Abstract
Caloric restriction (CR) is cited as the most robust means of increasing lifespan across a range of taxa, yet there is a high degree of variability in the response to CR, both within and between species. To examine the intraspecific evolutionary conservation of lifespan extension by CR, we tested the effects of chronic caloric restriction (CCR) at multiple food levels and of intermittent fasting (IF) in twelve isolates from the Brachionus plicatilis species complex of monogonont rotifers. While CCR generally increased or did not change lifespan and total fecundity, IF caused increased, unchanged, or decreased lifespan, depending upon the isolate, and decreased total fecundity in all but one isolate. Lifespan under ad libitum (AL) feeding varied among isolates and predicted the lifespan response to CR: longer-lived isolates under AL were less likely to have a significant increase in lifespan under CCR and were more likely to have a significantly shortened lifespan under IF. Lifespan under AL conditions and the response to CR were not correlated with hydroperiodicity of native habitat or with time in culture. Lack of trade-off between lifespan and fecundity under CCR, and differences in lifespan and fecundity under CCR and IF, even when average food intake was similar, suggest that longevity changes are not always directly determined by energy intake and that CCR and IF regimens extend lifespan through diverse genetic mechanisms.
Keywords: Caloric restriction, Evolution of aging, Rotifer, Resource allocation, Trade-off, Intermittent fasting
1. Introduction
Caloric restriction (CR) is frequently cited as the most reliable means of extending lifespan across diverse taxa and is frequently employed as a means to investigate the mechanisms of aging. Studies of CR in non-human model systems, with the goal of applying the results to better understand aging in humans, assume a common selective pressure as the origin of the CR response, yet the universality of this pressure is uncertain. A prevailing hypothesis is that the extension of lifespan due to CR is an evolutionary adaptation to low nutrient conditions, delaying senescence to increase the chance of reproducing when food again becomes available (Harrison and Archer, 1988). According to the life history theory, the timing and duration of key events in an organism's life—including development, age of maturity and first reproduction, parental investment, and senescence—are shaped by natural selection to maximize the number of viable offspring (Fisher, 1958; Stearns, 1989). In a natural extension of the life history theory, the disposable soma theory posits that in the face of limiting resources there is a trade-off between reproduction and maintenance of the adult body; plasticity in life history allows the optimal investment in somatic maintenance and reproduction with changes in the environment (Holliday, 1989; Kirkwood, 1977; Kirkwood, 2002). These theories predict that organisms experiencing high extrinsic mortality would not evolve the capacity to extend lifespan or alter reproduction in response to environmental changes, and that CR would have the greatest effect on species with short reproductive lifespan and little effect on long-lived species like humans (Harrison and Archer, 1988; Phelan and Rose, 2005). Phelan and Austad (1989) pointed out that reproductive senescence, the end of the reproductive period, may not be relevant to evolution in natural systems if post-reproductive individuals are rare in the wild, but mounting evidence suggests that senescence is common in natural populations (Nussey et al., 2013). Over the years, resource allocation theories have been augmented by aspects of the hormesis hypothesis: the idea that stressors activate repair mechanisms, providing widespread repair and protection and possibly even increasing lifespan. Food limitation may be a stressor in itself, or increased food searching behavior due to CR could expose an organism to increased stressors, so upregulating stress-defense mechanisms would increase the chance of survival and secondarily extend lifespan (Masoro, 2007; Masoro and Austad, 1996; Phelan and Austad, 1989).
The more that CR is studied the more variability is found in its effect on lifespan, both within and among species. For example, wild mice under CR do not extend lifespan as strongly as laboratory mice, suggesting that laboratory conditions select for the CR effect, that there is genetic variability in the ability to respond to CR in wild populations that is not seen in inbred lines, or that the wild mice were simply not restricted at the correct level to see an effect (Harper et al. 2006). In a survey of 40 laboratory mouse strains, CR had no significant effect on the majority of strains, extended female lifespan in about one fifth of strains, and decreased female lifespan in about a quarter of strains; there was no correlation between lifespan under ad libitum (AL) and CR food conditions (Liao et al., 2010).
Different modes of imposing CR lead to variable results in the delay of age-related diseases, increased longevity, and changes in fecundity (Anson et al., 2003; Anson et al., 2005; Cleary and Grossmann, 2011; Dogan et al., 2011; Greer and Brunet, 2009; Gribble and Mark Welch, 2013). Recent studies suggest that long-lived primates subjected to CR may have increased lifespan and/or delayed onset of age related diseases in some but not all cases, differences that may be due to variation in the CR regimen (Mattison et al., 2012). Distinct genetic mechanisms have been associated with different CR regimens and species, highlighting the possible lack of a single selective pressure that gave rise to CR-mediated lifespan extension (Greer and Brunet, 2009; Kenyon, 2010). Additionally, expected trade-offs between fecundity and lifespan are not always seen either in long or short-lived animals (Gribble and Mark Welch, 2013; Johnston et al., 2006; Kirk, 2001; Sawada and Enesco, 1984). Exploring this variability in the CR response provides another means of probing the mechanisms and origins of aging.
In this study we investigated the effects of chronic caloric restriction (CCR) and intermittent fasting (IF) on the lifespan and fecundity of 12 isolates from the Brachionus plicatilis species group of monogonont rotifers. Rotifers have long been used for aging research and currently are being revived as a relevant model for phenotypic and molecular genetic research on aging. Studies on an array of rotifer species demonstrated that some increase, while others decrease, lifespan in response to CR (Fanestil et al., 1965; Gribble and Mark Welch, 2013; Kaneko et al., 2010; Kirk, 2001; Meadows and Barrows, 1971; Stelzer, 2001; Weithoff, 2007).
Rotifers are microscopic, aquatic, basally-branching triploblasts, with a number of advantages as an animal system for the study of aging (Austad, 2009). Their small size and ease of culturing allows testing of multiple treatments with a high degree of replication. As facultatively sexual animals, monogonont rotifers generally reproduce asexually, with a diploid female producing diploid eggs by mitosis. These eggs hatch into asexual (amictic) females, giving rise to a clonal population. In response to a quorum sensing mechanism, sexual (mictic) females are produced that generate haploid eggs through meiosis. If unfertilized, these haploid eggs hatch into males that can fertilize other haploid gametes to produce diploid, diapausing eggs. Males do not feed, so studies of CR are conducted on females.
The cultures used in this study were isolated from brackish ponds from around the world that varied in hydroperiodicity (the duration a wetland is covered by water) from ephemerally to permanently hydrated (Table 1, see table for hydroperiodicity definitions from Brock et al., 2003). All isolates belong to the B. plicatilis cryptic species complex, made up of at least 13 morphologically similar but genetically distinct groups (Gómez et al., 2002; Mills et al., 2007) that have been the focus of several recent aging studies (Gribble and Mark Welch, 2013; Kaneko et al., 2010; Oo et al., 2010; Snell et al., 2012). Ten of the 12 isolates used in this study were from a single clade (Clade A) within the species complex and are able to interbreed, suggesting any differences in CR have evolved recently (Gribble and Mark Welch, 2012; Snell, 1989; Snell and Hawkinson, 1983; Suatoni et al., 2006). The other two isolates, Brachionus sp. Tiscar and Brachionus sp. Towerinninesis, are members of a different clade (Clade B) and cannot interbreed with the other isolates in this study (Gribble and Mark Welch, 2012; Mills et al., 2007; Suatoni et al., 2006).
Table 1.
Species designation, culture origin, habitat type, and time in culture for 12 isolates from the Brachionus plicatilis species complex. Hydroperiodicity of native habitat is designated by E, ephemeral (fills for a few days after unpredictable rainfall); S, seasonal (alternately wet and dry every year, according to season); SP, semi-permanent (usually holds some water but occasionally dries); P, permanent (always holds water). Definitions from Brock et al. (2003).
| Isolate | Species | Origin | Habitat | Years in culture |
|---|---|---|---|---|
| BmanL5 | B. manjavacas | Manjavacas (Cuenca), Spain | SP | 14 |
| BmanMAN | B. manjavacas | Manjavacas (Cuenca), Spain | SP | 2 |
| BmanRUS | B. manjavacas | Azov Sea, Russia | SP | 30 |
| MNCHU008 | B. plicatilis Austria | Chuluutiin Tsagaan Nuur, Mongolia | E | 6 |
| Bp HOS | B. plicatilis sensu stricto | El Hondo Sur lagoon, Spain | SP | 2 |
| BpL1 | B. plicatilis sensu stricto | Torreblanca Sur (Castellon), Spain | S | 10 |
| BpL3 | B. plicatilis sensu stricto | Torreblanca Sur (Castellon), Spain | S | 10 |
| BpSAL | B. plicatilis sensu stricto | Salobrejo Pond, Spain | SP | 2 |
| CGAL6 | B. plicatilis sensu stricto | Clot de Galvany (Alicante), Spain | SP | 14 |
| USGET006 | B. plicatilis sensu stricto | Eel Culture Pond, Mie Province, Japan | P | 40 |
| JPNAG023 | B. sp. Tiscar | ? | ? | ? |
| AUYEN020 | B. sp. Towerinniensis | Yenyenning Lakes, Australia | SP | 5 |
The goal of this study was to explore the degree of conservation in the response to CR among closely related rotifer species and to determine if differences might be predictably related to environmental conditions including habitat stability or time in culture. Examination of variability in the lifespan and fecundity responses of closely related rotifer species derived from different environments provides a comparative system of “natural mutants” with which to investigate possible trade-offs between lifespan and reproduction and the universality of CR-mediated lifespan extension.
2. Materials and methods
Culturing and experimental conditions followed Gribble and Mark Welch (2013) and are described briefly below.
2.1. Cultures
The chlorophyte Tetraselmis suecica was maintained in 2 L flasks of bubbled 15 ppt artificial seawater (ASW) f/2 medium (Guillard, 1975) and was used as food for rotifer cultures. Both rotifer and algae cultures were kept at 21 °C on a 12:12 h light:dark cycle. Cultures of T. suecica used for CR studies were maintained in semi-continuous log phase growth by daily removal of approximately 20% of the culture and replacement with f/2 medium. Maternal Brachionus spp. females were maintained in ad libitum (AL) food conditions for at least one week prior to experiments to prevent known maternal effects of CR on offspring (Kaneko et al., 2010). Origins of isolates and species designations are given in Table 1. Data for the B. manjavacas RUS isolate were previously published (Gribble and Mark Welch, 2013).
2.2. Experimental conditions
In this study, we conducted life table experiments to examine the effects of different food concentrations on lifespan and reproduction of 12 isolates from the B. plicatilis cryptic species complex. Amictic eggs were removed from mature amictic females by vortexing, isolated by micropipette, and allowed to hatch for 5 h. Neonates were individually isolated into 1 ml of T. suecica at an AL concentration of 6 × 105 cells ml−1 (referred to as 100% of AL) in 24-well plates. The AL concentration was chosen based on previous studies that showed maximal ingestion and specific growth rates in Brachionus rotifers under comparable food conditions (Awaïss et al., 1993; Montagnes et al., 2001) and ingestion rate experiments in B. manjavacas in our laboratory. All individuals were fed at AL concentrations for the first 24 h then maintained in 1 ml volumes in individual wells of 24-well tissue culture plates at the treatment food concentrations for the duration of lifespan. Caloric restriction was attained by diluting algae with 15 ppt ASW to 75%, 50%, 25%, or 10% of AL concentrations, or by transferring animals to ASW without food (0% of AL). Intermittently fasted (IF) individuals were fed on alternate days at 100% and 0% of AL. Experiments were performed initially at all seven food levels in two isolates. When the effects of CCR were found to be nonsignificant at levels from 25%–75% of AL concentrations, life table experiments were scaled back to four treatments for five isolates, then to three treatments for another five isolates. Using fewer food concentrations allowed experimentation on more isolates. All lifetable experiments were performed once for each isolate; the number of individuals in each treatment (n) is given in Table 2.
Table 2.
Lifespan and fecundity of Brachionus isolates at a range of food concentrations from 100% of ad libitum (6 × 105 cells/ml; AL) to 0% of AL and under intermittent fasting (IF). Lifespan is reported in days; fecundity is reported as offspring per individual; bold denotes statistically significant difference (P < 0.05) between the treatment and the AL food level by the Krusal–Wallis test with Dunn's multiple comparison post-hoc test; test statistics are given in Supplementary Table 1.
| Clone | CR regimen | Mean lifespan (SEM) |
Median lifespan | Maximum lifespan (95th percentile) |
Mean lifetime fecundity (SEM) |
Median fecundity | Maximum fecundity (95th percentile) |
n |
|---|---|---|---|---|---|---|---|---|
| BmanL5 | AL | 10.00 (0.30) | 10 | 13 | 26.68 (0.54) | 27 | 30.85 | 22 |
| 10 | 11.35 (0.49) | 11 | 17 | 25.64 (1.3) | 27 | 29.85 | 20 | |
| IF | 10.52 (0.57) | 10 | 16 | 22.08 (0.76) | 21.5 | 28 | 23 | |
| BmanMAN | AL | 11.82 (0.39) | 12 | 17 | 30.14 (0.37) | 30 | 33 | 22 |
| 50 | 13.11 (0.53) | 13 | 19 | 31.32 (0.84) | 31 | 45 | 19 | |
| 10 | 12.70 (0.44) | 13 | 17 | 29.83 (0.46) | 30 | 33 | 23 | |
| IF | 9.43 (0.39) | 10 | 13 | 17.90 (0.77) | 17 | 24 | 21 | |
| BmanRUS | AL | 8.74 (0.22) | 8 | 14 | 32.24 (0.35) | 32 | 34 | 49 |
| 75 | 8.47 (0.28) | 8 | 15 | 30.91 (0.55) | 32 | 34 | 47 | |
| 50 | 9.19 (0.35) | 9 | 17 | 31.07 (0.56) | 32 | 34 | 43 | |
| 25 | 9.57 (0.30) | 9 | 17 | 30.35 (0.99) | 32 | 34 | 49 | |
| 10 | 10.33 (0.26) | 10 | 15 | 31.12 (0.63) | 32 | 34 | 51 | |
| IF | 12.33 (0.28) | 12 | 16 | 22.18 (0.56) | 23 | 27 | 40 | |
| 0 | 5.08 (0.12) | 5 | 7 | 3.85 (0.12) | 4 | 6 | 53 | |
| MNCHU008 | AL | 12.87 (0.43) | 13 | 18 | 21.89 (0.81) | 23 | 25 | 51 |
| 10 | 14.43 (0.64) | 16 | 21 | 20.52 (1.7) | 24 | 25.65 | 48 | |
| IF | 16.00 (0.67) | 17 | 22 | 19.09 (0.91) | 21 | 24 | 46 | |
| Bp HOS | AL | 8.69 (0.46) | 9 | 12 | 22.22 (0.91) | 23 | 28 | 16 |
| 10 | 11.05 (0.59) | 10 | 17 | 23.58 (0.57) | 25 | 27 | 19 | |
| IF | 10.06 (0.93) | 11 | 14 | 19.20 (1.7) | 21 | 27 | 16 | |
| BpL1 | AL | 10.05 (0.63) | 10 | 18 | 24.90 (0.56) | 25 | 28.95 | 20 |
| 50 | 11.58 (0.89) | 12 | 21 | 22.75 (1.1) | 23.5 | 28 | 24 | |
| 10 | 10.35 (0.73) | 11 | 19 | 23.91 (1.1) | 26 | 28.8 | 23 | |
| IF | 10.21 (0.60) | 11 | 16 | 20.75 (1.2) | 21 | 28.75 | 24 | |
| BpL3 | AL | 8.68 (0.84) | 10 | 15 | 16.21 (2.3) | 21 | 27 | 20 |
| 10 | 10.56 (0.81) | 12 | 16 | 19.45 (1.6) | 21 | 30.7 | 18 | |
| IF | 10.95 (0.91) | 13 | 17 | 15.59 (1.4) | 16 | 27.4 | 21 | |
| BpSAL | AL | 10.73 (0.57) | 10 | 15 | 25.13 (4.6) | 25.5 | 28 | 16 |
| 10 | 11.90 (0.45) | 12 | 17 | 25.45 (0.38) | 25.5 | 28 | 22 | |
| IF | 9.83 (0.57) | 10 | 16 | 22.04 (0.84) | 21 | 32.75 | 24 | |
| CGAL6 | AL | 11.62 (0.59) | 13 | 18 | 16.30 (0.96) | 18.5 | 22 | 39 |
| 75 | 10.43 (0.72) | 10 | 20 | 15.25 (1.0) | 16 | 24.95 | 40 | |
| 50 | 11.98 (0.85) | 12 | 30 | 16.45 (0.86) | 18 | 22.75 | 44 | |
| 25 | 13.05 (0.94) | 13 | 32 | 17.73 (0.98) | 19 | 25 | 41 | |
| 10 | 10.95 (0.62) | 10 | 21 | 16.38 (0.93) | 16 | 25.85 | 42 | |
| IF | 6.05 (0.33) | 6 | 14 | 6.55 (0.36) | 6 | 12.25 | 44 | |
| 0 | 4.65 (0.09) | 5 | 6 | 2.96 (0.13) | 3 | 4.65 | 46 | |
| USGET006 | AL | 9.60 (0.54) | 10 | 13 | 26.30 (1.4) | 27 | 39.6 | 20 |
| 50 | 9.65 (0.43) | 10 | 14 | 26.15 (1.1) | 28 | 31.95 | 20 | |
| 10 | 9.18 (0.69) | 10.5 | 14 | 21.77 (2.0) | 26.5 | 31.7 | 22 | |
| IF | 8.84 (0.29) | 9 | 10 | 14.74 (1.1) | 16 | 22 | 19 | |
| JPNAG023 | AL | 7.76 (0.44) | 8 | 11 | 16.83 (1.3) | 18.5 | 22 | 21 |
| 50 | 9.20 (0.69) | 8 | 17 | 16.60 (0.80) | 16.5 | 24.85 | 20 | |
| 10 | 9.70 (0.84) | 9 | 14 | 16.60 (1.4) | 17.5 | 22 | 10 | |
| IF | 6.17 (0.82) | 7 | 12 | 6.56 (1.3) | 6.5 | 18 | 18 | |
| AUYEN020 | AL | 4.54 (0.23) | 4 | 6 | 7.54 (0.69) | 6 | 13.5 | 24 |
| 50 | 5.41 (0.45) | 5 | 10 | 8.96 (1.1) | 7 | 20 | 22 | |
| 10 | 6.35 (0.51) | 6 | 11 | 10.74 (1.3) | 9 | 24.8 | 23 | |
| IF | 7.12 (0.60) | 7 | 11 | 14.71 (1.9) | 14 | 25 | 17 |
Every 24 h, lifespan, reproductive status (pre-reproductive, reproductive, or post-reproductive), and number of offspring (including both neonates and detached eggs) were scored for each individual, and the original female was moved to a new well with clean water and T. suecica at the treatment concentration. As rotifers have direct development, without separate larval or juvenile stages, lifespan was measured from the time of hatching to the time of death. Death was defined as a lack of movement of the cilia, mastax (food grinding organ) and foot. Mantel–Cox tests for significant differences between Kaplan–Meier survival curves were calculated using Prism 6.0b. Significance of differences between median lifespan or median reproduction values between 100% of AL and CR treatments were determined using Kruskal–Wallis tests with Dunn's post-test. The instantaneous mortality rate, calculated as μx = −ln(1 − qx) where qx is the age-specific probability of death in the interval Δx, was determined daily until only four individuals persisted. Gompertz curves were constructed from the log of the mortality rate, ln(μx). The significance of differences between correlations or between slopes or intercepts at different food concentrations was determined from linear regression using F-tests in Prism 6.0b. Linear regression provided a better fit to the data than Gomperz parameters estimated using maximum likelihood in Survomatic (http://spark.rstudio.com/bokov/sm/), likely due to the sample sizes and number of observations over the short lifespan of Brachionus rotifers.
2.3. Ingestion rate
Zooplankton are known to vary their ingestion (grazing) rates with the concentration of food, so we conducted experiments to measure clearance and ingestion rates over the range of T. suecica concentrations used in the lifetable experiments. Prior to ingestion rate experiments, rotifers to be tested at concentrations from 10% to 100% of AL levels were fed AL for four days. Rotifers to be tested for IF ingestion rates were fed AL and starved on alternate days for 4 days, and ingestion was measured at 100% of AL on the fifth day.
To measure ingestion rates, 10 rotifers/ml were introduced into 15 ml experimental tubes with known initial concentrations of T. suecica. Control tubes had only T. suecica and no rotifers. After 24 h, concentrations of T. suecica and of rotifers were counted in both experimental and control tubes. Ingestion was measured at approximately the same concentrations as those used in the CR life table experiments. Clearance rate (CLR) was calculated as ln(Cc/Cexp) · [V / (t · n)], where Cc and Cexp were the T. suecia concentrations at the end of the experiment in the control and experimental tubes, respectively, V was the volume, t was the duration of the experiment, and n the average number of rotifers per ml over the course of the experiment. The average rotifer and T. suecica concentrations were calculated as Cavg = (Cend − C0) / ln(Cend / C0), where C0 and Cend were the initial and final concentrations of rotifers or T. suecia in the experimental tubes. Ingestion rate was determined from CLR × Cavg. To compare mean food consumption of rotifers under IF with those fed continuously, we divided the 24 hour ingestion rate of IF rotifers at the 100% food concentration by 2 to give an average daily ingestion rate.
Ingestion rates at a given food concentration varied greatly among isolates. However, because the relationship between ingestion rate at a given T. suecica concentration and lifespan or fecundity varied among isolates (Supplementary Fig. 1), and because ingestion rate was positively correlated with T. suecica concentration for all isolates (Supplementary Fig. 2), we plotted lifespan and reproduction results against food exposure concentrations to simplify comparisons between isolates.
3. Results
3.1. Lifespan
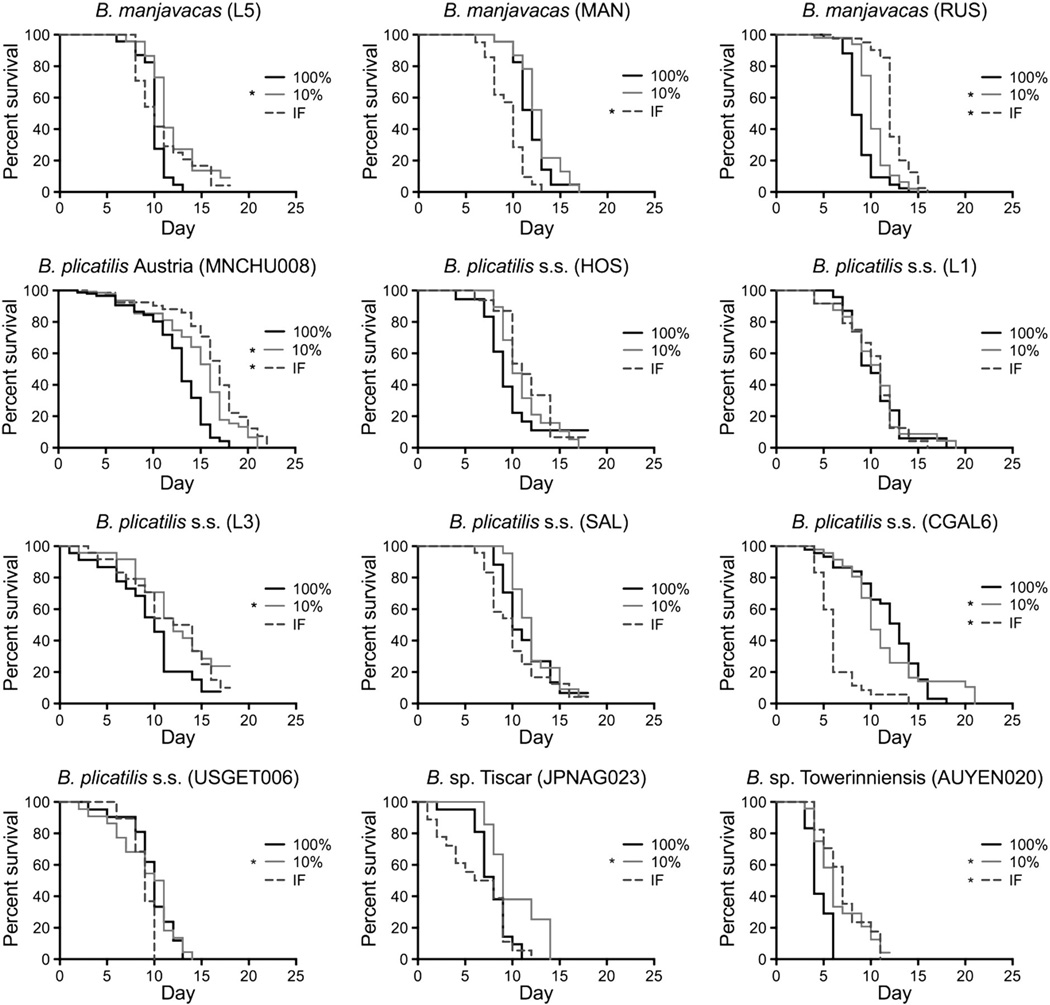
Under AL, lifespan varied from a minimum of 2 days to a maximum of 32 days; mean lifespan of isolates ranged from 4.5 to 12.9 days (Figs. 1 and 2, Table 2, Supplementary Figs. 3 and 4). Mean lifespan increased 5–50% under at least one level of CCR in eleven out of twelve isolates, with significant differences in median lifespan and/or Kaplan–Meier survival curves in eight isolates. The level of CCR maximizing median lifespan differed among isolates, but in no case was mean lifespan significantly decreased under any level of CCR. In contrast, under IF there was a significant increase in median lifespan in four isolates, no significant change in six isolates, and a significant decrease in two isolates.
Fig. 1.
Kaplan–Meier survival curves for 12 isolates of Brachionus spp. Isolates were fed at 100% and 10% of ad libitum food concentration or under intermittent fasting (IF). * denotes statistically significant difference between the treatment and the 100% food level (p < 0.05, Mantel–Cox test).
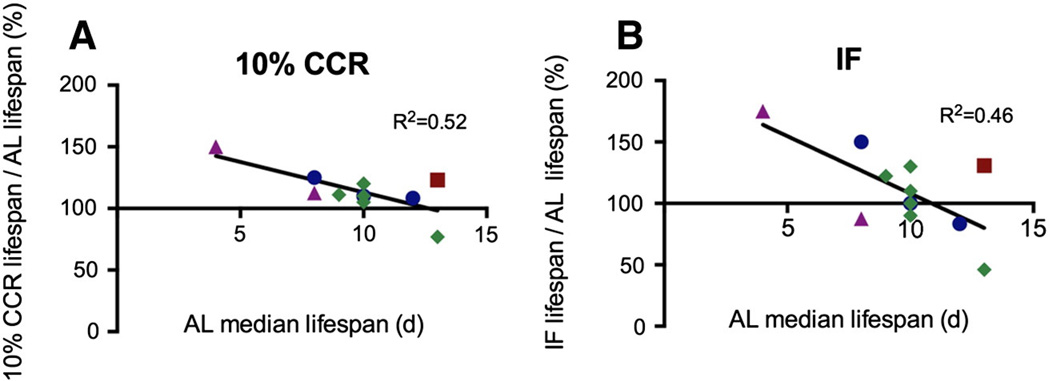
Fig. 2.
Lifespan responses to CR among isolates of Brachionus spp. Correlation between median lifespan under AL feeding and percent change in lifespan under (A) 10% CCR (rs = −0.54, p = 0.048, df = 10) and (B) IF (IF: rs = −0.47, p = 0.09, df = 10). Line indicates linear regression; R2 value shown. Blue circles denote B. manjavacas, green diamonds denote B. plicatilis sensu stricto, a red square denotes B. plicatilis Austria, all from Clade A; purple triangles denote Brachionus spp. from Clade B. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The longest-lived isolates under AL feeding were least able to increase lifespan under CCR or IF (Fig. 2). At 10% CCR, change in lifespan was significantly negatively correlated with median lifespan under AL; while all but one isolate had increased median lifespan (though not always significantly increased) the shortest lived under AL generally had the greatest lifespan extension. This negative correlation was present though less pronounced in the smaller number of isolates tested at 50% CCR (Supplementary Fig. 4). Under IF, five of eight isolates with mean lifespan ≥10 days under AL feeding had no change or a decrease in median lifespan, although the negative correlation did not reach statistical significance.
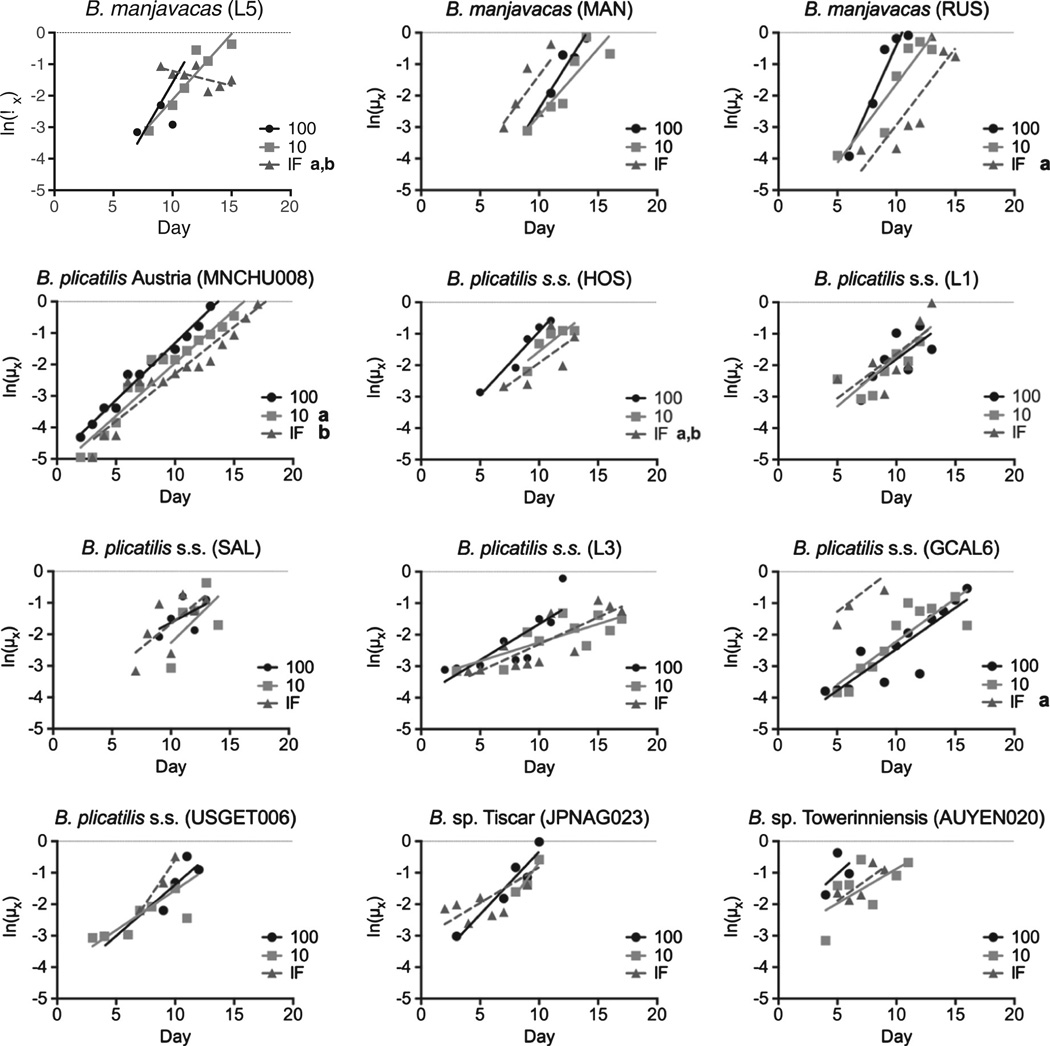
Gompertz curves of age specific instantaneous mortality rates (hazard rates) revealed an array of effects of CR on both the onset and rate of aging (Fig. 3). Because of the short lifespan of rotifers and the extremely high percent survival over the first several days of life, there were relatively few points dictating the linear regression in many cases; statistically significant differences between slopes and/or intercepts were thus not often achieved even where the trends appear strikingly different. Under IF conditions, changes in lifespan—positive or negative—could be attributed to significant shifts in the onset (the intercept of a Gompertz curve) or the rate (slope) of aging relative to AL conditions in three isolates. In an additional two isolates there were significant shifts in both the onset and rate of aging between IF and AL conditions.
Fig. 3.
Gomperz curves for 12 isolates of Brachionus spp. fed at 10% or 100% of ad libitum food concentration or under intermittent fasting (IF). Significant difference in slope from that at 100% food level denoted by a, significant difference in intercept denoted by b (p < 0.05, linear regression, F-test results in Supplementary Table 1).
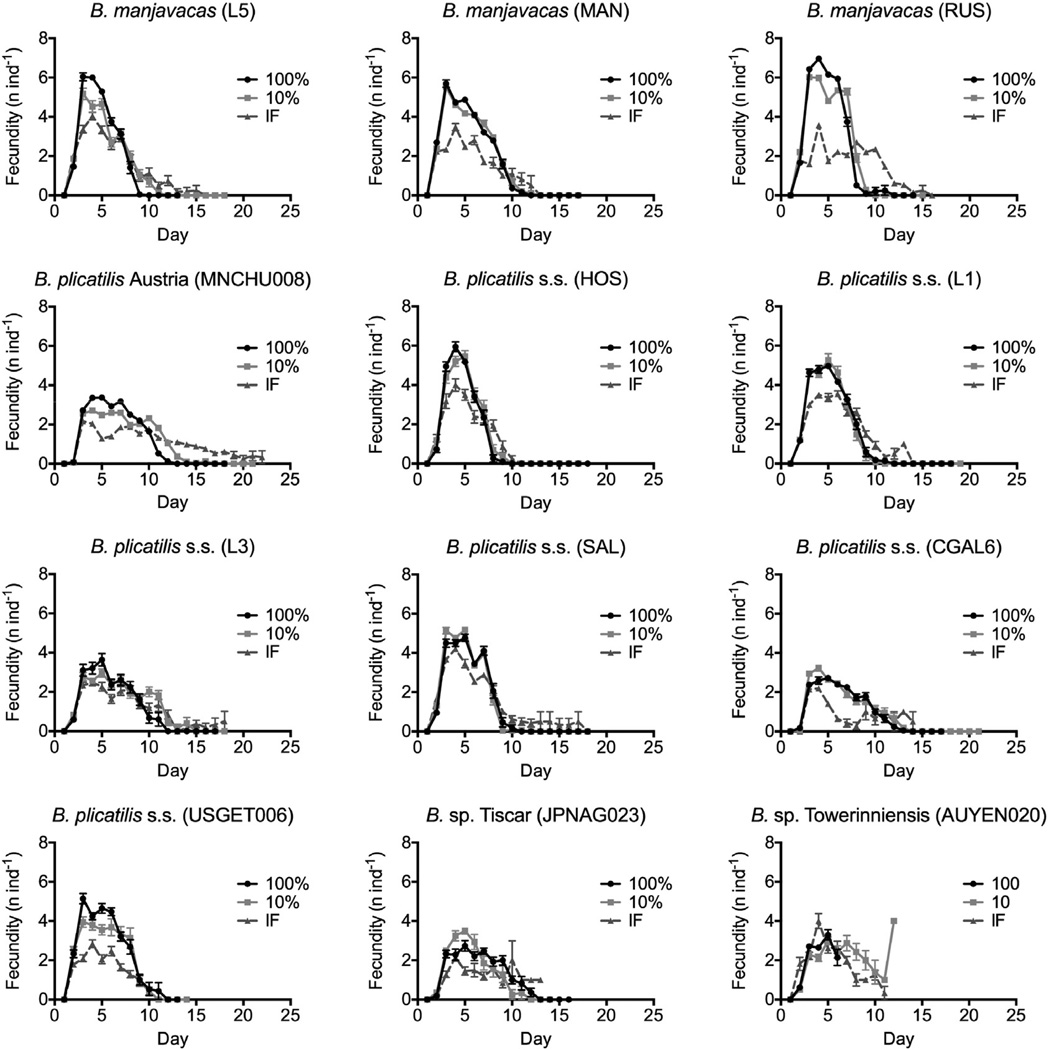
3.2. Fecundity
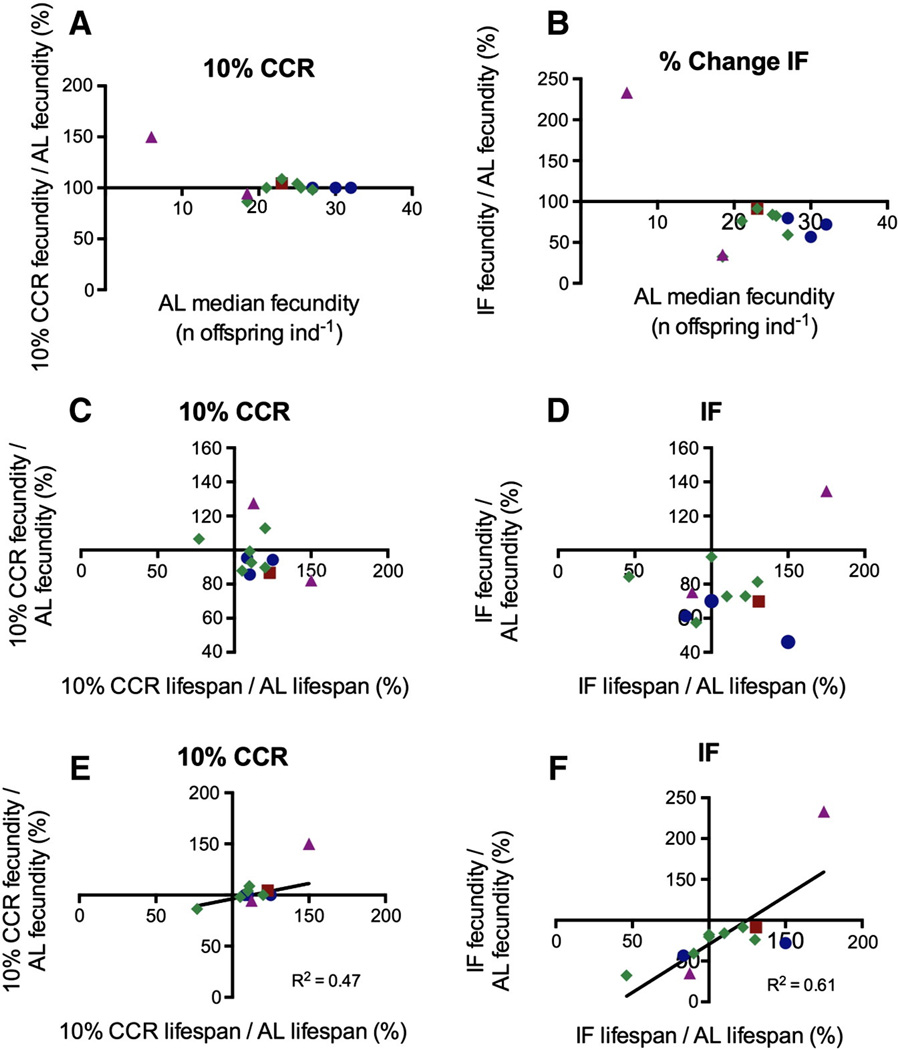
As with lifespan, mean lifetime fecundity (total number of offspring) varied greatly among isolates, with means of 7–32 offspring per individual under AL. Fecundity of each isolate, with one exception, was relatively constant across all levels of CCR and decreased under IF (Fig. 4A–B, Table 2; see Supplementary Fig. 4 for 50% CCR). The exception, B. sp. Towerinniensis AUYEN020, had a much lower fecundity than all other strains under AL but increased fecundity under CR conditions.
Fig. 4.
Fecundity responses to CR among isolates of Brachionus spp. (A–B) Relationship between mean lifetime fecundity (mean number of offspring per individual) under AL feeding relative to (A) 10% CCR, and (B) IF. There was no significant correlation for either treatment (10% CCR: rs = −0.098, p = 0.651, df = 10; IF: rs = −0.166, p = 0.595, df = 10). (C–D) Relationship between change from AL-fed lifespan and change in early reproduction, over the first 96 h of life for (C) 10% CCR and (D) IF. Spearman correlation was not significant for either treatment (10% CCR: −0.2842, p = 0.362, df = 10; IF: rs = 0.0210, p = 0.951, df = 10). (E–F) Relationship between the percent change from AL-fed lifespan and the percent change from AL-fed lifetime fecundity under (E) 10% CCR (Spearman correlation: rs = 0.564, p = 0.059, df = 10) and (F) IF (Spearman correlation: rs = 0.768, p = 0.005, df = 10), with linear regression and R2 value shown. Blue circles denote B. manjavacas, green diamonds denote B. plicatilis sensu stricto, a red square denotes B. plicatilis Austria, all from Clade A; purple triangles denote Brachionus spp. from Clade B. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
To examine the relationship between fecundity and lifespan we plotted change in fecundity against change in lifespan between treatment and AL for each isolate in Cartesian coordinates, such that quadrant I represents an increase in both lifespan and fecundity under treatment conditions and quadrant IV represents the classic trade-off between increased lifespan and decreased fecundity (Fig. 4C–F). Considering only early life reproduction, measured as total fecundity through the first 96 h, the fecundity–lifespan relationship of most isolates placed them in quadrant IV with reproduction slightly lowered for 10% CCR and greatly decreased for IF, but there was no significant correlation between increased lifespan and decreased fecundity for either regimen (Fig. 4C–D). Lifetime fecundity under CCR was nearly identical to AL, and no trade-off between lifespan and fecundity was evident (Fig. 4E). Lifetime fecundity under IF decreased in all isolates except B. sp. Towerinniensis, regardless of whether lifespan increased or decreased (Fig. 4F). The change in lifetime fecundity was positively correlated with change in lifespan under both 10% CCR and IF. Under 50% CCR lifetime fecundity was not significantly correlated with AL lifespan, AL median fecundity, or change in lifespan (Supplementary Fig. 4).
Analysis of daily reproduction showed that an isolate had very similar levels and patterns of daily reproduction under AL and 10% CCR conditions, with maximum reproduction at days 3–4 and a long post-reproductive period of up to 38% of lifespan (Fig. 5). In contrast, under IF, maximum reproduction on days 3–4 was repressed, and the reproductive period was often extended, with a larger proportion of individuals still reproductive at the time of death. Across all isolates, 20% of AL rotifers, 12% of CCR rotifers, and 30% of IF rotifers were reproductive within 24 h of death. Under 10% CCR, eight of twelve isolates had <10% of individuals die while reproductive, while under IF all but two isolates had >20% of individuals die while reproductive.
Fig. 5.
Daily reproduction (mean offspring individual−1) for 12 isolates of Brachionus spp. under AL (100%) feeding, 10% CCR, and IF.
Caloric restriction led to few changes in the age of first reproduction among isolates, and changes in the age of first reproduction were independent of the direction of change in lifespan (Supplementary Fig. 5). In several isolates the age of first egg production was significantly earlier under IF than AL; the age of first egg production was significantly delayed under IF in only one isolate. Earlier egg production led to earlier hatching in a single isolate; in all other cases, the time of first hatching was unchanged or increased under IF.
3.3. CCR versus IF regimens
The impact of CR on lifespan was not simply a matter of the average amount of food ingested (Supplementary Fig. 1). In some cases, average daily ingestion rates were very similar between a CCR treatment and an IF treatment for a given isolate, but lifespan and fecundity were very different between these treatments (e.g. B. manjavacas BmanRUS, B. plicatilis Austria MNCHU008, and B. sp. Towerinniensis). Some isolates (e.g. B. manjavacas BmanRUS, BmanMAN; B. plicatilis s. s. BpL3, USGET006) were able to adjust their ingestion rates quickly to switch from no food to AL food conditions and thus compensate for days of starvation on days when food was present. Other isolates were unable to upregulate ingestion rates on fed days (e.g. B. plicatilis Austria MNCHU008, B. plicatilis s. s. CGAL6). There was no relationship between the level of compensation and the resulting lifespan (Supplementary Figs. 1, 6). That is, some isolates under IF conditions that were able to increase food consumption on fed days had increased lifespan while others had decreased lifespan, relative to AL-fed rotifers. Similarly, isolates with increased or decreased lifespan could be found among the group that did not increase ingestion rates to compensate for starved days under IF.
3.4. Habitat and culture conditions
The isolates have been in laboratory culture for 2–40 years (Table 1). There was no co-variation between years in culture and mean lifespan under AL (Supplementary Fig. 7). There was a slight non-significant negative trend between years in culture and change in lifespan under 50% and 10% CCR, but no correlation between time in culture and change in lifespan under IF.
The isolates were collected from brackish water habitats with hydration periods (hydroperiodicity) ranging from ephemeral to permanent (Table 1). No correlation was seen between the type of native habitat and the mean lifespan under AL or change in lifespan under CCR (Supplementary Fig. 8). A wide range in lifespan and CR response was seen even among the majority of isolates that were collected from semi-permanent habitats. In two cases, two isolates of the same species originated from the same pond (Table 1): The isolates from the same semi-permanent habitat had significantly different lifespans under AL feeding, but similar responses to 10% CCR and IF. The isolates from the same seasonal habitat had lifespans that were not significantly different at 100% food levels, but had differences in their response to CCR and IF. The fact that the isolates from the same semi-permanent habitat were isolated 12 years apart while those from the same seasonal habitat were brought into culture the same year confounded attempts to draw meaning from the habitat, lifespan and CR response results.
4. Discussion
Previous studies of aging interventions in rotifers have examined the effects of one or two levels of CR on single, or multiple but distantly related, species of monogonont and bdelloid rotifers. This study represents the first effort to quantify the response to CR in multiple isolates of the same or closely related species of monogonont rotifers under the same food conditions. Examination of the lifespan and fecundity effects of a range of food concentrations in twelve natural isolates of five closely-related species revealed that the effect of restricted energy consumption on lifespan is indirect and does not always appear to act through a resource allocation trade-off with fecundity in rotifers.
4.1. Variability and patterns in aging and the response to CR
The twelve isolates from the B. plicatilis species complex exhibited large differences in mean lifespan and fecundity in food-replete (AL) conditions as well as in their responses to both chronic and intermittent food restriction. This variation was often greater between isolates of a single species than between species, and isolates of the same species collected from the same location at the same time differed significantly. This high variability suggests a need to be cautious about generalizing between isolates of the same species, to say nothing of between distantly related species. Within the variability observed, several patterns emerged that provide insight into the mechanisms and origins of the CR response.
Our results show that, in rotifers, longer lifespan under AL conditions is correlated with a decreased ability to extend lifespan under CR. CCR and IF had the greatest beneficial impact on the shortest-lived isolates, and the least (or even negative) impact on the longest lived isolates. The large differences in lifespan changes in any given isolate across the range of food concentrations tested, and among isolates at any particular food concentration, suggests that there is not a single food level at which maximum lifespan will be achieved; the optimal food concentration is different for each isolate, even for isolates of the same species.
In assessing the role of CR on lifespan it is important to ensure that high food concentrations do not negatively impact lifespan and reproduction and thereby conflate the beneficial effects of low food concentrations. Ingestion and daily reproductive rates were at a maximum at the highest food concentrations for 11 of 12 isolates, suggesting that there is not a toxic effect of the algal food that is removed when the algae is diluted at lower food concentrations. Additionally, there is no difference in longevity in male B. manjavacas, which do not eat, when grown in high versus low T. suecica concentrations or in artificial seawater in the absence of algae (K. Gribble, unpublished data: 100% T. suecica, male median lifespan = 5.7 days, 50% T. suecica, male median lifespan = 5.7 days, 10% T. suecica male median lifespan = 6.0 days, 0% T. suecica, male median lifespan = 5.7 days; n = 48 for each treatment, observation interval = 12 h), indicating that T. suecica does not release an allelopathic or toxic substance into the media.
4.2. Evolution of the CR response
It is unclear why rotifers should have developed the ability to increase lifespan in response to CR. Relative to other animals, these are short-lived species likely to have high extrinsic mortality from predation, competition, and the ephemeral nature of their habitats. Accordingly, they maximize reproduction early in life. They also have an alternative reproductive strategy that results in a diapausing embryo that can desiccate and overwinter, allowing survival through unfavorable environmental conditions without the need to extend lifespan for later reproduction. Thus, there does not seem to be a benefit to extending lifespan to outlast food restriction. Yet, most isolates in this study and most rotifer species tested to date increase lifespan at least under some types of food limitation.
The response of Brachionus spp. rotifers to CCR does not match the predictions of the disposable soma theory, which hypothesizes that lifespan extension during CR is the result of selection to postpone reproductive senescence, increasing the chance of reproduction if food resources are renewed later in life (Fisher, 1958; Holliday, 1989; Kirkwood, 1977; Kirkwood, 2002; Stearns, 1989). One might thus predict a delay in the age of first reproduction, to free energetic resources for somatic maintenance, with the extension of lifespan as a secondary consequence (Phelan and Austad, 1989). We observed a delay in only one isolate, under only IF conditions. In contrast, first egg production and first neonate hatching under CCR was unchanged or occurred earlier in all other isolates, and under all treatments was maximized in the first few days of life. Similarly, a high reproductive rate early in life did not preclude extended lifespan, and decreased reproduction early in life did not guarantee increased lifespan—that is, we did not see a cost of early reproduction on lifespan. Given that all treatments involved keeping individuals at AL food levels for the first 24 h of life, and that egg production began 24–48 h after hatching, the effect of a change in food concentration on changes in the age of first reproduction was extremely rapid. CR from the moment of hatching might shift the timing of reproduction.
Alternatively, one might predict that late life reproduction would be decreased to allow for, or as a consequence of, extended lifespan. This would seem to have minimal fitness benefits, however, since any additional lifespan extension during late life would be relatively small, the possibility of restoring reproduction would be more difficult, and the impact of restoring food on fecundity would be relatively minor. Indeed, we saw no decline in late-life reproduction under CCR or IF.
Rotifers under CCR had the same or even higher lifetime fecundity than those fed AL. The reproductive period increased in proportion with the lifespan, and was relatively constant across CCR and AL treatments. Conversely, under IF, there was a sharp decline in the daily reproductive rate and an extended reproductive period, whether lifespan was or was not extended. The post-reproductive period decreased under IF largely because a higher proportion of rotifers died while still reproductive. Changing the feeding regimen—for example, feeding rotifers AL for the first several days of life and then restricting food or resuming feeding late in life after early CR—may change these results. Under food limitation, there may be trade-offs in aspects of fitness that were not measured in this study, including body size, swimming speed, or fertility of sexual females.
4.3. Impact of caloric restriction regimen
The differences we observed in lifespan and reproduction under CCR versus IF, even when food consumption was similar on average, suggest that different mechanisms underlie the response to the two CR regimens. For example, decreased fecundity was only seen under IF, even when lifespan did not increase. The short-term starvation of IF may put into motion the same set of energy-sensing molecular mechanisms in all isolates, but isolates with higher energy requirements may have insufficient resources for extended lifespan. Alternatively isolates may have evolved different links between energy-sensing and lifespan-extending genetic mechanisms. Identifying what these molecular mechanisms are, and what mechanisms allow or preclude increased lifespan under IF is an ongoing subject of investigation.
The different outcomes under CCR and IF suggest that the effects of CR on aging may be indirectly controlled by energy input, at least under certain conditions. This supports the idea that aging may be regulated by a small set of molecular pathways that are, in some cases, independent of fitness (Johnston et al., 2006; Partridge et al., 2005). While nutrient sensing pathways are likely involved under both CR regimens, it may be that stress-response mechanisms are up-regulated under a given CR regimen, and metabolic and energy allocation pathways impacting reproductive mechanisms are altered under another CR regimen. Evidence continues to emerge that diverse pathways are involved in different types of food restriction (Greer and Brunet, 2009; Greer et al., 2007). Our results show that these varied mechanisms result in different outcomes for reproduction as well as lifespan.
4.4. Effect of native habitat and laboratory culture on CR response
If the anti-aging effect of CR is an evolutionarily adaptive response to outlast limited food availability in the environment, then the habitat of any given isolate should select for the response to CR. Environments with fluctuating food supply would select for individuals with short lifespan, early reproduction, and the ability to extend lifespan in the face of food shortage to allow later reproduction (Harrison and Archer, 1988). Isolates from stable environments with constant food supply would not be under selective pressure to evolve such traits. An examination of four Daphnia species at a range of temperature and food concentrations showed a correlation between senescence with ecological variation rather than taxonomy (Dudycha, 2003). Additional evidence from diverse taxa supports the evolutionary theory of aging: an isolated population of opossums has lower age-specific mortality and reproductive rates than a population exposed to higher predation (Austad, 1993); high-altitude grasshoppers subject to increased mortality from severe winters have shorter lifespan (Tatar et al., 1997); and eusocial ant taxa living in well-protected colonies have longer lifespan than solitary taxa (Keller and Genoud, 1997).
Rotifers have short reproductive lifespans and live in variable environments, and thus may be under increased selective pressure for mechanisms that extend life in response to resource deprivation, since many droughts or food shortages would outlast maximum reproductive lifespan (Harrison and Archer, 1988). In this study, we found no strong association between habitat hydoperiodicity and either mean lifespan under AL conditions or the response to CR. The wide range of lifespan and CR responses for the many isolates from semi-permanent environments may simply reflect the large variability in environmental stability within that hydroperiodicity category. Alternatively, lifespan phenotypes may be linked more directly to other factors such as food (phytoplankton and bacterial) or predator dynamics in the isolates' habitats. However, the variable CR responses we observe among Brachionus isolates suggest a disconnect between CR-induced lifespan extension and selection for maximum reproductive potential in dynamically irregular food environments.
We found no correlation between time in culture and mean lifespan under AL or the lifespan effects of CR. Some have hypothesized that laboratory conditions select for strains with high early fecundity and shortened lifespan (Linnen et al., 2001; Promislow et al., 1999) and for lifespan extension in response to CR (Harper et al., 2006). A recent meta-analysis of CR data from a large number of model and non-model organisms supports this hypothesis across species (Nakagawa et al., 2012). That study equated “non-model” with “short time in culture,” however, and provided no data for the number of generations that each taxon or strain had been resident in the laboratory, so assessment of selection over time was not possible. Additionally, the higher number of samples and independent studies for model organisms generated smaller confidence intervals for these species, making them more likely than non-models to fall within a range where CR explains the life-extending effect. As more strains of both laboratory and wild populations are studied under a wider range of CR conditions, more evidence accumulates that there is a high degree of variability in the CR response in both natural and laboratory strains (Liao et al., 2010; Nussey et al., 2013).
5. Conclusions
Collectively, the individual and intraspecific variability in the response to CR; the general lack of reproductive trade-off under CCR conditions; the decrease in fecundity under IF conditions, independent of whether or not lifespan is extended; and the difference in lifespan effects when similar amounts of food are ingested under different restriction regimens imply that the longevity changes in response to decreased food availability are not always directly related to the amount of energy ingested.
Examination of natural mutants provides a powerful system for characterizing the degree of conservation of lifespan extension under CR. The intraspecific variability in longevity and CR response in rotifers suggests that lifespan is a rapidly evolving trait that may be shaped by differences in food dynamics and predation pressure among the isolates' native habitats. Differences in reproductive allocation and fecundity under CCR and IF, even when lifespan extension was similar, imply that varied CR regimens extend lifespan through diverse genetic mechanisms.
Supplementary Material
Acknowledgments
We thank Scott Mills, Manuel Serra, Eduardo Garcia, Evangelina Michaloudi and Terry Snell for cultures. We thank Christian Jersabek for habitat information and Bette Hecox-Lea and Martha Bock for laboratory assistance. The critiques of two anonymous reviewers greatly improved the manuscript. This work was supported by the Ellison Medical Foundation/American Federation for Aging Research Post-doctoral Fellows in Aging Research Program and by the National Institute on Aging Division of Aging Biology (R01 AG037960-01).
Abbreviations
- CR
caloric restriction
- CCR
chronic caloric restriction
- IF
intermittent fasting
- AL
ad libitum
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.exger.2013.12.005.
Conflict of interest
The authors have no conflicts of interests.
References
- Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from caloric intake. Proc. Natl. Acad. Sci. U.SA. 2003;100:6216–6220. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anson RM, Jones B, de Cabo R. The diet restriction paradigm: a brief review of the effects of every-other-day feeding. Age. 2005;27:17–25. doi: 10.1007/s11357-005-3286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austad SN. Retarded senescence in an insular population of Virginia opossomus (Didelphis virginiana) J. Zool. 1993;229:695–708. [Google Scholar]
- Austad SN. Is there a role for new invertebrate models for aging research? J. Gerontol. 2009;64A:192–194. doi: 10.1093/gerona/gln059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awaïss A, Kestemont P, Micha JC. An investigation into the mass production of the freshwater rotifer Brachionus calyciflorus Pallas. 1. An eco-physiological approach to nutrition. Aquaculture. 1993;105:325–336. [Google Scholar]
- Brock MA, Nielsen DL, Shiel RJ, Green JD, Langley JD. Drought and aquatic community resilience: the role of eggs and seeds in sediments of temporary wetlands. Freshw. Biol. 2003;48:1207–1218. [Google Scholar]
- Cleary MP, Grossmann ME. The manner in which calories are restricted impacts mammary tumor cancer prevention. J. Carcinog. 2011;10:21. doi: 10.4103/1477-3163.85181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan S, Johannsen AC, Grande JP, Cleary MP. Effects of intermittent and chronic calorie restriction on mammalian target of rapamycin (mTOR) and IGF-1 signaling pathways in mammary fat pad tissues and mammary tumors. Nutr. Cancer. 2011;63:389–401. doi: 10.1080/01635581.2011.535968. [DOI] [PubMed] [Google Scholar]
- Dudycha JL. A multi-environment comparison of senescence between sister species of Daphnia. Oecologia. 2003;135:555–563. doi: 10.1007/s00442-003-1230-7. [DOI] [PubMed] [Google Scholar]
- Fanestil DD, Barrows J, Charles H. Aging in the rotifer. J. Gerontol. 1965;20:462–469. [PubMed] [Google Scholar]
- Fisher R. The Genetical Theory of Natural Selection. New York, Dover, New York: 1958. [Google Scholar]
- Gómez A, Serra M, Carvalho GR, Lunt DH. Speciation in ancient cryptic species complexes: evidence from the molecular phylogeny of Brachionus plicatilis (Rotifera) Evolution. 2002;56:1431–1444. doi: 10.1111/j.0014-3820.2002.tb01455.x. [DOI] [PubMed] [Google Scholar]
- Greer EL, Brunet A. Different dietary restriction regimens extend lifespan by both independent and overlapping genetic pathways in C. elegans. Aging Cell. 2009;8:113–127. doi: 10.1111/j.1474-9726.2009.00459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr. Biol. 2007;17:1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble KE, Mark Welch DB. The mate recognition protein gene mediates reproductive isolation and speciation in the Brachionus plicatilis cryptic species complex. BMC Evol. Biol. 2012;12:134. doi: 10.1186/1471-2148-12-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gribble KE, Mark Welch DB. Life-span extension by caloric restriction is determined by type and level of food reduction and by reproductive mode in Brachionus manjavacas (Rotifera) J. Gerontol. A: Biol. Sci. 2013;68:349–358. doi: 10.1093/gerona/gls170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillard RRL. Culture of phytoplankton for feeding marine invertebrates. In: ed^eds, editor. Culture of Marine Invertebrates. New York, New York: Plenum Publishing Corporation; 1975. [Google Scholar]
- Harper JM, Leathers CW, Austad SN. Does caloric restriction extend life in wild mice? Aging Cell. 2006;5:441–449. doi: 10.1111/j.1474-9726.2006.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DE, Archer JR. Natural selection for extended longevity from food restriction. Growth Dev. Aging. 1988;52:65. [PubMed] [Google Scholar]
- Holliday R. Food, reproduction and longevity: is the extended lifespan of calorie-restricted animals an evolutionary adaptation? BioEssays. 1989;10:125–127. doi: 10.1002/bies.950100408. [DOI] [PubMed] [Google Scholar]
- Johnston SL, Grune T, Bell LM, Murray SJ, Souter DM, Erwin SS, Yearsly JM, Gordon IJ, Illius AW, Kyriazakis I, Speakman JR. Having it all: historical energy intakes do not generate the anticipated trade-offs in fecundity. Proc. R. Soc. Lond. B Biol. Sci. 2006;273:1369–1374. doi: 10.1098/rspb.2005.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko G, Yoshinaga T, Yanagawa Y, Ozaki Y, Tsukamoto K, Watabe S. Calorie restriction-induced maternal longevity is transmitted to their daughters in a rotifer. Funct. Ecol. 2010 http://dx.doi.org/10.1111/j.1365-2435.2010.01773.x. [Google Scholar]
- Keller L, Genoud M. Extraordinary lifespans in ants: a test of evolutionary theories of aging. Nature. 1997;389:958–960. [Google Scholar]
- Kenyon C. The genetics of aging. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Kirk KL. Dietary restriction and aging: comparative tests of evolutionary hypotheses. J. Gerontol. 2001;56A:B123–B129. doi: 10.1093/gerona/56.3.b123. [DOI] [PubMed] [Google Scholar]
- Kirkwood TBL. Evolution of aging. Nature. 1977;270:301–304. doi: 10.1038/270301a0. [DOI] [PubMed] [Google Scholar]
- Kirkwood TBL. Evolution of ageing. Mech. Ageing Dev. 2002;123:737–745. doi: 10.1016/s0047-6374(01)00419-5. [DOI] [PubMed] [Google Scholar]
- Liao C-Y, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 2010;9:92–95. doi: 10.1111/j.1474-9726.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnen C, Tatar M, Promislow DE. Cultural artifacts: a comparison of senescence in natural, laboratory-adapted and artificially selected lines of Drosophila melanogaster. Evol. Ecol. Res. 2001;3:877–888. [Google Scholar]
- Masoro EJ. Role of hormesis in life extension by caloric restriction. Dose–Res. 2007;5:163–173. doi: 10.2203/dose-response.06-005.Masoro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ, Austad SN. The evolution of the antiaging action of dietary restriction: a hypothesis. J. Gerontol. Biol. Sci. 1996;51A:B387–B391. doi: 10.1093/gerona/51a.6.b387. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, Barnard D, Ward WF, Qi W, Ingram DK, de Cabo R. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows ND, Barrows CH., Jr Studies on aging in a bdelloid rotifer. II The effects of various environmental conditions and maternal age on longevity and fecundity. J. Gerontol. 1971;26:302–309. doi: 10.1093/geronj/26.3.302. [DOI] [PubMed] [Google Scholar]
- Mills S, Lunt DH, Gómez A. Global isolation by distance despite strong relational phylogeography in a small metazoan. BMC Evol. Biol. 2007;7:225. doi: 10.1186/1471-2148-7-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagnes D, Kimmance S, Tsounis G, Gumbs J. Combined effect of temperature and food concentration on the grazing rate of the rotifer Brachionus plicatilis. Mar. Biol. 2001;139:975–979. [Google Scholar]
- Nakagawa S, Lagisz M, Hector KL, Spencer HG. Comparative and meta-analytic insights into life extension via dietary restriction. Aging Cell. 2012;11:401–409. doi: 10.1111/j.1474-9726.2012.00798.x. [DOI] [PubMed] [Google Scholar]
- Nussey DH, Froy H, Lemaitre J-F, Gaillard J-M, Austad SN. Senescence in natural populations of animals: widespread evidence and its implications for bio-gerontology. Ageing Res. Rev. 2013;12:214–225. doi: 10.1016/j.arr.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oo AKS, Kaneko G, Hirayama M, Kinoshita S, Watabe S. Identification of genes differentially expressed by calorie restriction in the rotifer (Brachionus plicatilis) J. Comp. Physiol. B. 2010;180:105–116. doi: 10.1007/s00360-009-0389-6. [DOI] [PubMed] [Google Scholar]
- Partridge L, Gems D, Withers DJ. Sex and death: what is the connection? Cell. 2005;120:461–472. doi: 10.1016/j.cell.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Phelan JP, Austad SN. Natural selection, dietary restriction, and extended longevity. Growth Dev. Aging. 1989;53:4–6. [PubMed] [Google Scholar]
- Phelan JP, Rose MR. Why dietary restriction substantially increases longevity in animal models but won't in humans. Ageing Res. Rev. 2005;4:339–350. doi: 10.1016/j.arr.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Promislow DE, Tatar M, Pletcher S, Carey JR. Below-threshold mortality: implications for studies in evolution, ecology and demography. J. Evol. Biol. 1999;12:314–328. [Google Scholar]
- Sawada M, Enesco HE. A study of dietary restriction and lifespan in the rotifer Asplanchna brightwellii monitored by chronic neutral red exposure. Exp. Gerontol. 1984;19:329–334. doi: 10.1016/0531-5565(84)90006-8. [DOI] [PubMed] [Google Scholar]
- Snell TW. Systematics, reproductive isolation and species boundaries in rotifers. Hydrobiologia. 1989;186/187:299–310. [Google Scholar]
- Snell TW, Fields AM, Johnston RK. Antioxidants can extend lifespan of Brachionus manjavacas (Rotifera), but only in a few combinations. Biogerontology. 2012;13:261–275. doi: 10.1007/s10522-012-9371-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell TW, Hawkinson CA. Behavioral reproductive isolation among populations of the rotifer Brachionus plicatilis. Evolution. 1983;37:1294–1305. doi: 10.1111/j.1558-5646.1983.tb00245.x. [DOI] [PubMed] [Google Scholar]
- Stearns SC. Trade-offs in life-history evolution. Funct. Ecol. 1989;3:259–268. [Google Scholar]
- Stelzer C-P. Resource limitation and reproductive effort in a planktonic rotifer. Ecology. 2001;82:2521–2533. [Google Scholar]
- Suatoni E, Vicario S, Rice S, Snell T, Acaccone A. An analysis of species boundaries and biogeographic patterns in a cryptic species complex: the rotifer— Brachionus plicatilis. Mol. Phylogenet. Evol. 2006;41:86–98. doi: 10.1016/j.ympev.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Tatar M, Gray DW, Carey JR. Altitudinal variation for senescence in Melanoplus grasshoppers. Oecologia. 1997;111:357–364. doi: 10.1007/s004420050246. [DOI] [PubMed] [Google Scholar]
- Weithoff G. Dietary restriction in two rotifer species: the effect of the length of food deprivation on lifespan and reproduction. Oecologia. 2007;153:303–308. doi: 10.1007/s00442-007-0739-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.