, suggesting persistent neuroprotective actions. A limited period of artemin treatment elicits disease modification by promoting sensory reinnervation of distal territories and restoring pre-injury sensory thresholds.
Keywords: Artemin, peripheral nerve injury, sensory neurons, hyperalgesia, regeneration
Introduction
Incomplete recovery from nerve damage often leads to loss of sensory function that may be paradoxically accompanied by severe, intractable neuropathic pain [1; 24]. Exogenously administered GDNF or of artemin has promoted neuronal regeneration in conditions of neuropathic pain [7; 11; 27] and brachial avulsion [23]. However, clinical trials have demonstrated that systemic GDNF administration produces substantial side effects such as pain, weight loss, bowel urgency and paraesthesias [15; 19], that may stem from the relatively broad distribution of GDNF receptors. Artemin, a neurotrophin in the family of GDNF ligands currently under clinical investigation for treatment of sciatica (clinicaltrials.gov), preferentially binds to the GDNF family receptor (GFR) GFRα3, which is expressed primarily on nociceptive sensory neurons within the dorsal root ganglion (DRG) [21]. Such restricted expression may be advantageous in allowing for systemic administration within the clinical setting with diminished side effects.
Previous studies have shown that artemin both prevented and reversed nerve injury-induced thermal and tactile hypersensitivity and blocked multiple nerve-injury induced neurochemical changes during drug administration [9]. In addition, recent studies demonstrated that artemin promotes regeneration of injured primary afferent fibers through the dorsal root entry zone and into the spinal cord after dorsal root crush [11; 27]. These changes were accompanied by long-term recovery of synaptic function along with normalization of sensory responses to tactile and thermal stimuli as well as locomotor activity, effects that persisted across 6 months following a 2 week period of intermittent systemic artemin treatment [27]. In the present investigation, we compared the effects of systemic artemin on nerve-injury induced hypersensitivity (i.e., “pain”) and neurochemical changes, including possible regeneration of peripheral nerves to distal territories, across multiple models of nerve-injury induced pain.
Methods
Animals
Male, Sprague-Dawley rats (Harlan, Indianapolis, IN), weighing 175–250g at the time of surgery, were housed in a temperature-controlled room on a 12-h light/dark cycle. Food and water were available ad libitum. All testing was performed in accordance with the policies and recommendations of the International Association for the Study of Pain (IASP) and the National Institutes of Health (NIH) guidelines for the handling and use of laboratory animals and received approval from the Institutional Animal Care and Use Committee (IACUC) of the University of Arizona.
Spinal nerve injuries
Anesthesia was induced with 4% isoflurane in air and maintained with 2% isoflurane in air delivered at a rate of 1 L/min. Separate groups of rats underwent 1) transection and removal of a 2–3 mm portion of the L5 spinal nerve (axotomy), 2) tight ligation of the L5 spinal nerve with 4-0 silk thread, 3) repetitive crush of the L5 spinal nerve for 60 seconds with #7 head-bent forceps or 4) sham surgery. On completion of the surgery, hemostasis was confirmed, the muscles sutured using silk thread, and the skin closed with metal clips. Postoperatively, all rats were individually housed, and any rats with motor deficits were euthanized.
Artemin administration
Rat artemin (113 amino acids) was refolded from Escherichia coli inclusion bodies and purified to >98% homogeneity. Purified artemin migrated as a reducible dimer by SDS-PAGE and eluted as a single peak (24 kDa) by size exclusion chromatography and reverse-phase high-performance liquid chromatography. The purified product was confirmed to contain the characteristic cysteine-knot disulfide pattern seen in GDNF, and to be fully active in vitro by assaying receptor binding, cell-based c-RET kinase activation and sensory neuronal survival as previously described [9; 27]. Artemin (1 mg/kg) was injected subcutaneously on a Monday, Wednesday and Friday schedule across 14 days beginning immediately after surgery (i.e., 6 total injections) as described previously [9; 27]. Vials containing artemin or vehicle were prepared and coded and the experiments performed by a different investigator blinded to the codes; the labels opened at the termination of the study.
Behavioral observations
Tactile sensory thresholds were determined by the withdrawal threshold of the left paw in response to probing with a series of eight calibrated von Frey filaments (Stoelting, Wooddale, IL) in logarithmically spaced increments ranging from 0.41 to 15 g (4–150 N) as previously described [9; 27]. Thermal sensory thresholds were evaluated by the determination of paw withdrawal latency from an infrared radiant heat source [9; 27]. All behavioral assessments were performed in a blinded fashion. Eight rats per treatment group were used by this procedure.
Neuronal tracing
For tracer injections into the sciatic nerve to evaluate nerve regeneration, rats were anesthetized at 5 weeks post-spinal nerve injury with 4% isofluorane and the sciatic nerves at mid-thigh region ipsilateral to spinal nerve injury were exposed under aseptic conditions. A 5 µl solution of 0.5% CTB (Cholera Toxin B subunit, low salt; List Labs) or 10% tetramethylrhodamine-dextran (3000 MW, Molecular Probes) were injected into the sciatic nerve at 3–5 sites using a Hamilton syringe. After injections, the muscles and skin were closed with 4-0 silk suture. For tracer injections into the dermis to evaluate possible nerve reinnervation, a 50 µl solution of 0.5% CTB or of 10% tetramethylrhodamine-dextran (3000 MW, Molecular Probes) was injected intradermally into both plantar and dorsum of the ipsilateral hindpaw at 5–6 sites using a Hamilton syringe. One week following tracer injection into the sciatic nerve or dermis (six weeks post-spinal nerve injury), rats underwent intracardiac perfusions and ipsilateral L5 spinal nerves and DRGs were harvested for immunohistochemical evaluation.
Immunohistochemistry
Frozen sliced sections of DRG (10 µm) and spinal nerve (10 µm) were serially mounted. Selected sections (at least 120 µm apart from each other if from the same DRG) were incubated with primary reagents 24 h at 4 °C, and with the secondary antibody for 2 hrs at room temperature. Primary antisera were: monoclonal mouse anti-neurofilament 200 (1:5,000; Sigma, MO), polyclonal goat anti-CTB IgG (1:5,000; List, CA), and polyclonal rabbit anti-CGRP/NPY (1:10,000; Peninsula Laboratories), goat anti-Caspase-3 (1:5,000; Abcam), polyclonal rabbit anti-ATF3 (1:10,000; Santa Cruz Biotec., CA). Secondary antisera were Cy3-conjugated goat anti-rabbit IgG (1:1000; Jackson Laboratories, West Grove, PA), Alexa Fluor 488 conjugated goat anti-mouse or donkey anti-goat IgG (1:1,000; Molecular Probes). For IB4 binding, tissue sections were incubated with a FITC-conjugated IB4 (1:1000; Vector Laboratories, Burlingame, CA) in PBS for 2 hrs at room temperature. Following PBS washes, sections were dried and sealed with fluorescent mounting medium (Vector Laboratories). Fluorescence images were acquired with a Hamamatsu digital camera attached to an Olympus fluorescence microscope and saved as TIFF files. Counting was performed with MetaMorph image analysis software (Molecular Devices, Sunnyvale, CA).
Nickel-enhanced DAB was used for caspase-3 labeling. Sections were washed in PBS for 30 min after primary incubation, then incubated in 1% NGS-PBS with 1:500 biotinylated horse anti-goat IgG secondary antiserum (Vector Labs Inc., Burlingame, CA) 2hr, then avidin-biotin horseradish peroxidase complex (ABC Kit, Vector) for 90 min. The reaction product was developed in diaminobenzidine (SigmaFast, DAB tablets with metal enhancer, Sigma Chem. Co., St. Louis, MO) solution for 10 min, then washed repeatedly in PBS, ethanol dehydrated, cleared in xylene, and coverslipped with permount.
Image analysis
For counts of the percentage of the labeled neurons in DRG sections, three sections (apart at least 120 µm) from each DRG were randomly selected. In each section, a minimum of 150 cells were counted and the percentage of cells expressing each label were determined by counting the number of immunoreactive profiles and the total number of neuronal profiles as determined by DAPI nuclear staining. The mean percent and SEM were thus determined based on these samples. To determine the size of CTB and tetramethylrhodamine-dextran labled sensory neurons, three sections (apart at least 120 µm) from each DRG were randomly selected. In each section, a minimum of 50 labeled cells with clear nuclei were assessed and then the mean diameter was averaged by the longest and the shortest diameters [25]. A total of three animals per treatment group were studied by an experimenter under blinded conditions.
Data analysis
For behavioral studies, comparisons within treatments over time were performed by ANOVA followed by Fisher’s least significant difference test. Significant decreases in withdrawal thresholds to mechanical stimuli or latencies to thermal stimuli from the baseline values indicated tactile and thermal hypersensitivity, respectively. Comparisons between treatments were performed by 2-factor ANOVA. For imaging studies, counts of profiles for each biomarker obtained from DRG were averaged for each treatment group. Differences from the sham-operated, vehicle-treated group were detected by ANOVA followed by Fisher’s least significant difference test. In all analyses, significance was determined at p ≥ 0.05.
Results
Systemic artemin reverses nerve injury-induced hypersensitivity and promotes the recovery of mechanical responsiveness after nerve crush
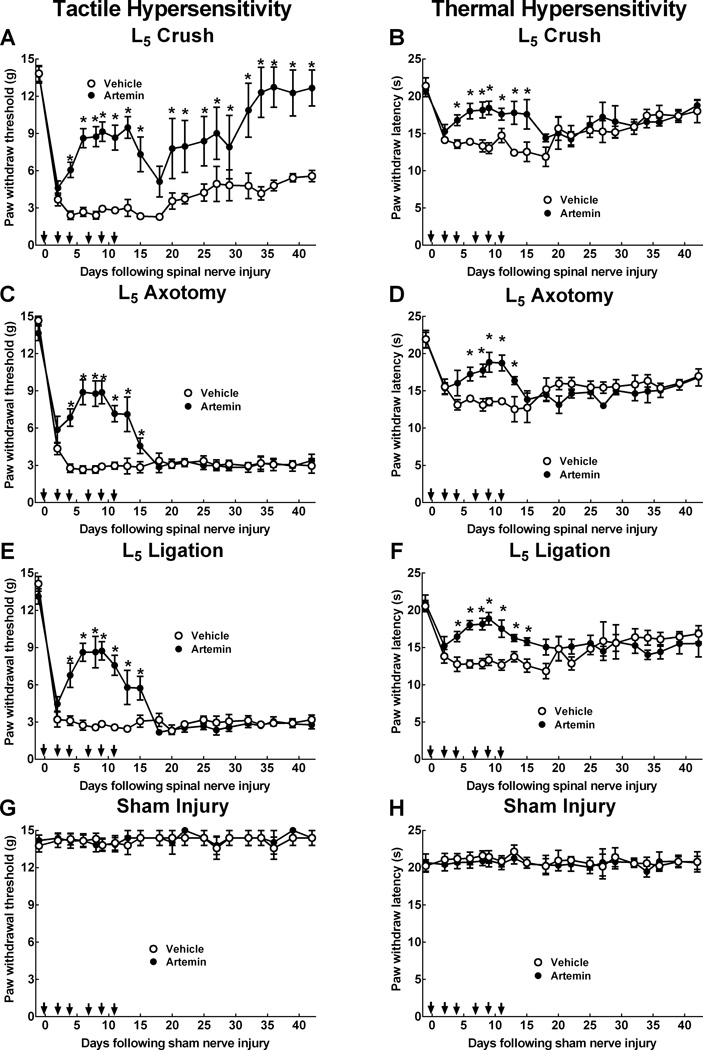
L5 spinal nerve crush, axotomy or ligation induced tactile (Fig. 1A,C,E) and thermal (Fig. 1B,D,F) hypersensitivity within 2 days after the injury. While tactile hypersensitivity persisted for the duration of the study, thermal hypersensitivity gradually progressed towards returned to pre-injury values by the end of the testing period, consistent with our previous findings [26]. Sensory thresholds increased towards pre-injury (baseline) levels within the first week of artemin treatment in each injury model; however, this effect was temporary since injury-induced tactile and thermal hypersensitivity were reestablished within 1 week of discontinuation of the artemin treatment. Continued testing revealed a time-dependent normalization of tactile sensory thresholds in rats treated with artemin with tactile sensory thresholds returning to pre-injury values by 6 weeks post-injury following nerve crush (Fig. 1A). This effect was not observed in artemin treated rats with nerve axotomy or ligation (Fig. 1C,E). In contrast, thermal hypersensitivity during this time period was not altered by the earlier artemin treatment after crush, axotomy or ligation injury (Fig. 1B,D,F). Sham-operated rats did not demonstrate any significant changes in tactile or thermal sensory thresholds (Fig. 1G, H).
Figure 1.
Systemic artemin promotes recovery of tactile and thermal hypersensitivity following peripheral nerve crush, axotomy or ligation. The rats received subcutaneous injections of 1mg/kg artemin on a schedule of MWF (arrows) or vehicle for two weeks. Artemin treated rats showed partial reversal of L5 nerve-crush induced tactile (A) and thermal hypersensitivity (B) by the end of the first week of treatment. Tactile hypersensitivity returned by the first week following termination of artemin treatment, followed by a gradual recovery of sensory thresholds to pre-injury values across the remaining test period (A). Thermal hypersensitivity returned by the first week following termination of artemin treatment, followed by a gradual return of sensory thresholds towards pre-injury values in both vehicle and artemin treated rats (B). Artemin treated rats showed partial reversal of L5 axotomy-induced tactile (C) and thermal (D) hypersensitivity by the end of the first week of treatment. Tactile and thermal hypersensitivity returned following termination of artemin treatment and persisted for the remainder of the testing period. Artemin treated rats showed partial reversal of L5 ligation-induced tactile (E) and thermal (F) hypersensitivity by the end of the first week of treatment. Tactile and thermal hypersensitivity returned following termination of artemin treatment and persisted for the remainder of the testing period. sham-operated rats did not demonstrate any significant changes in tactile (G) or thermal (H) sensory thresholds either with artemin or vehicle treatment. Error bars indicate mean ± SEM. n = 8 per group. Asterisks indicate significance (P < 0.05) between artemin and vehicle treated groups.
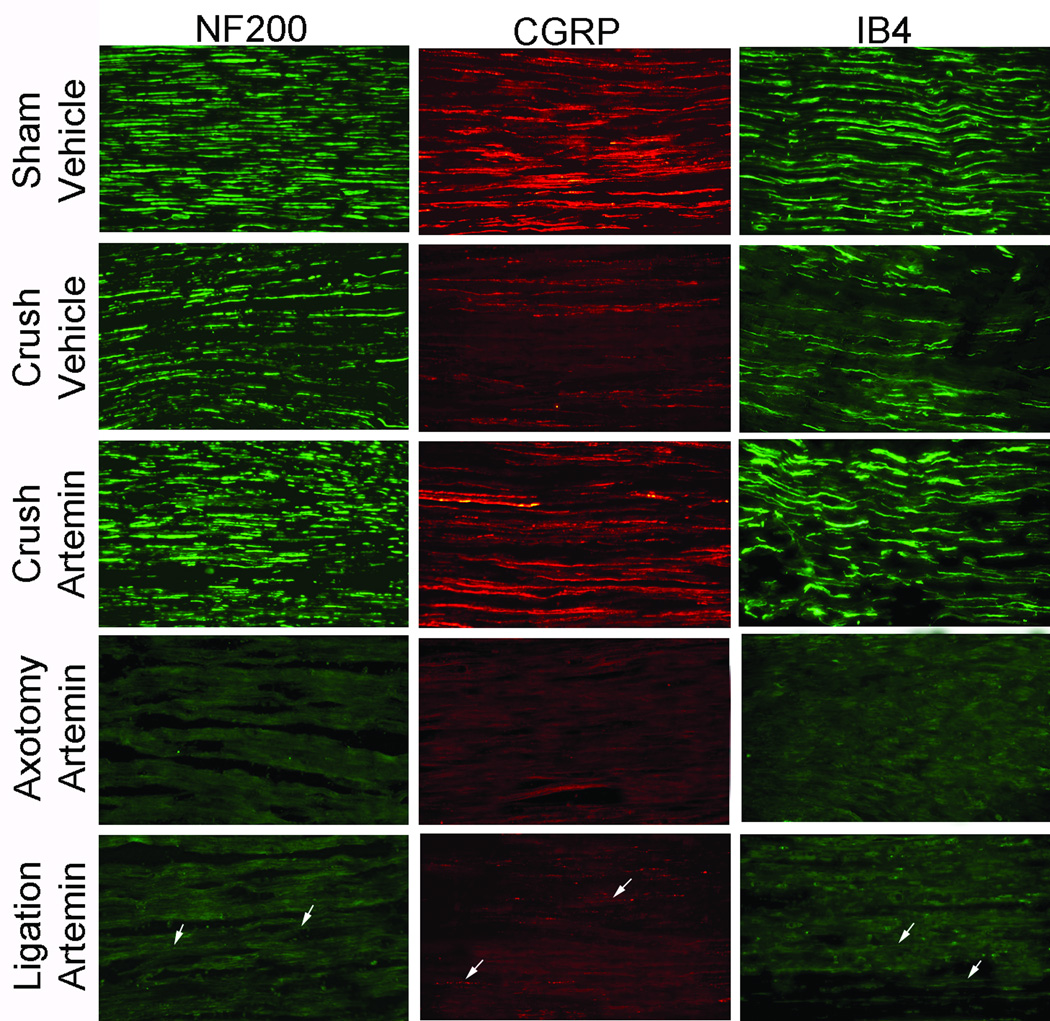
Systemic artemin normalizes nerve-crush induced fiber labeling distal to injury site
Six weeks following nerve crush injury, vehicle treated rats had diminished IB4, CGRP and NF200 labeling of the L5 spinal nerve distal to injury compared to the sham-operated control group (Fig. 2, rows 1 and 2). Tissues from artemin treated rats showed increased NF200, CGRP, and IB4 immunolabeling in nerve sections distal to crush injury (Fig. 2, Row 3). However, an absence (Fig. 2, rows 4) or very low level (Fig. 2, rows 5) of immunolabeling for NF200, CGRP or IB4 was observed in the L5 spinal nerve distal to the injury site following axotomy or ligation, respectively, even with artemin-treatment (Fig. 2, rows 4 and 5).
Figure 2.
Systemic artemin across 2 weeks immediately following injury restores neurochemical alterations in sensory fibers 6 weeks post- L5 spinal nerve crush. Artemin treatment after nerve crush increased NF200, CGRP and IB4 labeled fibers observed in L5 spinal nerve distal to crush region. Axotomy or nerve ligation of the L5 spinal nerve greatly diminished NF200, CGRP and IB4 labeling in the spinal nerve distal to nerve injury. Labeling after L5 ligation was sparse after artemin treatment, indicated by arrows, and labeling after axotomy was not affected by artemin treatment. Scale bar indicates 20 µm for all images.
Systemic artemin normalizes markers of nerve crush-induced axon injury and apoptosis
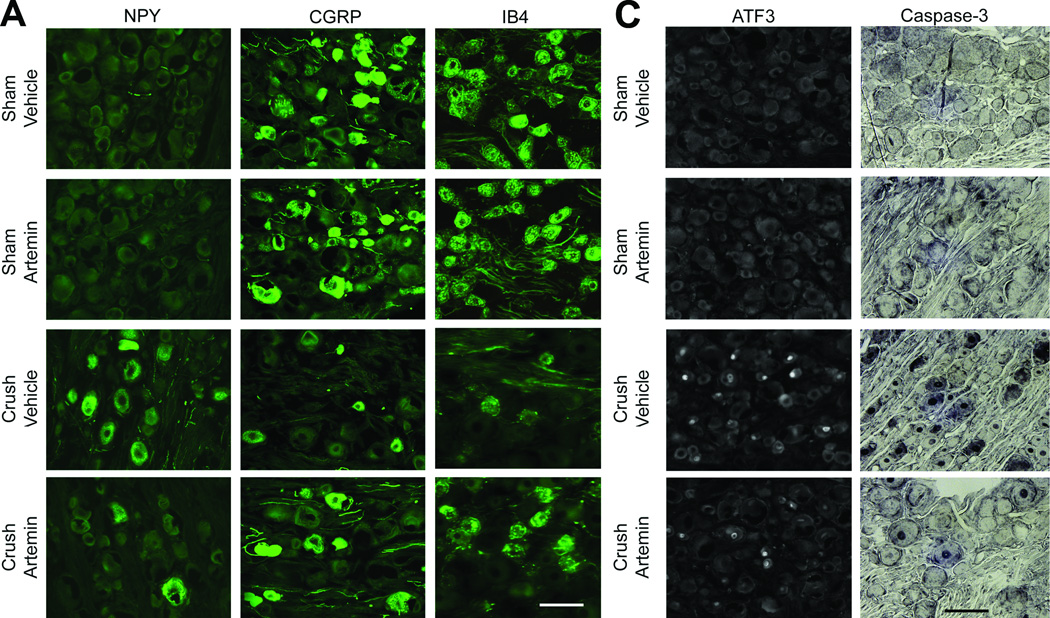
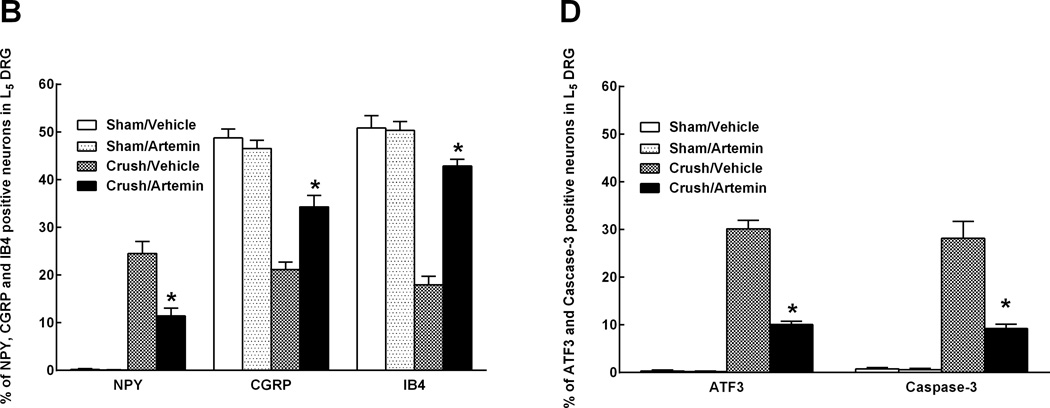
Nerve crush injury of the L5 spinal nerve resulted in apparent de novo expression of NPY in the L5 DRG, along with fewer neuronal profiles expressing CGRP or IB4 (Fig. 3A). Artemin treatment reduced the number of neuronal profiles expressing NPY and increased the number of neuronal profiles expressing CGRP and IB4 in rats with nerve crush (Fig. 3A, graph). Artemin did not alter expression of NPY, CGRP or IB4 in L5 DRG of sham-treated rats (Fig. 3A).
Figure 3.
Artemin blocks neurochemical signs in the DRG of nerve crush-induced neuropathy 6 weeks following injury. A. Nerve crush resulted in increased NPY labeling and decreased CGRP as well as IB4 labeling within the ipsilateral L5 DRG. Artemin attenuated the nerve-crush induced increase in NPY and the decreased CGRP and IB4 labeling. B. The graph shows quantification of NPY, CGRP and IB4 labeled neuronal profiles in the ipsilateral L5 DRG. The counts confirm that artemin ameliorated the nerve-crush induced increase in NPY and the decreased CGRP and IB4 labeling. Asterisks indicate significant differences (P < 0.05) compared to vehicle treatment after nerve crush. C. Nerve crush induced ATF3 labeled neuronal profiles were markedly reduced with artemin treatment. A similar decrease in nerve-crush induced caspase-3 labeled neuronal profiles was observed in artemin treated rats. Scale bar indicates 50 µm for all images. D. The graph shows quantification of ATF3 and caspase-3 labeled neuronal nuclei from counted sections confirming that artemin decreased the percentage of ATF3 and caspase-3 labeled neurons following nerve crush. Asterisks indicates significant differences (P < 0.05) compared to vehicle treatment after nerve crush.
Nerve crush injury of the L5 spinal nerve resulted in increased expression of both ATF3, a marker of axon-injured sensory neurons, and caspase-3, a marker for apoptosis and programmed cell death, in L5 DRG neuronal profiles (Fig. 3B). Artemin treatment decreased the number of neuronal profiles expressing ATF3 and caspase-3 (Fig. 3B, graph). ATF3 or caspase-3 was absent in L5 DRG obtained from sham-operated rats irrespective of treatment (Fig. 3B).
Systemic artemin promotes axonal regeneration of sensory fibers across nerve crush site
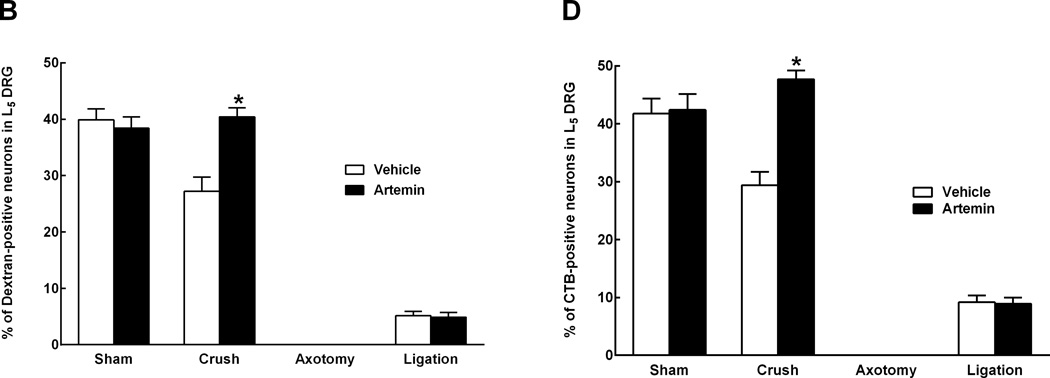
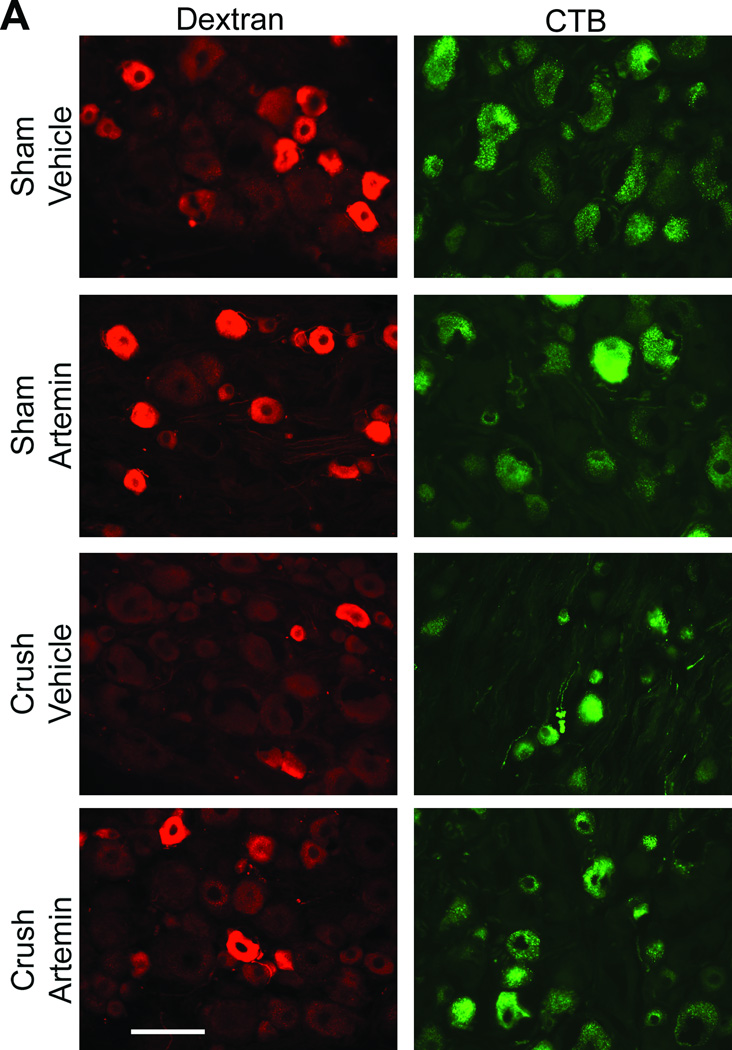
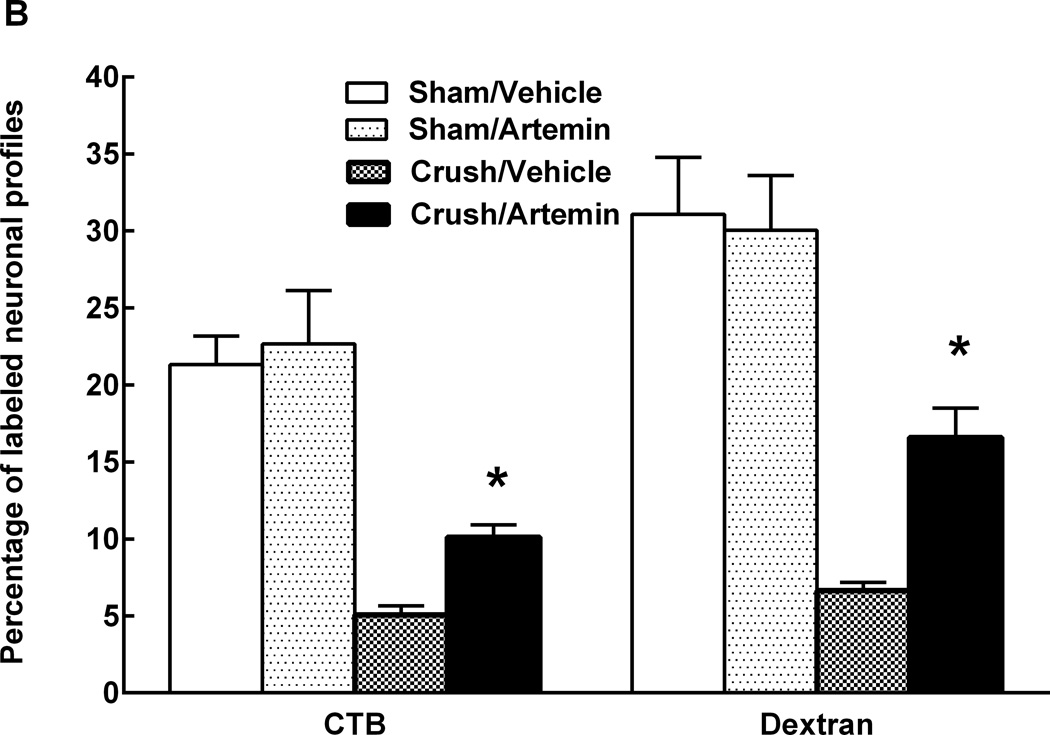
In animals with L5 crush and receiving rhodamine-dextran or CTB injection to the sciatic nerve, artemin treatment returned L5 DRG expression of both rhodamine-dextran (Fig 4A) and CTB (Fig 4B) to levels seen in sham-operated rats. Artemin failed to alter the diminished labeling of rhodamine-dextran or CTB within the L5 DRG of axotomy or ligation treated rats (Fig 4A,B). Counts of neuronal profiles labeled with rhodamine-dextran (Fig 4A, graph) or CTB (Fig 4B, graph) confirmed that artemin selectively restored labeling of the retrograde markers in the rats with L5 crush injury. Artemin did not alter rhodamine-dextran or CTB labeling in sham-operated rats. Approximately 92% and 90% of the CTB-labeled DRG neurons obtained from sham-operated rats receiving saline or artemin treatment, respectively, were greater than 30 µm in diameter, suggesting that nearly all of these neurons were medium to large myelinated afferents. Nerve crush injury reduced the proportion of CTB-labeled neurons greater than 30 µm in diameter to 51% in saline treated rats, suggesting an increase in small-diameter fibers taking up CTB relative to the medium to large-diameter myelinated afferents. Approximately 76% of the CTB-labeled DRG neurons were greater than 30 µm in diameter in the artemin-treated group, suggesting a normalization of these populations by artemin. In contrast, the proportions of DRG neurons labeled with rhodamine-dextran applied distal to the crush injury did not change. Neurons with diameters less than 30 µm accounted for 79% and 76% of the rhodamine-dextran labeled L5 DRG cells obtained from vehicle- or artemin-treated sham-operated rats. After nerve crush injury with saline or artemin treatment, the proportions of rhodamine-labeled neurons less than 30 µm in diameter were 82% and 77%, respectively. Possible regeneration of sensory fibers into target cutaneous tissues was determined by intradermal administration of rhodamine-dextran or CTB into the hindpaw ipsilateral to the nerve injury. Artemin attenuated nerve crush-induced decreases in rhodamine-dextran and CTB labeling in L5 DRG neuronal profiles (Fig. 5A). Quantification of labeled neuronal profiles confirmed artemin attenuated crush-induced reduction in rhodamine-dextran and CTB expression following crush (Fig. 5B).
Figure 4.
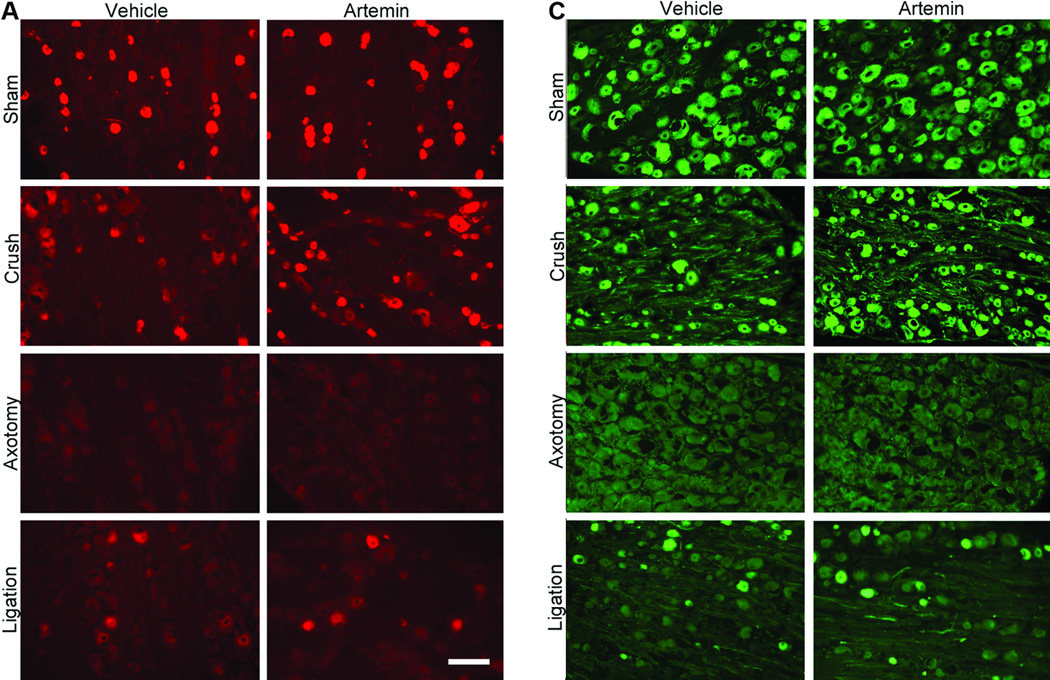
Artemin promotes regeneration of non-myelinated and myelinated axons through nerve crush injury into sciatic nerve 6 weeks post L5 spinal nerve crush. A. Artemin or vehicle treatment did not markedly change the number of cell bodies within the L5 DRG showing retrograde labeling of dextran from sciatic nerve in sham-operated rats, corresponding to non-myelinated fibers. Artemin treatment increased dextran labeling in L5 DRG of rats treated with nerve crush, but not in rats that had undergone axotomy or nerve ligation. Scale bar indicates 50 µm for all images. B. The graph shows quantification of dextran-labeled neurons from counted sections in percentages. Asterisks indicates significant differences (P < 0.05) compared to vehicle treatment with the same surgery. C. Artemin or vehicle treatment did not markedly change the number of cell bodies within the L5 DRG showing retrograde labeling of CTB from sciatic nerve in sham-operated rats, corresponding to myelinated fibers. Artemin treatment increased CTB labeling in L5 DRG of rats treated with nerve crush, but not in rats that had undergone axotomy or nerve ligation. Scale bar indicates 50 µm for all images. D. The graph shows quantification of CTB-labeled neurons from counted sections in percentages. Asterisks indicate significant differences (P < 0.05) compared to vehicle treatment with the same surgery.
Figure 5.
Artemin promotes reinnervation by non-myelinated and myelinated fibers of peripheral cutaneous tissues. A. Rhodamine-dextran or CTB labeling in L5 DRG 1 week following intradermal administration into the ipsilateral hindpaw, 6 weeks following sham or nerve crush. Artemin treatment did not alter rhodamine-dextran or CTB labeling in sham-operated rats. Artemin treatment increased rhodamine-dextran as well as CTB labeling in L5 DRG of nerve crush treated rats compared to vehicle treated rats. Scale bar indicates 50 µm for all images. B. Quantification of rhodamine-dextran and CTB labeled neurons from L5 DRG sections confirm an increase in the percentage of cells labeled for rhodamine-dextran or CTB with artemin treatment. Asterisks indicate significant differences (P < 0.05) compared to vehicle treatment after nerve crush.
Discussion
These findings demonstrate that intermittent artemin administration over 2 weeks 1) transiently reverses nerve injury induced thermal and tactile hypersensitivity during treatment following nerve crush, axotomy or ligation; 2) promotes normalization of neurochemical markers of neuropathic pain following nerve crush; 3) blocks markers of axonal damage and apoptosis in cell bodies of the ipsilateral DRG following nerve crush, and 4) promotes peripheral nerve regeneration by 4 weeks post-artemin treatment following nerve crush. These effects are accompanied by a time-dependent functional recovery as illustrated by reversal of tactile hypersensitivity 6 weeks following crush injury but not axotomy or ligation injury. This time-point corresponds to the normalization of neurochemical markers and evidence of peripheral nerve regeneration.
Artemin-induced transient reversal of nerve-injury induced thermal and tactile hypersensitivity in the groups with axotomy or nerve ligation suggests that the mechanisms that promote these responses are unrelated to nerve regeneration to distal territories. This conclusion is supported by the persistent re-establishment of tactile and thermal hypersensitivity following termination of artemin treatment after either nerve ligation or axotomy and the absence of immunohistochemical evidence of peripheral nerve regeneration at 6 weeks following axotomy.It should be noted that immunofluorescent imaging also revealed that a few peripheral fibers appear to escape axotomy during the ligation injury and thus apparently can be rescued by artemin treatment, as indicated by label for NF200, CGRP or IB4 distal to the ligation in tissue from these animals. However, such labeling is sparse after ligation whereas it is abundant after crush injury. These findings are consistent with previous reports demonstrating that artemin prevents or reverses neuropathic pain behaviors induced by nerve injury during artemin treatment [9; 27]. Artemin treatment has been demonstrated to normalize both peripheral and central neurochemical changes associated with nerve injury at time-points corresponding to alleviation of evoked hypersensitivity [9]. In our previous studies [9], artemin normalized spinal nerve ligation-induced neurochemical changes in sensory fibers proximal to the DRG. Notably, these effects of artemin were observed on multiple fiber types, including small, unmyelinated C-fibers, with normalization both in CGRP containing peptidergic as well IB-4 binding and P2X3 expressing non-peptidergic fibers [9]. Normalization of neurochemical changes was also observed in myelinated fibers demonstrating increased NPY expression [9], which is selectively up-regulated in medium to large diameter sensory neurons and implicated in tactile hypersensitivity [22]. These results contrast with the observations of Bennett and colleagues [5], who found that artemin did not change NPY upregulation in large-diameter DRG neurons. However, in that study, human, instead of rat, artemin was given by spinal infusion for 2 weeks and nerve injury was induced by L5 ligation and axotomy [5]. In the present study, the rat homolog was given on a repeated systemic injection schedule. These methodological differences may explain the differences in outcomeOne possible mechanism that may underlie these transient functional effects of artemin could be related to remodeling of the nerve injured membrane, perhaps through effects on channels mediating excitability including the NaV1.8 sodium channel [9] that has been implicated in driving nerve-injury induced pain [10; 16]. Within the spinal dorsal horn, artemin was previously shown to normalize nerve injury-induced enhanced capsaicin-evoked release of CGRP [9], indicating blockade of amplified signaling within the spinal cord as well as to normalize nerve-injury induced up-regulation of spinal dynorphin [9] implicated in maintaining neuropathic pain [17; 18]. We suggest that the normalization of such endpoints associated with the neuropathic pain state likely distinguishes artemin from systemically administered drugs that do not reverse injury induced pathophysiological changes [9; 24].
Although artemin-induced alleviation of hypersensitivity and normalization of pathophysiological changes appear to occur only during treatment in the ligation and axotomy treatment groups, a gradual and persistent effect of the 2-week treatment was observed in animals that had received nerve crush. Thus, a limited period of artemin administration likely promotes a time-related peripheral nerve regeneration and re-innervation to cutaneous tissue within the ipsilateral hindpaw that corresponds to time-points with recovery of nerve-injury induced hypersensitivity. These findings extend previous reports demonstrating that artemin treatment following dorsal root crush promotes regeneration of sensory nerve fibers through the dorsal root entry zone into the spinal cord [27]. Moreover, systemic artemin restores synaptic function of sensory fibers within the spinal cord that corresponds to recovery of sensory and motor function [27]. Subsequent studies demonstrated that administration of artemin following dorsal root crush promotes topographically specific regeneration, with sensory neurons projecting to appropriate lamina within the spinal cord [11]. While the present study did not include definitive measures to determine whether artemin induced a true nerve regeneration effect in contrast to a nerve-sparing effect preventing degeneration, the previous investigations taken together support a likely regenerative effect of artemin [9; 11; 27], and argue in favor of this interpretation.
The mechanisms underlying artemin-induced regeneration of injured axons are unknown. Artemin binds preferentially to the GFRα3 receptor forming a complex with the RET tyrosine kinase, and thus activate intracellular signaling cascades to promote nerve growth [2; 3; 21; 24]. Artemin supports survival of cultured sensory and sympathetic neurons [24], and promotes regeneration of peripheral sensory or motor nerves following injury [8; 9; 11; 27; 29]. Consistent with this, artemin blocked expression of caspase-3, a marker of apoptosis, as well as ATF3, a marker of axonal damage following nerve crush in the present investigation. Since the expression of the GFRα3 co-receptor is largely restricted to the peripheral nervous system and expressed primarily on unmyelinated, small-diameter nociceptive fibers [2; 3; 21], it may seem unlikely that artemin may be expected to promote regeneration or recovery of large-fiber primary afferents. However, several studies employing either immunohistochemistry or in situ hybridization techniques for message for GFRα3 indicate that 1% to 14% of large-fiber myelinated peripheral DRG neurons express this receptor [4; 11; 13; 21; 27]. In addition, peripheral nerve injury is associated with a marked upregulation of GFRα3, of up to 59%, and that this increase occurs across all classes of peripheral nerves, including the large-diameter neurons [4; 9; 12; 28]. In a recent study, we showed regeneration of large-diameter myelinated fibers through the DREZ and to the nucleus cuneatus, a target of the peripheral Aβ fibers [28]. This regeneration was accompanied by recovery of behavioral and electrophysiologic measures of sensorimotor function, thus clearly showing an effect of artemin on large-fiber function [28]. An additional possibility is that Schwann cells, which also express the GFRα3 as well as the GFRα1 receptor [20] and support peripheral nerve regeneration in part by the secretion of artemin and GDNF in the region of the growth cone [8] might also play a role in artemin-mediated nerve regeneration by stimulation of release of these neurotrophic factors. Finally, whereas the GFRα3 receptor is the preferred receptor for artemin, artemin can nonetheless activate the GFRα1 receptor as well, although to a lesser extent [2; 3]. For example, artemin supports the growth of GFRα1 receptor-expressing neurons, and activates the GFRα1-RET receptor complex as well as the GFRα3-Ret receptor complex in transfected cells [2; 3]. Since nerve injury substantially upregulates GFRα1 receptors in DRG neurons, including large-diameter neurons, then artemin might promote regeneration of large diameter nerves through the increased availability of the GFRα1, as well as by acting on the small population of large diameter neurons expressing GFRα3. Our results demonstrate that intermittent administration of artemin across 2 weeks blocks nerve-injury induced tactile and thermal hypersensitivity and promotes peripheral nerve regeneration following nerve crush. These effects of artemin on sensory neurons, paired with the restricted expression of GFRα3 to nociceptive neurons, indicate that artemin might be an effective treatment for nerve-injury induced pain, without having broader effects that may lead to side effects observed following GDNF administration [15; 19; 24]. The protective effects of artemin in conjunction with reversal of nerve-injury induced neurochemical changes indicate that artemin goes beyond symptom-based treatment observed with currently available drugs for neuropathic pain. Moreover, it is emphasized that most peripheral nerve injures in humans are partial in nature more closely resembling the crush, rather than axotomy injury used here [14]. For this reason, in situations where regeneration is possible, our data indicate that artemin will promote regeneration of peripheral nerves resulting in persistent recovery of function and alleviation of pain.
Acknowledgments
DWYS and AR were previously employed by BiogenIdec Inc. This research was supported in part by a NIH/NINDS grant R01NS/066958 to FP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare that there are no conflicts of interest.
Systemic artemin normalized sensory responses and neuronal immunofluorescent markers in rats with sciatic nerve crush. Labeling indicated regeneration of peripheral fibers across the injury site.
References
- 1.Allodi I, Udina E, Navarro X. Specificity of peripheral nerve regeneration: interactions at the axon level. Progress in neurobiology. 2012;98(1):16–37. doi: 10.1016/j.pneurobio.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 2.Baloh RH, Gorodinsky A, Golden JP, Tansey MG, Keck CL, Popescu NC, Johnson EM, Jr, Milbrandt J. GFRalpha3 is an orphan member of the GDNF/neurturin/persephin receptor family. Proc Natl Acad Sci U S A. 1998;95(10):5801–5806. doi: 10.1073/pnas.95.10.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baloh RH, Tansey MG, Lampe PA, Fahrner TJ, Enomoto H, Simburger KS, Leitner ML, Araki T, Johnson EM, Jr, Milbrandt J. Artemin, a novel member of the GDNF ligand family, supports peripheral and central neurons and signals through the GFRalpha3-RET receptor complex. Neuron. 1998;21(6):1291–1302. doi: 10.1016/s0896-6273(00)80649-2. [DOI] [PubMed] [Google Scholar]
- 4.Bennett DL, Boucher TJ, Armanini MP, Poulsen KT, Michael GJ, Priestley JV, Phillips HS, McMahon SB, Shelton DL. The glial cell line-derived neurotrophic factor family receptor components are differentially regulated within sensory neurons after nerve injury. J Neurosci. 2000;20(1):427–437. doi: 10.1523/JNEUROSCI.20-01-00427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennett DL, Boucher TJ, Michael GJ, Popat RJ, Malcangio M, Averill SA, Poulsen KT, Priestley JV, Shelton DL, McMahon SB. Artemin has potent neurotrophic actions on injured C-fibres. J Peripher Nerv Syst. 2006;11(4):330–345. doi: 10.1111/j.1529-8027.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- 6.Bespalov MM, Sidorova YA, Tumova S, Ahonen-Bishopp A, Magalhaes AC, Kulesskiy E, Paveliev M, Rivera C, Rauvala H, Saarma M. Heparan sulfate proteoglycan syndecan-3 is a novel receptor for GDNF, neurturin, and artemin. The Journal of cell biology. 2011;192(1):153–169. doi: 10.1083/jcb.201009136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boucher TJ, Okuse K, Bennett DL, Munson JB, Wood JN, McMahon SB. Potent analgesic effects of GDNF in neuropathic pain states. Science. 2000;290(5489):124–127. doi: 10.1126/science.290.5489.124. [DOI] [PubMed] [Google Scholar]
- 8.Fontana X, Hristova M, Da Costa C, Patodia S, Thei L, Makwana M, Spencer-Dene B, Latouche M, Mirsky R, Jessen KR, Klein R, Raivich G, Behrens A. c-Jun in Schwann cells promotes axonal regeneration and motoneuron survival via paracrine signaling. The Journal of cell biology. 2012;198(1):127–141. doi: 10.1083/jcb.201205025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gardell LR, Wang R, Ehrenfels C, Ossipov MH, Rossomando AJ, Miller S, Buckley C, Cai AK, Tse A, Foley SF, Gong B, Walus L, Carmillo P, Worley D, Huang C, Engber T, Pepinsky B, Cate RL, Vanderah TW, Lai J, Sah DW, Porreca F. Multiple actions of systemic artemin in experimental neuropathy. Nat Med. 2003;9(11):1383–1389. doi: 10.1038/nm944. [DOI] [PubMed] [Google Scholar]
- 10.Gold MS, Weinreich D, Kim CS, Wang R, Treanor J, Porreca F, Lai J. Redistribution of Na(V)1.8 in uninjured axons enables neuropathic pain. J Neurosci. 2003;23(1):158–166. doi: 10.1523/JNEUROSCI.23-01-00158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvey P, Gong B, Rossomando AJ, Frank E. Topographically specific regeneration of sensory axons in the spinal cord. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(25):11585–11590. doi: 10.1073/pnas.1003287107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harvey P, Gong B, Rossomando AJ, Frank E. Topographically specific regeneration of sensory axons in the spinal cord. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(25):11585–11590. doi: 10.1073/pnas.1003287107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Josephson A, Widenfalk J, Trifunovski A, Widmer H-R, Olson L, Spenger C. GDNF and NGF family members and receptors in human fetal and adult spinal cord and dorsal root ganglia. The Journal of Comparative Neurology. 2001;440(2):204–217. doi: 10.1002/cne.1380. [DOI] [PubMed] [Google Scholar]
- 14.Kim DH, Murovic JA, Tiel R, Kline DG. Management and outcomes in 353 surgically treated sciatic nerve lesions. Journal of neurosurgery. 2004;101(1):8–17. doi: 10.3171/jns.2004.101.1.0008. [DOI] [PubMed] [Google Scholar]
- 15.Kordower JH, Palfi S, Chen EY, Ma SY, Sendera T, Cochran EJ, Cochran EJ, Mufson EJ, Penn R, Goetz CG, Comella CD. Clinicopathological findings following intraventricular glial-derived neurotrophic factor treatment in a patient with Parkinson's disease. Annals of neurology. 1999;46(3):419–424. doi: 10.1002/1531-8249(199909)46:3<419::aid-ana21>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 16.Lai J, Gold MS, Kim CS, Bian D, Ossipov MH, Hunter JC, Porreca F. Inhibition of neuropathic pain by decreased expression of the tetrodotoxin-resistant sodium channel, NaV1.8. Pain. 2002;95(1–2):143–152. doi: 10.1016/s0304-3959(01)00391-8. [DOI] [PubMed] [Google Scholar]
- 17.Lai J, Luo MC, Chen Q, Ma S, Gardell LR, Ossipov MH, Porreca F. Dynorphin A activates bradykinin receptors to maintain neuropathic pain. Nat Neurosci. 2006;9(12):1534–1540. doi: 10.1038/nn1804. [DOI] [PubMed] [Google Scholar]
- 18.Luo MC, Chen Q, Ossipov MH, Rankin DR, Porreca F, Lai J. Spinal dynorphin and bradykinin receptors maintain inflammatory hyperalgesia. J Pain. 2008;9(12):1096–1105. doi: 10.1016/j.jpain.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER, Jr, Lozano AM, Penn RD, Simpson RK, Jr, Stacy M, Wooten GF. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60(1):69–73. doi: 10.1212/wnl.60.1.69. [DOI] [PubMed] [Google Scholar]
- 20.Onochie CI, Korngut LM, Vanhorne JB, Myers SM, Michaud D, Mulligan LM. Characterisation of the human GFRalpha-3 locus and investigation of the gene in Hirschsprung disease. Journal of medical genetics. 2000;37(9):674–679. doi: 10.1136/jmg.37.9.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orozco OE, Walus L, Sah DW, Pepinsky RB, Sanicola M. GFRalpha3 is expressed predominantly in nociceptive sensory neurons. Eur J Neurosci. 2001;13(11):2177–2182. doi: 10.1046/j.0953-816x.2001.01596.x. [DOI] [PubMed] [Google Scholar]
- 22.Ossipov MH, Zhang ET, Carvajal C, Gardell L, Quirion R, Dumont Y, Lai J, Porreca F. Selective mediation of nerve injury-induced tactile hypersensitivity by neuropeptide Y. J Neurosci. 2002;22(22):9858–9867. doi: 10.1523/JNEUROSCI.22-22-09858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramer MS, Priestley JV, McMahon SB. Functional regeneration of sensory axons into the adult spinal cord. Nature. 2000;403(6767):312–316. doi: 10.1038/35002084. [DOI] [PubMed] [Google Scholar]
- 24.Sah DW, Ossipov MH, Rossomando A, Silvian L, Porreca F. New approaches for the treatment of pain: the GDNF family of neurotrophic growth factors. Curr Top Med Chem. 2005;5(6):577–583. doi: 10.2174/1568026054367593. [DOI] [PubMed] [Google Scholar]
- 25.Shehab SA, Spike RC, Todd AJ. Evidence against cholera toxin B subunit as a reliable tracer for sprouting of primary afferents following peripheral nerve injury. Brain Res. 2003;964(2):218–227. doi: 10.1016/s0006-8993(02)04001-5. [DOI] [PubMed] [Google Scholar]
- 26.Wang R, King T, De Felice M, Guo W, Ossipov MH, Porreca F. Descending facilitation maintains long-term spontaneous neuropathic pain. J Pain. doi: 10.1016/j.jpain.2013.02.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang R, King T, Ossipov MH, Rossomando AJ, Vanderah TW, Harvey P, Cariani P, Frank E, Sah DW, Porreca F. Persistent restoration of sensory function by immediate or delayed systemic artemin after dorsal root injury. Nat Neurosci. 2008;11(4):488–496. doi: 10.1038/nn2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang R, King T, Ossipov MH, Rossomando AJ, Vanderah TW, Harvey P, Cariani P, Frank E, Sah DW, Porreca F. Persistent restoration of sensory function by immediate or delayed systemic artemin after dorsal root injury. Nature neuroscience. 2008;11(4):488–496. doi: 10.1038/nn2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Widenfalk J, Wu W, Hao J, Person JK, Wiesenfeldt-Hallin Z, Risling M. Treatment of transected peripheral nerves with artemin improved motor neuron regeneration, but did not reduce nerve injury-induced pain behaviour. Scand J Plast Reconstr Surg Hand Surg. 2009;43(5):245–250. doi: 10.3109/02844310903259082. [DOI] [PubMed] [Google Scholar]