Abstract
Purpose
Anti-tumor necrosis factor-alpha (TNF-α) medications represent a major advancement in the management of chronic inflammatory diseases. However, these agents are associated with increased risks of tuberculosis (TB) and other serious infections. The aim of this study was to evaluate the incidences of such disease among tertiary hospitals in Korea.
Materials and Methods
We retrospectively studied patients who received anti-TNF-α therapy; we reviewed serious infections including TB that developed within 6 months after initiation of anti-TNF-α therapy. Data concerning patient demographics, types of anti-TNF-α agents, concomitant immunosuppressive drugs use, and infection details were collected.
Results
A total 175 patients treated with infliximab (n=72) or adalimumab (n=103) with the following conditions were enrolled: Crohn's disease, 34 (19.4%); ulcerative colitis, 20 (11.4%); ankylosing spondylitis, 82 (46.9%); and rheumatoid arthritis, 39 (22.2%). There were 18 cases (6.0%) of serious infections. The most common site of serious infection was the intra-abdomen (n=6), followed by TB (n=3), skin and soft tissue (n=3), bone and joints (n=2), ocular neurons (n=2), lower respiratory tract (n=1), and urinary tract (n=1). Of the 175 patients, only 3 cases showed development of TB. Furthermore, of all those who developed TB, none had taken anti-TB chemoprophylaxis prior to treatment with an anti-TNF agent due to negative screening results.
Conclusion
Serious infections with anti-TNF-α therapy were uncommon among tertiary hospitals in Korea; TB was the second most frequent infection. Nevertheless, there were no TB reactivations after anti-TB chemoprophylaxis. Accordingly, physicians should be aware of TB in subjects undergoing anti-TNF-α therapy, especially in countries with a high prevalence of TB.
Keywords: Tumor necrosis factor-alpha, tuberculosis, infection
INTRODUCTION
Tumor necrosis factor alpha (TNF-α) is an important immune modulator, and neutralization of this cytokine significantly suppresses immune responses.1 Anti-TNF-α agents are widely used in gastroenterology (for inflammatory bowel disease), rheumatology (for rheumatoid arthritis, psoriatic arthritis and spondyloarthropathies) and dermatology (for psoriasis). Treatment of chronic inflammatory disease with anti-TNF-α agents has allowed for improved control of disease progression.2-4 To treat such diseases, several different types of anti-TNF-α monoclonal antibodies are used: 1) Infliximab, a chimeric (human murine) monoclonal antibody against TNF-α, for rheumatoid arthritis5,6 and Crohn's disease6; 2) adalimumab, a fully humanized monoclonal antibody that recently received approval for the treatment of rheumatoid arthritis7; and 3) etanercept, a fusion protein composed of the ligand-binding portions of two human 75-kDa TNF receptors and the Fc portion of IgG1, used in the treatment of rheumatoid arthritis,5 juvenile chronic polyarthritis, psoriatic arthritis, and ankylosing spondylitis.8
Increased use of this therapy to treat chronic diseases, such as rheumatoid arthritis and Crohn's disease, has led to an increase in the number of infections [mainly Tuberculosis (TB)] after anti-TNF-α therapy in recent years. In particular, anti-TNF-α therapy has been shown to be associated with an approximately 14-fold greater incidence of TB reactivation compared to healthy controls.9-11 Thus, the increased risk of TB associated with anti-TNF-α therapy may indicate a need for screening for active and latent TB prior to anti-TNF-α therapy administration. Nevertheless, there are no reports of a consistent pattern regarding the risk of serious infections associated with anti-TNF-α therapy in clinical trials in the literature. Some studies have reported no increased risk of serious infections in anti-TNF-α treatment groups compared with placebo groups,12-15 while other studies throughout numerous regions worldwide have suggested that such treatment possibly increases the risk of serious infections.16,17
So far, there is limited data concerning the association between occurrences of serious infections and reactivation and newly developed TB in Korea. Therefore, the aim of this study was to examine the incidence of serious infections and TB in patients receiving anti-TNF-α therapy in Korea.
MATERIALS AND METHODS
Participants
This retrospective study enrolled 183 patients who received anti-TNF-α therapy including infliximab or adalimumab at Korea University Hospitals in Anam and Ansan from January 1, 2005 to December 31, 2011. We excluded 8 patients with Kawasaki syndrome, a systemic vasculitis disease that affects children. The remaining 175 patients were evaluated, diagnosed, treated and followed by the Departments of Gastroenterology or Rheumatology.
Study design
We evaluated infections and their association with the drugs at several steps. Demographic data as well as clinical and outcome data were collected from complete medical records, including all inpatient and outpatient records (Table 1). A variety of information was obtained via a thorough review of physicians' notes and medication histories. In order to define each incident case, every case of prior disease was exhaustively evaluated.
Table 1.
Baseline Characteristics
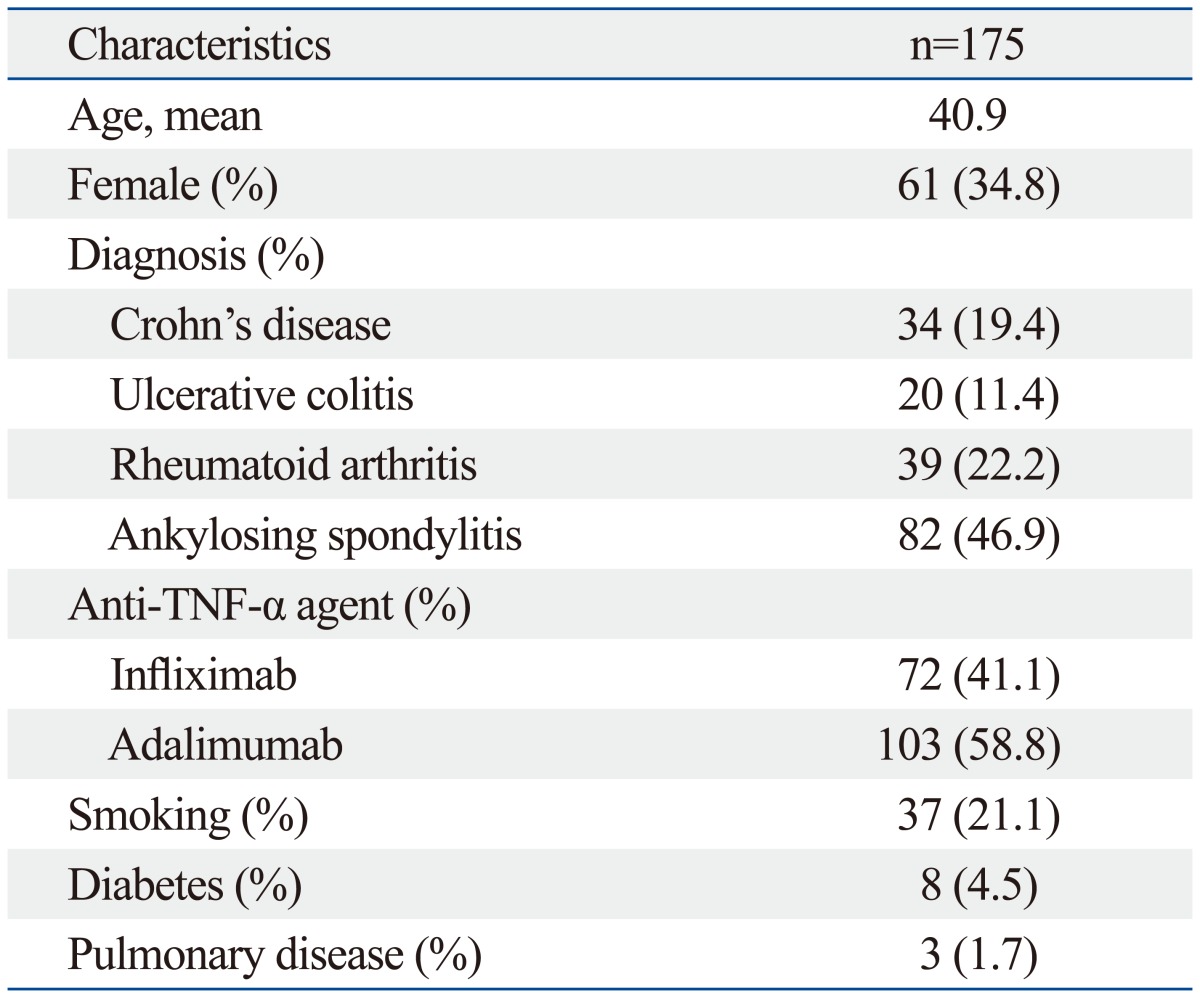
TNF-α, tumor necrosis factor alpha.
Cases of serious infection and TB in patients receiving anti-TNF-α therapy were analyzed. Before evaluating TB due to anti-TNF-α therapy, we reviewed the rates of latent TB infection diagnosed by a latent screening strategy. Patients with active TB detected on chest radiograph or by clinical examination were treated according to the national guidelines for the treatment of TB. After excluding active TB patients, those who tested positive for latent TB upon screening were given chemoprophylaxis before the administration of anti-TNF agents. The chemoprophylaxis regimen consisted of isoniazid 300 mg/day for 9 months from the time of diagnosis, followed by anti-TNF-α treatment 3 weeks later. These regimens were based on the "Korean Guidelines for Tuberculosis".18
Definition of serious infection
Serious infections were defined as infections that were 1) life threatening, 2) required hospitalization, 3) treated with intravenous antibiotics, or 4) lead to significant disability/incapacity.13,14
These infections included infections of the lower respiratory tract, intra-abdomen, skin and soft tissue, bone and joints, urinary tract, and ocular neurons.
TB screening and prophylaxis
Patients should be screened for active or latent TB infections before the prescription of anti-TNF-α agents.
Definition of active TB infection
Active TB was defined on the basis of acid-fast bacilli positivity, clinical suspicion, or imaging, including consolidation, endobronchial spread pattern, or tree-in-bud opacities.19 In patients with active TB detected on a chest radiograph or CT scan or on histopathology, anti-TNF agent therapy was delayed until they had been treated according to the national guidelines for the treatment of TB for 6 months.18
Definition of latent TB infection
Latent TB infection was defined as the presence of at least one positive result on a tuberculin skin test (TST), interferon-γ release assay (IGRA), or chest X-ray.
Tuberculin skin test
The TST was performed using the Mantoux method, in which five tuberculin test units of purified protein derivative were injected intradermally into the volar surface of forearm.20 The test was analyzed within 48-72 hours for a maximum transverse diameter of induration. Diameters ≥10 and ≥5 mm in patients without or with human immunodeficiency virus (HIV) infection, respectively, were considered positive.
Interferon-γ release assay
IGRA test was performed using the QuantiFERON®-TB Gold In-Tube (Cellestis, Melbourne, Vic., Australia) kit. Whole blood IGRA based on the Mycobacterium tuberculosis peptides ESAT-6, CFP-10 and TB7.7 was performed. This test is the test of choice for detecting TB because it is sensitive and does not exhibit a booster effect.10
Chest radiograph
Findings on chest X-ray indicative of latent TB included calcified granulomas, pleural scarring, apical densities, and/or hilar lymphadenopathy.
Data collection
The following patient information was collected from complete reviews of medical records:
Demographics: age at time of initial anti-TNF-α agent use, race and gender.
Possible causes of infection: we searched the records for evidence of diabetes mellitus, pulmonary disease, and HIV infection.
Cigarettes and alcohol: we checked medical records for the use of cigarettes and alcohol abuse (i.e., current/ever/never).
Medications: types of anti-TNF-α agents and concomitant immunosuppressive drugs such as disease-modifying antirheumatic drugs (DMARD), sulfasalazines, and steroids were included.
Duration for diagnosis infection: we recorded the first and last date of anti-TNF-α agent use and the date of diagnosis of the infection. Disease duration was defined as the last date that anti-TNF-α agents were used to the date that infection developed.
Cause of infection: site specific infections were recorded based on principal discharge diagnosis.
Statistical analysis
Serious infections stratified by site were included in the analysis. Person-years were calculated from the first day of anti-TNF-α therapy to the date of serious infection occurrence in patients taking anti-TNF agents. Rates of serious infections are presented as events/1000 person-years and 95% confidence intervals (95% CIs). CIs were calculated by comparing two rates. Categorical and continuous data were analyzed by χ2 analysis and unpaired, two-tailed Student's t-tests. The level of significance was set at p<0.05. All analyses were performed using SPSS software, version 18.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
A total of 175 patients were enrolled: 72 and 103 in the infliximab and adalimumab groups, respectively. The baseline characteristics of the patients are shown in Table 1. Their ages ranged from 18 to 86 years (mean, 40), the proportion of women was 34.8%. All patients were Asian, and none had HIV infection. Within these patient groups there were 34 cases of Crohn's disease (19.4%), 20 of ulcerative colitis (11.4%), 39 of rheumatoid arthritis (22.2%), and 82 of ankylosing spondylitis (46.9%). Patients were divided into two groups according to the type of anti-TNF-α therapy: infliximab or adalimumab. Table 2 shows the characteristics of the patient groups along with infection rates and drugs. Patients in the infliximab group were significantly younger than in those in the adalimumab group (33.9 years vs. 41.2 years, p=0.009). The proportions of women were similar in both groups at 50%.
Table 2.
Rates of All Serious Infections According to Anti-TNF-α Agent
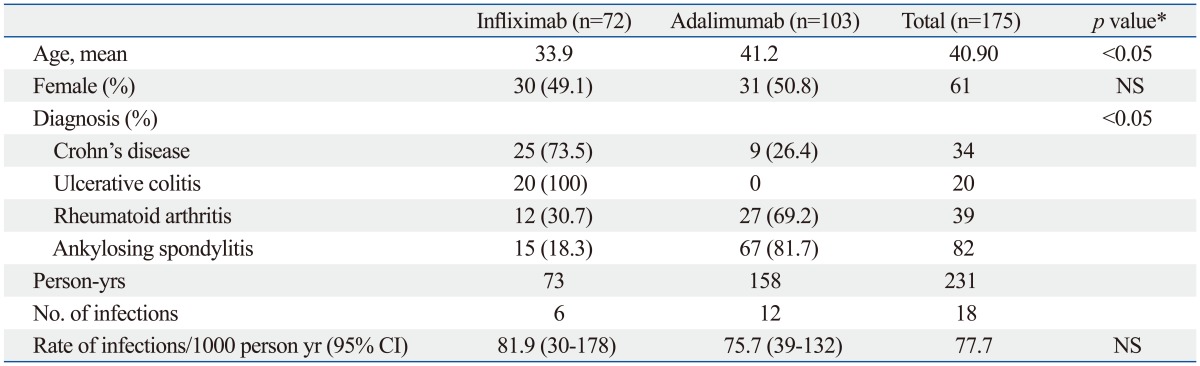
NS, not significant; CI, confidence interval.
*Statistical significance between groups was tested by Student's t-test or χ2 analysis.
Overview of serious infection risk with anti-TNF-α drugs
A total of 18 (6.0%) cases of serious infection occurred in the present study. Infection rates are reported in terms of 1000 person-years. There were no significant differences in infection rates between the infliximab and adalimumab groups (81.9 vs. 75.7, p=0.87).
The most common site of serious infection in anti-TNF-α treated patients was the intra-abdomen (26/1000 person-years), followed by TB (17/1000 person-years), skin and soft tissue (13/1000 person-years), bone and joints (9/1000 person-years), ocular neurons (9/1000 person-years), and the urinary tract (4/1000 person-years).
Screening for latent TB
One hundred fifty-nine of the 175 patients with inflammatory disease (159/175) underwent baseline screening before anti-TNF-α therapy administration. Sixteen patients did not undertake a purified protein derivative (PPD), chest X-ray or QuantiFERON test at all. Fifty-nine patients who met the criteria were diagnosed with latent TB. Of these 59 cases, 56 were positive on either PPD test or QuantiFERON test, and three patients were diagnosed with latent TB by chest X-ray only.
During the study, 55 patients completed the anti-TB chemoprophylaxis regimen. There were four patients who did not receive chemoprophylaxis, despite positive results for latent TB infection, including three patients who were diagnosed with latent TB by chest X-ray only and one patient who was diagnosed with latent TB only by TST. However, all four of them did not show TB reactivation.
Incidence rate of TB in anti-TNF-α-treated patients
We stratified patients into two groups according to the presence of latent TB infection. In the patients with latent TB infection, 55/59 patients completed chemoprophylaxis. There were no TB reactivations with the application of chemoprophylaxis. Among the patients who showed negative results upon screening, three (1.7%) newly developed TB. Of the 3 patients diagnosed with active TB: two had negative latent TB test results; one patient showed negative chest X-ray and QuantiFERON test results; however, the PPD test reaction measured 5 mm, which was considered negative for a non-HIV patient. The details of these patients are listed in Table 3. None of these three patients took anti-TB chemoprophylaxis before taking anti-TNF agents due to a negative result upon screening. For these 3 patients, the period of starting drug treatment to diagnosis of TB was 1 month, 3 months, and 22 months, respectively. All patients treated with infliximab received steroids or DMARDs, such as immunosuppressants.
Table 3.
Details of TB Infection
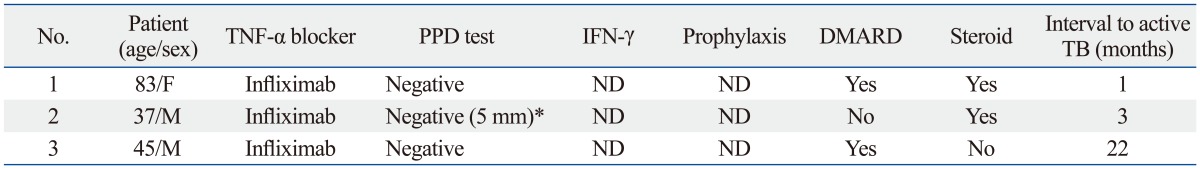
DMARD, disease-modifying antirheumatic drug; IFN-γ, interferon gamma; TNF-α, tumor necrosis factor alpha; PPD, purified protein derivative; TB, tuberculosis; ND, not done.
*A PPD reaction measuring below 10 mm was considered negative.
Cancer and mortality
There was only one incidence of cancer in our study. This patient was diagnosed with pancreatic cancer 1 year after adalimumab administration. However, any correlations between cancer and the anti-TNF-α agent remain unclear.
One patient who took infliximab died during the study period due to intra-abdominal infection. This patient was admitted to the hospital and presented with hematochezia caused by the worsening of ulcerative colitis. The patient was injected with infliximab on the second day of admission and underwent colectomy during hospitalization. However, the disease worsened, and the patient eventually died of sepsis despite being treated for more than 3 months.
DISCUSSION
In the present study, 18 cases (6.0%) of serious infection were linked to anti-TNF-α therapy. Additionally, the present results show that TB was the second most frequent infection among serious infections in patients who received anti-TNF-α therapy (1300/100000 person-years).
The incidence of TB associated with anti-TNF-α agents was 18-fold greater than that of TB in the general Korean population (72.6/100000 person-years).21 TB is significantly more common in Korea than other developed countries. Moreover, the actual incidence of TB in Korea may be higher than the reported incidence rate due underreporting.22 In the USA, the incidence rate of TB was 3.4 per 100000 in 2011 and is currently in decline.23 Even in Spain, which has one of the highest incidence rates of TB in Europe, there were only 18 cases of TB per 100000 in 2010.24 Therefore, we hypothesized that anti-TNF-α therapy is strongly associated with TB reactivation or the development of new TB despite the availability of anti-TB chemoprophylaxis in Korea. However, contrary to our expectations, 55 patients who received latent TB chemoprophylaxis did not develop reactivation.25
Sichletidis, et al.25 studied patients who received anti-TNF-α therapy for rheumatic diseases and reported that 19% (7/36) developed TB after receiving an adequate chemoprophylaxis. In the present study, of the 55 patients who were properly receiving or who had completed the chemoprophylaxis regimen, none of them developed TB reactivation (0/55), whereby the incidence of active TB in the study population was calculated to be 1300/100000 person-years. These differences can be explained in two ways. First, the effectiveness of chemoprophylaxis is limited not only by the efficacy of the drug, but also by adherence to therapy. Nearly all patients (55/59, 93%) properly adhered to chemoprophylaxis in the present study. Comparatively, Sichletidis, et al.25 reported that only 36 of 45 patients (80%) completed the chemoprophylaxis regimen. Second, we administered a chemoprophylaxis regimen of "9-months isoniazid" after screening for latent TB, while the patients in a study by Sichletidis, et al.25 received regimens of either "3-months isoniazid and rifampin" or "isoniazid for 6 months". Some studies have reported that isoniazid therapy for 6 or 12 months in patients with latent TB is superior to placebo treatment, with maximum benefits obtained with 9 months of therapy.26 These results suggest that a chemoprophylaxis regimen of a shorter duration may be less effective.
All 3 patients who newly developed TB received DMARDs or steroids. Because of the combined use of immunosuppressive drugs, it is unclear whether these three cases of TB infection were exclusively due to anti-TNF-α therapy. Additionally, one of these patients developed an infection 22 months after infliximab administration. Most clinical studies have reported TB occurring in patients shortly after receiving the anti-TNF-α monoclonal antibody infliximab (i.e., 12-21 weeks), indicating the reactivation of latent TB infection. The fact that a patient developed TB after 22 months in the present study demonstrates new development of TB due to causes other than infliximab.
The role of TNF-α in the human immune response to Mycobacterium tuberculosis is unclear.27 However, in vitro studies suggest that TNF-α plays an important role in the regulation of granuloma formation, which serves to restrict bacterial growth.1,28 TNF-α, a pleiotropic cytokine produced by infected and activated macrophages and proinflammatory T cells,29,30 enhances macrophage activation,31 chemokine production by macrophages,32 and immune cell recruitment during M. tuberculosis infection.33 Anti-TNF-α monoclonal antibody administration may subsequently result in the dissolution of intact granulomas, the release of viable mycobacteria, and disease reactivation.34 This can explain the higher incidence of TB observed in patients receiving anti TNF-α treatment. Therefore, screening for and management of latent TB are crucial before administering anti-TNF-α treatments.
From our study, it would be difficult to state with certainty the actual increased risk of TB in anti-TNF-α treatments. However, more careful surveillance for latent TB prior to initiation of infliximab treatment may be warranted in countries with high prevalences of TB. Before administering anti-TNF-α treatment, physicians should be aware of the increased risk of TB development among patients receiving infliximab and other immunosuppressive agents. It is crucial to evaluate TB in patients on anti-TNF-α treatment by thoroughly reviewing patient histories, TST results, interferon gamma assay results, and chest X-ray findings, as well as effectively treating patients with latent TB infections. It is also important that patients properly adhere to the chemoprophylaxis.
The most common site of serious infection in anti-TNF-α-treated patients in the present study was the intra abdomen, followed by TB and skin and soft tissue. TNF-α may act differentially at different anatomic sites.35 Taking this into account, further exploration of infection sites in anti-TNF-α-treated patients may reveal important physiological roles for TNF-α in host defense. For example, we reported that the third most common site of infection was the skin and soft tissue. TNF-α plays a key role in cutaneous immunity and inflammation.36 TNF-α is also a key cytokine responsible for cutaneous endothelial activation and thus the recruitment of inflammatory cells to the skin.37 Accordingly, TNF-α inhibition in the skin may result in both decreased sensing of and response to infection. Further studies are required to evaluate the site specific mechanisms of TNF-α.
Limitation
The major limitation of the present study is its retrospective nature. Since this was a retrospective medical record-based study, the follow-up duration and our ability to ascertain serious infection rates varied. Thus, the cumulative probability of serious infection should be considered a lower-range estimate. However, it is worth mentioning that there were no cases of TB reactivation in patients receiving chemoprophylaxis in Korea, which has a high TB burden. Another limitation is the lack of a control group including patients with chronic inflammatory diseases who were not treated with anti-TNF-α agents. In other words, we could not estimate the relative risk of serious infection in patients receiving anti-TNF-α agents compared to those in a control group.
In conclusion, the present results provide data on anti-TNF-α therapy in patients with chronic inflammatory diseases in Korea. There were 18 cases of severe infection, where the most common site was the intra-abdomen. TB was the second most frequent type of infection. However, there was no TB reactivation after anti-TB chemoprophylaxis. Therefore, physicians should be vigilant against TB in patients on anti-TNF-α therapy, especially in countries with high rates of TB. Physicians should also perform proper surveillance and administer anti-TB chemoprophylaxis when appropriate.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Lin PL, Myers A, Smith L, Bigbee C, Bigbee M, Fuhrman C, et al. Tumor necrosis factor neutralization results in disseminated disease in acute and latent Mycobacterium tuberculosis infection with normal granuloma structure in a cynomolgus macaque model. Arthritis Rheum. 2010;62:340–350. doi: 10.1002/art.27271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goekoop-Ruiterman YP, de Vries-Bouwstra JK, Allaart CF, van Zeben D, Kerstens PJ, Hazes JM, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): A randomized, controlled trial. Arthritis Rheum. 2008;58(2 Suppl):S126–S135. doi: 10.1002/art.23364. [DOI] [PubMed] [Google Scholar]
- 3.Heiberg MS, Kaufmann C, Rødevand E, Mikkelsen K, Koldingsnes W, Mowinckel P, et al. The comparative one-year performance of anti-tumor necrosis factor alpha drugs in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: results from a longitudinal, observational, multicenter study. Ann Rheum Dis. 2007;66:1038–1042. doi: 10.1002/art.23333. [DOI] [PubMed] [Google Scholar]
- 4.Weaver AL. The impact of new biologicals in the treatment of rheumatoid arthritis. Rheumatology (Oxford) 2004;43(Suppl 3):iii17–iii23. doi: 10.1093/rheumatology/keh203. [DOI] [PubMed] [Google Scholar]
- 5.Finckh A, Simard JF, Duryea J, Liang MH, Huang J, Daneel S, et al. The effectiveness of anti-tumor necrosis factor therapy in preventing progressive radiographic joint damage in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2006;54:54–59. doi: 10.1002/art.21491. [DOI] [PubMed] [Google Scholar]
- 6.Kornbluth A. Infliximab approved for use in Crohn's disease: a report on the FDA GI Advisory Committee conference. Inflamm Bowel Dis. 1998;4:328–329. doi: 10.1002/ibd.3780040415. [DOI] [PubMed] [Google Scholar]
- 7.Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50:1400–1411. doi: 10.1002/art.20217. [DOI] [PubMed] [Google Scholar]
- 8.Baraliakos X, Brandt J, Listing J, Haibel H, Sörensen H, Rudwaleit M, et al. Outcome of patients with active ankylosing spondylitis after two years of therapy with etanercept: clinical and magnetic resonance imaging data. Arthritis Rheum. 2005;53:856–863. doi: 10.1002/art.21588. [DOI] [PubMed] [Google Scholar]
- 9.Dixon WG, Hyrich KL, Watson KD, Lunt M, Galloway J, Ustianowski A, et al. Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR) Ann Rheum Dis. 2010;69:522–528. doi: 10.1136/ard.2009.118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marehbian J, Arrighi HM, Hass S, Tian H, Sandborn WJ. Adverse events associated with common therapy regimens for moderate-to-severe Crohn's disease. Am J Gastroenterol. 2009;104:2524–2533. doi: 10.1038/ajg.2009.322. [DOI] [PubMed] [Google Scholar]
- 11.Tubach F, Salmon D, Ravaud P, Allanore Y, Goupille P, Bréban M, et al. Risk of tuberculosis is higher with anti-tumor necrosis factor monoclonal antibody therapy than with soluble tumor necrosis factor receptor therapy: The three-year prospective French Research Axed on Tolerance of Biotherapies registry. Arthritis Rheum. 2009;60:1884–1894. doi: 10.1002/art.24632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genovese MC, Bathon JM, Fleischmann RM, Moreland LW, Martin RW, Whitmore JB, et al. Longterm safety, efficacy, and radiographic outcome with etanercept treatment in patients with early rheumatoid arthritis. J Rheumatol. 2005;32:1232–1242. [PubMed] [Google Scholar]
- 13.Klareskog L, van der Heijde D, de Jager JP, Gough A, Kalden J, Malaise M, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363:675–681. doi: 10.1016/S0140-6736(04)15640-7. [DOI] [PubMed] [Google Scholar]
- 14.Maini RN, Breedveld FC, Kalden JR, Smolen JS, Furst D, Weisman MH, et al. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum. 2004;50:1051–1065. doi: 10.1002/art.20159. [DOI] [PubMed] [Google Scholar]
- 15.Grijalva CG, Chen L, Delzell E, Baddley JW, Beukelman T, Winthrop KL, et al. Initiation of tumor necrosis factor-α antagonists and the risk of hospitalization for infection in patients with autoimmune diseases. JAMA. 2011;306:2331–2339. doi: 10.1001/jama.2011.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keystone EC, Schiff MH, Kremer JM, Kafka S, Lovy M, DeVries T, et al. Once-weekly administration of 50 mg etanercept in patients with active rheumatoid arthritis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2004;50:353–363. doi: 10.1002/art.20019. [DOI] [PubMed] [Google Scholar]
- 17.van de Putte LB, Atkins C, Malaise M, Sany J, Russell AS, van Riel PL, et al. Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed. Ann Rheum Dis. 2004;63:508–516. doi: 10.1136/ard.2003.013052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park JS. Korean guidelines for the treatment of tuberculosis. Korean J Med. 2012;82:269–273. [Google Scholar]
- 19.Im JG, Itoh H, Shim YS, Lee JH, Ahn J, Han MC, et al. Pulmonary tuberculosis: CT findings--early active disease and sequential change with antituberculous therapy. Radiology. 1993;186:653–660. doi: 10.1148/radiology.186.3.8430169. [DOI] [PubMed] [Google Scholar]
- 20.Malaviya AN, Kapoor S, Garg S, Rawat R, Shankar S, Nagpal S, et al. Preventing tuberculosis flare in patients with inflammatory rheumatic diseases receiving tumor necrosis factor-alpha inhibitors in India -- An audit report. J Rheumatol. 2009;36:1414–1420. doi: 10.3899/jrheum.081042. [DOI] [PubMed] [Google Scholar]
- 21.Korean National Tuberculosis Association. Trend of case notification rate per 100,000 by year, 2004-2011. [accessed on 2013 Feb 25]. Available at: http://www.knta.or.kr.
- 22.Hong SJ, Park YS, An H, Kang SM, Cho EH, Shin SS. Factors leading to under-reporting of tuberculosis in the private sector in Korea. Int J Tuberc Lung Dis. 2012;16:1221–1227. doi: 10.5588/ijtld.11.0782. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. Reported tuberculosis in the United States. 2011. [accessed on 2013 Feb 25]. Available at: http://www.cdc.gov.
- 24.United Nations, MDG Indicators. Tuberculosis incidence rate per year per 100,000 population (upper bound) [accessed on 2013 Feb 25]. Available at: http://mdgs.un.org/unsd/mdg/Default.aspx.
- 25.Sichletidis L, Settas L, Spyratos D, Chloros D, Patakas D. Tuberculosis in patients receiving anti-TNF agents despite chemoprophylaxis. Int J Tuberc Lung Dis. 2006;10:1127–1132. [PubMed] [Google Scholar]
- 26.Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999 This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC) This statement was endorsed by the Council of the Infectious Diseases Society of America (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med. 2000;161(4 Pt 2):S221–S247. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 27.Kisich KO, Higgins M, Diamond G, Heifets L. Tumor necrosis factor alpha stimulates killing of Mycobacterium tuberculosis by human neutrophils. Infect Immun. 2002;70:4591–4599. doi: 10.1128/IAI.70.8.4591-4599.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clay H, Volkman HE, Ramakrishnan L. Tumor necrosis factor signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death. Immunity. 2008;29:283–294. doi: 10.1016/j.immuni.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saunders BM, Briscoe H, Britton WJ. T cell-derived tumour necrosis factor is essential, but not sufficient, for protection against Mycobacterium tuberculosis infection. Clin Exp Immunol. 2004;137:279–287. doi: 10.1111/j.1365-2249.2004.02518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 31.Harris J, Hope JC, Keane J. Tumor necrosis factor blockers influence macrophage responses to Mycobacterium tuberculosis. J Infect Dis. 2008;198:1842–1850. doi: 10.1086/593174. [DOI] [PubMed] [Google Scholar]
- 32.Algood HM, Lin PL, Yankura D, Jones A, Chan J, Flynn JL. TNF influences chemokine expression of macrophages in vitro and that of CD11b+ cells in vivo during Mycobacterium tuberculosis infection. J Immunol. 2004;172:6846–6857. doi: 10.4049/jimmunol.172.11.6846. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Z, Connell MC, MacEwan DJ. TNFR1-induced NF-kappaB, but not ERK, p38MAPK or JNK activation, mediates TNF-induced ICAM-1 and VCAM-1 expression on endothelial cells. Cell Signal. 2007;19:1238–1248. doi: 10.1016/j.cellsig.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 34.Keane J, Bresnihan B. Tuberculosis reactivation during immunosuppressive therapy in rheumatic diseases: diagnostic and therapeutic strategies. Curr Opin Rheumatol. 2008;20:443–449. doi: 10.1097/BOR.0b013e3283025ec2. [DOI] [PubMed] [Google Scholar]
- 35.Dixon WG, Watson K, Lunt M, Hyrich KL, Silman AJ, Symmons DP. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2006;54:2368–2376. doi: 10.1002/art.21978. [DOI] [PubMed] [Google Scholar]
- 36.Groves RW, Allen MH, Ross EL, Barker JN, MacDonald DM. Tumour necrosis factor alpha is pro-inflammatory in normal human skin and modulates cutaneous adhesion molecule expression. Br J Dermatol. 1995;132:345–352. doi: 10.1111/j.1365-2133.1995.tb08666.x. [DOI] [PubMed] [Google Scholar]
- 37.Vestergaard C, Johansen C, Otkjaer K, Deleuran M, Iversen L. Tumor necrosis factor-alpha-induced CTACK/CCL27 (cutaneous T-cell-attracting chemokine) production in keratinocytes is controlled by nuclear factor kappaB. Cytokine. 2005;29:49–55. doi: 10.1016/j.cyto.2004.09.008. [DOI] [PubMed] [Google Scholar]


