Abstract
Significance: Radiation therapy is widely used for treatment of prostate cancer. Radiation can directly damage biologically important molecules; however, most effects of radiation-mediated cell killing are derived from the generated free radicals that alter cellular redox status. Multiple proinflammatory mediators can also influence redox status in irradiated cells and the surrounding microenvironment, thereby affecting prostate cancer progression and radiotherapy efficiency. Recent Advances: Ionizing radiation (IR)–generated oxidative stress can regulate and be regulated by the production of proinflammatory mediators. Depending on the type and stage of the prostate cancer cells, these proinflammatory mediators may lead to different biological consequences ranging from cell death to development of radioresistance. Critical Issues: Tumors are heterogeneous and dynamic communication occurs between stromal and prostate cancer cells, and complicated redox-regulated mechanisms exist in the tumor microenvironment. Thus, antioxidant and anti-inflammatory strategies should be carefully evaluated for each patient at different stages of the disease to maximize therapeutic benefits while minimizing unintended side effects. Future Directions: Compared with normal cells, tumor cells are usually under higher oxidative stress and secrete more proinflammatory mediators. Thus, redox status is often less adaptive in tumor cells than in their normal counterparts. This difference can be exploited in a search for new cancer therapeutics and treatment regimes that selectively activate cell death pathways in tumor cells with minimal unintended consequences in terms of chemo- and radio-resistance in tumor cells and toxicity in normal tissues. Antioxid. Redox Signal. 20, 1481–1500.
Introduction
Cancer is a major health issue throughout the world and accounts for about 25% of all deaths in the United States. Prostate cancer has accounted for ∼29% of newly diagnosed cancer cases and 9% of cancer deaths in men in 2012 (167). The common forms of treatment for prostate cancer are surgery, radiation, chemotherapy, and hormone management (181). Radiation therapy can be used to treat localized disease or as part of a curative therapy to prevent cancer recurrence after surgical removal of the primary tumor. Unfortunately, the disease recurs and progresses to an advanced stage in as many as 30%–40% of prostate cancer patients treated with radiation (181). Contributing factors that influence radiation therapy outcomes are as follows: the presence of radiation-resistant prostate cancer cells and cancer stem cells; the complexity of the tumor microenvironment, such as hypoxia; increased inflammatory cytokine and growth factor secretion; and elevated relevant receptor expression.
Consideration of the effects of radiation therapy should not be limited to isolated cells since the entire tissue plays a role in determining the response of individual cells to any regulatory or damaging signals (13, 148). The localized release of radiation energy generates free radicals, mainly by ionization of water, which constitutes about 80% of cell mass, and produces various reactive oxygen species (ROS). The ROS can then rapidly diffuse and react with other molecules to damage DNA, protein, and lipid targets. This ROS-mediated effect of ionizing radiation (IR) is suspected to have caused a majority of radiation-induced damage (13, 66). Different types of cells in tumor tissues are subjected to complex regulatory mechanisms depending on their interactions with other cells and cellular products in the microenvironment, such as interleukin-1β (IL-1β), IL-6, IL-8, tumor necrosis factor-alpha (TNF-α), and transforming growth factor-beta (TGF-β). Altered cytokine expression can alter many signaling pathways that converge on a few important transcription factors, including nuclear factor kappa B (NF-κB), activator protein-1 (AP-1), and signal transducers and activators of transcription (STATs). These transcription factors also upregulate the expression of several cytokines, such as IL-1β and TNF-α (105). Such positive feedback loops amplify radiation- or oxidative-stress-induced inflammation, which may persist chronically (156). Because ROS play crucial dual roles in inducing cancer development (initiation, promotion, and progression) and maintaining metabolic homeostasis, both prooxidant- and antioxidant-based agents have been developed for cancer prevention and treatment (63, 183).
This article reviews commonly elevated cytokines and growth factors, such as IL-6, IL-8, TNF-α, and TGF-β, as major mediators of IR response found in prostate cancer after radiation therapy, and discusses different redox signaling pathways and redox-sensitive transcription factors controlled by these proteins. The biological significance of this information can be particularly useful in understanding the development of cancer radioresistance and improving radiation therapeutic effects in humans.
Radiation in Prostate Cancer Treatment
IR and radiation therapy
Cancer radiotherapy is the medical use of IR to control or kill malignant cells. For prostate cancer treatment, radiation is most commonly given by an external source (external beam radiotherapy), but it may also be administered by inserting small radioactive seeds directly into the tumor (brachytherapy), which is appropriate for some men with early prostate cancer (181). Delivery of a lethal dose of radiation to a tumor lesion while minimizing damage to normal surrounding tissues is one of the major challenges of radiotherapy. The accuracy and precision of radiotherapy has improved as imaging technology has improved, especially the use of 3-dimensional conformal radiation therapy and intensity-modulated radiation therapy (164).
External beam radiotherapy can deliver two types of radiation that damage DNA and other macromolecules of cancerous cells: photons, such as X-rays and γ-rays, and charged particles, such as protons and electrons. X-rays are generated extranuclearly from X-ray machines whereas γ-rays are produced intranuclearly from radioactive materials. While they differ in the source of generation, X-rays and γ-rays share the same radiophysical properties (66, 113, 148) (Fig. 1). Direct action of IR is the interaction of radiation beams or particles with critical target molecules in cells, such as DNA, to cause various types of damage in DNA structure, leading to lethal chromosome aberrations (113). Indirect action of radiation is a multicellular effect by water radiolysis that produces free radicals, such as hydrated electrons (e−aq), ionized water (H2O+), hydroperoxyl radical (HO2•), hydrogen radical (H•), and hydroxyl radical (•OH), which can diffuse far enough to reach and damage the DNA, protein, and lipid targets (13, 66, 113, 148). Direct action and indirect action of IR are closely linked; for example, direct damage to DNA by IR can induce ROS generation via histone H2AX-mediated mechanisms that involve NADPH oxidase 1 (NOX1) and Ras-related C3 botulinum toxin substrate 1 (Rac1) GTPase (83). To a large extent, it is these free radicals that break chemical bonds, produce chemical changes, and initiate the chain of events that results in the final expression of biological damage.
FIG. 1.
Electromagnetic spectrum of radiation and medical use of ionizing radiation (IR). Direct action of IR leads to damages in DNA structure. Indirect action of radiation results from water radiolysis, which produces free radicals that can diffuse far enough to reach and damage the DNA.
It has been reported that intracellular ROS levels are quickly increased after exposure to IR and that elevated levels of ROS are sustained for several hours after initial IR exposure (32, 179). NOX is responsible, in part, for a late increase in intracellular superoxide generation after exposure to IR (32, 179). IR-induced mitochondrial dysfunction, especially decreased electron transport chain complex I activity, produces a feed forward loop that contributes to persistent oxidative stress after irradiation (198). Since the mitochondrion is the most important energy-generating organelle, mitochondrial dysfunction due to direct effects of IR or indirect effects mediated by ROS may result in alteration or adaptive responses of metabolic pathways involved in cancer development. Free radicals may amplify and prolong the deleterious effects of radiation, leading to chronic oxidative stress, alteration of multiple metabolic pathways, normal tissue injury, cell death, and other bystander effects [reviewed in (142)].
Radioresistance: an important impediment to prostate cancer treatment
The development of resistance to radiation is one of the worst obstacles of prostate cancer radiotherapy. Some of the molecular entities associated with radioresistance have been identified in prostate cancer (71, 88, 95, 171); however, the underlying mechanisms are still not well understood. While it is possible to increase radiation doses to a level that ensures complete irradiation of cancer, the use of higher doses of radiation may cause unacceptable serious side effects to normal tissues. Thus, it is important to develop strategies that can sensitize tumor cells to radiation treatment and/or can protect normal tissue from radiation damage.
Radiotherapy mainly induces cell death by generating oxidative stress, and cellular antioxidant status also affects normal tissue injury and tumor sensitivity to radiation treatment [reviewed in (71)]. Inhibiting prooxidant enzymes, such as cyclooxygenase-2 (75), and overexpression or upregulation of antioxidant enzymes, such as extracellular superoxide dismutase (ECSOD) (84) and heme oxygenase-1 (200), have been shown to protect against radiation-induced thoracic, lung, and skin injuries (141). Radiation-resistant mice have been shown to have higher levels of superoxide dismutase (SOD) and catalase activities compared with radiation-sensitive mice (69). Manganese superoxide dismutase (MnSOD) upregulation has been implicated in adaptive response induced by low or fractionated doses of IR, leading to radioresistance (71, 73). MnSOD is one of the most important antioxidant enzymes located exclusively in mitochondria, the main source of ROS (115). The levels and activities of MnSOD modulate cellular redox status and influence the effects of chemotherapy and radiotherapy; therefore, MnSOD may confer radioresistance through its antioxidant enzyme activity. Our previous studies have demonstrated that selective inhibition of RelB-induced MnSOD after irradiation can sensitize prostate cancer cells to radiation treatment (71, 79), confirming the importance of MnSOD in radioresistance.
Depending on the level of tolerance to oxidative stress, different cancer cell types may exhibit an opposite response to ROS elevation. For example, the anticancer drug 2-methoxyestradiol (2-ME) is associated with upregulation of MnSOD as an adaptive response that protects pancreatic cancer cells from increased ROS (202). In contrast, 2-ME can sensitize radioresistant MCF-7/FIR breast cancer cells by activating apoptosis, arresting the cell cycle, and further enhancing radiation-induced ROS (152). Therefore, applying redox-modulating reagents, such as ascorbate (49), arsenic trioxide (34), selenite (76), or a metalloporphyrin antioxidant mimetic (MnTE-2-PyP) (112), in combination with IR can either increase the cell-killing effect of IR or protect against radiation-induced oxidative stress to surrounding normal tissues.
The neuroendocrine differentiation (NED) of prostate cancer cells is closely correlated with radioresistance (43, 185). In the prostate gland, neuroendocrine (NE) cells are <1% of total epithelial cells; however, the number of NE-like cells increases in advanced prostate cancer (129). Fractionated IR can induce NED in the LNCaP prostate cancer cell line by activation of the cAMP response element binding protein (CREB) and cytoplasmic sequestration of activating transcription factor 2 (ATF2) (43). NE-like cells can dedifferentiate to a proliferating state, which may contribute to tumor recurrence (43, 44). Interestingly, by utilizing LNCaP cell clones with stably overexpressed MnSOD with lower superoxide levels and higher H2O2 levels, Quiros-Gonzalez et al. showed that MnSOD upregulation was sufficient to drive NE differentiation, resulting in androgen independence and cell survival in prostate cancer cells (145). It has been proposed that the balance between O2•− and H2O2 can determine pathways that drive the NED process (145). Thus, it is conceivable that MnSOD might affect NED by modulating the rate of H2O2 production and the balance between O2•− and H2O2. Further investigation of the roles of MnSOD in regulating prostate cancer cell NED and the significance of NED in prostate cancer radioresistance and recurrence may lead to discoveries that can be explored to overcome radioresistance.
ROS and Prostate Cancer
Reactive species, which include ROS and reactive nitrogen species (RNS), are categorized into two groups: free radicals that contain one or more unpaired electrons, such as superoxide (O2•−), •OH, and nitric oxide (NO•), and nonradicals, such as hydrogen peroxide (H2O2). Biological organisms are able to maintain a delicate redox homeostasis because they contain a complex intracellular “redox buffer” network, including both enzymatic and nonenzymatic antioxidants. The major enzyme defense system against ROS includes SOD, catalase, glutathione peroxidase, peroxiredoxin, and glutathione S-transferase (GST) (74). In addition to these antioxidant enzymes, small thiol-containing peptides, such as glutathione (GSH), glutaredoxin, and thioredoxin (Trx) systems, also help to scavenge ROS and maintain appropriate redox homeostasis (157).
The redox status (oxidizing/reducing conditions) of cells can regulate various transcription factors/activators, such as AP-1, NF-κB, and p53, thereby influencing target gene expression and modulating cellular signaling pathways. Requisite levels of ROS and RNS must be present for normal physiological function of living organisms (177). An increase in production of reactive species and/or a decrease in antioxidants can lead to oxidative stress, which can damage DNA, inhibit cellular enzyme activities, and induce cell death through activation of kinases and caspase cascades (154). Oxidative stress resulting from an imbalance between prooxidants and antioxidants that favors the former is believed to play a critical role in prostate carcinogenesis and prostate cancer progression [reviewed in (64)].
Sources of ROS
ROS derived from incomplete reduction of oxygen can be produced either endogenously (e.g., mitochondria respiration) or exogenously (e.g., IR) (80, 125) (Fig. 2). The most important endogenous source of ROS is the mitochondrial electron transport chain (ETC). The electrons that leak from some components of mitochondrial ETC lead to a one-electron reduction of O2 and generation of superoxide radicals (O2•−). Superoxide can be dismutated by SOD to yield hydrogen peroxide and O2. In the presence of transition metal ions, especially iron ions, hydrogen peroxide is subsequently converted through Fenton and Haber-Weiss reactions to a hydroxyl radical, which is the most toxic form of ROS, leading to various types of lipid peroxidation, protein modification, and particularly oxidative DNA damages, such as 8-hydroxy-deoxyguanosine (148). Somatic mutations in the mitochondrial genome are relatively frequent events in prostate cancer. Compared with nuclear DNA, mitochondrial DNA (mtDNA) is more susceptible to radiation-induced loss of integrity due, in part, to the lack of protective histones, an inefficient DNA repair system, and continuous exposure to the mutagenic effect of ROS (187), which is exacerbated by GSH depletion in mitochondria (120). ROS-induced mtDNA damage can alter polypeptides encoded by mtDNA for respiratory complexes, resulting in additional decreased electron transfer activity and increased ROS generation, thereby establishing a vicious cycle of oxidative stress (102) and decline in mitochondria energy production after initial oxidative damage of mtDNA (101). Mitochondrial dysfunction that causes persistent oxidative stress may contribute to radiation-induced genomic instability (198).
FIG. 2.
Scheme of cellular reactive oxygen species (ROS) generation and the antioxidant system. ROS generated from extracellular or intracellular sources can damage nuclear and mitochondrial DNA. Various transcription factors, such as nuclear factor kappa B (NF-κB), p53, activator protein-1 (AP-1), signal transducers and activators of transcription (STAT)3, Nrf2, HIF-1α, Sp1, PPARγ, and Ref1 are modulated by ROS. Examples of extracellular sources of ROS: radiation, carcinogens, inflammation, and hypoxia. Intracellular sources of ROS: ETC, electron transport chain; XO, xanthine oxidase; LPX, lipoxygenase; P450, cytochrome P450; COX, cyclooxygenase. Antioxidant system: MnSOD; Cu/ZnSOD; ECSOD, extracellular superoxide dismutase; GSH, glutathione; GPx, glutathione peroxidase; Prx, peroxiredoxin; GR, glutathione reductase; TrxR, thioredoxin reductase; TRXox, oxidized thioredoxin; TRXre, reduced thioredoxin; HIF-1α, hypoxia-inducible factor 1-alpha; PPARγ, peroxisome proliferator-activated receptor gamma.
ROS can also be generated by other enzymes, such as xanthine oxidase, membrane-associated NOX, and cytochrome P450 in endoplasmic reticulum, and oxidases in peroxisomes (97). The association of NOX enzymes with prostate cancer growth and malignant phenotypes has been extensively reviewed (86, 98).
ROS and prostate cancer progression
Increased oxidative stress plays a significant role in several physiological/pathological situations, such as aging and aging-associated diseases. Prostate cancer is closely associated with aging as it occurs frequently in older men. Prostate cancer cells generally have a higher level of oxidative stress compared with normal prostate cells, and the level of oxidative stress is associated with prostate cancer occurrence, recurrence, and progression (10, 21, 98). It has been demonstrated that, at an early stage of cancer development, tumor cells are exposed to high oxidative stress, in part due to inhibition of various antioxidant enzyme activities (Fig. 3). Lower levels of antioxidant enzymes, such as MnSOD, copper/zinc SOD, and catalase (10, 21) and defects in several classes of GSTs (22) have been observed repeatedly in prostate adenocarcinoma compared with benign prostate cells and tissues. However, after cancer has progressed, ROS partially render cancer cells more dependent on the function of antioxidant enzymes, such as SODs, to protect against damages caused by increased levels of superoxide radicals (115). Our laboratory has provided in vivo evidence and has proposed a model for the underlying molecular mechanism by which p53 differentially regulates MnSOD expression between early and advanced stages of cancer (46).
FIG. 3.
Role of oxidative stress in cancer development and radioresistance. IR-induced genomic instability and oxidative stress are closely related to each other. Many human cancer cells harbor low levels of antioxidants at early stages of a tumor, whereas cancer cells may eventually become resistant to radiation treatment and/or chemotherapy and possess high levels of antioxidants at advanced stages of a tumor. With cancer development, tumor cells are continuously facing increased oxidative stress and risk of genomic instability.
The protein levels of some antioxidant enzymes and signaling molecules are associated with cellular redox status. The proteins play a more dominant role when activation/inhibition of enzymatic activity and redox modification during redox signaling, or in response to cellular redox change in specific cellular compartments, occurs (Table 1). For example, it has been suggested that redox-sensitive molecule Trx1 functions as a protective cellular antioxidant and its upregulation protects cancer cells from oxidative stress (122). However, despite a significant increase in its protein level, oxidation of nuclear Trx1 resulted in a loss of antioxidant activity, which clearly demonstrates that when redox imbalance occurs, prostate cancer cells adapt to oxidative stress (161). Therefore, characterization of redox-sensitive protein structure and cellular localization, identification of potential redox modifications based on structure information and modeling strategies, and investigation of different functions before and after modification will provide insightful knowledge of cellular redox status at each stage of cancer development.
Table 1.
Roles of MnSOD Expression or Activity and MnSOD-Regulated Cellular Redox Status in Different Stages of Tumor Development
| Tumor development | Normal cells | Early stage of cancer | Advanced stage of cancer |
|---|---|---|---|
| MnSOD level or activity | High | Low | Very high |
| ↓prooxidant | ↑prooxidant | ↑prooxidant | |
| Cellular redox status | ↑antioxidant | ↓antioxidant | ↑antioxidant |
| Low oxidative stress | Modest oxidative stress | High oxidative stress | |
| Sensitivity to ROS | ++ | +++ | + |
| Implications in radiotherapy | Protect normal cells from oxidative insults such as radiation by inducing adaptive responses | Promote radiation-mediated cell killing due to low level of antioxidant capacity | Protect tumor cells from radiation and modulate neuroendocrine-like differentiation leading to radioresistance |
Based on the biomedical property of increased ROS and altered redox status in cancer cells, many avenues of research have been proposed to modulate the unique redox regulatory mechanisms of cancer cells for therapeutic benefits (183). Mitochondrial ROS have been shown to promote proinflammatory cytokine expression (27) and NLRP3 inflammasome activity (174). Blocking androgen-induced ROS production by inhibiting polyamine oxidase could delay prostate cancer progression and prolong survival of animals when spontaneous prostate cancer develops (14). A highly oxidizing condition is strongly cytotoxic and is the primary mechanism for tumor cell killing by radiation therapy and some chemotherapeutics, such as Taxol and Adriamycin. Since tumor cells are under more oxidative stress and normal cells usually carry higher redox-buffering capacity, specific mild prooxidants, such as parthenolide, have been shown to be redox-modulating reagents capable of selectively pushing tumor cells beyond their tolerance to oxidative stress and sensitizing cancer cells to radiation-induced cell killing (178).
ROS are not only involved in radioresistance but also are implicated in prostate cancer progression and castration resistance. Growth and proliferation of castration-resistant prostate cancer are mediated by gain-of-function changes in the androgen receptor (AR) and AR reactivation. MnSOD downregulation is directly responsible for AR reactivation in prostate cancer and occurs through an ROS-mediated mechanism (163). Shiota et al. have extensively reviewed both the effects of AR signaling on oxidative stress and the effects of oxidative stress on AR signaling in the context of prostate cancer, especially castration-resistant prostate cancer (165). Castration-induced oxidative stress may promote AR overexpression through transcription factor Twist1 overexpression, which may result in a gain of castration resistance (166). Thus, modulating redox status to sensitize cells and overcome radioresistance may result in castration resistance, which diminishes the therapeutic benefits of redox modulation. Thus, the stage of prostate cancer development and AR signaling must be carefully determined before introducing redox intervention strategies.
Role of ROS in the interaction between stroma and prostate cancer cells
It is becoming increasingly clear that the microenvironment is crucial to prostate cancer cell survival, progression, metastasis [reviewed in (20)], and resistance to chemotherapy and/or radiotherapy. Redox status within such a microenvironment is complicated at all stages of prostate cancer development, due to the considerable heterogeneity of the cellular composition of stroma and tumors. IR generates highly reactive free radicals, and it has been well documented that stromal components, such as cancer-associated fibroblasts (CAFs), tumor-associated macrophages (TAMs), and endothelial cells, enhance oxidative stress, which promotes tumor progression (2, 50).
As the most abundant cell type in the microenvironment of solid tumors, fibroblasts are particularly prominent in prostatic carcinoma (Fig. 4). The origin of CAFs and their significance in determining cancer aggressiveness have been elegantly demonstrated previously (109, 134). Cunha and colleagues have shown that CAFs contribute to prostate tumor growth and metastatic potential. Human prostatic CAFs grown using initiated human prostatic epithelial cells dramatically stimulated the growth and altered histological characteristics of the epithelial cell population. However, this effect was not observed when normal prostatic fibroblasts were grown using initiated epithelial cells under the same experimental conditions (134).
FIG. 4.
Radiotherapy-induced cell killing and unintended effects on tumor stromal components leading to inflammatory mediator secretion. Inflammatory mediators induced by IR include epidermal growth factor (EGF), fibroblast growth factor (FGF), interferon-γ (IFN-γ), transforming growth factor-beta (TGF-β), interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and IL-8. Both radiation treatment and IR-induced inflammatory mediators increase ROS levels within a tumor microenvironment, which contributes to radioresistance and recurrence of cancer.
It has been observed that ROS formation increased immediately after irradiation and continued for several hours, resulting in the production of 8-oxoguanine, which is a product of oxidative DNA damage (132). Oxidative damage in DNA is repaired mainly via the base excision repair (BER) pathway (42). The BER pathway is initiated by removal of the base by DNA glycosylases, leaving an intact abasic (AP) site. Subsequently, AP endonuclease 1 (APE1/Ref1) nicks the damaged DNA strand upstream of the AP site, creating a 3′-hydroxyl terminus and a 5′-deoxyribose phosphate group flanking the gap (172). APE1/Ref-1 (APE1) possesses not only DNA repair functions but also transcriptional regulatory activities, controlling cellular response to oxidative stress (180). APE1 has been identified as a protein with nuclear redox activity, inducing the DNA binding activity of several transcription factors, such as AP-1, NF-κB, hypoxia-inducible factor-1α, p53, Myb, and the ATF/CREB family [reviewed in (180)]. Thus, while IR-induced ROS lead to oxidative DNA damage, their repair can also contribute to cellular redox status, at least in part, through APE1/Ref-1 functions.
Due to diffusibility and abundance, multiple ROS and inflammatory mediators associated with aging, infection, or IR exposure may provide a permissive environment for cancer development. Compelling experimental and clinical evidence indicates that ROS-mediated stromal–epithelial interactions in both normal and malignant prostatic environments involve a number of soluble factors and their corresponding receptors (37). ECSOD plays predominant roles in scavenging superoxide in the extracellular space where redox state regulates intracellular signaling or tumor growth. ECSOD-derived H2O2 can promote vascular endothelial growth factor (VEGF) signaling in caveolin-enriched lipid rafts and stimulate endothelial cell migration and proliferation through oxidative inactivation of protein tyrosine phosphatases (PTPs), such as density-enhanced protein tyrosine phosphatase-1 (DEP-1) and PTP1B (135). VEGF is critical for not only angiogenesis but also prostate-cancer-mediated osteoblastic activity (93). IR modulates VEGF expression through multiple mitogen activated protein kinases (MAPK)–dependent pathways (137) and enhances glioma cell motility through vascular endothelial growth factor receptor 2 (VEGFR2) signaling pathways (87). Since prostate cancer cells lack expression of specific VEGF receptors, especially VEGFR2, IR-induced VEGFs are more likely to promote prostate cancer progression indirectly through their functions in stromal cells, in particular, endothelial cell survival, and as a chemotactic agent within the tumor microenvironment (93). In addition to endothelial cells, CAFs can also exert their cancer-promoting roles through release of growth factors, such as TGF-β and epidermal growth factor (EGF), as well as chemokines (2). Oxidative-stress-dependent monocarboxylate transporter 4 expression in CAFs is closely involved in stromal–epithelial lactate shuttling (190). According to a recently proposed model, an increase of ROS in CAFs drives tumor-stroma coevolution, DNA damage, and aneuploidy in cancer cells (109). Pavlides et al. have provided detailed information on how CAFs accelerate tumor growth and metastasis via oxidative stress, mitophagy, and aerobic glycolysis (138). The multiple roles of ROS in these new metabolic coupling interactions and models suggest that certain redox-modulation-based therapeutic methods can be helpful when used in combination with traditional radiotherapy in prostate cancer treatment.
The radiation-induced bystander effect, mediated through gap junctions and inflammatory responses, is defined as the response of cells to their irradiated neighbors (142). Many types of cancer-infiltrating immune cells, such as macrophages, dendritic cells, and T cells, are important stromal components of a prostate tumor as well as being prominent bystander targets of radiotherapy. More information on the mechanisms by which IR influences tumor-associated immune responses and various immune cells to secrete different inflammatory mediators appear in recent reviews (38, 123). Thus, activated immune cells are not limited to induction of antitumor immunity; they are also involved in creating an immunosuppressive and prooxidant network that promotes tumor progression and facilitates immune evasion. Since tumor cells are often under higher oxidative stress with disregulated and/or less adaptive redox buffering capacity than their normal counterparts, tumor cells are probably less able to cope with additional incremental increases in oxidative stress than normal cells are, which can be explored to enhance antitumor immunity while minimizing the possibility of unintended tumor progression and evasion.
Radiotherapy-Induced Inflammatory Mediator Secretion
When cells are exposed to IR, DNA damage generated from either direct or indirect effects of IR induces a multicellular program through a variety of signaling pathways to start DNA repair and prevent the proliferation of damaged cells. Such programs are usually mediated by soluble factors composed of cytokines, growth factors, and chemokines, which perform functions in both tumors and stroma to determine the fate of affected cells (13). IR exposure commonly induces stromal cells, especially CAFs, into a senescence-like phenotype in an altered tumor microenvironment. The so-called senescence-activated secretory pathways in senescent stromal fibroblasts generate an inflammatory environment through the secretion of proinflammatory cytokines and proteases (50). These soluble factors can exert paracrine or autocrine functions mediated by their respective receptors or interactive partners to promote prostate cancer progression and to create a continuous loop that pushes prostate cancer to a more aggressive state.
Chronic inflammatory mediator secretion associated with aging has been involved in the etiology and progression of prostate cancer. A chronic inflammatory microenvironment leads to an increased fraction of epithelial cells that proliferate in local atrophy lesions, an event called proliferative inflammatory atrophy (PIA) (149). PIA may progress to high-grade intraepithelial neoplasia and prostate cancer (96). That a relationship exists between a chronic inflammatory microenvironment and prostate cancer is gaining wide acceptance (39, 67). The evidence that the microenvironment is altered as a result of radiotherapy, especially that various types of cytokines are generated, has been elegantly reviewed (13, 142). There are many types of mediators induced by IR (13), including EGF (45), fibroblast growth factor (13), interferon-γ (54), TGF-β (78), proinflammatory cytokines IL-6 (35), TNF-α (201), the chemokine IL-8 (128), and others (111). A range of studies has shown that a clear difference exists in the level of circulating cytokines in prostate cancer patients compared with normal or benign controls and changes in levels of circulating cytokines after radiation exposure and/or androgen deprivation therapy (26). For the purpose of brevity, we will only highlight the signaling pathways mediated by IL-6, IL-8, TNF-α, and TGF-β induced by IR, as well as their implications in prostate cancer malignancy and their potential significance in radiotherapy of prostate cancer.
Interleukin-6
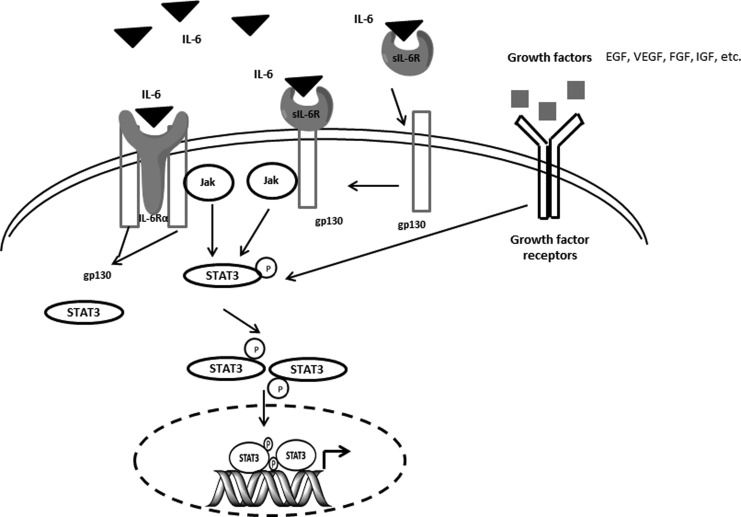
IL-6 is a multifunctional cytokine that signals through a cell-surface type 1 cytokine receptor complex composed of the ligand-binding protein of IL-6Rα (also called CD126) and the signal-transducing component gp130 (CD130) (62). Another type of receptor for IL-6 is a soluble IL-6 receptor (sIL-6R) that lacks a membrane-signaling domain but can bind with IL-6 and then with the membrane receptor β chain (gp130) to mediate the intracellular signaling pathways (153). IL-6 mainly activates the Janus kinase (JAK)/STAT3 signaling pathway (110), but it also participates in the MAPK and phosphatidyl inositol 3-kinase (PI3K)/Akt pathways to influence a wide range of biological activities in tumor cells (150). IL-6 also acts as an autocrine and/or paracrine proliferative factor in prostate cancer cell lines (133). IL-6 treatment not only stimulates the IL-6 autocrine loop but also activates insulin-like type I growth factor receptor (IGF-1R) signaling. This STAT3-mediated cooperation between IL-6 signaling and IGF-1R signaling in the prostate plays a critical role in facilitating prostate malignancy and epithelial–mesenchymal transition (EMT). STAT3 has been shown to promote oncogenesis in human cancer through Src oncogene transformation of STAT3 cells (199). In addition to its classical role in the nucleus, STAT3 modified by serine phosphorylation augments oxidative phosphorylation in mitochondria and supports cellular transformation by the oncogene Ras (58). Considering the different cellular localization of STAT3 implicated in intracellular energy metabolism and a variety of redox-sensitive genes, it will be interesting to investigate mitochondrial functions and cellular transformation under IR-induced IL-6 activation (Fig. 5).
FIG. 5.
IL-6-mediated Jak-STAT3 signaling pathway. IL-6 signals through a cell-surface type 1 cytokine receptor complex composed of the ligand-binding protein of IL-6Rα and the signal-transducing component gp130 (glycoprotein of 130 kDa). IL-6 can also bind to soluble IL-6 receptor (sIL-6R), which lacks a membrane-signaling domain, and then with gp130 to mediate Jak phosphorylation and activation. Activated Jak family tyrosine kinases further phosphorylate STAT3, which in turn translocate to the nucleus and regulate target gene transcription. Many types of growth factors, such as EGF, vascular endothelial growth factor (VEGF), FGF, and insulin-like type I growth factor (IGF), can aggregate with respective receptors and activate STAT3 signaling pathways.
Different cell types, such as B and T cells, macrophages, monocytes, fibroblasts, and certain tumor cells, can synthesize IL-6 (92), which regulates various cellular functions, including immune response, proliferation, apoptosis, angiogenesis, and differentiation (41). Several clinical studies have reported that elevated serum levels of IL-6 and sIL-6R are associated with metastasis and castration resistance, suggesting that IL-6 correlates with prostate cancer progression and patient morbidity (1, 25, 126). Most clinical data support the biological role of the IL-6 pathway in prostate cancer, especially in an advanced castration-resistant prostate cancer patient where the significance of the IL-6 pathway is mediated by crosstalk between IL-6 and the AR pathway (9). Under androgen deprivation conditions, IL-6 is able to promote intracellular synthesis of androgens in the prostate (36), resulting in AR activation and upregulation of AR-targeted prostate specific antigen (PSA) expression, via STAT3 and MAPK signaling pathways (9), as well as an androgen enhancer region within the human PSA promoter (184).
Even though increased IL-6 may indicate the presence of an advanced prostate cancer tumor in a patient or in in vivo experiments, some in vitro results that support the significance of the IL-6 pathway in the growth of prostate cancer cells are still controversial. IL-6 can act as either a growth inducer (144) or inhibitor (70) in androgen-dependent LNCaP cells (9). It is possible that IL-6-induced growth arrest may be associated with NED (175). The presence of NE-like cells has been correlated with a radioresistant phenotype and an unfavorable prognosis (43, 185).
IL-6 signaling is tightly regulated by several negative feedback inhibitors, including suppressors of cytokine signaling, Src-homology 2 containing protein tyrosine phosphatases, and protein inhibitors of activated STATs (40, 99). The mechanisms by which these inhibitors regulate IL-6 intracellular signaling pathways have been reviewed previously in detail (40). Since IL-6 also plays an inhibitory role in prostate cancer cells depending on signaling crosstalk and the difference between the cancer and normal cells in redox status and adaptive response to oxidative stress may influence the signaling crosstalk, blocking IL-6 with antibody or signaling inhibitors may instead promote prostate cancer progression. Thus, it is necessary to identify the role of IL-6 signaling in specific situations before applying anti-IL-6-related therapy.
Interleukin-8
IL-8, also known as CXCL8, is a chemoattractant chemokine. IL-8 is usually associated with inflammation that predisposes cells to produce different chemokines for malignant transformation or progression (170). IL-8 secretion is increased by oxidative stress from either intracellular or extracellular sources. IL-8 can stimulate the recruitment of inflammatory cells, which further elevates oxidant stress mediators, thereby making IL-8 a key parameter in localized inflammation (186). Two cell-surface G protein-coupled receptors, CXCR1 and CXCR2 (72), are responsible for the binding of IL-8 and regulating target gene expression through downstream signaling pathways, such as activation of serine/threonine kinases, protein tyrosine kinases, and Rho-GTPases (189). Depletion of CXCR1 leads to inhibition of IL-8-mediated androgen-independent tumor growth by increasing proapoptotic proteins and decreasing antiapoptotic proteins (160).
Increased IL-8 expression is associated with both a high Gleason score and a tumor pathologic stage (56). Elevation of IL-8 expression has been linked to various markers of the progression of prostate cancer up to an advanced stage, such as castration resistance, metastasis, and enhanced angiogenesis in vitro (7, 159), in vivo (7, 197), and in human patients (30). It has been shown that IL-8 signaling, which is endogenous and induced by a TNF-related apoptosis-inducing ligand, can modulate the extrinsic apoptosis pathway in prostate cancer cells through direct transcriptional regulation of c-FLIP, an endogenous caspase-8 inhibitor, and reduce the propensity of prostate cancer cells to undergo apoptosis (194). Therefore, inhibiting IL-8 signaling may be a promising strategy to sensitize advanced prostate cancer to chemotherapy. The reduction of intrinsic IL-8 potentiates ansamycin-based heat shock protein 90 cytotoxicity by several mechanisms, including inhibition of IL-8-induced NF-κB activity (158), cell cycle arrest at the G1/S boundary, and increased spontaneous apoptosis as well as enhancement of the efficacy of multiple chemotherapeutic drugs, such as Docetaxel, Staurosporine, and Rapamycin (168).
There are currently seven known CXC chemokine receptors in mammals, named CXCR1 through CXCR7. Various types of crosstalk exist between different chemokine-mediated signaling pathways due to remarkable redundancy within chemokines with multiple chemokine bindings to similar or the same receptor(s) and multiple receptors binding with similar or the same chemokine(s) (53). It has been shown that IL-8 can upregulate CXCR7 expression and the ligand-independent functions of CXCR7, which usually binds to CXCL11 and CXCL12 ligands to promote the growth, proliferation, and angiogenesis of prostate cancer cells by increasing epidermal growth factor receptor (EGFR) and ERK1/2 phosphorylation (169). Therefore, the effects of IL-8/IL-8 receptor signaling pathways in prostate cancer progression and radiation sensitivity may be orchestrated by communication and/or interaction with many chemokines and their receptors, such as CXCR1–7.
The correlation between IL-8 signaling and AR signaling pathways has been reviewed previously (170, 189). Proteomic data illustrate that the androgen-stimulated LNCaP cells have increased expression of IL-8 (55), which is dependent on AR. Additionally, IL-8 signaling also increases AR expression and alters the distribution and transcriptional activation of AR, leading to increased expression of AR-targeted genes (159). Since the transition of prostate cancer to an androgen-independent state is partially due to IL-8-signaling-induced AR activation, targeting IL-8 expression and signaling pathways may significantly enhance the efficacy of androgen ablation therapy.
In addition to establishing the importance of IL-8 in developing chemoresistance (193), our laboratory found that the RelB-mediated NF-κB alternative pathway plays a crucial role in IL-8 upregulation, which enhances the radioresistance of prostate cancer cells (196). This result is consistent with the observation that RelB promotes prostate cancer progression and radioresistance (197). The relationship of the NF-κB pathway, increased antioxidant capacity, and resistance to radiation treatment in many tumor cell types has been well documented (61, 147). It has been demonstrated, and reviewed, that RelB regulates MnSOD gene expression and the radioresistance of prostate cancer cells (71, 79). Selective inhibition of the RelB-mediated NF-κB alternative pathway can, to a remarkable degree, sensitize prostate cancer cells to IR-induced killing (71). Thus, it will be interesting to investigate the relationship of radiosensitizing effects of IL-8 signaling blockage with either inhibitors of IL-8 receptors or monoclonal antibodies against IL-8. This strategy may synergistically facilitate the killing of castration-resistant and/or radiation-resistant prostate cancer cells.
Tumor necrosis factor-alpha
TNF-α is synthesized as a 26 kDa (233 amino acids) membrane-bound pro-peptide (pro-TNF) and is released as a 17 kDa soluble polypeptide (157 amino acids) after cleavage by the TNF-converting enzyme (119). The action of TNF-α is mediated by two distinct receptors, TNF-receptor I (55 kDa, TNFRI) (106), which mediates the majority of TNF-α biological activities, and receptor II (75 kDa, TNFRII) (173), with both having an affinity for TNF-α in human tissues. An imbalance between prosurvival and apoptosis signals by TNF-α-initiated signaling pathways (Fig. 6) has been implicated in malignancies of the colon (52), ovary (151), breast (149), and prostate (117).
FIG. 6.
TNFα-regulated major cellular signaling pathways. Binding of TNFα to TNFR1/2 leads to the rapid phosphorylation of the NF-κB, ERK, p38, and JNK pathways, and activates a group of transcription factors, such as NF-κB, Elk1, and AP-1, in the nucleus. In addition to these pro-survival pathways, TNF-α can induce apoptosis through receptor-mediated caspase activation, and caspase-dependent and -independent components of the mitochondrial cell death pathway. A balance between these intracellular signaling pathways determines whether cells will die or survive after exposure to TNF-α. TNFα-mediated ROS generation is mainly derived from mitochondria and membrane-associated NADPH oxidase, which contributes to signaling pathways. JNK, c-Jun N-terminal Kinase.
TNF-α is one of the central factors involved in stress responses, including response to radiation exposure, because of its ability to induce rapid hemorrhagic necrosis via selective destruction of tumor blood vessels and generate specific T-cell antitumor immunity (100). Antagonists of TNF-α action have been developed for the treatment of rheumatoid arthritis and other inflammatory diseases (11). When present chronically in the tumor microenvironment, TNF-α is a major mediator of cancer-related inflammation. In addition to maintaining homeostasis of the immune system, inflammation, and host defense, TNF-α also plays paradoxical roles in cancer promotion and progression pathways leading to activation of NF-κB and AP-1 transcription factor complexes [reviewed by Balkwill (11)]. Circulating TNF-α is normally not detectable in healthy individuals but can be detected in some cancer patients (25, 117). While it remains to be determined whether TNF-α elevation in prostate cancer patients is the cause or consequence of cancer development and progression, a relatively consistent association between increased TNF-α and cachexia in patients with prostate carcinoma (127, 140) has been determined, and it is one of the most devastating conditions of late stages of cancer. TNF-α plays multiple roles in changes related to cancer cachexia, such as altered nitrogen metabolism associated with cachexia (3), blockage of muscle differentiation associated with muscle tissue regeneration (29), and activation of transcription factors NF-κB and AP-1 to increase proteolysis (182) [reviewed in (8)].
TNF-α is often produced in response to oxidative stress and it acts, at least in part, by causing oxidative stress in its target cells. Mitochondria are the primary generators of ROS, which contribute to the TNF-α-initiated signaling pathway (81). TNF-α-induced ROS, which can be inhibited by mitochondrial-specific MnSOD overexpression, may oxidize and inhibit c-Jun N-terminal Kinase (JNK)-inactivating phosphatases, and sustained JNK activation is required for cytochrome c release and caspase 3 cleavage as well as necrotic cell death (81). NOX activation is involved in TNF-α-induced ROS production, depending on cell type and the extent of TNF-α exposure (90, 104). It has been shown that acute TNF-α exposure induces rapid (within 5 min) p47phox phosphorylation and increases p47phox-TNF-α receptor-associated factor 4 association and membrane translocation, which further mediates p47phox-p22phox complex formation, leading to NADPH-dependent O2•− production (104). The binding of TNF-α to TNFR1 can activate NOX1 or NOX2 to generate ROS in early endosome (131). During TNF-α-induced necrotic cell death, Nox1 is activated by forming a complex with TRADD (TNF-receptor-associated protein with death domain), RIP1 (Receptor Interacting Protein 1), and Rac1 (90). This NOX-dependent redox-regulated mechanism plays a key role in TNF-α-induced necrotic cell death.
Most studies referenced before used a relatively high dose of TNF-α to induce an acute and pro-cell death response. However, chronic elevation of TNF-α at a relatively low level can result in cytoprotection, which is related to increased levels of antioxidant, antiapoptotic, and other defense proteins, such as thioredoxins and MnSOD. Increased mitochondrial ROS production induced by TNF-α leads to activation of nuclear genes, especially NF-κB. In human and mouse ovarian cancer, TNF-α maintains TNFR1-dependent IL-17 production by CD4+ cells, which leads to myeloid cell recruitment into the tumor microenvironment and enhances tumor growth (31). TNF-α can be produced when NF-κB is activated and TNF-α is also an important stimulus of NF-κB signaling and additional cytokine production. The NF-κB signaling pathway is critical in supporting cancer-related inflammation and malignant progression as well as maintaining the immunosuppressive phenotype of TAMs (65). TNF-α may play a role in the initiation of an androgen-independent state in prostate cancer through its ability to inhibit AR sensitivity (118). The interplay of NF-κB and B-myb contributes to negative regulation of AR expression by TNF-α (94). Immunohistochemistry results show that nuclear localization of NF-κB family member p65 is associated with PSA relapse, the first sign of prostate cancer recurrence, while cytoplasmic expression does not (47). Our laboratory demonstrated that the RelB-mediated alternative NF-κB pathway is involved in prostate cancer aggressiveness and radiation resistance (71, 79, 197). TNF-α functions as a potent inducer of the NF-κB signaling pathway and mediates the crosstalk between the classical and alternative NF-κB signaling pathways, as well as interactions with AR (according to our unpublished data). Thus, it is important to investigate in prostate cancer the effects of TNF-α production after chemo/radiotherapy and the potential influences of TNF-α on the activation of the RelB-mediated alternative NF-κB pathway.
The expression and activation of several genes and kinases, such as cyclooxygenase-2, Cyclin D1, the Bcl-2 family, survivin, Akt, and EGFR, are regulated by NF-κB in various tumor cells (103). The therapeutic potential and benefit of targeting NF-κB in cancer and the possible complications and pitfalls associated with NF-κB modulation have been reviewed and explored (15). Inhibition of NF-κB has been proposed as a means to treat cancer or to overcome chemoresistance and radioresistance in cancer therapy (103). Inhibition of IR-induced NF-κB activation sensitizes Ki-Ras transformed prostate epithelial cells (267b1/K-Ras) to IR (88). Selective inhibition of RelB nuclear activation and downregulation of RelB-targeted MnSOD gene expression improve IR-induced killing of PC3 cells (71).
Novel strategies have been proposed to target TNF-α-mediated signaling for treatment of human prostate cancer. For example, Gambogic acid can inhibit TNF-α-induced invasion of human prostate cancer PC3 cells in vitro by inhibiting the PI3K/Akt and NF-κB pathways (107). TNF-α induces MnSOD expression, which mediates delayed radioprotection (124) through an NF-κB binding site located within the second intron of the sod2 gene (115). The natural compound curcumin acts as a potent radiosensitizer in PC3 cells by inhibiting TNF-α-mediated NF-κB activity, resulting in bcl-2 protein downregulation (33). There is a caveat to targeting TNF-α in prostate cancer therapy. TNF-α synergizes with γ-irradiation to induce apoptosis in LNCaP cells through a mechanism that may involve increased production of ceramide at 48–72 h after exposure (91). Anti-TNF-α treatment may mitigate the effect of γ-irradiation. Depending on TNF-α dose and prostate cancer cell type, different isoforms of C/EBPβ may regulate cell growth and confer TNF-α resistance to prostate cancer cells (89). Although TNF-α is clearly linked with prostate cancer progression and radioresistance, it may also contribute to tumor immune surveillance and apoptosis-mediated antitumor pathways. Since TNF-α expression is subjected to redox regulation, the difference between tumor and normal cells in their redox regulation and adaptive redox buffering capacity can be exploited to shift the paradoxical effects TNF-α toward increased immune surveillance and tumor cell apoptosis.
Transforming growth factor-beta
TGF-β is a ubiquitous cytokine that plays a critical role in numerous pathways regulating homeostasis and injury response as well as in the progression of human cancer. Prior to tumor initiation and during early phases of tumor progression, TGF-β acts as a tumor suppressor. At later stages of cancer development, TGF-β promotes processes associated with tumor aggressiveness, such as cell invasion, dissemination, and immune invasion (48, 114). In mammals, there are three TGF-β isoforms: TGF-β1, TGF-β2, and TGF-β3. With the assistance of the coreceptors endoglin and betaglycan (known as type III receptors or TGFβRIII), active TGF-β binds to cell surface type I (TGFβRI) and type II (TGFβRII) serine/threonine kinase receptors, which phosphorylate and activate the Smad family of signal transducers (48) (Fig. 7).
FIG. 7.
TGF-β-mediated classical Smads signaling pathway. With the assistance of TGFβRIII, active TGF-β (three isoforms, i.e., TGF-β1, TGF-β2, and TGF-β3) binds to cell surface TGFβRI and TGFβRII, which phosphorylate and activate the Smad family of signal transducers.
Once activated by TGF-β binding to the receptors, Smad2 and Smad3 associate with Smad4 and translocate to the nucleus where they regulate the transcription of genes involved in cell cycle arrest and apoptosis, which are essential to the tumor suppressor role of the TGF-βs in normal epithelial cells and at early stages of carcinogenesis (18, 19). TGF-β-induced growth arrest is mediated by the inhibition of cyclin-dependent kinases and the downregulation of myelocytomatosis oncogene cellular homolog [reviewed in (48)]. Mutational inactivation of TGF-β signal-transduction components, such as the TGF-β type II receptor (TGFβRII) (60) or its mediators, Smad2 and Smad4, leads to defective TGF-β signaling in some cancers (17, 59). Pu et al. developed a transgenic adenocarcinoma of mouse prostate-based prostate cancer transgenic mouse model that harbors the dominant negative mutant TGF-β type II receptor in epithelial cells to characterize the in vivo consequences of inactivated TGF-β signaling on prostate tumor initiation and progression, and found that disruption of TGF-β signaling in vivo accelerated pathologic malignant changes in the prostate by altering the kinetics of prostate growth and inducing EMT (143). These findings indicate that TGF-β exerts its tumor suppressor functions through inhibition of cell proliferation, induction of apoptosis, and regulation of autophagy.
TGF-β is expressed at high levels in the later stages of tumor development (192), during which it is utilized as a potent promoter of cell motility, invasion, metastasis, and tumor stem cell maintenance, as demonstrated in experimental prostate cancer models (121). Local TGF-β1 elevation has been associated with tumor grade, pathologic stage, and lymph node metastasis in prostate cancer patients (162). Although some investigators were not able to find a discriminative difference in the serum concentration of TGF-β1 in benign prostate hyperplasia and prostate cancer (195), elevated levels of plasma TGF-β1 (77), TGF-β2 (139), and urinary TGF-β1 (139) were found in patients with prostate cancer.
TGF-β1 plays a critical role in tumor–stromal cell interactions and modulates the growth of prostate cancer, either positively or negatively, through the balance between the amounts of IGF-1 and IGF binding protein-3 (85). Resistance to TGF-β-mediated growth arrest results in highly malignant phenotypes with increased EMT, tumor invasion, metastatic dissemination, and evasion of immune surveillance (114). Interestingly, TGF-β1 activates IL-6, which has been implicated in the malignant progression of prostate cancers, as described earlier, via multiple signaling pathways, including Smad2, NF-κB, JNK, and Ras (136). Zhu and Kyprianou have provided a detailed description of the crosstalk between AR and growth factors, including TGF-β-mediated signaling pathways, in prostate cancer cells (203). Smad3, a downstream mediator of the TGF-β signaling pathway, can function as a coregulator to enhance AR-mediated transactivation and increase AR-targeted PSA gene expression (82). Considering the correlation between increased circulating levels of TGF-β1 with invasion (77), metastasis (77), and poor prognosis in patients with prostate cancer (162), TGF-β1 could be an additional serum marker for prostate cancer (16, 176).
TGF-β acts as an important mediator for response to IR, and its signaling is tightly regulated by redox status within tumor cells and the tumor microenvironment. IR has been shown to induce the release and activation of TGF-β in cells and tissues (13). A mechanistic study in a cell-free system demonstrated that oxidation of the TGF-β latent complex acts as a sensor of oxidative stress to mediate the release and activation of TGF-β1 and orchestrate cellular responses to damage (12). More aspects of TGF-β biology, particularly its involvement in the microenvironmental response to IR, have been described elegantly (13). Intracellular redox equilibrium is essential for constitutive AP-1-dependent TGF-β1 expression (57). Nitric oxide downregulates TGF-β1 expression in prostate cancer cells at the transcriptional level by suppressing the de novo synthesis of TGF-β1 mRNA (188). TGF-β1 induces a stromal oxidant/antioxidant imbalance as a result of elevated NOX4-dependent ROS production and inhibits the expression of the MnSOD and catalase (116) that may be critical in the acquisition of epithelial migratory properties (23). In addition, TGF-β1 decreases ETC complex IV activity by decreasing phosphorylation of the subunit 6b of glycogen synthase kinase 3, which contributes to senescence-associated mitochondrial ROS generation (28). The significant roles of TGF-β in modulating tumor intracellular and extracellular redox statuses suggest that TGF-β signaling is involved in mediating cell autonomous, local, and systemic responses, which together regulate the initiation, promotion, progression, and prognosis of prostate cancer.
Radiotherapy-induced TGF-β activation may have undesirable side effects that are implicated in late tissue damage, such as fibrosis (6, 108). Several studies support the use of TGF-β inhibitors to ameliorate IR toxicity to normal tissues (51, 146). Anticancer therapies, such as IR or doxorubicin, may accelerate the steps of tumor progression, such as EMT and metastasis, due to the promoting effect of TGF-β within the tumor microenvironment (4, 130). This effect can be abrogated by administration of a pan-TGF-β neutralizing antibody (19). Current strategies to target TGF-β in radiotherapy mainly focus on general inhibition of TGF-β signaling. It has been shown that blockade of TGF-β signaling prior to irradiation attenuates DNA damage responses, increases clonogenic cell death, and promotes tumor growth delay and, thus, enhances radiation response and prolonged survival in patients with breast cancer (24) and glioblastoma (68), but renders a lung cancer cell line more radioresistant (191). Genetic differences and tumor specificity can be important factors in determining the radiosensitizing effect of TGF-β inhibition in radiotherapy. For an example, a hypofunctional genetic haplotype of the TGFB1 gene encoding TGF-β1 is associated with lower TGF-β1 plasma concentrations and increased sensitivity to radiation-induced chromosomal aberrations and apoptosis in lymphoid cells (155). There are three major approaches to inhibit TGF-β signaling: targeting TGF-β synthesis using antisense molecules and using ligand traps that sequester TGF-β and small-molecule inhibitors that hinder the kinase activity of TGF-β receptors [reviewed in (5, 114)]. Since IR-induced TGF-β may not only provide a survival benefit to cancer cells that are radioresistant but also accelerate tumor progression, targeted disruption of the TGF-β signaling pathway for therapeutic intervention may be an effective adjuvant in cancer radiotherapy.
Conclusion and Future Perspectives
Radiation therapy is generally used to treat early stage and inoperable locally advanced prostate cancer. Radiation kills prostate cancer cells and extends long-term patient survival by direct and indirect actions leading to macromolecule damages and altered redox signaling. However, IR is also responsible for the induction of neoplastic transformation and tumor progression as well as normal tissue injuries. The development of radioresistance is a significant impediment to prostate cancer treatment. The side effects and late complications that result from IR exposure limit the full potential of radiotherapy efficacy. Considering the heterogeneity of tumors, dynamic communications between stromal and prostate cancer cells as well as the complicated redox-regulated mechanisms within the tumor microenvironment—simply applying generalized anti-inflammatory strategies—might result in unintended adverse effects. Thus, it is important to develop individualized treatment regimes that will be most effective and will not disrupt antitumor immunity in individual patients. Additionally, redox-dependent proinflammatory mediator production from the directly exposed cells and their neighboring nonirradiated cells, as the bystander effect of radiotherapy, may play a critical role in the response of cells and tissues to IR. The key roles of IR-induced cytokines and growth factors and their interference with prostate cancer radiotherapy have been extensively discussed in this review with an emphasis on IL-6, IL-8, TNF-α, and TGF-β. These major cytokines, which are induced by IR in prostate cancer treatment, are not only involved in modulating redox balance but are also subjected to regulation by various oxidative stresses. Compared and contrasted to normal cells, tumor cells are usually under a higher oxidative stress and secrete more proinflammatory mediators. An incremental increase in oxidative stress to the extent that is still within the adaptive redox buffering capability of normal cells may overwhelm the less adaptive redox buffering capability of cancer cells, thereby selectively disrupting the redox state in tumor cells and activating the apoptotic or necrotic pathway, which leads to selective killing of tumor cells. Thus, modulation of IR-induced oxidative stress and inflammatory cytokine signaling may provide a better basis for enhancing radiation-mediated killing in prostate cancer treatment with minimal normal tissue damage.
Abbreviations Used
- 2-ME
2-methoxyestradiol
- 3DCRT
3-dimensional conformal radiation therapy
- AP-1
activator protein-1
- APE1
AP endonuclease 1
- AP site
abasic site
- AR
androgen receptor
- ATF
activating transcription factor
- BER
base excision repair
- CAF
cancer associated fibroblast
- COX-2
cyclooxygenase-2
- CREB
cAMP response element binding protein
- Cu/ZnSOD
copper/zinc superoxide dismutase
- DEP-1
density-enhanced protein tyrosine phosphatase-1
- ECSOD
extracellular superoxide dismutase
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- EMT
epithelial mesenchymal transition
- ETC
mitochondrial electron transport chain
- FGF
fibroblast growth factor
- GPx
glutathione peroxidase
- GR
glutathione reductase
- Grx
glutaredoxin
- GSH
glutathione
- GST
glutathione S-transferase
- HIF-1α
hypoxia-inducible factor-1alpha
- HO-1
heme oxygenase-1
- IGF
insulin-like type I growth factor
- IGF-1R
insulin-like type I growth factor receptor
- IL-1β
interleukin-1beta
- IL-6
interleukin-6
- IL-8
interleukin-8
- IR
ionizing radiation
- Jak
Janus kinase
- JNK
c-Jun N-terminal kinase
- LPX
lipoxygenase
- MAPK
mitogen-activated protein kinase
- MnSOD
manganese superoxide dismutase
- mtDNA
mitochondrial DNA
- MYC
myelocytomatosis oncogene cellular homolog
- NE
neuroendocrine
- NED
neuroendocrine differentiation
- NF-κB
nuclear factor kappa B
- NOX
NADPH oxidase
- PPARγ
peroxisome proliferator-activated receptor gamma
- PI3K
phosphatidyl inositol 3-kinase
- PIA
proliferative inflammatory atrophy
- PIAS
protein inhibitors of activated STATs
- Prx
peroxiredoxin
- PSA
prostate-specific antigen
- PTP
protein tyrosine phosphatase
- Rac1
ras-related C3 botulinum toxin substrate 1
- RIP1
receptor Interacting Protein 1
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SH2
Src-homology 2
- sIL-6R
soluble IL-6 receptor
- SOD
superoxide dismutase
- STAT
signal transducers and activators of transcription
- TACE
TNF-converting enzyme
- TAM
tumor-associated macrophage
- TGF-β
transforming growth factor-beta
- TNAF4
TNF-α receptor-associated factor 4
- TNF-α
tumor necrosis factor-alpha
- TNFRI
TNF-receptor I
- TRAIL
TNF-related apoptosis-inducing ligand
- TRAMP
transgenic adenocarcinoma of mouse prostate
- Trx
thioredoxin
- TRXox
oxidized thioredoxin
- TrxR
thioredoxin reductase
- TRXre
reduced thioredoxin
- VEGF
vascular endothelial growth factor
- VEGFR2
vascular endothelial growth factor receptor 2
- XO
xanthine oxidase
Acknowledgments
This work was supported by NIH grants CA 115801 and CA 143428 and the Edward P. Evans Foundation.
References
- 1.Adler HL, McCurdy MA, Kattan MW, Timme TL, Scardino PT, and Thompson TC. Elevated levels of circulating interleukin-6 and transforming growth factor-beta1 in patients with metastatic prostatic carcinoma. J Urol 161: 182–187, 1999 [PubMed] [Google Scholar]
- 2.Allen M. and Louise Jones J. Jekyll and Hyde: the role of the microenvironment on the progression of cancer. J Pathol 223: 162–176, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Alvarez B, Quinn LS, Busquets S, Quiles MT, Lopez-Soriano FJ, and Argiles JM. Tumor necrosis factor-alpha exerts interleukin-6-dependent and -independent effects on cultured skeletal muscle cells. Biochim Biophys Acta 1542: 66–72, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Andarawewa KL, Erickson AC, Chou WS, Costes SV, Gascard P, Mott JD, Bissell MJ, and Barcellos-Hoff MH. Ionizing radiation predisposes nonmalignant human mammary epithelial cells to undergo transforming growth factor beta induced epithelial to mesenchymal transition. Cancer Res 67: 8662–8670, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Andarawewa KL, Paupert J, Pal A, and Barcellos-Hoff MH. New rationales for using TGFbeta inhibitors in radiotherapy. Int J Radiat Biol 83: 803–811, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Anscher MS, Thrasher B, Rabbani Z, Teicher B, and Vujaskovic Z. Antitransforming growth factor-beta antibody 1D11 ameliorates normal tissue damage caused by high-dose radiation. Int J Radiat Oncol Biol Phys 65: 876–881, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Araki S, Omori Y, Lyn D, Singh RK, Meinbach DM, Sandman Y, Lokeshwar VB, and Lokeshwar BL. Interleukin-8 is a molecular determinant of androgen independence and progression in prostate cancer. Cancer Res 67: 6854–6862, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Argiles JM, Busquets S, Toledo M, and Lopez-Soriano FJ. The role of cytokines in cancer cachexia. Curr Opin Support Palliat Care 3: 263–268, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Azevedo A, Cunha V, Teixeira AL, and Medeiros R. IL-6/IL-6R as a potential key signaling pathway in prostate cancer development. World J Clin Oncol 2: 384–396, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker AM, Oberley LW, and Cohen MB. Expression of antioxidant enzymes in human prostatic adenocarcinoma. Prostate 32: 229–233, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer 9: 361–371, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Barcellos-Hoff MH. and Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol 10: 1077–1083, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Barcellos-Hoff MH, Park C, and Wright EG. Radiation and the microenvironment—tumorigenesis and therapy. Nat Rev Cancer 5: 867–875, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Basu HS, Thompson TA, Church DR, Clower CC, Mehraein-Ghomi F, Amlong CA, Martin CT, Woster PM, Lindstrom MJ, and Wilding G. A small molecule polyamine oxidase inhibitor blocks androgen-induced oxidative stress and delays prostate cancer progression in the transgenic adenocarcinoma of the mouse prostate model. Cancer Res 69: 7689–7695, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baud V. and Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov 8: 33–40, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bensalah K, Lotan Y, Karam JA, and Shariat SF. New circulating biomarkers for prostate cancer. Prostate Cancer Prostatic Dis 11: 112–120, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Bierie B. and Moses HL. TGF-beta and cancer. Cytokine Growth Factor Rev 17: 29–40, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Bierie B. and Moses HL. Tumour microenvironment: TGFbeta: the molecular Jekyll and Hyde of cancer. Nat Rev Cancer 6: 506–520, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Biswas S, Guix M, Rinehart C, Dugger TC, Chytil A, Moses HL, Freeman ML, and Arteaga CL. Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Invest 117: 1305–1313, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonfil RD, Chinni S, Fridman R, Kim HR, and Cher ML. Proteases, growth factors, chemokines, and the microenvironment in prostate cancer bone metastasis. Urol Oncol 25: 407–411, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Bostwick DG, Alexander EE, Singh R, Shan A, Qian J, Santella RM, Oberley LW, Yan T, Zhong W, Jiang X, and Oberley TD. Antioxidant enzyme expression and reactive oxygen species damage in prostatic intraepithelial neoplasia and cancer. Cancer 89: 123–134, 2000 [PubMed] [Google Scholar]
- 22.Bostwick DG, Meiers I, and Shanks JH. Glutathione S-transferase: differential expression of alpha, mu, and pi isoenzymes in benign prostate, prostatic intraepithelial neoplasia, and prostatic adenocarcinoma. Hum Pathol 38: 1394–1401, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Boudreau HE, Casterline BW, Rada B, Korzeniowska A, and Leto TL. Nox4 involvement in TGF-beta and SMAD3-driven induction of the epithelial-to-mesenchymal transition and migration of breast epithelial cells. Free Radic Biol Med 53: 1489–1499, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bouquet F, Pal A, Pilones KA, Demaria S, Hann B, Akhurst RJ, Babb JS, Lonning SM, DeWyngaert JK, Formenti SC, and Barcellos-Hoff MH. TGFbeta1 inhibition increases the radiosensitivity of breast cancer cells in vitro and promotes tumor control by radiation in vivo. Clin Cancer Res 17: 6754–6765, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouraoui Y, Ricote M, Garcia-Tunon I, Rodriguez-Berriguete G, Touffehi M, Rais NB, Fraile B, Paniagua R, Oueslati R, and Royuela M. Pro-inflammatory cytokines and prostate-specific antigen in hyperplasia and human prostate cancer. Cancer Detect Prev 32: 23–32, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Bower JE, Ganz PA, Tao ML, Hu W, Belin TR, Sepah S, Cole S, and Aziz N. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clin Cancer Res 15: 5534–5540, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, and Siegel RM. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J Exp Med 208: 519–533, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Byun HO, Jung HJ, Seo YH, Lee YK, Hwang SC, Hwang ES, and Yoon G. GSK3 inactivation is involved in mitochondrial complex IV defect in transforming growth factor (TGF) beta1-induced senescence. Exp Cell Res 318: 1808–1819, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Carbo N, Busquets S, van Royen M, Alvarez B, Lopez-Soriano FJ, and Argiles JM. TNF-alpha is involved in activating DNA fragmentation in skeletal muscle. Br J Cancer 86: 1012–1016, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caruso DJ, Carmack AJ, Lokeshwar VB, Duncan RC, Soloway MS, and Lokeshwar BL. Osteopontin and interleukin-8 expression is independently associated with prostate cancer recurrence. Clin Cancer Res 14: 4111–4118, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charles KA, Kulbe H, Soper R, Escorcio-Correia M, Lawrence T, Schultheis A, Chakravarty P, Thompson RG, Kollias G, Smyth JF, Balkwill FR, and Hagemann T. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J Clin Invest 119: 3011–3023, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Q, Chai YC, Mazumder S, Jiang C, Macklis RM, Chisolm GM, and Almasan A. The late increase in intracellular free radical oxygen species during apoptosis is associated with cytochrome c release, caspase activation, and mitochondrial dysfunction. Cell Death Differ 10: 323–334, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chendil D, Ranga RS, Meigooni D, Sathishkumar S, and Ahmed MM. Curcumin confers radiosensitizing effect in prostate cancer cell line PC-3. Oncogene 23: 1599–1607, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Chiu HW, Chen YA, Ho SY, and Wang YJ. Arsenic trioxide enhances the radiation sensitivity of androgen-dependent and -independent human prostate cancer cells. PloS One 7: e31579, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chou CH, Chen PJ, Lee PH, Cheng AL, Hsu HC, and Cheng JC. Radiation-induced hepatitis B virus reactivation in liver mediated by the bystander effect from irradiated endothelial cells. Clin Cancer Res 13: 851–857, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Chun JY, Nadiminty N, Dutt S, Lou W, Yang JC, Kung HJ, Evans CP, and Gao AC. Interleukin-6 regulates androgen synthesis in prostate cancer cells. Clin Cancer Res 15: 4815–4822, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chung LW, Baseman A, Assikis V, and Zhau HE. Molecular insights into prostate cancer progression: the missing link of tumor microenvironment. J Urol 173: 10–20, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Condeelis J. and Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell 124: 263–266, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Coussens LM. and Werb Z. Inflammation and cancer. Nature 420: 860–867, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Culig Z. and Puhr M. Interleukin-6: a multifunctional targetable cytokine in human prostate cancer. Mol Cell Endocrinol 360: 52–58, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Culig Z, Steiner H, Bartsch G, and Hobisch A. Interleukin-6 regulation of prostate cancer cell growth. J Cell Biochem 95: 497–505, 2005 [DOI] [PubMed] [Google Scholar]
- 42.David SS, O'Shea VL, and Kundu S. Base-excision repair of oxidative DNA damage. Nature 447: 941–950, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deng X, Elzey BD, Poulson JM, Morrison WB, Ko SC, Hahn NM, Ratliff TL, and Hu CD. Ionizing radiation induces neuroendocrine differentiation of prostate cancer cells in vitro, in vivo and in prostate cancer patients. Am J Cancer Res 1: 834–844, 2011 [PMC free article] [PubMed] [Google Scholar]
- 44.Deng X, Liu H, Huang J, Cheng L, Keller ET, Parsons SJ, and Hu CD. Ionizing radiation induces prostate cancer neuroendocrine differentiation through interplay of CREB and ATF2: implications for disease progression. Cancer Res 68: 9663–9670, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dent P, Yacoub A, Fisher PB, Hagan MP, and Grant S. MAPK pathways in radiation responses. Oncogene 22: 5885–5896, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Dhar SK, Tangpong J, Chaiswing L, Oberley TD, and St Clair DK. Manganese superoxide dismutase is a p53-regulated gene that switches cancers between early and advanced stages. Cancer Res 71: 6684–6695, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Domingo-Domenech J, Mellado B, Ferrer B, Truan D, Codony-Servat J, Sauleda S, Alcover J, Campo E, Gascon P, Rovira A, Ross JS, Fernandez PL, and Albanell J. Activation of nuclear factor-kappaB in human prostate carcinogenesis and association to biochemical relapse. Br J Cancer 93: 1285–1294, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drabsch Y. and ten Dijke P. TGF-beta signalling and its role in cancer progression and metastasis. Cancer Metastasis Rev 31: 553–568, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Epperly MW, Osipov AN, Martin I, Kawai KK, Borisenko GG, Tyurina YY, Jefferson M, Bernarding M, Greenberger JS, and Kagan VE. Ascorbate as a “redox sensor” and protector against irradiation-induced oxidative stress in 32D CL 3 hematopoietic cells and subclones overexpressing human manganese superoxide dismutase. Int J Radiat Oncol Biol Phys 58: 851–861, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Fiaschi T. and Chiarugi P. Oxidative stress, tumor microenvironment, and metabolic reprogramming: a diabolic liaison. Int J Cell Biol 2012: 762825, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flechsig P, Dadrich M, Bickelhaupt S, Jenne J, Hauser K, Timke C, Peschke P, Hahn EW, Grone HJ, Yingling J, Lahn M, Wirkner U, and Huber PE. LY2109761 attenuates radiation-induced pulmonary murine fibrosis via reversal of TGF-beta and BMP-associated proinflammatory and proangiogenic signals. Clin Cancer Res 18: 3616–3627, 2012 [DOI] [PubMed] [Google Scholar]
- 52.Flores MB, Rocha GZ, Damas-Souza DM, Osorio-Costa F, Dias MM, Ropelle ER, Camargo JA, de Carvalho RB, Carvalho HF, Saad MJ, and Carvalheira JB. Obesity-induced increase in tumor necrosis factor-alpha leads to development of colon cancer in mice. Gastroenterology 143: 741–753e1–e4,2012 [DOI] [PubMed] [Google Scholar]
- 53.Gahan JC, Gosalbez M, Yates T, Young EE, Escudero DO, Chi A, Garcia-Roig M, Satyanarayana R, Soloway MS, Bird VG, and Lokeshwar VB. Chemokine and chemokine receptor expression in kidney tumors: molecular profiling of histological subtypes and association with metastasis. J Urol 187: 827–833, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gallet P, Phulpin B, Merlin JL, Leroux A, Bravetti P, Mecellem H, Tran N, and Dolivet G. Long-term alterations of cytokines and growth factors expression in irradiated tissues and relation with histological severity scoring. PloS One 6: e29399, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gannon PO, Godin-Ethier J, Hassler M, Delvoye N, Aversa M, Poisson AO, Peant B, Alam Fahmy M, Saad F, Lapointe R, and Mes-Masson AM. Androgen-regulated expression of arginase 1, arginase 2 and interleukin-8 in human prostate cancer. PloS One 5: e12107, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gladson CL. and Welch DR. New insights into the role of CXCR4 in prostate cancer metastasis. Cancer Biol Ther 7: 1849–1851, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonzalez-Ramos M, Mora I, de Frutos S, Garesse R, Rodriguez-Puyol M, Olmos G, and Rodriguez-Puyol D. Intracellular redox equilibrium is essential for the constitutive expression of AP-1 dependent genes in resting cells: studies on TGF-beta1 regulation. Int J Biochem Cell Biol 44: 963–971, 2012 [DOI] [PubMed] [Google Scholar]
- 58.Gough DJ, Corlett A, Schlessinger K, Wegrzyn J, Larner AC, and Levy DE. Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science 324: 1713–1716, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Govinden R. and Bhoola KD. Genealogy, expression, and cellular function of transforming growth factor-beta. Pharmacol Ther 98: 257–265, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Grady WM, Myeroff LL, Swinler SE, Rajput A, Thiagalingam S, Lutterbaugh JD, Neumann A, Brattain MG, Chang J, Kim SJ, Kinzler KW, Vogelstein B, Willson JK, and Markowitz S. Mutational inactivation of transforming growth factor beta receptor type II in microsatellite stable colon cancers. Cancer Res 59: 320–324, 1999 [PubMed] [Google Scholar]
- 61.Guo G, Yan-Sanders Y, Lyn-Cook BD, Wang T, Tamae D, Ogi J, Khaletskiy A, Li Z, Weydert C, Longmate JA, Huang TT, Spitz DR, Oberley LW, and Li JJ. Manganese superoxide dismutase-mediated gene expression in radiation-induced adaptive responses. Mol Cell Biol 23: 2362–2378, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guo Y, Xu F, Lu T, Duan Z, and Zhang Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev 38: 904–910, 2012 [DOI] [PubMed] [Google Scholar]
- 63.Gupta SC, Hevia D, Patchva S, Park B, Koh W, and Aggarwal BB. Upsides and downsides of reactive oxygen species for cancer: the roles of reactive oxygen species in tumorigenesis, prevention, and therapy. Antioxid Redox Signal 16: 1295–1322, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gupta-Elera G, Garrett AR, Robison RA, and O'Neill KL. The role of oxidative stress in prostate cancer. Eur J Cancer Prev 21: 155–162, 2012 [DOI] [PubMed] [Google Scholar]
- 65.Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, Robinson SC, and Balkwill FR. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med 205: 1261–1268, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hall EJ. Radiobiology for the Radiologist. Philadelphia: JB Lippincott Company, 1994 [Google Scholar]
- 67.Hanahan D. and Weinberg RA. Hallmarks of cancer: the next generation. Cell 144: 646–674, 2011 [DOI] [PubMed] [Google Scholar]
- 68.Hardee ME, Marciscano AE, Medina-Ramirez CM, Zagzag D, Narayana A, Lonning SM, and Barcellos-Hoff MH. Resistance of glioblastoma-initiating cells to radiation mediated by the tumor microenvironment can be abolished by inhibiting transforming growth factor-beta. Cancer Res 72: 4119–4129, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hardmeier R, Hoeger H, Fang-Kircher S, Khoschsorur A, and Lubec G. Transcription and activity of antioxidant enzymes after ionizing irradiation in radiation-resistant and radiation-sensitive mice. Proc Natl Acad Sci U S A 94: 7572–7576, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hobisch A, Ramoner R, Fuchs D, Godoy-Tundidor S, Bartsch G, Klocker H, and Culig Z. Prostate cancer cells (LNCaP) generated after long-term interleukin 6 (IL-6) treatment express IL-6 and acquire an IL-6 partially resistant phenotype. Clin Cancer Res 7: 2941–2948, 2001 [PubMed] [Google Scholar]
- 71.Holley AK, Xu Y, St Clair DK, and St Clair WH. RelB regulates manganese superoxide dismutase gene and resistance to ionizing radiation of prostate cancer cells. Ann N Y Acad Sci 1201: 129–136, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Holmes WE, Lee J, Kuang WJ, Rice GC, and Wood WI. Structure and functional expression of a human interleukin-8 receptor. Science 253: 1278–1280, 1991 [DOI] [PubMed] [Google Scholar]
- 73.Hosoki A, Yonekura S, Zhao QL, Wei ZL, Takasaki I, Tabuchi Y, Wang LL, Hasuike S, Nomura T, Tachibana A, Hashiguchi K, Yonei S, Kondo T, and Zhang-Akiyama QM. Mitochondria-targeted superoxide dismutase (SOD2) regulates radiation resistance and radiation stress response in HeLa cells. J Radiat Res 53: 58–71, 2012 [DOI] [PubMed] [Google Scholar]
- 74.Huang LE, Arany Z, Livingston DM, and Bunn HF. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J Biol Chem 271: 32253–32259, 1996 [DOI] [PubMed] [Google Scholar]
- 75.Hunter NR, Valdecanas D, Liao Z, Milas L, Thames HD, and Mason KA. Mitigation and treatment of radiation-induced thoracic injury with a cyclooxygenase-2 inhibitor, celecoxib. Int J Radiat Oncol Biol Phys 85: 472–476, 2012 [DOI] [PubMed] [Google Scholar]
- 76.Husbeck B, Peehl DM, and Knox SJ. Redox modulation of human prostate carcinoma cells by selenite increases radiation-induced cell killing. Free Radic Biol Med 38: 50–57, 2005 [DOI] [PubMed] [Google Scholar]
- 77.Ivanovic V, Melman A, Davis-Joseph B, Valcic M, and Geliebter J. Elevated plasma levels of TGF-beta 1 in patients with invasive prostate cancer. Nat Med 1: 282–284, 1995 [DOI] [PubMed] [Google Scholar]
- 78.Iyer R, Lehnert BE, and Svensson R. Factors underlying the cell growth-related bystander responses to alpha particles. Cancer Res 60: 1290–1298, 2000 [PubMed] [Google Scholar]
- 79.Josson S, Xu Y, Fang F, Dhar SK, St Clair DK, and St Clair WH. RelB regulates manganese superoxide dismutase gene and resistance to ionizing radiation of prostate cancer cells. Oncogene 25: 1554–1559, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kakkar P. and Singh BK. Mitochondria: a hub of redox activities and cellular distress control. Mol Cell Biochem 305: 235–253, 2007 [DOI] [PubMed] [Google Scholar]
- 81.Kamata H, Honda S, Maeda S, Chang L, Hirata H, and Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120: 649–661, 2005 [DOI] [PubMed] [Google Scholar]
- 82.Kang HY, Lin HK, Hu YC, Yeh S, Huang KE, and Chang C. From transforming growth factor-beta signaling to androgen action: identification of Smad3 as an androgen receptor coregulator in prostate cancer cells. Proc Natl Acad Sci U S A 98: 3018–3023, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kang MA, So EY, Simons AL, Spitz DR, and Ouchi T. DNA damage induces reactive oxygen species generation through the H2AX-Nox1/Rac1 pathway. Cell Death Dis 3: e249, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kang SK, Rabbani ZN, Folz RJ, Golson ML, Huang H, Yu D, Samulski TS, Dewhirst MW, Anscher MS, and Vujaskovic Z. Overexpression of extracellular superoxide dismutase protects mice from radiation-induced lung injury. Int J Radiat Oncol Biol Phys 57: 1056–1066, 2003 [DOI] [PubMed] [Google Scholar]
- 85.Kawada M, Inoue H, Arakawa M, and Ikeda D. Transforming growth factor-beta1 modulates tumor-stromal cell interactions of prostate cancer through insulin-like growth factor-I. Anticancer Res 28: 721–730, 2008 [PubMed] [Google Scholar]
- 86.Khandrika L, Kumar B, Koul S, Maroni P, and Koul HK. Oxidative stress in prostate cancer. Cancer Lett 282: 125–136, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kil WJ, Tofilon PJ, and Camphausen K. Post-radiation increase in VEGF enhances glioma cell motility in vitro. Radiat Oncol 7: 25, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim BY, Kim KA, Kwon O, Kim SO, Kim MS, Kim BS, Oh WK, Kim GD, Jung M, and Ahn JS. NF-kappaB inhibition radiosensitizes Ki-Ras-transformed cells to ionizing radiation. Carcinogenesis 26: 1395–1403, 2005 [DOI] [PubMed] [Google Scholar]
- 89.Kim MH, Minton AZ, and Agrawal V. C/EBPbeta regulates metastatic gene expression and confers TNF-alpha resistance to prostate cancer cells. Prostate 69: 1435–1447, 2009 [DOI] [PubMed] [Google Scholar]
- 90.Kim YS, Morgan MJ, Choksi S, and Liu ZG. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol Cell 26: 675–687, 2007 [DOI] [PubMed] [Google Scholar]
- 91.Kimura K, Bowen C, Spiegel S, and Gelmann EP. Tumor necrosis factor-alpha sensitizes prostate cancer cells to gamma-irradiation-induced apoptosis. Cancer Res 59: 1606–1614, 1999 [PubMed] [Google Scholar]
- 92.Kishimoto T, Akira S, Narazaki M, and Taga T. Interleukin-6 family of cytokines and gp130. Blood 86: 1243–1254, 1995 [PubMed] [Google Scholar]
- 93.Kitagawa Y, Dai J, Zhang J, Keller JM, Nor J, Yao Z, and Keller ET. Vascular endothelial growth factor contributes to prostate cancer-mediated osteoblastic activity. Cancer Res 65: 10921–10929, 2005 [DOI] [PubMed] [Google Scholar]
- 94.Ko S, Shi L, Kim S, Song CS, and Chatterjee B. Interplay of nuclear factor-kappaB and B-myb in the negative regulation of androgen receptor expression by tumor necrosis factor alpha. Mol Endocrinol 22: 273–286, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kong Z, Xie D, Boike T, Raghavan P, Burma S, Chen DJ, Habib AA, Chakraborty A, Hsieh JT, and Saha D. Downregulation of human DAB2IP gene expression in prostate cancer cells results in resistance to ionizing radiation. Cancer Res 70: 2829–2839, 2010 [DOI] [PubMed] [Google Scholar]
- 96.Koul HK, Kumar B, Koul S, Deb AA, Hwa JS, Maroni P, van Bokhoven A, Lucia MS, Kim FJ, and Meacham RB. The role of inflammation and infection in prostate cancer: importance in prevention, diagnosis and treatment. Drugs Today 46: 929–943, 2010 [DOI] [PubMed] [Google Scholar]
- 97.Kryston TB, Georgiev AB, Pissis P, and Georgakilas AG. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res 711: 193–201, 2011 [DOI] [PubMed] [Google Scholar]
- 98.Kumar B, Koul S, Khandrika L, Meacham RB, and Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res 68: 1777–1785, 2008 [DOI] [PubMed] [Google Scholar]
- 99.Larsen L. and Ropke C. Suppressors of cytokine signalling: SOCS. APMIS 110: 833–844, 2002 [DOI] [PubMed] [Google Scholar]
- 100.Lejeune FJ. Clinical use of TNF revisited: improving penetration of anti-cancer agents by increasing vascular permeability. J Clin Invest 110: 433–435, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lenaz G. and Genova ML. Structure and organization of mitochondrial respiratory complexes: a new understanding of an old subject. Antioxid Redox Signal 12: 961–1008, 2010 [DOI] [PubMed] [Google Scholar]
- 102.Levine AJ, Momand J, and Finlay CA. The p53 tumour suppressor gene. Nature 351: 453–456, 1991 [DOI] [PubMed] [Google Scholar]
- 103.Li F. and Sethi G. Targeting transcription factor NF-kappaB to overcome chemoresistance and radioresistance in cancer therapy. Biochim Biophys Acta 1805: 167–180, 2010 [DOI] [PubMed] [Google Scholar]
- 104.Li JM, Fan LM, Christie MR, and Shah AM. Acute tumor necrosis factor alpha signaling via NADPH oxidase in microvascular endothelial cells: role of p47phox phosphorylation and binding to TRAF4. Mol Cell Biol 25: 2320–2330, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Linard C, Ropenga A, Vozenin-Brotons MC, Chapel A, and Mathe D. Abdominal irradiation increases inflammatory cytokine expression and activates NF-kappaB in rat ileal muscularis layer. Am J Physiol Gastrointest Liver Physiol 285: G556–G565, 2003 [DOI] [PubMed] [Google Scholar]
- 106.Loetscher H, Pan YC, Lahm HW, Gentz R, Brockhaus M, Tabuchi H, and Lesslauer W. Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell 61: 351–359, 1990 [DOI] [PubMed] [Google Scholar]
- 107.Lu L, Tang D, Wang L, Huang LQ, Jiang GS, Xiao XY, and Zeng FQ. Gambogic acid inhibits TNF-alpha-induced invasion of human prostate cancer PC3 cells in vitro through PI3K/Akt and NF-kappaB signaling pathways. Acta Pharmacol Sin 33: 531–541, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Martin M, Lefaix J, and Delanian S. TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys 47: 277–290, 2000 [DOI] [PubMed] [Google Scholar]
- 109.Martinez-Outschoorn UE, Balliet RM, Rivadeneira DB, Chiavarina B, Pavlides S, Wang C, Whitaker-Menezes D, Daumer KM, Lin Z, Witkiewicz AK, Flomenberg N, Howell A, Pestell RG, Knudsen ES, Sotgia F, and Lisanti MP. Oxidative stress in cancer associated fibroblasts drives tumor-stroma co-evolution: a new paradigm for understanding tumor metabolism, the field effect and genomic instability in cancer cells. Cell Cycle 9: 3256–3276, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Masuda M, Wakasaki T, Suzui M, Toh S, Joe AK, and Weinstein IB. Stat3 orchestrates tumor development and progression: the Achilles' heel of head and neck cancers? Curr Cancer Drug Targets 10: 117–126, 2010 [DOI] [PubMed] [Google Scholar]
- 111.McBride WH, Chiang CS, Olson JL, Wang CC, Hong JH, Pajonk F, Dougherty GJ, Iwamoto KS, Pervan M, and Liao YP. A sense of danger from radiation. Radiat Res 162: 1–19, 2004 [DOI] [PubMed] [Google Scholar]
- 112.Mehrotra S, Pecaut MJ, Freeman TL, Crapo JD, Rizvi A, Luo-Owen X, Slater JM, and Gridley DS. Analysis of a metalloporphyrin antioxidant mimetic (MnTE-2-PyP) as a radiomitigator: prostate tumor and immune status. Technol Cancer Res Treat 11: 447–457, 2012 [DOI] [PubMed] [Google Scholar]
- 113.Mettler F. and Upton A. Medical Effects of Ionizing Radiation. Philadelphia, PA: Saunders Elsvier, 2008 [Google Scholar]
- 114.Meulmeester E. and Ten Dijke P. The dynamic roles of TGF-beta in cancer. J Pathol 223: 205–218, 2011 [DOI] [PubMed] [Google Scholar]
- 115.Miao L. and St Clair DK. Regulation of superoxide dismutase genes: implications in disease. Free Radic Biol Med 47: 344–356, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Michaeloudes C, Sukkar MB, Khorasani NM, Bhavsar PK, and Chung KF. TGF-beta regulates Nox4, MnSOD and catalase expression, and IL-6 release in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 300: L295–L304, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Michalaki V, Syrigos K, Charles P, and Waxman J. Serum levels of IL-6 and TNF-alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. Br J Cancer 90: 2312–2316, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mizokami A, Gotoh A, Yamada H, Keller ET, and Matsumoto T. Tumor necrosis factor-alpha represses androgen sensitivity in the LNCaP prostate cancer cell line. J Urol 164: 800–805, 2000 [DOI] [PubMed] [Google Scholar]
- 119.Mocellin S, Rossi CR, Pilati P, and Nitti D. Tumor necrosis factor, cancer and anticancer therapy. Cytokine Growth Factor Rev 16: 35–53, 2005 [DOI] [PubMed] [Google Scholar]
- 120.Morales A, Miranda M, Sanchez-Reyes A, Biete A, and Fernandez-Checa JC. Oxidative damage of mitochondrial and nuclear DNA induced by ionizing radiation in human hepatoblastoma cells. Int J Radiat Oncol Biol Phys 42: 191–203, 1998 [DOI] [PubMed] [Google Scholar]
- 121.Morton DM. and Barrack ER. Modulation of transforming growth factor beta 1 effects on prostate cancer cell proliferation by growth factors and extracellular matrix. Cancer Res 55: 2596–2602, 1995 [PubMed] [Google Scholar]
- 122.Mukherjee A. and Martin SG. The thioredoxin system: a key target in tumour and endothelial cells. Br J Radiol 81Spec No 1: S57–S68, 2008 [DOI] [PubMed] [Google Scholar]
- 123.Multhoff G. and Radons J. Radiation, inflammation, and immune responses in cancer. Front Oncol 2: 58, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Murley JS, Kataoka Y, Baker KL, Diamond AM, Morgan WF, and Grdina DJ. Manganese superoxide dismutase (SOD2)-mediated delayed radioprotection induced by the free thiol form of amifostine and tumor necrosis factor alpha. Radiat Res 167: 465–474, 2007 [DOI] [PubMed] [Google Scholar]
- 125.Naka K, Muraguchi T, Hoshii T, and Hirao A. Regulation of reactive oxygen species and genomic stability in hematopoietic stem cells. Antioxid Redox Signal 10: 1883–1894, 2008 [DOI] [PubMed] [Google Scholar]
- 126.Nakashima J, Tachibana M, Horiguchi Y, Oya M, Ohigashi T, Asakura H, and Murai M. Serum interleukin 6 as a prognostic factor in patients with prostate cancer. Clin Cancer Res 6: 2702–2706, 2000 [PubMed] [Google Scholar]
- 127.Nakashima J, Tachibana M, Ueno M, Miyajima A, Baba S, and Murai M. Association between tumor necrosis factor in serum and cachexia in patients with prostate cancer. Clin Cancer Res 4: 1743–1748, 1998 [PubMed] [Google Scholar]
- 128.Narayanan PK, LaRue KE, Goodwin EH, and Lehnert BE. Alpha particles induce the production of interleukin-8 by human cells. Radiat Res 152: 57–63, 1999 [PubMed] [Google Scholar]
- 129.Nelson EC, Cambio AJ, Yang JC, Ok JH, Lara PN, Jr., and Evans CP. Clinical implications of neuroendocrine differentiation in prostate cancer. Prostate Cancer Prostatic Dis 10: 6–14, 2007 [DOI] [PubMed] [Google Scholar]
- 130.Nguyen DH, Oketch-Rabah HA, Illa-Bochaca I, Geyer FC, Reis-Filho JS, Mao JH, Ravani SA, Zavadil J, Borowsky AD, Jerry DJ, Dunphy KA, Seo JH, Haslam S, Medina D, and Barcellos-Hoff MH. Radiation acts on the microenvironment to affect breast carcinogenesis by distinct mechanisms that decrease cancer latency and affect tumor type. Cancer Cell 19: 640–651, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Oakley FD, Abbott D, Li Q, and Engelhardt JF. Signaling components of redox active endosomes: the redoxosomes. Antioxid Redox Signal 11: 1313–1333, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ogawa Y, Kobayashi T, Nishioka A, Kariya S, Hamasato S, Seguchi H, and Yoshida S. Radiation-induced reactive oxygen species formation prior to oxidative DNA damage in human peripheral T cells. Int J Mol Med 11: 149–152, 2003 [PubMed] [Google Scholar]
- 133.Okamoto M, Lee C, and Oyasu R. Interleukin-6 as a paracrine and autocrine growth factor in human prostatic carcinoma cells in vitro. Cancer Res 57: 141–146, 1997 [PubMed] [Google Scholar]
- 134.Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, and Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res 59: 5002–5011, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Oshikawa J, Urao N, Kim HW, Kaplan N, Razvi M, McKinney R, Poole LB, Fukai T, and Ushio-Fukai M. Extracellular SOD-derived H2O2 promotes VEGF signaling in caveolae/lipid rafts and post-ischemic angiogenesis in mice. PloS One 5: e10189, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Park JI, Lee MG, Cho K, Park BJ, Chae KS, Byun DS, Ryu BK, Park YK, and Chi SG. Transforming growth factor-beta1 activates interleukin-6 expression in prostate cancer cells through the synergistic collaboration of the Smad2, p38-NF-kappaB, JNK, and Ras signaling pathways. Oncogene 22: 4314–4332, 2003 [DOI] [PubMed] [Google Scholar]
- 137.Park JS, Qiao L, Su ZZ, Hinman D, Willoughby K, McKinstry R, Yacoub A, Duigou GJ, Young CS, Grant S, Hagan MP, Ellis E, Fisher PB, and Dent P. Ionizing radiation modulates vascular endothelial growth factor (VEGF) expression through multiple mitogen activated protein kinase dependent pathways. Oncogene 20: 3266–3280, 2001 [DOI] [PubMed] [Google Scholar]
- 138.Pavlides S, Vera I, Gandara R, Sneddon S, Pestell RG, Mercier I, Martinez-Outschoorn UE, Whitaker-Menezes D, Howell A, Sotgia F, and Lisanti MP. Warburg meets autophagy: cancer-associated fibroblasts accelerate tumor growth and metastasis via oxidative stress, mitophagy, and aerobic glycolysis. Antioxid Redox Signal 16: 1264–1284, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Perry KT, Anthony CT, Case T, and Steiner MS. Transforming growth factor beta as a clinical biomarker for prostate cancer. Urology 49: 151–155, 1997 [DOI] [PubMed] [Google Scholar]
- 140.Pfitzenmaier J, Vessella R, Higano CS, Noteboom JL, Wallace D, Jr., and Corey E. Elevation of cytokine levels in cachectic patients with prostate carcinoma. Cancer 97: 1211–1216, 2003 [DOI] [PubMed] [Google Scholar]
- 141.Ping X, Junqing J, Junfeng J, and Enjin J. Radioprotective effects of troxerutin against gamma irradiation in mice liver. Int J Radiat Biol 88: 607–612, 2012 [DOI] [PubMed] [Google Scholar]
- 142.Prise KM. and O'Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer 9: 351–360, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pu H, Collazo J, Jones E, Gayheart D, Sakamoto S, Vogt A, Mitchell B, and Kyprianou N. Dysfunctional transforming growth factor-beta receptor II accelerates prostate tumorigenesis in the TRAMP mouse model. Cancer Res 69: 7366–7374, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Qiu Y, Robinson D, Pretlow TG, and Kung HJ. Etk/Bmx, a tyrosine kinase with a pleckstrin-homology domain, is an effector of phosphatidylinositol 3'-kinase and is involved in interleukin 6-induced neuroendocrine differentiation of prostate cancer cells. Proc Natl Acad Sci U S A 95: 3644–3649, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Quiros-Gonzalez I, Sainz RM, Hevia D, and Mayo JC. MnSOD drives neuroendocrine differentiation, androgen independence, and cell survival in prostate cancer cells. Free Radic Biol Med 50: 525–536, 2011 [DOI] [PubMed] [Google Scholar]
- 146.Rabbani ZN, Anscher MS, Zhang X, Chen L, Samulski TV, Li CY, and Vujaskovic Z. Soluble TGFbeta type II receptor gene therapy ameliorates acute radiation-induced pulmonary injury in rats. Int J Radiat Oncol Biol Phys 57: 563–572, 2003 [DOI] [PubMed] [Google Scholar]
- 147.Reuter S, Gupta SC, Chaturvedi MM, and Aggarwal BB. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic Biol Med 49: 1603–1616, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Riley PA. Free radicals in biology: oxidative stress and the effects of ionizing radiation. Int J Radiat Biol 65: 27–33, 1994 [DOI] [PubMed] [Google Scholar]
- 149.Rivas MA, Carnevale RP, Proietti CJ, Rosemblit C, Beguelin W, Salatino M, Charreau EH, Frahm I, Sapia S, Brouckaert P, Elizalde PV, and Schillaci R. TNF alpha acting on TNFR1 promotes breast cancer growth via p42/P44 MAPK, JNK, Akt and NF-kappa B-dependent pathways. Exp Cell Res 314: 509–529, 2008 [DOI] [PubMed] [Google Scholar]
- 150.Rose-John S. Coordination of interleukin-6 biology by membrane bound and soluble receptors. Adv Exp Med Biol 495: 145–151, 2001 [DOI] [PubMed] [Google Scholar]
- 151.Rzymski P, Opala T, Wilczak M, Wozniak J, and Sajdak S. Serum tumor necrosis factor alpha receptors p55/p75 ratio and ovarian cancer detection. Int J Gynaecol Obstet 88: 292–298, 2005 [DOI] [PubMed] [Google Scholar]
- 152.Salama S, Diaz-Arrastia C, Patel D, Botting S, and Hatch S. 2-Methoxyestradiol, an endogenous estrogen metabolite, sensitizes radioresistant MCF-7/FIR breast cancer cells through multiple mechanisms. Int J Radiat Oncol Biol Phys 80: 231–239, 2011 [DOI] [PubMed] [Google Scholar]
- 153.Scheller J, Ohnesorge N, and Rose-John S. Interleukin-6 trans-signalling in chronic inflammation and cancer. Scand J Immunol 63: 321–329, 2006 [DOI] [PubMed] [Google Scholar]
- 154.Scherz-Shouval R. and Elazar Z. ROS, mitochondria and the regulation of autophagy. Trends Cell Biol 17: 422–427, 2007 [DOI] [PubMed] [Google Scholar]
- 155.Schirmer MA, Brockmoller J, Rave-Frank M, Virsik P, Wilken B, Kuhnle E, Campean R, Hoffmann AO, Muller K, Goetze RG, Neumann M, Janke JH, Nasser F, Wolff HA, Ghadimi BM, Schmidberger H, Hess CF, Christiansen H, and Hille A. A putatively functional haplotype in the gene encoding transforming growth factor beta-1 as a potential biomarker for radiosensitivity. Int J Radiat Oncol Biol Phys 79: 866–874, 2011 [DOI] [PubMed] [Google Scholar]
- 156.Schreiber S, Nikolaus S, and Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut 42: 477–484, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell 10: 175–176, 2006 [DOI] [PubMed] [Google Scholar]
- 158.Seaton A, Maxwell PJ, Hill A, Gallagher R, Pettigrew J, Wilson RH, and Waugh DJ. Inhibition of constitutive and cxc-chemokine-induced NF-kappaB activity potentiates ansamycin-based HSP90-inhibitor cytotoxicity in castrate-resistant prostate cancer cells. Br J Cancer 101: 1620–1629, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Seaton A, Scullin P, Maxwell PJ, Wilson C, Pettigrew J, Gallagher R, O'Sullivan JM, Johnston PG, and Waugh DJ. Interleukin-8 signaling promotes androgen-independent proliferation of prostate cancer cells via induction of androgen receptor expression and activation. Carcinogenesis 29: 1148–1156, 2008 [DOI] [PubMed] [Google Scholar]
- 160.Shamaladevi N, Lyn DA, Escudero DO, and Lokeshwar BL. CXC receptor-1 silencing inhibits androgen-independent prostate cancer. Cancer Res 69: 8265–8274, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shan W, Zhong W, Zhao R, and Oberley TD. Thioredoxin 1 as a subcellular biomarker of redox imbalance in human prostate cancer progression. Free Radic Biol Med 49: 2078–2087, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shariat SF, Shalev M, Menesses-Diaz A, Kim IY, Kattan MW, Wheeler TM, and Slawin KM. Preoperative plasma levels of transforming growth factor beta(1) (TGF-beta(1)) strongly predict progression in patients undergoing radical prostatectomy. J Clin Oncol 19: 2856–2864, 2001 [DOI] [PubMed] [Google Scholar]
- 163.Sharifi N, Hurt EM, Thomas SB, and Farrar WL. Effects of manganese superoxide dismutase silencing on androgen receptor function and gene regulation: implications for castration-resistant prostate cancer. Clin Cancer Res 14: 6073–6080, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sheets NC, Goldin GH, Meyer AM, Wu Y, Chang Y, Sturmer T, Holmes JA, Reeve BB, Godley PA, Carpenter WR, and Chen RC. Intensity-modulated radiation therapy, proton therapy, or conformal radiation therapy and morbidity and disease control in localized prostate cancer. JAMA 307: 1611–1620, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shiota M, Yokomizo A, and Naito S. Oxidative stress and androgen receptor signaling in the development and progression of castration-resistant prostate cancer. Free Radic Biol Med 51: 1320–1328, 2011 [DOI] [PubMed] [Google Scholar]
- 166.Shiota M, Yokomizo A, Tada Y, Inokuchi J, Kashiwagi E, Masubuchi D, Eto M, Uchiumi T, and Naito S. Castration resistance of prostate cancer cells caused by castration-induced oxidative stress through Twist1 and androgen receptor overexpression. Oncogene 29: 237–250, 2010 [DOI] [PubMed] [Google Scholar]
- 167.Siegel R, Naishadham D, and Jemal A. Cancer statistics, 2012. CA Cancer J Clin 62: 10–29, 2012 [DOI] [PubMed] [Google Scholar]
- 168.Singh RK. and Lokeshwar BL. Depletion of intrinsic expression of interleukin-8 in prostate cancer cells causes cell cycle arrest, spontaneous apoptosis and increases the efficacy of chemotherapeutic drugs. Mol Cancer 8: 57, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Singh RK. and Lokeshwar BL. The IL-8-regulated chemokine receptor CXCR7 stimulates EGFR signaling to promote prostate cancer growth. Cancer Res 71: 3268–3277, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Singh RK, Sudhakar A, and Lokeshwar BL. Role of chemokines and chemokine receptors in prostate cancer development and progression. J Cancer Sci Ther 2: 89–94, 2010 [DOI] [PubMed] [Google Scholar]
- 171.Skvortsova I, Skvortsov S, Stasyk T, Raju U, Popper BA, Schiestl B, von Guggenberg E, Neher A, Bonn GK, Huber LA, and Lukas P. Intracellular signaling pathways regulating radioresistance of human prostate carcinoma cells. Proteomics 8: 4521–4533, 2008 [DOI] [PubMed] [Google Scholar]
- 172.Slupphaug G, Kavli B, and Krokan HE. The interacting pathways for prevention and repair of oxidative DNA damage. Mutat Res 531: 231–251, 2003 [DOI] [PubMed] [Google Scholar]
- 173.Smith CA, Davis T, Anderson D, Solam L, Beckmann MP, Jerzy R, Dower SK, Cosman D, and Goodwin RG. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science 248: 1019–1023, 1990 [DOI] [PubMed] [Google Scholar]
- 174.Sorbara MT. and Girardin SE. Mitochondrial ROS fuel the inflammasome. Cell Res 21: 558–560, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Spiotto MT. and Chung TD. STAT3 mediates IL-6-induced neuroendocrine differentiation in prostate cancer cells. Prostate 42: 186–195, 2000 [DOI] [PubMed] [Google Scholar]
- 176.Steuber T, O'Brien MF, and Lilja H. Serum markers for prostate cancer: a rational approach to the literature. Eur Urol 54: 31–40, 2008 [DOI] [PubMed] [Google Scholar]
- 177.Sun Y. and Oberley LW. Redox regulation of transcriptional activators. Free Radic Biol Med 21: 335–348, 1996 [DOI] [PubMed] [Google Scholar]
- 178.Sun Y, St Clair DK, Xu Y, Crooks PA, and St Clair WH. A NADPH oxidase-dependent redox signaling pathway mediates the selective radiosensitization effect of parthenolide in prostate cancer cells. Cancer Res 70: 2880–2890, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tateishi Y, Sasabe E, Ueta E, and Yamamoto T. Ionizing irradiation induces apoptotic damage of salivary gland acinar cells via NADPH oxidase 1-dependent superoxide generation. Biochem Biophys Res Commun 366: 301–307, 2008 [DOI] [PubMed] [Google Scholar]
- 180.Tell G, Quadrifoglio F, Tiribelli C, and Kelley MR. The many functions of APE1/Ref-1: not only a DNA repair enzyme. Antioxid Redox Signal 11: 601–620, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Thompson I, Thrasher JB, Aus G, Burnett AL, Canby-Hagino ED, Cookson MS, D'Amico AV, Dmochowski RR, Eton DT, Forman JD, Goldenberg SL, Hernandez J, Higano CS, Kraus SR, Moul JW, and Tangen CM. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol 177: 2106–2131, 2007 [DOI] [PubMed] [Google Scholar]
- 182.Tisdale MJ. Mechanisms of cancer cachexia. Physiol Rev 89: 381–410, 2009 [DOI] [PubMed] [Google Scholar]
- 183.Trachootham D, Alexandre J, and Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 8: 579–591, 2009 [DOI] [PubMed] [Google Scholar]
- 184.Tsui KH, Lin YF, Chen YH, Chang PL, and Juang HH. Mechanisms by which interleukin-6 regulates prostate-specific antigen gene expression in prostate LNCaP carcinoma cells. J Androl 32: 383–393, 2011 [DOI] [PubMed] [Google Scholar]
- 185.Valerie NC, Casarez EV, Dasilva JO, Dunlap-Brown ME, Parsons SJ, Amorino GP, and Dziegielewski J. Inhibition of neurotensin receptor 1 selectively sensitizes prostate cancer to ionizing radiation. Cancer Res 71: 6817–6826, 2011 [DOI] [PubMed] [Google Scholar]
- 186.Vlahopoulos S, Boldogh I, Casola A, and Brasier AR. Nuclear factor-kappaB-dependent induction of interleukin-8 gene expression by tumor necrosis factor alpha: evidence for an antioxidant sensitive activating pathway distinct from nuclear translocation. Blood 94: 1878–1889, 1999 [PubMed] [Google Scholar]
- 187.Wallace DC. Diseases of the mitochondrial DNA. Annu Rev Biochem 61: 1175–1212, 1992 [DOI] [PubMed] [Google Scholar]
- 188.Wang D, Lu S, and Dong Z. Regulation of TGF-beta1 gene transcription in human prostate cancer cells by nitric oxide. Prostate 67: 1825–1833, 2007 [DOI] [PubMed] [Google Scholar]
- 189.Waugh DJ. and Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res 14: 6735–6741, 2008 [DOI] [PubMed] [Google Scholar]
- 190.Whitaker-Menezes D, Martinez-Outschoorn UE, Lin Z, Ertel A, Flomenberg N, Witkiewicz AK, Birbe RC, Howell A, Pavlides S, Gandara R, Pestell RG, Sotgia F, Philp NJ, and Lisanti MP. Evidence for a stromal-epithelial “lactate shuttle” in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle 10: 1772–1783, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wiegman EM, Blaese MA, Loeffler H, Coppes RP, and Rodemann HP. TGFbeta-1 dependent fast stimulation of ATM and p53 phosphorylation following exposure to ionizing radiation does not involve TGFbeta-receptor I signalling. Radiother Oncol 83: 289–295, 2007 [DOI] [PubMed] [Google Scholar]
- 192.Wikstrom P, Stattin P, Franck-Lissbrant I, Damber JE, and Bergh A. Transforming growth factor beta1 is associated with angiogenesis, metastasis, and poor clinical outcome in prostate cancer. Prostate 37: 19–29, 1998 [DOI] [PubMed] [Google Scholar]
- 193.Wilson C, Purcell C, Seaton A, Oladipo O, Maxwell PJ, O'Sullivan JM, Wilson RH, Johnston PG, and Waugh DJ. Chemotherapy-induced CXC-chemokine/CXC-chemokine receptor signaling in metastatic prostate cancer cells confers resistance to oxaliplatin through potentiation of nuclear factor-kappaB transcription and evasion of apoptosis. J Pharmacol Exp Ther 327: 746–759, 2008 [DOI] [PubMed] [Google Scholar]
- 194.Wilson C, Wilson T, Johnston PG, Longley DB, and Waugh DJ. Interleukin-8 signaling attenuates TRAIL- and chemotherapy-induced apoptosis through transcriptional regulation of c-FLIP in prostate cancer cells. Mol Cancer Ther 7: 2649–2661, 2008 [DOI] [PubMed] [Google Scholar]
- 195.Wolff JM, Fandel TH, Borchers H, and Jakse G. Serum concentrations of transforming growth factor-beta 1 in patients with benign and malignant prostatic diseases. Anticancer Res 19: 2657–2659, 1999 [PubMed] [Google Scholar]
- 196.Xu Y, Fang F, St Clair DK, and St Clair WH. Inverse relationship between PSA and IL-8 in prostate cancer: an insight into a NF-kappaB-mediated mechanism. PloS One 7: e32905, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Xu Y, Josson S, Fang F, Oberley TD, St Clair DK, Wan XS, Sun Y, Bakthavatchalu V, Muthuswamy A, and St Clair WH. RelB enhances prostate cancer growth: implications for the role of the nuclear factor-kappaB alternative pathway in tumorigenicity. Cancer Res 69: 3267–3271, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Yoshida T, Goto S, Kawakatsu M, Urata Y, and Li TS. Mitochondrial dysfunction, a probable cause of persistent oxidative stress after exposure to ionizing radiation. Free Radic Res 46: 147–153, 2012 [DOI] [PubMed] [Google Scholar]
- 199.Yu H. and Jove R. The STATs of cancer—new molecular targets come of age. Nat Rev Cancer 4: 97–105, 2004 [DOI] [PubMed] [Google Scholar]
- 200.Zhang S, Song C, Zhou J, Xie L, Meng X, Liu P, Cao J, Zhang X, Ding WQ, and Wu J. Amelioration of radiation-induced skin injury by adenovirus-mediated heme oxygenase-1 (HO-1) overexpression in rats. Radiat Oncol 7: 4, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhou H, Ivanov VN, Gillespie J, Geard CR, Amundson SA, Brenner DJ, Yu Z, Lieberman HB, and Hei TK. Mechanism of radiation-induced bystander effect: role of the cyclooxygenase-2 signaling pathway. Proc Natl Acad Sci U S A 102: 14641–14646, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhou J. and Du Y. Acquisition of resistance of pancreatic cancer cells to 2-methoxyestradiol is associated with the upregulation of manganese superoxide dismutase. Mol Cancer Res 10: 768–777, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Zhu ML. and Kyprianou N. Androgen receptor and growth factor signaling cross-talk in prostate cancer cells. Endocr-Relat Cancer 15: 841–849, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]