Abstract
Hybrid dysgenesis is manifested as a group of correlated aberrant genetic traits such as sterility, increased mutation rate, and male recombination. Previous work has shown that it appears when males of strains carrying either of two independent families of transposable elements called I and P factors are hybridized with females of susceptible strains called R and M, respectively. Here the results of an extensive survey for dysgenic potential in Drosophila melanogaster strains are reported. Striking temporal trends in the distribution of strains were observed with respect to the two transposable element systems; in particular, the frequency of R and M strains is positively correlated with laboratory age. In recent tests of strain samples, those collected from nature about 50 years ago were the earliest observed to possess I characteristics. The I type was increasingly frequent in samples from strains more recently originating in the wild. This type is apparently ubiquitous in present day natural populations. the P type was not found in strain samples collected before 1950, and collections made subsequently showed increasing frequencies of P-factor activity with decreasing laboratory age. Marked geographical patterns are documented in the contemporary worldwide distribution of variant strains within the P-M system. M strains are currently fairly common in natural populations from various parts of the world, except on the American continent where they are rare. The degree and distribution of quantitative variation within M and P strain categories is related to their time of origin in the wild. The implications of these results are discussed in relation to the hypothesis that hybrid dysgenesis determinants have evolved recently in natural populations and to an alternative hypothesis of laboratory evolution.
Keywords: transposable elements, non-Mendelian inheritance, geographic variation, speciation
Full text
PDF
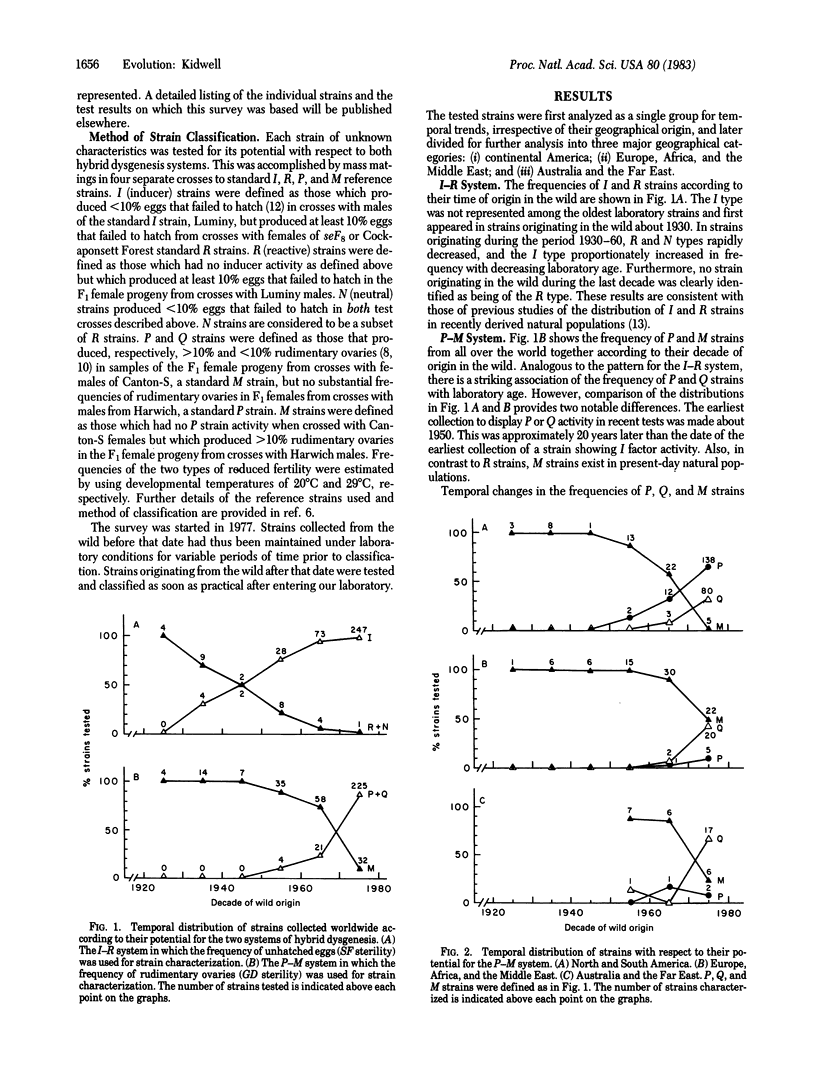
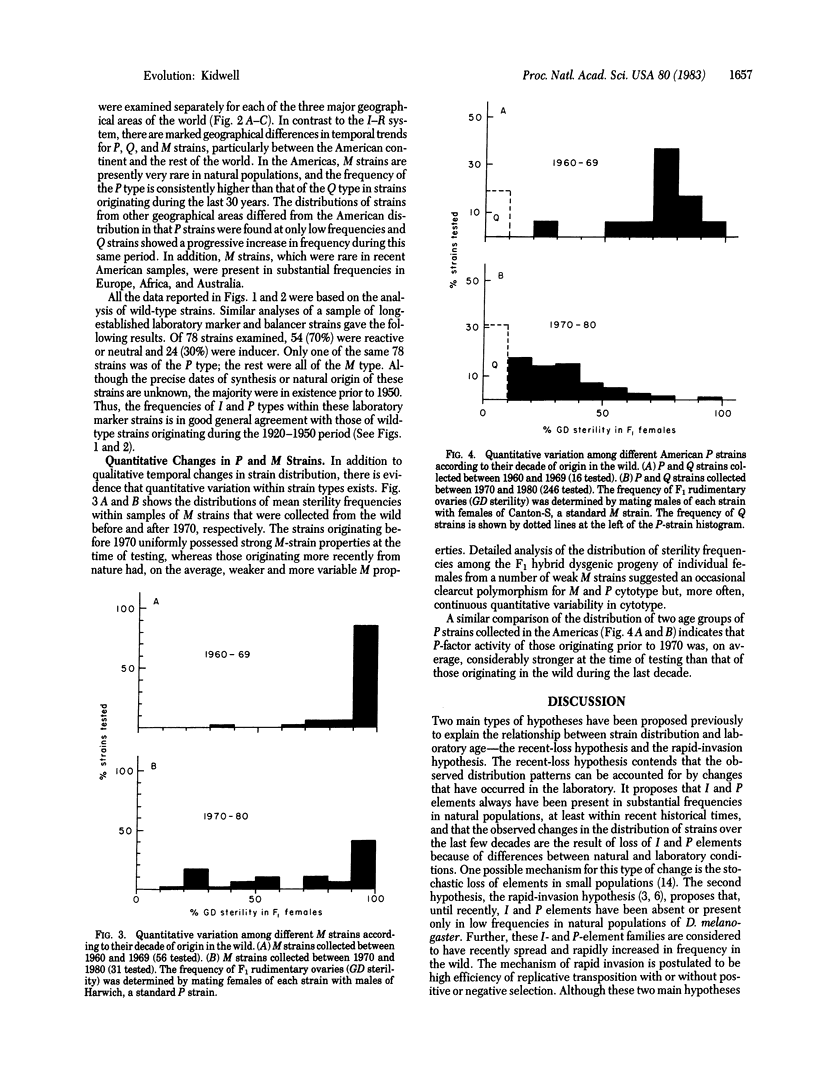


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bingham P. M., Kidwell M. G., Rubin G. M. The molecular basis of P-M hybrid dysgenesis: the role of the P element, a P-strain-specific transposon family. Cell. 1982 Jul;29(3):995–1004. doi: 10.1016/0092-8674(82)90463-9. [DOI] [PubMed] [Google Scholar]
- Bucheton A., Lavige J. M., Picard G., L'Heritier P. Non-mendelian female sterility in Drosophila melanogaster: quantitative variations in the efficiency of inducer and reactive strains. Heredity (Edinb) 1976 Jun;36(3):305–314. doi: 10.1038/hdy.1976.38. [DOI] [PubMed] [Google Scholar]
- Engels W. R. Extrachromosomal control of mutability in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4011–4015. doi: 10.1073/pnas.76.8.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels W. R. Hybrid dysgenesis in Drosophila and the Stochastic loss hypothesis. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):561–565. doi: 10.1101/sqb.1981.045.01.072. [DOI] [PubMed] [Google Scholar]
- Engels W. R., Preston C. R. Components of hybrid dysgenesis in a wild population of Drosophila melanogaster. Genetics. 1980 May;95(1):111–128. doi: 10.1093/genetics/95.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels W. R., Preston C. R. Hybrid dysgenesis in Drosophila melanogaster: the biology of female and male sterility. Genetics. 1979 May;92(1):161–174. doi: 10.1093/genetics/92.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey D. A. Selfish DNA: a sexually-transmitted nuclear parasite. Genetics. 1982 Jul-Aug;101(3-4):519–531. doi: 10.1093/genetics/101.3-4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell M. G., Kidwell J. F., Sved J. A. Hybrid Dysgenesis in DROSOPHILA MELANOGASTER: A Syndrome of Aberrant Traits Including Mutation, Sterility and Male Recombination. Genetics. 1977 Aug;86(4):813–833. doi: 10.1093/genetics/86.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidwell M. G., Novy J. B., Feeley S. M. Rapid unidirectional change of hybrid dysgenesis potential in Drosophila. J Hered. 1981 Jan-Feb;72(1):32–38. doi: 10.1093/oxfordjournals.jhered.a109422. [DOI] [PubMed] [Google Scholar]
- Kidwell M. G., Novy J. B. Hybrid Dysgenesis in DROSOPHILA MELANOGASTER: Sterility Resulting from Gonadal Dysgenesis in the P-M System. Genetics. 1979 Aug;92(4):1127–1140. doi: 10.1093/genetics/92.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M., Kidwell M. G., Bingham P. M. The molecular basis of P-M hybrid dysgenesis: the nature of induced mutations. Cell. 1982 Jul;29(3):987–994. doi: 10.1016/0092-8674(82)90462-7. [DOI] [PubMed] [Google Scholar]
- Rubin G. M., Spradling A. C. Genetic transformation of Drosophila with transposable element vectors. Science. 1982 Oct 22;218(4570):348–353. doi: 10.1126/science.6289436. [DOI] [PubMed] [Google Scholar]
- Schaefer R. E., Kidwell M. G., Fausto-Sterling A. Hybrid Dysgenesis in DROSOPHILA MELANOGASTER: Morphological and Cytological Studies of Ovarian Dysgenesis. Genetics. 1979 Aug;92(4):1141–1152. doi: 10.1093/genetics/92.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A. C., Rubin G. M. Drosophila genome organization: conserved and dynamic aspects. Annu Rev Genet. 1981;15:219–264. doi: 10.1146/annurev.ge.15.120181.001251. [DOI] [PubMed] [Google Scholar]
- Thompson J. N., Jr, Woodruff R. C. Increased mutation in crosses between geographically separated strains of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1059–1062. doi: 10.1073/pnas.77.2.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]


