Abstract
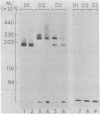
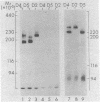
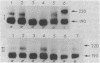
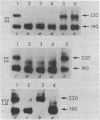
The human erythrocyte receptor for the major activation fragments of the third and fourth components of complement (HuE-C3bR) was isolated from individual donors. Erythrocytes were surface labeled with 125I and solubilized in Nonidet P-40.HuE-C3bR was purified by using C3-Sepharose affinity chromatography and analyzed by autoradiography of NaDodSO4/polyacrylamide gels. Three distinct receptor patterns were demonstrated. Type a had a single major band with Mr of 190,000, type b had a single major band with Mr of 220,000, and type c had two major bands of Mr 190,000 and 220,000. In all three types, a minor band accounting for less than 25% of the total radioactivity was usually observed at a Mr 15,000 greater than that of each major band. Identical autoradiographic patterns were obtained by affinity chromatography using methylamine-inactivated C4-Sepharose or by immunoprecipitation of solubilized membranes with a monoclonal antibody against HuE-C3bR. All three types were distinct after reduction and alkylation, although the apparent Mr uniformly increased by approximately equal to 30,000. Characterization of HuE-C3bR types in 33 unrelated individuals demonstrated that 23 had type a, 1 had type b, and 9 had type c. Family studies provide evidence for transmission by two codominant alleles. Thus, in the normal population two alleles appear to control expression of HuE-C3bR phenotypes and account for the polymorphism of this integral membrane glycoprotein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger M., Gaither T. A., Hammer C. H., Frank M. M. Lack of binding of human C3, in its native state, to C3b receptors. J Immunol. 1981 Oct;127(4):1329–1334. [PubMed] [Google Scholar]
- Bolotin C., Morris S., Tack B., Prahl J. Purification and structural analysis of the fourth component of human complement. Biochemistry. 1977 May 3;16(9):2008–2015. doi: 10.1021/bi00628a039. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Dixit R., Schneider R., Law S. K., Kulczycki A., Jr, Atkinson J. P. Ligand binding specificity of a rabbit alveolar macrophage receptor for C3b. J Biol Chem. 1982 Feb 25;257(4):1595–1597. [PubMed] [Google Scholar]
- Dobson N. J., Lambris J. D., Ross G. D. Characteristics of isolated erythrocyte complement receptor type one (CR1, C4b-C3b receptor) and CR1-specific antibodies. J Immunol. 1981 Feb;126(2):693–698. [PubMed] [Google Scholar]
- Fearon D. T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980 Jul 1;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T., Kaneko I., Thomson G. G. Membrane distribution and adsorptive endocytosis by C3b receptors on human polymorphonuclear leukocytes. J Exp Med. 1981 Jun 1;153(6):1615–1628. doi: 10.1084/jem.153.6.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Regulation of the amplification C3 convertase of human complement by an inhibitory protein isolated from human erythrocyte membrane. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5867–5871. doi: 10.1073/pnas.76.11.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J., Naiem M., Mason D. Y., Stein H. Human complement (C3b) receptors defined by a mouse monoclonal antibody. Immunology. 1982 Apr;45(4):645–653. [PMC free article] [PubMed] [Google Scholar]
- Iida K., Mornaghi R., Nussenzweig V. Complement receptor (CR1) deficiency in erythrocytes from patients with systemic lupus erythematosus. J Exp Med. 1982 May 1;155(5):1427–1438. doi: 10.1084/jem.155.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida K., Nussenzweig V. Complement receptor is an inhibitor of the complement cascade. J Exp Med. 1981 May 1;153(5):1138–1150. doi: 10.1084/jem.153.5.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isenman D. E., Cooper N. R. The structure and function of the third component of human complement--I. The nature and extent of conformational changes accompanying C3 activation. Mol Immunol. 1981 Apr;18(4):331–339. doi: 10.1016/0161-5890(81)90057-2. [DOI] [PubMed] [Google Scholar]
- Isenman D. E., Kells D. I., Cooper N. R., Müller-Eberhard H. J., Pangburn M. K. Nucleophilic modification of human complement protein C3: correlation of conformational changes with acquisition of C3b-like functional properties. Biochemistry. 1981 Jul 21;20(15):4458–4467. doi: 10.1021/bi00518a034. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kulczycki A., Jr, Krause V., Killion C. C., Atkinson J. P. Improved cell surface radioiodination of macrophages. J Immunol Methods. 1980;37(2):133–138. doi: 10.1016/0022-1759(80)90198-2. [DOI] [PubMed] [Google Scholar]
- Kulczycki A., Jr, Krause V., Killion C. C., Atkinson J. P. Purification of Fc gamma receptor from rabbit alveolar macrophages that retains ligand-binding activity. J Immunol. 1980 Jun;124(6):2772–2779. [PubMed] [Google Scholar]
- Kulczycki A., Jr, Parker C. W. The cell surface receptor for immunoglobulin E. I. The use of repetitive affinity chromatography for the purification of a mammalian receptor. J Biol Chem. 1979 May 10;254(9):3187–3193. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Law S. K., Lichtenberg N. A., Levine R. P. Covalent binding and hemolytic activity of complement proteins. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7194–7198. doi: 10.1073/pnas.77.12.7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa Y., Yamada A., Kosaka K., Tsuda F., Kosugi E., Mayumi M. Defective immune-adherence (C3b) receptor on erythrocytes from patients with systemic lupus erythematosus. Lancet. 1981 Sep 5;2(8245):493–497. doi: 10.1016/s0140-6736(81)90882-5. [DOI] [PubMed] [Google Scholar]
- Nicholson-Weller A., Burge J., Fearon D. T., Weller P. F., Austen K. F. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982 Jul;129(1):184–189. [PubMed] [Google Scholar]
- Rothman I. K., Gelfand J. A., Fauci A. S., Frank M. M. The immune adherence receptor: dissociation between the expression of erythrocyte and mononuclear cell C3b receptors. J Immunol. 1975 Nov;115(5):1312–1315. [PubMed] [Google Scholar]
- Schneider R. J., Kulczycki A., Jr, Law S. K., Atkinson J. P. Isolation of a biologically active macrophage receptor for the third component of complement. Nature. 1981 Apr 30;290(5809):789–792. doi: 10.1038/290789a0. [DOI] [PubMed] [Google Scholar]
- Schreiber R. D., Pangburn M. K., Müller-Eberhard H. J. C3 modified at the thiolester site: acquisition of reactivity with cellular C3b receptors. Biosci Rep. 1981 Nov;1(11):873–880. doi: 10.1007/BF01114821. [DOI] [PubMed] [Google Scholar]
- Tack B. D., Prahl J. W. Third component of human complement: purification from plasma and physicochemical characterization. Biochemistry. 1976 Oct 5;15(20):4513–4521. doi: 10.1021/bi00665a028. [DOI] [PubMed] [Google Scholar]
- Wilson J. G., Wong W. W., Schur P. H., Fearon D. T. Mode of inheritance of decreased C3b receptors on erythrocytes of patients with systemic lupus erythematosus. N Engl J Med. 1982 Oct 14;307(16):981–986. doi: 10.1056/NEJM198210143071604. [DOI] [PubMed] [Google Scholar]
- von Zabern I., Gigli I. Conformational changes in complement component C4 induced by activation, treatment with amines, chaotropes, or freezing-thawing, detectable by radioiodination using lactoperoxidase. J Immunol. 1982 Mar;128(3):1439–1442. [PubMed] [Google Scholar]