Abstract
In this second of two articles on second messenger/signal transduction cascades in major mood disorders, we will review the evidence in support of intracellular dysfunction and its rectification in the etiopathogenesis and treatment of bipolar disorder (BD). The importance of these cascades is highlighted by lithium’s (the gold standard in BD psychopharmacology) ability to inhibit multiple critical loci in second messenger/signal transduction cascades including protein kinase C (involved in the IP3/PIP2 pathway) and GSK-3β (canonically identified in the Wnt/Fz/Dvl/GSK-3β cascade). As a result, and like major depressive disorder (MDD), more recent pathophysiological studies and rational therapeutic targets have been directed at these and other intracellular mediators. Even in the past decade, intracellular dysfunction in numerous neuroprotective/apoptotic cascades appears important in the pathophysiology and may be a future target for pharmacological interventions of BD.
Keywords: Bipolar disorder, signal transduction, second messenger, intracellular cascades, mood stabilizers, lithium
Introduction
Like the other major mood disorder, major depressive disorder (MDD), pathophysiological and psychopharmacological research in bipolar disorder (BD) have encompassed monoamine (serotonin, norepinephrine, dopamine) and amino acid (γ-aminobutyric acid, glutamate)–based neurotransmission. However, as early as the 1970s, lithium (a cell-permeant cation with minimal reactivity at cell surface receptors) reduced brain inositol levels1 [presumably via the inhibition of protein kinase C (PKC)] and was speculated to have state-specific, mood-stabilizing effects on second messenger/signal transduction cascades. Lithium is still the standard of care in the treatment of BD, especially in antimanic and maintenance therapies.2 The antiepileptic drugs valproic acid and carbamazepine, although chemically dissimilar to lithium, also have profound effects on intracellular pathways to stabilize mood.3–6
Like other neuropsychiatric disorders, BD arises from complex and still poorly understood abnormalities at the molecular, cellular, and circuit levels. As in other neuropsychiatric disorders, these multitiered abnormalities are likely responsible for BD’s signs and symptoms: elevated/expansive or irritable mood, impaired circadian rhythmicity, increased goal-directed activity, decreased cognitive control, increased impulsivity, and frequent risk-taking behaviors, eg, sexual indiscretions and reckless substance misuse. As was done with the first review in this series, this article will summarize our current state of knowledge of second messenger/signal transduction cascades in the etiopathogenesis of BD. We will then discuss what is known about the mechanism of action of the aforementioned mood stabilizers. Finally, we will illuminate potential future directions and rational therapeutic targets in BD.
Second Messenger/Signal Transduction Cascades
The following second messenger/signal transduction cascades will be discussed in sequence: cyclic adenosine monophosphate (cAMP)/protein kinase A (PKA)/cAMP-response element binding protein (CREB); extracellular regulated kinase (ERK)/mitogen-activated protein kinase (MAPK); phosphoinositide (PI)/protein kinase C (PKC); Wnt/frizzled (Fz)/disheveled (Dvl)/glycogen synthase kinase-3 beta (GSK-3β); and mitochondrial (pro- and anti-apoptotic) cascades. Although it certainly is as important in bipolar as in unipolar depression, we will not discuss neurotrophic signaling here in detail other than as an extracellular stimuli for intracellular cascades; the interested reader is referred to our extensive discussion of neutrophins in the first article in this series. And, as in the first part, we will also not discuss extracellular neurotransmission (via classic neurotransmitters, neuropeptides, or other neuroendocrine mechanisms) except as the means of stimulating or inhibiting intracellular signal transduction/second messenger pathways.
cAMP/PKA/CREB
As may be surmised from the wealth of data in unipolar depression and preclinical models of despair, the cAMP/PKA/CREB pathway is also affected in BD. However, in contrast to preclinical models and unipolar depression, this cascade is upregulated/overactive in BD, especially in mania (Table 1). Levels of the stimulatory G protein linked to this cascade, Gs, are increased in postmortem bipolar brain (Table 1).7,8 As detected by coimmunoprecipitation with total brain homogenates, there is increased heterotrimeric G protein complex (Gαβγ) association relative to age, sex, and postmortem-interval matched controls.8 The increased levels/activity of this cascade have also been replicated in peripheral samples.9–11 Unfortunately, some of the aforementioned studies report conflicting results both based on the phase of illness and the specific patient population. Next, adenylyl cyclase activity (both basal and stimulated) is enhanced in postmortem samples from bipolar patients, which increases production of the second messenger cyclic adenosine monophosphate (cAMP). The catalytic subunit of protein kinase A (PKA) and cAMP-stimulated PKA activity are also increased in BD brain.12,13 Like linked G proteins, increased PKA activity has been observed in peripheral blood platelets and lymphoblasts, even in the absence of mood stabilizers.14,15 CREBP1 [a cAMP-response element binding (CREB) protein interactor] expression is reduced in BD postmortem orbitofrontal cortex,16 but, to date, there have been no reports of CREB levels and/or transcriptional activity in BD or animal models of the disorder, eg, psychostimulant-induced hyperlocomotion. Finally, a recent multiple rare variant genetic analysis identified several single nucleotide polymorphisms (SNPs) in related signaling genes [including several variants of phosphodiesterase (PDE)10A] in bipolar I disorder (BDI). Moreover, several SNP × SNP interactions among these signaling genes multiplicatively increased the genetic risk of BDI in this sample.17 However, the relationship of these PDE10A SNPs is speculative at best without evidence of dysregulation in cAMP levels and impairment in cAMP-stimulated PKA activity.
Table 1.
Pathophysiological impairments of second messenger/signal transduction cascades in bipolar disorder (BD)
| cAMP/PKA/CREB |
|
| ERK/MAPK |
|
| PI/PKC |
|
| Wnt/Fz/Dvl/GSK-3b |
|
| Mitochondria/Cell Survival |
|
Traditional mood stabilizers normalize activity in the cAMP/PKA/CREB second messenger/signal transduction cascade. Lithium and the antiepileptic drug carbamazepine promote the cytosol-to-plasma membrane translocation of G-protein receptor kinase-3 (GRK3), a serine/threonine kinase implicated in the homologous desensitization of G-protein coupled receptors.5 GRK3’s plasma membrane translocation may dampen receptor overactivation in bipolar brain. Chronic lithium administration also affects adenylyl cyclase activity, ie, an increase in basal activity while inhibiting receptor-mediated overactivation.18 (Of note, these are total cytosolic AC activity assays and may not reflect differences in activity in specific AC subtypes and/or in different subcellular microdomains.19) There are conflicting results of lithium’s effect on CREB in preclinical studies.20,21 (This intersects with our discussion of the ERK/MAPK second messenger/signal transduction cascade below to affect CREB phosphorylation, nuclear translocation, and CRE-mediated gene transcription.)
Although structurally dissimilar to lithium, valproic acid’s antimanic effects may also result from alterations in the cAMP/PKA/CREB second messenger/signal transduction cascade. Chronic valproic acid administration decreased the expression of β1-adrenergic receptors and postreceptor-mediated cAMP generation.3 In a microarray study of rats exposed to intraperitoneal valproic acid (200 mg/kg), many genes implicated in G-protein–mediated signaling (including the catalytic subunit of PKA and CREB) were up- or downregulated at least 1.4-fold relative to untreated controls.4 Real-time quantitative polymerase chain reaction (PCR) in an independent sample validated these microarray expression differences. Nonetheless, the aforementioned studies were conducted in cell lines and rodents, and these results have yet to be translated into bipolar patients. Therefore, valproic acid’s antimanic mechanism of action in humans still remains poorly understood.
Albeit equally dissimilar in structure to lithium and valproic acid, carbamazepine has analogous biochemical and cell biological effects. In addition to promoting the cytosol-to-cell membrane translocation of GRK-3, carbamazepine has been shown to decrease basal and stimulated cAMP production in rodents.22,23 Also, like valproic acid, chronic administration in rodents decreased dopamine (D2) receptor activity (as displayed by quinpirole-mediated inhibition of arachidonic acid production/signaling).24 Finally, in mania, carbamazepine decreased cAMP levels in cerebrospinal fluid.6
ERK/MAPK
The ERK/MAPK pathway has also been investigated in BD and preclinical models. As mentioned, there is significant overlap in the cAMP/PKA/CREB and ERK/MAPK second messenger/signal transduction cascades to converge on CREB phosphorylation and CRE-mediated gene transcription. Unfortunately, there has been minimal research to date on ERK/MAPK dysregulation in the pathophysiology of BD (Table 1). However, there have been numerous pharmacological investigations into the mechanism of action of mood stabilizing medications. In an immortalized human cell line (SH-SY5Y) and in primary neuronal cultures, both lithium and valproic acid stimulate the ERK/MAPK cascade in contrast to other mood stabilizers (carbamazepine and lamotrigine).25 Valproic acid induces microglial apoptosis in vitro, which relies on p38-stimulated MAPK phosphorylation [in contrast to other MAPK isoforms, phospho-ERK and phosphoc-Jun activated kinase (JNK)].26 Lithium also enhances the phosphorylation of p38-MAPK, p53 downregulation, and the reversal of cell cycle arrest at G2/M in rat fibroblasts and an immortalized p53 mutant cell line.27 Next, lithium and valproic acid increased levels of phospho-ERK in the rodent frontal cortex and hippocampus, and ERK inhibitors have stimulatory effects similar to D-amphetamine administration (a rodent model of mania) that are reversed by lithium pretreatment.28 As in other psychiatric and nonpsychiatric disorders, eg, oncology, the ERK/MAPK cascade is a central regulator of cell survival and proliferation, which provides novel hypotheses into the mechanistic underpinnings of the neuroprotective and mitogenic effects of mood stabilization.
PI/PKC
Phosphoinositide (PI) levels are decreased in BD postmortem prefrontal cortex,29 and stimulated PI turnover is reduced (∼50% at all tested concentrations of GTPγs) in fractionated occipital cortical membranes from BD vs. controls (Table 1).30 There is evidence of altered PI signaling in peripheral tissue as well. Interestingly, medication-free bipolar subjects in a current manic or depressive episode display higher phosphatidylinositol-4,5-bisphophate (PIP2) levels in platelets.31 A genetic association between BD and the PI/PKC pathway has also been suggested. In a genome-wide association study (GWAS) of common SNPs there was a strong correlation between BD diagnosis and the first intron of diacylglycerol kinase eta (DGKH),32 a regulator of PIP2 and diacylglycerol (DAG) production to stimulate PKC and modulate the expression of members of the transient receptor potential cation channel family.33,34 Total PKC levels, cytosol-to-plasma membrane translocation, and enzymatic activity were also increased in postmortem BD frontal cortex.35 The same research group also discovered a facilitated interaction of PKC with the receptor for activated protein kinase C (RACK-1) in the frontal cortex.36 PKC activity and membrane translocation are also increased in platelets from patients in a current manic episode.37,38 Other groups, however, have reported conflicting observations of PKC. PKC isozyme levels and activity were decreased with concomitant increases in other members of this pathway, ie, myristoylated alanine-rich C-kinase substrate (MARCKS), in membrane and cytosolic fractions from platelets of unmedicated bipolar patients relative to unmedicated MDD and nondepressed healthy volunteers.39 In pediatric BD, peripheral PKC isozyme levels were reduced at baseline with concomitant increased activity alone (not isozyme levels) after successful mood stabilization.37
Reduced inositol monophosphatase (IMPase) activity and elevated basal intracellular calcium (iCa21) have been observed in B lymphoblast cell lines (BLCLs) in BDI. Interestingly, BDI males with higher basal serum Ca21 have lower levels of IMPase mRNA relative to male BDI subjects with normal serum Ca21, female BDIs, and healthy volunteers. Postmortem IMPase levels in the temporal cortex, in contrast, were higher in male BDI subjects relative to age-matched male postmortem temporal cortex.40 PKC overactivation (both increased activity and membrane localization) and phosphorylation of downstream targets, eg, GAP43, have been observed in psychostimulant-induced psychomotor activation. Although these observations are excitingly suggestive of PI dysfunction, it is important to note that all studies to date have been performed on relatively small numbers of subjects. It is therefore imperative to obtain in vivo evidence of pathway dysfunction in the bipolar brain before definitive conclusions can be drawn.
As mentioned above, mood stabilizer pharmacology has been intimately tied to the PI/PKC cascade. Lithium-mediated reduction in central inositol levels has been one of the most formative observations in BD pharmacology. Lithium was shortly thereafter discovered to be a potent PKC inhibitor with concomitant decreased phosphorylation of downstream targets (Figure 1). Preclinical studies have elucidated some of the molecular players involved in lithium and other mood stabilizers’ biochemical and molecular effects. Chronic lithium treatment reduced PKC isozymes (α and ε) in the hippocampus and the frontal cortex.41,42 Next, downstream levels and/or activity of PKC substrates are reduced with chronic lithium treatment, eg, MARCKS.43 Lithium decreased PKC-induced phosphorylation of neurogranin and excitatory glutamatergic N-methyl-D-aspartate (NMDA) receptors and 2-amino-3-(3-hydroxy-5-methylisoxazol-4-yl)propanoic acid (AMPA) receptors in the prefrontal cortex of psychostimulant-exposed rodents. Consistent with the “kindling hypothesis” of BD pathophysiology, lithium may exert antimanic effects partially by decreasing excessive glutamatergic neurotransmission.44 Finally, valproic acid had similar biochemical effects to lithium,45,46 but chronic carbamazepine treatment has been reported to increase neocortical MARCKS expression.47
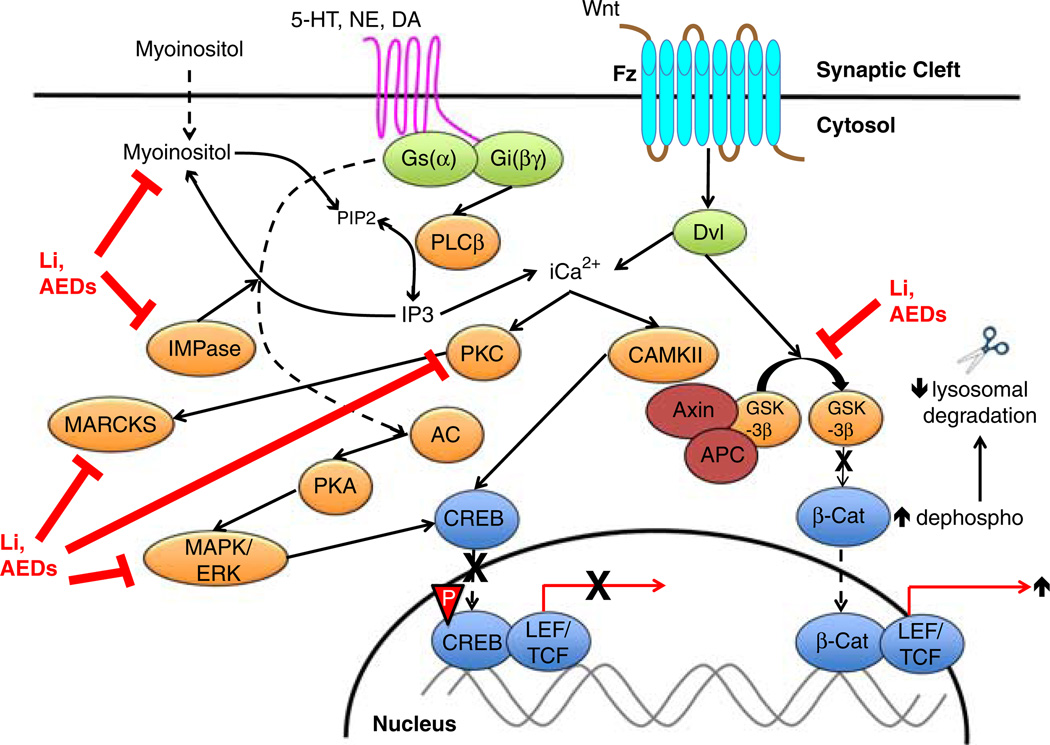
Figure 1.
Canonical signal transduction cascades and mood stabilizer targets in bipolar disorder. On the left side of the figure, monoaminergic (serotonin, norepinephrine, and dopamine) neurotransmitter receptors activates the intracellular phosphoinositide second messenger/signal transduction cascade. Phospholipases enzymatically degrade phosphoinositides to inositol triphosphate (IP3) and diacylglycerol (DAG). Lithium (and potentially mood-stabilizing antiepileptic drugs) noncompetitively inhibits inositol monophosphatase (IMPase), which decreases myoinositol uptake and phosphoinositide production in the brain and/or peripheral tissues. Mood stabilizers also inhibit multiple other overactive nodes in intracellular pathways: protein kinase C (PKC), myristolated alanine-rich C kinase substrate (MARCKS), and extracellular regulated protein kinase (ERK)/mitogen-activated protein kinase (MAPK), which coalesces to decrease cAMP-response element binding protein (CREB) phosphorylation and CRE-mediated gene transcription. On the right side of the figure, overactivation of the canonical Wnt/Fz/Dvl/GSK-3β signaling pathway in BD commences when Wnt glycoprotein extracellularly binds to its cognate Frizzled (Fz) receptor. Intracellular Fz activation stimulates Disheveled and GSK-3β phosphorylation, which phosphorylates β-catenin and promotes the formation of a lysosomal destruction complex with the other proteins (axin/APC/GSK-3β/β-catenin). Lithium is a potent GSK-3β inhibitor. Therefore, lithium reduces GSK-3β phosphorylation, decreases β-catenin phosphorylation, and dissociates the destruction complex, which stabilizes β-catenin for nuclear translocation and increases the transcription of β-catenin target genes. Li, lithium; AED, antiepileptic mood stabilizer medication; IMPase, inositol monophosphatase; 5-HT, 5-hydroxytryptamine (serotonin); NE, norepinephrine; DA, dopamine; PLCβ, phospholipase C-beta; IP3, inositol triphosphate; PIP2, phosphoinositide-4,5-bisphosphate; PKC, protein kinase C; CAMKII, calcium-calmodulin dependent protein kinase II; PKA, protein kinase A; MAPK, mitogen-activated protein kinase; ERK, extracellular-regulated kinase; CREB, cyclic adenosine monophosphate (cAMP)-response element binding protein; LEF/TCF, lymphoid enhancer factor/T-cell factor; Fz, frizzled; Dvl, disheveled; APC, adenosis polyposis coli; GSK-3b, glycogen synthase kinase-3 beta; β-Cat, beta-catenin.
Due to lithium’s ability to inhibit PKC, more selective PKC inhibitors have been sought in BD. Intrerestingly, tamoxifen is the only central nervous system (CNS)-penetrant medication currently available with high selectivity for PKC. Tamoxifen attenuated both the behavioral (decreased locomotion) and biochemical (blunted GAP43 phosphorylation) effects of acute psychostimulants.48 In translation, tamoxifen initially demonstrated efficacy in two small trials for acute mania.49,50 Then, in two larger, single-site, double-blind, placebo-controlled mania trials, tamoxifen had a large treatment effect within only a few days of initiation; it was also well-tolerated at relatively high doses.51,52 Unfortunately, no study to date has included an active comparator, ie, an approved antimanic agent such as lithium or valproic acid. Chronic tamoxifen treatment is also not without side effects. Nonetheless, we await larger, multisite, placebo-controlled trials of tamoxifen as either monotherapy (with an active comparator arm) or adjunctive treatment to traditional mood stabilizers. Although it is an attractive explanation, it is currently unknown if tamoxifen’s seemingly antimanic effects are dependent on PKC inhibition. Tamoxifen is also a powerful antagonist of estrogen receptor stimulation, which is crucial for its mechanism of action in the treatment of breast and other reproductive cancers. These anti-apoptotic or even other unidentified effects might also be critical in mood stabilization. Finally, other alternative strategies for PKC inhibition, eg, omega-3 fatty acid dietary supplementation, have been studied in BD with mixed results53.
Based on the initial studies with lithum discussed above, IMPase inhibition has been proposed to induce myoinositol depletion in the bipolar brain. However, there is little in vitro/vivo evidence to support this hypothesis. Lithium and antiepileptic mood stabilizers also inhibit the sodium-dependent myoinositol transporter (SMIT).54 Consistent with this biochemical effect, unmedicated BD patients have elevated SMIT levels in peripheral neutrophils, which were reduced with both chronic lithium and valproic acid therapy.55 On the other hand, in rodents, SMIT haploinsufficiency did not cause inositol depletion nor alter lithium-sensitive behaviors, eg, decreased immobilization on the forced swim test.56 As a result of these conflicting observations, the jury remains out on the ultimate importance of myoinositol, IMPase, and SMIT in the pathophysiology and treatment of BD.
Wnt/Fz/Dvl/GSK-3β
The Wnt/Fz/Dvl/GSK-3β pathway has been implicated in the etiopathogenesis and treatment of BD. In addition to its potent PKC inhibition (of note, there is PI crosstalk with the Wnt/Fz/Dvl/GSK-3β pathway), lithium is a powerful inhibitor of GSK-3β phosphorylation (via its competition with magnesium at an allosteric site).57 Valproic acid58 and electro-convulsive seizures59 also inhibit GSK-3β in mice. Interestingly, mice with a GSK-3β serine-to-alanine knock-in mutation have increased susceptibility to amphetamine-induced hyperlocomotion and stress-induced despair.60 The same study also demonstrated impaired GSK-3β phosphorylation in stressed wild-type mice and peripheral samples from bipolar patients.60 GSK-3β also plays a crucial role in circadian rhythmicity, which is often impaired in the earliest stages of hypo/mania. Therefore, like lithium itself, pharmacologic or genetic manipulations of GSK-3β may have antimanic, antidepressant, and/or maintenance effects depending on type of episode.
GSK-3β inhibition results in decreased phosphorylation/stabilization of β-catenin. This normalizes transcription of multifarious messenger ribonucleic acids (mRNAs) that affect synaptic transmission, postsynaptic signaling, and cytoskeletal reorganization in BD brain. Like GSK-3β modulation, the over-expression of dephosphorylated β-catenin in rodents had mood stabilizing effects analogous to lithium.61 As a result of these findings, more selective GSK-3β inhibitors, agents to promote dephosphorylated β-catenin accumulation, and/or the manipulation of upstream targets in this cascade, ie, Wnt-neutralizing antibodies, and/or Fz receptor antagonists, are potentially novel molecular targets in BD treatment.
Mitochondria/cell survival
In the past decade, there has been a burst of interest in mitochondrial-based cell signaling pathways in BD (Table 1). BD is associated with increased intracellular Ca2+, which may be released from intracellular stores, eg, endoplasmic reticulum and mitochondria, and/or influx through the stimulation of cell surface receptors. Excessive NMDA receptor activation via glutamate promotes neuronal cell death (“excitotoxicity”) (Figure 2). A recent microarray screen in postmortem BD hippocampus identified the upregulation of numerous pro-apototic genes and downregulation of antioxidant and anti-apoptotic genes62,63 (Table 1). In an independent sample of BD patients, the phosphorylation of the glucocorticoid receptor was decreased (pro-apoptotic), heat shock protein (Hsp-70) levels were decreased (pro-apoptotic), cytosolic Bax expression was decreased (anti-apoptotic), and cytosolic cytochrome C protein was increased (pro-apoptotic) in manic, depressed, and euthymic cohorts, which suggests a complex relationship to intracellular apoptotic cascades.64 The anti-apoptotic gene B-cell lymphoma-2 (Bcl-2), a mitochondrial CREB-responsive gene that prevents the release of cytochrome C and concomitant caspase (proteolytic enzyme) activation, has been genetically associated with BD in several studies by our group. First, lymphoblasts from bipolar subjects with the Bcl-2 SNP rs956572AA displayed decreased Bcl-2 expression and increased IP3-mediated Ca2+-release relative to the AG/GG genotypes.65 The same SNP was also associated with increased Glx (combined glutamate and glutamine)/creatine ratio in the anterior cingulate cortex in euthymic BDI,66 which again supports the kindling hypothesis with a further provocative hypothesis that there may be ongoing excitotoxic cell damage even when not manic or depressed. Next, a polymorphism in the promoter (−116G/C) of the Ca2+-responsive endoplasmic reticulum stress gene, XBP1, has been implicated in the pathophysiology and treatment of BD.67 This polymorphism affects transcription in response to stress; valproic acid induces the transcription of the upstream gene ATF6, which may result in the downregulation of XBP1 expression with effective treatment.67 Although several other studies have confirmed decreased stress-induced XBP1 expression in peripheral samples from bipolar patients,68,69 there is conflicting data on the XBP1 −116C/G SNP and impaired stress-related transcription in BD.68,70
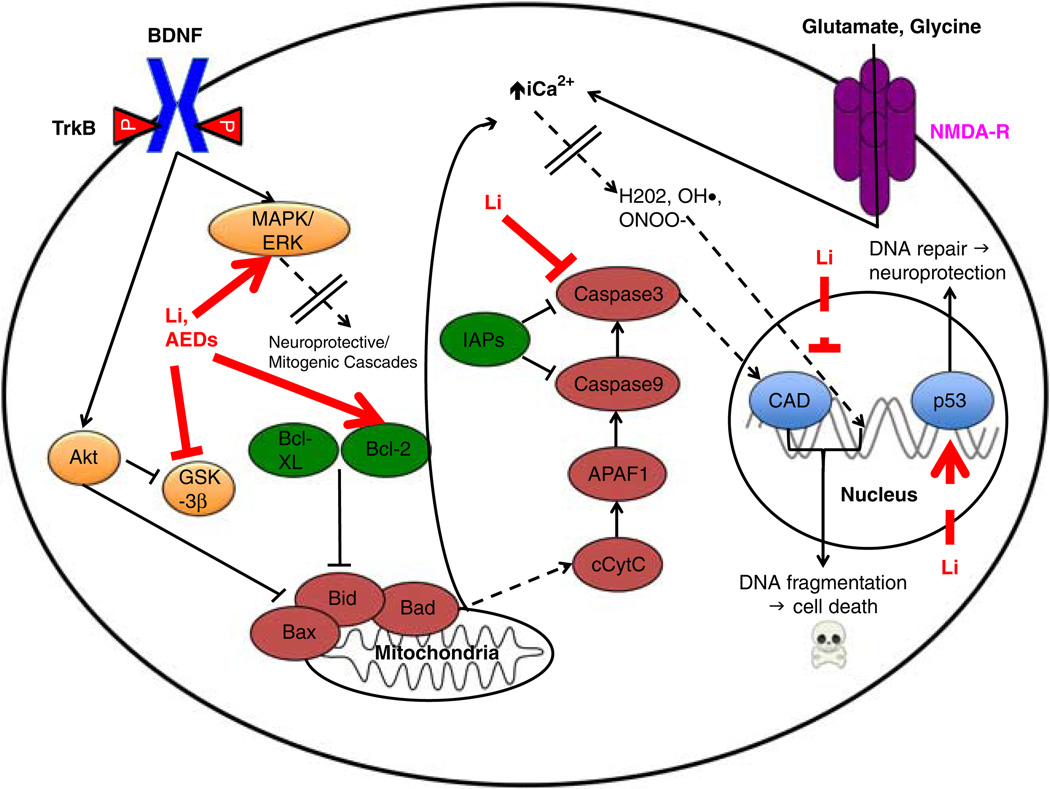
Figure 2.
Neuroprotective/apoptotic intracellular cascades at baseline and as mitigated by mood stabilizers in BD. The canonical brain-derived neurotrophic factor (BDNF)/TrkB signaling pathway is presented to highlight its potent neurotrophic/mitogenic effects, which occurs partially via the inhibition of apoptosis. As described in Part I: Figure 1, BDNF binds to TrkB, which stimulates a panoply of intracellular modulators that culminates in the transcription of neuroprotective genes (for the sake of presentation, these effects are collapsed by a dashed line with doubled dividers). On the pro-apoptotic end, increased intracellular calcium overloads mitochondrial calcium homeostatic regulatory mechanisms. This impairs the respiratory chain (complex I function), eventually resulting in decreased ATP levels and increasing reactive oxygen species (ROS) production (again, for the sake of presentation, the intermediaries between increased intracellular calcium and ROS have been collapsed by a dashed line with double dividers). ROS not only exert genotoxic effects but also act on a number of ion channels. This vicious cycle further increases intracellular calcium influx and apoptosis. Lithium and mood stabilizing antiepileptic drugs activate the ERK/MAPK cascade, thereby increasing the expression of B-cell lymphoma 2 (Bcl-2), a critical anti-apoptotic regulator. In the presence of neuropsychiatric insults (“death signals”), eg, chronic stress or prolonged sleep deprivation, increased extrasynaptic glutamate “spill-over” increases intracellular calcium (again, due to the loss of mitochondrial membrane integrity and release from intracellular stores). As a result, many destructive cascades commence and converge on final common pathways, ie, the activation of nucleic acid-cleaving caspases and excessive ROS production. Mitochondrial membrane damage also releases cytochrome C, which promotes apoptosis by caspase stimulation and the inhibition of the basal inhibitors of apoptosis (IAPs). Lithium decreases caspase3 transcription, which has powerful pro-apoptotic effects. In sum, lithium and other mood stabilizing drugs display potent pharmacological properties that either stimulate or inhibit the expression of many anti- or pro-apoptotic genes, respectively. Pro-apoptotic proteins are represented by maroon, and anti-apoptotic proteins are in green. Li, lithium; AED, antiepileptic mood stabilizer drugs; BDNF, brain-derived neurotrophic factor; MAPK, mitogen-activated protein kinase; ERK, extracellular-regulated kinase; GSK-3β, glycogen synthase kinase-3 beta; Bcl, B-cell lymphoma; Bax, Bcl-associated X-protein; Bad, Bcl-associated death promoter; Bid, BH3 domain-interacting death agonist; cCytC, cytosolic cytochrome C; APAF1, apoptotic protease activating factor 1; IAPs, inhibitors of apoptosis; iCa2+, intracellular calcium cation; H202, hydrogen peroxide; OH•, hydroxyl radical; ONOO−, peroxynitrite; CAD, caspase-activated DNase; p53, tumor suppressor protein of 53 kilodaltons; NMDA-R, N-methyl-D-aspartate receptor.
Although there are reports of pro-apoptotic effects of lithium in rodents,71,72 the preponderance of data support a neuroprotective role. In valinomycin (a potassium ionophore)-treated human SY5Y neuroblastoma cells, lithium decreased the expression of the pro-apoptotic caspase-3.73 In a recent microarray study, lithium responders were found to selectively downregulate pro-apoptotic transcription, ie, Bax1 and Bad, and upregulate anti-apoptotic gene expression, ie, Bcl-2 and IRS2, after only one month of treatment.74 Interestingly, the expression profile of lithium-resistance was the converse.74 Pramipexole, a dopamine receptor agonist and downstream inducer of Bcl-2, had efficacy over placebo in a randomized, double-blind, placebo-controlled trial in BDII patients maintained on therapeutic levels of lithium or valproic acid.75 Several pharmaceutical companies are attempting to develop inhibitors of apoptosis for many neuropsychiatric and medical disorders, which may ultimately find utility in the treatment of BD. Next, pro-and anti-apoptotic gene regulation may be a useful pharmacogenetic biomarker of treatment response, which warrants further investigation earlier in the course of lithium and anti-epileptic mood stabilizer therapy.
Conclusions/Future Directions
In this second of two articles, we have reviewed our current understanding of intracellular second messenger/signal transduction pathways in the pathophysiology and treatment of BD. We have surveyed evidence in support (and, in some cases, in refutation) of dysfunction in the following intracellular second messenger/signal transduction cascades: cAMP/PKA/CREB, ERK/MAPK, PI/PKC, Wnt/Fz/Dvl/GSK-3β, and anti- and pro-apoptotic pathways. There are several nodes of overlap and discrepancy with MDD and mouse models of despair, ie, PKC down-regulation in MDD/preclinical models and upregulation in BD. These differences warrant future diagnostic exploration and may eventually be exploited by novel treatments such as more selective PKC inhibitors. As a potential caveat, there have been relatively few studies directly demonstrating signal transduction impairment in the bipolar brain and/or response to effective treatment. Many of these studies have been conducted with rodent models, eg, psychostimulant-induced hyperlocomotion, or, when studied in humans, have occurred in small samples with carefully selected patients, eg, on a particular mood stabilizer, preserved psychosocial functioning, and no comorbid substance abuse. Therefore, although promising, these animal and clinical samples may not pertain to typical community bipolar patients. Moreover, our current technologies also limit our ability to directly test intracellular pathway dysfunction in the living human brain.
Apoptotic signaling cascades may be uniquely impaired in BD relative to other neuropsychiatric disorders such as schizophrenia.62 Nevertheless, there have been few studies that have demonstrated apoptosis in the human bipolar brain, ie, Kim et al’s63 revelation of increased Bax/Bak and decreased Bcl-2 expression in postmortem BD brain. Expression differences, however, do not necessarily translate to increased apoptosis, which has been exemplified by non-apoptotic roles for Bcl-2.76
In conclusion, our increasing understanding of intracellular second messenger/signal transduction pathway dysfunction in BD may eventually lead to improved diagnostic markers, better predictors of treatment response, and exciting future therapeutic targets. As a single example, the genetic manipulation of and/or small-molecule, membrane-permeant inhibitors of apoptosis are such novel therapeutic targets for future drug discovery and development.
Clinical Implications.
Bipolar disorder (BD) is a complex, highly-heritable major mood disorder characterized by episodes of hypo/mania and depression. Intracellular second messenger/signal transduction dysfunction in BD was first suggested by lithium’s ability to inhibit protein kinase C (PKC).
Including the aforementioned, some of the intracellular second messenger/signal transduction cascades that have been implicated in BD are the following: cAMP/PKA/CREB, ERK/MAPK, p11, PI/PKC, and Wnt/Fz/Dvl/GSK-3(beta).
Apoptotic/cell survival dysfunction in BD has excited much interest in mitochondrial-based mechanisms of disease. Albeit preliminary, BCL-2 and XBP1 polymorphisms may be influential in BD.
As in the other major mood disorder, major depressive disorder (MDD), intracellular second messenger/signal transduction abnormalities in BD and their reversal with successful treatment may be nosological endophenotypes and biomarkers of response to, respectively, improve diagnostics and further development of mood stabilizers with novel mechanisms of action.
Acknowledgments
The authors gratefully acknowledge the support of the IRP-NIMH/NIH, and the NARSAD Independent Investigator Award and Brain and Behavior Foundation Bipolar Research Award (C.A.Z.). Salary support was also provided by the IRP-NIMH/NIH (M.J.N., D.F.I., D.C.M., and E.M.R.). Dr. Zarate is listed as a co-inventor on a patent application for the use of ketamine and its metabolites in major depression. Dr. Zarate has assigned his rights in the patent to the U.S. government but will share a percentage of any royalties that may be received.
Footnotes
Disclosures
Drs. Niciu, Ionescu, Mathews, and Richards have no potential financial conflicts of interest to disclose.
References
- 1.Allison JH, Stewart MA. Reduced brain inositol in lithium-treated rats. Nat New Biol. 1971;233(43):267–268. doi: 10.1038/newbio233267a0. [DOI] [PubMed] [Google Scholar]
- 2.Machado-Vieira R, Manji HK, Zarate CA., Jr The role of lithium in the treatment of bipolar disorder: convergent evidence for neurotrophic effects as a unifying hypothesis. Bipolar Disord. 2009;11(suppl 2):92–109. doi: 10.1111/j.1399-5618.2009.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen G, Manji HK, Wright CB, Hawver DB, Potter WZ. Effects of valproic acid on beta-adrenergic receptors, G-proteins, and adenylyl cyclase in rat C6 glioma cells. Neuropsychopharmacology. 1996;15(3):271–280. doi: 10.1016/0893-133X(95)00207-T. [DOI] [PubMed] [Google Scholar]
- 4.Bosetti F, Bell JM, Manickam P. Microarray analysis of rat brain gene expression after chronic administration of sodium valproate. Brain Res Bull. 2005;65(4):331–338. doi: 10.1016/j.brainresbull.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Ertley RN, Bazinet RP, Lee HJ, Rapoport SI, Rao JS. Chronic treatment with mood stabilizers increases membrane GRK3 in rat frontal cortex. Biol Psychiatry. 2007;61(2):246–249. doi: 10.1016/j.biopsych.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 6.Post RM, Ballenger JC, Uhde TW, et al. Effect of carbamazepine on cyclic nucleotides in CSF of patients with affective illness. Biol Psychiatry. 1982;17(9):1037–1045. [PubMed] [Google Scholar]
- 7.Young LT, Li PP, Kish SJ, Siu KP, Warsh JJ. Postmortem cerebral cortex Gs alpha-subunit levels are elevated in bipolar affective disorder. Brain Res. 1991;553(2):323–326. doi: 10.1016/0006-8993(91)90843-k. [DOI] [PubMed] [Google Scholar]
- 8.Friedman E, Wang HY. Receptor-mediated activation of G proteins is increased in postmortem brains of bipolar affective disorder subjects. J Neurochem. 1996;67(3):1145–1152. doi: 10.1046/j.1471-4159.1996.67031145.x. [DOI] [PubMed] [Google Scholar]
- 9.Young LT, Li PP, Kamble A, Siu KP, Warsh JJ. Mononuclear leukocyte levels of G proteins in depressed patients with bipolar disorder or major depressive disorder. Am J Psychiatry. 1994;151(4):594–596. doi: 10.1176/ajp.151.4.594. [DOI] [PubMed] [Google Scholar]
- 10.Schreiber G, Avissar S, Danon A, Belmaker RH. Hyperfunctional G proteins in mononuclear leukocytes of patients with mania. Biol Psychiatry. 1991;29(3):273–280. doi: 10.1016/0006-3223(91)91289-4. [DOI] [PubMed] [Google Scholar]
- 11.Spleiss O, van Calker D, Scharer L, et al. Abnormal G protein alpha(s)- and alpha(i2)-subunit mRNA expression in bipolar affective disorder. Mol Psychiatry. 1998;3(6):512–520. doi: 10.1038/sj.mp.4000393. [DOI] [PubMed] [Google Scholar]
- 12.Fields A, Li PP, Kish SJ, Warsh JJ. Increased cyclic AMP-dependent protein kinase activity in postmortem brain from patients with bipolar affective disorder. J Neurochem. 1999;73:1704–1710. doi: 10.1046/j.1471-4159.1999.731704.x. [DOI] [PubMed] [Google Scholar]
- 13.Chang A, Li PP, Warsh JJ. Altered cAMP-dependent protein kinase subunit immunolabeling in post-mortem brain from patients with bipolar affective disorder. J Neurochem. 2003;84(4):781–791. doi: 10.1046/j.1471-4159.2003.01605.x. [DOI] [PubMed] [Google Scholar]
- 14.Tardito D, Mori S, Racagni G, et al. Protein kinase A activity in platelets from patients with bipolar disorder. J Affect Disord. 2003;76(1–3):249–253. doi: 10.1016/s0165-0327(02)00065-4. [DOI] [PubMed] [Google Scholar]
- 15.Karege F, Schwald M, Papadimitriou P, Lachausse C, Cisse M. The cAMP-dependent protein kinase A and brain-derived neurotrophic factor expression in lymphoblast cells of bipolar affective disorder. J Affect Disord. 2004;79(1–3):187–192. doi: 10.1016/S0165-0327(02)00463-9. [DOI] [PubMed] [Google Scholar]
- 16.Ryan MM, Lockstone HE, Huffaker SJ, et al. Gene expression analysis of bipolar disorder reveals downregulation of the ubiquitin cycle and alterations in synaptic genes. Mol Psychiatry. 2006;11(10):965–978. doi: 10.1038/sj.mp.4001875. [DOI] [PubMed] [Google Scholar]
- 17.McDonald ML, Macmullen C, Liu DJ, Leal SM, Davis RL. Genetic association of cyclic AMP signaling genes with bipolar disorder. Transl Psychiatry. 2012;2:e169. doi: 10.1038/tp.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manji HK, Lenox RH. Signaling: cellular insights into the pathophysiology of bipolar disorder. Biol Psychiatry. 2000;48(6):518–530. doi: 10.1016/s0006-3223(00)00929-x. [DOI] [PubMed] [Google Scholar]
- 19.Sadana R, Dessauer CW. Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knockout and overexpression studies. Neurosignals. 2009;17(1):5–22. doi: 10.1159/000166277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozaki N, Chuang DM. Lithium increases transcription factor binding to AP-1 and cyclic AMP-responsive element in cultured neurons and rat brain. J Neurochem. 1997;69(6):2336–2344. doi: 10.1046/j.1471-4159.1997.69062336.x. [DOI] [PubMed] [Google Scholar]
- 21.Tardito D, Tiraboschi E, Kasahara J, Racagni G, Popoli M. Reduced CREB phosphorylation after chronic lithium treatment is associated with down-regulation of CaM kinase IV in rat hippocampus. Int J Neuropsychopharmacol. 2007;10(4):491–496. doi: 10.1017/S1461145706007140. [DOI] [PubMed] [Google Scholar]
- 22.Ferrendelli JA, Kinscherf DA. Inhibitory effects of anticonvulsant drugs on cyclic nucleotide accumulation in brain. Ann Neurol. 1979;5(6):533–538. doi: 10.1002/ana.410050606. [DOI] [PubMed] [Google Scholar]
- 23.Van Calker D, Steber R, Klotz KN, Greil W. Carbamazepine distinguishes between adenosine receptors that mediate different second messenger responses. Eur J Pharmacol. 1991;206(4):285–290. doi: 10.1016/0922-4106(91)90111-t. [DOI] [PubMed] [Google Scholar]
- 24.Basselin M, Chang L, Chen M, Bell JM, Rapoport SI. Chronic carbamazepine administration attenuates dopamine D2-like receptor-initiated signaling via arachidonic acid in rat brain. Neurochem Res. 2008;33(7):1373–1383. doi: 10.1007/s11064-008-9595-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Daniel E, Mudge AW, Maycox PR. Comparative analysis of the effects of four mood stabilizers in SH-SY5Y cells and in primary neurons. Bipolar Disord. 2005;7(1):33–41. doi: 10.1111/j.1399-5618.2004.00164.x. [DOI] [PubMed] [Google Scholar]
- 26.Xie N, Wang C, Lin Y, et al. The role of p38 MAPK in valproic acid induced microglia apoptosis. Neurosci Lett. 2010;482(1):51–56. doi: 10.1016/j.neulet.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Tsui MM, Tai WC, Wong WY, Hsiao WL. Selective G2/M arrest in a p53(Val135)-transformed cell line induced by lithium is mediated through an intricate network of MAPK and beta-catenin signaling pathways. Life Sci. 2012;91(9–10):312–321. doi: 10.1016/j.lfs.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 28.Einat H, Yuan P, Gould TD, et al. The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J Neurosci. 2003;23(19):7311–7316. doi: 10.1523/JNEUROSCI.23-19-07311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimon H, Agam G, Belmaker RH, Hyde TM, Kleinman JE. Reduced frontal cortex inositol levels in postmortem brain of suicide victims and patients with bipolar disorder. Am J Psychiatry. 1997;154(8):1148–1150. doi: 10.1176/ajp.154.8.1148. [DOI] [PubMed] [Google Scholar]
- 30.Jope RS, Song L, Li PP, et al. The phosphoinositide signal transduction system is impaired in bipolar affective disorder brain. J Neurochem. 1996;66(6):2402–2409. doi: 10.1046/j.1471-4159.1996.66062402.x. [DOI] [PubMed] [Google Scholar]
- 31.Brown AS, Mallinger AG, Renbaum LC. Elevated platelet membrane phosphatidylinositol-4,5-bisphosphate in bipolar mania. Am J Psychiatry. 1993;150(8):1252–1254. doi: 10.1176/ajp.150.8.1252. [DOI] [PubMed] [Google Scholar]
- 32.Baum AE, Akula N, Cabanero M, et al. A genome-wide association study implicates diacylglycerol kinase eta (DGKH) and several other genes in the etiology of bipolar disorder. Mol Psychiatry. 2008;13(2):197–207. doi: 10.1038/sj.mp.4002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu C, Li PP, Cooke RG, et al. TRPM2 variants and bipolar disorder risk: confirmation in a family-based association study. Bipolar Disord. 2009;11(1):1–10. doi: 10.1111/j.1399-5618.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- 34.Xu C, Warsh JJ, Wang KS, Mao CX, Kennedy JL. Association of the iPLA2beta gene with bipolar disorder and assessment of its interaction with TRPM2 gene polymorphisms. Psychiatr Genet. In press doi: 10.1097/YPG.0b013e32835d700d. [DOI] [PubMed] [Google Scholar]
- 35.Wang HY, Friedman E. Enhanced protein kinase C activity and translocation in bipolar affective disorder brains. Biol Psychiatry. 1996;40(7):568–575. doi: 10.1016/0006-3223(95)00611-7. [DOI] [PubMed] [Google Scholar]
- 36.Wang H, Friedman E. Increased association of brain protein kinase C with the receptor for activated C kinase-1 (RACK1) in bipolar affective disorder. Biol Psychiatry. 2001;50(5):364–370. doi: 10.1016/s0006-3223(01)01147-7. [DOI] [PubMed] [Google Scholar]
- 37.Friedman E, Hoau Yan W, Levinson D, Connell TA, Singh H. Altered platelet protein kinase C activity in bipolar affective disorder, manic episode. Biol Psychiatry. 1993;33(7):520–525. doi: 10.1016/0006-3223(93)90006-y. [DOI] [PubMed] [Google Scholar]
- 38.Wang HY, Markowitz P, Levinson D, Undie AS, Friedman E. Increased membrane-associated protein kinase C activity and translocation in blood platelets from bipolar affective disorder patients. J Psychiatr Res. 1999;33(2):171–179. doi: 10.1016/s0022-3956(98)90057-7. [DOI] [PubMed] [Google Scholar]
- 39.Pandey GN, Dwivedi Y, SridharaRao J, et al. Protein kinase C and phospholipase C activity and expression of their specific isozymes is decreased and expression of MARCKS is increased in platelets of bipolar but not in unipolar patients. Neuropsychopharmacology. 2002;26(2):216–228. doi: 10.1016/S0893-133X(01)00327-X. [DOI] [PubMed] [Google Scholar]
- 40.Yoon IS, Li PP, Siu KP, et al. Altered IMPA2 gene expression and calcium homeostasis in bipolar disorder. Mol Psychiatry. 2001;6(6):678–683. doi: 10.1038/sj.mp.4000901. [DOI] [PubMed] [Google Scholar]
- 41.Manji HK, Etcheberrigaray R, Chen G, Olds JL. Lithium decreases membrane-associated protein kinase C in hippocampus: selectivity for the alpha isozyme. J Neurochem. 1993;61(6):2303–2310. doi: 10.1111/j.1471-4159.1993.tb07474.x. [DOI] [PubMed] [Google Scholar]
- 42.Chen G, Masana MI, Manji HK. Lithium regulates PKC-mediated intracellular cross-talk and gene expression in the CNS in vivo. Bipolar Disord. 2000;2(3 pt. 2):217–236. doi: 10.1034/j.1399-5618.2000.20303.x. [DOI] [PubMed] [Google Scholar]
- 43.Lenox RH, Watson DG, Patel J, Ellis J. Chronic lithium administration alters a prominent PKC substrate in rat hippocampus. Brain Res. 1992;570(1–2):333–340. doi: 10.1016/0006-8993(92)90598-4. [DOI] [PubMed] [Google Scholar]
- 44.Szabo ST, Machado-Vieira R, Yuan P, et al. Glutamate receptors as targets of protein kinase C in the pathophysiology and treatment of animal models of mania. Neuropharmacology. 2009;56(1):47–55. doi: 10.1016/j.neuropharm.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen G, Manji HK, Hawver DB, Wright CB, Potter WZ. Chronic sodium valproate selectively decreases protein kinase C alpha and epsilon in vitro. J Neurochem. 1994;63(6):2361–2364. doi: 10.1046/j.1471-4159.1994.63062361.x. [DOI] [PubMed] [Google Scholar]
- 46.Watson DG, Watterson JM, Lenox RH. Sodium valproate down-regulates the myristoylated alanine-rich C kinase substrate (MARCKS) in immortalized hippocampal cells: a property of protein kinase C-mediated mood stabilizers. J Pharmacol Exp Ther. 1998;285(1):307–316. [PubMed] [Google Scholar]
- 47.Hasegawa H, Osada K, Misonoo A, et al. Chronic carbamazepine treatment increases myristoylated alanine-rich C kinase substrate phosphorylation in the rat cerebral cortex via down-regulation of calcineurin A alpha. Brain Res. 2003;994(1):19–26. doi: 10.1016/j.brainres.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 48.Einat H, Yuan P, Szabo ST, Dogra S, Manji HK. Protein kinase C inhibition by tamoxifen antagonizes manic-like behavior in rats: implications for the development of novel therapeutics for bipolar disorder. Neuropsychobiology. 2007;55(3–4):123–131. doi: 10.1159/000106054. [DOI] [PubMed] [Google Scholar]
- 49.Bebchuk JM, Arfken CL, Dolan-Manji S, et al. A preliminary investigation of a protein kinase C inhibitor in the treatment of acute mania. Arch Gen Psychiatry. 2000;57(1):95–97. doi: 10.1001/archpsyc.57.1.95. [DOI] [PubMed] [Google Scholar]
- 50.Kulkarni J, Garland KA, Scaffidi A, et al. A pilot study of hormone modulation as a new treatment for mania in women with bipolar affective disorder. Psychoneuroendocrinology. 2006;31(4):543–547. doi: 10.1016/j.psyneuen.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 51.Zarate CA, Jr, Singh JB, Carlson PJ, et al. Efficacy of a protein kinase C inhibitor (tamoxifen) in the treatment of acute mania: a pilot study. Bipolar Disord. 2007;9(6):561–570. doi: 10.1111/j.1399-5618.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- 52.Yildiz A, Guleryuz S, Ankerst DP, Ongur D, Renshaw PF. Protein kinase C inhibition in the treatment of mania: a double-blind, placebo-controlled trial of tamoxifen. Arch Gen Psychiatry. 2008;65(3):255–263. doi: 10.1001/archgenpsychiatry.2007.43. [DOI] [PubMed] [Google Scholar]
- 53.Sylvia LG, Peters AT, Deckersbach T, Nierenberg AA. Nutrient-based therapies for bipolar disorder: a systematic review. Psychother Psychosom. 2013;82(1):10–19. doi: 10.1159/000341309. [DOI] [PubMed] [Google Scholar]
- 54.van Calker D, Belmaker RH. The high affinity inositol transport system—implications for the pathophysiology and treatment of bipolar disorder. Bipolar Disord. 2000;2(2):102–107. doi: 10.1034/j.1399-5618.2000.020203.x. [DOI] [PubMed] [Google Scholar]
- 55.Willmroth F, Drieling T, Lamla U, et al. Sodium-myoinositol co-transporter (SMIT-1) mRNA is increased in neutrophils of patients with bipolar 1 disorder and down-regulated under treatment with mood stabilizers. Int J Neuropsychopharmacol. 2007;10(1):63–71. doi: 10.1017/S1461145705006371. [DOI] [PubMed] [Google Scholar]
- 56.Shaldubina A, Johanson RA, O’Brien WT, et al. SMIT1 haploinsufficiency causes brain inositol deficiency without affecting lithium-sensitive behavior. Mol Genet Metab. 2006;88(4):384–388. doi: 10.1016/j.ymgme.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 57.Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93(16):8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen G, Huang LD, Jiang YM, Manji HK. The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. J Neurochem. 1999;72(3):1327–1330. doi: 10.1046/j.1471-4159.2000.0721327.x. [DOI] [PubMed] [Google Scholar]
- 59.Roh MS, Kang UG, Shin SY, et al. Biphasic changes in the Ser-9 phosphorylation of glycogen synthase kinase-3beta after electroconvulsive shock in the rat brain. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(1):1–5. doi: 10.1016/s0278-5846(02)00307-x. [DOI] [PubMed] [Google Scholar]
- 60.Polter A, Beurel E, Yang S, et al. Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances. Neuropsychopharmacology. 2010;35(8):1761–1774. doi: 10.1038/npp.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gould TD, Einat H, O’Donnell KC, et al. Beta-catenin overexpression in the mouse brain phenocopies lithium-sensitive behaviors. Neuropsychopharmacology. 2007;32(10):2173–2183. doi: 10.1038/sj.npp.1301338. [DOI] [PubMed] [Google Scholar]
- 62.Benes FM, Matzilevich D, Burke RE, Walsh J. The expression of proapoptosis genes is increased in bipolar disorder, but not in schizophrenia. Mol Psychiatry. 2006;11(3):241–251. doi: 10.1038/sj.mp.4001758. [DOI] [PubMed] [Google Scholar]
- 63.Kim HW, Rapoport SI, Rao JS. Altered expression of apoptotic factors and synaptic markers in postmortem brain from bipolar disorder patients. Neurobiol Dis. 2010;37(3):596–603. doi: 10.1016/j.nbd.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bei E, Salpeas V, Pappa D, et al. Phosphorylation status of glucocorticoid receptor, heat shock protein 70, cytochrome c and Bax in lymphocytes of euthymic, depressed and manic bipolar patients. Psychoneuroendocrinology. 2009;34(8):1162–1175. doi: 10.1016/j.psyneuen.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 65.Machado-Vieira R, Pivovarova NB, Stanika RI, et al. The Bcl-2 gene polymorphism rs956572AA increases inositol 1,4,5-trisphosphate receptor-mediated endoplasmic reticulum calcium release in subjects with bipolar disorder. Biol Psychiatry. 2011;69(4):344–352. doi: 10.1016/j.biopsych.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soeiro-de-Souza MG, Salvadore G, Moreno RA, et al. Bcl-2 rs956572 polymorphism is associated with increased anterior cingulate cortical glutamate in euthymic bipolar I disorder. Neuropsychopharmacology. 2013;38(3):468–475. doi: 10.1038/npp.2012.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kakiuchi C, Iwamoto K, Ishiwata M, et al. Impaired feedback regulation of XBP1 as a genetic risk factor for bipolar disorder. Nat Genet. 2003;35(2):171–175. doi: 10.1038/ng1235. [DOI] [PubMed] [Google Scholar]
- 68.So J, Warsh JJ, Li PP. Impaired endoplasmic reticulum stress response in B-lymphoblasts from patients with bipolar-I disorder. Biol Psychiatry. 2007;62(2):141–147. doi: 10.1016/j.biopsych.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 69.Hayashi A, Kasahara T, Kametani M, et al. Aberrant endoplasmic reticulum stress response in lymphoblastoid cells from patients with bipolar disorder. Int J Neuropsychopharmacol. 2009;12(1):33–43. doi: 10.1017/S1461145708009358. [DOI] [PubMed] [Google Scholar]
- 70.Kim B, Kim CY, Lee MJ, Joo YH. Preliminary evidence on the association between XBP1-116C/G polymorphism and response to prophylactic treatment with valproate in bipolar disorders. Psychiatry Res. 2009;168(3):209–212. doi: 10.1016/j.psychres.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 71.Song L, Zhou T, Jope RS. Lithium facilitates apoptotic signaling induced by activation of the Fas death domain-containing receptor. BMC Neurosci. 2004;5:20. doi: 10.1186/1471-2202-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gomez-Sintes R, Lucas JJ. NFAT/Fas signaling mediates the neuronal apoptosis and motor side effects of GSK-3 inhibition in a mouse model of lithium therapy. J Clin Invest. 2010;120(7):2432–2445. doi: 10.1172/JCI37873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li R, El-Mallahk RS. A novel evidence of different mechanisms of lithium and valproate neuroprotective action on human SY5Y neuroblastoma cells: caspase-3 dependency. Neurosci Lett. 2000;294(3):147–150. doi: 10.1016/s0304-3940(00)01559-7. [DOI] [PubMed] [Google Scholar]
- 74.Lowthert L, Leffert J, Lin A, et al. Increased ratio of anti-apoptotic to pro-apoptotic Bcl2 gene-family members in lithium-responders one month after treatment initiation. Biol Mood Anxiety Disord. 2012;2(1):15. doi: 10.1186/2045-5380-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zarate CA, Jr, Payne JL, Singh J, et al. Pramipexole for bipolar II depression: a placebo-controlled proof of concept study. Biol Psychiatry. 2004;56(1):54–60. doi: 10.1016/j.biopsych.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 76.Danial NN, Gimenez-Cassina A, Tondera D. Homeostatic functions of BCL-2 proteins beyond apoptosis. Adv Exp Med Biol. 2010;687:1–32. doi: 10.1007/978-1-4419-6706-0_1. [DOI] [PubMed] [Google Scholar]