Abstract
Clinical depression is a serious mental disorder characterized by low mood, anhedonia, loss of interest in daily activities, and other symptoms, and is associated with severe consequences including suicide and increased risk of cardiovascular events. Depression affects nearly 15% of the population. The standard of care for the last 50 years has focused on monoamine neurotransmitters, including such treatments as selective serotonin reuptake inhibitors (SSRIs) and serotonin–norepinephrine reuptake inhibitors (SNRIs). However, these treatments have significant limitations: they can take weeks before showing mood-altering effects, and only one to two out of ten patients shows clinical effects beyond those associated with placebo. A major paradigm shift in research into the treatment of depression is underway, based on promising results with the glutamatergic NMDA receptor antagonist ketamine. Further research has demonstrated the significance of glutamatergic pathways in depression and the association of this system with the stress pathway and magnesium homeostasis. Treatment with NMDA receptor antagonists and magnesium have shown the ability to sprout new synaptic connections and reverse stress-induced neural changes, opening up promising new territory for the development of drugs to meet the unmet need in patients with clinical depression.
Keywords: depression, SSRI, SNRI, ketamine, glutamate antagonist, NMDA, scopolamine, magnesium, glutamine, CP-AMPA
Introduction
Research into the glutamatergic mechanism of depression is an important avenue to identify new treatments for depression. Several recent developments have come together to enable this. Fundamental findings in this area date back to the early 20th century. An early report on the mechanism of a compound, which has since been identified as an N-methyl-D-aspartate (NMDA) antagonist, was published in 1921 by Weston et al.1 for the treatment of agitation, mostly in depressed patients. Much later, treatment with amantadine2 demonstrated beneficial effects.3 However, at that time glutamate—and hence the NMDA receptor—was not regarded as a neurotransmitter, and it was only accepted as such in the early 1980s.4
A clear theoretical foundation for the use of NMDA receptor antagonists in depression has been developed since 1990.3 However, these early findings did not significantly influence antidepressant drug development until recently, as it had been almost exclusively informed by the monoamine hypothesis of depression.5 In the early 21st century, the clinical limitations of the monoaminergic approach became apparent with the recognition of the relatively low efficacy of current treatment strategies.6,7
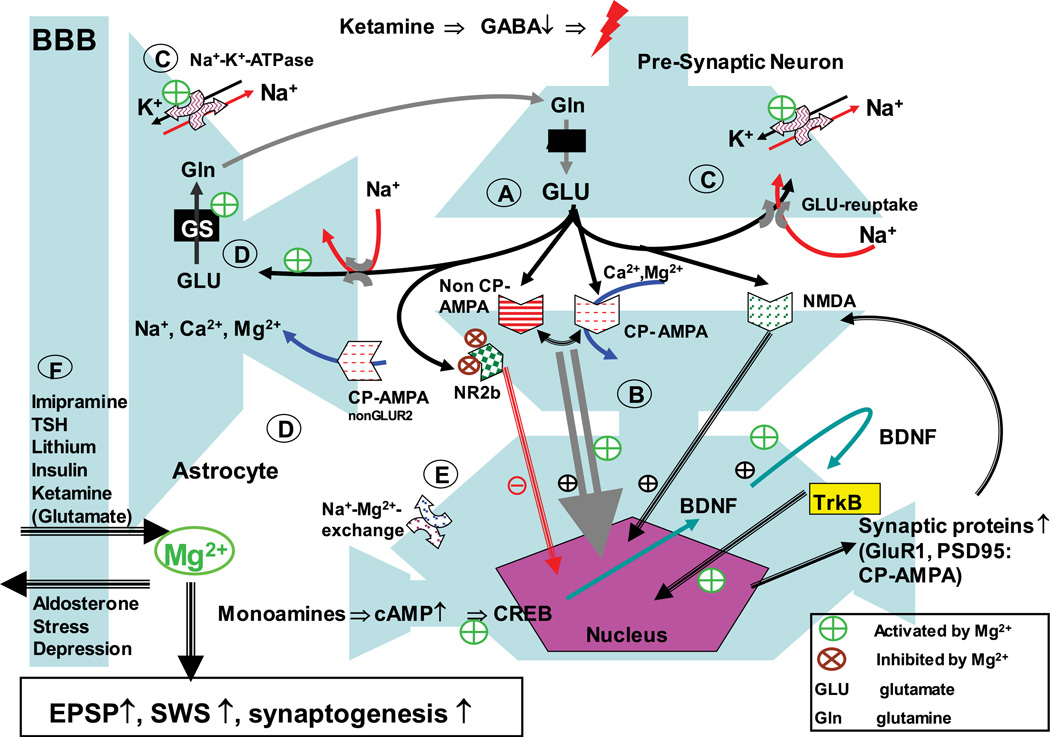
The serendipitous observation by Berman et al.8 of the rapid response of ketamine in depression, later confirmed by Zarate,9 led to the current focus in this area in both academia and industry. Extensive preclinical characterization of the effects of ketamine has partly illuminated its mechanism of action,10 though how ketamine’s activity is linked to known pathophysiological changes in depression has just begun to be understood. For example, animal models that focus on glutamatergic overactivity may be helpful in understanding vulnerability to depression and the specific biological and behavioral features that match the pharmacology of glutamatergic compounds.11 One model in this context is the magnesium (Mg2+)-depletion model (Fig. 1).12 Magnesium depletion leads to NMDA overactivity and, as a consequence, to depression and anxiety-like symptoms, neuroendocrine changes including increased cortisol levels, sleep disturbances—including a reduction of slow-wave sleep—and increased inflammatory markers. This model covers not only selected aspects but the whole variety of biological changes observed in certain patients with depression. This raises the question of the role of Mg2+ itself in the pathophysiology of depression; for example, can depression be induced by nutritional Mg2+ deficit? Moreover, Mg2+ may additionally be considered as a mediator of established treatments—including imipramine, lithium, and thyroid stimulating hormone (TSH)11 treatment—that boosts improvement in refractory patients, which may open up the possibility for development of a new class of compounds based on their capability to increase neuronal and astrocyte Mg2+ content.
Figure 1.
Magnesium involvement in ketamine-induced pathways. Ketamine administration leads to a cascade of events finally resulting in modifications of glutamatergic receptor profile and synaptogenesis. Functional consequences are increased excitatory postsynaptic potentials (EPSP) and increased slow-wave sleep; both phenomena are also induced by Mg2+. In detail: (A) ketamine inhibits GABAergic interneurons and therefore activates the release of glutamate downstream in the context of partial NR2b receptor blockade. (B) AMPA mediates BDNF expression and release. BDNF activates TrkB receptor, which induces changes in gene expression. BNDF induces its own expression. NMDA receptor activation is facilitating this process.33 This could constitute a feedforward mechanism, explaining the long-term effect of ketamine administration. Further, the expression of synaptic proteins is induced, in particular GluR1 and PSD-95, which constitute the synaptic expression of Ca2+-permeable AMPA receptors (CP-AMPA).34 Importantly, CP-AMPA are permeable for Mg2+. (C) Glutamate is taken up quickly by neurons and astrocytes. This is of importance as high concentrations of glutamate can spill over to extrasynaptic NMDA receptors, which appear to be primarily from the NR2b type. Their activation can block synaptogenesis and lead to cell damage. Rapid reuptake of glutamate prevents this spillover. Glutamate reuptake is an energy-dependent process driven by the Na+-gradient over the membrane,35 which in itself is driven by the Na+–K+–ATPase. The Na+–K+–ATPase is dependent on Mg2+, therefore increased Mg2+ availability supports its activity and secondarily glutamate clearance. (D) In parallel glutamate receptors at astrocytes are activated. CP-AMPA receptor activation can lead to an increase in astrocytic Ca2+35 and Mg2+36 A potential consequence of increased Mg2+ in astrocytes is activation of glutamine synthetase (GS), which is Mg2+ dependent.37,38 (E) A neuronal Na+–Mg2+–exchange mechanism regulates intracellular Mg2+ concentration. Imipramine appears to block Na+–Mg2+ exchange, preventing the efflux of Mg2+ from the neuron. (F) Magnesium uptake into the brain has been described with compounds, which are known to be efficacious in treatment-refractory depression, i.e., ketamine (via its glutamate-releasing capability), TSH, lithium, imipramine, and potentially an insulin-related mechanism (metformin, glitazones). On the other hand, stress and refractory depression are linked to lower Mg2+ levels in the brain, which may in part be mediated via an aldosterone mediated mechanism. Adapted with permission from Ref. 11.
On March 25, 2013, the New York Academy of Sciences hosted the conference “Treatment-Resistant Depression” that attracted attendees from industry, academia, and governmental agencies and focused on current research that seeks to move beyond the traditional monoamine neurotransmitter–focused treatment for patients suffering from depression and related disorders.
Developing novel treatments: use of rapid-acting antidepressants and biomarkers of treatment response
Carlos A. Zarate, Jr. (National Institute of Mental Health) began the meeting with a discussion of the current horizons of development of drugs for the treatment of depression and biomarkers that could be used to evaluate patients’ responses to treatment. Despite considerable effort over the last several decades, little progress has been made in developing more effective antidepressants that the current armamentarium. The first antidepressant drug, discovered by serendipity, eventually led to a multitude (over 30 specific compounds currently) of other antidepressants drugs that were little more than molecular refinements of the initial prototype drug, whose mode of action was to modulate the effects of serotonin and norepinephrine. Over the decades, drug discovery and development for depression have proceeded largely on two fronts: mimic what earlier ones do—modulate serotonin and norepinephrine—or explore new therapeutic targets for depression.
Concerning the measure of success along the first front, none of the developed compounds have demonstrated a significant advantage in terms of efficacy over earlier antidepressants, owing to the fact that all of these second and third generation drugs remain either primarily serotonergically- or noradrenergically-based (and known as selective serotonin reuptake inhibitors (SSRIs) or serotonin–norepinephrine reuptake inhibitors (SNRIs)). Rather than having greater efficacy, the drugs are, for the most part, better tolerated than the original tricyclic antidepressants and monoamine oxidase inhibitors.
Success along the second front of drug discovery, based on identifying novel therapeutic targets that result in new treatments, has unfortunately failed miserably. Several new compounds that seemed to show improvement in animal models were subsequently shown to have little effect in humans (phase I and II studies). Reasons for this lack of success in developing new and improved antidepressant have been discussed.13,14
One strategy that has been increasingly used in drug discovery and development strategies is the incorporation of biomarkers that either signal drug effects (target or functional engagement) or are used in treatment-response paradigms. This strategy has been encouraged by many groups, including the Institute of Medicine (IOM).15 Technologies that have begun to be incorporated in treatment-response studies include positron emission tomography (PET); functional magnetic resonance imaging (fMRI); brain proton magnetic resonance spectroscopy (1H-MRS); neurophysiology measures such as sleep electroencephalography and magnetoencephalography (MEG); peripheral blood, plasma, and urine markers; cerebrospinal fluid (CSF); and genetics, proteomics, and metabolomics, to name several. Many of these technologies are still being refined.
One paradigm which is now being utilized to enhance drug development efforts for depression and bipolar disorder is the study of interventions that are radically distinct, in some clinically useful way, from existing treatments. The study of ketamine and scopolamine exemplify this different paradigm. First, both of these drugs have been demonstrated, in at least two controlled trials, to have much more rapid antidepressant and antisuicidal effects than existing treatments; within a few hours for ketamine and a few days for scopolamine.16,17 Second, ketamine is effective in patients who have failed a multitude of antidepressants as well as electroconvulsive therapy, which is the most effective current treatment. Third, these two drugs, unlike traditional antidepressants, appear to more directly modulate two distinct neurotransmitter systems, the glutamatergic and muscarinic neurotransmitter systems. Finally, because of their rapid antidepressant effects, these drugs appear to be useful tools for synchronizing possible biomarkers within a relatively short period of time, in contrast to the complexity and cost of studying existing treatments (for review see Ref. 17).
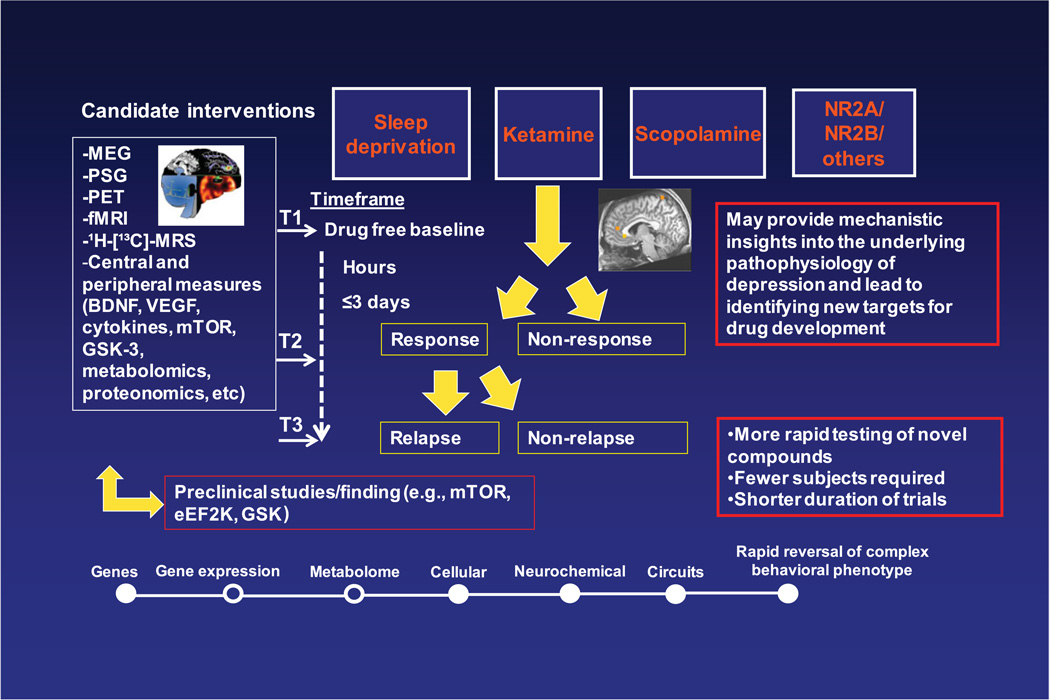
Figure 2 illustrates the new paradigm in the study of biomarkers with rapid-acting antidepressants—early work has begun to yield promising putative biomarkers predictive of rapid antidepressant response.18,19 This model investigates a multitude of biomarkers (e.g., fMRI, MEG, PSG, brain-derived neurotrophic factor (BDNF), SNPs) during interventions that produce rapid antidepressant clinical effects, for example, within 72 hours. Bio-signatures of response, non-response, and relapse are generated from the integration of biological findings. These results can then inform and guide drug discovery and development efforts.
Figure 2.
Conceptual framework for advancing translational findings across a systems level of biomarkers of response/relapse to develop rapid-acting antidepressants.
Indeed, similar strategies are already being conducted on a preclinical level, where the molecular and cellular signatures of response to ketamine and scopolamine are being compared and contrasted. The antidepressant actions of scopolamine, a muscarinic antagonist, and ketamine, an NMDA antagonist, require the mammalian target of rapamycin (mTOR) signaling.20 Such strategies that can assess the efficacy of rapid-acting antidepressants on micro and macro levels—across systems levels—may be more likely to generate important insights for developing the next generation of treatments that, hopefully, will act more rapidly and will be more effective than existing antidepressants. Such next-generation treatments may even put psychiatry on par with other areas of medicine, by allowing physicians to intervene with treatment that rapidly prevents or reverses major depressive episodes or prevents suicide by rapidly eliminating suicidal ideation.
Ketamine treatment as a new paradigm for the treatment of affective disorders: preclinical studies
Ronald S. Duman (Yale University School of Medicine) further explored the potential of ketamine as a treatment for depression and other affective disorders. Recent molecular and cellular studies have demonstrated that stress and antidepressants exert opposing effects on the expression of neurotrophic factors that result in structural alterations of neurons, including regulation of dendrite complexity and spine density in the prefrontal cortex (PFC) and the hippocampus. The deleterious effects of stress may contribute to the reduced volume of the PFC and the hippocampus in depressed patients. Conversely, the actions of antidepressants could be mediated in part by blocking or reversing the atrophy caused by stress and depression.
Recent studies have demonstrated that ketamine produces rapid (within hours) antidepressant responses in treatment-resistant depressed patients, thus addressing amajor limitation of currently available agents (i.e., delayed onset of action and low response rates). The discovery that ketamine produces rapid and efficacious antidepressant effects by a mechanism completely different from conventional antidepressants (blockade of NMDA receptors (NMDARs)) represents one of the most important discoveries in the field of depression in over 50 years. Preclinical studies in rodent models have begun to unravel the molecular and cellular mechanisms underlying the rapid actions of NMDAR antagonists. Duman’s presentation discussed recent work demonstrating that ketamine causes a rapid induction of spine density in the medial PFC through activation of neurotrophic factor signaling and mTOR, which regulate the translation of synaptic proteins (reviewed in Ref. 21). The roles of mTOR signaling and synaptogenesis in the response to scopolamine were also discussed by Duman.
The ability of a single dose of ketamine to cause a rapid antidepressant response suggests that this agent produces rapid effects on neuronal function, possibly through regulation of spine synapses. To directly test this possibility, Duman’s group examined the influence of ketamine administration on spine number and function in layer V pyramidal neurons in slices of the PFC using single-cell patch clamp electrophysiology combined with confocal imaging of neurobiotin-labeled neurons. They found that a single dose of ketamine increased the amplitude and frequency of 5-HT– and hypocretin-induced excitatory postsynaptic currents (EPSCs) in layer V neurons.22 Moreover, they found that ketamine increased the density of spine synapses, as well as the number of mature mushroom spines (i.e., increased spine head diameter), in both the proximal and distal dendrite branches of layer V neurons. At a behavioral level, they found that ketamine also produced a rapid antidepressant response in the forced-swim and novelty-suppressed feeding tests, and blocked anhedonia (decreased sucrose preference) caused by chronic stress exposure. The latter results provide a rigorous test and confirmation of the rapid actions of ketamine, compared to the requirement for long-term treatment (three weeks) of a typical reuptake inhibitor antidepressant to produce similar effects.
The rapid synaptogenic action of ketamine is similar to what is observed in cellular models of learning and memory, where a burst of glutamate—the major excitatory neurotransmitter in the brain—results in the release of BDNF and activation of mTOR signaling, which leads to increased translation of synaptic proteins required for synaptogenesis. Previous studies demonstrated that ketamine rapidly increases glutamate release in the PFC, possibly through inhibition of tonic firing of GABAergic interneurons, providing support for this hypothesis.23 Duman’s group found that ketamine causes a rapid (30 min) activation of mTOR signaling in the PFC, as measured by increased levels of the phosphorylated and activated forms of mTOR and S6 kinase. A role for mTOR signaling was further supported by studies demonstrating that pretreatment with rapamycin, a selective mTOR inhibitor, completely blocked the induction of spine synapses and the behavioral actions of ketamine.22,24 Duman’s group also tested the role of BDNF in mice with a knock-in of the Val66Met BDNF polymorphism, where the Met allele blocks activity-dependent release of BDNF. The ability of ketamine to increase spine synapses and produce a rapid antidepressant behavioral response was completely blocked in the BDNFVal66Met mice.25 Clinical studies have demonstrated that patients carrying the BDNFVal66Met allele have a significantly decreased response to ketamine; thus the Val66Met polymorphism serves as a genetic marker for ketamine treatment response.
Based on these findings, Duman and colleagues have also examined the role of mTOR signaling and synaptogenesis in the actions of scopolamine, another treatment that produces rapid antidepressant actions.19 The results thus far have demonstrated that a single dose of scopolamine also rapidly increases spine number and function in layer V neurons and increases mTOR signaling in the PFC. In addition, scopolamine produces rapid antidepressant actions in the forced swim test that are blocked by pretreatment with rapamycin. Preliminary evidence indicates that scopolamine also increases glutamate release in the PFC.
Together these findings suggest a common mechanism for the effects of rapid-acting antidepressants, including a burst of glutamate transmission that causes release of BDNF, stimulation of mTOR signaling, and increased spine number and function. The induction of spine synpases blocks or reverses the atrophy and loss of connections in cortical and limbic circuits caused by chronic stress, thereby causing reinstatement of normal circuit-connection control of mood and emotion.21
Targeting glutamatergic receptors
Jorge Quiroz (Roche) discussed work underway to develop antidepressant treatments targeting the glutamatergic pathway. Well-powered and adequately controlled studies have failed to demonstrate the efficacy of newer pharmacological interventions; this, in addition to high placebo-response ratios, has motivated a major withdrawal of the pharmaceutical industry from basic and clinical research in neuroscience. Despite this trend, the deepened understanding of mood disorder pathophysiology, including the better characterization of depression endophenotypes and the improvement of mechanistic and circuitry-based understanding of these diseases, has enabled investigational efforts beyond the classic monoaminergic approach for the treatment of major depression.
It is noteworthy that advances in the physiological understanding of the glutamatergic neurotransmitter system have demonstrated the modulatory controls over emotional processing and have therefore increased our capacity for neurobiological tractability in mood disorders. Quiroz presented the rationale for targeting the mGlu2 and mGlu5 receptors, which offer novel treatment approaches that address both the depressive symptomatology and the cognitive deficits associated with depression. Consequently, Roche is currently conducting two proof-of-concept studies (clinicaltrials.gov; NCT01483469) in major depressive disorder with mGlu negative allosteric modulators as adjunctive treatment in patients with inadequate response to SSRIs and SNRIs. These therapies are being developed for the treatment of depression with the hope of improving remission rates, speed of onset, and overall quality of life for patients suffering from these devastating diseases.
Magnesium for treatment-resistant unipolar depression
Guosong Liu (Tsinghua University) shifted the discussion from antidepressant drug development toward treatments focusing on the magnesium-depletion model of depression. Patients with major depressive disorder (MDD) express strong negative emotions such as anxiety, feelings of worthlessness, helplessness, and anhedonia, as well as reduction of executive functions such as difficulty in concentrating, remembering, or making decision. Currently available drugs that target monoaminergic systems have a delayed onset of action and significant limitations in efficacy. Several studies show that MDD patients have significant synapse loss in the PFC. Since the PFC is a brain region critical for cognitive abilities and emotional control, synapse loss in the PFC might underlie the reduction of cognitive abilities and dysfunction of emotional control. Promoting synaptogenesis in the PFC might become a novel therapeutic strategy for treating MDD.
Liu’s laboratory has been studying the principles governing synapse organization on the dendrite.26 One of their findings is that elevation of extracellular Mg2+ concentration promotes synaptogenesis and enhances synaptic plasticity.27 Mechanistically, they show that, in vitro, elevation of the extracellular concentration of Mg2+ can selectively reduce Ca2+ influx through NMDARs near resting membrane potential. This reduction of basal Ca2+ leads to a compensatory upregulation of NR2B-containing NMDARs, resulting in enhancement of synaptic plasticity.
To investigate the role of brain magnesium on synapse density and plasticity, Liu suggested that one needs to find a way to elevate brain Mg2+ effectively. Unfortunately, common magnesium compounds have poor bioavailability and, importantly, they fail to deliver magnesium to the brain in an efficient and safe manner. Taking on this challenge, Liu’s group, after extensive screening in rodents, identified Mg-L-threonate (Magtein™) as a bioavailable magnesium compound that, importantly, could efficiently elevate brain magnesium concentrations.
Having demonstrated that Magtein treatment effectively increases brain magnesium levels, Liu and colleagues studied the effects of elevated brain magnesium on synapse density and plasticity in brain regions critical for cognitive abilities and emotions. They found that Magtein treatment induces a unique regional-specific pattern of action, enhancing NMDAR signaling and synaptic plasticity in the PFC and hippocampus, but not in the basolateral amygdala. The increase in synapse density and plasticity in the PFC and hippocampus correlated with enhancement of learning and memory in young and aged rats.28,29 Interestingly, elevation of brain magnesium remain effective in preventing and reversing cognitive deficit in an Alzheimer’s disease mouse model.30 These results confirm that the level of brain magnesium is critical for cognitive functions.
Liu and colleagues have also studied the effects of elevation of brain magnesium on emotional control. Anxiety is one of major symptoms of MDD. In the clinic, cognitive therapy is effective in treating anxiety. However, many cases relapse or resist this therapy. Therefore, considerable research has been conducted to identify cognitive enhancers that promote extinction of fear memory. Fear memories are formed in the amygdala. Following extinction, fear expression is modulated by other brain regions such as the PFC and hippocampus. Therefore, enhancement of PFC and hippocampal functions without simultaneously enhancing or impairing amygdala functions would boost the efficacy of cognitive therapy for anxiety. Indeed, Magtein treatment enhances the retention of extinction and prevents spontaneous recovery and renewal of extinguished fear memories without enhancing, impairing, or erasing the amygdala-dependent fear memory.29
In animal models of depression-like behavior, Magtein treatment reduced the immobility time in the forced-swimming test (in a dosedependent manner) and reversed the escape deficits in a learned-helplessness paradigm. Furthermore, Magtein treatment reduced anxiety-like behavior in three models of anxiety: elevated plusmaze, novelty-suppressed feeding test, and open field.
Liu concluded by stating that if elevation of brain magnesium can enhance cognitive abilities and reduce anxiety and helplessness simultaneously, treatments that increase brain magnesium concentrations may offer a novel approach for treating depression with improved therapeutic benefits.
NMDA receptor over-reactivity as a model for unraveling biochemical pathways contributing to enhanced depression-related behavior
Simone Sartori (University of Innsbruck) further emphasized the suggestion that the glutamatergic pathways, in particular the NMDAR, may represent a novel approach to the treatment of depression, although the role of the NMDAR in the pathophysiology of affective disorders is not clear. In order to address this question, one strategy to mildly modulate central NMDAR activity is to target its voltage-dependent magnesium (Mg2+) block. For example, feeding mice a low Mg2+–containing diet (50–120 mg/kg food) for three weeks causes a reduction in brain and plasma Mg2+ levels compared with mice on a control diet;12,30,31 a similar condition was also reported in some, though not all, patients with depression.1 Although Mg2+-deficient animals appear normal (e.g., they have shiny fur and normal gain in body weight, circadian activity rhythm, and locomotor performances12), their body temperature increased.32 Mg2+-deficient mice display signs of behavioral despair, as indicated by high immobility times in the forced-swim and/or tail-suspension tests, and signs of anhedonia (Sah, Sartori, Singewald, unpublished), both of which are core symptoms of patients with depression. In addition, anxiety-related behavior is increased in Mg2+-deficient mice.12,32 The pro-depressive effects of dietary-induced hypomagnesemia are observed in several mouse strains with divergent levels of depression/anxiety-related behavior including the C57/Bl6, Balb/c, and CD-1 strains, as well as in rats. While these results have been replicated in different laboratories, the anxiogenic effects of hypomagnesemia in humans seem to be more variable, reflecting the clinical setting where a comorbid anxiety disorder is diagnosed in about 60% of depressed individuals. Chronic oral administration of antidepressant drugs (paroxetine, desipramine, or St. John’s Wort) normalizes the enhanced depression-and/or anxiety-related behavior displayed by Mg2+-deficient mice.12,31,32
Despite the ubiquitous distribution of Mg2+ in the brain, Sartori and colleagues observed stress-induced neuronal hyperactivation in the amygdala (Singewald et al., unpublished) and the paraventricular hypothalamic nucleus.32 Using unbiased proteomics, only four out of more than 300 soluble proteins analyzed were identified as altered in the amygdala following Mg2+-deficiency. Among these, changes in N,N–dimethylarginindimethylaminohydrolase 1 and manganese superoxide dismutase point toward the possibility of upregulated NMDAR/nitric oxide (NO) pathway signaling.31 Furthermore, preliminary data from Sartori’s group shows that the Mg2+-deficiency–induced enhanced depression-related behavior is blocked after a single application of the NMDAR antagonist ketamine, as well as in heterozygous neuronal nitric oxide synthase knockout mice (unpublished data). These findings indicate that a hyperactive NMDAR/NO signaling pathway plays a key role in mediating the behavioral effects of diet-induced Mg2+ deficiency. Sartori also reported that in Mg2+-deficient mice, increased transcription of the pre–pro-corticotropin releasing hormone in the paraventricular hypothalamic nucleus leads to increased release of the stress hormones ACTH32 and aldosterone, indicating dysfunction in the neuroendocrine stress axis that is known to control mood and emotions as well as the immune system. Correspondingly, immune responses have been shown to be altered in Mg2+-deficient rodents.1 Chronic antidepressant treatment normalizes the Mg2+-deficiency–induced dysfunctions in the neuroendocrine system and NMDAR/NO signaling pathways in parallel with the observed behavioral effects.12,31,32
Overall, rodents with dietary-induced Mg2+ deficiency highly reflect patients with depression in terms of deranged physiological homeostasis, behavior, neurobiology, and antidepressant treatment responses. Despite or even because of the multifunctional properties of Mg2+ in the brain,1 the Mg2+ deficiency is a model for studying neurobiological mechanisms beyond the monoaminergic systems leading from chronic (over-) activation of the NMDAR and stress pathways to enhanced depression- and anxiety-related behavior. This is pivotal knowledge for the development of novel pharmacotherapies. However, because no novel antidepressant with greatly improved pharmacological properties has been approved by the FDA in the last 30 years, refinement of drug development at early preclinical stages is recommended by using well-characterized model organisms with high face, construct, and predictive validity to the human disorder. Accordingly, the rodent Mg2+-deficiency model is clinically relevant for testing potential novel drug targets with fast-acting antidepressant properties. For an overview of the discussed effects, see Table 1.
Table 1.
Comparison of ketamine- and magnesium-induced changes with reference to those observed in major depression
| Major depression (human) | Magnesium administration | Ketamine | |
|---|---|---|---|
| PFC-NR1 | ⇔39 | ⇔26 | ⇑40 |
| PFC-NR2A | ⇓39 | ⇔26 | ⇔40 |
| PFC-NR2B | ⇓39 | ⇑26 | ⇑40,41 |
| Cortical (PFC)–GluR1 | ? | ? | ⇑24,42 (after CUS) |
| Cortical (PFC)–GluR2 | ⇑43 | ? | ? |
| PFC–BNDF | ? | ⇑26 | ⇑44 |
| PFC–PSD-95 | ⇓39 | ? | ⇑24 (after CUS) |
| Cortical | ⇓45,46 | ⇑47 | ⇑44,48 |
| P-CREB | ⇔26 | ||
| PFC/ACC–glutamine/GS activity | ⇓46 | ⇑49 | ⇑50 |
| Evoked synaptic potentials (cortex) | ? | ⇑26 | ⇑24 (after CUS) |
| Cortical synaptogenesis | ? | ⇑26 | ⇑24 (after CUS) |
Adapted with permission from Ref. 11. CUS, chronic unpredictable stress.
Conclusion
While the standard of care for people suffering from clinical depression has not changed significantly since the introduction of SSRIs and SNRIs decades ago, current research has explored novel paradigms with the potential to develop new treatments to address the unmet need in depression patients. This research has been facilitated by the development of biomarkers and technologies that signal drug effects or can be used to gauge patient responses. The rapid response observed in patients taking the muscarinic antagonist scopolamine and the glutamatergic antagonist ketamine revealed these pathways to be potent targets for antidepressant drug development. Glutamatergic antagonism reverses the atrophy and loss of connections in cortical and limbic circuits caused by chronic stress, reestablishing normal circuit-connection control of mood and emotion. While ongoing developmental research aims to develop optimal antagonists and allosteric inhibitors of the glutamatergic pathway, other work is exploring magnesium homeostasis in the brain: magnesium levels modulate glutamatergic activity, and low brain magnesium levels correspond to animal models of depression, while high levels increase synaptogenesis and synaptic plasticity. The synthesis of research exploring how modifying these systems changes the structure and function of neurons and pathways with development of treatments targeting these pathways offers hope for improving the speed of response, remission, and quality of life for patients suffering from depression.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest. J.Q. is employed by Roche, and H.M. is employed by Covance.
References
- 1.Eby GA, Eby KL, Murck H. Magnesium and major depression. In: Vink R, Nechifor M, editors. Magnesium in the Central Nervous System. Adelaide: University of Adelaide Press; 2011. pp. 313–330. [PubMed] [Google Scholar]
- 2.Vale S, Espejel MA, Dominguez JC. Amantadine in depression. Lancet. 1971;2:437. doi: 10.1016/s0140-6736(71)90153-x. [DOI] [PubMed] [Google Scholar]
- 3.Paul IA, Skolnick P. Glutamate and depression: clinical and preclinical studies. Ann. N. Y. Acad. Sci. 2003;1003:250–272. doi: 10.1196/annals.1300.016. [DOI] [PubMed] [Google Scholar]
- 4.Watkins JC, Jane DE. The glutamate story. Br. J. Pharmacol. 2006;147:S100–S108. doi: 10.1038/sj.bjp.0706444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am. J. Psychiatry. 1965;122:509–522. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- 6.Kirsch I, Moore TJ, Scoboria A, Nicholls SS. The emperors´s new drugs: an analysis of antidepressant medication data submitted to the U.S. food and drug administration. Prevention and Treatment. 2002;5:1–10. [Google Scholar]
- 7.Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am. J. Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 8.Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 9.Zarate CA, Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 10.Duman RS, Li N, Liu RJ, et al. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62:35–41. doi: 10.1016/j.neuropharm.2011.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murck H. Ketamine, magnesium and major depression – From pharmacology to pathophysiology and back. J. Psychiatr. Res. 2013;47:955–965. doi: 10.1016/j.jpsychires.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Singewald N, Sinner C, Hetzenauer A, et al. Magnesium-deficient diet alters depression- and anxietyrelated behavior in mice–influence of desipramine and Hypericum perforatum extract. Neuropharmacology. 2004;47:1189. doi: 10.1016/j.neuropharm.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 13.Miller G. Is pharma running out of brainy ideas? Science. 2010;329:502–504. doi: 10.1126/science.329.5991.502. [DOI] [PubMed] [Google Scholar]
- 14.Hyman S. Revolution stalled. Sci. Transl. Med. 2012;4:155. doi: 10.1126/scitranslmed.3003142. [DOI] [PubMed] [Google Scholar]
- 15.Institute of Medicine. Neuroscience Biomarkers and Biosignatures: Converging Technologies, Emerging Partnerships, Workshop Summary. Washington (DC): 2008. [PubMed] [Google Scholar]
- 16.Zarate CA, Jr, Brutsche NE, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol. Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zarate CA, Jr, Mathews DC, Furey ML. Human biomarkers of rapid antidepressant effects. Biol. Psychiatry. 2013;73:1142–1155. doi: 10.1016/j.biopsych.2012.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salvadore G, Cornwell BR, et al. Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology. 2010;35:1415–1422. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furey ML, Drevets WC, et al. Potential of pretreatment neural activity in the visual cortex during emotional processing to predict treatment response to scopolamine in major depressive disorder. JAMA Psychiatry. 2013;70:280–290. doi: 10.1001/2013.jamapsychiatry.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voleti A, Navarria A, et al. Scopolamine rapidly increases mammalian target of rapamycin complex 1 signaling, synaptogenesis, and antidepressant behavior response. Biol. Psychiatry. in press doi: 10.1016/j.biopsych.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: Novel therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J. Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li N, Liu RJ, Dwyer JM, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol. Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu RJ, Lee FS, Li X-Y, et al. BDNF Met allele decreases the density and function of spine/synapses in the prefrontal cortex and blocks the effects of ketamine. Biological Psychiatry. 2012;71:996–1005. doi: 10.1016/j.biopsych.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abumaria N, Yin B, Zhang L, et al. Effects of elevation of brain magnesium on fear conditioning, fear extinction, and synaptic plasticity in the infralimbic prefrontal cortex and lateral amygdala. J. Neurosci. 2011;31:14871–14881. doi: 10.1523/JNEUROSCI.3782-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li W, Yu J, Liu Y, et al. Elevation of brain magnesium prevents and reverses cognitive deficits and synaptic loss in Alzheimer’s disease mouse model. J. Neurosci. 2013;33:8423–8441. doi: 10.1523/JNEUROSCI.4610-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.Liu G. Local structural balance and functional interaction of excitatory and inhibitory synapses in hippocampal dendrites. Nat. Neurosci. 2004;7:373–379. doi: 10.1038/nn1206. [DOI] [PubMed] [Google Scholar]
- 29.Slutsky I, Sadeghpour S, Li B, Liu G. Enhancement of synaptic plasticity through chronically reduced Ca(2+) flux during uncorrelated activity. Neuron. 2004;44:835–849. doi: 10.1016/j.neuron.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Whittle N, et al. Changes in brain protein expression are linked to magnesium restriction-induced depression-like behavior. Amino Acids. 2011;40:1231–1248. doi: 10.1007/s00726-010-0758-1. [DOI] [PubMed] [Google Scholar]
- 31.Eby GA, Eby KL, Murck H. Magnesium and major depression. In: Vink R, Nechifor M, editors. Magnesium in the Central Nervous System. Adelaide: University of Adelaide Press; 2011. pp. 303–312. [PubMed] [Google Scholar]
- 32.Sartori SB, et al. Magnesium deficiency induces anxiety and HPA axis dysregulation: modulation by therapeutic drug treatment. Neuropharmacology. 2012;62:304–312. doi: 10.1016/j.neuropharm.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiong H, Futamura T, Jourdi H, et al. Neurotrophins induce BDNF expression through the glutamate receptor pathway in neocortical neurons. Neuropharmacology. 2002;42:903–912. doi: 10.1016/s0028-3908(02)00043-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fortin DA, Srivastava T, Dwarakanath D, et al. Brain-derived neurotrophic factor activation of CaM-kinase kinase via transient receptor potential canonical channels induces the translation and synaptic incorporation of GluA1-containing calcium-permeable AMPA receptors. J. Neurosci. 2012;32:8127–8137. doi: 10.1523/JNEUROSCI.6034-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magistretti PJ. Role of glutamate in neuronglia metabolic coupling. Am. J. Clin. Nutr. 2009;90:875S–880S. doi: 10.3945/ajcn.2009.27462CC. [DOI] [PubMed] [Google Scholar]
- 36.Muller A, Gunzel D, Schlue WR. Activation of AMPA/kainate receptors but not acetylcholine receptors causes Mg2+ influx into Retzius neurones of the leech Hirudo medicinalis. J. Gen. Physiol. 2003;122:727–739. doi: 10.1085/jgp.200308851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greenberg J, Lichtenstein N. Effect of manganous and magnesium ions concentration on glutamine synthetase and glutamotransferase of sheep brain. J. Biol. Chem. 1959;234:2337–2339. [PubMed] [Google Scholar]
- 38.Maurizi MR, Pinkofsky HB, McFarland PJ, Ginsburg A. Mg2+ is bound to glutamine synthetase extracted from bovine or ovine brain in the presence of L-methionine-S-sulfoximine phosphate. Arch. Biochem. Biophys. 1986;246:494–500. doi: 10.1016/0003-9861(86)90496-0. [DOI] [PubMed] [Google Scholar]
- 39.Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33:70–75. doi: 10.1016/j.pnpbp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chatterjee M, Verma R, Ganguly S, Palit G. Neurochemical and molecular characterization of ketamineinduced experimental psychosis model in mice. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.05.041. [DOI] [PubMed] [Google Scholar]
- 41.Burgdorf J, Zhang XL, Nicholson KL, et al. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology. 2013;38:729–742. doi: 10.1038/npp.2012.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamada S, Yamamoto M, Ozawa H, et al. Reduced phosphorylation of cyclic AMP-responsive element binding protein in the postmortem orbitofrontal cortex of patients with major depressive disorder. J. Neural Transm. 2003;110:671. doi: 10.1007/s00702-002-0810-8. [DOI] [PubMed] [Google Scholar]
- 43.Teyssier JR, Ragot S, Chauvet JC, Gelinier, et al. Activation of a DeltaFOSB dependent gene expression pattern in the dorsolateral prefrontal cortex of patients with major depressive disorder. J. Affect Disord. 2011;133:174–178. doi: 10.1016/j.jad.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 44.Reus GZ, Stringari RB, Ribeiro KF, et al. Ketamine plus imipramine treatment induces antidepressant-like behavior and increases CREB and BDNF protein levels and PKA and PKC phosphorylation in rat brain. Behav. Brain Res. 2011;221:166–171. doi: 10.1016/j.bbr.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 45.Dwivedi Y, Rao JS, Rizavi HS, et al. Abnormal expression and functional characteristics of cyclic adenosine monophosphate response element binding protein in postmortem brain of suicide subjects. Arch. Gen. Psychiatry. 2003;60:273–282. doi: 10.1001/archpsyc.60.3.273. [DOI] [PubMed] [Google Scholar]
- 46.Choudary PV, Molnar M, Evans SJ, et al. Altered cortical glutamatergic and GABAergic signal transmission with glial involvement in depression. Proc. Natl. Acad. Sci. USA. 2005;102:15653–15658. doi: 10.1073/pnas.0507901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang CY, Liou YF, Chung SY, et al. Role of ERK signaling in the neuroprotective efficacy of magnesium sulfate treatment during focal cerebral ischemia in the gerbil cortex. Chin. J. Physiol. 2010;53:299–309. doi: 10.4077/cjp.2010.amk063. [DOI] [PubMed] [Google Scholar]
- 48.Shu L, Li T, Han S, et al. Inhibition of neuronspecific CREB dephosphorylation is involved in propofol and ketamine-induced neuroprotection against cerebral ischemic injuries of mice. Neurochem. Res. 2012;37:49–58. doi: 10.1007/s11064-011-0582-3. [DOI] [PubMed] [Google Scholar]
- 49.Maurizi MR, Pinkofsky HB, McFarland PJ, Ginsburg A. Mg2+ is bound to glutamine synthetase extracted from bovine or ovine brain in the presence of L-methionine-S-sulfoximine phosphate. Arch. Biochem. Biophys. 1986;246:494–500. doi: 10.1016/0003-9861(86)90496-0. [DOI] [PubMed] [Google Scholar]
- 50.Rowland LM, Bustillo JR, Mullins PG, et al. Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. Am. J. Psychiatry. 2005;162:394–396. doi: 10.1176/appi.ajp.162.2.394. [DOI] [PubMed] [Google Scholar]