Abstract
Dynamin proteins are involved in vesicle generation, providing mechanical force to excise newly formed vesicles from membranes of cellular compartments. In the brain, dynamin-1, -2 and -3 have been well-studied; however, their function in the retina remains elusive. A retina-specific splice-variant of dynamin-1 interacts with the photoreceptor-specific protein Tubby-like Protein 1 (Tulp1), which when mutated causes an early-onset form of autosomal recessive retinitis pigmentosa. Here we investigated the role of the dynamins in the retina, using immunohistochemistry to localize dynamin-1, -2 and -3 and immunoprecipitation followed by mass spectrometry to explore dynamin-1 interacting proteins in mouse retina. Dynamin-2 is primarily confined to the inner segment compartment of photoreceptors, suggesting a role in outer segment protein transport. Dynamin-3 is present in the terminals of photoreceptors and dendrites of second-order neurons, but is most pronounced in the inner plexiform layer where second-order neurons relay signals from photoreceptors. Dynamin-1 appears to be the dominant isoform in the retina and is present throughout the retina and in multiple compartments of the photoreceptor cell. This suggests that it may function in multiple cellular pathways. Surprisingly, dynamin-1 expression and localization did not appear to be disrupted in tulp1−/− mice. Immunoprecipitation experiments reveal that dynamin-1 associates primarily with proteins involved in cytoskeletal-based membrane dynamics. This finding is confirmed by western blot analysis. Results further implicate dynamin-1 in vesicular protein transport processes relevant to synaptic and post-Golgi pathways and indicate a possible role in photoreceptor stability.
Keywords: photoreceptor, Dynamin-1, Tulp1, proteomics, vesicle
Introduction
The classical dynamins belong to a superfamily of GTPases that includes dynamin-like proteins (Ferguson & De Camilli, 2012). Dynamins participate in diverse membrane dynamics and vesicle trafficking functions, contributing mechanical force to excise newly formed vesicles from membranes of cellular components (McNiven et al., 2000). Three genes in mammals encode the dynamin proteins: DNM1, DNM2 and DNM3 (Cao et al., 1998). Of these, dynamin-2 is the only member that is ubiquitously expressed. Dynamin-1 is neuron-specific, while dynamin-3 expression is tissue specific, reportedly found in brain, testis, lung and heart (Cao et al., 1998). All three dynamin isoforms are present in neuronal tissue. Determining the physiological role of the dynamins is a significant challenge as there are overlapping functions of the isoforms (Ferguson et al., 2007; Raimondi et al., 2011) yet splice variants of each isoform may perform separate cellular tasks (Cao et al., 1998; McNiven et al., 2000).
Dynamin -1, -2, and -3 proteins have been identified in mouse photoreceptor cells of the retina (Liu et al., 2007); however, the extent of their expression in the retina has not been fully investigated. Only two diseases are associated with mutations in dynamin genes and both have a visual defect. Mutations in DNM2 underlie an autosomal dominant form of intermediate Charcot-Marie-Tooth disease, a peripheral neuropathy that can result in vision loss (Zuchner et al., 2005) and autosomal dominant centronuclear myopathy, which can induce a paralysis of the extraocular muscles (Jeub et al., 2008). Given the importance of dynamins in the central nervous system, it would not be surprising if additional defects in dynamin gene products affect retinal physiology and induce ocular phenotypes. The photoreceptor cells have an extremely high energy requirement due in part to tonic synaptic signaling and extensive vesicular protein transport (Linton et al., 2010). To meet this high demand, photoreceptors have developed specialized mechanisms to handle the continuous cycling of vesicles. How the dynamins contribute to these specialized features remains to be determined.
We previously identified a splice-variant of dynamin-1, termed dynamin-1 (a,c), which colocalizes and interacts with the photoreceptor-specific Tubby-like protein 1 (Tulp1) (Xi et al., 2007). Mutations in TULP1 cause a form of autosomal recessive retinitis pigmentosa, a type of inherited retinal degeneration which leads to blindness (Hagstrom et al., 1998). As an approach to better understanding dynamin function in photoreceptor cells, we report here the characterization of dynamin isoform expression and localization in the mouse retina and the identification of possible dynamin-1 interacting proteins.
Methods
Mice
Generation and maintenance of tulp1−/− mice on a C57BL/6 background has been described previously (Hagstrom et al., 1999). Control C57BL/6 mice were obtained from The Jackson Laboratory. Mice were euthanized by CO2 inhalation followed by cervical dislocation. All animal experiments were approved by the Institutional Animal Care and Use Committee of the Cleveland Clinic and were performed in compliance with the National Institutes of Health guidelines.
Immunohistochemistry
Eyes from P16 mice were prepared as previously described (Xi et al., 2007; Grossman et al., 2011). Briefly, after removal of the cornea and lens, the posterior poles were fixed in 4% paraformaldehyde in PBS for 3 h. The eye cups were then immersed through a graded series of sucrose solutions: 10% for 1 h, 20% for 1 h and 30% overnight. The posterior pole was embedded in OCT freezing medium, flash frozen on powderized dry ice and immediately transferred to −80°C. The tissue was sectioned at 10-μm thickness using a cryostat (Leica, Wetzlar, Germany) at −30°C. For each genotype (wt and tulp1−/−), a minimum number of four sections from five different mice were examined for each antibody. Retinal sections were blocked in 5% bovine serum albumin and 1% normal goat serum with 0.1% Triton X-100 for 1 h before incubation with primary antibodies overnight at 4°C. A panel of well-characterized antibodies was used for immunostaining. Table 1 contains a complete list of antibodies, immunogens, sources, host species and dilutions used. After washing 3 times in PBS, sections were incubated in fluorescent secondary antibodies at room temperature for 1 h. Secondary antibodies were: Alexa Fluor ® 488 goat anti-rabbit IgG and goat anti-mouse IgG; Alexa Fluor® 594 goat anti-rabbit IgG and goat anti-mouse IgG (Invitrogen, Carlsbad, California, USA). The sections were then rinsed 3 times with PBS followed by a dH2O rinse and coverslipped with Vectashield® mounting media with DAPI (Vector Laboratories, Burlingame, California, USA). Sections were imaged using an Olympus BX-61 fluorescent microscope (Olympus, Tokyo, Japan), equipped with a CCD monochrome camera (Hamamatsu Photonics, Bridgewater, New Jersey, USA).
Table 1.
Primary Antibody Characterization
| Protein | Immunogen | Technique/Dilution | Source | Host | Reference |
|---|---|---|---|---|---|
| Dynamin-1 | Synthetic peptide corresponding to AA residues 633–647 from rat/mouse/human dynamin-1 (EKASETEENGSDSF) | IHC 1:200, WB 1:1,000 and IP 10μg per 500μl retinal lysate | Thermo Scientific, (Rockford, Illinois; PA1-660) | rabbit polyclonal | (Kinuta et al., 2002; Ferguson et al., 2007) |
| Dynamin-2 | Synthetic peptide corresponding to AA residues 760–779 from rat/mouse/human dynamin-2 (SPTPQRRPVSSIHPPGRPPA) | IHC 1:200 and WB 1:1,000 | Thermo Scientific, (Rockford, Illinois; PA1-661) | rabbit polyclonal | (Sun et al., 2002; Lundmark & Carlsson, 2003; Kelly et al., 2005) |
| Dynamin-3 | Synthetic C-terminal peptide, specific to dynamin-3 (RLTLSAPLPRPASSRGPAPAIPSPGPHS) | IHC 1:200 and WB 1:1,000 | Dr. Mark A. McNiven, (Mayo Clinic, Minneapolis, MN) | rabbit polyclonal | (Cao et al., 1998; Gray et al., 2003; Vaid et al., 2007) |
| Pan-Dynamin | Synthetic peptide corresponding to AA residues 2–17 of dynamin 1,2 & 3 in rat/mouse (GNRGMEDLIPLVNRLQ) | IHC 1:100 | Synaptic Systems, (Gottingen, Germany; 115 002) | rabbit polyclonal | (Holroyd et al., 2002; Ferguson et al., 2007; Rozas et al., 2011) |
| Tulp1 | Synthetic peptide corresponding to N-terminal AA residues 1–201 of mouse Tulp1 | WB 1:1,000 | Dr. Stephanie A. Hagstrom, (Mass. Eye and Ear Infirmary, Boston, MA) | rabbit polyclonal | (Hagstrom et al., 2001; Xi et al., 2007; Grossman et al., 2009) |
| Peripherin | AA residues of the C-terminus of mouse Peripherin (CVEAEGADAGPAPEAG) | IHC 1:500 and WB 1:500 | Dr. Andrew F.X. Goldberg, (Oakland Univ., Rochester, MI) | rabbit polyclonal | (Goldberg et al., 2007) |
| Clathrin | Full length native bovine protein (purified), which recognizes AAs 207–264 of bovine clathrin heavy chains | WB 1:500 | Novus Biologicals (Littleton, CO;NB120-11331 | mouse monoclonal | (http://www.novusbio.com/Clathrin-heavy-chain-Antibody-TD1_NB120-11331.html) |
| Lima1 | AA residues 700–759 corresponding to human Lima1 | WB 1:500 | Bethyl Laboratories (Montgomery, TX; 0-103A) | rabbit polyclonal | (Han et al., 2007) |
| Myelin Basic Protein | Myelin basic protein isolated from human brain | WB 1:200 | Dako (Carpinteria, CA; A0623) | rabbit polyclonal | (Werner et al., 2007) |
| Myosin 10 | Synthetic peptide corresponding to the C-terminus of human Myosin 10 (Myosin IIB) | WB 1:500 | Cell Signaling Technology (Boston, MA;3404S) | rabbit polyclonal | (Daley et al., 2009) |
| Rhodopsin | 15 AA residues of N-terminal of bovine rhodopsin (MNGTEGPNFYVPFSN) | WB 1:1,000 | Dr. Paul Hargrave (University of Florida, Gainesville, FL;R2-15N) | mouse monoclonal | (Adamus et al., 1991) |
| Spectrin | cell membranes purified by hypotonic lysis and mechanical enucleation | WB 1:400 | EMD Millipore (Billerica, MA; MAB1622-Clone AA6) | mouse monoclonal | (Nakajima et al., 2011) |
| Rootletin | AA residues 1819–2006 of mouse rootletin | WB 1:1000 | Dr. Tiansen Li, (National Eye Institute, Bethesda, MD) | rabbit polyclonal | (Yang et al., 2002) |
Western Blot
Western blot analysis was performed as previously described with P16 mouse tissues from wt and tulp1−/− mice (n=2 for each genotype) (Hagstrom et al., 2001; Xi et al., 2005; Xi et al., 2007). Briefly, proteins were separated on SDS-PAGE gels and electroblotted to PVDF membranes. Membranes were incubated with primary antibodies (Table 1), followed by peroxidase-conjugated secondary antibodies, and were detected by chemiluminescence.
Immunoprecipitation and Protein Identification
Immunoprecipitation (IP) with Dynabeads was performed according to the manufacturer (Dynabeads Protein A 100.06D, Invitrogen, Carlsbad, California, USA). IPs followed by protein identification by liquid chromatography tandem mass spectrometry (LC MS/MS) were performed using mouse retina and brain tissue. In separate experiments, IPs followed by Western blot analysis were performed using bovine retinal tissue or mouse brain and retinal tissue. Briefly, adult bovine retina (600 mg: ~1 retina), P16-P60 mouse retinas (300 mg: 30 retinas) or P16-P60 mouse brains (300 mg: ~ 1 brain) were homogenized in 3 mL of lysis buffer (50mM Tris pH 8.0, 150 mM NaCl, 10% glycerol, 0.5% Triton X-100, 0.1% NP40) supplemented with protease inhibitors (Roche Applied Science, Indianapolis, Indiana, USA). Homogenization was accomplished by freezing the tissue and manually grinding with a disposable pestle within a micro-centrifuge tube. This was repeated two additional cycles. The suspension was centrifuged 9,000 rpm for 15 min, and the supernatant containing the retinal lysates were transferred to a separate tube and the protein concentration (bovine: 4 μg/μl; mouse: 1.2 μg/μl) was determined by the BCA (bicinchoninic acid) assay (Thermo Scientific, Rockford, Illinois, USA). For IP, 10 μg of polyclonal anti-dynamin-1 antibody (Thermo Scientific, Rockford, Illinois, USA), or non-specific rabbit IgG (Southern Biotech, Birmingham, Alabama, USA) was used for each 500 μl of retinal lysate. Immediately prior to IP, the antibody was crosslinked to the beads using BS3 (Bis[sulfosuccinimidyl] suberate) (Thermo Scientific, Rockford, Illinois, USA) for 30 min at room temperature. The IP binding reactions proceeded overnight at 4°C, followed by 3 washes with buffer. Proteins were then eluted with 20 μl of SDS sample buffer. Immunoprecipitated antigens were fractionated by SDS-PAGE on a 4–20% Tris-glycine 5-well gel (Invitrogen) with 60 μl of each IP product loaded per gel lane. The gel was then stained with colloidal Coomassie blue (Gel Code Blue, Pierce, Rockford, IL) and a disposable gridcutter (Gel Company Inc, San Francisco, California, USA) was used to excise 2 mm serial sections from each lane containing the IP products. Each gel sample was digested in situ with trypsin and proteins identified by LC MS/MS using a quadrupole time-of-flight (QTOF) instrument and Cap LC system (CapLC System; Waters Corporation, Milford, Massachusetts, USA), as described previously (Crabb et al., 2002; Xi et al., 2003; Xi et al., 2005; Xi et al., 2007). Protein identifications utilized ProteinLynx Global Server software (Waters Corporation), Mascot 2.2 (Matrix Science, Boston, Massachusetts, USA) and the Swiss-Protein murine sequence database (May 2012 release, 16529 total mouse sequences). Bioinformatic functional analyses were performed with Ingenuity Pathway Analysis (Summer Release, June 2012, Ingenuity Systems, Redwood City, California, USA, www.ingenuity.com), and the UniProtKB/Swiss-Prot protein database (web.expasy.org/docs/)
Results
All dynamin isoforms are expressed in the mouse retina
Western blot analysis shows that all three dynamin isoforms are expressed in the mouse retina (Fig. 1). All studies were conducted at P16, an age at which all of the cell types of the retina are present in wt mice, but which precedes photoreceptor cell death in tulp1−/− mice (Hagstrom et al., 1999; Grossman et al., 2009; Grossman et al., 2011). Dynamin-1, the neuronal-specific isoform, was detected in the retina and brain at its known molecular weight of 100 kDa, but not in testis (Fig. 1A). This is in agreement with previous findings (Cao et al., 1998; Ferguson et al., 2007). In western blots probed with dynamin-2 antibodies, immunoreactive bands were present at ~100 kDa in all tissues examined (Fig. 1B), in agreement with studies supporting the ubiquitous expression of this isoform (Cao et al., 1998; Ferguson et al., 2007). We detected dynamin-3 as a component of the retina, as well as the brain and testis (Fig. 1C).
Figure 1.

Multi-tissue analysis indicating that all three dynamin isoforms are present in the P16 mouse retina. (A) Western blot probed with dynamin-1 antibodies. (B) Western blot probed with dynamin-2 antibodies. (C) Western blot probed with dynamin-3 antibodies. Actin is shown in lower blots (~45 kDa) as a control for loading equivalency.
Because of the association between Tulp1 and dynamin-1 (a,c) in the retina (Xi et al., 2007), we examined whether dynamin-1 expression is altered in the absence of Tulp1. Western blot analysis of wt and tulp1−/− retinal lysates probed with a dynamin-1 specific antibody identified one band at approximately 100 kDa in both lysates (Fig. 2A). Probing with antibodies against actin supported equal protein loading across lanes. Our results suggest that dynamin-1 protein expression levels are not severely altered in the absence of Tulp1. Western blots of wt and tulp1−/− retinal lysates probed with dynamin-2 (Fig. 2B) and dynamin-3 (Fig. 2C) antibodies also showed that their levels were not grossly affected by the absence of Tulp1.
Figure 2.

All three dynamin isoforms are expressed in the P16 wt and tulp1−/− mouse retina at similar levels, respectively. (A) Western blot of wt and tulp1−/− retinal homogenates probed with dynamin-1 antibodies. In both samples, a single band is present at ~100 kDa at similar levels. (B) Western blot of wt and tulp1−/− retinal homogenates probed with dynamin-2 antibodies. In both samples, a major band is present at ~100 kDa at similar levels. (C) Western blot of wt and tulp1−/− retinal homogenates probed with dynamin-3 antibodies. In both samples, a single band is present at ~100 kDa at similar levels. Actin is shown in lower blots (~45 kDa) as a control for loading equivalency.
Differential localization of dynamin isoforms in the mouse retina
The distribution of the dynamin isoforms in P16 mouse retinas was examined by immunohistochemistry (IHC) (Fig. 3). A minimum of four sections from five different mouse retinas were examined for each experiment. Figure 3A shows that dynamin-1 is localized to the inner segment (IS), cell bodies and axons of the outer nuclear layer (ONL) and synaptic terminals of the photoreceptor cells in wt mouse retina. Immunoreactivity was also detected in other portions of the retina, including the outer plexiform layer (OPL), around cell bodies in the inner nuclear layer (INL), the inner plexiform layer (IPL) and ganglion cell layer (GCL) (Fig. 3A). High magnification shows that staining was present throughout the OPL, indicating that dynamin-1 is localized to the photoreceptor terminals as well as the postsynaptic dendrites of the second-order neurons.
Figure 3.
Immunofluorescent localization of dynamin isoforms in P16 mouse retinal sections. Images of dynamin proteins (first column and pseudo-colored red in the merged images) and nuclei labeled with DAPI (second column and pseudo-colored blue in the merged images) compare the localization of dynamin isoforms. (A) In the wt retina, dynamin-1 staining is present in the IS, ONL, OPL, INL, IPL and GCL. In the fourth column, showing a magnified view, staining is present throughout the OPL, indicating that dynamin-1 is localized to the photoreceptor terminals as well as the postsynaptic dendrites of the second-order neurons. (B) In the tulp1−/− retina, dynamin-1 staining exhibits the same pattern as in the wt, signifying that dynamin-1 localization is unaffected in the absence of tulp1. (C) Dynamin-2 immunoreactivity is detected in the IS, OPL, IPL and GCL; however, the magnified view shows that the signal is most concentrated above the myoid region of the IS. (D) Dynamin-3 expression is predominantly localized to the IPL. Less signal is detected in the IS, OPL, INL and GCL. (E) A pan-dynamin antibody that was raised against an epitope shared by all three isoforms shows a staining pattern similar to that of dynamin-1, with immunoreactivity in the IS, ONL, OPL, INL, IPL and GCL. Scale bars = 50 μm.
Since an association exists between Tulp1 and dynamin-1 (Xi et al., 2007), we examined dynamin-1’s localization in the tulp1−/− retina (Fig. 3B). Figure 3B indicates that the localization of dynamin-1 in the tulp1−/− retina appears similar to that in the wt retina with immunoreactivity seen in the IS, ONL, OPL, INL, IPL and GCL. These results imply that the absence of Tulp1 does not grossly affect the retinal distribution of dynamin-1.
In contrast to the broad staining pattern of dynamin-1 in the wt retina, dynamin-2 immunoreactivity was found predominantly above the myoid region of the IS (Fig. 3C). The myoid region is the proximal portion of the IS which houses the machinery for protein translation including the Golgi apparatus and endoplasmic reticulum. High magnification indicates that dynamin-2 localizes near the region of the connecting cilium (CC), the intracellular link between the functionally different compartments of the IS and OS (Fig. 3C). This is in agreement with proteomic data from CC preparations (Liu et al., 2007). There was also dynamin-2 staining in the OPL, IPL and GCL, but much less pronounced as compared to dynamin-1. Dynamin-3 localization in the wt retina was most apparent in the IPL (Fig. 3D). Immunoreactivity was also detected in the IS, OPL, INL and GCL. Finally, a pan-dynamin antibody appears to label much of the neural retina, similar to dynamin-1 (Fig. 3E). The localization patterns of dynamin isoforms in wt and tulp1−/− mice were similar at earlier (P13) and later ages (P21) (data not shown).
Dynamin-1 interacting proteins in the mouse retina
To identify dynamin-1 interacting proteins, we performed IPs from wt mouse retinal homogenates using a dynamin-1 specific antibody. Two independent IP experiments were performed and figure 4 shows a representative SDS-PAGE result. The IP and control lanes were excised into 31 fractions and proteins in each were identified by LC MS/MS. The major Coomassie blue band at ~100 kDa (Fig. 4A) was identified in both independent experiments as dynamin-1 (based on 83% sequence coverage). In addition, western blot analysis with anti-dynamin-1 also identified the 100 kDa band as dynamin-1 (Fig. 4B). Subsequent western blot analysis with anti-Tulp1 supported Tulp1 as a constituent of a dynamin-1 protein complex in the wt mouse retina but not in the tulp1−/− retinal lysate (Fig. 4C). In a separate experiment, Tulp1 was also detected by western blot analysis of dynamin-1 IP products from bovine retinal homogenates (data not shown). Tulp1 was not identified in an IP from wt retina using non-specific IgG antibodies or in an IP from tulp1−/− retina using dynamin-1 antibodies (Fig. 4D).
Figure 4.
Immunoprecipitation of a dynamin-1 complex from the mouse retina. (A) Gel Code Blue stain of an SDS-PAGE gel separating retinal lysates incubated with dynamin-1 antibodies. Lane 1: Proteins immunoprecipitated with dynamin-1 antibodies. Lane 2: BSA standard. Lane 3: Proteins immunoprecipitated with rabbit IgG antibodies, serving as a negative control. Lanes 1 and 3 were excised into 2mm segments, digested with trypsin, and the peptides identified by LC MS/MS. (B) Western blot of immunoprecipitation experiment shown in A probed with dynamin-1 antibodies. An arrow points to a major band at ~100 kDa, corresponding to the correct size of the dynamin-1 protein. This band was identified as dynamin-1 by LC MS/MS analysis. The band in lanes 1 and 3 at ~50 kDa was identified as IgG heavy chain. (C) Western blot of a dynamin-1 immunoprecipitation experiment probed with Tulp1 antibodies. Lane 1: Proteins immunoprecipitated with dynamin-1 antibodies. Lane 2: tulp1−/− retinal lysate. Lane 3: wt retinal lysate. An arrow points to a major band at ~78 KDa, corresponding to the correct size of the Tulp1 protein, in both the immunoprecipitation product and wt retinal lysate. This band is not seen in the tulp1−/− retinal lysate. (D) Western blot of control IP experiments probed with Tulp1 antibodies. Lane 1: Proteins immunoprecipitated with rabbit IgG antibodies from wt retina. Lane 2: Proteins immunoprecipitated with Dynamin-1 antibodies from tulp1−/− retina. Lane 3: wt retinal lysate.
Proteomic analyses of the anti-dynamin-1 IP products from mouse retina identified 128 proteins (Supplementary Table 1). Table 2 presents a summary of the 50 proteins identified in two IP experiments that were not identified in control IP experiments using non-specific IgG. These 50 proteins were classified into nine functional groups using bioinformatic methods, with several proteins categorized into more than one group (Fig. 5). The three most prevalent functional categories were cytoskeletal-associated, membrane dynamics and transport, and synaptic processes (Fig. 5). Sixteen proteins were classified as members of the cytoskeleton, fourteen were involved in membrane dynamics and vesicular protein transport and nine were synaptic proteins. Other categories containing fewer proteins included mRNA processing, cell adhesion, ion transport, translation and mitochondrial-associated.
Table 2.
Proteins identified in two anti-dynamin-1 immunoprecipitations from mouse retina
| Proteins | Accesion No. | Matches* | Function | |
|---|---|---|---|---|
| 1 | 60 kDa heat shock protein, mitochondrial | P63038 | 13 | MC |
| 2 | 60S acidic ribosomal protein P0 | P14869 | 5 | TL |
| 3 | 60S ribosomal protein L6 | P47911 | 3 | TL |
| 4 | 60S ribosomal protein L7 | P14148 | 3 | TL |
| 5 | 60S ribosomal protein L18 | P35980 | 2 | TL |
| 6 | Actin-gamma | P63260 | 92 | CYT |
| 7 | Actin-alpha 2 | P62737 | 49 | CYT |
| 8 | Cadherin-2 | P15116 | 12 | CA |
| 9 | Cadherin-4 | P39038 | 4 | CA |
| 10 | Calcium-binding mitochondrial carrier protein Aralar1 | Q8BH59 | 4 | MC |
| 11 | Catenin alpha-2 | Q61301 | 19 | CYT, CA |
| 12 | Catenin beta-1 | Q02248 | 15 | CA, mRNA, CYT |
| 13 | Catenin delta-2 | O35927 | 7 | CA, mRNA |
| 14 | Clathrin heavy chain 1 | Q68FD5 | 9 | MT, SP |
| 15 | Cytochrome c oxidase subunit 2 | P00405 | 7 | MC |
| 16 | Dynamin-1 | P39053 | 306 | MT, SP |
| 17 | Dynamin-3 | Q8BZ98 | 106 | MT, SP |
| 18 | Hemoglobin subunit alpha | P01942 | 8 | OX |
| 19 | Heterogeneous nuclear ribonucleoprotein M | Q9D0E1 | 10 | mRNA |
| 20 | IQ motif and SEC7 domain-containing protein 3 | Q3TES0 | 11 | MT, CYT |
| 21 | Lima1 | Q9ERG0 | 2 | CYT |
| 22 | Microtubule-associated protein 1B | P14873 | 3 | CYT, SP, MT |
| 23 | Myosin regulatory light chain 12B | Q3THE2 | 2 | MT |
| 24 | Myosin-Va | Q99104 | 23 | MT |
| 25 | Myosin-VI | Q64331 | 21 | MT |
| 26 | Myosin-9 | Q8VDD5 | 17 | MT |
| 27 | Myosin-10 | Q61879 | 35 | MT |
| 28 | Myosin-14 | Q6URW6 | 19 | MT |
| 29 | Nestin | Q6P5H2 | 2 | MT, CYT, NG |
| 30 | Neurofilament light polypeptide | P08551 | 3 | CYT, MT |
| 31 | 6-phosphofructokinase, liver type | P12382 | 9 | GL |
| 32 | Plakophilin-4 | Q68FH0 | 9 | CA, CYT |
| 33 | Protein KIAA1967 homolog | Q8VDP4 | 6 | mRNA |
| 34 | RNA-binding protein 14 | Q8C2Q3 | 3 | mRNA |
| 35 | Rhodopsin | P15409 | 4 | PT |
| 36 | Rho GTPase-activating protein 32 | Q811P8 | 8 | SP, IS, MT |
| 37 | Rootletin | Q8CJ40 | 7 | CYT |
| 38 | Serine/arginine-rich splicing factor 1 | Q6PDM2 | 5 | mRNA |
| 39 | Serine/threonine-protein phosphatase PGAM5, mitochondrial | Q8BX10 | 6 | MC |
| 40 | Sodium/potassium-transporting ATPase subunit alpha-2 | Q6PIE5 | 4 | IT |
| 41 | Spectrin (fodrin) alpha chain, brain | P16546 | 26 | CYT, SP |
| 42 | Spectrin (fodrin) beta chain, brain 1 | Q62261 | 25 | CYT, SP |
| 43 | SRC kinase signaling inhibitor 1 | Q9QWI6 | 29 | CYT, SP |
| 44 | Testican-2 | Q9ER58 | 2 | NG |
| 45 | Transcriptional activator protein Pur-beta | O35295 | 3 | mRNA |
| 46 | Tropomodulin-2 | Q9JKK7 | 5 | CYT |
| 47 | Tubulin alpha-4A chain | P68368 | 14 | CYT |
| 48 | V-type proton ATPase 116 kDa subunit a isoform 1 | Q9Z1G4 | 12 | IT, SP |
| 49 | V-type proton ATPase catalytic subunit A | P50516 | 6 | IT |
| 50 | V-type proton ATPase subunit d 1 | P51863 | 8 | IT |
Peptide hits which did not reach statistical significance as determined by Matrix Science Mascot were omitted from the results. All Keratins and Histone H2B types (10 types) were omitted from the results.
Highest matches within experiments reported
AM: anti-microbial, AP: apoptosis, CA: cell adhesion, CM: cellular metabolism, CJ: cell junction; CP: complement, CYT; cytoskelton,
GL: glycolysis, IS: intracellular signaling, IT: ion transport, MC: Mitochondria, mRNA: mRNA processing,
MT: membrane dynamics and transport, NG: neurogenesis, OX: oxygen binding, PT: phototransduction, SP: synpase, SR: stress response, TL: translation
Figure 5.
Bar graph representing the potential dynamin-1 interacting proteins organized by cellular function. Classifications were determined using IPA software and review of protein databases. Each category originates from Table 2 and represents the proteins that were identified in both immunoprecipitation experiments but not in the control experiment. Some proteins are encompassed in more than one functional class. The three most prevalent categories were cytoskeletal, membrane dynamics and transport and synaptic-associated.
To determine whether these potential dynamin-1 interactions are retina-specific or ubiquitous, we conducted parallel IPs in wt mouse brain tissue. Two independent brain IP experiments were performed and non-specific IgG was used as a control. As performed in retinal experiments, the IP and control lanes were excised into 31 fractions and proteins in each were identified by LC MS/MS. Proteomic analyses of the anti-dynamin-1 IP products from mouse brain identified 132 proteins (Supplementary Table 2). Nineteen proteins were identified in two IP experiments that were not identified in control IP experiments using non-specific IgG (Supplementary Table 3). Of these, seven proteins were identical between retina and brain. These include actin-alpha 2, cadherin 2, catenin alpha-2, catenin beta-1, dynamin-1, dynamin-3 and SRC kinase signaling inhibitor 1. All seven proteins can be classified into the functional categories of cytoskeletal-associated or membrane dynamics and transport.
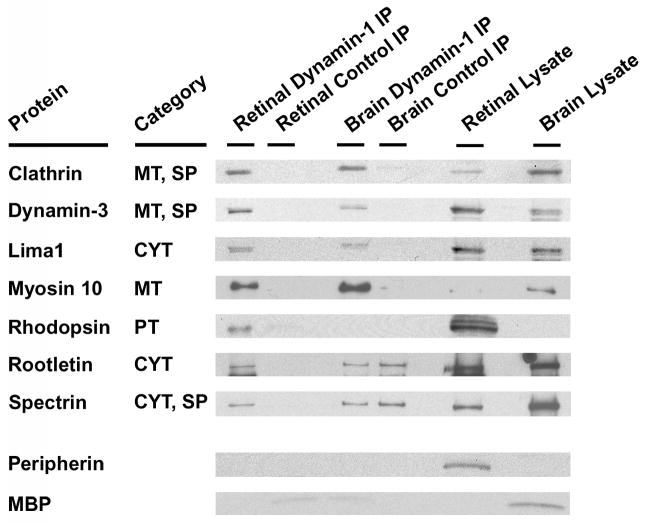
We further validated seven identified protein partners of dynamin-1 in the retina: clathrin, dynamin-3, lima1, myosin 10, rhodopsin and spectrin by western blot analysis of IP complexes (Fig. 6). These are representative proteins of the three major functional classes of dynamin-1 interacting partners – cytoskeletal, membrane dynamics and transport and synaptic. All seven proteins were clearly detected in the retinal IP samples but not in the control IP samples, indicating that these proteins interact with dynamin-1. The abundant brain protein, myelin basic protein (MBP), was used as a brain-specific control; peripherin was used as retina-specific control. In agreement with our proteomic data, peripherin and MBP were not detected in the IP product lanes of their respective tissues; however, they were present in the appropriate lysate preparations (Fig. 6).
Figure 6.
Western blot analysis validating seven dynamin-1 protein binding partners identified in proteomic analysis. Select proteins from the three most prevalent functional categories are highlighted. The brain and retinal IP complexes were separated by SDS-PAGE and then immunoblotted with the different antibodies as indicated. The category for each protein is indicated. Myelin basic protein (MBP) was used as a brain-specific control; peripherin was used as retina-specific control. CYT: cytoskeleton. MT: membrane dynamics and transport. PT: phototransduction. SP: synapse.
Discussion
Dynamins play important roles in neurons of the central nervous system, where they generate vesicles from membranes (Ferguson & De Camilli, 2012). Herein, we confirm that dynamins -1, -2, and -3 are expressed in the retina and are present in multiple classes of retinal neurons. Furthermore, our results indicate that each isoform has a distinct pattern of distribution throughout the retina.
The broad cellular localization of dynamin-1 is similar to that seen using a pan-dynamin antibody (Fig. 3A and E), indicating that dynamin-1 is the dominant isoform in the mouse retina, as has been postulated for the mouse brain (Ferguson et al., 2007). In contrast to dynamin-1, dynamin-2 localization is highly concentrated above the myoid region of the IS near the CC (Fig. 3C). The CC represents a transition zone between the IS and OS and plays a key role in the transport of proteins as they are synthesized in the IS and trafficked to the OS (Insinna & Besharse, 2008). This suggests that dynamin-2 plays a role in intracellular transport in the photoreceptor cell, as has been proposed for other cell types (Cao et al., 1998). Dynamin-3 expression is most pronounced in the IPL where synaptic terminals of second order bipolar neurons synapse with amacrine and ganglion cells (Fig. 3D). Dynamin-3 is also present throughout the OPL, and appears to be expressed in photoreceptor terminals and the dendrites of second order neurons. These results suggest that dynamin-3 plays a critical role in retinal synaptic processing.
Dynamin-1 and Tulp1 interact in photoreceptor cells and co-localize in the IS, ONL and OPL (Xi et al., 2007). Although Tulp1 was below detection limits by mass spectroscopy in mouse retina, we confirm the interaction of these two proteins by western blot analysis in both mouse and bovine retinas (Fig. 4). Tulp1 is a photoreceptor–specific protein which associates with the cytoskeleton and plays a role in intracellular protein transport (Hagstrom et al., 1999; Xi et al., 2005; Xi et al., 2007; Grossman et al., 2009). Tulp1−/− mice develop early-onset photoreceptor degeneration with defects in protein vesicular transport (Hagstrom et al., 1999; Hagstrom et al., 2001; Grossman et al., 2009; Grossman et al., 2011). Several proteins are not transported correctly to the OS in tulp1−/− retinas (Grossman et al., 2011); therefore, we investigated whether dynamin-1 localization was influenced in the absence of Tulp1. Surprisingly, dynamin-1 expression and localization did not appear to be affected in tulp1−/− retinas (Figs. 2 and 3). In fact, all three dynamin isoform protein levels were comparable in wt and tulp1−/− retinas, thus excluding dynamin-1 mislocalization as part of the retinal degenerative process observed in tulp1−/− mice.
Fifty proteins were identified in two retinal anti-dynamin-1 IPs, suggesting that these proteins may interact with dynamin-1 in mouse retina in vivo (Table 2). Of these, 30 were either constituents of the cytoskeleton or involved in membrane dynamics and transport (Fig. 5). This is not surprising given that there is a bi-directional interaction between cytoskeleton proteins and cellular membranes (Doherty & McMahon, 2008). Furthermore, the dynamin proteins have been shown to function in vesicle movement in association with cytoskeleton elements in neurons of the central nervous system (Ferguson et al., 2007; Jaiswal et al., 2009). Our protein interaction results suggest that a similar model applies to retinal neurons. Although there are differences between the dynamin-1 protein constituents between retina and brain, the majority of the proteins identified in both retina and brain are involved in either membrane dynamics and transport or associated with the cytoskeleton (Supplementary Tables 2 and 3). The cytoskeleton is the cellular scaffolding composed of microfilaments, intermediate filaments and microtubules that is critical in membrane dynamics such as endocytosis at the synapse (Mooren et al., 2012), vesicle generation at the trans-Golgi face (Stow & Heimann, 1998) and motor-driven intracellular vesicular protein transport (Anitei & Hoflack, 2012). We identified several potential retinal dynamin-1 interacting proteins that comprise the filamentous scaffolding of the cytoskeleton including actin, tubulin, neurofilament polypeptide, rootletin and lima1, as well as proteins which modulate or stabilize these networks, such as nestin, spectrin, MAP1B and catenin. Several of these proteins were validated by western blot analysis indicating that these proteins likely interact with dynamin-1 in vivo (Fig. 6)
A graphic depiction of the highly polarized and compartmentalized photoreceptor cell is shown in Figure 7. The three regions where dynamin-1 and Tulp1 co-localize (Fig. 7, shaded in orange) exhibit high levels of cytoskeleton-dependent vesicular transport and extensive membrane dynamics. The compartment-specific biological processes include OS protein transport (Fig. 7A), axonal transport (Fig. 7B), and synaptic vesicle transport and membrane retrieval (Fig. 7C).
Figure 7.
A graphic depiction of the photoreceptor cell, highlighting the three areas where there are high levels of cytoskeletal-dependent vesicular transport and extensive membrane remodeling. These compartment-specific functions represent the largest categories of dynamin-1 interacting partners, and are active in the regions where dynamin-1 and Tulp1 colocalize (shaded in orange). (A) The inner segment (IS) is the compartment in which outer segment (OS) proteins are processed and packaged into post-Golgi vesicles for transport through the IS and connecting cilium (CC) to the OS disks. (B) Anterograde and retrograde axonal transport allows nascent protein complexes to be trafficked to the photoreceptor synapse and cellular components to be transported back towards the cell body for degradation. (C) At the terminal, endocytosis replenishes synaptic vesicles and compensates for increases in membrane area due to exocytosis.
Photoreceptor OS-specific proteins are packaged into vesicles generated at the trans-Golgi face and transported through the CC (Fig. 7A). All three dynamin isoforms have been shown to function in vesicle generation at the trans-Golgi face (Cao et al., 1998). In support of this role, we identified members of the spectrin protein family as dynamin-1 binding partners. Spectrins associate with the cytoskeleton and play a role in protein transport from the trans-Golgi network (Fath et al., 1997). In mammalian photoreceptors, there appears to be several distinct post-Golgi vesicle transport pathways (Grossman et al., 2011), some of which are linked to myosin-based movement (Liu et al., 1999). The myosin family is composed of actin-based motor proteins that are responsible for many diverse functions including intracellular transport (Syamaladevi et al., 2012). We identified several members of the myosin family as potential dynamin-1 retinal binding partners, including myosin VI. Myosin VI is localized to the IS region and has been implicated in cargo sorting and vesicle formation at the trans-Golgi face (Warner et al., 2003; Kitamoto et al., 2005). Important to both vesicle generation and transport in the IS, the non-muscle cell α and γ actin isoforms were identified in our IP analyses. Actin microfilaments perform many cellular functions not the least of which is to provide tracks for the myosins to transport cargo. In addition to the actin-based transport pathways, there are distinct microtubule networks in the IS and CC of the photoreceptor which are critical in the transport of OS proteins (Whitehead et al., 1999). Previous studies suggest that the dynamins stabilize microtubule networks (Tanabe & Takei, 2009). We identified α-tubulin, a component of microtubules, as a possible dynamin-1 binding partner.
A second region where there is vesicular transport in the photoreceptor cell is throughout the axon (Fig. 7B). Protein transport in the axon is bi-directional and relies on microtubule-based motor proteins (Schwartz, 1979). Here too, the dynamins may regulate the stability of axonal microtubule networks (Tanabe & Takei, 2009). Pertinent to this role, we identified microtubule-associated protein 1B (MAP1B) as a potential dynamin-1 interacting protein. MAP1B is expressed primarily in neurons where it functions in stabilizing microtubules (Halpain & Dehmelt, 2006). We have localized MAP1B to the ONL (Grossman et al., 2012a), where it may interact with Tulp1 (Xi et al., 2003). Interestingly, Tulp1 exhibits a genetic interaction with a gene for the related protein, MAP1A, whereby an allele of the gene significantly attenuates photoreceptor degeneration in Tulp1 mutant mice (Maddox et al., 2012). Both MAP1A and Tulp1 have been shown to bind tubulin and actin, linking the microtubule networks to the microfilament cytoskeleton (Noiges et al., 2002; Xi et al., 2003; Xi et al., 2005). These results indicate that interactions between dynamin-1, MAPs and Tulp1 may contribute to the stability of these networks and modulate protein movement along these networks.
The synaptic terminal is also reliant upon continuous membrane remodeling and vesicle cycling (Fig. 7C). Synaptic membrane events are highly coordinated by the cytoskeleton to replenish synaptic vesicles and compensate for increases in membrane area due to exocytosis. Synaptic proteins were the third largest category of potential dynamin-1 interacting proteins in our study, and included proteins exclusive to synaptic vesicle transport and membrane retrieval such as src kinase signaling inhibitor 1 and V-type proton ATPase 116 kDa subunit a isoform 1.
The dynamins have long been associated with clathrin-mediated endocytosis (CME) and clathrin-independent endocytosis (van der Bliek & Meyerowitz, 1991). The dynamin-1 (a,c) retinal splice-variant has a frameshift mutation which terminates the protein at amino acid 814 (Xi et al., 2007), eliminating the last 53 amino acids in the proline-rich domain (PRD). Many proteins of the CME pathway bind to the PRD of dynamin, including the amphiphysins, endophilins and syndapins (Anggono & Robinson, 2007). In agreement with this model, none of these proteins were detected in this study. However, rho GTPase-activating protein 32 was identified, which has been shown to regulate clathrin-independent endocytic pathways (Qualmann & Mellor, 2003; Lundmark et al., 2008).
Although our study focused primarily on dynamin-1, dynamin-3 may also perform important functions in the retina. Dynamin-3 staining was strongest in the IPL, but immunoreactivity was also seen in the IS and at the photoreceptor terminal (Fig. 3D). Self-assembly of dynamin polymers has been shown to involve the GTPase, middle and GED domains (Smirnova et al., 1999), all of which are present in the retina-specific dynamin-1 splice-variant (Xi et al., 2007). Therefore, we hypothesize that this dynamin-1 isoform maintains this essential ability. In our study, dynamin-3 was identified and confirmed as a dynamin-1 binding partner, suggesting that different isoforms may have in vivo interactions in the retina. Indeed, there is recent evidence that dynamin-1 and -3 isoforms cooperate in promoting activity-dependent synaptic vesicle endocytosis in neurons of the brain (Raimondi et al., 2011).
Twenty other apparent dynamin-1 interacting proteins fell into additional categories. Several are important to mitochondrial function. Although not previously attributed to dynamin-1, members of the dynamin superfamily are known to be involved in mitochondrial fission and fusion (Ferguson & De Camilli, 2012). Proteins involved in cell adhesion were also identified. This is not surprising, given that there is an association between the cytoskeleton and cell adhesion matrices (Kaibuchi et al., 1999). Finally, although the dynamins are not known to function in translation or transcription, we identified several proteins in these groups, perhaps indicating expanded retinal roles for this isoform.
In conclusion, all three dynamin isoforms are expressed in the mouse retina with distinct localization patterns. Dynamin-1 exhibits the broadest localization and appears to be the primary isoform in the retina. Dynamin-1 localizes to specialized compartments of the photoreceptor and many of the potential interacting proteins can be grouped into cytoskeleton and membrane modeling categories, participating in intracellular transport processes relevant to endocytic and post-Golgi pathways. In addition, dynamin-1 may be important for photoreceptor cellular stability. Nevertheless, several of these proteins are specific to distinct pathways, suggesting that dynamin-1 may have compartment-specific interactomes. This has recently been proposed for Tulp1 (Grossman et al., 2012b). Additional analyses are necessary to support this idea and to expand upon the role of dynamins in the retina, including differentiating cell-type-specific functions and splice-variant contributions.
Supplementary Material
References
- Adamus G, Zam ZS, Arendt A, Palczewski K, McDowell JH, Hargrave PA. Anti-rhodopsin monoclonal antibodies of defined specificity: characterization and application. Vision Res. 1991;31:17–31. doi: 10.1016/0042-6989(91)90069-h. [DOI] [PubMed] [Google Scholar]
- Anggono V, Robinson PJ. Syndapin I and endophilin I bind overlapping proline-rich regions of dynamin I: role in synaptic vesicle endocytosis. J Neurochem. 2007;102:931–943. doi: 10.1111/j.1471-4159.2007.04574.x. [DOI] [PubMed] [Google Scholar]
- Anitei M, Hoflack B. Bridging membrane and cytoskeleton dynamics in the secretory and endocytic pathways. Nat Cell Biol. 2012;14:11–19. doi: 10.1038/ncb2409. [DOI] [PubMed] [Google Scholar]
- Cao H, Garcia F, McNiven MA. Differential distribution of dynamin isoforms in mammalian cells. Mol Biol Cell. 1998;9:2595–2609. doi: 10.1091/mbc.9.9.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc Natl Acad Sci U S A. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daley WP, Gulfo KM, Sequeira SJ, Larsen M. Identification of a mechanochemical checkpoint and negative feedback loop regulating branching morphogenesis. Dev Biol. 2009;336:169–182. doi: 10.1016/j.ydbio.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty GJ, McMahon HT. Mediation, modulation, and consequences of membrane-cytoskeleton interactions. Annu Rev Biophys. 2008;37:65–95. doi: 10.1146/annurev.biophys.37.032807.125912. [DOI] [PubMed] [Google Scholar]
- Fath KR, Trimbur GM, Burgess DR. Molecular motors and a spectrin matrix associate with Golgi membranes in vitro. J Cell Biol. 1997;139:1169–1181. doi: 10.1083/jcb.139.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Brasnjo G, Hayashi M, Wolfel M, Collesi C, Giovedi S, Raimondi A, Gong LW, Ariel P, Paradise S, O’Toole E, Flavell R, Cremona O, Miesenbock G, Ryan TA, De Camilli P. A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science. 2007;316:570–574. doi: 10.1126/science.1140621. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, De Camilli P. Dynamin, a membrane-remodelling GTPase. Nat Rev Mol Cell Biol. 2012 doi: 10.1038/nrm3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray NW, Fourgeaud L, Huang B, Chen J, Cao H, Oswald BJ, Hemar A, McNiven MA. Dynamin 3 is a component of the postsynapse, where it interacts with mGluR5 and Homer. Curr Biol. 2003;13:510–515. doi: 10.1016/s0960-9822(03)00136-2. [DOI] [PubMed] [Google Scholar]
- Grossman GH, Beight C, Ebke LE, Hagstrom SA. Interaction of Tulp1 and the Microtubule-associated Proteins in the Murine Retina. 2012a. [DOI] [PubMed] [Google Scholar]
- Grossman GH, Pauer GJ, Hoppe G, Hagstrom SA. Isolating photoreceptor compartment-specific protein complexes for subsequent proteomic analysis. Adv Exp Med Biol. 2012b;723:701–707. doi: 10.1007/978-1-4614-0631-0_89. [DOI] [PubMed] [Google Scholar]
- Grossman GH, Pauer GJ, Narendra U, Peachey NS, Hagstrom SA. Early synaptic defects in tulp1−/− mice. Invest Ophthalmol Vis Sci. 2009;50:3074–3083. doi: 10.1167/iovs.08-3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman GH, Watson RF, Pauer GJ, Bollinger K, Hagstrom SA. Immunocytochemical evidence of Tulp1-dependent outer segment protein transport pathways in photoreceptor cells. Exp Eye Res. 2011;93:658–668. doi: 10.1016/j.exer.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstrom SA, Adamian M, Scimeca M, Pawlyk BS, Yue G, Li T. A role for the Tubby-like protein 1 in rhodopsin transport. Invest Ophthalmol Vis Sci. 2001;42:1955–1962. [PubMed] [Google Scholar]
- Hagstrom SA, Duyao M, North MA, Li T. Retinal degeneration in tulp1−/− mice: vesicular accumulation in the interphotoreceptor matrix. Invest Ophthalmol Vis Sci. 1999;40:2795–2802. [PubMed] [Google Scholar]
- Hagstrom SA, North MA, Nishina PL, Berson EL, Dryja TP. Recessive mutations in the gene encoding the tubby-like protein TULP1 in patients with retinitis pigmentosa. Nat Genet. 1998;18:174–176. doi: 10.1038/ng0298-174. [DOI] [PubMed] [Google Scholar]
- Halpain S, Dehmelt L. The MAP1 family of microtubule-associated proteins. Genome Biol. 2006;7:224. doi: 10.1186/gb-2006-7-6-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MY, Kosako H, Watanabe T, Hattori S. Extracellular signal-regulated kinase/mitogen-activated protein kinase regulates actin organization and cell motility by phosphorylating the actin cross-linking protein EPLIN. Mol Cell Biol. 2007;27:8190–8204. doi: 10.1128/MCB.00661-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd P, Lang T, Wenzel D, De Camilli P, Jahn R. Imaging direct, dynamin-dependent recapture of fusing secretory granules on plasma membrane lawns from PC12 cells. Proc Natl Acad Sci U S A. 2002;99:16806–16811. doi: 10.1073/pnas.222677399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insinna C, Besharse JC. Intraflagellar transport and the sensory outer segment of vertebrate photoreceptors. Dev Dyn. 2008;237:1982–1992. doi: 10.1002/dvdy.21554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal JK, Rivera VM, Simon SM. Exocytosis of post-Golgi vesicles is regulated by components of the endocytic machinery. Cell. 2009;137:1308–1319. doi: 10.1016/j.cell.2009.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeub M, Bitoun M, Guicheney P, Kappes-Horn K, Strach K, Druschky KF, Weis J, Fischer D. Dynamin 2-related centronuclear myopathy: clinical, histological and genetic aspects of further patients and review of the literature. Clin Neuropathol. 2008;27:430–438. doi: 10.5414/npp27430. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells. Annu Rev Biochem. 1999;68:459–486. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- Kelly BL, Vassar R, Ferreira A. Beta-amyloid-induced dynamin 1 depletion in hippocampal neurons. A potential mechanism for early cognitive decline in Alzheimer disease. J Biol Chem. 2005;280:31746–31753. doi: 10.1074/jbc.M503259200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinuta M, Yamada H, Abe T, Watanabe M, Li SA, Kamitani A, Yasuda T, Matsukawa T, Kumon H, Takei K. Phosphatidylinositol 4,5-bisphosphate stimulates vesicle formation from liposomes by brain cytosol. Proc Natl Acad Sci U S A. 2002;99:2842–2847. doi: 10.1073/pnas.261715599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto J, Libby RT, Gibbs D, Steel KP, Williams DS. Myosin VI is required for normal retinal function. Exp Eye Res. 2005;81:116–120. doi: 10.1016/j.exer.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Linton JD, Holzhausen LC, Babai N, Song H, Miyagishima KJ, Stearns GW, Lindsay K, Wei J, Chertov AO, Peters TA, Caffe R, Pluk H, Seeliger MW, Tanimoto N, Fong K, Bolton L, Kuok DL, Sweet IR, Bartoletti TM, Radu RA, Travis GH, Zagotta WN, Townes-Anderson E, Parker E, Van der Zee CE, Sampath AP, Sokolov M, Thoreson WB, Hurley JB. Flow of energy in the outer retina in darkness and in light. Proc Natl Acad Sci U S A. 2010;107:8599–8604. doi: 10.1073/pnas.1002471107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Tan G, Levenkova N, Li T, Pugh EN, Jr, Rux JJ, Speicher DW, Pierce EA. The proteome of the mouse photoreceptor sensory cilium complex. Mol Cell Proteomics. 2007;6:1299–1317. doi: 10.1074/mcp.M700054-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Udovichenko IP, Brown SD, Steel KP, Williams DS. Myosin VIIa participates in opsin transport through the photoreceptor cilium. J Neurosci. 1999;19:6267–6274. doi: 10.1523/JNEUROSCI.19-15-06267.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundmark R, Carlsson SR. Sorting nexin 9 participates in clathrin-mediated endocytosis through interactions with the core components. J Biol Chem. 2003;278:46772–46781. doi: 10.1074/jbc.M307334200. [DOI] [PubMed] [Google Scholar]
- Lundmark R, Doherty GJ, Howes MT, Cortese K, Vallis Y, Parton RG, McMahon HT. The GTPase-activating protein GRAF1 regulates the CLIC/GEEC endocytic pathway. Curr Biol. 2008;18:1802–1808. doi: 10.1016/j.cub.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox DM, Ikeda S, Ikeda A, Zhang W, Krebs MP, Nishina PM, Naggert JK. An allele of microtubule-associated protein 1A (Mtap1a) reduces photoreceptor degeneration in Tulp1 and Tub mutant mice. Invest Ophthalmol Vis Sci. 2012 doi: 10.1167/iovs.11-8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNiven MA, Cao H, Pitts KR, Yoon Y. The dynamin family of mechanoenzymes: pinching in new places. Trends Biochem Sci. 2000;25:115–120. doi: 10.1016/s0968-0004(99)01538-8. [DOI] [PubMed] [Google Scholar]
- Mooren OL, Galletta BJ, Cooper JA. Roles for actin assembly in endocytosis. Annu Rev Biochem. 2012;81:661–686. doi: 10.1146/annurev-biochem-060910-094416. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Ochi S, Oda C, Ishii M, Ogawa K. Ischemic preconditioning attenuates of ischemia-induced degradation of spectrin and tau: implications for ischemic tolerance. Neurol Sci. 2011;32:229–239. doi: 10.1007/s10072-010-0359-5. [DOI] [PubMed] [Google Scholar]
- Noiges R, Eichinger R, Kutschera W, Fischer I, Nemeth Z, Wiche G, Propst F. Microtubule-associated protein 1A (MAP1A) and MAP1B: light chains determine distinct functional properties. J Neurosci. 2002;22:2106–2114. doi: 10.1523/JNEUROSCI.22-06-02106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qualmann B, Mellor H. Regulation of endocytic traffic by Rho GTPases. Biochem J. 2003;371:233–241. doi: 10.1042/BJ20030139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondi A, Ferguson SM, Lou X, Armbruster M, Paradise S, Giovedi S, Messa M, Kono N, Takasaki J, Cappello V, O’Toole E, Ryan TA, De Camilli P. Overlapping role of dynamin isoforms in synaptic vesicle endocytosis. Neuron. 2011;70:1100–1114. doi: 10.1016/j.neuron.2011.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozas JL, Gomez-Sanchez L, Tomas-Zapico C, Lucas JJ, Fernandez-Chacon R. Increased neurotransmitter release at the neuromuscular junction in a mouse model of polyglutamine disease. J Neurosci. 2011;31:1106–1113. doi: 10.1523/JNEUROSCI.2011-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JH. Axonal transport: components, mechanisms, and specificity. Annu Rev Neurosci. 1979;2:467–504. doi: 10.1146/annurev.ne.02.030179.002343. [DOI] [PubMed] [Google Scholar]
- Smirnova E, Shurland DL, Newman-Smith ED, Pishvaee B, van der Bliek AM. A model for dynamin self-assembly based on binding between three different protein domains. J Biol Chem. 1999;274:14942–14947. doi: 10.1074/jbc.274.21.14942. [DOI] [PubMed] [Google Scholar]
- Stow JL, Heimann K. Vesicle budding on Golgi membranes: regulation by G proteins and myosin motors. Biochim Biophys Acta. 1998;1404:161–171. doi: 10.1016/s0167-4889(98)00055-x. [DOI] [PubMed] [Google Scholar]
- Sun TX, Van Hoek A, Huang Y, Bouley R, McLaughlin M, Brown D. Aquaporin-2 localization in clathrin-coated pits: inhibition of endocytosis by dominant-negative dynamin. Am J Physiol Renal Physiol. 2002;282:F998–1011. doi: 10.1152/ajprenal.00257.2001. [DOI] [PubMed] [Google Scholar]
- Syamaladevi DP, Spudich JA, Sowdhamini R. Structural and functional insights on the Myosin superfamily. Bioinform Biol Insights. 2012;6:11–21. doi: 10.4137/BBI.S8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K, Takei K. Dynamic instability of microtubules requires dynamin 2 and is impaired in a Charcot-Marie-Tooth mutant. J Cell Biol. 2009;185:939–948. doi: 10.1083/jcb.200803153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaid KS, Guttman JA, Babyak N, Deng W, McNiven MA, Mochizuki N, Finlay BB, Vogl AW. The role of dynamin 3 in the testis. J Cell Physiol. 2007;210:644–654. doi: 10.1002/jcp.20855. [DOI] [PubMed] [Google Scholar]
- van der Bliek AM, Meyerowitz EM. Dynamin-like protein encoded by the Drosophila shibire gene associated with vesicular traffic. Nature. 1991;351:411–414. doi: 10.1038/351411a0. [DOI] [PubMed] [Google Scholar]
- Warner CL, Stewart A, Luzio JP, Steel KP, Libby RT, Kendrick-Jones J, Buss F. Loss of myosin VI reduces secretion and the size of the Golgi in fibroblasts from Snell’s waltzer mice. EMBO J. 2003;22:569–579. doi: 10.1093/emboj/cdg055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner HB, Kuhlmann K, Shen S, Uecker M, Schardt A, Dimova K, Orfaniotou F, Dhaunchak A, Brinkmann BG, Mobius W, Guarente L, Casaccia-Bonnefil P, Jahn O, Nave KA. Proteolipid protein is required for transport of sirtuin 2 into CNS myelin. J Neurosci. 2007;27:7717–7730. doi: 10.1523/JNEUROSCI.1254-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead JL, Wang SY, Bost-Usinger L, Hoang E, Frazer KA, Burnside B. Photoreceptor localization of the KIF3A and KIF3B subunits of the heterotrimeric microtubule motor kinesin II in vertebrate retina. Exp Eye Res. 1999;69:491–503. doi: 10.1006/exer.1999.0724. [DOI] [PubMed] [Google Scholar]
- Xi Q, Pauer GJ, Ball SL, Rayborn M, Hollyfield JG, Peachey NS, Crabb JW, Hagstrom SA. Interaction between the photoreceptor-specific tubby-like protein 1 and the neuronal-specific GTPase dynamin-1. Invest Ophthalmol Vis Sci. 2007;48:2837–2844. doi: 10.1167/iovs.06-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Q, Pauer GJ, Marmorstein AD, Crabb JW, Hagstrom SA. Tubby-like protein 1 (TULP1) interacts with F-actin in photoreceptor cells. Invest Ophthalmol Vis Sci. 2005;46:4754–4761. doi: 10.1167/iovs.05-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi Q, Pauer GJ, West KA, Crabb JW, Hagstrom SA. Retinal degeneration caused by mutations in TULP1. Adv Exp Med Biol. 2003;533:303–308. doi: 10.1007/978-1-4615-0067-4_37. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu X, Yue G, Adamian M, Bulgakov O, Li T. Rootletin, a novel coiled- coil protein, is a structural component of the ciliary rootlet. J Cell Biol. 2002;159:431–440. doi: 10.1083/jcb.200207153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuchner S, Noureddine M, Kennerson M, Verhoeven K, Claeys K, De Jonghe P, Merory J, Oliveira SA, Speer MC, Stenger JE, Walizada G, Zhu D, Pericak-Vance MA, Nicholson G, Timmerman V, Vance JM. Mutations in the pleckstrin homology domain of dynamin 2 cause dominant intermediate Charcot-Marie-Tooth disease. Nat Genet. 2005;37:289–294. doi: 10.1038/ng1514. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.