Abstract
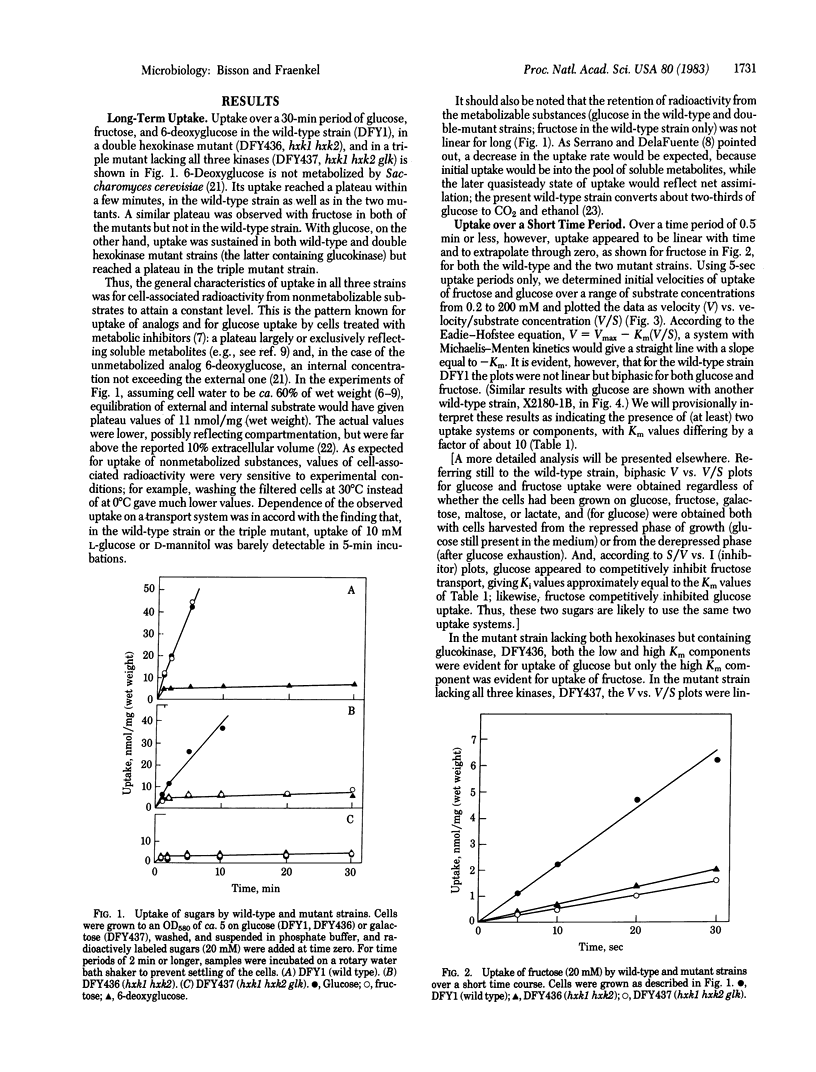
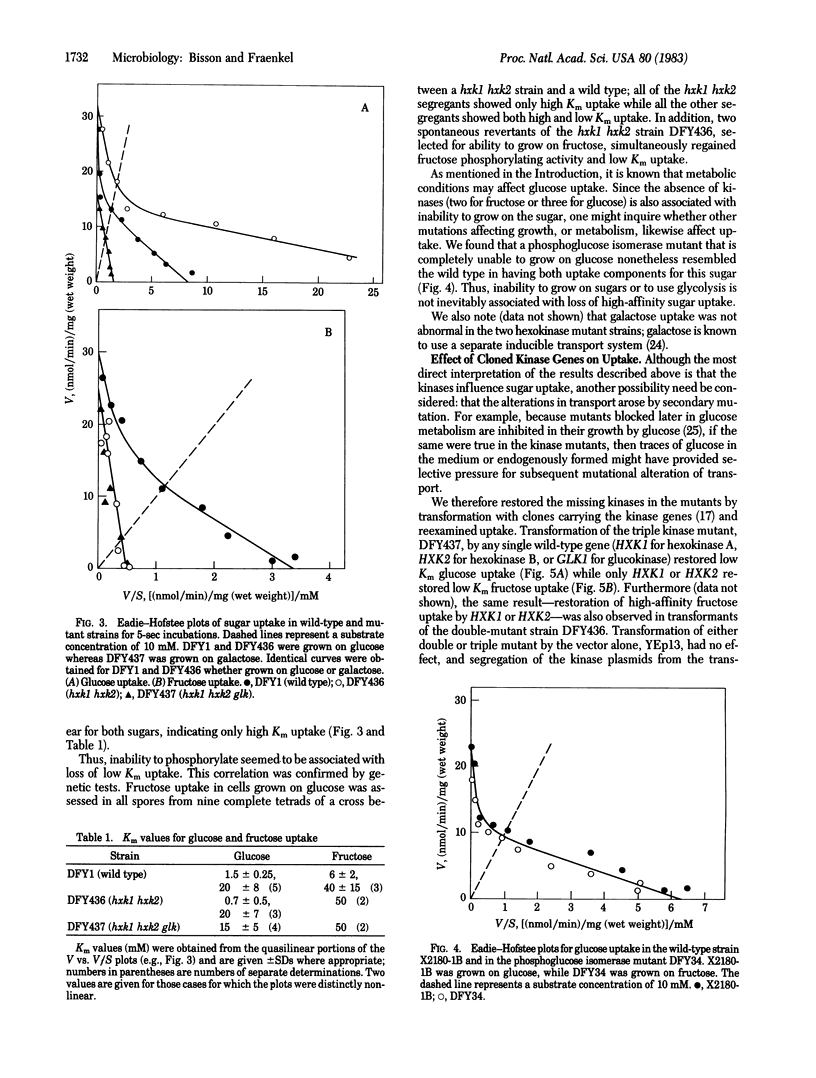
Uptake of glucose, fructose, and the nonmetabolizable analog 6-deoxyglucose was measured in wild-type Saccharomyces cerevisiae and two mutant strains, one (hxk1 hxk2) lacking both hexokinase A(P-I) and B(P-II) but containing glucokinase (and hence able to grow on glucose but not fructose) and the other (hxk1 hxk2 glk) also lacking glucokinase (and not able to grow on glucose either). Uptake of the nonmetabolized substances (i.e., 6-deoxyglucose in all three strains, fructose in the two mutants, and glucose in the triple mutant) reached a plateau at or below the external concentration. The kinetic characteristics of uptake were determined from 5-sec incubations by plotting velocity (V) vs. velocity/substrate concentration (V/S) curves. According to such plots, in the wild-type strain uptake had two components, "high affinity uptake" with Km values of ca. 1 mM for glucose and 6 mM for fructose and "low affinity uptake" with Km values of ca. 20 and 50 mM, respectively. The double kinase mutant showed both components for glucose but only the high Km component for fructose, while the triple kinase mutant showed only high Km uptake for both glucose and fructose. Genetic analysis showed that only in strains lacking both hexokinases (hxk1 hxk2) was the low Km system for fructose absent. Low Km uptake was restored to the triple mutant by introduction of the cloned wild-type genes: HXK1 or HXK2, for fructose uptake, and HXK1, HXK2, or GLK1, for glucose uptake. A phosphoglucose isomerase mutant had both low and high Km uptake for glucose. These results indicate the presence of two types of uptake mechanism for glucose and fructose in yeast, the functioning of one of which, the low Km system, is influenced by the cognate kinases.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker J. U., Betz A. Membrane transport as controlling pacemaker of glycolysis in Saccharomyces carlsbergensis. Biochim Biophys Acta. 1972 Aug 9;274(2):584–597. doi: 10.1016/0005-2736(72)90205-2. [DOI] [PubMed] [Google Scholar]
- Beggs J. D. Transformation of yeast by a replicating hybrid plasmid. Nature. 1978 Sep 14;275(5676):104–109. doi: 10.1038/275104a0. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Strathern J. N., Hicks J. B. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979 Dec;8(1):121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- CIRILLO V. P. Mechanism of glucose transport across the yeast cell membrane. J Bacteriol. 1962 Sep;84:485–491. doi: 10.1128/jb.84.3.485-491.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONWAY E. J., DOWNEY M. An outer metabolic region of the yeast cell. Biochem J. 1950 Sep;47(3):347–355. doi: 10.1042/bj0470347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo V. P. Relationship between sugar structure and competition for the sugar transport system in Bakers' yeast. J Bacteriol. 1968 Feb;95(2):603–611. doi: 10.1128/jb.95.2.603-611.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirillo V. P. The role of membrane carriers in the regulation of the free glucose pool in metabolizing cells. J Protozool. 1970 May;17(2):178–181. doi: 10.1111/j.1550-7408.1970.tb02351.x. [DOI] [PubMed] [Google Scholar]
- Clifton D., Fraenkel D. G. Mutant studies of yeast phosphofructokinase. Biochemistry. 1982 Apr 13;21(8):1935–1942. doi: 10.1021/bi00537a037. [DOI] [PubMed] [Google Scholar]
- Clifton D., Weinstock S. B., Fraenkel D. G. Glycolysis mutants in Saccharomyces cerevisiae. Genetics. 1978 Jan;88(1):1–11. doi: 10.1093/genetics/88.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzusoff A., Cirillo V. P. Uptake and phosphorylation of 2-deoxy-D-glucose by wild-type and single-kinase strains of Saccharomyces cerevisiae. Biochim Biophys Acta. 1982 Jun 14;688(2):295–304. doi: 10.1016/0005-2736(82)90340-6. [DOI] [PubMed] [Google Scholar]
- Gancedo J. M., Clifton D., Fraenkel D. G. Yeast hexokinase mutants. J Biol Chem. 1977 Jul 10;252(13):4443–4444. [PubMed] [Google Scholar]
- Heredia C. F., Sols A., DelaFuente G. Specificity of the constitutive hexose transport in yeast. Eur J Biochem. 1968 Aug;5(3):321–329. doi: 10.1111/j.1432-1033.1968.tb00373.x. [DOI] [PubMed] [Google Scholar]
- Horák J., Kotyk A. Isolation of a glucose-binding lipoprotein from yeast plasma membrane. Eur J Biochem. 1973 Jan 3;32(1):36–41. doi: 10.1111/j.1432-1033.1973.tb02575.x. [DOI] [PubMed] [Google Scholar]
- Jaspers H. T., van Steveninck J. Transport-associated phosphorylation of 2-deoxy-D-glucose in Saccharomyces fragilis. Biochim Biophys Acta. 1975 Oct 17;406(3):370–385. doi: 10.1016/0005-2736(75)90017-6. [DOI] [PubMed] [Google Scholar]
- Kliman H. J., Steck T. L. Association of glyceraldehyde-3-phosphate dehydrogenase with the human red cell membrane. A kinetic analysis. J Biol Chem. 1980 Jul 10;255(13):6314–6321. [PubMed] [Google Scholar]
- Kopperschläger G., Hofmann E. Uber multiple Formen der Hexokinase in Hefe. Eur J Biochem. 1969 Jun;9(3):419–423. doi: 10.1111/j.1432-1033.1969.tb00625.x. [DOI] [PubMed] [Google Scholar]
- Kotyk A., Michaljanicová D., Veres K., Soukupová V. Transport of 4-deoxy- and 6-deoxy-D-glucose in baker's yeast. Folia Microbiol (Praha) 1975;20(6):496–503. doi: 10.1007/BF02891709. [DOI] [PubMed] [Google Scholar]
- Kotyk A. Properties of the sugar carrier in baker's yeast. II. Specificity of transport. Folia Microbiol (Praha) 1967;12(2):121–131. doi: 10.1007/BF02896872. [DOI] [PubMed] [Google Scholar]
- Kou S. C., Christensen M. S., Cirillo V. P. Galactose transport in Saccharomyces cerevisiae. II. Characteristics of galactose uptake and exchange in galactokinaseless cells. J Bacteriol. 1970 Sep;103(3):671–678. doi: 10.1128/jb.103.3.671-678.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindén M., Gellerfors P., Nelson B. D. Pore protein and the hexokinase-binding protein from the outer membrane of rat liver mitochondria are identical. FEBS Lett. 1982 May 17;141(2):189–192. doi: 10.1016/0014-5793(82)80044-6. [DOI] [PubMed] [Google Scholar]
- Lobo Z., Maitra P. K. Genetics of yeast hexokinase. Genetics. 1977 Aug;86(4):727–744. doi: 10.1093/genetics/86.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo Z., Maitra P. K. Physiological role of glucose-phosphorylating enzymes in Saccharomyces cerevisiae. Arch Biochem Biophys. 1977 Aug;182(2):639–645. doi: 10.1016/0003-9861(77)90544-6. [DOI] [PubMed] [Google Scholar]
- Meredith S. A., Romano A. H. Uptake and phosphorylation of 2-deoxy-D-glucose by wild type and respiration-deficient bakers' yeast. Biochim Biophys Acta. 1977 May 26;497(3):745–759. doi: 10.1016/0304-4165(77)90295-1. [DOI] [PubMed] [Google Scholar]
- Muratsubaki H., Katsume T. Distribution of hexokinase isoenzymes depending on a carbon source in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1979 Feb 28;86(4):1030–1036. doi: 10.1016/0006-291x(79)90220-1. [DOI] [PubMed] [Google Scholar]
- Schneider R. P., Wiley W. R. Kinetic characteristics of the two glucose transport systems in Neurospora crassa. J Bacteriol. 1971 May;106(2):479–486. doi: 10.1128/jb.106.2.479-486.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R., Delafuente G. Regulatory properties of the constitutive hexose transport in Saccharomyces cerevisiae. Mol Cell Biochem. 1974 Dec 20;5(3):161–171. doi: 10.1007/BF01731379. [DOI] [PubMed] [Google Scholar]


