Abstract
Objective
To identify the processes surgeons use to establish patient buy-in to postoperative treatments.
Background
Surgeons generally believe they confirm the patient's commitment to an operation and all ensuing postoperative care, before surgery. How surgeons get buy-in and whether patients participate in this agreement is unknown.
Methods
We used purposive sampling to identify three surgeons from different subspecialties who routinely perform high-risk operations at each of three distinct medical centers (Toronto, ON; Boston, MA; Madison, WI). We recorded preoperative conversations with three to seven patients facing high-risk surgery with each surgeon (n = 48) and used content analysis to analyze each preoperative conversation inductively.
Results
Surgeons conveyed the gravity of high-risk operations to patients by emphasizing the operation is “big surgery” and that a decision to proceed invoked a serious commitment for both the surgeon and the patient. Surgeons were frank about the potential for serious complications and the need for intensive care. They rarely discussed the use of prolonged life-supporting treatment, and patients’ questions were primarily confined to logistic or technical concerns. Surgeons regularly proceeded through the conversation in a manner that suggested they believed buy-in was achieved, but this agreement was rarely forged explicitly.
Conclusions
Surgeons who perform high-risk operations communicate the risks of surgery and express their commitment to the patient's survival. However, they rarely discuss prolonged life-supporting treatments explicitly and patients do not discuss their preferences. It is not possible to determine patients’ desires for prolonged postoperative life support based on these preoperative conversations alone.
In the event of a postoperative complication requiring prolonged aggressive treatments, surgeons often delay or deny requests by patients or their surrogates to withdraw life support.1-6 This can undermine patient autonomy and lead to conflict between care providers in the intensive care unit (ICU)7, 8 which has adverse effects on patient safety, quality of care,9-11 and can significantly reduce health related quality of life measures for survivors.12 One author argues that this reticence to withdraw life support can be attributed to the surgeon's desire for psychological self-protection from the inevitable bad outcome.1 Others describe a covenantal relationship between surgeons and patients whereby patients permit the surgeon to operate on their bodies and in turn, the surgeon promises not to let them die.2 Our research has identified a third contributor; surgeons assert that they preoperatively establish the patient's commitment to an operation as well as to the ensuing postoperative care, including prolonged life-supporting treatments.13 We call this implicitly understood contract “surgical buy-in.”
Surgeons note that “...during a big operation surgeons feel that there is a commitment made by both the patient and the surgeon to get through the operation as well as all of the postoperative issues that come up.”13 This position is grounded in the surgeon's sense of personal responsibility for outcomes and fear of being “the agent” of a patient's death.13 In a survey of 900 surgeons using a clinical vignette, 63% of respondents favored not withdrawing life support on postoperative day 7 for a patient with a stroke and respiratory failure who requests withdrawal. Ninety-four percent of these surgeons reported that preoperative discussions with the patient or family were a significant factor in their decision making.14
Although surgeons generally believe they establish the patient's commitment to the operation and all ensuing postoperative care before surgery, how surgeons establish this agreement is unknown. We performed a qualitative study to identify the process surgeons use to establish buy-in and to determine whether patients participate in the agreement that surgeons describe.
Methods
Study subjects
We used purposive sampling to identify surgeons in Toronto, ON, Boston, MA, and Madison, WI. We sampled surgeons who were considered by peers to have good communication skills, hypothesizing that surgeons who communicate well with patients would be most likely to explore patients’ buy-in to postoperative life support. In addition, we selected surgeons from subspecialties where surgeons routinely perform operations that are considered “high-risk”15 to include surgeons who regularly discuss the use of postoperative intensive care with patients.
We excluded trauma surgeons because the preponderance of emergency procedures might preclude an extensive discussion about postoperative life support. We also excluded transplant surgeons as their unique duty to allocate scarce resources might present an extreme case of preoperative buy-in. Three surgeons from each site participated. This sample included vascular (1), hepato-biliary (1), cardiac (4), thoracic (1), and neurosurgeons (2).
We asked surgeons to identify patients with appointments scheduled to discuss a high-risk operation. We audio taped and transcribed verbatim, one preoperative outpatient conversation with 3 to 7 patients per surgeon. Each patient completed a short demographic survey at the end of the visit.
Analysis
We used content analysis to analyze each transcript inductively.16 We coded the first ten transcripts using the technique of constant comparison to develop an overall coding taxonomy.17 For all 48 transcripts, four coders independently coded the transcript and then met as a group of at least three to discuss each code and achieve consensus. We continued this process until no new codes or coding refinements surfaced and specific codes were saturated and appeared with a degree of regularity. Higher-level analysis proceeded simultaneously as we used the group process to explore the content of communication about postoperative treatment revealed by the coding. We used a context chart to characterize major themes in order to ensure maximal fit and faithful data representation.18 We used NVIVO (QSR International-Melbourne) to catalogue coded transcripts.
Researchers
All four investigators coding transcripts have extensive professional experience with postoperative patients in the intensive care unit, although they come from diverse backgrounds: surgery (MLS, KJB), medicine (JMK), palliative care (KJB), and nursing (KEP). The process of sorting through varied interpretations of the preoperative visit revealed assumptions based on professional identities, allowing us to attend to researcher biases throughout the analysis.
This study was approved by the Institutional Review Boards of the University of Wisconsin, Partners Health Care System and The University Health Network in Toronto. All surgeons, patients and associates present gave informed consent for participation.
Results
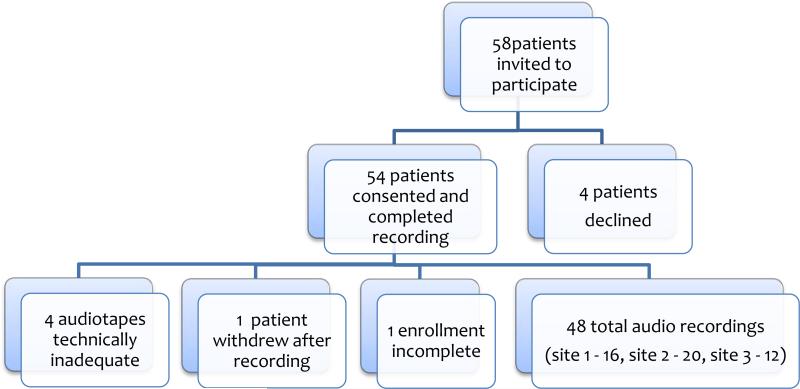
Fifty-eight patients were invited to participate, 4 declined, 5 audio tapes were either incomplete or not technically adequate for transcription, one patient withdrew after taping leaving 48 conversations for analysis. (Figure) The patients ranged in age from 26 to 94, had a broad spectrum of educational backgrounds, and 83% had undergone a previous operation. (Table 1) In 71% of cases a definitive decision to proceed with surgery was made. In the remaining cases, the surgeon delayed decision making in order to gain additional testing (6 patients), smoking cessation (1 patient) or observe a trial of non-surgical therapy (1 patient). Two patients wanted time to think over the decision to have surgery and one preferred to delay surgery indefinitely. In two cases, surgeons considered an operation but ultimately did not offer it to the patient. The operations under consideration included abdominal aortic aneurysm repair, brain tumor resection, coronary artery bypass grafting (CABG), esophagectomy, lung volume reduction, and hepatobiliary resections for tumor.
Figure.
Patient recruitment and enrollment
Table 1.
Participant Characteristics (N=48)
| No. (%) | |
|---|---|
| Male | 28 (58) |
| Age (years) | |
| 20-39 | 4 (8) |
| 40-59 | 17 (35) |
| 60-79 | 19 (40) |
| 80+ | 7 (15) |
| Race | |
| White or Caucasian | 39 (81) |
| Black or African American | 2 (4) |
| Hispanic | 2 (4) |
| Asian | 5 (10) |
| Education | |
| Some high school or less | 10 (21) |
| High school diploma or GED | 9 (19) |
| Vocational degree or some college | 8 (17) |
| College degree | 11 (23) |
| Professional or graduate degree | 10 (21) |
| Lives in a nursing home or assisted living facility | 1 (2) |
| Needs assistance reading instructions, pamphlets or other written materials from doctor or pharmacy26 | |
| Never | 36 (75) |
| Rarely | 7 (15) |
| Often | 4 (8) |
| Always | 1 (2) |
| Has had a previous operation | 40 (83) |
The median conversation length was 22 minutes (range: 7 – 78 minutes). In all cases, surgeons talked for the majority of the time, accounting for 70-75% of the conversation (range: 52% -90%). Each visit included an extensive discussion about the patient's disease, the procedure proposed, the surgeon's decision to offer surgery, and, for cases when surgery was agreed upon, informed consent.
Big Surgery
When surgeons discussed high-risk operations, they regularly emphasized that it was “big surgery”. “Big surgery” or analogs such as “a big deal” or “not small surgery” were used to stress to patients that the operation proposed could have profound consequences. Surgeons distinguished “big surgery” from more routine operations, “It's not day surgery,” and expressed concern about the potential for complications, “Now what can go wrong? Lots can go wrong. This is heart surgery...” In some conversations “big surgery” meant that the operation was painful or the recovery would be arduous. In other instances, “big surgery” was technically difficult, “...we have to make an abdominal incision and then we have to make incisions in each groin. Sometimes we clean those arteries out, sometimes we have to bring the graft down actually into the groin and do a bypass. It's a pretty big operation to go through.”
Surgeons stressed that their decision to take on “big surgery” required the patient to understand the gravity of the operation and prepare for potentially serious consequences. Thus, “big surgery” should not be performed without careful deliberation by both the surgeon and the patient. Ultimately, a decision to proceed with “big surgery” invoked a serious commitment for both surgeon and patient. For example, “It's a reality when you have a big surgery. This is inherently dangerous business. My job is to make it turn out right. That's what we do, but it's still dangerous to go through it. Just so you understand that.”
In contrast to discussions about “big surgery” that provided a vivid description about the gravity of surgery, a more systematic informed consent conversation typically occurred at the end of the visit. The possible complications were named, but not described, although surgeons often qualified that they were “serious, awful, real, important” and “something to worry about”. Surgeons presented specific complications in a list with near universal disclosure of death. For example, “The big risk that we worry about with surgery, it may sound terrible, you know, heart attack, stroke, bleeding, infection, death, kidney trouble.”
Agency
Surgeons regularly and clearly expressed that they were personally responsible for the outcome of the operation; they are agents through which the goals of surgery are obtained but also agents of possible suffering. Therefore, the decision to perform an operation was first and foremost the surgeon's to make and was determined by careful calculation of the trade-offs involved. As one surgeon said, “I think I'll get you through the operation. The bigger concern I have is whether or not I make you feel any better.” Since surgeons see themselves as the treatment and they are the arbiter of the procedure, performing surgery was dependent on the surgeon's assessment that the goals of surgery were achievable, “... but I would only do it if I really thought that was the right thing and it would help you.”
Surgeons assumed primary responsibility for the patient's wellbeing and survival. They stressed their role as a surgeon required total control of all aspects of care to ensure safety, “...because I'm a beating heart surgeon, I like to do it on people if it's safe” and that by offering surgery, success is expected, “If I didn't think he was going to pull through, I probably would not offer him surgery.” Furthermore, surgeons acknowledged ownership for harms resulting from surgery, “...it's a specific surgery on the brain. I go past balance and hearing. It's possible I could damage those” and noted that death was a failure of the surgeon's duty, “Well my job is to get you through it in one piece.” Surgeons frequently described the emotional impact of the outcomes of surgery for themselves. They specifically told patients that good outcomes would inspire their own happiness or prompt a “day of joy” whereas bad outcomes or complications would cause the surgeon to be “very upset”.
Use of Life Support
Surgeons frequently explained the routine use of postoperative intensive care. For example, “So go from the operating room to the ICU, everybody.” Although a few surgeons were clear about the need for postoperative ventilatory support, they emphasized that the need was temporary, “You're in the ICU for a day or two afterwards, usually a couple days. Many times we let you stay on the ventilator overnight so you can wake up gradually and it's not too stressful. Then that evening or the next morning we take the breathing tube out of your throat.”
Most surgeons did not mention the use of prolonged life support. In turn, patients rarely engaged the surgeon in a discussion of their feelings about such treatments. One patient expressed concern about discomfort with routine postoperative intubation:
Patient: When you have all these tubes in you and everything, are you conscious? Do you know all that?
Doctor: Yes.
Patient: That's bad . . .
Doctor: It seems to work out.
A few patients made more general comments about aggressive care; however the meaning of their statements was unclear. For example one patient said, “Like I tell the girls, if you're going to tell me its 80/20 and I got a year to go, I'll go the way I am now and die happy.” Overall, a clear discussion about the potential use of prolonged life support and the patient's corresponding preferences did not occur.
Only one surgeon discussed the potential risk of postoperative stroke and subsequent need for prolonged life-supporting treatment in every recorded conversation. He said, “... So I operate on you. The next morning I come in, Why haven't you woken him up yet? We tried. He didn't wake up...So we wait another day. Still hasn't woken up. Still hasn't woken up the third day. And after a few weeks ...they come to us and they shrug their shoulders... Breathing machine, in a coma.” Another surgeon had a similar conversation with only one patient. For both surgeons, this conversation was not used to establish the patient's commitment to postoperative treatments but rather to encourage the patient to discuss his or her preferences with family members in the event of a devastating outcome.
Patient Concerns
Nearly all surgeons encouraged patients to ask questions after discussing risks of surgery. Patients most often responded to the surgeon's request with logistic or technical concerns. For example:
Doctor: This is an area for vision, smell, for function of legs and bladder. You could even have a stroke...or damage to the vision. You could have an infection or trouble with the healing...What questions do you have for me?
Patient: Washing my hair was one.
Doctor: I want you to wash your hair with baby shampoo on the third day...Don't scrub the stitches.
Family: Stitches or staples?
Other logistic concerns that patients commonly posed during the discussion of surgical risk included the length of the operation, the date or the time of day of surgery, where to go for testing, the need to travel to and from the hospital, the ability to wear pajamas, and rules about visiting hours.
Patients frequently noted that they were worried about being anxious, having pain, or whether the surgery would be successful. However, these concerns were stated as general fears about having surgery rather than explicit concerns about specific outcomes or treatments:
Doctor: What other questions do you have for me?
Patient: Don't know that I have any more. Kind of scares me, but that's going to be a natural feeling.
Doctor: If you weren't concerned, you wouldn't be normal. It's a big deal.
Establishing Buy-In
On occasion, we observed surgeons forging a clear agreement with the patient to participate in postoperative life-supporting treatments. (Table 2) In one instance the surgeon explicitly told the patient that the performance of surgery required a commitment from the patient to participate in postoperative treatments. This was described as a “verbal contract” that “everybody would do their utmost” to achieve the goals of surgery. In three conversations buy-in was implicitly solicited when the surgeon expressed expectations that the patient would participate in prolonged or unexpected treatments if necessary. For example, “Here we would say, ‘No, let's put you on a mechanical support device until either your heart recovers from the surgery, or we get you a new heart.’ ”
Table 2.
Levels of Surgeon-Perceived Preoperative Buy-in for Postoperative Treatments
| Types of Buy-in | Surgeon | Patient | ||
|---|---|---|---|---|
| Description | Representative Quotes | Description | Representative Quotes | |
| Explicit buy-in | Surgeon states the performance of surgery is contingent on a preoperatively determined agreement between surgeon and patient about participation in postoperative treatments. | MD: “If we decide we're going to be there, then you and I have sort of a verbal contract that we are going to do everything that we can to have that outcome that we want. So if we're going to go forward with that, then we have an understanding that everybody is going to do their utmost.” | Patient states that the surgeon can do whatever is necessary around the time of surgery. | PT: “You do what you have to do,” MD: ...It's understandable to be nervous because it's a big surgery. PT: But I have complete faith in you. MD: Okay, I know you're anxious about this but we have to take it a step at a time. PT: I understand. You're the boss! |
| Implicit buy-in | Surgeon emphasizes that the patient will participate in prolonged or unexpected treatments if necessary. | MD: “We are going to do the operation that Friday and then you're here in the hospital as long as you need to be, and that's the bottom line, right? If you need to be here longer, you're here longer...” MD: “Sometimes it turns into a war and you have to fight battles to win the war, so you have to be prepared for that on some level.” |
Patient notes a general recognition that death and other complications will be accepted or tolerated. | PT: “That's God's will, I've led a good life.” PT: “Yeah, okay. So that's it. The lifestyle changes, you adjust to it, you know.” |
| Assumed buy-in | Surgeon assumes that the patient agrees to postoperative care by acknowledging surgical risk. | MD: “And this is a form that basically says that we've gone over the risks.... And that you agree to go forward. It's not a contract, by any means. You can always just not show up.” | Patient states a willingness to proceed with surgery and acknowledgment that risks exist. | MD: “Yeah, and I think... I mean there are risks to it of course...” PT: “Yeah. There's a risk to everything.” PT: “I think I'd like to have you do the surgery.” |
Aside from these instances, we regularly observed surgeons proceeding through the conversation in a manner that suggested they believed buy-in was achieved. This was characterized by an assumption that the patient was committed to the operation as well as postoperative life support when the patient acknowledged the risks of surgery. For example, one surgeon noted the consent form was not a contract because the patient “could always not show up” suggesting that if the patient did present for surgery, the patient was committed to the entire process.
Patients frequently made statements that surgeons could interpret as an agreement to postoperative life support. Patients’ assertions varied from blanket permission for any necessary procedure, to a tacit agreement to proceed with surgery. (Table 2) Two patients explicitly offered complete faith in the surgeon to direct their care and “do what you have to do” to get the job done. Seven patients expressed a general acceptance of risk, that they would adjust to complications or that it was “God's will” if death or other complications occurred. These statements were non-specific and did not provide information about the patient's preferences for prolonged life support. For example:
Doctor: But we know that some patients’ complications mount up and they don't survive it... I'm not too worried about that, but you know, it's not zero.
Patient: Yeah, exactly; reality.
Doctor: It's a reality when you have a big surgery. You know, we have a team that works on you for pre and post-op care...My job is to make it turn out right.
Patient: I don't have a problem with this at all. If something goes wrong, it goes wrong...I've led a good life.”
Most patients did not make clear statements signaling that they had bought in to postoperative treatments. Instead, patients simply acknowledged risk and expressed a willingness to have surgery. Patients noted they recognized surgery could have complications, “There's a risk to everything” and reported their eagerness to have surgery for example, “Well, that's pretty good odds. Let's go for it.” Although there was consensus that the patient understood the risks and was ready to proceed, there was no specific agreement about the use of postoperative life support.
Discussion
During preoperative conversations, surgeons use “big surgery” to stress that the operation is a serious commitment with potentially grave consequences and convey expectations that patients would participate in postoperative care. However, surgeons in our study rarely discussed the use of prolonged life support and the patients did not offer insight about whether they would want aggressive treatment in the event of a poor outcome.
While some surgeons made efforts to establish a preoperative commitment to postoperative life support, in most cases, this was not clearly confronted. Given the processes observed in these conversations, including the surgeon's assumption of responsibility for outcomes, expectation of success and disclosure of specific risks, it is understandable that surgeons would assume that buy-in has been achieved. Although surgeons were frank about the potential for serious complications, patients’ questions about surgery were primarily confined to logistic or technical concerns. Therefore, it is not possible to determine patients’ preferences for prolonged life support based on these preoperative conversations alone. As such, physicians caring for patients who need prolonged or unanticipated postoperative interventions will need to revisit the patient's willingness to pursue subsequent aggressive treatments.
These findings are significant because they question the reliability of the preoperative conversation to form the basis for a surgeon's postoperative decision-making and raise concerns about whether patients fully understand the potential use of prolonged life support after high-risk surgery. These observations have important implications for surgeons, intensivists and patients.
For surgeons who have made significant effort to describe the gravity of surgery, it may be surprising that patients have not clearly bought in to the use of prolonged life support. Although surgeons regularly described the need for postoperative intensive care and consistently disclosed serious complications associated with the use of prolonged life support, naming the complication without describing its treatment requires patients to infer the interventions used in such settings. We did not find evidence that patients made this link or explicitly agreed to such treatments.
Surgeons were also clear about their commitment to the patient's survival and their responsibility for reaching this goal, a stance that is likely reassuring to a patient facing a difficult operation.19 Given the serious nature of the operations under consideration, surgeons may encrypt details about treatment of bad outcomes within “big surgery” in order to avoid a more vivid discussion about unwanted events that may be difficult for patients to tolerate preoperatively.
For intensivists, knowledge about the content of preoperative discussions between surgeons and patients can inform postoperative decision-making. Understanding that the surgeon believes the patient has bought in to postoperative life support and that the details of this commitment are, at best, incomplete can assist the ICU team as they navigate the use of prolonged life support with the patient, surrogates and surgeons.
For patients, it is difficult to know whether these preoperative conversations satisfy their informational needs. Although surgeons in our study met the requirements of informed consent and gave patients ample opportunity to ask questions, the patients rarely interrogated the use of aggressive treatments beyond the operating room. It is possible that patients assumed they were unlikely to need such treatments, were too anxious to inquire about the treatment of serious complications20, 21 or did not know what questions to ask. While engaging patients in conversations about “states worse than death”22 is likely onerous for a patient facing major surgery, a thorough discussion about the patient's fears, goals, and leeway to achieve these goals23 could enable the surgeon to advocate more accurately for his patient's preferences postoperatively.
This study raises ethical questions about the boundaries of surgical informed consent. For operations under consideration in this study, e.g. CABG, consent for some postoperative treatments such as mechanical ventilation is necessary and likely covered by the surgical consent. However, consent to prolonged aggressive treatment should not be assumed or required. Patients who consent to surgery should be able to refuse postoperative treatments if they are overly burdensome or the original goals of surgery are no longer possible.
Our study has both strengths and limitations. By using qualitative methods to examine surgeons and patients in action as they discuss the use of high-risk surgery, this study makes an important contribution to the debate about the boundaries of surgical informed consent.4 Our multi-site study was designed to minimize bias stemming from geographic variation of practice style.24 Furthermore, our strategy of sampling surgeons who are reportedly good communicators suggests that a clear conversation about the use of prolonged life support does not regularly occur even in this exemplary cohort.
Our analysis was limited to verbal communication at one preoperative visit so it is possible we missed non-verbal communication contributing to these agreements. Although a definitive decision to proceed to surgery followed the majority of our recorded conversations, others have shown that elements of complex decision-making increase over multiple preoperative visits.25 Additional information may have been exchanged after the conversation we audio taped (via telephone or in other settings). As such, it is possible that conversations about the use of prolonged life support and patients’ preferences were missed. In addition, our qualitative methods do not allow us to determine the exact proportion or the true prevalence of surgeons who explicitly solicit patient buy-in for postoperative life support as a condition for performing surgery. While we have carefully reported the statements patients make to surgeons about their willingness to undergo aggressive postoperative interventions, our study design limits our ability to determine exactly what the patients believe they have agreed to.
Conclusion
In preoperative discussions about high-risk surgery, surgeons explained the risks of the procedure, their commitment to patient survival, and the seriousness of the patient's decision to proceed. While these efforts may confer a sense that the patient has agreed to participate in all postoperative life support, evidence is limited that patients have bought in to the use of aggressive interventions beyond the operation itself. This study questions the assumption that patients who agree to high-risk operations also consent to the next level of care and suggests that reconsideration of patients treatment preferences is required if subsequent interventions are unexpected or prolonged.
Acknowledgments
The authors would like to thank Amber Barnato, MD, MPH, MAS, University of Pittsburgh, and Caprice Greenberg, MD, MPH, Wisconsin Surgical Outcomes Research Program, University of Wisconsin, for their thoughtful review of this manuscript. We also appreciate the feedback received from Nora Jacobson, PhD, School of Nursing, University of Wisconsin, the Qualitative Research Group supported by the Institute for Clinical and Translational Research (ICTR) at the University of Wisconsin, and technical assistance from Eva Chittenden, MD, Palliative Care Service, Massachusetts General Hospital.
Source of Funding: The project described was supported by the Clinical and Translational Science Award (CTSA) program, previously through the National Center for Research Resources (NCRR) grant 1UL1RR025011, and now by the National Center for Advancing Translational Sciences (NCATS), grant 9U54TR000021 (Dr. Schwarze). This project was also supported by the Greenwall (Kornfeld) Program for Bioethics and Patient Care (Dr. Schwarze), and the American Geriatrics Society Jahnigen Career Development Award, grant 1R03AG042361-01 NIH (Dr. Cooper). These funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; and preparation, review, or approval of the manuscript for publication.
Footnotes
Conflicts of Interest For the remaining authors no conflicts were declared.
References
- 1.Bosk CL. Conclusion. Forgive and Remember. The University of Chicago Press; Chicago: 1979. p. 172. [Google Scholar]
- 2.Cassell J, Buchman TG, Streat S, et al. Surgeons, intensivists, and the covenant of care: administrative models and values affecting care at the end of life--Updated. Crit Care Med. 2003;31(5):1551–7. [PubMed] [Google Scholar]
- 3.Tulsky JA. Beyond advance directives: importance of communication skills at the end of life. JAMA. 2005;294(3):359–65. doi: 10.1001/jama.294.3.359. [DOI] [PubMed] [Google Scholar]
- 4.D'Amico TA, Krasna MJ, Krasna DM, et al. No heroic measures: how soon is too soon to stop? Ann Thorac Surg. 2009;87(1):11–8. doi: 10.1016/j.athoracsur.2008.09.075. [DOI] [PubMed] [Google Scholar]
- 5.Belanger S. Check your advance directive at the door: transplantation and the obligation to live. Am J Bioeth. 2010;10(3):65–6. doi: 10.1080/15265160903581809. [DOI] [PubMed] [Google Scholar]
- 6.Buchman TG, Cassell J, Ray SE, et al. Who should manage the dying patient?: Rescue, shame, and the surgical ICU dilemma. J Am Coll Surg. 2002;194(5):665–73. doi: 10.1016/s1072-7515(02)01157-2. [DOI] [PubMed] [Google Scholar]
- 7.Danjoux Meth N, Lawless B, Hawryluck L. Conflicts in the ICU: perspectives of administrators and clinicians. Intensive Care Med. 2009;35(12):2068–77. doi: 10.1007/s00134-009-1639-5. [DOI] [PubMed] [Google Scholar]
- 8.Larochelle MR, Rodriguez KL, Arnold RM, et al. Hospital staff attributions of the causes of physician variation in end-of-life treatment intensity. Palliat Med. 2009;23(5):460–70. doi: 10.1177/0269216309103664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fassier T, Azoulay E. Conflicts and communication gaps in the intensive care unit. Curr Opin Crit Care. 2010 doi: 10.1097/MCC.0b013e32834044f0. [DOI] [PubMed] [Google Scholar]
- 10.Azoulay E, Timsit JF, Sprung CL, et al. Prevalence and factors of intensive care unit conflicts: the conflicus study. Am J Respir Crit Care Med. 2009;180(9):853–60. doi: 10.1164/rccm.200810-1614OC. [DOI] [PubMed] [Google Scholar]
- 11.Embriaco N, Papazian L, Kentish-Barnes N, et al. Burnout syndrome among critical care healthcare workers. Curr Opin Crit Care. 2007;13(5):482–8. doi: 10.1097/MCC.0b013e3282efd28a. [DOI] [PubMed] [Google Scholar]
- 12.Lemiale V, Kentish-Barnes N, Chaize M, et al. Health-related quality of life in family members of intensive care unit patients. J Palliat Med. 2010;13(9):1131–7. doi: 10.1089/jpm.2010.0109. [DOI] [PubMed] [Google Scholar]
- 13.Schwarze ML, Bradley CT, Brasel KJ. Surgical “buy-in”: the contractual relationship between surgeons and patients that influences decisions regarding life-supporting therapy. Crit Care Med. 2010;38(3):843–8. doi: 10.1097/CCM.0b013e3181cc466b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwarze ML, Redmann AJ, Brasel KJ, et al. The role of surgeon error in withdrawal of postoperative life support. Ann Surg. 2012;256(1):10–5. doi: 10.1097/SLA.0b013e3182580de5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364(22):2128–37. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kondracki NL, Wellman NS, Amundson DR. Content analysis: review of methods and their applications in nutrition education. J Nutr Educ Behav. 2002;34(4):224–30. doi: 10.1016/s1499-4046(06)60097-3. [DOI] [PubMed] [Google Scholar]
- 17.Murphy E, Dingwall R, Greatbatch D, et al. Qualitative research methods in health technology assessment: a review of the literature. Health Technol Assess. 1998;2(16) [PubMed] [Google Scholar]
- 18.Miles M, Huberman A. Within-Case Displays: Exploring and Describing. Qualitative Data Analysis. SAGE Publications; Thousand Oaks, CA: 1994. pp. 102–105. [Google Scholar]
- 19.McKneally MF, Martin DK. An entrustment model of consent for surgical treatment of life-threatening illness: perspective of patients requiring esophagectomy. J Thorac Cardiovasc Surg. 2000;120(2):264–9. doi: 10.1067/mtc.2000.106525. [DOI] [PubMed] [Google Scholar]
- 20.Aquilina R, Baldacchino D. An exploratory study of Maltese patients’ perceptions of their preparation for total joint replacement at the pre-admission clinic. Journal of Orthopaedic Nursing. 2007;11(3–4):194–203. [Google Scholar]
- 21.Edwards C. Exploration of the orthopaedic patient's ‘need to know’. Journal of Orthopaedic Nursing. 2003;7(1):18–25. [Google Scholar]
- 22.Patrick DL, Starks HE, Cain KC, et al. Measuring preferences for health states worse than death. Med Decis Making. 1994;14(1):9–18. doi: 10.1177/0272989X9401400102. [DOI] [PubMed] [Google Scholar]
- 23.Sudore RL, Fried TR. Redefining the “planning” in advance care planning: preparing for end-of-life decision making. Ann Intern Med. 2010;153(4):256–61. doi: 10.1059/0003-4819-153-4-201008170-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnato AE, Herndon MB, Anthony DL, et al. Are regional variations in end-of-life care intensity explained by patient preferences?: A Study of the US Medicare Population. Med Care. 2007;45(5):386–93. doi: 10.1097/01.mlr.0000255248.79308.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Etchells E, Ferrari M, Kiss A, et al. Informed decision-making in elective major vascular surgery: analysis of 145 surgeon-patient consultations. Can J Surg. 2011;54(3):173–8. doi: 10.1503/cjs.047709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeppesen KM, Coyle JD, Miser WF. Screening questions to predict limited health literacy: a cross-sectional study of patients with diabetes mellitus. Ann Fam Med. 2009;7(1):24–31. doi: 10.1370/afm.919. [DOI] [PMC free article] [PubMed] [Google Scholar]