Abstract
Use of soluble signals for modulation of bone formation has become a significant clinical market in recent years. Improvements in implant site preparation and osseointegration have already been achieved with the use of recombinant PDGF and BMP on osteogenic scaffolds. Other states of insufficient bone such as osteoporosis are widely treated with inhibitors of osteoclast function or osteoblast anabolic agents. However, despite promising therapies targeting the osteoblast and osteoclast directly, therapies utilizing indirect regulation through secondary cellular nodes of control (NOC) are just beginning to emerge. This article will review current strategies for regulation of bone formation by targeting two primary NOCs, the osteoblast and osteoclast, as well as four secondary NOCs, the vascular, hematopoietic, mesenchymal and neural.
Keywords: tissue engineering, bone, dental implant, regeneration, cell signaling, growth factors, differentiation
CURRENT STATE OF THE FIELD
Significant advancements in the fields of tooth replacement and bone tissue engineering have been made since the first characterization of the bone morphogenetic family of proteins (BMPs) in 1965 (1). This discovery coincides with the placement of the first human titanium root-form dental implant that had significant research to support its use (2). Though they originated at the same time, the first publications suggesting combined use of soluble factors such as BMPs and dental implants did not occur until 28 years later in 1993 (3). Even without additional osteogenic factors, the success of osseointegrated titanium implants has reached levels of 95.6–97.5% in settings where the majority (up to 83.5%) of patients are medically compromised, active, or former smokers (4, 5). For those who are candidates for dental implants, this success rate is extraordinary. As such, technological advancements to bring implant therapy to those previously excluded as candidates for reasons related to insufficient bone quality or volume are desired. This article will address current therapeutics designed to address osseous deficiencies and outline novel strategies that may prove beneficial for future implant therapeutics.
INTRODUCTION TO THE BONE MARROW
Largely due to the use of marrow as a food source, it has long been recognized that bone marrow composition varies by site with the more red marrow being confined toward the middle of the animal and the yellow, or fatty, marrow existing toward the periphery. Indeed, the initial inclusion of meat into the hominid diet 2.6 million years ago was inferred due to evidence of bone marrow removal shown by impact fractures from stone tools on sections of ungulate limb bones (6). We have since learned that the primary function of the adult bone marrow is blood cell formation, or hematopoiesis, and in 1968 the transplantation of bone marrow to rescue patients with serious illnesses such as leukemia was realized (7, 8). The forty years following this first successful bone marrow transplant have been spent uncovering and characterizing the many cell types and signaling molecules present in this complex microenvironment. Application of this knowledge to the regeneration of the marrow’s supporting osseous and cartilaginous structures is a primary goal of the field of bone tissue engineering.
To emphasize the complexity of this challenge, it is now appreciated that over thirty distinct cell populations reside in close proximity to osteoblasts and osteoclasts (9, 10). These include hematopoietic lineage cells, mesenchymal lineage cells, blood vessels, and neural tissue. Each of these cell populations, alone or in combination, possesses the capacity to influence bone growth and regeneration. If we consider that the sequence of signaling between cells can vary (e.g., B cell → T cell → Osteoblast vs T cell → B cell → Osteoblast) there exist at minimum 2.65*1032 unique sequences of cells/signals that could potentially regulate bone formation (often with redundancies in signaling pathways). This is without even considering the important signaling role of the extracellular matrix in this environment. Though overwhelming, this complexity reaffirms the need to continue our exploration with an open mind.
THE OSTEOBLAST
Current strategies for induction of bone formation at implant sites revolve around two primary ‘nodes of control’ (NOC) in the bone marrow microenvironment. The first node encompasses modulation of the osteoblast. Osteoblasts are specialized cells that reside on the bone surface and are responsible for production and mineralization of bone matrix. Osteoblasts are derived from mesenchymal stem cells (MSCs) when differentiation is induced and transcription factors such as Runx2 are activated (11). Increased bone formation can be achieved by (1) increasing the activity of mature osteoblasts, (2) increasing the number of osteoblast precursor cells, (3) stimulating differentiation of new cells from osteoblast precursors, and (4) inhibiting osteoblast cell death.
Modulation of osteoblast formation and function is currently the most exploited strategy for bone tissue engineering and represents a prime target for augmenting osseointegration. Clinicians can now offer BMP-2 laced collagen sponges to their patients as an alternative grafting protocol for approximately $870 per 0.5cc (Medtronic, INFUSE® Bone Graft). Though not yet available to dentists, BMP-7, referred to as osteogenic protein one (OP-1®), has also been approved under the humanitarian device exemption by the FDA as of October 17th 2001 (Stryker, OP-1®). Currently, the cost is $5000 per 3.3mg dose to be applied for spinal fusion or fracture repair when an autograft is not feasible and other treatments have failed. BMPs are the prototypical bone inducing soluble factors that were first identified as present within the bone matrix. As early as 1889 it was shown that decalcified bone could be used to heal lesions due to osteomyelitis (12). Forty-nine years later, studies revealed that intramuscular injection of an acid-alcohol extract of bone could induce a mix of bone and cartilage in rabbits (13, 14). And now, seventy-two years later, BMP family members have been identified (1), cloned (15, 16), synthetically-produced (17), and delivered to patients to enhance periodontal tissue regeneration. When administered locally to a bone defect, BMP-2 and BMP-7 can induce the differentiation of progenitor cells into chondrocytes and osteoblasts and enhance bone formation (18–20). There are currently two ongoing clinical trials testing localized alveolar ridge augmentation with the BMP-2 INFUSE® bone graft for implant placement both at the time of the graft and six months after graft placement (21). The expected completion date for these studies is December 2010.
Growth and differentiation factor 5 (GDF-5) is closely related to BMP and is also a member of the transforming growth factor-beta (TGF-β) superfamily of proteins. GDF-5 stimulates limb mesenchyme aggregation and chondrogenesis and regulates skeletal development (22). A human recombinant form of GDF-5 coated onto beta-tricalcium phosphate (β-TCP) (Scil Technology GmbH, MD05®) is undergoing clinical trials as an osteoinductive and osteoconductive bone graft material for use in dental and maxillofacial applications (21, 23). Results in the rat calvarial defect model demonstrated a five-fold enhancement in bone histomorphometrical parameters with GDF-5/β-TCP over β-TCP alone (23).
In addition to BMPs, there are a variety of growth factors that are being pursued as pro-osteogenic agents. Platelet derived growth factor (PDGF) on β-TCP (Osteohealth, GEM21S®) can be purchased for $299 per 0.5cc and is advertised for use in periodontal, furcation, and intrabony defects. PDGF is known to stimulate bone cell growth, promote division of precursor cells, and increase type I collagen synthesis by osteoblasts (24). In addition to clinical trials for bone formation, administration of PDGF in protein or adenoviral vector (gene therapy) form at the time of implant placement enhances osseointegration in rats (25). Fibroblast growth factor (FGF), like PDGF, can increase cell division of osteoblast precursors and other cells and enhance local bone formation. However, overexpression of FGF-2 in mice causes osteopenia which emphasizes the need to selectively regulate dose and site selection (26). The main advantage of mitogenic factors such as PDGF and FGF is that after increasing precursor cell numbers the body can then take over and regenerate structures in a way that we can not yet mimic in the laboratory environment. Unlike predominantly bone forming BMPs, local application of FGF-2 has facilitated regeneration of new periodontal ligament, cementum, and bone simultaneously in canine periodontal defects (27). The drug Trafermin® (Kaken Pharmaceutical), a human recombinant FGF-2 protein, is currently in phase III clinical trials for periodontal tissue regeneration (21). In addition, FGF coated onto the surface of titanium implants in a calcium phosphate matrix can enhance osseointegration in animal models and a slowly-degrading FGF-gelatin hydrogel complex for repair of fenestrated implants has been successful in dogs (28, 29).
Though not yet used in dental applications, injection of anabolic parathyroid hormone (PTH) is widely used as a treatment for osteoporosis. Recombinant versions of the N-terminal segment (Eli Lilly, Forteo®) or full-length recombinant protein (NPS Pharmaceuticals, Preos®) have been tested in humans. However, only Forteo® is currently approved for use by the United States FDA due to unresolved concerns about development of hypercalcemia with Preos®. Anabolic PTH has multiple actions that include enhancement of osteoblast proliferation and differentiation, increased engraftment of hematopoietic stem cells (HSCs) in new bone, and induction of bone formation (30, 31). Studies with ectopic ossicles in mice suggest that anabolic PTH (N-terminal segment) can significantly enhance formation of new bone with daily subcutaneous injection (32). Phase I clinical trials testing Forteo® for augmentation of regeneration after periodontal surgery have been completed with results anticipated to be published in 2010.
Insulin-like growth factor (IGF) has two identified isoforms (IGF-I and –II) that are the most abundant growth factors in bone tissue (26). IGF-I has a slight mitogenic effect on osteoblast precursors and a significant anti-apoptotic function that helps to increase the total osteoblast pool without dramatically regulating differentiation (26). IGF-I can also enhance osteoblast synthesis of collagen I (33). However, this effect is balanced by increases in RANKL production and modulation of osteoclastogenesis (34). Our basic understanding of IGF-I function and its diverse systemic effects have limited its use for bone tissue engineering, however, human recombinant IGF-I has been available since 1986 and was approved for use in the US in 2005 to treat IGF-I deficiency (35). Clinical trials to treat children of short stature associated with IGF-I deficiency have demonstrated mild improvement in height with once daily subcutaneous injection of 60 μg/kg IGF-I (Tercica, Mecasermin) over a period of 86 weeks (21, 35). Production of IGF by the liver is regulated by growth hormone (GH) secreted by the pituitary, and lack of GH significantly impairs skeletal development (36). Systemic GH has effects on many tissues, including induction of osteoblast and osteoclast activity and subsequent increases of bone remodeling (36). Use of recombinant GH to augment fracture healing has been proposed since the 1950s (37). Recent completion of a human clinical trial revealed a 26% decrease in closed tibial fracture healing time with daily injection of 60ug/kg GH (Novo Nordisk, Norditropin® SimpleXx®) (38). GH did not significantly alter healing time of open fractures, or closed fractures at doses less than 60ug/kg (38). Successful use of IGF-I or GH alone for bone tissue engineering or periodontal regeneration is unlikely, but may prove useful in combination with other factors.
THE OSTEOCLAST
The second primary NOC that is highly targeted in osteoporosis therapies, but rarely utilized for bone tissue engineering, involves treatment to decrease both the activity and formation of osteoclasts. Bisphosphonates such as zoledronic acid are prototypical anti-resorptive agents and have a half-life in bone potentially exceeding 10 years (39). Bisphosphonates block the cytoskeletal organization of osteoclasts, inhibit formation of resorption pits, and induce osteoclast apoptosis (40, 41). Bisphosphonates are used in the treatment of osteoporosis, bone metastasis, multiple myeloma, and Paget’s disease. Initial treatment allows osteoblasts to continue to function resulting in a net gain in bone volume. However, after approximately two years for many of the bisphosphonates, the osteoblast activity decreases due to lack of feedback signals from the osteoclast resorption pits (42). In healing bone, such as during fracture repair, uncoupling of bone formation and resorption is undesirable since activity of the osteoclast is necessary to properly remodel the bone and direct osteoblast activity at the site of injury. However, use of anti-resorptives for temporal regulation of osteoclast remodeling may prove beneficial for future periodontal tissue engineering and implant therapy.
Newer medications are being designed to inhibit bone resorption without the untoward effect of decreasing osteoblast activity. Upon the observation that bone resorption increases during fasting, including at night on a circadian rhythm, administration of glucagon-like peptide 2 (GLP-2) was tested for treatment of osteoporosis (43). GLP-2 is a 33 amino acid peptide produced by the intestine in response to food intake that has been assessed as a treatment for short bowel syndrome, Crohn’s disease, and osteoporosis. Daily subcutaneous injection of GLP-2 before bedtime in post-menopausal women decreased serum markers of osteoclast activity for up to eight hours without a change in markers of osteoblast function (43). At the lowest effective dose of GLP-2 there was no increase in adverse side effects over the placebo control (43). A second method involves inhibition of cathepsin K (CatK), a protein expressed in lysosomes of osteoclasts that degrades type I collagen after acidification of the resorption pit (42). Administration of a CatK inhibitior (Merck, Odanacatib®) weekly for two years in post-menopausal women resulted in a 5.5% BMD increase in the lumbar spine without dose-related trends in adverse events (44). This increase is similar to the 4.3–5.1% BMD increase achieved with the bisphosphonate zoledronic acid in a similar post-menopausal population but may not have the extended uncoupling effects due to the long half-life of the bisphosphonate (45). In addition, in mice CatK inhibitors were successfully used in combination with anabolic PTH suggesting the potential for combination therapy. This may be considered for clinical trials in regenerative medicine and dental implant therapy in anatomic locations with less than adequate bone quality and volume.
SECONDARY NODES OF CONTROL
When we move beyond the osteoblast and osteoclast it readily becomes apparent that there are many other cell types and signaling pathways in the bone marrow microenvironment that may be considered to enhance bone formation. Most factors that regulate osteoblast cell function also have effects on surrounding populations such as vascular endothelial cells, hematopoietic lineages, mesenchymal lineages, and neural cells. Thus, four secondary NOCs should be considered for future therapeutic advantage: the vascular, the hematopoietic, the mesenchymal, and the neural.
Vascular Node of Control
To maximize formation of new bone around implant sites, the cells must receive a steady nutritional supply as well as have access to a conduit to remove metabolic waste from the actively healing wound. These processes require establishment of a vascular bed in close contact with bone to maintain skeletal integrity, a concept that has been recognized since the 1700s (46). In 1963 it was proposed that a vascular stimulating factor (VSF) was released at osseous fracture sites (47). It is now understood that the main regulators of new vessel formation include vascular endothelial growth factor (VEGF), basic FGF, hypoxia-inducible transcription factor (HIF), PDGF, IGF-I/II, and angiopoietin (46). VEGFs are thought to be the main regulators of angiogenesis and VEGF in plasmid or protein form has been tested in clinical trials for treatment of peripheral artery disease, limb ischemia, chronic diabetic foot ulcers, and myocardial ischemia (21). Crosstalk between VEGF and HIF producing osteoblasts and surrounding endothelial cells is critical for coupling angiogenesis and osteogenesis during bone formation (48, 49). Reciprocal studies establish that endothelial cells have the ability to modulate osteoblast differentiation and enhance bone formation (50). VEGF produced by osteoblasts can upregulate BMP-2 in microvascular endothelial cells emphasizing the close relationship between these two cell types (51, 52). Release of VEGF alone or in combination with BMP-4 from biomimetic scaffolds can significantly enhance bone regeneration in rodent models (53, 54). Despite success, VEGF therapy has not yet been applied in human clinical trials for bone regeneration. FGF-2 and PDGF, discussed above, are also capable of stimulating angiogenesis in addition to their pro-osteogenic effects (55, 56).
Hematopoietic Node of Control
Materials such as titanium and β-TCP are carefully screened for biocompatibility and developed using good manufacturing practice before being used in humans. Thus, at its most basic level, modulation of the immune response is essential for successful engraftment of foreign material or tissue into the body. However, direct regulation of the blood cells of the marrow may provide additional benefits to bone formation if we can determine the proper signals. One of the reasons BMPs are pro-osteogenic is that they help to maintain the hematopoietic stem cell (HSC) niche and establish a fully functional marrow cavity in newly formed bone (57). PTH was also able to regulate HSC recruitment to newly formed bone in an ectopic ossicle model in mice (31).
Mature lineages derived from the HSC also play a significant role in bone homeostasis. The recently termed osteal tissue macrophages or ‘OsteoMacs’ are the resident macrophage population of the bone marrow. Studies both in vitro and in vivo have shown that depletion of this population dramatically impairs osteoblast function and can cause complete loss of osteoblast bone-forming surface (58). Implant placement is followed by a robust inflammatory response in which macrophages help to guide osseointegration (59). However, in cases where the inflammation becomes chronic, implant loss may occur. Temporal regulation of the inflammatory healing response with implant surface modification, growth factors, and cytokines may allow facilitated increases in healing rate and bone formation (59).
A common issue in dental treatment is the presence of infection. It is well appreciated that the success rate of a bone graft or osseointegration of a dental implant will decrease if an infection is present. Indeed, preliminary evidence shows that immunocompromised mice which lack both T cells and B cells have enhanced fracture healing (60). Additional research in the field of osteoimmunology has uncovered that T cells are potent regulators of osteoclastogenesis via their expression of the pro-osteoclastogenic factor Receptor Activator of Nuclear Factor κB Ligand (RANKL) (61). The presence of inflammation increases the local concentration of inflammatory cytokines such as TNF-α. Infliximab (Centocor, Remicade®), a monoclonal antibody against TNF-α, has been approved by the US FDA for treatment of psoriatic and rheumatoid arthritis and effectively reduces bone destruction in inflamed joints (61). Etanercept (Amgen/Wyeth, Enbrel®), a fusion protein inhibitor of TNF is also clinically available with similar prescribing indications. Targeting the mechanisms behind these hematopoietic-osseous interactions may provide new tools to deal with infection-induced immune responses at the implant site.
Mesenchymal Node of Control
Though modulation of osteoblast function falls under this NOC, the function of three other mesenchymal lineage cells should be considered when engineering bone. The first is the adipocyte. As mentioned above, the number of fat cells present in the marrow at any one period of time varies depending on area of the skeleton, age of the host, obesity, or disease state. The mandible and maxilla generally contain very little adipose tissue, however, manipulation of this area with soluble factors or radiation can significantly change the ratio of red to yellow marrow. For example, irradiation of the bone at a dose designed to prepare the host for a bone marrow transplant results in a transient filling of the bone cavity with adipose tissue for one to two weeks (62). Blocking fat accumulation with a small molecule inhibitior of peroxisome proliferator-activated receptor-γ (PPAR-γ), a transcription factor required for adipocyte differentiation, significantly enhances engraftment of new HSCs as well as trabecular bone formation (62).
The chondrocyte is a mesenchymal derivative that plays a major role in formation of bone during endochrondral ossification, a mechanism that is used during development of bones excluding the clavicle and most bones of the skull. Regardless of developmental site, this process of forming bone with a cartilage intermediate is in some cases recapitulated during bone tissue engineering by addition of factors such as BMP-2 (63). Endochondral ossification begins with recruitment and differentiation of MSCs into chondroblasts. This is followed by chondroblast proliferation and organization over the first 1–3 weeks. The calcifying hypertrophic chondrocytes are then removed and subsequent osteoblast recruitment and mineralization results in new bone formation (64). This process allows relatively rapid establishment of a soft callus that is subsequently replaced by woven bone. Since cartilage is naturally an avascular tissue, factors that induce endochondral ossification instead of osteoblast stimulation directly may help keep the bioengineered graft viable while angiogenesis occurs.
Osteocytes are a third MSC derived lineage that are embedded into the bone matrix and communicate via an extensive canalicular network of cell processes. Osteocytes are able to respond to changes in force in the external environment by changing the activity of the Wnt/β-catenin pathway, this then signals to the bone surface cells to regulate bone formation (65). Without knowing the mechanism, we have long recognized that repeated loading of bone through regular weight bearing activity helps to maintain and increase bone density. Though the jaws are not as accessible for loading protocols as the limbs, newer technologies are seeking to stimulate cells of the marrow with ultrasound and pulsed electromagnetic fields (PEMF). In human clinical trials low-intensity pulsed ultrasound has enhanced repair and regeneration of fractured bone by up to 40% (66) and extension of ultrasound’s anabolic effects has also been proposed for tooth repair (67). The exact mechanism behind these effects is unclear, but direct regulation of precursor cell differentiation and expression of osteocalcin, VEGF, and the integrin family of mechanoreceptors may play a role (see (66) for review).
Neural Node of Control
Neural regulation of bone formation has been inferred for multiple decades due to observations of osteopenia secondary to neural disorders including reflex sympathetic dystrophy syndrome, stroke, spinal cord injury, and peripheral neuropathy (68). Central regulation of calcium and phosphate serum levels requires neural control of bone remodeling that is at least party regulated through hypothalamic signal integration and sympathetic output (68). Hypothalamic regulators of BMD include leptin, neuropeptide Y, cannabinoid receptors, cocaine and amphetamine-regulated transcript (CART), and melanocortin 4 receptor (see (68) for review). Direct targeting of hypothalamic centers for tissue engineering may seem a subject of the distant future, but indirect targets are already under investigation. As discussed above, GLP-2 is being tested as a novel therapy for osteoporosis. GLP-2 functions by dampening normal circadian rhythm induced night-time bone remodeling (43). β-blockers which inhibit neurons downstream of the hypothalamus are commonly used to treat high blood pressure and migraine headaches. However, administration of β-blockers such as propranolol can additionally increase bone formation and inhibit resorption in rodent models (68, 69) implying that β-blockers may have previously unrealized therapeutic value for bone formation.
FUTURE DIRECTIONS
Though osteogenic factor saturated scaffolds have proven to be useful, they are restricted to local actions on surrounding cells. In situations where the site lacks sufficient stem and progenitor cells such as with extensive trauma, radiation therapy or advanced age, recruitment of stem cells from remote sites to aid regeneration and repair would be beneficial. This would mimic adding cells that have been previously harvested from the patient and expanded in vitro or augmenting with tissue from another site, but eliminates the need for extended culture time or additional surgical manipulation. Two human clinical trials have applied this concept to the treatment of ischemic heart disease. The first trial examined the ability of VEGF and granulocyte colony stimulating factor (G-CSF) to mobilize stem cells from the bone for repair of heart muscle. The second tested the ability of GH to mobilize endothelial progenitor cells. VEGF gene transfer and recombinant G-CSF in combination increased the number of circulating CD34+ progenitor cells nearly 10-fold, but this did not improve myocardial perfusion after three months (70). Growth hormone increased the number of circulating endothelial progenitors by 1.5-fold without a change in CD34+ cells, functional significance was not analyzed (71).
These clinical trials have successfully forced progenitor cells into the circulation, but the circulating cells are not yet receiving the molecular instructions necessary to perform the desired function. Co-administration of an instructing factor may overcome this barrier and enhance treatment. The concept of combination therapy is certainly important. For example, since PDGF has agonist effects both on the osteoblast and the osteoclast, combination therapy with an anti-resorptive bisphosphonate increases bone density two-fold over PDGF alone when administered systemically to rats (24). Another popular combination pairs a cell mitogen, such as FGF or PDGF, with an anabolic agent such as BMP. Basic FGF and BMP-2 combined therapy significantly increases bone formation and osseointegration of dental implants placed in rabbits when compared to BMP-2 alone after four weeks of healing (72). Another strategy involves enhancing the actions of osteogenic factors that we know work well, such as BMP. For example, there are at least ten characterized secreted BMP antagonists (see (26) for review). Administration of BMP protein with a corresponding block to its signaling antagonist could both reduce the required dose of BMP and increase its effects at the local site. Along these same lines of inhibiting the inhibitors, human neutralizing antibodies to sclerostin, a pro-osteogenic Wnt signaling antagonist have gone through phase I clinical trials and can increase BMD in humans (26). A second trial is anticipated to start in May 2010 (21). Lastly, in mice, CatK inhibitors were successfully used in combination with anabolic PTH suggesting the potential for future combination therapy (73).
In addition to re-creating native proteins, small molecule inhibitors and biomimetic scaffolds are viable alternatives that may reduce costs due to elimination of the need for biological synthesis and protein folding in a host cell. A small molecule antagonist of secreted frizzled receptor-1 has been reported to enhance Wnt signaling and bone formation (74). Bisphenol A diglycidyl ether (Tocris Bioscience, BADGE) is a PPARy antagonist that when administered after radiation therapy in mice can enhance engraftment of new marrow and subsequent bone formation (62). See (75) for a review of scaffold design strategies and use for tissue engineering.
Developing a new technology for clinical use requires a significant amount of time, but of the soluble signals already discussed, several may be available in the next five years. FGF-2 is beginning phase III clinical trials for periodontal tissue regeneration, successful completion of these studies will allow the company to seek FDA approval for clinical marketing. Anabolic PTH therapy, though only in Phase I trials for dental use, may move toward clinical use rapidly if successful since the drug Forteo® is already widely used for osteoporosis. Combination therapies pairing an already approved osteogenic factor (e.g. BMP) with a cell mitogen (e.g. PDGF, FGF-2) may also become popular. Lastly, due to robust success in animals and preliminary clinical trials the approach to soluble factor delivery may begin to shift to genetic regulation of factor production. This would potentially allow for increased local concentrations of factor to be produced for a longer period of time. See (76) for a review of gene therapy in craniofacial applications. Myocardial injection of VEGF165 plasmid has successfully passed safety standards in human Phase II clinical trials (70) and though significant clinical improvement was not achieved with this application – animal studies suggest that VEGF gene therapy may allow for significant augmentation of periodontal tissue regeneration (53).
One main challenge of regenerative medicine is to be able to replace normal tissue at a specific location without toxic or other negative side effects. Activation of the Wnt pathway is a popular target for osteoblast differentiation, however, over-activation of canonical Wnt signaling through β-catenin has been linked to tumor formation in extra-skeletal tissues (77). As a second example, development of the CatK inhibitor balicatib was suspended due to an unexpected incidence of skin reactions and other side effects (42). In addition to safety concerns, costs and insurance coverage drive clinical use of dental therapeutics. At $299 per 0.5cc PDGF (GEM21S®) is the most reasonably priced, use of BMP-2 (INFUSE®) grafts is more expensive at $870 per 0.5cc but still manageable in some circumstances. Injectable agents like Forteo® and Remicade® can cost up to $900–1500 per month and may require multiple months of use but in the future costs may be mitigated by health insurance coverage. Despite the required safety profile and costs involved, there are seemingly limitless possibilities for protein, small molecule, and scaffold combinations to enhance bone formation and implant osseointegration. Optimization and individual customization of these therapies will require the combined effort and imagination of many investigators to best satisfy the needs of those that require implant therapy.
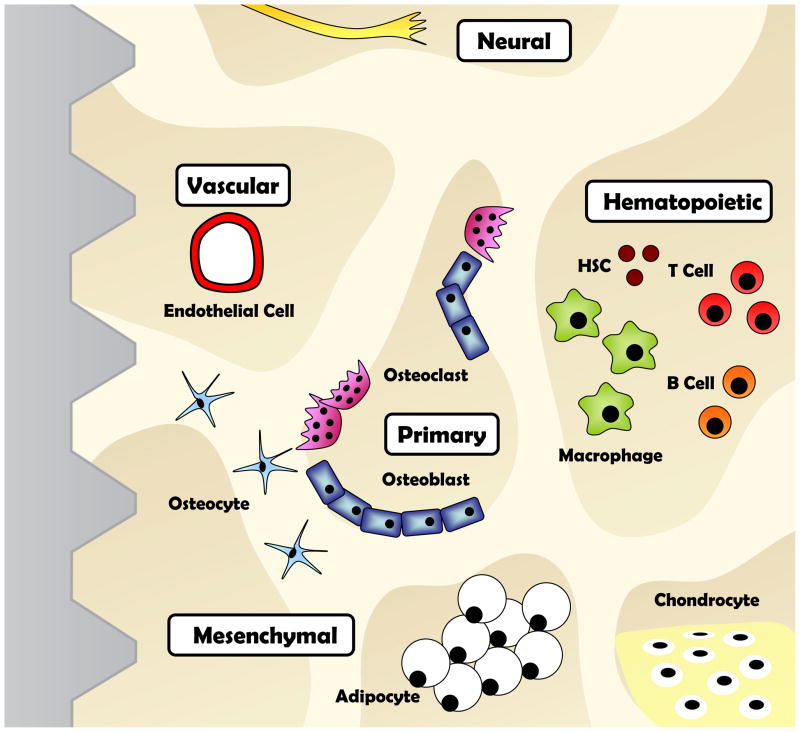
Figure 1. Theoretical Diagram of the Marrow Space Adjacent to an Implant.
The surface of an implant is surrounded by a complex bone marrow microenvironment. Over thirty unique cell types exist in a complex matrix that can regulate bone formation. A subset of these cells are depicted here with emphasis on the two primary nodes of control, the osteoblast and osteoclast, and the four secondary nodes of control, the vascular, hematopoietic, mesenchymal, and neural.
Table 1. Summary of Clinical Development of Recombinant Proteins.
Major anabolic therapies undergoing testing for medical and dental use. Phase I–III indicates level of human clinical trials currently underway for the indicated use (21).
| Signal | Summary of Action | Clinical Status |
|---|---|---|
| BMP-2 | MSC Survival and differentiation (Edgar 2007, Solmesky 2009) Initiation of Fracture Repair (Tsuji 2006) Endochrondal ossification (Sasano 1997) Maintenance of the HSC niche (Zhang 2003) |
Medtronic, INFUSE® Available for Dental Use |
| BMP-7 | Differentiation of MSCs (Asahina 1996) Activity of mature osteoblasts (Asahina 1996) |
Stryker, OP-1® Available for Medical Use |
| GDF-5 | Skeletal patterning and limb development (Hotten 1996) Enhanced bone formation in skeletal defects (Poehling 2006) |
Scil Technology, MD05® Phase II for Dental Use |
| PDGF | Increase osteoblast precursor population (Mitlak 1996) Induces osteoclastogenesis (Mitlak 1996) Induction of VEGF production (Cooke 2006) |
Osteohealth, GEM21S® Available for Dental Use |
| FGF-2 | Increase cell division and osteoblast precursor (Murakami 2003) Stimulation of angiogenesis (Montesano 1986) |
Kaken Pharm, Trafermin® Phase III for Dental Use |
| PTH | Osteoblast proliferation and differentiation (Pettway 2008) Increase engraftment of HSCs in new bone (Song 2010) |
Eli Lilly, Forteo® Available for Medical Use Phase I for Dental Use |
| IGF-I | Slightly mitogenic, enhances osteoblast function (Canalis 1980) Decrease osteoblast apoptosis (Canalis 2009) Induces osteoclastogenesis via RANKL (Mochizuki 1992) Induction of VEGFa (Akeno 2002) |
Tercica, Mecasermin® Available for Medical Use |
| GH | Induction of IGF-I production by the liver (Olney 2003) Promotes lipolysis and protein synthesis (Olney 2003) |
Novo Nordisk, SimpleXx® Phase II for Frature Repair |
| VEGF | Regulation of angiogenesis (Kanczler 2008) HSC and monocyte recruitment, bone formation (Kanczler 2008) |
VEGF165 Plasmid/Protein Phase III for Medical Use |
Contributor Information
Erica L Scheller, University of Michigan DDS/PhD Student, Department of Biologic and Materials Sciences, 1011 N University Ave, Ann Arbor MI 48109.
Paul H Krebsbach, University of Michigan, Department of Biologic and Materials Sciences, 1011 N University Ave, Ann Arbor MI 48109.
References
- 1.Urist MR. Bone: formation by autoinduction. Science (New York, NY. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 2.Pilcher E. The History of Dental Implants. 2010. [Google Scholar]
- 3.Kawai T, Mieki A, Ohno Y, Umemura M, Kataoka H, Kurita S, et al. Osteoinductive activity of composites of bone morphogenetic protein and pure titanium. Clinical orthopaedics and related research. 1993:296–305. [PubMed] [Google Scholar]
- 4.Andreana S, Beneduce C, Buhite R. Implant success rate in dental school setting: retrospective study. The New York state dental journal. 2008;74:67–70. [PubMed] [Google Scholar]
- 5.Romeo E, Lops D, Margutti E, Ghisolfi M, Chiapasco M, Vogel G. Long-term survival and success of oral implants in the treatment of full and partial arches: a 7-year prospective study with the ITI dental implant system. The International journal of oral & maxillofacial implants. 2004;19:247–259. [PubMed] [Google Scholar]
- 6.Krech S, McNeill JR, Merchant C. Encyclopedia of world environmental history. Vol. 3. Routledge: 2004. [Google Scholar]
- 7.Thomas DB, Smith CM, Sumpster JM. The proliferation of transplanted haematopoietic cells derived from bone marrow and foetal liver. Journal of anatomy. 1970;107:194–195. [PubMed] [Google Scholar]
- 8.Gatti RA, Meuwissen HJ, Allen HD, Hong R, Good RA. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968;2:1366–1369. doi: 10.1016/s0140-6736(68)92673-1. [DOI] [PubMed] [Google Scholar]
- 9.Metcalf D. Concise review: hematopoietic stem cells and tissue stem cells: current concepts and unanswered questions. Stem cells (Dayton, Ohio) 2007;25:2390–2395. doi: 10.1634/stemcells.2007-0544. [DOI] [PubMed] [Google Scholar]
- 10.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem cells (Dayton, Ohio) 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 11.Granero-Molto F, Weis JA, Longobardi L, Spagnoli A. Role of mesenchymal stem cells in regenerative medicine: application to bone and cartilage repair. Expert opinion on biological therapy. 2008;8:255–268. doi: 10.1517/14712598.8.3.255. [DOI] [PubMed] [Google Scholar]
- 12.Senn N. On the Healing of Aseptic Bone Cavities by Implantation of Antiseptic Decalcified Bone. Annals of surgery. 1889;10:352–368. doi: 10.1097/00000658-188907000-00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levander G. A study of bone regeneration. Surg Gynecol Obstet. 1938;67:705–714. [Google Scholar]
- 14.Dimitriou R, Giannoudis PV. Discovery and development of BMPs. Injury. 2005;36 (Suppl 3):S28–33. doi: 10.1016/j.injury.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 15.Rosen V, Wozney JM, Wang EA, Cordes P, Celeste A, McQuaid D, et al. Purification and molecular cloning of a novel group of BMPs and localization of BMP mRNA in developing bone. Connective tissue research. 1989;20:313–319. doi: 10.3109/03008208909023902. [DOI] [PubMed] [Google Scholar]
- 16.Celeste AJ, Iannazzi JA, Taylor RC, Hewick RM, Rosen V, Wang EA, et al. Identification of transforming growth factor beta family members present in bone-inductive protein purified from bovine bone. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:9843–9847. doi: 10.1073/pnas.87.24.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubler NR, Reuther JF, Faller G, Kirchner T, Ruppert R, Sebald W. Inductive properties of recombinant human BMP-2 produced in a bacterial expression system. International journal of oral and maxillofacial surgery. 1998;27:305–309. doi: 10.1016/s0901-5027(05)80621-6. [DOI] [PubMed] [Google Scholar]
- 18.Edgar CM, Chakravarthy V, Barnes G, Kakar S, Gerstenfeld LC, Einhorn TA. Autogenous regulation of a network of bone morphogenetic proteins (BMPs) mediates the osteogenic differentiation in murine marrow stromal cells. Bone. 2007;40:1389–1398. doi: 10.1016/j.bone.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosen V. BMP2 signaling in bone development and repair. Cytokine & growth factor reviews. 2009;20:475–480. doi: 10.1016/j.cytogfr.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Solmesky LJ, Abekasis M, Bulvik S, Weil M. Bone morphogenetic protein signaling is involved in human mesenchymal stem cell survival in serum-free medium. Stem cells and development. 2009;18:1283–1292. doi: 10.1089/scd.2009.0020. [DOI] [PubMed] [Google Scholar]
- 21.NIH. ClinicalTrials.gov. US National Institutes of Health; 2010. [Google Scholar]
- 22.Hotten GC, Matsumoto T, Kimura M, Bechtold RF, Kron R, Ohara T, et al. Recombinant human growth/differentiation factor 5 stimulates mesenchyme aggregation and chondrogenesis responsible for the skeletal development of limbs. Growth factors (Chur, Switzerland) 1996;13:65–74. doi: 10.3109/08977199609034567. [DOI] [PubMed] [Google Scholar]
- 23.Poehling S, Pippig SD, Hellerbrand K, Siedler M, Schutz A, Dony C. Superior effect of MD05, beta-tricalcium phosphate coated with recombinant human growth/differentiation factor-5, compared to conventional bone substitutes in the rat calvarial defect model. Journal of periodontology. 2006;77:1582–1590. doi: 10.1902/jop.2006.050328. [DOI] [PubMed] [Google Scholar]
- 24.Mitlak BH, Finkelman RD, Hill EL, Li J, Martin B, Smith T, et al. The effect of systemically administered PDGF-BB on the rodent skeleton. J Bone Miner Res. 1996;11:238–247. doi: 10.1002/jbmr.5650110213. [DOI] [PubMed] [Google Scholar]
- 25.Chang PC, Seol YJ, Cirelli JA, Pellegrini G, Jin Q, Franco LM, et al. PDGF-B gene therapy accelerates bone engineering and oral implant osseointegration. Gene therapy. 2010;17:95–104. doi: 10.1038/gt.2009.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canalis E. Growth factor control of bone mass. Journal of cellular biochemistry. 2009;108:769–777. doi: 10.1002/jcb.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murakami S, Takayama S, Kitamura M, Shimabukuro Y, Yanagi K, Ikezawa K, et al. Recombinant human basic fibroblast growth factor (bFGF) stimulates periodontal regeneration in class II furcation defects created in beagle dogs. Journal of periodontal research. 2003;38:97–103. doi: 10.1034/j.1600-0765.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- 28.Akagawa Y, Kubo T, Koretake K, Hayashi K, Doi K, Matsuura A, et al. Initial bone regeneration around fenestrated implants in Beagle dogs using basic fibroblast growth factor-gelatin hydrogel complex with varying biodegradation rates. Journal of prosthodontic research. 2009;53:41–47. doi: 10.1016/j.jpor.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Lee IS, Cui FZ, Choi SH. The biocompatibility of nanostructured calcium phosphate coated on micro-arc oxidized titanium. Biomaterials. 2008;29:2025–2032. doi: 10.1016/j.biomaterials.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Pettway GJ, Meganck JA, Koh AJ, Keller ET, Goldstein SA, McCauley LK. Parathyroid hormone mediates bone growth through the regulation of osteoblast proliferation and differentiation. Bone. 2008;42:806–818. doi: 10.1016/j.bone.2007.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song J, Kiel MJ, Wang Z, Wang J, Taichman RS, Morrison SJ, et al. An in vivo model to study and manipulate the hematopoietic stem cell niche. Blood. 2010;115:2592–2600. doi: 10.1182/blood-2009-01-200071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schneider A, Taboas JM, McCauley LK, Krebsbach PH. Skeletal homeostasis in tissue-engineered bone. J Orthop Res. 2003;21:859–864. doi: 10.1016/S0736-0266(03)00042-1. [DOI] [PubMed] [Google Scholar]
- 33.Akeno N, Robins J, Zhang M, Czyzyk-Krzeska MF, Clemens TL. Induction of vascular endothelial growth factor by IGF-I in osteoblast-like cells is mediated by the PI3K signaling pathway through the hypoxia-inducible factor-2alpha. Endocrinology. 2002;143:420–425. doi: 10.1210/endo.143.2.8639. [DOI] [PubMed] [Google Scholar]
- 34.Mochizuki H, Hakeda Y, Wakatsuki N, Usui N, Akashi S, Sato T, et al. Insulin-like growth factor-I supports formation and activation of osteoclasts. Endocrinology. 1992;131:1075–1080. doi: 10.1210/endo.131.3.1505451. [DOI] [PubMed] [Google Scholar]
- 35.Fintini D, Brufani C, Cappa M. Profile of mecasermin for the long-term treatment of growth failure in children and adolescents with severe primary IGF-1 deficiency. Therapeutics and clinical risk management. 2009;5:553–559. doi: 10.2147/tcrm.s6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olney RC. Regulation of bone mass by growth hormone. Medical and pediatric oncology. 2003;41:228–234. doi: 10.1002/mpo.10342. [DOI] [PubMed] [Google Scholar]
- 37.Tran GT, Pagkalos J, Tsiridis E, Narvani AA, Heliotis M, Mantalaris A, et al. Growth hormone: does it have a therapeutic role in fracture healing? Expert opinion on investigational drugs. 2009;18:887–911. doi: 10.1517/13543780902893069. [DOI] [PubMed] [Google Scholar]
- 38.Raschke M, Rasmussen MH, Govender S, Segal D, Suntum M, Christiansen JS. Effects of growth hormone in patients with tibial fracture: a randomised, double-blind, placebo-controlled clinical trial. European journal of endocrinology/European Federation of Endocrine Societies. 2007;156:341–351. doi: 10.1530/EJE-06-0598. [DOI] [PubMed] [Google Scholar]
- 39.Khan SA, Kanis JA, Vasikaran S, Kline WF, Matuszewski BK, McCloskey EV, et al. Elimination and biochemical responses to intravenous alendronate in postmenopausal osteoporosis. J Bone Miner Res. 1997;12:1700–1707. doi: 10.1359/jbmr.1997.12.10.1700. [DOI] [PubMed] [Google Scholar]
- 40.Luckman SP, Hughes DE, Coxon FP, Graham R, Russell G, Rogers MJ. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13:581–589. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 41.Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, et al. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J Bone Miner Res. 1995;10:1478–1487. doi: 10.1002/jbmr.5650101008. [DOI] [PubMed] [Google Scholar]
- 42.Deal C. Future therapeutic targets in osteoporosis. Current opinion in rheumatology. 2009;21:380–385. doi: 10.1097/BOR.0b013e32832cbc2a. [DOI] [PubMed] [Google Scholar]
- 43.Henriksen DB, Alexandersen P, Hartmann B, Adrian CL, Byrjalsen I, Bone HG, et al. Disassociation of bone resorption and formation by GLP-2: a 14-day study in healthy postmenopausal women. Bone. 2007;40:723–729. doi: 10.1016/j.bone.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 44.Bone HG, McClung MR, Roux C, Recker RR, Eisman JA, Verbruggen N, et al. Odanacatib, a Cathepsin-K Inhibitor for Osteoporosis: A Two-Year Study in Postmenopausal Women With Low Bone Density. J Bone Miner Res. 2009 doi: 10.1359/jbmr.091035. [DOI] [PubMed] [Google Scholar]
- 45.Reid IR, Brown JP, Burckhardt P, Horowitz Z, Richardson P, Trechsel U, et al. Intravenous zoledronic acid in postmenopausal women with low bone mineral density. The New England journal of medicine. 2002;346:653–661. doi: 10.1056/NEJMoa011807. [DOI] [PubMed] [Google Scholar]
- 46.Kanczler JM, Oreffo RO. Osteogenesis and angiogenesis: the potential for engineering bone. European cells & materials. 2008;15:100–114. doi: 10.22203/ecm.v015a08. [DOI] [PubMed] [Google Scholar]
- 47.Trueta J, Buhr AJ. The Vascular Contribution to Osteogenesis. V. the Vasculature Supplying the Epiphysial Cartilage in Rachitic Rats. J Bone Joint Surg Br. 1963;45:572–581. [PubMed] [Google Scholar]
- 48.Kaigler D, Krebsbach PH, Polverini PJ, Mooney DJ. Role of vascular endothelial growth factor in bone marrow stromal cell modulation of endothelial cells. Tissue engineering. 2003;9:95–103. doi: 10.1089/107632703762687573. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR, et al. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. The Journal of clinical investigation. 2007;117:1616–1626. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaigler D, Krebsbach PH, Wang Z, West ER, Horger K, Mooney DJ. Transplanted endothelial cells enhance orthotopic bone regeneration. Journal of dental research. 2006;85:633–637. doi: 10.1177/154405910608500710. [DOI] [PubMed] [Google Scholar]
- 51.Bouletreau PJ, Warren SM, Spector JA, Peled ZM, Gerrets RP, Greenwald JA, et al. Hypoxia and VEGF up-regulate BMP-2 mRNA and protein expression in microvascular endothelial cells: implications for fracture healing. Plastic and reconstructive surgery. 2002;109:2384–2397. doi: 10.1097/00006534-200206000-00033. [DOI] [PubMed] [Google Scholar]
- 52.Kaigler D, Krebsbach PH, West ER, Horger K, Huang YC, Mooney DJ. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. Faseb J. 2005;19:665–667. doi: 10.1096/fj.04-2529fje. [DOI] [PubMed] [Google Scholar]
- 53.Huang YC, Kaigler D, Rice KG, Krebsbach PH, Mooney DJ. Combined angiogenic and osteogenic factor delivery enhances bone marrow stromal cell-driven bone regeneration. J Bone Miner Res. 2005;20:848–857. doi: 10.1359/JBMR.041226. [DOI] [PubMed] [Google Scholar]
- 54.Kaigler D, Wang Z, Horger K, Mooney DJ, Krebsbach PH. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J Bone Miner Res. 2006;21:735–744. doi: 10.1359/jbmr.060120. [DOI] [PubMed] [Google Scholar]
- 55.Montesano R, Vassalli JD, Baird A, Guillemin R, Orci L. Basic fibroblast growth factor induces angiogenesis in vitro. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:7297–7301. doi: 10.1073/pnas.83.19.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cooke JW, Sarment DP, Whitesman LA, Miller SE, Jin Q, Lynch SE, et al. Effect of rhPDGF-BB delivery on mediators of periodontal wound repair. Tissue engineering. 2006;12:1441–1450. doi: 10.1089/ten.2006.12.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 58.Chang MK, Raggatt LJ, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, et al. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 2008;181:1232–1244. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- 59.Stanford CM. Surface modification of biomedical and dental implants and the processes of inflammation, wound healing and bone formation. International journal of molecular sciences. 2010;11:354–369. doi: 10.3390/ijms11010354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gaston MS, Noble BS, Simpson AHRW. Deficiency of the Specific Immune System Enhances Fracture Repair In-Vivo. Journal of Bone and Joint Surgery - British Volume. 2008;90-B:365. [Google Scholar]
- 61.Takayanagi H. Inflammatory bone destruction and osteoimmunology. Journal of periodontal research. 2005;40:287–293. doi: 10.1111/j.1600-0765.2005.00814.x. [DOI] [PubMed] [Google Scholar]
- 62.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460:259–263. doi: 10.1038/nature08099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sasano Y, Mizoguchi I, Takahashi I, Kagayama M, Saito T, Kuboki Y. BMPs induce endochondral ossification in rats when implanted ectopically within a carrier made of fibrous glass membrane. The Anatomical record. 1997;247:472–478. doi: 10.1002/(SICI)1097-0185(199704)247:4<472::AID-AR5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 64.Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury. 2005;36:1392–1404. doi: 10.1016/j.injury.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 65.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42:606–615. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pounder NM, Harrison AJ. Low intensity pulsed ultrasound for fracture healing: a review of the clinical evidence and the associated biological mechanism of action. Ultrasonics. 2008;48:330–338. doi: 10.1016/j.ultras.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 67.Scheven BA, Shelton RM, Cooper PR, Walmsley AD, Smith AJ. Therapeutic ultrasound for dental tissue repair. Medical hypotheses. 2009;73:591–593. doi: 10.1016/j.mehy.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 68.Patel MS, Elefteriou F. The new field of neuroskeletal biology. Calcified tissue international. 2007;80:337–347. doi: 10.1007/s00223-007-9015-3. [DOI] [PubMed] [Google Scholar]
- 69.Sato T, Arai M, Goto S, Togari A. Effects of Propranolol on Bone Metabolism in Spontaneously Hypertensive Rats. The Journal of pharmacology and experimental therapeutics. 2010 doi: 10.1124/jpet.110.167643. [DOI] [PubMed] [Google Scholar]
- 70.Ripa RS, Wang Y, Jorgensen E, Johnsen HE, Hesse B, Kastrup J. Intramyocardial injection of vascular endothelial growth factor-A165 plasmid followed by granulocyte-colony stimulating factor to induce angiogenesis in patients with severe chronic ischaemic heart disease. European heart journal. 2006;27:1785–1792. doi: 10.1093/eurheartj/ehl117. [DOI] [PubMed] [Google Scholar]
- 71.Devin JK, Vaughan DE, Blevins LS, Jr, Chen Q, Covington J, Verity DK, et al. Low-dose growth hormone administration mobilizes endothelial progenitor cells in healthy adults. Growth Horm IGF Res. 2008;18:253–263. doi: 10.1016/j.ghir.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 72.Lan J, Wang Z, Wang Y, Wang J, Cheng X. The effect of combination of recombinant human bone morphogenetic protein-2 and basic fibroblast growth factor or insulin-like growth factor-I on dental implant osseointegration by confocal laser scanning microscopy. Journal of periodontology. 2006;77:357–363. doi: 10.1902/jop.2006.050016. [DOI] [PubMed] [Google Scholar]
- 73.Gauthier JY, Chauret N, Cromlish W, Desmarais S, Duong le T, Falgueyret JP, et al. The discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K. Bioorganic & medicinal chemistry letters. 2008;18:923–928. doi: 10.1016/j.bmcl.2007.12.047. [DOI] [PubMed] [Google Scholar]
- 74.Bodine PV, Stauffer B, Ponce-de-Leon H, Bhat RA, Mangine A, Seestaller-Wehr LM, et al. A small molecule inhibitor of the Wnt antagonist secreted frizzled-related protein-1 stimulates bone formation. Bone. 2009;44:1063–1068. doi: 10.1016/j.bone.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 75.Scheller EL, Krebsbach PH, Kohn DH. Tissue engineering: state of the art in oral rehabilitation. Journal of oral rehabilitation. 2009;36:368–389. doi: 10.1111/j.1365-2842.2009.01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scheller EL, Krebsbach PH. Gene therapy: design and prospects for craniofacial regeneration. Journal of dental research. 2009;88:585–596. doi: 10.1177/0022034509337480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lustig B, Behrens J. The Wnt signaling pathway and its role in tumor development. Journal of cancer research and clinical oncology. 2003;129:199–221. doi: 10.1007/s00432-003-0431-0. [DOI] [PubMed] [Google Scholar]