Abstract
Purpose
To analyze which sonographic features of thyroid nodules with macrocalcifications were predictable of thyroid malignancy.
Materials and Methods
We reviewed sonographic findings of 854 macrocalcified thyroid nodules in patients who underwent fine needle aspiration biopsy between December 2009 and January 2011. There were 171 non-diagnostic aspirations, 34 nodules with category 3, 4, 5 based on Bethesda system, which were not confirmed by surgery, and these nodules were excluded from the analysis. Sonographic characteristics of the macrocalcifications including its thickness, interruption, and existence of soft tissue rim outside the macrocalcification were analyzed. Other sonographic characteristics of nodules such as shape, margin, composition, echo pattern, vascularity, and underlying parenchymal echogenicity were also evaluated. The correlation of sonographic features with cytopathologic results and the diagnostic performance of sonographic features for the prediction of malignancy were analyzed.
Results
Among 649 nodules, 179 (27.6%) nodules were malignant and 470 (72.4%) nodules were benign. Among the features of the macrocalcification, interruption, irregular thickness, or the presence of soft tissue outside calcification rim were associated with malignancy (p<0.001). A high sensitivity and negative predictive values for the prediction of malignancy was found in sonographic characteristics of irregular thickness (92.2% and 91.0%, respectively) and the presence of soft tissue (88.5% and 88.8%, respectively).
Conclusion
Sonographic characteristics of macrocalcification such as interruption, irregular thickness and the presence of soft tissue rim were associated with malignancy in thyroid nodules with macrocalcifications.
Keywords: Sonography, papillary thyroid cancer, macrocalcification
INTRODUCTION
Evaluation of a thyroid nodule with sonography (US) is essential to determine whether it is likely to be benign or malignant and patients with malignant nodules can be properly diagnosed and treated. Several US characteristics that have been reported as potential predictors of thyroid malignancy include irregular margins, hypoechogenicity, absence of a halo, a predominantly solid composition, or presence of calcification.1 It is well known that microcalcifications are associated with thyroid malignancy. However, there have been controversies about the interpretation of macrocalcifications.1,2
US-guided fine-needle aspiration biopsy (FNAB) has been proven accurate for the diagnosis of thyroid cancer and majority of FNAB is adequate for a cytological diagnosis. However, 5-20% of FNAB results in inadequate sampling and the rate of non-diagnostic cytology are reported to be higher in nodules with macrocalcification.3 Thus, FNAB in nodules with macrocalcification is challenging for accurate diagnoses.4-6
Only a few reports about US findings of macrocalcified nodules are associated with malignancy.1,2 Moreover, insufficient data was used to suggest which type of macrocalcifications was suggestive of malignancy.
The purpose of our study was to investigate which US findings were associated with thyroid carcinoma in thyroid nodules with macrocalcification.
MATERIALS AND METHODS
This retrospective study was conducted with institutional review board approval and a waiver of patient informed consent.
Patients
Between December 2009 and January 2012, 2664 consecutive patients with 3012 thyroid nodules who had undergone FNAB at our institution were considered for the study. An retrospective review of our database about the US findings of the lesions in 2664 patients was performed to search nodules with macrocalcification. Macrocalcification was defined as echogenic foci of calcification larger than 1 mm at the longest diameter. These included nodules with complete or near complete peripheral calcification. When microcalfications, which were defined as multiple punctate bright echoes of less than 1 mm with or without acoustic shadowing, were present, the lesions were excluded from the study. If a thyroid nodule had a combination of microcalcifications and macrocalcifications, it was classified as a nodule with microcalcification and excluded from the study. Thus, 854 macrocalcified nodules from 845 patients were found, and 34 nodules with FNAB results of Bethesda system category 3 (atypia of undetermined significance), 4 (suspicious for a follicular neoplasm) or 5 (suspicious for malignancy) were excluded from the analysis due to the lack of definitive pathologic result. There were 171 (20.8%) non-diagnostic aspirations after FNAB that did not undergo subsequent surgery or without other cytologic results on repeat FNAB, and these nodules were excluded from the analysis. A total of 649 nodules from 654 (535 women, 119 men, mean age 52 years±12.1) patients were included in the study for analysis.
Ultrasound imaging
All US examinations were performed with HDI 5000 (Philips Advanced Technology Laboratories, Bothell, WA, USA), iU22 (Philips Medical Systems, Bothell, WA, USA) or SuperSonic Imagine (Aix-en-Provence, France). Board-certified radiologists specialized in thyroid imaging evaluated and recorded the following US findings of the lesion before FNAB and performed US-guided FNAB: size (maximal dimension), shape (ovoid to round, taller-than-wide, or irregular), margin (well-defined smooth, or irregular), composition (solid, cystic, mixed, or spongiform), echo pattern (hyperechoic, isoechoic, hypoechoic, or markedly hypoechoic), vascularity (peripheral, central, both, or absent) and underlying parenchymal echogenicity (homogenous or heterogenous). The composition of a nodule was categorized according to the ratio of the cystic portion to the solid portion in the nodule. A solid nodule was defined when more than 90% of the nodule was solid, and a cystic nodule was defined when more than 90% of the nodule was cystic. A spongiform appearance was defined as the aggregation of multiple microcystic components. A mixed nodule was defined when the nodule did not meet the criteria of solid, cystic, or spongiform nodule. Echo pattern of solid portion was assessed with respect to the thyroid parenchyma and strap muscles and was classified as markedly hypoechoic (when a nodule showed a relatively hypoechoic pattern in regard to the adjacent strap muscle), hypoechoic, isoechoic, or hyperechoic (when a nodule showed a relatively hypoechoic, isoechoic, or hyperechoic pattern in regard to the normal thyroid parenchyma). Vascularity was assessed in respect to its location and was classified as peripheral, central, both, or absence of vascularity. Underlying parenchymal echogenicity was categorized as homogeneous or heterogeneous.
Fine-needle aspiration cytology
US-guided FNAB was performed to localize the lesion. Free hand FNAB was performed by radiologists specialized in thyroid imaging with a 23- to 25-gauge needle and a 10-mL syringe. Ultrasonographic guidance was used to confirm the correct placement of the needle in the nodules. At least two passes were made per nodule. Specimens were smeared on a slide, fixed in 95% ethanol immediately, and stained by the Papanicolaou method. Radiologists determined the adequacy of the specimen and the number of passes.
Three pathologists with more than 6 years of experience in thyroid cytopathology interpreted the cytologic findings. FNAB cytologic diagnosis was made based on the Bethesda system; Nodules were classified as category 1 nondiagnostic, category 2 benign, category 3 atypia of undetermined significance, category 4 suspicious for a follicular neoplasm/follicular neoplasm, category 5 suspicious for malignancy, or category 6 malignant.7
Image interpretation
Two radiologists (K.J.A and P.Y.J) blinded to the cytopathologic diagnosis retrospectively reviewed US images of macrocalcified nodules. The following findings of macrocalcifications were analyzed for each nodule: thickness of calcification (regular or irregular), presence of interruption (present or absent), and existence of soft tissue outside the calcification if the calcification has a shell appearance (present or absent). Regularity of the calcification thickness was assessed subjectively by consensus. Presence of interruption referred to loss of continuance of hyperechoic structures or a loss of approximation in the alignment of the macrocalcification. Existence of more than 1 mm thickness of a soft tissue rim outside the calcification was defined as presence of soft tissue echogenicity outside the macrocalcification. Diagnostic impression regarding the US findings were classified into three categories (probably benign, low suspicious for malignancy, or suspicious for malignancy) by consensus.
Statistical analysis
Each of the US characteristics was analyzed to determine its association with a cytopathologic diagnosis or surgical pathology. When FNAB cytology and the pathology of surgical specimen were discordant, the pathology of surgical specimen was regarded as the standard histologic diagnosis.
Statistical comparisons were performed using the chi-square or Fisher's exact tests for categoric data, and the Kruskal-Wallis test for continuous data. Multiple logistic regression analysis with a forward stepwise method for selection of significant variables was performed to determine independent US predictors for malignancy from the US characteristics that showed statistical significance (p<0.05). For all analyses, results were considered statistically significant if the p value was 0.05 or less. Diagnostic performance of US characteristics for the prediction of malignancy was assessed using sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV), and accuracy. Statistical analyses were performed using PASW Statistics, version 18.0.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Pathologic diagnosis
Out of 649 nodules, 197 nodules were pathologically confirmed by surgical specimen, 18 nodules were proved benign, and 179 nodules were malignant.
Moreover, 452 nodules out of 649 nodules were diagnosed by FNAB cytology, and category 2 benign according to two consecutive FNAB cytologies.
The diagnoses of malignancy at histologic examination included papillary carcinomas (n=177), follicular carcinomas (n=1), and medullary carcinomas (n=1). Diagnosis of benign lesions included nodular hyperplasia (n=12), follicular adenoma (n=389), and chronic lymphocytic thyroiditis (n=69).
US findings
The size of nodules ranged from 2 mm to 70 mm (mean size, 12.2±8.8 mm). The size, shape, margin, composition, echo-pattern, vascularity, and underlying parenchymal echogenicity showed significant association with malignancy (p<0.05). Among the US characteristics of macrocalcification, irregularity of thickening, interruption and soft tissue rim showed significant association with malignancy (Table 1).
Table 1.
Correlation of US Characteristics of Macrocalcification with Pathologic Diagnosis
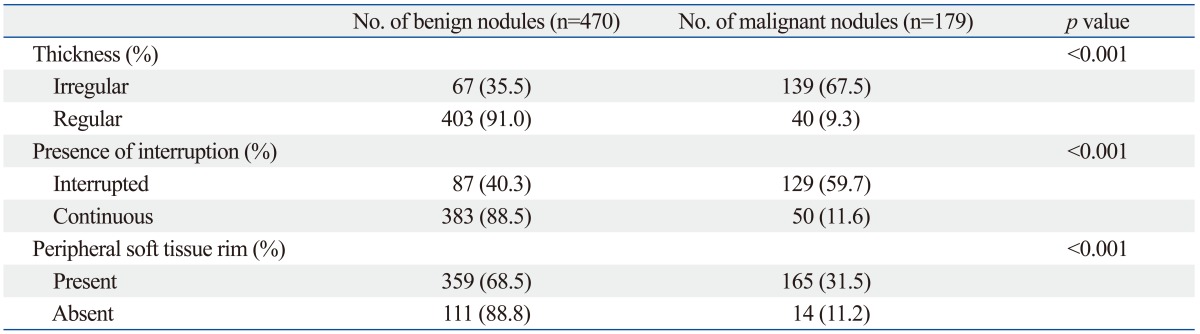
p-value is by chi-square analysis.
A multivariate analysis with multiple logistic regression analysis was performed to determine independent US predictors for malignancy. Five US criteria including taller-than-wide or irregular shape, irregular margin of nodules, irregular thickness of calcification, interruption of calcification, or presence of soft tissue outside the calcification showed significant association with thyroid malignancy (p<0.05) (Table 2, Figs. 1 and 2).
Table 2.
Multiple Logistic Regression Analysis of Malignancy Rate According to the US Features of Macrocalcified Thyroid Nodules
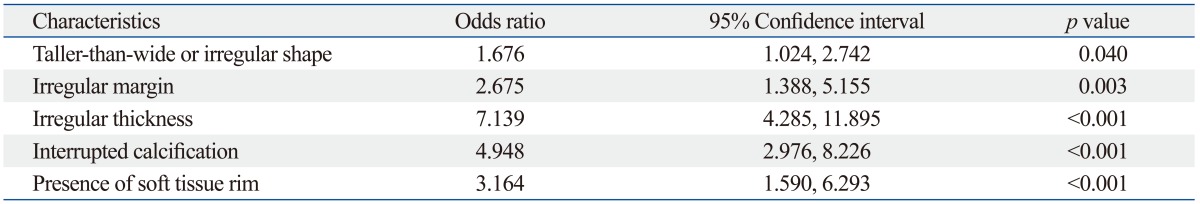
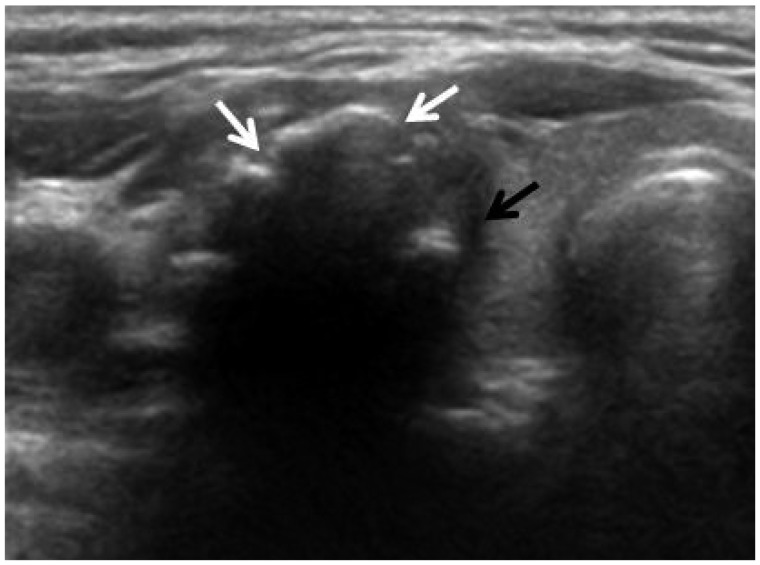
Fig. 1.
US findings of malignant thyroid nodule with macrocalcification. Transverse US image in a 42-year-old woman shows a nodule with interrupted macrocalcification (white arrows), irregular thickness and soft tissue rim outside the calcification (black arrow) and the nodule was diagnosed as papillary thyroid carcinoma by fine needle aspiration cytology.
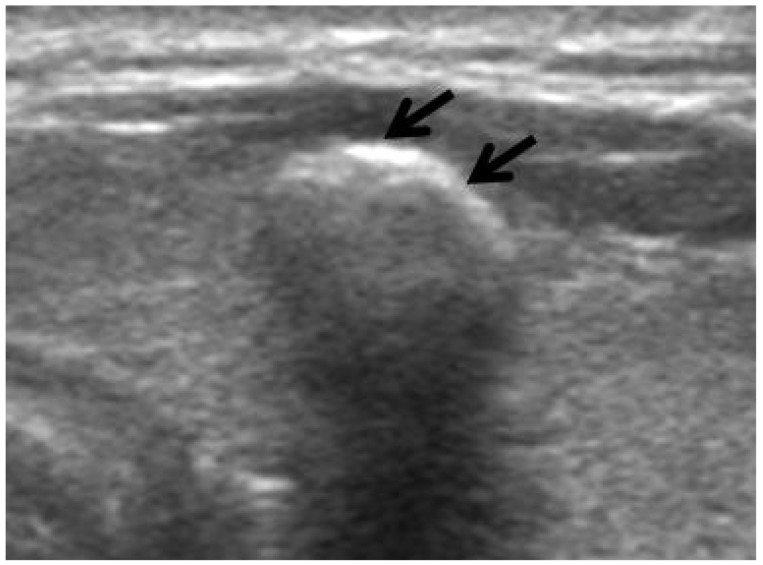
Fig. 2.
US findings of benign thyroid nodule with macrocalcification. Transverse US image of a 54-year-old woman shows a nodule with macrocalcification (arrows) which has regular margin and regular thickness without interruption. The soft tissue rim outside of the macrocalcification was not visible and the nodule was diagnosed as adenomatous hyperplasia by fine needle aspiration biopsy.
Sensitivity, specificity, NPV, PPV and accuracy of the US characteristics of macrocalcificationfor the prediction of malignancy are listed in Table 3.
Table 3.
Diagnostic Performance of US Characteristics for the Prediction of Malignancy in Thyroid Nodules with Macrocalcification
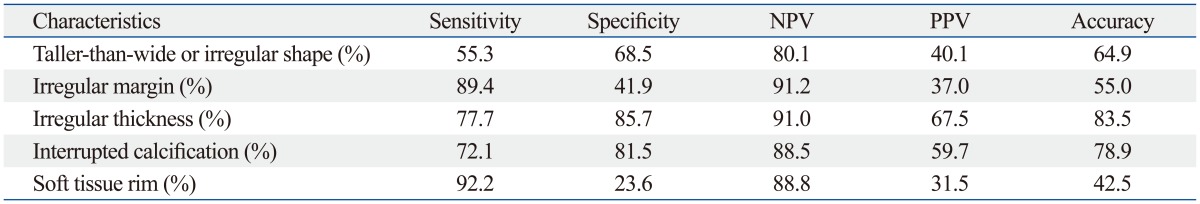
NPV, negative predictive value; PPV, positive predictive value.
DISCUSSION
FNAB cytology has a sensitivity of 71-83% and a specificity of 96%.6 However, regardless of the diagnostic performance of the FNAB cytology, 5-20% still remain insufficient for diagnosis.4,8-12 Moreover, macrocalcification of thyroid nodules is frequently associated with inadequate sampling of FNAB.3 The portion of nondiagnostic aspirates are larger in nodules with macrocalcification ranging from 7.5% to 26.8% and these nodules are frequently sampled inadequately by the second FNAB.5,6,13,14 In this study, we observed a similar rate of 21% of inadequate FNAB cytology in nodules with macrocalcification. This might be explained by the procedural difficulty that a needle cannot break through the stiff calcification and approach the soft tissue component of the nodule, especially when there is no soft tissue rim located outside the calcification. Therefore, US-guided FNAB is a challenging diagnostic modality for macrocalcified thyroid nodules according to these limitations.
Calcifications can be present in both benign and malignant thyroid nodules. It was reported that thyroid nodules with microcalcifications (1 mm or less in size) were associated with thyroid malignancy.1,15-18 The results for thyroid nodules with macrocalcifications (larger than 1 mm at the longest diameter) are controversial. In macrocalcified thyroid nodules, peripheral rim or eggshell calcification has been considered associated with multinodular goiters and has been generally considered as an indicator of a benign nodule.19-21 There are a few reports suggesting that a considerable portion of the macrocalcified nodules are malignant. Taki, et al.2 reported that 43% (6/14) of nodules with peripheral calcification were histopathologically proved to be papillary carcinoma. Kim, et al.1 evaluated 174 nodules with macrocalcification and 66% (116/174) proved to be malignant. In our study, 27.6% (179/649) nodules were diagnosed as malignant. These results suggested that macrocalcification of the nodule was not infrequently associated with the thyroid malignancy.
Therefore, preoperative US features suggesting malignancy can provide useful information for preoperative prediction of pathology and can further lead to the proper management of the macrocalcified thyroid nodules. To our knowledge, only a few studies have reported the US findings of the thyroid nodules associated with macrocalcification. Kim, et al.1 classified the macrocalcification into three subtypes (solitary, eggshell, and coarse not otherwise specified) and reported no significant difference in the prevalence of malignancy according to the types of calcification. Yoon, et al.5 divided the peripheral macrocalcification into stippled, smooth curvilinear, and irregular curvilinear calcification, but did not find any significant differences in the risk of malignancy.
We specifically classified the subtypes of macrocalcification in thyroid nodules by their regularity of thickness, presence of interruption, and existence of soft tissue rim peripheral to the calcification. We observed that thyroid nodules with macrocalcification showing irregular thickness, interruption or soft tissue outside the calcification rim showed significantly higher rate of malignancy than nodules with other features with odds ratios of 7.139, 4.948, and 3.164, respectively (Table 2). Irregular thickness of the macrocalcification showed high sensitivity, specificity, NPV, and diagnostic accuracy for malignancy (77.7%, 85.7%, 91.0%, and 83.5%, respectively). Interrupted macrocalfication showed overall diagnostic accuracy that exceeded 75.0% with the specificity and NPV of 81.5% and 88.5%, respectively. This result can be explained by the pathology that an interruption of peripheral calcifications demonstrated tumor infiltration through the broken calcification rim.6 The presence of soft tissue outside calcification rim was a sensitive finding for malignancy with the sensitivity of 92.2% and showed high NPV of 88.8% (Table 3).
Other US findings of the thyroid nodules associated with macrocalcification were also evaluated for its value in predicting malignancy. Previously reported US features predictive of malignancy included the presence of microcalcification, hypoechogenicity, irregular margins, and the absence of a halo.22 Park, et al.6 suggested that decreased internal echogenicity of the nodule with peripheral macrocalcification was associated with malignancy. Kim, et al.1 reported that macrocalcified nodules with at least one of the following triple criteria (hypoechogenicity, irregular or microlobulated margins, or taller-than-wide shape) showed a significantly higher rate of malignancy than in cases without any triple criteria. In our study, nodules with taller-than-wide or irregular shape and irregular margin showed a significantly higher rate of malignancy than nodules with other features with an odds ratio of 1.676 and 2.675, respectively. Among the previously reported US features that are predictive of malignancy,22 only the finding of taller-than-wide or irregular shape and irregular margin were significantly associated with malignancy in our study and the other findings including hypoechogenicity and the absence of a halo were not associated with malignancy in nodules with macrocalcification.
There are some limitations in our study. First, our study was retrospective and there could be selection bias. In our study, patients with benign findings at US usually did not undergo biopsy or surgery, which might have resulted in relatively fewer benign nodules in our study. This limitation could be overcome by a large-scale prospective study. Second, in clinical practice, FNAB is recommended in nodules with its diameter larger than 5 mm, but in our series, a nodule of 2 mm in diameter was included due to its posterior location; therefore, detailed evaluation was not available. In addition, we did not correlate the US findings and the pathologic findings nodule by nodule. Further study with detailed correlation of US and pathologic findings could give supporting pathologic bases for the significant US findings.
In conclusion, US findings predictive of malignancy in thyroid nodules with macrocalcification were an interruption of macrocalcification, irregular thickness of macrocalcification, the presence of soft tissue outside the macrocalfication rim, taller-than-wide or irregular shape, and irregular margin of the thyroid nodules. Our study provides useful information about thyroid nodules with macrocalcification, and might be especially helpful in cases with inadequate FNAB cytology for predicting malignancy with US.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Kim MJ, Kim EK, Kwak JY, Park CS, Chung WY, Nam KH, et al. Differentiation of thyroid nodules with macrocalcifications: role of suspicious sonographic findings. J Ultrasound Med. 2008;27:1179–1184. doi: 10.7863/jum.2008.27.8.1179. [DOI] [PubMed] [Google Scholar]
- 2.Taki S, Terahata S, Yamashita R, Kinuya K, Nobata K, Kakuda K, et al. Thyroid calcifications: sonographic patterns and incidence of cancer. Clin Imaging. 2004;28:368–371. doi: 10.1016/S0899-7071(03)00190-6. [DOI] [PubMed] [Google Scholar]
- 3.Choi SH, Han KH, Yoon JH, Moon HJ, Son EJ, Youk JH, et al. Factors affecting inadequate sampling of ultrasound-guided fine-needle aspiration biopsy of thyroid nodules. Clin Endocrinol (Oxf) 2011;74:776–782. doi: 10.1111/j.1365-2265.2011.04011.x. [DOI] [PubMed] [Google Scholar]
- 4.Alexander EK, Heering JP, Benson CB, Frates MC, Doubilet PM, Cibas ES, et al. Assessment of nondiagnostic ultrasound-guided fine needle aspirations of thyroid nodules. J Clin Endocrinol Metab. 2002;87:4924–4927. doi: 10.1210/jc.2002-020865. [DOI] [PubMed] [Google Scholar]
- 5.Yoon DY, Lee JW, Chang SK, Choi CS, Yun EJ, Seo YL, et al. Peripheral calcification in thyroid nodules: ultrasonographic features and prediction of malignancy. J Ultrasound Med. 2007;26:1349–1355. doi: 10.7863/jum.2007.26.10.1349. [DOI] [PubMed] [Google Scholar]
- 6.Park M, Shin JH, Han BK, Ko EY, Hwang HS, Kang SS, et al. Sonography of thyroid nodules with peripheral calcifications. J Clin Ultrasound. 2009;37:324–328. doi: 10.1002/jcu.20584. [DOI] [PubMed] [Google Scholar]
- 7.Cibas ES, Ali SZ NCI Thyroid FNA State of the Science Conference. The Bethesda System For Reporting Thyroid Cytopathology. Am J Clin Pathol. 2009;132:658–665. doi: 10.1309/AJCPPHLWMI3JV4LA. [DOI] [PubMed] [Google Scholar]
- 8.Carmeci C, Jeffrey RB, McDougall IR, Nowels KW, Weigel RJ. Ultrasound-guided fine-needle aspiration biopsy of thyroid masses. Thyroid. 1998;8:283–289. doi: 10.1089/thy.1998.8.283. [DOI] [PubMed] [Google Scholar]
- 9.Danese D, Sciacchitano S, Farsetti A, Andreoli M, Pontecorvi A. Diagnostic accuracy of conventional versus sonography-guided fine-needle aspiration biopsy of thyroid nodules. Thyroid. 1998;8:15–21. doi: 10.1089/thy.1998.8.15. [DOI] [PubMed] [Google Scholar]
- 10.Goellner JR, Gharib H, Grant CS, Johnson DA. Fine needle aspiration cytology of the thyroid, 1980 to 1986. Acta Cytol. 1987;31:587–590. [PubMed] [Google Scholar]
- 11.Rago T, Vitti P. Role of thyroid ultrasound in the diagnostic evaluation of thyroid nodules. Best Pract Res Clin Endocrinol Metab. 2008;22:913–928. doi: 10.1016/j.beem.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 12.Bastin S, Bolland MJ, Croxson MS. Role of ultrasound in the assessment of nodular thyroid disease. J Med Imaging Radiat Oncol. 2009;53:177–187. doi: 10.1111/j.1754-9485.2009.02060.x. [DOI] [PubMed] [Google Scholar]
- 13.Seningen JL, Nassar A, Henry MR. Correlation of thyroid nodule fine-needle aspiration cytology with corresponding histology at Mayo Clinic, 2001-2007: an institutional experience of 1,945 cases. Diagn Cytopathol. 2012;40(Suppl 1):E27–E32. doi: 10.1002/dc.21566. [DOI] [PubMed] [Google Scholar]
- 14.Renshaw AA. Histologic follow-up of nondiagnostic thyroid fine needle aspirations: implications for adequacy criteria. Diagn Cytopathol. 2012;40(Suppl 1):E13–E15. doi: 10.1002/dc.21492. [DOI] [PubMed] [Google Scholar]
- 15.Berker D, Isik S, Ozuguz U, Tutuncu YA, Kucukler K, Akbaba G, et al. Prevalence of incidental thyroid cancer and its ultrasonographic features in subcentimeter thyroid nodules of patients with hyperthyroidism. Endocrine. 2011;39:13–20. doi: 10.1007/s12020-010-9405-6. [DOI] [PubMed] [Google Scholar]
- 16.Qian M, Wang J, Qiu Y. [The significance of calcification in the thyroid papillary carcinoma] Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011;25:673–675. [PubMed] [Google Scholar]
- 17.Petrone L, Mannucci E, De Feo ML, Parenti G, Biagini C, Panconesi R, et al. A simple ultrasound score for the identification of candidates to fine needle aspiration of thyroid nodules. J Endocrinol Invest. 2012;35:720–724. doi: 10.3275/7978. [DOI] [PubMed] [Google Scholar]
- 18.Ozel A, Erturk SM, Ercan A, Yılmaz B, Basak T, Cantisani V, et al. The diagnostic efficiency of ultrasound in characterization for thyroid nodules: how many criteria are required to predict malignancy? Med Ultrason. 2012;14:24–28. [PubMed] [Google Scholar]
- 19.Chen CY, Tseng HS, Lee CH, Chan WP. Primary squamous cell carcinoma of the thyroid gland with eggshell calcification: sonographic and computed tomographic findings. J Ultrasound Med. 2010;29:1667–1670. doi: 10.7863/jum.2010.29.11.1667. [DOI] [PubMed] [Google Scholar]
- 20.Yaturu S, Rainer L. Thyroid nodule with eggshell calcification and oncocytic thyroid cancer. Med Sci Monit. 2010;16:CS25–CS28. [PubMed] [Google Scholar]
- 21.Gooding GA. Ultrasonic appearance of a thyroid nodule invested in eggshell calcification. J Clin Ultrasound. 1978;6:41–43. doi: 10.1002/jcu.1870060112. [DOI] [PubMed] [Google Scholar]
- 22.Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, et al. Benign and malignant thyroid nodules: US differentiation--multicenter retrospective study. Radiology. 2008;247:762–770. doi: 10.1148/radiol.2473070944. [DOI] [PubMed] [Google Scholar]